Abstract
Purpose
R1507 is a selective, fully human, recombinant monoclonal antibody (immunoglobulin G1 subclass) against insulin-like growth factor-1 receptor (IGF-1R). The strong preclinical evidence supporting coinhibition of IGF-1R and epidermal growth factor receptor (EGFR) as anticancer therapy prompted this study.
Patients and Methods
Patients with advanced-stage non–small-cell lung cancer (NSCLC) with progression following one or two prior regimens, Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, and measurable disease were eligible. Patients were randomly assigned to receive erlotinib (150 mg orally once a day) in combination with either placebo, R1507 9 mg/kg weekly, or R1507 16 mg/kg intravenously once every 3 weeks. Treatment cycles were repeated every 3 weeks. The primary end point was comparison of the 12-week progression-free survival (PFS) rate.
Results
In all, 172 patients were enrolled: median age, 61 years; female, 33%; never-smokers, 12%; and performance status 0 or 1, 88%. The median number of R1507 doses was six for the weekly arm and 3.5 for the every-3-weeks arm. Grades 3 to 4 adverse events occurred in 37%, 44%, and 48% of patients with placebo, R1507 weekly, and R1507 every 3 weeks, respectively. The 12-week PFS rates were 39%, 37%, and 44%, and the median overall survival was 8.1, 8.1, and 12.1 months for the three groups, respectively, with statistically nonsignificant hazard ratios. The 12-week PFS rate in patients with KRAS mutation was 36% with R1507 compared with 0% with placebo.
Conclusion
The combination of R1507 with erlotinib did not provide PFS or survival advantage over erlotinib alone in an unselected group of patients with advanced NSCLC. Predictive biomarkers are essential for further development of combined inhibition of IGF-1R and EGFR.
INTRODUCTION
Erlotinib, an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase, is used for the treatment of patients with advanced non–small-cell lung cancer (NSCLC) with progression following one or two prior chemotherapy regimens. This is based on a phase III study that demonstrated improved overall survival with erlotinib over placebo in this setting.1 The molecular determinants of sensitivity to erlotinib include primarily the EGFR mutation status but also the KRAS genotype.2,3 Regardless of the extent of initial response to erlotinib, patients invariably develop resistance. One well-described mechanism of resistance is the activation of the insulin-like growth factor-1 receptor (IGF-1R) pathway.4,5
The IGF-1R signaling pathway plays an important role in various aspects of neoplastic transformation, including cell proliferation, differentiation, and apoptosis.6 Therefore, it has emerged as a novel target for the treatment of cancer. Several studies have demonstrated an interaction between IGF-1R and EGFR signaling. Activation of the IGF-1R pathway has been noted as a consequence of EGFR inhibition in a variety of NSCLC cell lines, leading to cellular proliferation and evasion of apoptosis.7 Furthermore, coinhibition of EGFR and IGF-1R resulted in synergistic growth inhibition of H1299 NSCLC xenografts in vivo compared with treatment with erlotinib alone.8 Studies have also documented heterodimerization of EGFR and IGF-1R in response to stimulation with either EGF or IGF-1, the ligands for the two receptors.9 In addition, transphosphorylation of EGFR, mediated by IGF-1R is another mechanism of resistance to gefitinib.10 Taken together, combined inhibition of EGFR and IGF-1R is a rational approach to overcome resistance and enhance the efficacy of EGFR inhibitors in patients with NSCLC.
R1507 is a fully human immunoglobulin G1–type monoclonal antibody against IGF-1R. It binds to the extracellular domain of IGF-1R with high selectivity and inhibits receptor activation and function.11 It has demonstrated anticancer activity against a variety of cancers including NSCLC in preclinical models.12,13 In a phase I study of R1507,14 weekly administration at 9 mg/kg was tolerated well without any dose-limiting toxicity. Two patients with Ewing sarcoma achieved partial responses. Notably, only two of the 37 patients developed hyperglycemia, and neither was of grade 3 or 4 severity. An alternate schedule of R1507 at 16 mg/kg given every 3 weeks has also demonstrated safety without dose-limiting toxicity or drug-related serious adverse events.15 In preclinical studies, the combination of R1507 and erlotinib resulted in enhanced growth inhibition and induction of apoptosis compared with either agent alone.12 Cell lines with high levels of total IGF-1R and higher gene copy numbers were moderately sensitive to R1507 alone.12 On the basis of these preclinical data, we conducted a randomized phase II study of erlotinib in combination with either R1507 or placebo in patients with advanced-stage NSCLC.
PATIENTS AND METHODS
The study was designed to compare the efficacy of erlotinib in combination with placebo or one of two schedules of R1507 (weekly or every 3 weeks). Patients were randomly assigned in a 1:1 ratio to either the weekly or the every-3-weeks schedule on an open-label basis. Subsequently, they were further randomly assigned in a 2:1 ratio to receive either R1507 or placebo on each schedule in a blinded manner (Fig 1).
Fig 1.
CONSORT diagram for treatment assignment. R1507, selective, fully human, recombinant monoclonal antibody against insulin-like growth factor-1 receptor.
Inclusion Criteria
Patients with stage IIIB or IV NSCLC (American Joint Committee on Cancer [AJCC] TNM staging system, version 6) who had progressed following one or two prior chemotherapy regimens were included. Other salient inclusion criteria were availability of archived formalin-fixed tumor tissue, age older than 18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 to 2, presence of measureable disease, hemoglobin A1c less than 7%, life expectancy more than 12 weeks, serum creatinine less than 1.5 × institutional upper limit of normal (ULN), absolute neutrophil count ≥ 1.5 × 109/L, platelet count ≥ 100 × 109/L, serum hemoglobin ≥ 9 g/dL, serum bilirubin ≤ 1.5 × ULN, serum aminotransferases ≤ 2.5 × ULN (in case of liver metastasis, ≤ 5 × ULN), serum calcium ≤ 1.1 × ULN, serum international normalized ratio (INR), partial thromboplastin time ≤ 1.5 × ULN (unless on anticoagulant therapy), and willingness to sign informed consent. Patients with brain metastasis were eligible if the lesions remained stable for at least 4 weeks after radiotherapy. Prior therapy with an EGFR- or IGF-1R–targeted agent was not allowed. At least 3 weeks should have elapsed after prior chemotherapy and 2 weeks after prior surgery. A 4-week interval after prior radiotherapy or 2-week interval after radiotherapy to extrathoracic bony sites was required. Patients with history of allogeneic bone marrow transplantation or organ transplantation, ophthalmic abnormalities of the surface of the eye, another active cancer, or concurrent serious illness were excluded. Patients receiving high-dose therapy with corticosteroids (defined as > 20 mg/d of dexamethasone or equivalent for > 7 days) were excluded. Pregnant and lactating women were not included. Men and women of reproductive age were required to use contraception. Patients unable to ingest oral medications because of dysphagia or other GI disorders were excluded. The study protocol was approved by the institutional review board at each participating site.
Study Treatments
Erlotinib was given at 150 mg/d on a continuous daily schedule. R1507 was given at 9 mg/kg/wk or 16 mg/kg every 3 weeks as a 60- to 90-minute intravenous infusion. Matching placebo was used for both schedules. Treatment cycles were repeated every 21 days. Study therapy was continued until progression of disease, unacceptable toxicity, withdrawal of informed consent, or development of serious intercurrent illness. Routine use of premedications was not required. Patients were allowed to take appropriate supportive care measures for management of symptoms of disease and toxicity. Patients receiving anticoagulation with warfarin were monitored closely for changes in INR or changed to low-molecular-weight heparin therapy.
Toxicity was graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. For toxicity related to R1507, the dose of the drug was held for grades 3 or 4 events until recovery to grade 2 or lower, up to a maximum of 4 weeks. Dose was recalculated for R1507 only if the patient's body weight changed by 10% or more. The dose of erlotinib was decreased by 50 mg/d decrements for toxicity up to a maximum of two dose reductions. For grades 1 and 2 toxicities, appropriate supportive care measures were initiated. In the event of grade 3 toxicity, erlotinib was withheld until recovery to grade 1 or lower (grade 2 or lower for skin toxicity) and reintroduced at a lower dose. For patients with skin rash requiring a dose reduction, re-escalation was allowed after optimal control of symptoms. If any of the drugs was withheld, the other agent was continued according to study calendar. Patients requiring more than 4 weeks hold for either of the two agents were removed from study therapy.
Study Assessments
All screening tests including radiographic studies for baseline tumor assessment were performed within 4 weeks before registration. Baseline assessments included vital signs, history and physical examination, performance status, CBC, serum chemistry (blood urea nitrogen, creatinine, bilirubin, aminotransferases, alkaline phosphatase, partial thromboplastin time, INR, calcium, electrolytes, and albumin), concomitant medications, and a 12-lead ECG. Within 7 days before registration, patients underwent urinalysis, fasting blood glucose, and hemoglobin A1c assessment. Serum pregnancy test was done for women of reproductive age. Computed tomography scan or magnetic resonance imaging was done for measurement of disease at baseline. Tumor response was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.16 Archived tumor samples were required from all patients.
After initiation of study therapy, CBC and blood chemistry tests were done every week for the first two cycles and then on day 1 of each cycle thereafter. Radiographic tumor assessments were performed every 6 weeks, regardless of the study therapy schedule, and the same method that was used for baseline assessment was performed at each instance. Physical examination was done on day 1 of each cycle. A poststudy visit was done 30 days after the last dose of treatment and included vital signs, history and physical examination, CBC, blood chemistry, and assessment of PS. To assess compliance with erlotinib, pill counts were done by the study team at the end of each cycle. For patients with controlled diabetes mellitus, hemoglobin A1c was measured every 12 weeks during the study.
KRAS and EGFR Mutation Evaluation
Macrodissection of the archived tumor tissues was performed on formalin-fixed paraffin-embedded samples. Tumor DNA was extracted from these samples by using QIAamp DNA Mini Kit (Qiagen, Santa Clarita, CA). Exons 1 and 2 of the KRAS gene and exons 18 to 21 of the EGFR gene were amplified by polymerase chain reaction by using nested primers and 5 ng template DNA. DNA was treated with uracil N-glycosylase to reduce artifacts that may occur during formalin fixation of the tumor tissues and to prevent carryover contaminations. Polymerase chain reaction products were purified and subjected to Big Dye Terminator cycle sequencing reactions, which were then analyzed on ABI3730XL DNA Analyzers (Vernon Hills, IL). Sequencing results were evaluated by using Phred Phrap Polyphred software and reviewed visually in the sequence viewer Consed. Multiple independent products were sequenced on both strands of each amplicon.
Statistical Methods
The primary end point of the study was to determine the 12-week progression-free survival (PFS) rate for each treatment arm. The median PFS with erlotinib was expected to be 2.5 months, and the desired PFS for the study arms was 3.3 months, corresponding to a hazard ratio of 0.76. This would correspond to a 12-week PFS rate of 43% in the control arm and 53% in the treatment arms. The type I (one-sided) and type II error rates were set at 10% with the probability of making the correct decision at 75%. The estimated sample size was 150 patients, with at least 43 evaluable patients required for each arm study. This included a projected rate of 14% for patients who were not evaluable. The secondary end points included assessment of overall survival, PFS, response rate, and toxicity for the three arms of the study. A safety analysis was conducted after six patients were treated with both erlotinib and R1507 for at least 21 days to evaluate for safety and overall tolerability of the combination. All time-dependent secondary end points were summarized by using the Kaplan-Meier survival curves. All treated patients were included in the analysis. Radiographic assessments were done by the investigator.
RESULTS
This multicenter study was conducted in sites across in the United States, Canada, Europe, and Australia. Patients were enrolled from November 2008 until June 2009. Of the 172 patients enrolled, one patient randomly assigned to the weekly R1507 arm did not initiate study therapy.
Baseline Characteristics
The patient characteristics were even between the three arms of the study. Approximately two thirds were male and more than 95% were white. There was a slightly higher proportion of never-smokers and patients with adenocarcinoma in the placebo arm (Table 1). Approximately 10% of the patients had a history of brain metastasis. A majority of the patients had one prior chemotherapy regimen before entry to the study. The most commonly used prior chemotherapy agents included the platinum compounds, taxanes, gemcitabine, and pemetrexed. Less than 10% of the patients had received prior treatment with molecularly targeted agents such an antiangiogenic therapy. The median time from diagnosis of NSCLC to study entry was 10, 12, and 13 months for the placebo, weekly and every-3-weeks R1507 arms, respectively.
Table 1.
Patient Baseline Characteristics
| Parameter | Placebo(n = 57) |
R1507 (weekly;n = 57) |
R1507 (every 3 weeks;n = 57) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Female | 20 | 35 | 18 | 32 | 19 | 33 |
| Median age, years | 62 | 63 | 62 | |||
| Never-smoker | 9 | 16 | 8 | 14 | 5 | 9 |
| Race/ethnicity | ||||||
| White | 55 | 96 | 55 | 96 | 56 | 98 |
| Black | 1 | 2 | 1 | 2 | 1 | 2 |
| Hispanic | 1 | 2 | 1 | 2 | — | |
| Histology | ||||||
| Adenocarcinoma | 36 | 63 | 26 | 46 | 25 | 44 |
| Squamous cell carcinoma | 12 | 21 | 15 | 26 | 16 | 28 |
| Other | 9 | 16 | 16 | 28 | 16 | 28 |
| Stage IV | 46 | 81 | 50 | 88 | 50 | 88 |
| No. of prior regimens | ||||||
| 1 | 43 | 75 | 44 | 77 | 39 | 68 |
| 2 | 14 | 25 | 13 | 23 | 18 | 32 |
| KRAS mutation | 8 | 19 | 16 | 27 | 12 | 36 |
| EGFR mutation | 3 | 2 | 1 | |||
Abbreviation: R1507, selective, fully human, recombinant monoclonal antibody against insulin-like growth factor-1 receptor.
Treatment Delivery and Safety
The median number of doses of R1507 administered in the weekly and every-3-weeks arms were six (range, three to 11) and four (range, one to 22), respectively. For the placebo arm, 6.5 (range, two to 54) and two (range, one to 19) cycles were administered for the two arms, respectively. The median dose intensity of erlotinib was 150 mg/d in the placebo arm and 146.78 mg/d in the R1507 arms.
The overall incidence of adverse events was higher with the combination regimen (Table 2). Treatment discontinuation due to adverse events was more common with R1507, which accounted for seven and 10 patients in the weekly and every-3-weeks arms compared with two patients with placebo. The adverse events leading to discontinuation of study treatment with R1507 are described in Table 3. Deaths related to adverse events occurred in one, three, and five patients, respectively in the placebo, weekly, and every-3-weeks R1507 arms, respectively. Serious adverse events were noted in eight, 18, and 14 patients, respectively, in the three arms. Disease progression was the most common reason behind discontinuation of study therapy with 47, 38, and 42 patients on the placebo, weekly, and every-3-weeks R1507 arms, respectively. Notably, hyperglycemia was observed in only six patients treated with R1507.
Table 2.
Adverse Events*
| Adverse Event | Placebo(n = 57) |
R1507(weekly; n = 57) |
R1507(every 3 weeks; n = 57) |
P† |
||||
|---|---|---|---|---|---|---|---|---|
| All Grades | Grades 3 to 4 | All Grades | Grades 3 to 4 | All Grades | Grades 3 to 4 | All Grades | Grades 3 to 4 | |
| Anemia | 9 | 5 | 3 | 2 | 4 | — | .12 | .06 |
| Anorexia | 7 | — | 4 | 4 | 9 | 3 | .34 | .14 |
| Back pain | 7 | 4 | 2 | — | 5 | — | .23 | .03 |
| Constipation | 4 | — | 10 | — | 5 | — | .16 | 1.0 |
| Cough | 5 | — | 7 | — | 11 | — | .25 | 1.0 |
| Deep vein thrombosis | — | — | 7 | 5 | 2 | — | .01 | .01 |
| Dehydration | 4 | — | 5 | 2 | 7 | — | .72 | .33 |
| Diarrhea | 18 | 2 | 36 | 5 | 32 | 2 | .002 | .51 |
| Dyspepsia | 4 | — | 7 | — | 4 | 2 | .66 | .33 |
| Dyspnea | 9 | 7 | 2 | 2 | 7 | 4 | .08 | .24 |
| Fatigue | 20 | 7 | 37 | 13 | 40 | 7 | < .001 | .26 |
| Hyperglycemia | — | — | 2 | 2 | 4 | 4 | .17 | .17 |
| Mucositis | 5 | — | 5 | — | 11 | — | .19 | 1.0 |
| Muscle spasms | 4 | — | 8 | — | 7 | — | .56 | 1.0 |
| Nausea | 13 | — | 12 | 5 | 12 | — | 1.0 | .01 |
| Paronychia | 2 | — | 7 | — | 5 | — | .27 | 1.0 |
| Skin rash | 33 | 7 | 32 | 8 | 51 | 11 | < .001 | .64 |
| Stomatitis | 5 | — | 10 | 2 | 14 | — | .08 | .33 |
| Vomiting | 11 | — | 7 | 3 | 5 | — | .29 | .11 |
Abbreviation: R1507, selective, fully human, recombinant monoclonal antibody against insulin-like growth factor-1 receptor.
Adverse events reported in > 5% for any grade and > 3% for grades 3 to 4 toxicity.
Fisher's exact test.
Table 3.
Discontinuation of R1507 Because of Toxicity
| Event | Placebo |
R1507 (weekly) |
R1507 (every 3 weeks) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | Day of Onset* | No. of Patients | Day of Onset* | No. of Patients | Day of Onset* | |
| Any event | 2 | — | 7 | — | 10 | — |
| Infection | — | — | 2 | 5 | 2 | N/R |
| Respiratory failure | 1 | 4 | 1 | 2 | — | — |
| Pneumonitis | — | — | — | — | 1 | 16 |
| Dyspnea | — | — | — | — | 1 | 5 |
| Skin rash | — | — | 1 | 29 | 2 | 8,8 |
| Myocardial infarction | — | — | 1 | 94 | 1 | 6 |
| Stomatitis | — | — | 1 | 5 | — | |
| Failure to thrive | — | — | — | — | 1 | 52 |
| Lethargy | 1 | 35 | — | — | — | — |
| Gastroenteritis | — | — | 1 | 30 | — | — |
| Hemorrhage | — | — | — | 1 | 139 | |
| Declining overall condition | — | — | — | — | 1 | N/R |
Abbreviations: N/R, not reported; R1507, selective, fully human, recombinant monoclonal antibody against insulin-like growth factor-1 receptor.
Number of days since initiation of study therapy.
Efficacy
The 12-week PFS rate was 41% with erlotinib alone compared with 42% and 45%, respectively, on the weekly and every-3-weeks R1507 arms (Table 4). There was no statistically significant difference in the median PFS for patients on the two R1507 arms compared with placebo. The overall survival was 8.1, 8.1, and 12.1 months, respectively, with placebo, weekly, and every-3-weeks R1507, and the differences were not statistically significant. The objective response rates were also similar at 8.8%, 8.8%, and 7% for the three arms. The median duration of best response was 168, 120, and 164 days, respectively, with placebo, weekly, and every-3-weeks R1507. The median time to best response was 43, 66, and 85 days for the three treatment arms. The Kaplan-Meier curves for overall survival and PFS are represented in Figures 2A and 2B.
Table 4.
Efficacy
| Parameter | Erlotinib + Placebo(n = 57) |
Erlotinib + R1507 (weekly)(n = 57) |
Erlotinib + R1507 (every 3 weeks)(n = 57) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | 90% CI | % | HR | 90% CI | P | % | HR | 90% CI | P | |
| Response rate | 8.8 | 3.5 to 17.6 | 8.8 | 2.4 to 15.3 | 7 | 2.4 to 15.3 | ||||
| Stable disease rate | 40 | 29.4 to 52.1 | 40 | 29.4 to 52.1 | 49 | 37.6 to 60.7 | ||||
| 12-week PFS rate | 41 | 30 to 52 | 42 | 31 to 53 | 45 | 34 to 57 | ||||
| Median PFS, months | 1.5 | 1.45 to 2.91 | 1.87 | 1.41 to 2.91 | .67 | 2.7 | 2.1 to 3.9 | .73 | ||
| 1.09 | 0.79 to 1.50 | .66 | 0.93 | 0.68 to 1.29 | .73 | |||||
| Median OS, months | 8.1 | 4.8 to 10.3 | 8.1 | 6.0 to 10.0 | .48 | 12.1 | 7.8 to 15.2 | .04 | ||
| 0.84 | 0.58 to 1.21 | .43 | 0.72 | 0.53 to 0.99 | .09 | |||||
Abbreviations: HR, hazard ratio; OS, overall survival; PFS, progression-free survival; R1507, selective, fully human, recombinant monoclonal antibody against insulin-like growth factor-1 receptor.
Fig 2.
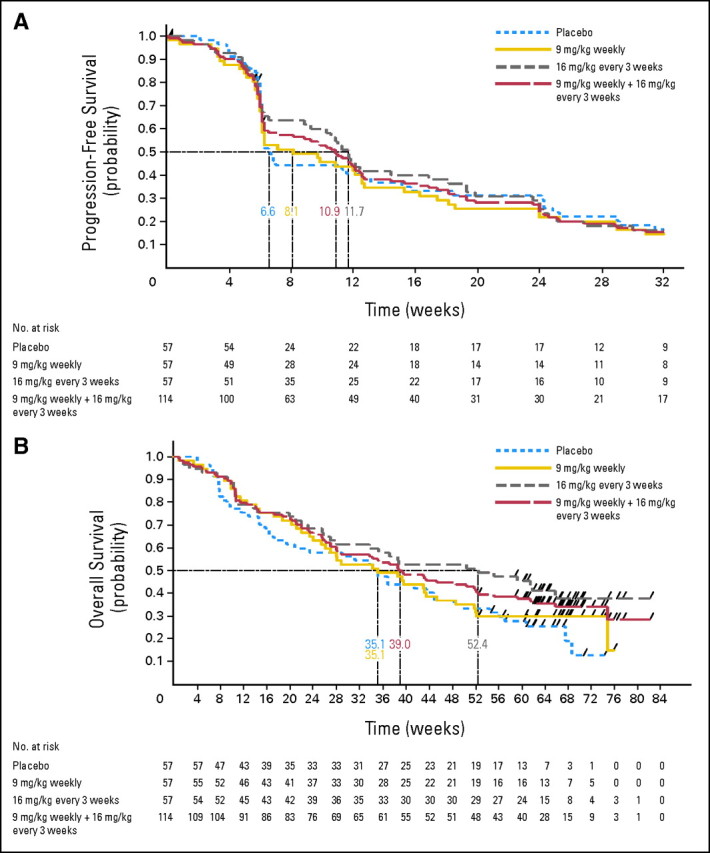
Kaplan-Meier curve for (A) progression-free survival and (B) overall survival.
KRAS and EGFR Mutation Analyses
KRAS genotype was analyzed in 132 tumor DNA samples. The overall mutation rate was 27%. The mutation rate for the placebo, weekly, and every-3-weeks arms were 19%, 37%, and 26%, respectively. The 12-week PFS rate for patients with a KRAS mutation was 0% with erlotinib compared with 36% with the combination (P = .039; Table 5). Conversely, for patients with wild-type KRAS, the 12-week PFS rate was 37% with erlotinib and 28% with the combination, although the difference was not statistically significant (P = .45). EGFR mutations were analyzed in 126 tumor DNA samples, and mutations were detected in six patients (4.5%). Three of the six patients with EGFR mutations received erlotinib plus placebo. The PFS rate at 12 weeks for these three patients was 100%, whereas the PFS rate was 25% for patients with EGFR wild-type with erlotinib alone (P = .0064).
Table 5.
Efficacy by KRAS Status
| Treatment Group | KRAS Mutation Status | No. of Patients | 12-Week PFS Rate (%) | P |
|---|---|---|---|---|
| Placebo | Wild type | 35 | 37 | .0390 |
| Mutated | 8 | 0 | ||
| R1507 weekly | Wild type | 27 | 30 | .7436 |
| Mutated | 16 | 25 | ||
| R1507 every 3 weeks | Wild type | 34 | 27 | .1350 |
| Mutated | 12 | 50 |
Abbreviations: PFS, progression-free survival; R1507, selective, fully human, recombinant monoclonal antibody against insulin-like growth factor-1 receptor.
DISCUSSION
Targeting the insulin-like growth factor has emerged as a novel strategy for the treatment of cancer. A randomized phase II study conducted in patients with advanced-stage NSCLC demonstrated promising efficacy with the combination of figitumumab, a monoclonal antibody against IGF-1R, and platinum-based chemotherapy.17 Objective responses were noted with figitumumab monotherapy in patients who crossed over from the control arm. However, a subsequent randomized phase III study18 in patients with nonsquamous NSCLC failed to demonstrate an improvement in overall survival with the combination of figitumumab with carboplatin and paclitaxel.
R1507 is an active anticancer agent as demonstrated by objective responses in patients with Ewing sarcoma.14 On the basis of promising preclinical data, we conducted this randomized study to evaluate the combination of R1507 with erlotinib in patients with advanced NSCLC. In contrast to the phase III study of figitumumab, all histologic subtypes of NSCLC were eligible for this study. A majority of the patients had received only one prior treatment regimen for advanced-stage NSCLC and had a good PS. The efficacy parameters in the control arm were consistent with data reported with erlotinib alone in randomized studies in the second- and third-line treatment settings.19 However, there was no improvement in any of the salient efficacy parameters with the addition of R1507 to erlotinib. Although the median PFS and overall survival were slightly higher with the every-3-weeks R1507 arm, the differences did not reach statistical significance. On the basis of these results, R1507 is not being developed further.
The absence of therapeutic benefit with erlotinib in combination with an IGF-1R–targeted agent was further substantiated by the recent announcement regarding a phase III study with figitumumab.20 In this study, patients with nonsquamous NSCLC were randomly assigned to therapy with erlotinib alone or in combination with figitumumab. The study was closed early because of a lack of benefit and an imbalance in toxicity profile for the combination. Furthermore, a study21 in patients with colon cancer involving the combination of IMC-A12, another monoclonal antibody against IGF-1R, and cetuximab, an antibody to EGFR, failed to demonstrate sufficient benefit. It was designed to evaluate whether the addition of an IGF-1R antibody would overcome resistance or refractoriness to prior EGFR-targeted therapy.
All of the studies referenced here with IGF-1R–targeted agents have been tested in a broad group of patients without specific molecular selection. It is conceivable that the use of biomarkers could identify a sensitive subgroup of patients. Gualberto et al22 observed a favorable outcome with figitumumab for patients with high circulating levels of the insulin-like growth factor. In this study, the presence of KRAS mutation was associated with a poor outcome with erlotinib but was predictive of a favorable outcome with the combination. On a similar note, AMG 479, another IGF-1R antibody, improved the efficacy of chemotherapy in patients with pancreatic cancer, a tumor characterized by KRAS mutation.23 Stearns et al24 noted IGF-1 dependence in RAS-mutated prostate cancer cells for inducing secretion of vascular endothelial growth factor. This interaction has been hypothesized to contribute to tumor growth and progression, which could be a potential explanation for the observations in our study. Given the small number of patients and the exploratory nature of our analysis, this observation requires further validation. However, these findings suggest a role for targeting IGF-1R in KRAS-driven tumors. Biomarkers related to the IGF-1R signaling pathway, including IGF-1R expression in the baseline tumor cells and circulating levels of IGF-1 or IGF-2 and IGF-binding protein, are being studied from specimens obtained in our patients and will be reported separately. The lower prevalence of EGFR mutation in our study is probably due to the small number of never-smokers.
The consistent lack of efficacy with IGF-1R monoclonal antibodies in combination with erlotinib illustrates that further studies in unselected patient populations are not warranted. Ongoing studies25 are evaluating the use of small-molecule IGF-1R tyrosine kinase inhibitors in combination with erlotinib. It remains to be seen whether this strategy might result in a different outcome from that with monoclonal antibodies. Given the strong scientific rationale for the combination approach, we believe that a subset of tumors with IGF-1R–driven EGFR resistance might still benefit from the combination, and further efforts are required to identify them.
Footnotes
Presented as a poster at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011, and as an oral presentation at the 14th World Conference on Lung Cancer, Amsterdam, the Netherlands, July 3-7, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00760929
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: David Chen, F. Hoffmann-La Roche (C); Kai Habben, Roche (C); Lian Liu, Roche (C); Carrie M. Brownstein, F. Hoffmann-La Roche (C) Consultant or Advisory Role: Suresh S. Ramalingam, Roche (C), Genentech (C), Astellas Pharma (C); David R. Spigel, Roche (U); Jeffrey A. Engelman, Genentech (C); Claus-Peter Schneider, Roche (C), AstraZeneca (C), Boehringer Ingelheim (C); Wilfried E.E. Eberhardt, Roche (C), Boehringer Ingelheim (C), GlaxoSmithKline (C), Pfizer (C), Novartis (C), Bayer HealthCare Pharmaceuticals (C), AstraZeneca (C), Amgen (C), sanofi-aventis (C), Eli Lilly (C); Pasi A. Jänne, Boehringer Ingelheim (C), Roche (C), Genentech (C), Abbott (C), AstraZeneca (C), Pfizer (C); Martin Reck, F. Hoffmann-La Roche (C), Eli Lilly (C), AstraZeneca (C), Pfizer (C), Bristol-Myers Squibb (C), Merck (C) Stock Ownership: David Chen, F. Hoffmann-La Roche; Kai Habben, Roche; Pasi A. Jänne, Gatekeeper Pharmaceuticals; Carrie M. Brownstein, F. Hoffmann-La Roche Honoraria: Suresh S. Ramalingam, Genentech; Martin B. Steins, Roche; Claus-Peter Schneider, Roche, Boehringer Ingelheim; Wilfried E.E. Eberhardt, Roche, Boehringer Ingelheim, AstraZeneca, Novartis, GlaxoSmithKline, Pfizer, Eli Lilly, Bayer HealthCare Pharmaceuticals, OSI Pharmaceuticals; Lucio Crino, Roche; Martin Reck, F. Hoffmann-La Roche, Eli Lilly, Merck, AstraZeneca Research Funding: None Expert Testimony: None Other Remuneration: Pasi A. Jänne, Genzyme
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desbois-Mouthon C, Cacheux W, Blivet-Van Eggelpoël MJ, et al. Impact of IGF-1R/EGFR cross-talks on hepatoma cell sensitivity to gefitinib. Int J Cancer. 2006;119:2557–2566. doi: 10.1002/ijc.22221. [DOI] [PubMed] [Google Scholar]
- 6.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 7.Morgillo F, Kim WY, Kim ES, et al. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 8.Morgillo F, Woo JK, Kim ES, et al. Heterodimerization of insulin-like growth factor receptor/epidermal growth factor receptor and induction of survivin expression counteract the antitumor action of erlotinib. Cancer Res. 2006;66:10100–10111. doi: 10.1158/0008-5472.CAN-06-1684. [DOI] [PubMed] [Google Scholar]
- 9.Barnes CJ, Ohshiro K, Rayala SK, et al. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13:4291–4299. doi: 10.1158/1078-0432.CCR-06-2040. [DOI] [PubMed] [Google Scholar]
- 10.Jones HE, Goddard L, Gee JM, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 11.Heskamp S, van Laarhoven HW, Molkenboer-Kuenen JD, et al. ImmunoSPECT and immunoPET of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J Nucl Med. 2010;51:1565–1572. doi: 10.2967/jnumed.110.075648. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Yao E, Shen R, et al. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507) PLoS One. 2009;4:e7273. doi: 10.1371/journal.pone.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolb EA, Kamara D, Zhang W, et al. R1507, a fully human monoclonal antibody targeting IGF-1R, is effective alone and in combination with rapamycin in inhibiting growth of osteosarcoma xenografts. Pediatr Blood Cancer. 2010;55:67–75. doi: 10.1002/pbc.22479. [DOI] [PubMed] [Google Scholar]
- 14.Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 15.Rodon J, Patnaik A, Stein M, et al. A phase I study of q3W R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol. 2007;25(suppl):160s. abstr 3590. [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Karp DD, Paz-Ares LG, Novello S, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 18.Jassem J, Langer CJ, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28(suppl):539s. doi: 10.1200/JCO.2013.54.4932. abstr 7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfizer Discontinues a Phase 3 Study of Figitumumab. World Pharma News. 2010. Mar 12, http://www.worldpharmanews.com/pfizer/1152.
- 21.Reidy DL, Vakiani E, Fakih MG, et al. Randomized, phase II study of the insulin-like growth factor-1 receptor inhibitor IMC-A12, with or without cetuximab, in patients with cetuximab- or panitumumab-refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:4240–4246. doi: 10.1200/JCO.2010.30.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualberto A, Hixon ML, Karp DD, et al. Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab. Br J Cancer. 2011;104:68–74. doi: 10.1038/sj.bjc.6605972. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 24.Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–5958. [PubMed] [Google Scholar]
- 25.Macaulay VM, Middleton MR, Eckhardt SG, et al. Phase I study of OSI-906, dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR) in combination with erlotinib (E) in patients with advanced solid tumors. J Clin Oncol. 2010;28(suppl):237s. abstr 3016. [Google Scholar]