Abstract
Purpose
The number of long-term survivors after hematopoietic stem-cell transplantation (HSCT) for malignant and nonmalignant disorders is increasing, and late effects are gaining importance. Osteoporosis and fractures can worsen the quality of life of HSCT survivors, but the burden of the disease is unknown.
Patients and Methods
We conducted a retrospective study of patients older than age 18 years who underwent an HSCT at The University of Texas MD Anderson Cancer Center from January 1, 1997, to December 31, 2011, and were observed until December 31, 2013, to ascertain occurrence of fractures. Cumulative incidence rates of fractures were calculated with death as a competing risk. Age- and sex-specific incidence rates per person-year of fracture were compared with those of the US general population by using estimated rates from the 1994 National Health Interview Survey and the 2004 National Hospital Discharge Survey.
Results
A total of 7,620 patients underwent an HSCT from 1997 to 2011 at the MD Anderson Cancer Center of whom 602 (8%) developed a fracture. Age, underlying disease, and HSCT type were significantly associated with fracture. Age- and sex-specific fracture incidence rates after HSCT were significantly greater than those of the US general population in almost all subgroups. The striking difference was an approximately eight times greater risk in females and approximately seven to nine times greater risk in males age 45 to 64 years old when compared with the National Health Interview Survey and National Hospital Discharge Survey fracture rates.
Conclusion
The incidence of fractures is compellingly higher after HSCT.
INTRODUCTION
In the last two decades, the number of long-term survivors after autologous and allogeneic hematopoietic stem-cell transplantations (HSCTs) has been increasing. This increase has resulted in more people suffering from the long-term effects of HSCT and its associated treatment.1,2 Bone loss leading to fractures is one such late effect, which can lead to significant morbidity and mortality and worsen the quality of life of long-term survivors after HSCT.3,4 Factors that may contribute to the increased bone loss in patients after HSCT include intensive chemotherapy, total-body irradiation, and post-transplantation glucocorticoid use.5,6
The process of bone remodeling in the context of HSCT is a complex interplay between two cytokines belonging to the tumor necrosis factor family, the receptor activator of the nuclear factor kappa B (RANK) ligand, and osteoprotegerin. Osteoblasts produce the RANK ligand, which binds to RANK that is expressed on osteoclast cell surfaces, leading to bone resorption. Osteoblasts also produce a receptor called osteoprotegerin, which inhibits the interaction between RANK and the RANK ligand and thus decreases osteoclastic activity and enhances bone formation. An imbalance between these two processes is thought to be a major factor driving bone loss after HSCT.7
The temporal sequence of bone loss after HSCT is also complex. An early phase of bone mineral density (BMD) loss occurs within 6 to 12 months after transplantation at all skeletal sites. This is followed by initial recovery of BMD in the lumbar spine and a slower process of recovery in the femur neck, with bone loss persisting for 48 to 120 months. Not all patients return to their baseline BMD level, probably as a result of continued risk exposure and prolonged treatment with glucocorticoids.8 It should also be noted that BMD loss in HSCT patients does not always correlate with the risk of developing a fracture.
Transplantation of solid organs (eg, kidney, heart, liver, and lung) has also been associated with rapid loss in BMD and increased susceptibility to osteoporotic fragility fractures.9–12 Unlike bone loss after HSCT, bone loss associated with solid organ transplantation occurs mainly during the first year but is followed by recovery within the next year or two.13,14 Fracture incidence following solid organ transplantation ranges from 6% to 45% for recipients of kidney transplantation and from 22% to 42% for recipients of heart, lung, and liver transplantations.15 Both bone disease before transplantation and bone loss after transplantation as a result of the effects of immunosuppressive medications have been postulated to be involved in bone disease after solid organ transplantation. HSCT has many risk factors similar to those in recipients of solid organ transplantation; however, the magnitude of fracture in patients receiving HSCT remains largely unknown.16,17
The temporal sequence of bone loss following HSCT has been established, and it is well accepted that HSCT and its associated treatment lead to increased bone loss and osteoporosis. Patients also have associated comorbidities, lifestyle factors, and genetic predispositions that may increase the risk of fractures that may adversely affect the quality of life. Despite this, little is known of the incidence of fractures following HSCT. Therefore, the purpose of this study was to calculate the incidence of fractures, identify risk factors, and compare the rates of fractures following HSCT to the rates of fractures in the general population. Addressing this gap in knowledge would illustrate the true burden of fractures associated with HSCT and provide a better understanding of the risk of such fractures.
PATIENTS AND METHODS
Institutional review board approval was obtained before any data were collected for this study. The use of patient information complied with the Health Insurance Portability and Accountability Act, and sensitive patient data were protected in the data analysis.
Patients
We performed a retrospective chart review of patients older than age 18 years who had undergone HSCT at The University of Texas MD Anderson Cancer Center from January 1, 1997, to December 31, 2011. Patients were observed until December 31, 2013, for ascertainment of fracture occurrence. HSCT patients were identified by using billing codes, and HSCT was confirmed with electronic medical record documentation. Once this cohort of HSCT patients was identified, we ran another query, using International Classification of Diseases, 9th revision (ICD-9 codes 800 to 829, 733.10 to 733.19, 733.81, and 733.82) to identify patients in this group who had experienced a fracture. All fractures were verified by using both physician medical record documentation and radiographic reports and assessment and their location was documented. Information on each patient's age at the time of transplantation, sex, race, type of HSCT, and underlying indication for receiving an HSCT was obtained from the electronic medical records.
Data Analysis
Descriptive statistics such as mean plus or minus standard deviation were used to summarize patient's age at the time of HSCT. Frequencies and percentages were used for categorical variables. χ2 and Fisher's exact tests were used to compare categorical variables by location of fracture. Cumulative incidence rate of bone fracture after transplantation was calculated with death as a competing risk (Fig 1). A Cox proportional hazards regression model was used to model the cause-specific hazard of fracture (death treated as censored) and compare event rates among groups. The effects of covariates on the cumulative incidence function of fracture were evaluated in the univariable setting by using Gray's test.18 In the multivariable setting, Fine and Gray's method was used to model the probability subdistribution function of failure by applying decreasing weights to patients who died before experiencing a fracture.19,20 Validity of the proportional cause-specific hazards and subdistribution hazards assumptions were assessed by using the proportionality test on time-varying covariates.
Fig 1.
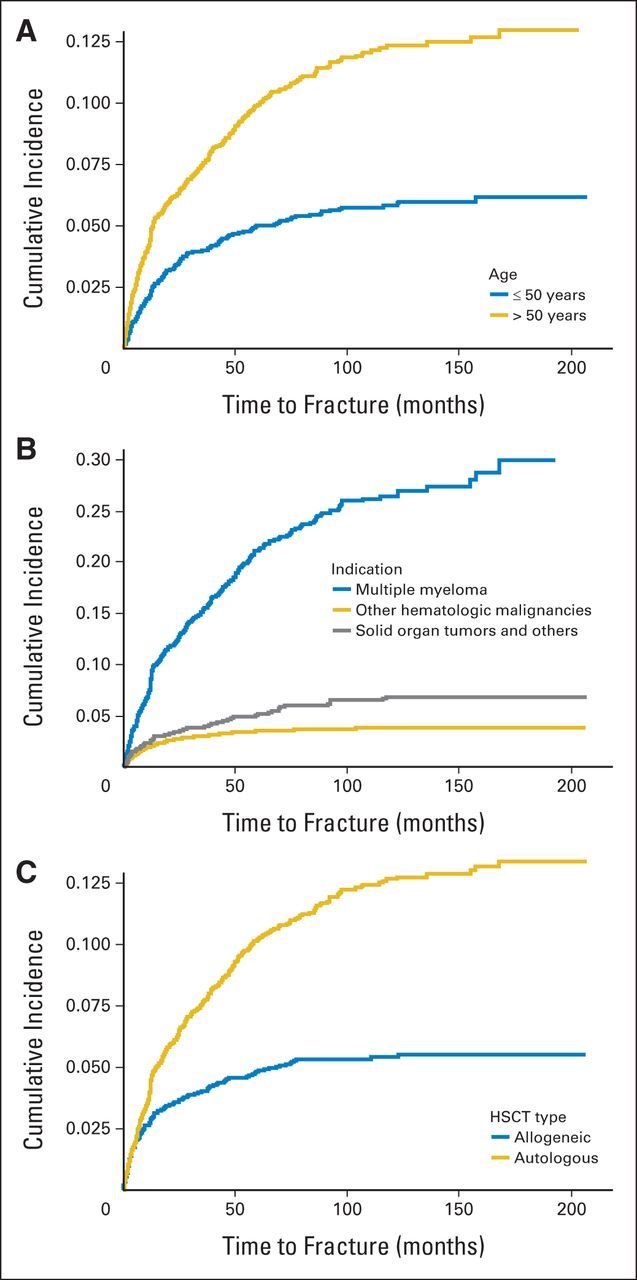
Cumulative incidence of fracture (A) by age at the time of hematopoietic stem-cell transplantation (HSCT), (B) by underlying malignancy, and (C) by type of HSCT.
Age- and sex-specific incidence rates per person-year of fracture were calculated by using the observed fracture frequency in the numerator and the sum of the survival times in the denominator. The time units were defined in years from the time of HSCT until the first fracture, death, or the last date of retrospective follow-up (December 31, 2013). Patients who did not experience a fracture and were alive were censored at the end of the follow-up period of the study.
We compared the rates of fractures with those of the US general population by using estimated rates from the 1994 National Health Interview Survey (NHIS) and the 2004 National Hospital Discharge Survey (NHDS). The NHIS is a personal interview survey of households that uses a nationwide multistage sample of 89,100 persons designed to represent the civilian noninstitutionalized population of the United States in which fractures were self-reported.17 The NHDS is a national probability survey designed to meet the need for information on the characteristics of 270,000 inpatients discharged from nonfederal short-stay hospitals in the United States. Information was gathered from medical transcriptions of hospital records. We chose to use NHDS estimates from the year 2004 (midpoint of our study time frame).21
The rates of fractures in the US general population estimated in the 1994 NHIS and in the 2004 NHDS were multiplied with the total person-years of observation to estimate the expected number of fractures. The ratio of observed and expected numbers of fractures was used to compare the number of fractures in our HSCT patient sample with that in the national surveys of the general population. Statistical significance was determined by using a two-sided P value of less than .05. Data were stored in a Microsoft Excel worksheet (Microsoft, Redmond, WA), and all analyses were conducted by using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Of 7,620 patients who underwent an HSCT from 1997 to 2011, 56% were male and a majority (75%) were white. The most common reason for undergoing an HSCT was a hematologic malignancy other than multiple myeloma (67%), followed by multiple myeloma (22%). Eleven percent (n = 801) underwent an HSCT for a primary solid tumor (most commonly breast [46%] or ovarian [27%]); other reasons for HSCT were diseases such as scleroderma and amyloidosis. Overall 8% (n = 602) developed a fracture: 11% of patients (n = 419) who received an autologous transplant and 5% of those (n = 183) who received an allogeneic transplant. The baseline demographic and transplant characteristics are provided in Table 1. More than 50% (n = 4,033) died before experiencing a fracture and 39% (n = 2,985) were censored at the end of the follow-up period of the study. The median follow-up time was 85 months (95% CI, 82 to 87 months).
Table 1.
Characteristics of HSCT Patients (N = 7,620)
| Characteristic | No. | % |
|---|---|---|
| Age at the time of HSCT, years | ||
| Mean ± SD | 49.3 ± 13.5 | |
| ≤ 50 | 3,623 | 47.5 |
| > 50 | 3,997 | 52.5 |
| Sex | ||
| Female | 3,393 | 44.5 |
| Male | 4,227 | 55.5 |
| Race | ||
| Asian | 179 | 2.3 |
| Black | 618 | 8.1 |
| Hispanic | 1,032 | 13.5 |
| White | 5,718 | 75.0 |
| Other/unknown | 73 | 1.0 |
| Indication for HSCT | ||
| Multiple myeloma | 1,685 | 22.1 |
| Hematologic malignancy other than multiple myeloma | 5,134 | 67.4 |
| Solid tumor and other | 801 | 10.5 |
| Type of HSCT | ||
| Autologous | 3,891 | 51.1 |
| Allogeneic | 3,729 | 48.9 |
Abbreviations: HSCT, hematopoietic stem-cell transplantation; SD, standard deviation.
Univariable cause-specific hazard models showed that age older than 50 years at the time of HSCT, multiple myeloma, solid organ tumors and other reasons, and autologous HSCT were associated with a higher hazard of developing a fracture. Because of a high correlation between indication for HSCT and the type of HSCT received, we considered analyzing their effects separately by using two multivariable models. Model 1 included all predictors of interest except type of HSCT, and model 2 included all but indication. Holding all other variables constant, the incidence of fracture was higher in patients older than age 50 years (the median age) than those who were younger in both models. Compared with patients with other hematologic malignancies, the hazards of fracture among patients with multiple myeloma are five time higher and they are 1.6 time higher among patients with solid organ and other tumors. Furthermore, model 2 shows that patients who underwent autologous transplantation are 45% more likely to develop a fracture than those who underwent an allogeneic transplantation, holding other variables constant (Table 2). The results of the univariable and multivariable Cox proportional subdistribution hazards models were in agreement with the cause-specific hazard models for fracture (Table 3). We explored an interaction between age and sex in all models and observed no interaction at a significance level of 0.05 (P > .40 for both cause-specific and subdistribution hazard models).
Table 2.
Cause-Specific Hazard Model for Fracture Among HSCT Patients (death treated as a censoring event)
| Parameter | Univariable |
Multivariable |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
||||||||||||||
| Estimate | SE | P | HR | 95% CI | Estimate | SE | P | HR | 95% CI | Estimate | SE | P | HR | 95% CI | |
| Age at time of HSCT, years | |||||||||||||||
| ≤ 50 | Ref | Ref | Ref | ||||||||||||
| > 50 | 0.76 | 0.09 | < .001 | 2.13 | 1.79 to 2.53 | 0.31 | 0.09 | .001 | 1.36 | 1.13 to 1.64 | 0.73 | 0.09 | < .001 | 2.07 | 1.74 to 2.47 |
| Sex | |||||||||||||||
| Female | Ref | Ref | Ref | ||||||||||||
| Male | 0.11 | 0.08 | .184 | 1.12 | 0.95 to 1.31 | 0.04 | 0.09 | .622 | 1.04 | 0.88 to 1.23 | 0.09 | 0.08 | .277 | 1.09 | 0.93 to 1.29 |
| Race | |||||||||||||||
| White | Ref | .159* | Ref | .655* | Ref | .153* | |||||||||
| Asian | −0.31 | 0.32 | .328 | 0.73 | 0.39 to 1.37 | −0.29 | 0.32 | .36 | 0.75 | 0.40 to 1.40 | −0.18 | 0.32 | .584 | 0.84 | 0.45 to 1.57 |
| Black | 0.30 | 0.13 | .024 | 1.35 | 1.04 to 1.76 | −0.10 | 0.14 | .474 | 0.91 | 0.70 to 1.18 | 0.27 | 0.13 | .046 | 1.31 | 1.01 to 1.70 |
| Hispanic | 0.09 | 0.12 | .442 | 1.10 | 0.87 to 1.39 | 0.06 | 0.12 | .614 | 1.06 | 0.84 to 1.35 | 0.21 | 0.12 | .086 | 1.23 | 0.97 to 1.56 |
| Other/unknown | 0.10 | 0.50 | .842 | 1.11 | 0.41 to 2.96 | 0.42 | 0.50 | .402 | 1.53 | 0.57 to 4.09 | 0.18 | 0.50 | .722 | 1.20 | 0.45 to 3.20 |
| Indication for HSCT | |||||||||||||||
| Hematologic malignancies other than multiple myeloma | Ref | < .001* | Ref | < .001* | |||||||||||
| Multiple myeloma | 1.69 | 0.09 | < .001 | 5.41 | 4.52 to 6.46 | 1.61 | 0.10 | < .001 | 5.00 | 4.14 to 6.03 | |||||
| Solid tumor and others | 0.41 | 0.16 | .01 | 1.51 | 1.10 to 2.06 | 0.47 | 0.17 | .005 | 1.60 | 1.16 to 2.21 | |||||
| Type of HSCT | |||||||||||||||
| Allogeneic | Ref | Ref | |||||||||||||
| Autologous | 0.45 | 0.09 | < .001 | 1.57 | 1.32 to 1.87 | 0.37 | 0.09 | < .001 | 1.45 | 1.22 to 1.73 | |||||
Abbreviations: HR, hazard ratio; HSCT, hematopoietic stem-cell transplantation; Ref, reference group.
P value for overall effects.
Table 3.
Subdistribution Hazard Model for Fracture Among HSCT Patients
| Parameter | Univariable |
Multivariable |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
||||||||||||||
| Estimate | SE | P | HR | 95% CI | Estimate | SE | P | HR | 95% CI | Estimate | SE | P | HR | 95% CI | |
| Age at time of HSCT, years | |||||||||||||||
| ≤ 50 | Ref | Ref | Ref | ||||||||||||
| > 50 | 0.72 | 0.09 | < .001 | 2.06 | 1.73 to 2.44 | 0.26 | 0.09 | .006 | 1.30 | 1.08 to 1.56 | 0.66 | 0.09 | < .001 | 1.94 | 1.63 to 2.31 |
| Sex | |||||||||||||||
| Female | Ref | Ref | Ref | ||||||||||||
| Male | 0.07 | 0.08 | .413 | 1.07 | 0.91 to 1.26 | 0.02 | 0.09 | .779 | 1.02 | 0.87 to 1.21 | 0.08 | 0.08 | .355 | 1.08 | 0.92 to 1.27 |
| Race | |||||||||||||||
| White | Ref | .116* | Ref | .797* | Ref | .218* | |||||||||
| Asian | −0.30 | 0.32 | .347 | 0.74 | 0.40 to 1.38 | −0.25 | 0.31 | .417 | 0.78 | 0.42 to 1.43 | −0.17 | 0.32 | .581 | 0.84 | 0.45 to 1.56 |
| Black | 0.32 | 0.13 | .016 | 1.38 | 1.06 to 1.79 | −0.11 | 0.14 | .413 | 0.90 | 0.69 to 1.17 | 0.24 | 0.13 | .071 | 1.27 | 0.98 to 1.66 |
| Hispanic | 0.06 | 0.12 | .632 | 1.06 | 0.84 to 1.34 | 0.06 | 0.12 | .634 | 1.06 | 0.84 to 1.34 | 0.19 | 0.12 | .119 | 1.21 | 0.95 to 1.53 |
| Other/unknown | −0.30 | 0.51 | .554 | 0.74 | 0.27 to 2.00 | 0.01 | 0.52 | .981 | 1.01 | 0.37 to 2.81 | −0.18 | 0.51 | .72 | 0.83 | 0.30 to 2.27 |
| Indication for HSCT | |||||||||||||||
| Hematologic malignancies other than multiple myeloma | Ref | < .001* | Ref | < .001* | |||||||||||
| Multiple myeloma | 1.93 | 0.09 | < .001 | 6.89 | 5.76 to 8.24 | 1.87 | 0.10 | < .001 | 6.50 | 5.37 to 7.86 | |||||
| Solid tumor and others | 0.53 | 0.16 | < .001 | 1.70 | 1.24 to 2.32 | 0.58 | 0.16 | < .001 | 1.79 | 1.29 to 2.46 | |||||
| Type of HSCT | |||||||||||||||
| Allogeneic | Ref | Ref | |||||||||||||
| Autologous | 0.80 | 0.09 | < .001 | 2.24 | 1.88 to 2.66 | 0.74 | 0.09 | < .001 | 2.09 | 1.75 to 2.49 | |||||
Abbreviations: HR, hazard ratio; HSCT, hematopoietic stem-cell transplantation; Ref, reference group.
P value for overall effects.
Age- and sex-specific fracture incidence rates in the US population from the NHIS 1994 and NHDS 2004 were used for comparison (Table 4). The female HSCT recipients age 45 to 64 years at MD Anderson Cancer Center had 7,565 person-years of observation. The estimated relative risk of fracture for that group was approximately eight times higher than in the general US female population (NHIS 1994 and NHDS 2004) of the same age. Similarly, male recipients age 45 to 64 years at MD Anderson Cancer Center had 8,693 person-years of observation, and the estimated relative risk of fracture was approximately seven to nine times higher in these male HSCT recipients than in the general US male population (NHIS 1994 and NHDS 2004) of the same age (Table 5). The age groups older than 65 years differed between the NHIS (age 65 to 69 years; no data for those older than age 70 years) and NHDS (age 65 to 74 and 75 to 84 years) groups; thus, the rates of fractures in those age groups in our study population differed as well. We observed a significantly higher risk of fracture in those receiving HSCT than in the US general population in both males and females in all age groups, except for males age 18 to 24 years, when using NHIS estimates.
Table 4.
Age- and Sex-Specific Fracture Rates in the US Population
| Age (years) | Females | Males |
|---|---|---|
| NHIS 1994* | ||
| 18-24 | 1.8 | 10.3 |
| 25-44 | 3.1 | 6.5 |
| 45-64 | 3 | 2.9 |
| 65-69 | 4.6 | 2.8 |
| NHDS 2004† | ||
| 18-24 | 12.8 | 44.8 |
| 25-44 | 14.2 | 32.3 |
| 45-64 | 30.1 | 39.6 |
| 65-74 | 108 | 60.1 |
| 75-84 | 249.6 | 138.8 |
Abbreviations: NHDS, National Hospital Discharge Survey; NHIS, National Health Interview Survey.
Age- and sex-specific fracture incidence rates in the US population; data from the National Center for Health Statistics, NHIS 1994. Rates are per 1,000 members of the population.
Age- and sex-specific fracture-related discharge rates in the US population; data from the 2004 NHDS. Rates are per 10,000 members of the population.
Table 5.
Age- and Sex-Specific Fracture Incidence Rates Among HSCT Patients Compared With NHIS and NHDS Estimates
| Age at Transplantation (years) | Comparison With NHIS Estimates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Females |
Males |
|||||||||
| Person-Years at Risk | Observed No. of Fractures | Fracture Incidence Rate per 1,000 Population | Expected No. of Fractures | Estimated Relative Risk | Person-Years at Risk | Observed No. of Fractures | Fracture Incidence Rate per 1,000 Population | Expected No. of Fractures | Estimated Relative Risk | |
| 18-24 | 787.9 | 3 | 3.8 | 1.4 | 2.1 | 966.3 | 8 | 8.3 | 10.0 | 0.8 |
| 25-44 | 4,136.7 | 45 | 10.9 | 12.8 | 3.5 | 3,969.6 | 41 | 10.3 | 25.8 | 1.6 |
| 45-64 | 7,565.1 | 182 | 24.1 | 22.7 | 8.0 | 8,693.6 | 227 | 26.1 | 25.2 | 9.0 |
| 65-69 | 623.7 | 22 | 35.3 | 2.9 | 7.7 | 1,146.7 | 42 | 36.6 | 3.2 | 13.1 |
| 70+ | 313.4 | 8 | 25.5 | — | — | 537.9 | 24 | 44.6 | — | — |
NOTE. Sex-specific fracture incidence rates for age > 70 years were not available in the National Health Interview Survey (NHIS).
Abbreviations: HSCT, hematopoietic stem-cell transplantation; NHDS, National Hospital Discharge Survey.
Of the 602 patients who experienced a fracture, there were slightly more vertebral (53%; n = 315) than nonvertebral (47%; n = 287) fractures. Nonvertebral fractures comprised clavicle/ribs (18%), upper limb (10%), femur (7%), lower limb other than femoral (7%), hip (3%), sacrum (1%), and other locations (1%). Males had more vertebral (57%) than nonvertebral fractures, and females tended to have more nonvertebral (53%) than vertebral fractures (χ2 = 6.14; P = .013). The majority of patients who experienced a fracture were older than age 50 years at the time of HSCT and had more vertebral fractures (54%); those age 50 years or younger were more likely to experience nonvertebral fractures (52%), but this difference was not statistically significant (χ2 = 2.17; P = .14). Of the 3,623 patients who were age 50 years or younger at the time of HSCT, 54% underwent an allogeneic transplantation. Of the 3,997 patients who were older than age 50 years, 44% underwent an allogeneic transplantation. Patients who had an autologous transplantation tended to have more vertebral fractures (54%), whereas those who underwent an allogeneic transplantation tended to have an equal number of vertebral and nonvertebral fractures, but this difference was not statistically significant (χ2 = 0.72; P = .40). Of the 372 patients with multiple myeloma who experienced a fracture, 65.3% had active disease or relapse, 13.2% were in complete remission, and 21.5% were in partial remission with low or stable disease at the time of fracture. Of the 180 patients with other hematologic malignancies who experienced a fracture, 66.6% were in complete remission and 33.3% had active disease or relapse at the time of fracture.
DISCUSSION
As the use of HSCT for the treatment of malignant and nonmalignant disease and the consequent number of long-term survivors have increased, the early and late complications of HSCT have gained attention. We discovered an increased risk of fracture at almost all ages in both males and females compared with the corresponding US general population. To the best of our knowledge, this study is the first to quantify the increased incidence of fractures in patients with cancer who undergo HSCT.
Loss of BMD following HSCT can be attributable to multiple factors, including myeloablative conditioning regimens, altered functioning of organs leading to a reduced intake and metabolism of calcium and vitamin D, high-dose steroids, graft-versus-host disease, and the use of cyclosporine A.22,23 In one study, a decrease of up to 25% in lumbar spine BMD and a 50% decrease in femoral neck BMD were observed after HSCT, and the bone loss after HSCT was shown to occur early and appeared to progress over the first 3 years before stabilizing.4
In addition to bone loss as a result of factors related to the primary disease and treatment modalities used, comorbid conditions may predispose patients to further bone loss and an increased risk of developing fractures such as sedentary lifestyle after the transplantation when coping with frequent infections, malabsorption, and graft-versus-host disease. Comorbid conditions were not assessed in our study to calculate fracture incidence but nevertheless need to be considered when evaluating risk factors for fracture development. Furthermore, an in vitro study showed a long-lasting decreased bone-forming capacity in patients who received stem-cell transplantation.24 Our study found significantly higher fracture rates following HSCT than in the general population. This is similar to the increased rates of fractures observed in patients undergoing solid organ (eg, kidney, liver and heart) transplantation.16,17 This similarity suggests that transplantation and the associated supportive therapies administered may play a key role in this increased risk of fracture.
We compared age- and sex-specific fracture rates of HSCT patients to those of the general population by using two separate databases (NHIS and NHDS). The rates of fractures differed somewhat between these databases, but this could be attributed to the mechanisms involved in obtaining the rates. The 1994 NHIS is a personal self-report survey and the NHDS contains data collected from hospital records. There are some differences in the types of fractures included in both databases compared with types included in our study (ie, our study used more ICD-9 codes to identify fractures). We may have observed exaggerated relative risks in older age groups compared with the NHIS estimates, but this maybe attributable to potential under-reporting of the self-reported fractures in older patients. Our fracture estimates were obtained by using a large cohort of 7,620 patients who underwent HSCT, but the true incidence may have been higher, because patients presenting to our center for treatment may have received care for their fractures at another institution. We could not control for this potential failure to capture all fractures because of the retrospective nature of our study. Nevertheless, we are confident that our estimates are close to the true rate of fractures because of the large sample size and long follow-up times observed in our study (median follow-up, 85 months). The possibility of misclassification of fractures in our study is low, because we assessed the medical records of all patients and rechecked and confirmed the findings with those of clinician notes and/or radiographic reports. There are multiple risk factors such as patient demographics, clinical factors related to the primary diagnosis, the treatment received, and comorbid conditions that need to be considered when evaluating treatment options for patients undergoing HSCT who present with evidence of bone loss. Although we could not address all these factors, our study does have some highlights. We observed that males and females seem to have a similar risk of fractures following HSCT, and no interaction was observed between age and sex. Being older than age 50 years at the time of transplantation and receiving an autologous transplantation place patients at a greater risk of having a fracture. Because 4,033 patients (53%) in the study cohort died before they experienced a fracture, simply censoring them would lead to a biased estimation of cumulative incidence. We conducted a competing risk analysis by using subdistribution hazard models with death as a competing risk for fracture in addition to the analyses done by using the Cox proportional hazards models (ie, cause-specific models). The hazard ratios differed slightly, but the conclusions did not change. Future large-scale prospective studies are imperative for identifying patients at high risk for developing fractures following HSCT so that screening and treatment can be instituted early on.
In conclusion, the incidence rate of fractures is significantly higher in patients who undergo HSCT than in the US general population. Patients undergoing or planning to undergo HSCT should have their bone health assessed early in their treatment and, if indicated, should start preventative therapy to prevent bone loss and fractures. All patients receiving an HSCT should be considered to be at risk for post-transplantation bone loss, because the risk factors for post-transplantation bone loss are still poorly identified. All patients should be counseled about current general preventative measures for bone loss and fractures such as physical exercise, fall prevention, and vitamin D and calcium supplementation. All patients should be encouraged to avoid tobacco and minimize excessive alcohol intake. We also recommend that patients undergoing an HSCT should have a dual energy x-ray absorptiometry scan performed at baseline and at 6 months following transplantation. A comprehensive assessment of risk factors involved in fracture development following HSCT still remains necessary.
Footnotes
Presented as a poster at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Xerxes N. Pundole, Heather Lin, Richard E. Champlin, Huifang Lu
Financial support: Huifang Lu
Administrative support: Richard E. Champlin
Provision of study materials or patients: Richard E. Champlin
Collection and assembly of data: Xerxes N. Pundole
Data analysis and interpretation: Xerxes N. Pundole, Andrea G. Barbo, Heather Lin
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Increased Incidence of Fractures in Recipients of Hematopoietic Stem-Cell Transplantation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Xerxes N. Pundole
No relationship to disclose
Andrea G. Barbo
No relationship to disclose
Heather Lin
No relationship to disclose
Richard E. Champlin
Consulting or Advisory Role: Takeda Pharmaceuticals, Celgene, Amgen, Alexion Pharmaceuticals, AiCuris, Actinium Pharmaceuticals
Huifang Lu
No relationship to disclose
REFERENCES
- 1.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClune BL, Majhail NS. Osteoporosis after stem cell transplantation. Curr Osteoporos Rep. 2013;11:305–310. doi: 10.1007/s11914-013-0180-1. [DOI] [PubMed] [Google Scholar]
- 3.Ebeling PR. Approach to the patient with transplantation-related bone loss. J Clin Endocrinol Metab. 2009;94:1483–1490. doi: 10.1210/jc.2009-0205. [DOI] [PubMed] [Google Scholar]
- 4.Serio B, Pezzullo L, Fontana R, et al. Accelerated bone mass senescence after hematopoietic stem cell transplantation. Transl Med UniSa. 2013;5:7–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Ebeling PR, Thomas DM, Erbas B, et al. Mechanisms of bone loss following allogeneic and autologous hemopoietic stem cell transplantation. J Bone Miner Res. 1999;14:342–350. doi: 10.1359/jbmr.1999.14.3.342. [DOI] [PubMed] [Google Scholar]
- 6.Yao S, McCarthy PL, Dunford LM, et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant. 2008;41:393–398. doi: 10.1038/sj.bmt.1705918. [DOI] [PubMed] [Google Scholar]
- 7.Lustberg MB, Reinbolt RE, Shapiro CL. Bone health in adult cancer survivorship. J Clin Oncol. 2012;30:3665–3674. doi: 10.1200/JCO.2012.42.2097. [DOI] [PubMed] [Google Scholar]
- 8.Tauchmanovà L, Colao A, Lombardi G, et al. Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2007;92:4536–4545. doi: 10.1210/jc.2006-2870. [DOI] [PubMed] [Google Scholar]
- 9.Leidig-Bruckner G, Hosch S, Dodidou P, et al. Frequency and predictors of osteoporotic fractures after cardiac or liver transplantation: A follow-up study. Lancet. 2001;357:342–347. doi: 10.1016/S0140-6736(00)03641-2. [DOI] [PubMed] [Google Scholar]
- 10.Krieg MA, Seydoux C, Sandini L, et al. Intravenous pamidronate as treatment for osteoporosis after heart transplantation: A prospective study. Osteoporos Int. 2001;12:112–116. doi: 10.1007/s001980170142. [DOI] [PubMed] [Google Scholar]
- 11.Pichette V, Bonnardeaux A, Prudhomme L, et al. Long-term bone loss in kidney transplant recipients: A cross-sectional and longitudinal study. Am J Kidney Dis. 1996;28:105–114. doi: 10.1016/s0272-6386(96)90138-9. [DOI] [PubMed] [Google Scholar]
- 12.Spira A, Gutierrez C, Chaparro C, et al. Osteoporosis and lung transplantation: A prospective study. Chest. 2000;117:476–481. doi: 10.1378/chest.117.2.476. [DOI] [PubMed] [Google Scholar]
- 13.Henderson NK, Sambrook PN, Kelly PJ, et al. Bone mineral loss and recovery after cardiac transplantation. Lancet. 1995;346:905. doi: 10.1016/s0140-6736(95)92748-4. [DOI] [PubMed] [Google Scholar]
- 14.Julian BA, Laskow DA, Dubovsky J, et al. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med. 1991;325:544–550. doi: 10.1056/NEJM199108223250804. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan V, Ranganna KM, Chauhan N, et al. Bone disease in organ transplant patients: Pathogenesis and management. Postgrad Med. 2012;124:80–90. doi: 10.3810/pgm.2012.05.2551. [DOI] [PubMed] [Google Scholar]
- 16.Edwards BJ, Desai A, Tsai J, et al. Elevated incidence of fractures in solid-organ transplant recipients on glucocorticoid-sparing immunosuppressive regimens. J Osteoporos. 2011;2011:591793. doi: 10.4061/2011/591793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey-Goldman R, Dunn JE, Dunlop DD, et al. Increased risk of fracture in patients receiving solid organ transplants. J Bone Miner Res. 1999;14:456–463. doi: 10.1359/jbmr.1999.14.3.456. [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Health data interactive report: Hospital discharges by first- and any-listed diagnosis: US, 1990-2010. http://205.207.175.93/HDI/TableViewer/tableView.aspx?ReportId=537.
- 22.Hautmann AH, Elad S, Lawitschka A, et al. Metabolic bone diseases in patients after allogeneic hematopoietic stem cell transplantation: Report from the Consensus Conference on Clinical Practice in Chronic Graft-Versus-Host Disease. Transpl Int. 2011;24:867–879. doi: 10.1111/j.1432-2277.2011.01264.x. [DOI] [PubMed] [Google Scholar]
- 23.Weilbaecher KN. Mechanisms of osteoporosis after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2000;6:165–174. doi: 10.1016/s1083-8791(00)70039-5. [DOI] [PubMed] [Google Scholar]
- 24.Tauchmanovà L, Serio B, Del Puente A, et al. Long-lasting bone damage detected by dual-energy x-ray absorptiometry, phalangeal osteosonogrammetry, and in vitro growth of marrow stromal cells after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2002;87:5058–5065. doi: 10.1210/jc.2002-020800. [DOI] [PubMed] [Google Scholar]


