Abstract
Lenalidomide is an orally active immunomodulatory drug that has direct antineoplastic activity and indirect effects mediated through multiple types of immune cells found in the tumor microenvironment, including B, T, natural killer (NK), and dendritic cells. Recently, the E3 ubiquitin ligase cereblon was identified as a molecular target that may underlie the effects of lenalidomide on tumor cells, as well as on cells in the tumor microenvironment. Decreases in cereblon attenuate these effects and also confer resistance to lenalidomide. Tumoricidal effects of lenalidomide are associated with reduced interferon regulatory factor 4, a downstream target of cereblon. Lenalidomide stimulates proliferation and activation of NK cells, thereby enhancing NK cell–mediated cytotoxicity and antibody-dependent cellular cytotoxicity. These effects appear to be secondary to cytokine production from T cells. Lenalidomide has been shown to produce synergistic effects in experimental models when evaluated in combination with rituximab, dexamethasone, bortezomib, and B-cell receptor signaling inhibitors, consistent with mechanisms complementary to these agents. These experimental findings have translated to the clinic, where single-agent use displays durable responses in relapsed/refractory non-Hodgkin lymphoma, and combination with rituximab and other agents leads to improved responses at first line and in relapsed/refractory disease. The activity of lenalidomide is evident across multiple lymphoma subtypes, including indolent and aggressive forms. The interaction among cell types in the immune microenvironment is increasingly recognized as important to tumor cell recognition and destruction, as well as to protection of normal immune cells, as reflected by lenalidomide studies across multiple types of B-cell lymphomas.
INTRODUCTION
B-cell non-Hodgkin lymphoma (NHL) comprises multiple clinico-pathologic subtypes, most commonly diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).1,2 First-line treatment typically consists of immunochemotherapy, which may be followed by rituximab-based maintenance therapy for FL, or consolidation with autologous stem-cell transplantation for mantle-cell lymphoma (MCL).3 For patients with relapsed or refractory NHL, a wide range of treatment options is available, although consensus on the best approach and sequence remains to be determined.
Chemotherapy has a broad impact on both malignant and healthy cells. Advances in delineating pathways involved in cell signaling and tumor growth have led to novel, molecularly-based treatments.4 The advent of rituximab provided proof-of-concept for targeted therapy in B-cell NHL. Since then, numerous novel agents have been evaluated, with favorable clinical activity portending improvements in patient outcome.5 One such agent is lenalidomide, an oral, immune modulator. Its antineoplastic effects include direct antineoplastic activity, immunologic effects mediated by inhibition of tumor cell proliferation and angiogenesis, and stimulation of cytotoxicity mediated by T cells and NK cells.6–13 Herein, we provide a comprehensive review of known mechanisms of action (MOAs) of lenalidomide in B-cell NHL. Lenalidomide was first approved for treatment of multiple myeloma, and much work has focused on its activity in this disease. Another immunomodulatory derivative of thalidomide family member, pomalidomide, has been approved for use in multiple myeloma, but it is not being explored in preclinical or clinical studies in lymphoma, and therefore this review focuses on lenalidomide only.
CEREBLON AS A DIRECT TARGET FOR LENALIDOMIDE
Cereblon is a ubiquitously expressed E3 ubiquitin ligase protein identified as the primary teratogenic target of thalidomide,14 and cereblon is also a direct and therapeutically important molecular target for lenalidomide. Direct binding of lenalidomide to endogenous cereblon isolated from cell line extracts and to recombinant cereblon–DNA damage-binding protein-1 complexes has been demonstrated in vitro.15 Ikaros and Aiolos, zinc finger–containing transcription regulators of B- and T-cell development, are selectively bound by cereblon.16–18 After direct binding, lenalidomide activates cereblon's E3 ligase activity, resulting in the rapid ubiquitination and degradation of Ikaros and Aiolos. Lenalidomide inhibits autoubiquitination of wild-type, but not mutant, cereblon protein. Zhu et al19 found that transfection of myeloma cell lines with lentiviral constructs targeting cereblon was cytotoxic, and surviving cells with stable cereblon depletion became lenalidomide resistant. Cereblon silencing in myeloma cells attenuated the antiproliferative effect of lenalidomide, induction of tumor suppressor p21WAF-1 expression, and decrease in interferon regulatory factor 4 (IRF4), and silencing in T cells decreased lenalidomide-induced interleukin (IL)-2 and tumor necrosis factor α (TNF-α) production.
Reduced or undetectable levels of cereblon were found in lenalidomide-resistant H929 and DF15R myeloma cells selected for incubation with increasing lenalidomide concentrations over extended periods,15 and in patients with myeloma, lower cereblon levels were associated with lenalidomide resistance.19 Translation of these findings to lymphoma remains to be shown.
EFFECT OF LENALIDOMIDE ON MALIGNANT B CELLS
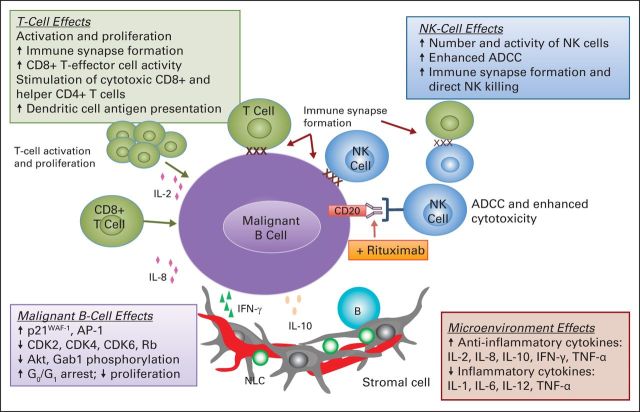
Lenalidomide exhibits in vitro and in vivo activity against malignant lymphoma B cells,6,11,12,20,21 and in specific tumor types, including DLBCL, FL, and MCL.10,13,22–24 Early preclinical evaluation showed antineoplastic and antiproliferative effects on malignant B-cell lines while sparing CD34+ progenitor and normal B cells (Fig 1).11 Lenalidomide increased the percentage of cells arrested in the G0-G1 phase, and there was a corresponding decrease in the S and G2-M phases. Lenalidomide upregulated protein and mRNA levels of p21WAF-1, a regulator of cyclin-dependent kinases (CDKs) important for G1-S progression, and promoted binding of p21WAF-1 to CDK2, CDK4, and CDK6 in malignant, but not normal, B cells. Upregulation of p21WAF-1 correlated with CDK inhibition, leading to hypophosphorylation of retinoblastoma protein, subsequent G1 cell-cycle arrest, and decreased cell proliferation. Lenalidomide inhibited protein kinase B (also known as Akt) and GRB2-associated binding protein 1 phosphorylation and enhanced activator protein-1 expression, suggesting that it, in part, exerts its antineoplastic and antiproliferative effects through kinase signaling pathways.7 Lenalidomide downregulates expression of checkpoint inhibitors, including programmed death-ligand 1 (PD-L1, CD274) on the surface of lymphoma cells.29 Lenalidomide upregulates expression of several genes involved in immune responses in MCL cells, including CD86, CD40, CD58, and CD1c.22
Fig 1.
Mechanisms of action of lenalidomide in lymphoma cells and the nodal microenvironment.6,9–13,25–28 ADCC, antibody-dependent cellular cytotoxicity; Akt, protein kinase B; AP-1, activator protein 1; CDK, cyclin-dependent kinase; Gab1, GRB2-associated binding protein 1; IFN, interferon; IL, interleukin; NK, natural killer; NLC, nurse-like cell; Rb, retinoblastoma; TNF, tumor necrosis factor.
Lenalidomide produces higher response rates in the activated B cell–like (ABC) subtype of DLBCL.30,31 Lenalidomide preferentially suppressed ABC DLBCL cell proliferation and delayed malignant growth in a human tumor xenograft model, while minimally affecting non-ABC DLBCL cells.24 The antineoplastic effects of lenalidomide in ABC DLBCL cells were associated with downregulation of IRF4 and, subsequently, B-cell receptor–dependent nuclear factor-κB (NF-κB) activity. Conversely, IRF4 overexpression led to enhanced NF-κB activation and a subsequent resistance to lenalidomide. Notably, cereblon expression was required for lenalidomide-induced downregulation of IRF4 and inhibition of B-cell receptor–mediated NF-κB signaling in ABC-type DLBCL cells.
A gain-of-function mutation of MYD88, an adaptor protein mediating Toll-like and IL-1 receptor signaling,32 is commonly observed in ABC DLBCL. MYD88 mutation promotes NF-κB and Janus kinase/signal transducer and activator of transcription (STAT) 3 signaling pathways to sustain ABC DLBCL viability, while also inducing interferon beta (IFN-β) production and autocrine signaling, paradoxically promoting cell-cycle arrest and apoptosis.33 On treatment of ABC DLBCL cells with lenalidomide, mRNA and protein levels of IRF4 and SPi-B (an Ets family transcription factor) were reduced in a cereblon-dependent manner. SPi-B acted together with IRF4 to prevent IFN-β production, allowing survival and proliferation of ABC DLBCL cells with MYD88 mutations. By blocking these transcription factors, lenalidomide augmented IFN-β production and promoted cytotoxicity against ABC DLBCL cells. The mRNA levels of CARD11, a transcription factor regulating the activity of IκB kinase in the NF-κB pathway, were reduced alongside IRF4 and SPi-B. Further examination of the pathways involved in lenalidomide's cytotoxic activity in ABC DLBCL cells showed decreased CARD11 and IκB kinase activity (and, thus, reduced NF-κB activity) with accompanying IRF4 and SPi-B downregulation.
MOAs OF LENALIDOMIDE IN THE LYMPH NODE MICROENVIRONMENT
Recent studies have emphasized the importance of crosstalk between malignant and surrounding nonmalignant cells within localized tumor niches and the bone marrow.34 Cells in the tumor microenvironment include macrophages, T cells, NK cells, dendritic cells, other myeloid-derived cells, and stromal cells. These cells not only provide a supportive network for tumor growth and progression but also can promote antitumor immune responses. Gene expression profiling (GEP) of 191 biopsy specimens from patients with FL who were treatment naive identified two immune response signature patterns (IR1 and IR2) predictive of survival.35 These signatures reflected the biologic characteristics of nonmalignant immune cells rather than the tumor cell of origin, and were independent of clinically prognostic variables. IR1 comprised genes generally highly expressed in T cells, whereas IR2 encompassed genes highly expressed in monocytes. The two signatures ranked patients by survival-predictor scores with clearly differentiated quartiles ranging from 13.6 to 3.9 years of survival time, illustrating unique biologic characteristics of the host immune system microenvironment, their influence on tumors, and their association with survival time.
Colocalization of FL cells with CD4+ T cells and follicular dendritic cells within follicular structures is necessary to support tumor cell proliferation.36 FL cells demonstrated reduced proliferative activity in interfollicular regions.37 Rather, FL cell proliferation depends on the surrounding immune system to support growth.38,39 FL cells adapt to a germinal center B-cell (GCB) –like phenotype, including their dependence on immune cell interactions within the follicular microenvironment. Immune cells are influenced by both positive and negative regulatory molecules, governing whether antitumor responses or supportive signals are available for tumor cell growth and proliferation.
EFFECT OF LENALIDOMIDE ON T CELLS
T cells in the lymph node are influenced by the presence of lymphoma and display altered GEP and decreased immune synapse (IS) formation and effector function. GEP analysis of highly purified CD4 and CD8 tumor-infiltrating lymphocytes from baseline lymph node biopsies in 172 patients with FL who were treatment naive was altered compared with healthy donor reactive tonsils and peripheral blood.40 Microarray analysis demonstrated multiple dysregulated genes in both CD4 and CD8. Multivariable analysis revealed that levels of expression of altered proteins on T cells were significantly prognostic for overall survival time and time to transformation in FL, further highlighting the role that lymphoma cells play in influencing the immune microenvironment and how this can affect outcome.
Tumor-infiltrating CD4+ and CD8+ T cells from lymphoma exhibit defective IS formation with antigen-presenting cells (APCs) compared with age-matched healthy donors,10 resulting in impaired antigen presentation.25,41 Ex vivo lenalidomide treatment of FL and autologous T cells repaired the F-actin IS activity and recruited tyrosine-phosphorylated protein independent of added antigen and irrespective of the patient's level of disease.10
When MCL and γδ T cells were cocultured, lenalidomide induced reorganization of the actin cytoskeleton and cell surface markers and enhanced γδ T-MCL cell IS formation, γδ T-cell expansion, and cytotoxicity against MCL cells. These findings suggest that lenalidomide may have multiple mechanisms against MCL cells, including increased CD1c expression and enhancement of γδ T cell–mediated cytotoxicity.
Although there is a considerable literature on the effects of lenalidomide on T-regulatory cells (Tregs), little has been published on lymphoma. In a murine model, lenalidomide was associated with reduced numbers of systemic Tregs, as well as myeloid-derived suppressor cells in tumor-bearing, but not naive, mice.42 In a phase II study, Tregs were increased in the peripheral blood of patients with MCL compared with that of healthy volunteers, and they rose more after lenalidomide treatment.43
EFFECT OF LENALIDOMIDE ON NK CELLS
NK cells are important contributors to the innate immune response, with vital roles in clearing viruses, regulating dendritic cells, and rejecting malignant cells.25 Lenalidomide treatment increased NK cell number, enhanced NK cell–induced cytotoxicity against cell lines,26 and enhanced antibody-dependent cellular cytotoxicity (ADCC). The effects of lenalidomide-induced NK cell cytotoxicity and ADCC may be mediated indirectly via IL-2 production by T cells, as shown via the abrogation of NK cytotoxicity when IL-2 was inhibited with IL-2 antibody.26
Lenalidomide enhanced NK cell–mediated ADCC in several rituximab-treated NHL cell lines; the effects were dependent on rituximab binding to Fc-γ receptors and either IL-2 or IL-12 production.12 Lenalidomide may stimulate NK cells by enhancing Fc-γ receptor signaling, which, in turn, elevates phosphorylated extracellular signal-regulated kinase and enhanced granzyme B and Fas ligand expression, contributing to enhanced ADCC.12
EFFECT OF LENALIDOMIDE ON DENDRITIC CELLS
Dendritic cells are APCs that are key messengers between the innate and adaptive immune systems, and function by processing and presenting antigens on their surface for recognition by T cells. Lenalidomide enhances expression of major histocompatibility complex class I and CD86 on bone marrow–derived murine dendritic cells, promotes uptake of tumor antigens by these APCs, and increases the efficiency of antigen presentation to naive CD8+ T cells.44 The enhancement of dendritic cell function by lenalidomide may be important during immunosurveillance of cancer cells. Moreover, these findings suggest that lenalidomide may be useful in dendritic cell–based vaccines. The impact of lenalidomide on stromal cells, angiogenesis, and myeloid-derived suppressor cells, which have all been studied in myeloma, has yet to be fully addressed in studies in lymphoma.
EFFECT OF LENALIDOMIDE ON NORMAL HEMATOPOIESIS
Lenalidomide spares CD34+ hematopoietic progenitor cells; indeed, lenalidomide has been shown to increase expansion of leukaphereses-derived CD34+ cells.11,45 The mechanism of lenalidomide-induced neutropenia has been associated with loss of PU.1, a key transcription factor involved in granulopoiesis.45 Downregulation of PU.1 resulted in transient arrest of neutrophil maturation alongside accumulation of immature myeloid precursors and subsequent neutropenia.
EFFECT OF LENALIDOMIDE ON INFLAMMATORY CYTOKINES
Cytokines secreted by hematopoietic and nonhematopoietic cells are important factors for mediating innate and adaptive immune responses. Lenalidomide decreases the production of several proinflammatory cytokines (eg, TNF-α, IL-1, IL-6, and IL-12) and increases production of anti-inflammatory cytokine IL-10.46,47 Modulation of these cytokines within the nodal microenvironment likely influences inflammatory responses, supports tumor growth and metastasis, and contributes to chemoresistance. The role of IL-6 was examined in preclinical studies of human MCL cells cocultured with peripheral blood mononuclear cells (PBMCs) or bone marrow–derived mononuclear cells.48 IL-6 receptor ligation initiates a downstream kinase signaling cascade (eg, STAT3, Ras, phosphoinositide 3-kinase [PI3K]/Akt) to promote tumorigenesis. In some MCL cells, IL-6 secretion provides an autocrine growth signal. Bone marrow stromal cells secrete high levels of IL-6, and PBMCs secrete both IL-6 and the soluble gp80 IL-6 receptor subunit.48 Because both stromal cells and PBMCs may be found in the MCL microenvironment, they may provide a paracrine source of IL-6 for supporting MCL growth. Consistent with this hypothesis, IL-6/gp80 knockdown effectively allows chemotherapy-induced apoptosis to occur on exogenous addition of IL-6 or gp80, rather than supporting tumor growth and proliferation. In line with IL-6 signaling, STAT3 phosphorylation and constitutive activation are dependent on autocrine and paracrine feedback loops for IL-6. The ability of lenalidomide to reduce IL-6 and STAT3 activity may provide mechanisms for reducing signaling within the MCL microenvironment, thereby inhibiting MCL cell growth and resistance to chemotherapy and promoting apoptosis.
Lenalidomide also stimulates production of IL-2 and other cytokines, including IFN-γ and TNF-α, and induces T-cell proliferation in the absence of CD28 stimulation.25,46,49 Because T-cell receptor and costimulatory signals are required for IL-2 production, these observations suggest that lenalidomide may activate costimulatory-dependent signaling normally triggered by CD28. Consistent with this hypothesis, lenalidomide increases tyrosine phosphorylation of CD28 in the intracellular domain of T cells in the absence of costimulatory molecules, and stimulates NF-κB activation downstream from CD28.50 Moreover, lenalidomide promotes nuclear translocation and binding of nuclear factor of activated T cells 2 and activator protein-1 to the IL-2 promoter, a process dependent on PI3K signaling, leading to enhanced IL-2 production.26
Although IL-2 and IL-12 are not required for monocyte-mediated cell lysis and ADCC for synergistic activity between lenalidomide and rituximab, enhancement of ADCC by lenalidomide is associated with increased cytokines on NK cells, including IL-8, monocyte chemotactic protein-1, RANTES (regulated on activation, normal T cell expressed and secreted), inducible protein-10, granulocyte-macrophage colony-stimulating factor, and with decreased IL-6.
COMBINATIONS OF LENALIDOMIDE WITH OTHER TREATMENTS
Lenalidomide may enhance or act synergistically with other treatments with complementary MOAs. Lenalidomide with dexamethasone and rituximab has been shown to synergistically inhibit growth and induce apoptosis of established MCL cell lines and ex vivo MCL cells from patients with relapsed or refractory MCL.13,23 Mechanistically, lenalidomide enhanced dexamethasone-induced G0-G1 cell-cycle arrest through an intrinsic mitochondrial pathway of apoptosis, evidenced by increased Bcl-2 phosphorylation; upregulation of the proapoptotic proteins Bax, BAD, and Bim; activation of caspase-3 and -9; and cleavage of poly(ADP-ribose) polymerase (Table 1). Lenalidomide enhanced rituximab-induced apoptosis by upregulating c-Jun N-terminal protein kinase phosphorylation and activating the mitochondrial-derived apoptotic pathway.13 In addition, lenalidomide increased the number of NK cells 10-fold and augmented rituximab-dependent NK cell–mediated cytotoxicity by increasing CD16 expression on a subset of NK cells considered key effector cells for ADCC in PBMCs. This increase was positively associated with elevated IFN-γ, TNF-α, and perforin expression. These preclinical findings translated into prolonged survival for severe combined immunodeficient mice inoculated with Mino MCL cells and treated with combined lenalidomide and rituximab; overall tumor burden was decreased two-fold (P < .01), and survival time significantly improved versus control or treatment with either single agent (P < .05). Further examination of tumor growth mechanisms demonstrated that after 21 days of treatment, lenalidomide increased the number of splenic NK cells 10-fold.
Table 1.
Preclinical Mechanistic Rationale for Combinations With Lenalidomide
| Combination Treatment | Known MOAs |
|---|---|
| Lenalidomide + rituximab13,23,51 | NK cell |
| ↑ NK-cell number and activity | |
| ↑ CD16 expression | |
| Malignant B cell | |
| G0-G1 cell cycle arrest | |
| ↑ p-Bcl-2, Bax, BAD, Bim | |
| Caspase-3 and -9 activation | |
| PARP cleavage | |
| p-JNK | |
| Antiangiogenic activity | |
| Microenvironment | |
| ↑ IFN-γ, MCP-1, TNF-α, and perforin | |
| Lenalidomide + XmAb5574 (MOR208)52 | NK cell |
| Enhanced ADCC | |
| Lenalidomide + SGN-4053 | NK cell |
| Enhanced ADCC | |
| Malignant B cell | |
| ↑ CD40 expression | |
| ↑ cytotoxicity | |
| Lenalidomide + bortezomib54 | Malignant B cell |
| ↑ cytotoxicity | |
| Lenalidomide + ibrutinib33 | Malignant B cell |
| ↓ IRF4 levels | |
| ↓ tumor growth | |
| Lenalidomide + anti–PD-1 or anti–PD-L129 | Proposed preclinical rationale for enhanced checkpoint control inhibition |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; IFN-γ, interferon-gamma; IRF4, interferon regulatory factor 4; JNK, c-Jun N-terminal protein kinase; MCP-1, monocyte chemotactic protein-1; MOAs, mechanisms of action; NK, natural killer; PARP, poly(ADP-ribose) polymerase; PD-1, programmed death 1; PD-L1, programmed death ligand 1; TNF-α, tumor necrosis factor-alpha.
The synergistic effects of lenalidomide with anti-CD20 monoclonal antibodies appear to be independent of CD20 expression and density on the surface of different types of lymphoma cells.27,51 In a severe combined immunodeficient mouse xenograft model bearing a disseminated Raji lymphoma, administration of lenalidomide significantly increased the number of circulating CD49b+ NK cells from day 5 to day 10, whereas depleting NK cells with anti–IL-2 receptor monoclonal antibody before inoculation with lymphoma cells abrogated the antitumor effects of lenalidomide with or without rituximab. In a subsequent study, lenalidomide increased the infiltration of NK cells into tumor sites compared with vehicle-treated animals. Notably, infiltration was directed into the central part of the tumor in lenalidomide-treated animals but confined to the tumor periphery in control animals.51 In an effort to explore other cellular effects in the immune microenvironment, NK cell activity was associated with dendritic cell stimulation and alterations in the dendritic cell cytokine milieu, as shown by increased monocyte chemotactic protein-1, TNF-α, and IFN-γ, collectively augmenting rituximab-mediated ADCC. Lenalidomide also exhibited antiangiogenic activity in the Raji xenograft model, as shown by significantly decreased tumor microvessel density compared with vehicle-treated animals (50 v 109 vessels/5 low-power fields; P = .009).
The ability to augment NK cells and enhance rituximab-mediated cytotoxic mechanisms suggests that lenalidomide may also work cooperatively in combination with monoclonal antibodies for other surface antigens. Targeting other cell surface antigens provides alternative pathways to engage, as well as strategies for overcoming potential adaptive or acquired resistance to rituximab (Table 1).55 In preclinical studies, ex vivo chronic lymphocytic leukemia (CLL) cells enhanced NK-mediated ADCC when lenalidomide was combined with XmAb5574 (MOR208), a humanized monoclonal antibody targeting CD19 found on the surface of normal and transformed B cells and involved in B-cell receptor signaling.52 Another example is CD40, a member of the TNF receptor superfamily mainly expressed on B cells and other APCs (eg, dendritic cells and macrophages).55 Lenalidomide increased CD40 expression and enhanced the direct cytotoxicity of anti-CD40 monoclonal antibody SGN-40 in CLL cells.53 Moreover, lenalidomide enhanced anti–CD40-mediated ADCC after treatment with NK cells or PBMCs isolated from patients with CLL.
Given its unique MOAs, lenalidomide is expected to provide complementary effects with treatments other than monoclonal antibodies. As mentioned previously, lenalidomide acted synergistically with dexamethasone in promoting growth inhibition and apoptosis in MCL cells.23 Similarly, lenalidomide synergistically enhanced bortezomib-induced cytotoxicity and apoptosis in FL and MCL cells.54 Lenalidomide also displayed synergistic activity in combination with ibrutinib, a Bruton's tyrosine kinase inhibitor, which blocks B-cell receptor signaling.33 Ibrutinib reduced IRF4 levels in ABC DLBCL cells, but when evaluated in combination with lenalidomide, IRF4 was decreased to undetectable levels. Lenalidomide and ibrutinib acted synergistically in inducing ABC DLCBL cell cytotoxicity in vitro, and the combination was effective in arresting tumor growth of OCI-Ly10 ABC DLBCL xenografts. These findings underscore the feasibility of lenalidomide combinations with other B-cell receptor pathway inhibitors, including the PI3Kδ inhibitor idelalisib and the spleen tyrosine kinase inhibitor entospletinib (GS-9973). Because checkpoint control inhibitors show activity in lymphoma, and because lenalidomide downregulates expression of PD-L1 on the surface of lymphoma cells,29 there is a rationale for exploration of combining lenalidomide with anti–PD-1 or anti–PD-L1 antibodies to attempt to fully block the pathway.
TRANSLATION OF PRECLINICAL DATA TO CLINICAL STUDIES
The MOAs of lenalidomide identified in experimental studies appear to translate into therapeutic relevance in the clinical setting, both as monotherapy and in combination with other agents. Single-agent lenalidomide produced durable responses in patients with relapsed/refractory indolent or aggressive NHL in several phase II trials.56–58 Subset analyses demonstrated that lenalidomide was active across multiple NHL subtypes; lenalidomide exhibited higher responses in non-GCB DLBCL compared with GCB30 and showed particularly promising activity in MCL.59,60 These latter findings led to a prospective international phase II trial known as MCL-001 (EMERGE [A Phase 2, Multicenter, Single-Arm, Open-Label Study to Determine the Efficacy and Safety of Single-Agent Lenalidomide (Revlimid) in Patients With Mantle Cell NHL Who Have Relapsed or Progressed After Treatment With Bortezomib or Are Refractory to Bortezomib]), which enrolled 134 patients with relapsed/refractory MCL.56 Lenalidomide produced a 28% overall response rate (8% complete response) in patients. The duration of response lasted for a median of 16.6 months, notable given that patients were heavily pretreated and 60% refractory to bortezomib. Pooled data analyses for patients with MCL from MCL-001 and earlier phase II studies (NHL-002 and NHL-003) confirmed the clinical activity of single-agent lenalidomide and supported its approval by the US Food and Drug Administration for relapsed or refractory MCL after two earlier therapies, one of which included bortezomib.56,59–61
Recent reports of an increased risk of second primary malignancies (SPMs) in patients with multiple myeloma after lenalidomide maintenance have piqued interest in understanding the underlying mechanism that contributes to the emergence of SPMs.62,63 Little has been reported in studies of lenalidomide in lymphoma because their follow-up times are shorter than those for multiple myeloma. The MCL-001 study of single-agent lenalidomide identified invasive SPM rates consistent with the expected background occurrences reported by the SEER program for individuals 65 years of age and older.56 Clear elucidation of the mechanisms involved in SPMs appears to be confounded by patients' prior exposure to multiple lines of therapy, making insights into the mechanisms involved speculative. Studies in multiple myeloma suggest that prior or concurrent exposure to the alkylating agent melphalan may increase the risk of developing SPMs through its DNA-damaging properties and potential synergy with lenalidomide's inhibition of DNA repair mechanisms (possibly via cereblon inhibition).62,63 An alternate potential mechanism might include disruption of viral latency, as has been suggested for Epstein-Barr virus in preclinical studies of B cells.64 For patients receiving lenalidomide for a long period of time, continued study is needed for better insight into the mechanisms involved in the development of SPMs. Clinically in lymphoma, a disease plagued by probable relapse, it is important to consider lenalidomide maintenance in the context of the risk to benefit ratio to the patient, as the risk of progressive disease or death is much greater than that of developing an SPM.
The single-agent activity of lenalidomide, combined with preclinical evidence of its ability to enhance the antitumor activity of rituximab, led to early trials of combination rituximab and lenalidomide (R2) therapy in first-line and relapsed settings. Enhanced activity has been observed with R2 in MCL,28,65 DLBCL,66–68 FL,69,70 and indolent NHL.71,72 A recently published study of R2 shows evidence of overcoming rituximab-resistance in indolent NHL and MCL.73 The feasibility of administering lenalidomide or R2 in combination with either dexamethasone or bortezomib in patients with MCL was also demonstrated.74–77 First-line R2 plus cyclophosphamide, doxorubicin, vincristine, prednisone (R2CHOP) produced encouraging response rates and progression-free survival times in patients with DLBCL and FL in several clinical trials, particularly when compared with historical data for R-CHOP alone.78–80 Notably, patients with GCB and non-GCB DLBCL phenotypes achieved similar objective response rates with R2CHOP.79 The combination of R2 with bendamustine is being explored as a first-line option in elderly patients with MCL (Table 2).81 Studies of R2 with multiple combination partners are ongoing in phase I and II trials. Recent findings on the combination of R2 with idelalisib in relapsed/refractory NHL (A051201; NCT01838434) indicate that combined mechanisms of action may not always be complementary.82 This triple combination led to unexpected toxicity suggestive of cytokine release syndrome (a rare event associated with rituximab), and the dosing regimen has been modified to include lenalidomide plus idelalisib without rituximab.
Table 2.
Ongoing Clinical Studies of Lenalidomide Combinations in Lymphoma
| Clinical Study | Type of NHL | Phase | Treatment | Primary End Point |
|---|---|---|---|---|
| Previously untreated patients | ||||
| R2-ibrutinib (NCT01829568) | FL | I | R2-ibrutinib | MTD |
| MCL4 (LENA-BERIT; NCT00963534) | MCL (age > 65 years) | I/II | R2-B | Ph I: MTD |
| Ph II: PFS | ||||
| Lenalidomide + DA-EPOCH-R (NCT02213913) | MYC-associated B-cell lymphoma | I/II | Lenalidomide + DA-EPOCH-R | Ph I: MTD |
| Ph II: PFS | ||||
| SAKK 35/10 (NCT01307605) | FL | II | R ± lenalidomide | CR |
| ECOG E1412 (NCT01856192) | DLBCL | II | R2CHOP v R-CHOP | PFS |
| ROBUST (DLC-002) (NCT02285062) | ABC-type DLBCL | III | R2CHOP v R-CHOP | PFS |
| LYSA SENIOR (NCT02128061) | CD20+ DLBCL, age ≥ 80 years | III | R2miniCHOP v R-miniCHOP (subcutaneous R) | OS |
| Maintenance | ||||
| Lenalidomide maintenance post-chemotherapy (NCT01035463) | Chemotherapy-resistant or high-risk NHL | I/II | Lenalidomide maintenance post-BEAM ± rituximab and ASCT | Ph I: MTD |
| Ph II: EFS, ORR, OS | ||||
| FIL R2-B (NCT01737177) | MCL | II | Induction: R2-B | CR, PFS |
| Maintenance: lenalidomide | ||||
| ECOG E1411 (NCT01415752) | MCL | II | Induction: RB v RBV | PFS |
| Maintenance: R2 v R | ||||
| 3-Arm randomized ECOG E2408 (NCT01216683) | High-risk stage II-IV FL | II | Arm I: BR to R | CR, DFS |
| Arm II: BVR to R | ||||
| Arm III: BR to lenalidomide to R | ||||
| RELEVANCE (NCT01650701) | FL | III | Induction: R2 v R-chemotherapy | CR/CRu at 30 months, PFS |
| Maintenance: R2 (post-R2) v R (post-R-chemotherapy) | ||||
| MCL R2 Elderly (NCT01865110) | Older MCL | III | Induction: R-CHOP + R-HAD v R-CHOP | PFS |
| Maintenance: R2 v R | ||||
| FIL MCL-0208 (NCT02354313) | Advanced MCL | III | Induction: R-high-dose chemotherapy and ASCT | PFS |
| Maintenance: lenalidomide v observation | ||||
| MAGNIFY (NHL-008) (NCT01996865) | FL, MZL, MCL | IIIb | Induction: R2 | PFS |
| Maintenance: lenalidomide v R | ||||
| Relapsed/refractory patients | ||||
| Lenalidomide + brentuximab vedotin (NCT02086604) | DLBCL | I | Lenalidomide + brentuximab vedotin | MTD |
| Lenalidomide + temsirolimus (NCT01076543) | NHL and HL | I/II | Lenalidomide + temsirolimus | Ph I: MTD |
| Ph II: ORR, CR | ||||
| R2 + chemotherapy (NCT01788189) | CD20+ NHL (not MCL) | I/II | R2 + methotrexate, leucovorin, and cytarabine | Ph I: MTD |
| Ph II: ORR | ||||
| R2 + carfilzomib (NCT01729104) | MCL | I/II | R2 + carfilzomib | Ph I: MTD |
| Ph II: ORR | ||||
| Lenalidomide ± idelalisib (NCT01838434) | MCL | I/II | R2 with idelalisib; amended to lenalidomide ± idelalisib | Ph I: MTD |
| Ph II: PFS | ||||
| LR-ESHAP (NCT02340936) | DLBCL | I/II | Salvage LR-ESHAP in candidates for HDT and ASCT | Ph I: MTD |
| Ph II: ORR | ||||
| Dose-finding lenalidomide + obinutuzuab (NCT01995669) | iNHL | I/II | Lenalidomide + obinutuzumab | Ph I: MTD |
| Ph II: ORR | ||||
| GALEN (NCT01582776) | DLBCL, MCL | Ib/II | Lenalidomide + obinutuzumab | Ph I: MTD |
| Ph II: ORR | ||||
| Lenalidomide + ibrutinib (NCT01955499) | NHL | I | Lenalidomide + ibrutinib | MTD |
| Lenalidomide + ibrutinib ± R (NCT02077166) | DLBCL | Ib/II | Lenalidomide + ibrutinib ± rituximab | Ph I: MTD |
| Ph II: ORR | ||||
| Lenalidomide + ibrutinib + DA-EPOCH-R (NCT02142049) | DLBCL | Ib/II | Lenalidomide + ibrutinib + DA-EPOCH-R | Ph I: MTD |
| Ph II: ORR | ||||
| Lenalidomide + romidepsin (NCT01755975) | Lymphoma and myeloma | Ib/IIa | Lenalidomide + romidepsin | MTD, safety |
| R2 + carfilzomib + romidepsin (NCT02341014) | B- and T-cell lymphoma | Ib/IIa | R2 + carfilzomib + romidepsin | Ph I: MTD |
| Ph II: ORR | ||||
| LEGEND (NCT02060656) | DLBCL | II | LR-GEM v R-GEM-P | CR |
| AUGMENT (NHL-007) (NCT01938001) | FL and MZL | III | R2 v R | PFS |
Abbreviations: ABC, activated B cell; ASCT, autologous stem-cell transplantation; B, bendamustine; BEAM, carmustine, etoposide, cytarabine, melphalan; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete response; CRu, CR unconfirmed; DA, dose adjusted; DFS, disease-free survival; DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; EFS, event-free survival; EPOCH, etoposide, prednisone, doxorubicin, cyclophosphamide, vincristine; ESHAP, etoposide, methylprednisolone, cisplatin, and cytarabine; FIL, Fondazione Italiana Linfomi; FL, follicular lymphoma; GEM(-P), gemcitabine, methylprednisolone (cisplatin); HAD, high-dose cytarabine and dexamethasone; HDT, high-dose therapy; iNHL, indolent non-Hodgkin lymphoma; LR, lenalidomide + rituximab; LYSA, Lymphoma Study Association; MCL, mantle-cell lymphoma; MTD, maximum tolerated dose; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Ph, phase; R, rituximab; R2, lenalidomide + rituximab; V, bortezomib.
Numerous clinical trials are currently underway to further elucidate how to best exploit lenalidomide pathways in NHL treatment (Table 2). RELEVANCE (NCT01650701) is a phase III open-label study comparing R2 with rituximab-based immunochemotherapy followed by rituximab or R2 maintenance in 1,000 previously untreated FLs. The primary outcomes are complete response rate at 30 months and progression-free survival time. GALEN (NCT01582776) is a phase IB/II study evaluating the combination of lenalidomide with obinutuzumab in relapsed or refractory FL, DLBCL, and MCL.83 The phase IB component recommended a dosage of 20 mg/d lenalidomide in combination with fixed-dose obinutuzumab in FLs; the ongoing phase II study will evaluate efficacy and safety in relapsed or refractory FLs and aggressive NHLs.
In conclusion, lenalidomide is an orally active immunomodulatory drug that has direct antineoplastic activity and indirect effects mediated through multiple types of immune cells found in the tumor microenvironment, including B, T, NK, and dendritic cells (Fig 1).6,9–13,25–28 Recently, the E3 ubiquitin ligase cereblon was identified as a molecular target that likely underlies the effects of lenalidomide on tumor cells as well as on cells in the tumor microenvironment. On the basis of its overall profile, lenalidomide was evaluated initially as monotherapy in patients with relapsed or refractory NHL and exhibited activity across multiple lymphoma subtypes. The observation of durable responses in patients with MCL provided a focus for clinical development and led to approval of lenalidomide for relapsed/refractory MCL. Preclinical studies have shown that lenalidomide has enhanced or synergistic activity with other agents, including rituximab, dexamethasone, bortezomib, and B-cell receptor pathway inhibitors, reflecting its unique mechanisms of action. These experimental observations, combined with the single-agent activity observed clinically, provided the basis for evaluation of R2 and other combination regimens across a variety of treatment phases for both indolent and aggressive NHL types. Clinical results highlight the potential activity for lenalidomide-based combinations. Continued understanding of the mechanisms of lenalidomide against tumor cells and cells in the tumor microenvironment will help optimize lenalidomide's therapeutic effects for patients with NHL overall and on an individual basis.
Acknowledgment
Supported by Bio Connections (editorial support) and funded by Celgene.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
John G. Gribben
Consulting or Advisory Role: Roche/Genentech, Gilead Sciences, Janssen Pharmaceuticals, Pharmacyclics, Celgene, Mundipharma, TG Therapeutics, Abbvie, AstraZeneca
Travel, Accommodations, Expenses: Gilead Sciences
Nathan Fowler
Consulting or Advisory Role: Celgene, Roche
Research Funding: Celgene, Roche
Franck Morschhauser
Consulting or Advisory Role: Gilead Sciences, Celgene, Mundipharma, Servier Laboratories, Takeda Pharmaceuticals, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: Genentech
REFERENCES
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes—Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012;380:848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology. Non-Hodgkin's Lymphomas, v1.2015. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 4.Soria JC, Blay JY, Spano JP, et al. Added value of molecular targeted agents in oncology. Ann Oncol. 2011;22:1703–1716. doi: 10.1093/annonc/mdq675. [DOI] [PubMed] [Google Scholar]
- 5.Ujjani C, Cheson BD. The current status and future impact of targeted therapies in non-Hodgkin lymphoma. Expert Rev Hematol. 2013;6:191–202. doi: 10.1586/ehm.13.6. [DOI] [PubMed] [Google Scholar]
- 6.Chang DH, Liu N, Klimek V, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: Therapeutic implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi AK, Kang J, Naziruddin S, et al. Lenalidomide inhibits proliferation of Namalwa CSN.70 cells and interferes with Gab1 phosphorylation and adaptor protein complex assembly. Leuk Res. 2006;30:849–858. doi: 10.1016/j.leukres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Gorgun G, Ramsay AG, Holderried TA, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci USA. 2009;106:6250–6255. doi: 10.1073/pnas.0901166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentzsch S, LeBlanc R, Podar K, et al. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17:41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: Implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhelle D, Corral LG, Wong K, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–559. doi: 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.) Br J Haematol. 2014;164:811–821. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanan-Khan AA, Padmanabhan S, Miller KC, et al. In vivo evaluation of immunomodulating effects of lenalidomide (L) on tumor cell microenvironment as a possible underlying mechanism of the antitumor effects observed in patients with chronic lymphocytic leukemia. Blood. 2005;106 (abstr 2975a) [Google Scholar]
- 21.Marriott JB, Dredge K, Dalgleish AG. Thalidomide derived immunomodulatory drugs (IMiDs) as potential therapeutic agents. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:181–186. doi: 10.2174/1568008033340207. [DOI] [PubMed] [Google Scholar]
- 22.Gaidarova S, Corral LG, Gleizer E, et al. Lenalidomide enhances anti-tumor effect of gamma delta T cells against mantle cell lymphoma. Blood. 2008;112 (abstr 2616a) [Google Scholar]
- 23.Qian Z, Zhang L, Cai Z, et al. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leuk Res. 2011;35:380–386. doi: 10.1016/j.leukres.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Zhang LH, Kosek J, Wang M, et al. Lenalidomide efficacy in activated B cell–like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol. 2013;160:487–502. doi: 10.1111/bjh.12172. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel JM, Pinilla-Ibarz J, Epling-Burnette PK. Molecular action of lenalidomide in lymphocytes and hematologic malignancies. Adv Hematol. 2012;2012:513702. doi: 10.1155/2012/513702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi T, Hideshima T, Akiyama M, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, et al. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: A phase 1/2 clinical trial. Lancet Oncol. 2012;13:716–723. doi: 10.1016/S1470-2045(12)70200-0. [DOI] [PubMed] [Google Scholar]
- 29.Ramsay AG, Clear AJ, Fatah R, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: Establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell–like than in germinal center B cell–like phenotype. Cancer. 2011;117:5058–5066. doi: 10.1002/cncr.26135. [DOI] [PubMed] [Google Scholar]
- 31.Czuczman MS, Davies A, Linton KM, et al. A phase 2/3 multicenter, randomized study comparing the efficacy and safety of lenalidomide versus investigator's choice in relapsed/refractory DLBCL. Blood. 2014;124 (abstr 628a) [Google Scholar]
- 32.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Shaffer AL, III, Emre NC, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell. 2012;21:723–737. doi: 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ame-Thomas P, Tarte K. The yin and the yang of follicular lymphoma cell niches: Role of microenvironment heterogeneity and plasticity. Semin Cancer Biol. 2014;24:23–32. doi: 10.1016/j.semcancer.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 36.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 37.Dogan A, Du MQ, Aiello A, et al. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood. 1998;91:4708–4714. [PubMed] [Google Scholar]
- 38.Johnson PW, Watt SM, Betts DR, et al. Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system. Blood. 1993;82:1848–1857. [PubMed] [Google Scholar]
- 39.Umetsu DT, Esserman L, Donlon TA, et al. Induction of proliferation of human follicular (B type) lymphoma cells by cognate interaction with CD4+ T cell clones. J Immunol. 1990;144:2550–2557. [PubMed] [Google Scholar]
- 40.Kiaii S, Clear AJ, Ramsay AG, et al. Follicular lymphoma cells induce changes in T-cell gene expression and function: Potential impact on survival and risk of transformation. J Clin Oncol. 2013;31:2654–2661. doi: 10.1200/JCO.2012.44.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linsley PS, Greene JL, Brady W, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 42.Sakamaki I, Kwak LW, Cha SC, et al. Lenalidomide enhances the protective effect of a therapeutic vaccine and reverses immune suppression in mice bearing established lymphomas. Leukemia. 2014;28:329–337. doi: 10.1038/leu.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eve HE, Carey S, Richardson SJ, et al. Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: Results from a UK phase II study suggest activity and possible gender differences. Br J Haematol. 2012;159:154–163. doi: 10.1111/bjh.12008. [DOI] [PubMed] [Google Scholar]
- 44.Henry JY, Labarthe MC, Meyer B, et al. Enhanced cross-priming of naive CD8+ T cells by dendritic cellss treated by the IMiDs immunomodulatory compounds lenalidomide and pomalidomide. Immunology. 2013;139:377–385. doi: 10.1111/imm.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal R, Monaghan SA, Hassett AC, et al. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood. 2010;115:605–614. doi: 10.1182/blood-2009-05-221077. [DOI] [PubMed] [Google Scholar]
- 46.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alfa. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 47.Kotla V, Goel S, Nischal S, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Yang J, Qian J, et al. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood. 2012;120:3783–3792. doi: 10.1182/blood-2012-04-424630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haslett PA, Corral LG, Albert M, et al. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187:1885–1892. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LeBlanc R, Hideshima T, Catley LP, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–1790. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 51.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 52.Awan FT, Lapalombella R, Trotta R, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115:1204–1213. doi: 10.1182/blood-2009-06-229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lapalombella R, Gowda A, Joshi T, et al. The humanized CD40 antibody SGN-40 demonstrates pre-clinical activity that is enhanced by lenalidomide in chronic lymphocytic leukaemia. Br J Haematol. 2009;144:848–855. doi: 10.1111/j.1365-2141.2008.07548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosenza M, Civallero M, Pozzi S, et al. In vitro combination of bortezomib with enzastaurin or lenalidomide enhances the cytotoxicity in B-cell lymphoma cell lines. Blood. 2012;120 doi: 10.1002/hon.2179. (abstr 2754a) [DOI] [PubMed] [Google Scholar]
- 55.Bello C, Sotomayor EM. Monoclonal antibodies for B-cell lymphomas: Rituximab and beyond. Hematology Am Soc Hematol Educ Program. 2007:233–242. doi: 10.1182/asheducation-2007.1.233. [DOI] [PubMed] [Google Scholar]
- 56.Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: Phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31:3688–3695. doi: 10.1200/JCO.2013.49.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 58.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol. 2011;22:1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 59.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 60.Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: Subset analysis of the NHL-003 study. Ann Oncol. 2013;24:2892–2897. doi: 10.1093/annonc/mdt366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.REVLIMID (lenalidomide) prescribing information. Celegne Corporation. https://www.celgene.com/content/uploads/revlimid_full_prescribing_info.pdf.
- 62.Landgren O, Mailankody S. Update on second primary malignancies in multiple myeloma: A focused review. Leukemia. 2014;28:1423–1426. doi: 10.1038/leu.2014.22. [DOI] [PubMed] [Google Scholar]
- 63.Shortt J, Hsu AK, Johnstone RW. Thalidomide-analogue biology: Immunological, molecular and epigenetic targets in cancer therapy. Oncogene. 2013;32:4191–4202. doi: 10.1038/onc.2012.599. [DOI] [PubMed] [Google Scholar]
- 64.Jones RJ, Kenney SC, Dawson C, et al. Thalidomide, lenalidomide and pomalidomide disrupt Epstein-Barr Virus (EBV) latency: Clinical implications. Blood. 2013;122 (abstr 3499a) [Google Scholar]
- 65.Ruan J, Martin P, Shah BD, et al. Sustained remission with the combination biologic doublet of lenalidomide plus rituximab as initial treatment for mantle cell lymphoma: A multicenter phase II study report. Blood. 2014;124 (abstr 625a) [Google Scholar]
- 66.Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: A phase II clinical trial. Leukemia. 2013;27:1902–1909. doi: 10.1038/leu.2013.95. [DOI] [PubMed] [Google Scholar]
- 67.Zinzani PL, Pellegrini C, Derenzini E, et al. Long-term efficacy of the combination of lenalidomide and rituximab in elderly relapsed/refractory diffuse large B-cell lymphoma patients. Hematol Oncol. 2013;31:223–224. doi: 10.1002/hon.2049. [DOI] [PubMed] [Google Scholar]
- 68.Zinzani PL, Pellegrini C, Gandolfi L, et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: A phase 2 trial. Clin Lymphoma Myeloma Leuk. 2011;11:462–466. doi: 10.1016/j.clml.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Leonard J, Jung SH, Johnson JL, et al. CALGB 50401: A randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma. J Clin Oncol. 2012;30:510s. doi: 10.1200/JCO.2014.59.9258. (abstr 8000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin P, Jung S, Johnson J, et al. CALGB 50803(ALLIANCE): A phase 2 trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma. Hematol Oncol. 2013;31(suppl I):117. abstr 063. [Google Scholar]
- 71.Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014;165:375–381. doi: 10.1111/bjh.12755. [DOI] [PubMed] [Google Scholar]
- 72.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: An open-label, phase 2 trial. Lancet Oncol. 2014;15:1311–1318. doi: 10.1016/S1470-2045(14)70455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chong EA, Ahmadi T, Aqui N, et al. Combination of lenalidomide and rituximab overcomes rituximab-resistance in patients with indolent B-cell and mantle cell lymphomas. Clin Cancer Res. 2015;21:1835–1842. doi: 10.1158/1078-0432.CCR-14-2221. [DOI] [PubMed] [Google Scholar]
- 74.Ahmadi T, Chong EA, Gordon A, et al. Combined lenalidomide, low-dose dexamethasone, and rituximab achieves durable responses in rituximab-resistant indolent and mantle cell lymphomas. Cancer. 2014;120:222–228. doi: 10.1002/cncr.28405. [DOI] [PubMed] [Google Scholar]
- 75.Flinn IW, Mainwaring M, Peacock N, et al. Rituximab, lenalidomide, and bortezomib in the first-line or second-line treatment of patients with mantle cell lymphoma: A phase I/II trial. Blood. 2012;120 (abstr 2748a) [Google Scholar]
- 76.Morrison VA, Jung SH, Johnson J, et al. Therapy with bortezomib plus lenalidomide for relapsed/refractory mantle cell lymphoma: Final results of a phase II trial (CALGB 50501) Leuk Lymphoma. 2015;56:958–964. doi: 10.3109/10428194.2014.938333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaja F, De Luca S, Vitolo U, et al. Salvage treatment with lenalidomide and dexamethasone in relapsed/refractory mantle cell lymphoma: Clinical results and effects on microenvironment and neo-angiogenic biomarkers. Haematologica. 2012;97:416–422. doi: 10.3324/haematol.2011.051813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: A phase II study. J Clin Oncol. 2014;33:251–257. doi: 10.1200/JCO.2014.55.5714. [DOI] [PubMed] [Google Scholar]
- 79.Vitolo U, Chiappella A, Franceschetti S, et al. Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: Results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol. 2014;15:730–737. doi: 10.1016/S1470-2045(14)70191-3. [DOI] [PubMed] [Google Scholar]
- 80.Tilly H, Morschhauser F, Casasnovas O, et al. Lenalidomide in combination with R-CHOP (R2-CHOP) in patients with high burden follicular lymphoma: Phase 2 study. Blood. 2013;122 (abstr 248a) [Google Scholar]
- 81.Jerkeman M, Albertsson-Lindblad A, Kolstad A, et al. Lenalidomide, bendamustine, and rituximab as first-line therapy for patients 65 years with mantle cell lymphoma: Preliminary results from the Nordic Lymphoma Group MCL4 (LENA-BERIT) phase I-II trial. Blood. 2013;122 (abstr 4377a) [Google Scholar]
- 82.Smith SM, Pitcher B, Jung SH, et al. Unexpected and serious toxicity observed with combined idelalisib, lenalidomide and rituximab in relapsed/refractory B cell lymphomas: Alliance A051201 and A051202. Blood. 2014;124 (abstr 3091a) [Google Scholar]
- 83.Morschhauser F, Salles G, Le Gouill S, et al. A phase Ib study of obinutuzumab combined with lenalidomide for relapsed/refractory follicular B-cell lymphoma. Blood. 2014;124 (abstr 4458a) [Google Scholar]