Abstract
Meta-analyses of association results for blood pressure using exome-centric single-variants and gene-based tests identified 31 novel loci in discovery among 146,562 individuals with follow-up and meta-analysis in 180,726 additional individuals (Ntotal=327,288). These blood pressure loci are enriched for known cardiometabolic trait variants. Associations were also observed for the aggregation of rare/low-frequency missense variants in three genes, NPR1, DBH, and PTPMT1. In addition, blood pressure associations at 39 previously reported loci were confirmed. The identified variants implicate biological pathways related to cardiometabolic traits, vascular function, and development. Several new variants are inferred to have roles in transcription or as hubs in protein-protein interaction networks. Genetic risk scores constructed from the identified variants were strongly associated with coronary disease and myocardial infarction. This large collection of blood pressure loci suggests new therapeutic strategies for hypertension emphasizing a link with cardiometabolic risk.
Hypertension (HTN) or high blood pressure (BP) is a major risk factor for cardiovascular disease, chronic kidney disease, and mortality1. To date, in addition to rare mutations that cause monogenic high or low BP disorders2–4, candidate gene studies, genome-wide association studies (GWAS), and admixture mapping approaches5–15 have identified variants at more than 60 genetic loci that are associated with BP or hypertension. Most of the known BP loci identified in large population-based studies are common non-coding variants with small effects on BP.
The Human Exome BeadChip (Exome Chip; Illumina, Inc., San Diego, CA) was designed to facilitate identification of functional variants that contribute to human traits, by focusing on variants that alter amino acid sequence. The Exome Chip includes 247,039 markers of which >90% are non-synonymous or splice modulating exonic variants that were not covered by previous genotyping arrays. While variants on previous GWAS arrays are largely common [minor allele frequency (MAF) ≥0.05], 83% of the Exome Chip variants are rare (MAF<0.01) and another 6% are low frequency (MAF 0.01 to 0.05). Only 11% of the Exome Chip variants are common, including a set of 5,542 (approximately 2% of overall array content) common variants that were drawn from the associations reported in the NHGRI GWAS Catalog16.
To identify functional coding variation associated with BP, we conducted a two-stage study in up to 327,288 individuals who were genotyped with the Exome Chip (Figure 1) for systolic and diastolic BP (SBP and DBP), pulse pressure (PP), mean arterial pressure (MAP), and HTN. We identified single variant associations at 31 novel loci and gene-based associations for three novel genes (two of which overlapped with the single variant loci) associated with BP phenotypes. About half of the novel BP variants identified in this study reside in loci that were previously reported in GWAS to be associated with lipids, immunologic diseases, and metabolic phenotypes, suggesting common etiologies of BP and metabolic risk factors and an opportunity to identify therapies that more broadly impact hypertension in the context of cardiometabolic risk.
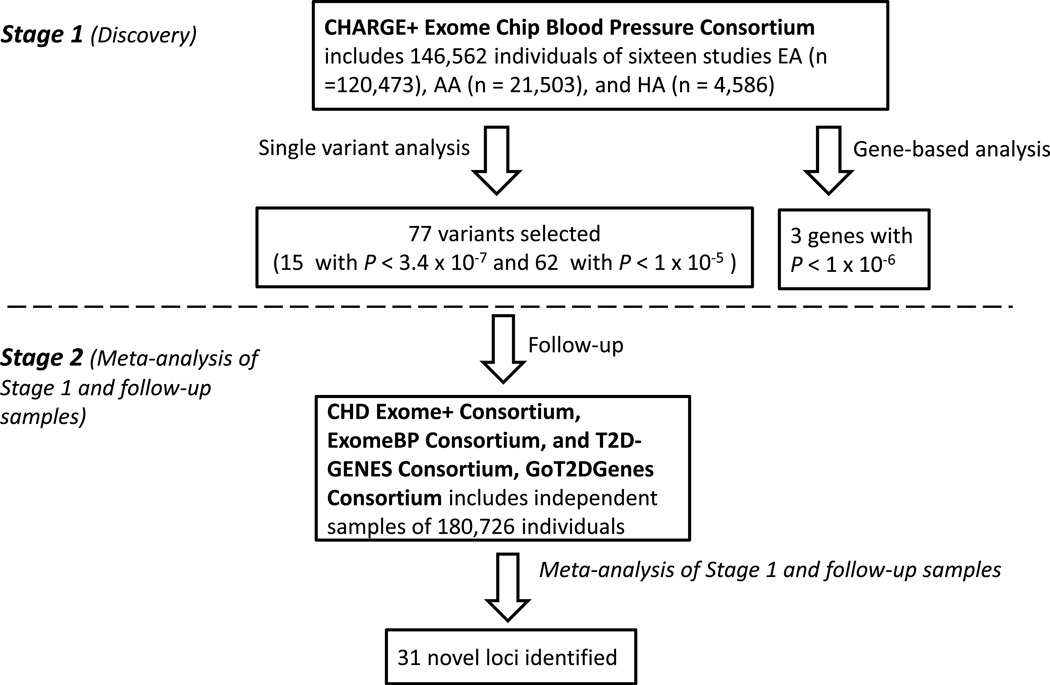
Figure 1. Overall study design.
In the discovery phase, single variant and gene-based analyses were performed for systolic and diastolic blood pressure, pulse pressure, mean arterial pressure, and hypertension among 146,562 individuals from the Cohorts for Heart and Aging Research in Genomic Epidemiology Plus (CHARGE+) Exome Chip Blood Pressure Consortium. Fifteen variants were significant (P<3.4×10−7) and 62 displayed P<1×10−5. In the follow-up phase, meta-analysis was performed for 77 variants with results from 180,726 individuals from the CHD Exome+ Consortium, ExomeBP Consortium, GoT2DGenes Consortium, T2D–GENES consortium.
New Loci Associated with BP by Single Variant Analyses
In the discovery stage (Stage 1), a total of 15 distinct novel candidate loci were associated (P<3.4×10−7) with at least one BP trait in a primary meta-analysis among samples of all ancestries and secondary meta-analyses among samples of European (EA) or African ancestry (AA) (Supplementary Table 1, Supplementary Figure 1). Meta-analysis using individuals from all ancestries identified 22 novel associations at 13 loci that met experiment-wide significance (Supplementary Table 1). All associations with P<1×10−4 for at least one trait in the primary analysis are listed in Supplementary Table 2. The sole locus that was identified in EA but not in the all-ancestry analysis was a rare missense variant rs3025380 in DBH [MAF 0.005, 0.001, and 0.003 in EA, AA, and Hispanic ancestry (HA) samples, respectively]. Meta-analysis of AA individuals identified a common missense variant rs12941884 in SEZ6 (MAF=0.21 and 0.12, respectively, in AA and EA) that was not identified in EA or all ancestry samples.
The Exome Chip contains 43 SNPs from loci previously identified in GWAS of BP5–15. Of these 43 loci, 39 were associated with at least one BP trait in Stage 1 analyses (P<0.05/43∼0.001) (Supplementary Table 3). Twenty-six of these SNPs met experiment-wide significance (P<3.4×10−7). Conditional analysis did not reveal any new independent variants at any of these previously identified loci5–15.
The 15 newly identified variants (P<3.4×10−7, Supplementary Table 1) and 62 additional variants (P <1×10−5 for at least one BP phenotype, Supplementary Table 2) from Stage 1 were selected for follow up in 180,726 independent individuals (Supplementary Methods). Of the 15 newly identified variants, 11 replicated (P<0.05/15∼0.0033) in the follow-up samples (Supplementary Tables 4, and 5). In Stage 2 analyses (i.e. joint meta-analysis of results from the Stage 1 and follow-up samples), we identified 48 novel BP variants at 31 loci (including the 11 replicated loci) associated with SBP, DBP, PP, or HTN at P<3.4×10−7 (MAP was not available in the follow-up analyses; Supplementary Tables 4 and 5). Among the top variants at the 31 loci, 13 were missense (Table 1). In Stage 2 analyses restricted to EA samples (Supplementary Table 4), all newly identified associations in EA samples meeting the significance threshold were also statistically significant in meta-analysis combining all ancestries (Supplementary Table 5) with the exception of rs1925153 in COL21A1. In addition, all of the variants except for the four that were nominated for follow up based on PP (SBP minus DBP) showed concordant directions of effects for SBP and DBP (Supplementary Table 6).
Table 1.
The newly identified significant blood pressure loci in meta-analysis of the discovery and follow-up samples(P<3.4 × 10−7)
| Discovery (n=146,562) |
Follow-up (n=180,726) |
Combined (n=327,288) |
ICBP Discovery (n=69,395) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Locus* | dbSNPID | Chr | Position | CA/ NCA |
CAF | Function† | Beta (SE) /Z score‡ |
P value | Beta (SE) /Z score‡ |
P value | Beta (SE)/Z score‡ | P value | IQ∥ or r2/IQ |
P value SBP/DBP |
|
Low-frequency variants (0.01 < MAF<0.05) | |||||||||||||||
| SBP | NPR1 | rs35479618 | 1 | 153662423 | A/G | 0.014 | E967K | 1.34(0.28) | 2.1 × 10−6 | 0.85(0.30) | 3.9 × 10−3 | 1.11(0.20) | 5.7×10−8 | n.a. | n.a. |
| SBP | SVEP1 | rs111245230 | 9 | 113169775 | C/T | 0.032 | D2702G | 0.94(0.18) | 2.9 × 10−7 | 0.44(0.19) | 2.2×10−2 | 0.70(0.13) | 1.2×10−7 | 1/0.91 | 0.009/0.003 |
| HTN | PTPMT1 | rs11537751 | 11 | 47587452 | T/C | 0.048 | S93L | 5.09 | 3.6 × 10−7 | 2.72 | 0.006 | 5.40 | 6.9×10−8 | 1/0.97 | 0.13/0.11 |
|
Common variants (MAF>0.05) | |||||||||||||||
| SBP | PRDM16 | rs2493292 | 1 | 3328659 | T/C | 0.151 | P633L | 0.42(0.09) | 4.0 × 10−6 | 0.32(0.09) | 7.2×10−4 | 0.37(0.07) | 1.4×10−8 | n.a. | n.a. |
| DBP | PABPC4 | rs4660293 | 1 | 40028180 | G/A | 0.208 | IN | 0.27(0.05) | 1.1 × 10−7 | 0.11(0.04) | 0.016 | 0.18(0.03) | 9.6×10−8 | 1∥ | 0.0030/0.0018 |
| SBP | SULT1C3 | rs6722745 | 2 | 108875244 | C/T | 0.338 | M194T | 0.28(0.08) | 3.3 × 10−4 | 0.26(0.07) | 9.0×10−5 | 0.27(0.05) | 1.1×10−7 | 0.99∥ | 0.37/0.37 |
| PP | CSNK1G3 | rs4530754 | 5 | 122855416 | G/A | 0.411 | IN | 0.22(0.05) | 4.5×10−6 | 0.13(0.04) | 2.5×10−3 | 0.17(0.03) | 9.9×10−8 | 1∥ | 0.03/0.46 |
| DBP | C5orf56 | rs2188962 | 5 | 131770805 | T/C | 0.366 | ncRNA_IN | −0.2(0.04) | 4.2×10−6 | −0.19(0.04) | 1.6×10−6 | −0.20(0.03) | 3.0×10−11 | 1∥ | 0.86/0.05 |
| DBP | SNORD32B | rs926552 | 6 | 29548089 | T/C | 0.111 | ITG | −0.31(0.07) | 8.5×10−6 | −0.22(0.07) | 1.6×10−3 | −0.26(0.05) | 7.2×10−8 | 0.88∥ | 0.44/0.45 |
| PP |
MSH5 SAPCD1 |
rs409558 | 6 | 31708147 | G/A | 0.176 | ncRNA_IN | −0.22(0.06) | 3.7×10−4 | −0.29(0.06) | 1.4×10−6 | −0.26(0.04) | 2.7×10−9 | 1/0.98 | 0.0019/0.10 |
| SBP | SLC22A7 | rs2270860 | 6 | 43270151 | T/C | 0.367 | SYN, splicing |
0.33(0.07) | 2.6×10−6 | 0.31(0.07) | 2.4×10−6 | 0.32(0.05) | 2.9×10−11 | 0.9∥ | 0.00013/0.037 |
| PP | COL21A1 | rs1925153§ | 6 | 56102780 | T/C | 0.445 | IN | −0.21(0.05) | 1.9×10−5 | −0.17(0.05) | 5.9×10−4 | −0.19(0.04) | 4.9×10−8 | 0.71∥ | 0.16/0.42 |
| DBP | PHIP | rs10943605 | 6 | 79655477 | A/G | 0.462 | IN | 0.18(0.04) | 1.2×10−5 | 0.15(0.04) | 5.4×10−5 | 0.16(0.03) | 3.3×10−9 | 1∥ | 0.05/0.01 |
| DBP | HOXA3 | rs6969780 | 7 | 27159136 | C/G | 0.125 | 5’UTR, splicing |
0.32(0.06) | 7.8×10−7 | 0.21(0.07) | 2.0×10−3 | 0.26(0.05) | 1.1×10−8 | 0.98∥ | 0.02/0.1 |
| PP | IGFBP3 | rs11977526 | 7 | 46008110 | A/G | 0.397 | ITG | −0.41(0.05) | 3.8×10−18 | −0.32(0.04) | 3.9×10−13 | −0.36(0.03) | 2.9×10−29 | 0.87∥ | 0.62/0.004 |
| DBP | NOS3 | rs891511 | 7 | 150704843 | A/G | 0.373 | IN | −0.25(0.04) | 1.8×10−8 | −0.26(0.04) | 2.0×10−9 | −0.26(0.03) | 2.0×10−16 | n.a. | n.a. |
| DBP | HRCT1 | rs76452347 | 9 | 35906471 | T/C | 0.191 | R63W | −0.25(0.05) | 1.1×10−6 | −0.20(0.05) | 1.1×10−4 | −0.23(0.04) | 6.8×10−10 | n.a. | n.a. |
| PP | PHF19 | rs1953126 | 9 | 123640500 | T/C | 0.331 | ITG | 0.27(0.05) | 6.3×10−8 | 0.10(0.05) | 0.035 | 0.17(0.03) | 1.8×10−7 | 0.99∥ | 0.11/0.86 |
| DBP | ADO | rs10995311 | 10 | 64564934 | G/C | 0.381 | P39A | −0.20(0.04) | 2.4×10−6 | −0.20(0.04) | 1.9×10−6 | −0.20(0.03) | 2.1×10−11 | n.a. | n.a. |
| DBP | CYP2C19 | rs4494250 | 10 | 96563757 | A/G | 0.319 | IN | 0.21(0.05) | 5.2×10−6 | 0.11(0.04) | 5.1×10−3 | 0.15(0.03) | 3.4×10−7 | 0.93/0.98 | 0.017/0.0030 |
| DBP | ARNTL | rs900145 | 11 | 13293905 | G/A | 0.336 | ITG | −0.25(0.05) | 9.1×10−7 | −0.15(0.05) | 0.002 | −0.20(0.03) | 1.8×10−8 | 1∥ | 0.0041/0.00087 |
| SBP | KCNJ11 | rs5219 | 11 | 17409572 | T/C | 0.320 | K23E | 0.48(0.07) | 1.8×10−11 | 0.21(0.06) | 9.4×10−4 | 0.32(0.05) | 4.9×10−12 | 0.94/1 | 0.00018/0.0023 |
| DBP | CERS5 | rs7302981 | 12 | 50537815 | A/G | 0.338 | C75R | 0.23(0.04) | 1.8×10−7 | 0.27(0.04) | 6.5×10−13 | 0.25(0.03) | 9.4×10−19 | 1∥ | 7.7×10−5/0.0053 |
| PP | MYH6 | rs452036 | 14 | 23865885 | A/G | 0.400 | IN | −0.23(0.05) | 1.6×10−6 | −0.31(0.05) | 1.4×10−11 | −0.27(0.03) | 2.4×10−16 | 0.89∥ | 0.64/0.094 |
| SBP | TNRC6A | rs11639856 | 16 | 24788645 | A/T | 0.193 | N185K | −0.37(0.08) | 7.7×10−6 | −0.30(0.08) | 3.6×10−4 | −0.34(0.06) | 1.3×10−8 | 0.99∥ | 0.068/0.54 |
| DBP | DPEP1 | rs1126464 | 16 | 89704365 | C/G | 0.215 | E351Q | 0.23(0.05) | 6.4×10−6 | 0.26(0.04) | 7.0×10−9 | 0.24(0.03) | 2.4×10−13 | 1/0.39 | 0.050/0.077 |
| DBP | TBX2 | rs8068318 | 17 | 59483766 | C/T | 0.350 | IN | −0.23(0.05) | 2.2×10−7 | −0.28(0.04) | 1.8×10−12 | −0.26(0.03) | 3.0×10−18 | 1∥ | 0.00080/9.0×10−6 |
| PP | DOT1L | rs2302061 | 19 | 2226772 | C/G | 0.163 | V1418L | 0.30(0.07) | 5.1×10−6 | 0.28(0.06) | 1.0×10−5 | 0.29(0.05) | 2.2×10−10 | 0.64∥ | 0.019/0.88 |
| PP | INSR | rs7248104 | 19 | 7224431 | A/G | 0.395 | IN | −0.20(0.05) | 1.8×10−5 | −0.20(0.04) | 3.3×10−6 | −0.20(0.03) | 2.6×10−10 | 1∥ | 0.16/0.43 |
| DBP | RGL3 | rs167479 | 19 | 11526765 | T/G | 0.448 | P162H | −0.26(0.04) | 6.4×10−10 | −0.33(0.04) | 3.8×10−20 | −0.30(0.03) | 4.2×10−28 | n.a. | n.a. |
| SBP | ZNRF3 | rs4823006 | 22 | 29451671 | G/A | 0.424 | 3’UTR | −0.33(0.07) | 8.7×10−7 | −0.20(0.06) | 9.2×10−4 | −0.26(0.05) | 7.9×10−9 | 0.98∥ | 0.29/0.093 |
CA/NCA, coded allele/non-coded allele; CAF: coded allele frequency; SYN, synonymous; IN, intronic; ITG, intergenic; UTR3, 3’ untranslated region; The discovery meta-analysis was performed in CHARGE+ Exome Chip BP Consortium samples (n=146,562); The follow-up meta-analysis was performed with samples from the CHD Exome+ Consortium, ExomeBP Consortium, GoT2DGenes Consortium, T2D–GENES consortium samples (n=180,726); The “combined” or joint meta-analysis was performed with both discovery and follow-up samples (n = 327,288); ICBP Discovery, the discovery sample for International Consortium for Blood Pressure; n.a, not available; IQ, Imputation quality; r2/IQ, linkage disequilibrium between the best proxy in ICBP and the one in “dbSNPID” column and imputation quality for the best proxy.
Loci are named according to the closest gene based on the position of the lead SNP.
Amino acid substitution is provided for a missense variant.
Meta-analysis used the inverse variance method for DBP, PP, and SBP and used the optimal Z score method for HTN.
rs1925153 was significant from joint meta-analysis of EA only samples, the rest were from samples of all ancestries.
The same variants in “dbSNPID” column were analyzed in ICBP.
Three of the 31 significant novel SNPs were low-frequency (MAF 0.01 to 0.05). These SNPs encode non-synonymous substitutions in the genes NPR1 (rs35479618), SVEP1 (rs111245230), and PTPMT1 (rs11537751). NPR1 encodes natriuretic peptide receptor 1 and has been reported to be associated with BP regulation in animal models17,18 but not previously in humans; SVEP1 and PTPMT1 are novel BP genes. The minor alleles of all three SNPs were associated with increased BP and had larger absolute effects on BP than the alleles of any of the newly identified common variants. For example, each minor allele of rs35479618 was associated with an increase of 0.85 mm Hg in SBP in the follow-up samples compared with a maximum absolute difference (per minor allele) among the novel common variants of 0.43 mm Hg in SBP (for rs8068318 in TBX2; Supplementary Table 5).
Of the 28 newly identified common variants for BP, 14 were genome-wide significant in prior GWAS of lipids19, immunologic disease20–22, diabetes23–25, kidney function26, age at menarche27, resting heart rate28, waist-hip ratio29, and homocysteine concentration30, but not BP (Table 2 and Supplementary Table 7). Six additional variants were reported for several phenotypes (Table 2) in previous candidate gene, patent filing or GWAS studies, but their P values were not specified or did not reach the genome-wide significance level31–36. By contrast, the remaining eight variants were missense SNPs that have not been reported in the NHGRI GWAS Catalog for any trait (Table 2). Several genes in Table 2 contain multiple variants showing distinct allelic roles. HOXA3 and NOS3, harbor variants rs17428471 (HOXA3)12 and rs3918226 (NOS3)10 with genome-wide significant BP association that are independent of the Exome Chip variants (r2=0.007 for rs17428471 with rs6969780 and r2=0.007 for rs3918226 with rs891511, respectively, in the 1000 Genomes data). A variant rs2651899 in PRDM16 has been reported to be associated with migraine37, but this variant is not in LD with the new BP variant rs2493292 (r2=0.01 in the 1000 Genomes data), suggesting predisposition to distinct vascular consequences for different variants at this locus. In addition, PRDM16 has been shown to play a critical role in vascular development38, adipocyte function in subcutaneous fat, and development of diabetes39. Finally, several variants in DOT1L were reported to be associated with cartilage thickness and hip osteoarthritis40. The new BP variant rs2302061, however, was not in LD with any of the prior identified signals at this locus40.
Table 2.
Novel common BP SNPs associated with non-BP traits
| Locus* (Function) | dbSNPID | Chr:Position | CA/NCA | CAF | GWAS Trait† | Amino Acid Substitution |
Literature Lab Term(s)‡ |
|---|---|---|---|---|---|---|---|
| SNPs not previously reported in GWAS | |||||||
| PRDM16 (NS) | rs2493292 | 1:3328659 | T/C | 0.15 | n.a. | Pro633Leu | |
| SULT1C3 (NS) | rs6722745 | 2:108875244 | C/T | 0.34 | n.a. | Met194Thr | |
| HRCT1 (NS) | rs76452347 | 9:35906471 | T/C | 0.19 | n.a. | Arg63Trp | |
| ADO (NS) | rs10995311 | 10:64564934 | G/C | 0.38 | n.a. | Pro39Ala | |
| CERS5 (NS) | rs7302981 | 12:50537815 | A/G | 0.34 | n.a. | Cys75Arg | |
| TNRC6A (NS) | rs11639856 | 16:24788645 | A/T | 0.19 | n.a. | Asn185Lys | |
| DOT1L (NS) | rs2302061 | 19:2226772 | C/G | 0.16 | n.a. | Val1418Leu | |
| RGL3 (NS) | rs167479 | 19:11526765 | T/G | 0.448 | n.a. | Pro162His | |
| SNPs previously reported to be significant in GWAS of other traits§ | |||||||
| PABPC4 (IN) | rs4660293 | 1:40028180 | G/A | 0.21 | HDL | ||
| CSNK1G3 (IN) | rs4530754 | 5:122855416 | G/A | 0.41 | LDL and TC | ||
| C5orf56 (IN) | rs2188962 | 5:131770805 | T/C | 0.35 | Crohn’s Disease | ||
| rs926552 | 6:29548089 | T/C | 0.11 | T1D | |||
|
MSH5-SAPCD1 (IN) |
rs409558 | 6:31708147 | G/A | 0.18 | SLE | ||
| IGFBP3 | rs11977526 | 7:46008110 | A/G | 0.40 | IGFBP3 | Insulin, 9%, IGF-1 signaling, 55% | |
|
PHF19 (5’ near gene) |
rs1953126 | 9:123640500 | T/C | 0.33 | RA | ||
| rs900145 | 11:13293905 | G/A | 0.34 | Age at Menarche | |||
| KCNJ11 (NS) | rs5219 | 11:17409572 | T/C | 0.32 | T2D | Lys23Glu | Insulin, 0.6%, T2D, 2.5% |
| MYH6 (IN) | rs452036 | 14:23865885 | A/G | 0.40 | Resting Heart Rate | Heart Development, 73%, Hypertrophy model, 83%, Cardiac muscle contraction, 84% | |
| DPEP1 (NS) | rs1126464 | 16:89704365 | C/G | 0.22 | Homocysteine Concentration |
Glu351Gln | |
| TBX2 (IN) | rs8068318 | 17:59483766 | C/T | 0.35 | Creatinine and eGFR |
Heart development, 17.5% | |
| INSR (IN) | rs7248104 | 19:7224431 | A/G | 0.395 | TG | Insulin, 90%, IGF-1 signaling, 45%, T2D, 93%, Hypertrophy model, 5.4% | |
| ZNRF3 (UTR3) | rs4823006 | 22:29451671 | G/A | 0.424 | WHR | ||
| SNPs previously reported in patent filing, candidate gene or GWAS∥ | |||||||
| SLC22A7 (SYN) | rs2270860 | 6:43270151 | T/C | 0.37 | HTN (patent filing) | ||
| COL21A1 (IN) | rs1925153 | 6:56102780 | T/C | 0.45 | Bipolar disease traits | ||
| PHIP (IN) | rs10943605 | 6:79655477 | A/G | 0.46 | Colon cancer (patent filing) |
||
| HOXA3 (UTR5) | rs6969780 | 7:27159136 | C/G | 0.13 | Hypospadias | ||
| NOS3 (IN) | rs891511 | 7:150704843 | A/G | 0.37 | Endothelium-dependent vasodilation | Heart Development, 6.7%, T2D, 3.9%, Cardiac muscle contraction, 14.5% | |
| CYP2C19 (IN) | rs4494250 | 10:96563757 | A/G | 0.32 | Breast cancer | ||
SNPs included in this table are common SNPs in Table 1. CA/NCA, coded allele/non-coded allele; CAF, coded allele frequency; IN, intron; NS, nonsynonymous; UTR3, 3’ upstream; UTR5, 5’ upstream; HDL/LDL, high/low- density cholesterol; TC, total cholesterol; T1D/T2D, Type I/Type 2 diabetes; SLE, systemic lupus erythematosus; IGFBP3, insulin-like growth factor-binding protein 3; RA, rheumatoid arthritis; TG, triglyceride; WHR, waist/hip ratio.
Loci are named according to closest gene based on the position of the index SNP.
Indicates whether a SNP was reported in previous genome-wide association studies (GWAS). n.a., not available.
Reported results were part of identifying biological and biochemical terms that were significantly associated with the investigated gene set using Literature Lab database. Percent shows relative weight of references to a BP candidate gene in relation to associated pathways / terms for the full gene set. Out of three classes of significances (STRONG, MODERATE and POSITIVE) above we reported only STRONG class.
Reported to be significant in GWAS using P<5 × 10−8 or pre-specified significance levels in the reported study. Details of association direction were included in Supplementary Table 7.
P values were not mentioned or did not reach the specified significance level.
Together, the 31 newly identified single variants explain 0.7% and 1.3% of inter-individual variation in SBP and DBP, respectively. The previously established and newly identified variants together explain 2.8% and 2.9% of phenotypic variation in SBP and DBP, respectively.
Gene Level Analyses
We considered the possibility that an aggregation of rare or low-frequency coding alleles at individual genes contributes to BP variation and tested specifically for effects of non-synonymous, stop codon, and splicing coding variants with MAF<0.05 (T5 test) or MAF<0.01 (T1 test) using the seqMeta package. The standard burden test41,42, which is sensitive for detecting association when all variants contribute effects on BP in a concordant direction, identified an aggregation of rare and low-frequency coding alleles in PTPMT1 that contribute to higher odds of HTN (experiment wide significance P<1×10−6, Table 3, Supplementary Table 8A). The SKAT test43, which is designed to detect effects of alleles that collectively contribute to higher and lower BP effects, identified significant BP associations for DBH (T1) and NPR1 (T5; Table 3, Supplementary Table 8A). Among additional individuals of European ancestry (up to 154,543 individuals) who were used for follow-up analysis, gene-based SKAT (with the RAREMETAL package) was performed for inverse normal transformed DBP, SBP, PP, and HTN (see Methods). The gene-based associations replicated in the follow-up samples at P<0.05/3∼0.017 for NPR1 (P=4.4×10−5 for SBP) and were marginally significant for PTPMT1 (P=0.019 for HTN) and DBH (P=0.053 for DBP) (Supplementary Table 8B).
Table 3.
CHARGE+ Exome Chip BP Consortium: significant genes in burden and sequence kernel association tests
| Gene | Chr | Test* | T1/T5† | Phenotype | Beta (SE) /Qmeta‡ |
P value§ | N Variants∥ | CAF |
|---|---|---|---|---|---|---|---|---|
| PTPMT1 | 11 | Burden | T5 | HTN | 0.05(0.01) | 3.5×10−7 | 4 | 0.053 |
| NPR1 | 1 | SKAT | T5 | MAP | 270678.8 | 4.4×10−8 | 14 | 0.025 |
| DBH | 9 | SKAT | T1 | MAP | 145331.4 | 9.2×10−7 | 27 | 0.028 |
CAF, cumulative coded allele frequency for variants used in an analysis. The experiment wide significance level for gene-based tests is P<1×10−6.
The standard burden test collapses the rare variants into a single variable and tests the association between this variable with a phenotype; the sequence kernel association test (SKAT) was designed to detect effects of alleles that collectively contribute to higher and lower BP effects.
Meta-analysis was conducted at the gene level to evaluate aggregate effects from multiple non-synonymous or splicing variants with MAFs<0.01 (T1) and <0.05 (T5).
The burden test yields beta/SE and the SKAT test provides Qmeta.
In pooled samples of all ancestries.
Number of variants used in analysis.
Twenty-eight previously reported genes associated with monogenic BP disorders3 contained at least two non-synonymous, stop codon, or splice-site coding variants with MAF <0.05 on the Exome Chip. Burden testing of these 28 genes identified a statistically significant association of SLC12A1 (26 variants all having MAFs<0.005) with SBP (P=0.0006<0.05/28; T1 test; Supplementary Table 9). Mutations in SLC12A1, the Na-K-2Cl co-transporter, cause Bartter’s syndrome, a Mendelian salt-wasting condition associated with hypotension44. The 26 variants in SLC12A1, however, did not overlap with the previously reported Bartter’s syndrome variants44. The other 27 monogenic BP genes did not reach statistical significance in standard burden testing. Additionally, none of the 28 genes showed significant association with BP using the SKAT test43 (all P>0.0006; Supplementary Table 9).
Inferred Function of the Identified BP Loci
We applied several computational strategies and conducted cis expression quantitative locus (eQTL) analysis to infer biological functions associated with genes at the 31 significant single variant BP loci (see details in Supplementary Methods).
Disease and pathway enrichment analysis
We examined functional annotations derived from pre-compiled gene sets in GeneGO and literature-based inference in Literature Lab45. In GeneGO biological processes, the 31 novel loci were enriched for cell signaling and development functions (e.g. “regulation of signaling”, “regulation of growth”) compared with largely cardiovascular functions (e.g. “negative regulation of [smooth] muscle contraction”, “blood circulation”) for the 39 validated BP loci (Supplementary Table 10). The novel loci were also enriched for several conditions related to cardiovascular and metabolic disease (e.g. “myocardial ischemia”, “congenital hyperinsulinism”, “acid-base imbalance”) whereas the validated loci were enriched for conditions more directly related to BP or cardiovascular conditions (e.g. “arrhythmias, cardiac”, “hypertension”, “hypotension”). Significant Literature Lab45 (Supplementary Table 11) pathways and disease MeSH headings were enriched for insulin-related terms (e.g. “IGF-1”, “type II diabetes”, “hyperinsulinism”) for the novel loci compared to BP-related terms (e.g. “cardiac muscle contraction”) and cardiovascular electrophysiology (e.g. “antiarrhythmics”) for the validated loci; both sets of loci were significant for “heart development”. In the Literature Lab45 anatomical annotations, the cardiovascular system (e.g. “myocardium”, “heart ventricles”) was highlighted for both the novel and validated SNPs, while the validated SNPs also associated with the renal system (e.g. “nephron”, “urinary tract”). Almost no annotations for either GeneGO or Literature Lab45 were unique to the set of combined novel and validated loci with the exception of a few terms predominantly related to BP or the renal system.
Protein-Protein Interaction Analysis
Using NCBI’s protein-protein interaction (PPI) network resources (Supplementary Methods), a total of 399 genes were found to be connected to at least one of the 31 novel BP genes (Supplementary Figure 2). Ordered on the basis of connectivity (“degree”; Supplementary Table 12), a measure that signifies a hub disposition in the PPI network, the top five BP candidate genes were INSR, PABPC4, NOS3, IGFBP3, and DOT1L. Based on “Google” page-rank, a connectivity measure that recognizes degree of connectivity while also emphasizing connections between highly connected nodes, the five top genes differed from ordering based on connectivity alone by the replacement of IGFBP3 by PTPMT1 (Supplementary Table 12).
ENCODE and Roadmap Epigenomics Analyses
RegulomeDB46 and HaploReg47 evaluations of potential cis regulatory functions identified rs8068318 (intronic to TBX2) as having the highest score among loci (or their LD proxies) that showed relatively strong evidence for a role in transcription (Supplementary Table 13). This SNP maps to an active TBX2 promoter histone mark in lung fibroblast and DNAse I hypersensitivity marks in seven cell types, while overlapping with five transcriptional regulatory motifs. TBX2 is a member of a highly conserved T-box family of transcription factors and has been implicated in cardiac developmental abnormalities48,49 and kidney function26.
cis-eQTL Analysis
The 31 newly identified BP variants were queried for cis-eQTL association (Supplementary Table 14) in over 5,000 participants from the Framingham Heart Study (FHS), using microarray-based transcriptomic profiling of RNA from whole blood. A total of 720 SNP-transcript pairs were tested. Forty-three pairs (representing 17 variants) were significant at FDR<10%, among which eight variants were cis-eQTLs for multiple gene transcripts. For example, rs1953126 (near the 5’-UTR of PHF19) is a cis-eQTL for PHF19 and for multiple nearby genes including C5, GSN, PSMD5, RAB14, FBXW2, and TRAF1. Query of publicly available eQTL databases via GRASP50 and recent publications51,52 based on profiling of whole blood or other tissue types51–58 yielded eQTL assignments that were concordant with the FHS findings for most variants listed in Supplementary Table 14.
Effects of BP-associated Variants on Clinical Outcomes
We considered the aggregate effects of the BP loci on BP-related clinical outcomes using new Exome Chip-based results for coronary artery disease/myocardial infarction (CAD/MI), including 42,335 cases and 78,239 controls59, and for renal function measured by glomerular filtration rate (GFR) in up to 111,655 individuals. For 59 of the 70 BP associated SNPs, alleles that were associated with higher BP were also associated with increased odds of CAD/MI (Supplementary Tables 15 and 16), a highly significant concordance with the known influence of BP on CAD/MI (sign test, binomial P=4.5×10−9). Similarly, genetic risk scores (GRS) constructed from the 70 BP SNPs using weights derived from their effects on SBP, DBP, and MAP were highly significantly associated with CAD/MI with odds-ratios (per 1 mm Hg increment in SNP-based BP) of 1.05 (P=8.6×10−44), 1.08 (P=1.9×10−41), and 1.06 (P=1.1×10−45) respectively (Supplementary Table 17, Supplementary Methods). GRSs constructed solely from the rare/low-frequency variants at the three loci with significant gene-based tests (DBH, NPR1, PTPMT1) were significant for CAD/MI using MAP-based weightings for DBH (P=0.026) and HTN-based weightings for PTPMT1 (P=0.003) with a non-significant concordant trend using MAP-based weightings for NPR1 (P=0.13; Supplementary Table 18). By contrast, BP-raising alleles for only 39 of the 70 BP associated SNPs were associated with diminished kidney function (CKD) as reflected by lower GFR, indicating a degree of concordance that was not significant (sign test, binomial P=0.40). A similar lack of association was observed for the BP GRS associations with GFR using weights for SBP (P=0.18), DBP (P=0.63), and MAP (P=0.31).
Discussion
Through a two-stage study design of discovery (n=146,562) followed by external look ups (n=180,726) and joint analysis (n=327,288), we identified single variant associations at 31 novel loci and gene-based associations for three novel genes (two of which overlapped with the single variant loci) associated with BP phenotypes. We also confirmed common variants at 39 previously reported BP loci, raising the number of statistically significant BP loci in our study to 71 and extended the number of non-monogenic BP-associated loci5–15 to over 90. The sample size for the joint analysis in this study is far larger than any prior genetic study of BP5–15. This large increase in sample size is an important reason for the discovery of many new BP loci and likely explains why some of the newly identified common loci were not discovered in previous BP GWAS. In addition, direct genotyping of coding variants likely added incremental power over imputed genotypes and tagging SNPs that were the basis of prior GWAS, suggesting that novel common variants will continue to be identified for BP phenotypes using the same set or similar set of samples with exome sequencing and whole genome sequencing. Furthermore, phenotypic and possibly genetic heterogeneity (due to additional samples in this study), differences in analysis plans, and the play of chance may be additional explanations of why some of the common variants identified in this study were not identified in prior BP GWAS.
Fourteen of the novel BP variants identified in the present study reside in loci that were previously reported in GWAS to be associated with lipids19, immunologic diseases20–22, and metabolic phenotypes23–25, 29 (Table 2 and Supplementary Table 7). Thirteen of the previously identified BP variants were also linked to non-BP traits/diseases (Supplementary Table 19). Considerable evidence has accumulated linking high BP to insulin resistance, altered lipid levels, inflammation, and other features of the metabolic syndrome60–65. Gene set enrichment, regulatory sequence variation, and PPI annotations of the new BP loci implicate genes that contribute to cardiac structure and function as well as insulin signaling and type 2 diabetes. In addition, among the previously reported BP genes that were confirmed in our study, ATXN2, GRB14, HECTD4, PTPN11, and SLC39A8 (Supplementary Table 3) have been proposed as candidate genes for metabolic syndrome based on their associations with metabolic traits and inflammatory biomarkers65.
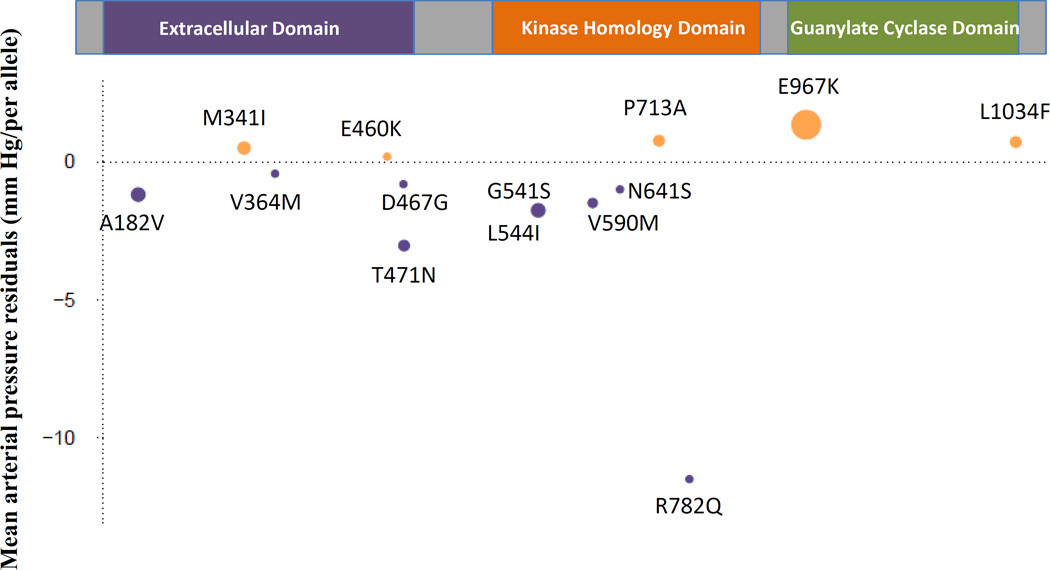
The NPR1 gene was associated with BP in both single variant and gene-based tests. This gene encodes the receptor for atrial and B-type natriuretic peptides, which regulate blood volume and BP17,18. The functional consequences of the Glu967Lys amino acid substitution that is encoded by rs35479618 (the significant NPR1 SNP in single variant analysis) is unknown, but the change results in opposite charge and a large difference in side chain volume, and is predicted to be possibly damaging (score=0.513) by Polyphen-266. The effects of the 13 rare and one low-frequency variants in NPR1 varied in directions, explaining why gene-based testing was significant using SKAT43, which is sensitive to BP-raising and lowering effects, rather than burden41,42 testing, which requires a consistent direction of BP effect, (Figure 2, Supplementary Figure 3). Of note, Npr1 knockout mice have hypertension, cardiac hypertrophy, and sudden death phenotypes17,18,67 and mice with only one copy of the Npr1 gene have salt-sensitive hypertension compared to wild type mice17. Future studies are warranted to determine if humans carrying the rare BP-increasing alleles of NPR1 also have salt-sensitive hypertension. We have previously demonstrated that common variation that raises atrial natriuretic peptides level lowers BP13, suggesting the potential for BP-lowering strategies that target natriuretic peptide interaction with natriuretic peptide receptors. Similarly, molecular mimicking of the action of BP-lowering alleles in NPR1 may be worth exploring as a novel BP treatment.
Figure 2. NPR1 Gene: Low-frequency and rare variants associated in aggregate with mean arterial pressure.
The NPR1 protein (1,061 amino acids) is comprised of three domains: extracellular domain, kinase homology domain, and guanylate cyclase domain. The effects of the 14 low-frequency and rare variants after adjustment for age, age2, sex, and body mass index on mean arterial pressure are shown for higher (tan) or lower (purple) values in mm Hg; dot area is proportional to the number of minor allele carriers. The minor allele of rs35479618 (MAF ∼ 0.012, E967K), was carried by 3,164 participants. The minor allele of rs201787421 (MAF ∼ 2.6×10−5 R782Q), was carried by 5 participants.
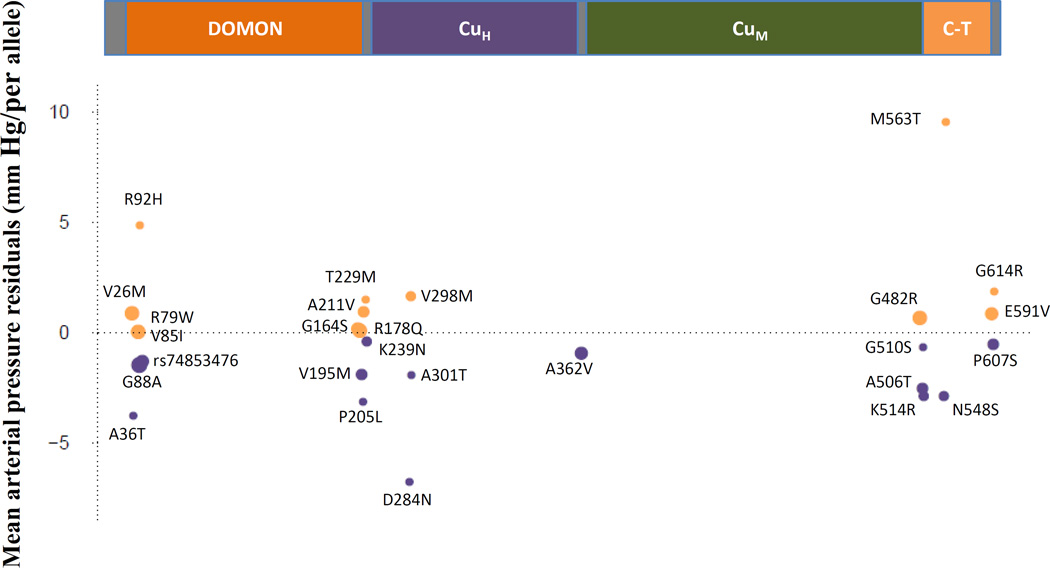
Both single variant and gene-based (T1) analysis in Stage 1 identified DBH as a BP gene (Figure 3). DBH codes the enzyme dopamine beta hydroxylase, which catalyzes the transformation of dopamine to norepinephrine. Both dopamine and norepinephrine act on the sympathetic nervous system, influencing a variety of complex traits including BP. Impaired dopamine beta hydroxylase activity has been identified in individuals with severe autonomic failure, including orthostatic hypotension68,69 , and mutation of DBH has been identified in two individuals with autonomic dysfunction70. The rare minor allele of rs3025380, encoding the Gly88Ala non-synonymous substitution, was associated with a comparatively large reduction of 1.81 mm Hg in MAP even though the amino acid change is predicted to be remote from the active site71. Inhibition of DBH has long been considered a potential target for anti-hypertensive therapy72 but these efforts have been undermined due to the broad involvement of catecholamines in a variety of critical biologic processes73,74 and the potential for undesirable side effects.
Figure 3. DBH Gene: Rare variants associated in aggregate with mean arterial pressure.
The DBH protein (617 amino acids) contains the dopamine β-monooxygenase N-terminal (DOMON) domain, the catalytic core (the CuH and CuM domains) and the C-terminal (C–T) domain. The effects of the 27 rare variants after adjustment for age, age2, sex, and body mass index on mean arterial pressure are shown for higher (tan) or lower (purple) values in mm Hg. The minor allele of rs74853476 (MAF ∼ 0.0015), a splicing variant, was carried by 291 participants. The minor allele of rs201681337 (MAF ∼ 7.9×10−5, A301T), was carried by 4 participants.
The remaining significant gene in gene-based testing was PTPMT1, which codes for mitochondrial protein tyrosine phosphatase 1. Knockdown of PTPMT1 expression in a rat pancreatic insulinoma cell line was found to enhance ATP production and insulin secretion75, which is closely aligned with the insulin and cardiometabolic regulatory features of many of the novel BP loci identified in this study. In addition, targeted burden testing of uncommon and rare variants in genes that cause monogenic BP disorders identified a significant BP association with SLC12A1, the Na-K-2Cl co-transporter that is well established to harbor rare mutations that cause Bartter’s syndrome, a salt wasting condition associated with hypotension44.
The Exome Chip array was designed to aid in the search for rare functional variants with large effect sizes. This study did not, however, identify any rare variants associated with BP phenotypes through single variant analyses, suggesting that rare variants with large effects on BP are an uncommon occurrence. With the current sample size, this study was not adequately-powered to identify rare variants with only modest effect sizes. Within the predominant class of variants studied (i.e. low-frequency and rare non-synonymous SNPs), there may not be a large enough number of variants or effects of sufficient size to account for a substantial proportion of the remaining missing heritability of BP. Nevertheless, this study greatly extends the number of known BP-associated loci and moreover demonstrates their potential relevance to cardiovascular disease. The discovery of a total of 32 new BP loci (31 from single variant tests, 1 from gene-based tests) and their overlap with other disease-related phenotypes suggest common etiologies of BP and metabolic risk factors and an opportunity to identify therapies that more broadly impact hypertension in the context of cardiometabolic risk.
Online Methods
Study Participants
A total of 146,562 individuals of European American (EA) (n=120,473), African American (AA) (n=21,503), and Hispanic American (HA) (n=4,586) contributed from 16 studies (Supplementary Table 20 and Supplementary Note) were included in the discovery stage association analyses. The entire discovery sample was also included in the meta-analyses of discovery and follow-up stage results (Figure 1). All study participants provided written informed consent for genetic research, with the exception of the BioVU biorepository, in which DNA was extracted from discarded blood collected during routine clinical testing and was linked to de-identified medical records. All studies received approval to conduct this research from their respective Institutional Review Boards. Studies contributing to the discovery analyses included a wide range of mean measured BP values (110 to 142 mm Hg for SBP and 69 to 84 mmHg for DBP), hypertension prevalence (2% to 77%), and proportion of individuals taking anti-hypertensive medications (0.6 to 63%) (Supplementary Table 20).
Genotyping and Quality Control
All samples were genotyped on the Illumina Infinium Human Exome Array v1.0 or v1.1 (Supplementary Table 21). Ten studies (51,106 individuals) were jointly called at the Human Genetics Center of the University of Texas Health Science Center in Houston76. Six additional studies followed genotyping calling protocols from Illumina or from the CHARGE consortium, and strand assignment for allele encoding specified by the CHARGE consortium76. All studies followed quality control guidelines recommended by the CHARGE analysis committee. Quality control procedures were further applied at the cohort level as described in Supplementary Table 21. Variants were removed for genotype call rate less than 95%, HWE p-value less than 1×10−6, and concordance rate (between overlapping variants from previous GWAS and the Exome Chip) less than 95%; individual samples were removed for call rate less than 95%, discordance rate less than 95% with GWAS data, or in the event of a suspected sample swap, sex mismatch, or heterozygosity F-value greater than 10.
BP Phenotypes
In the discovery stage, the BP phenotypes included were SBP, DBP, PP (SBP minus DBP), and MAP (1/3 SBP + 2/3 DBP). A participant was classified as having HTN if she/he had SBP ≥140 mm Hg, or DBP ≥90 mm Hg, or was taking anti-hypertensive medication. SBP and DBP values were obtained from the first examination attended for longitudinal studies; when available, the average of two single occasion measurements was used for SBP and DBP. To account for the reduction in BP due to medication use, all individuals taking BP lowering medication had15 mm Hg added to the measured SBP, and 10 mm Hg to the measured DBP15. The four continuous BP traits are moderately or highly correlated such that among the larger contributing cohorts, the ranges of correlations were: 0.70–0.82 (SBP-DBP), 0.92–0.95 (SBP-MAP), 0.73–0.89 (SBP-PP), 0.92–0.99 (DBP-MAP), 0.20–0.45 (DBP-PP), and 0.43–0.68 (MAP-PP). Such correlations appeared to be consistent across different ethnic populations within these same studies.
Association Analyses and Meta-analyses
Power Estimation
Nearly 90 percent of the markers on the Exome Chip are low-frequency (MAF 0.01–0.05) or rare (MAF <0.01) variants. Power for association was evaluated for MAP assuming a mean of 100 mm Hg with standard deviation of 10 mm Hg using QUANTO77 for a sample size n=150,000 at the significance level of 3.4 × 10−7 for a variant with MAF of 0.0005, 0.001, 0.005, or 0.01. To reach 80% power, an effect size of 5, 3.5, 1.6, or 1.1 mm Hg, is needed, respectively, for a variant with MAF=0.0005, 0.001, 0.005, or 0.01.
The Fraction of the Common Variants Tagged by the Exome Chip
We downloaded the phase 3 genotype data for the European ancestry from HapMap project. The phase 3 file “hapmap3_r2_b36_fwd.CEU.qc.poly” includes 1,416,121 variants (1,352,770 with MAF>0.01 and 1,223,919 with MAF> 0.05). We used the PLINK command “show-tags” to estimate the number of common variants (MAF>0.05) that can be tagged by Exome Chip variants. We estimated that 172,220 (linkage disequilibrium r2≥0.5) and 88,186 (linkage disequilibrium r2≥0.8) common SNPs (MAF >0.05) can be tagged by the Exome Chip variants. Compared to the number of variants tagged by a GWAS chip (e.g. Affymetrix 500K), the Exome Chip tags much fewer common variants.
Cohort-specific Analysis
Gene-based (or region-based) testing was performed using the seqMeta package78. Covariates included age, age-squared, sex, body mass index (BMI), and principle components (if applicable) to account for population structure. All variants were recoded to conform to the alleles specified in a “Recode” file distributed to each study. In all analyses, variant effects were modeled additively. Conditional analysis was performed to identify independent BP signals at previously reported BP loci5–15 using the seqMeta package78 by adjusting at the cohort level for the previously reported GWAS SNP with the smallest p-value in association analysis. Similarly, for any newly identified locus with multiple variants, conditional analysis was performed by adjusting for the most significant variant in the region to identify non-redundant signals.
Meta-analysis at the Single Variant Level
Meta-analysis of single variant associations from discovery and follow-up stage results was performed using the inverse variance weighted fixed-effects method79 implemented in the seqMeta package78. In the discovery stage, the primary meta-analysis was performed in all samples to identify variants showing consistent effects with BP traits across multiple ancestry groups. Secondary analysis was performed in each of the three ancestries separately to identify novel variants with different ancestral origin. Meta-analysis was also performed on results from conditional analysis and compared with the original meta-analysis to identify non-redundant signals. Although we performed association and meta-analysis on all genotyped variants that passed quality control, we only reported results from about 147,000 variants that had minor allele counts (MACs) ≥30 in meta-analyses of all samples. Since the BP traits are highly correlated, we used an array-wide Bonferroni-corrected significance threshold of 3.4 ×10−7 (=0.05/147,000). The Exome Chip array contains numerous previously published variants or their LD proxies, mostly from GWAS using imputed genotype information for a variety of human traits. Using exome chip experimental genotypes, associations from previous BP GWAS5–15 were considered significant with P values ≤ 0.05/n, where n is the number of previously identified SNPs or SNPs that showed at least moderate LD (r2≥0.3) on the Exome Chip.
Meta-analysis at the Gene Level
Meta-analysis was also conducted at the gene level to evaluate aggregate effects from multiple non-synonymous and splicing variants with MAFs ≤0.01 (T1) and ≤ 0.05 (T5) in a gene using both the sequence kernel association test (SKAT)43 and the standard burden test41,42 implemented in the seqMeta package78. The standard burden test collapses the rare variants and has optimal properties when these variants all have the same directionality and magnitude of effect on phenotype. In contrast, SKAT aggregates individual variant score test statistics and offers better power compared to the burden test when there are a variety of effect sizes and directions, e.g. both protective and deleterious effects in a gene43. Approximately 17,000 genes were included two or more non-synonymous variants in the primary meta-analysis of all study samples. An association was deemed to be signficant at P<1 ×10−6 for gene-based tests. Among up to 154,543 individuals of European ancestry from CHD Exome+ Consortium, ExomeBP Consortium, GoT2DGenes Consortium, T2D–GENES consortium (Supplementary Note), gene-based SKAT was applied to HTN and inverse normal transformed DBP, SBP, PP using the RAREMETAL software package80. We performed lookup in their SKAT results for the genes that displayed P<1 ×10−6 in Stage 1 analysis of this study.
The Follow-up Study at the Single Variant Level
The follow-up study was performed in external samples (follow-up samples) including a total of 180,726 individuals from the CHD Exome+ Consortium, ExomeBP Consortium, GoT2DGenes Consortium, T2D–GENES consortium (Supplementary Note). Summary information about participants, genotyping and quality control in the follow-up samples are presented in Supplementary Note. The follow-up samples provided SNP association statistics for DBP, PP, SBP, and HTN but not MAP for a total of 180,726 individuals. Significant variants (P ≤ 3.4 × 10−7) in the discovery samples were considered replicated in the follow-up samples with P ≤ 0.05/n with their pre-specified BP trait in the follow-up sample alone, where n was the number of variants tested in the follow-up samples. Both the significant variants from discovery and additional variants with P ≤ 1 × 10−5 from the discovery samples were selected for joint meta-analysis with the follow-up samples. The primary meta-analysis of the discovery and follow-up results was performed in individuals of all ancestries. The secondary meta-analysis was conducted in the EA only samples. The inverse variance weighted method was used in meta-analysis of the discovery and follow-up stage results for DBP, PP and SBP. Because the follow-up samples provided only z-scores and sample sizes for HTN, the optimally weighted z-score method81 was used in meta-analysis of HTN. The threshold of P ≤ 3.4 ×10−7 was required for significance in meta-analyses of the discovery and follow-up samples.
Functional Inference
We applied several computational strategies to infer biological functions associated with candidate genes of the 31 novel loci reaching P <3.4×10−7 (Table 1) and 39 validated loci (Supplementary Table 3): 1) To test whether SNPs in Table 1 and Supplementary Table 3 were significantly enriched among pre-specified gene sets defined in pathways, or by shared roles in particular diseases or biological processes, we performed gene pathway, disease, and Gene Ontology (GO) enrichment analysis using GeneGo software and Literature Lab45 data mining of literature (Supplementary Methods); 2) To investigate whether the coding and non-coding variants listed in Table 1 may influence the transcriptional regulation, we compared BP candidate SNPs with ENCODE and Roadmap Epigenomics regulome features summarized for mainly cis regulatory function in HaploReg47 and RegulomeDB46. The inclusion of coding variants in this analysis was justified by previous research showing that transcriptional regulation can be influenced by both non-coding and coding variations; a recent publication has shown that ∼15% of human codons simultaneously specify both amino acids and transcription factor recognition sites82; and 3) To identify genes that encode proteins especially connected to other proteins and therefore inferred to be important, we performed protein-protein interaction network analysis (PPI) on SNPs in Table 1. The PPI network was constructed using the NCBI PPI database information, which sources information from HPRD, BIND, BioGRID and EcoCys databases. By design, 2% of the Exome Chip variants were identified from previous GWAS. To investigate if these previous GWAS SNPs may artificially increase the extent of GeneGO enrichment in known functional classes, we performed GeneGO enrichment analysis on 10 randomly selected sets of genes from the Exome Chip (with replacement) with the size of new and previously BP candidates discovered. None of these random sets showed gene-set enrichment with significance comparable to the enrichment for the BP SNPs.
To further assess putative functionality for the novel loci, we performed cis-eQTL analysis between each of the newly identified variants with gene expression within 1 Mb flanking that variant in peripheral whole blood samples of ∼ 5000 individuals from the Framingham Heart Study (FHS). Statistical significance in the FHS expression data was evaluated at FDR<10% for newly identified variants83. We also searched for cis-associations between novel variants and gene transcripts within 1 Mb flanking the lead SNP based on databases of previously published expression quantitative trait locus (eQTL) analyses at the false discovery rate (FDR) <10%51,84.
Supplementary Material
Acknowledgments
We thank the two anonymous reviewers and editors for their helpful comments. Study-specific funding sources and acknowledgements are reported in Supplementary Note.
Footnotes
URLs
BIND, http://www.bind.ca
BioGRID, http://thebiogrid.org/
CHARGE+ Exome Chip, http://www.chargeconsortium.com/main/exomechip
EcoCys, http://www.ecocyc.org
GeneGO, http://lsresearch.thomsonreuters.com/
Literature Lab, http://www.acumenta.com/acumenta/overview/index.php
HaploReg, http://www.broadinstitute.org/mammals/haploreg/haploreg_v3.php
HPRD, http://www.hprd.org
NCBI PPI, ftp://ftp.ncbi.nih.gov/gene/GeneRIF/
NHGRI GWAS Catalog, http://www.genome.gov/gwastudies
Polyphen-2, http://genetics.bwh.harvard.edu/ggi/pph2/
RAREMETAL, http://genome.sph.umich.edu/wiki/RAREMETAL_Documentation
RegulomeDB, http://regulomedb.org/
Recode alleles, http://depts.washington.edu/chargeco/wiki/cgi_img_auth.php/c/c6/Recode_all.txt
Roadmap Epigenomics, http://www.roadmapepigenomics.org/
seqMeta package, http://cran.r-project.org/web/packages/seqMeta/index.html
Accession codes
The meta-analysis results at single variant level for SBP, DBP, MAP, PP and HTN can be downloaded at the dbGaP CHARGE Summary site phs000930.
Author contributions
Study design: A.T.K., C.L., N.F., G.B.E., C.N-C., J.I.R., B.M.P., D.L., D.I.C.
Phenotyping: E.B., V.G., B.M.P.;, D.L., D.R.W., A.C., W.P., M.D., R.R., W.H.S., P.M.R., A.P.R., J.R., C.K., N.F., K.L., C.B., Y.I.C., A.T.K., M.G.L., L.R., E.P.B., O.G., H.V., W.L., I.L., L.W.M., G.J.P.
Genotyping: E.B., D.L., A.P.R., C.K., Y.I.C., M.F., C.J.O., S.L.R.K., U.V., D.I.C., C.N-C., J.A.B., J.C.B., E.W.D., K.D.T., C.L., J.A.S., W.Z., J.D.F., Y.I.C., S.W., E.K., A.G.U., A.Y.C., F.G., P.L.A., M.L.G.
Quality control: A.P.R., D.I.C., C.N-C., J.A.B., J.C.B., E.W.D., K.D.T., C.L., S.H., J.A.S., W.Z, J.D.F., S.W., A.Y.C., F.G., P.L.A., M.L.G., M.D., H.V., G.B.E., A.C.M., J.J., A.V.S., L.L.
Software development: J.A.B., C.L., A.C, F.G., P.L.A., A.T.K., K.R., A.V., H.C., D.I.C.
Statistical analysis: A.P.R., D.I.C., C.N-C., G.K, J.A.B., J.C.B., C.L., J.A.S., W.Z., J.D.F., S.W., A.Y.C., F.G., P.L.A., G.B.E., A.C.M., J.J., A.V.S., L.L., T.H., A.G., C.K., N.F., A.T.K., M.G.L., S.G., E.S., K.R., H.M., X.G., J.Y., P.S., F.D., J.P.C., S.K., N.S., H.S., P.D., N.S., C.F., M.G., M.L., C.P.
Manuscript writing: C.L., A.T.K., J.A.S., N.F., J.J.C.B., Y.L., W.P., L.W.M., M.G.L., K.R., T.L.E., M.F., G.B.E., J.I.R., D.L., D.I.C.
Competing financial interests
B.M. P. serves on the DSMB for a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
Other authors declared no competing financial interests.
References
- 1.Lim SS, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toka HR, Luft FC. Monogenic forms of human hypertension. Semin Nephrol. 2002;22:81–88. doi: 10.1053/snep.2002.30206. [DOI] [PubMed] [Google Scholar]
- 3.Toka HR, Koshy JM, Hariri A. The molecular basis of blood pressure variation. Pediatr Nephrol. 2013;28:387–399. doi: 10.1007/s00467-012-2206-9. [DOI] [PubMed] [Google Scholar]
- 4.Garovic VD, Hilliard AA, Turner ST. Monogenic forms of low-renin hypertension. Nat Clin Pract Nephrol. 2006;2:624–630. doi: 10.1038/ncpneph0309. [DOI] [PubMed] [Google Scholar]
- 5.Zhu X, et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Hum Mol Genet. 2011;20:2285–2295. doi: 10.1093/hmg/ddr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tragante V, et al. Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am J Hum Genet. 2014;94:349–360. doi: 10.1016/j.ajhg.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wain LV, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padmanabhan S, Newton-Cheh C, Dominiczak AF. Genetic basis of blood pressure and hypertension. Trends Genet. 2012;28:397–408. doi: 10.1016/j.tig.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AD, et al. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson T, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89:688–700. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganesh SK, et al. Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum Mol Genet. 2013;22:1663–1678. doi: 10.1093/hmg/dds555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini N, et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet. 2013;93:545–554. doi: 10.1016/j.ajhg.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton-Cheh C, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehret GB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welter D, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliver PM, et al. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci U S A. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver PM, et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willer CJ, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–1283. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett JC, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando MM, et al. Transancestral mapping of the MHC region in systemic lupus erythematosus identifies new independent and interacting loci at MSH5, HLA-DPB1 and HLA-G. Ann Rheum Dis. 2012;71:777–784. doi: 10.1136/annrheumdis-2011-200808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plenge RM, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis--a genomewide study. N Engl J Med. 2007;357:1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippert C, et al. An exhaustive epistatic SNP association analysis on expanded Wellcome Trust data. Sci Rep. 2013;3:1099. doi: 10.1038/srep01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu L, et al. Quantitative assessment of the effect of KCNJ11 gene polymorphism on the risk of type 2 diabetes. PLoS One. 2014;9:e93961. doi: 10.1371/journal.pone.0093961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phani NM, et al. Population specific impact of genetic variants in KCNJ11 gene to type 2 diabetes: a case-control and meta-analysis study. PLoS One. 2014;9:e107021. doi: 10.1371/journal.pone.0107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers JC, et al. Genetic loci influencing kidney function and chronic kidney disease. Nat Genet. 2010;42:373–375. doi: 10.1038/ng.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elks CE, et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet. 2010;42:1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eijgelsheim M, et al. Genome-wide association analysis identifies multiple loci related to resting heart rate. Hum Mol Genet. 2010;19:3885–3894. doi: 10.1093/hmg/ddq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heid IM, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pare G, et al. Novel associations of CPS1, MUT, NOX4, and DPEP1 with plasma homocysteine in a healthy population: a genome-wide evaluation of 13 974 participants in the Women’s Genome Health Study. Circ Cardiovasc Genet. 2009;2:142–150. doi: 10.1161/CIRCGENETICS.108.829804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks JD, et al. Variants in tamoxifen metabolizing genes: a case-control study of contralateral breast cancer risk in the WECARE study. Int J Mol Epidemiol Genet. 2013;4:35–48. [PMC free article] [PubMed] [Google Scholar]
- 32.Geller F, et al. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat Genet. 2014;46:957–963. doi: 10.1038/ng.3063. [DOI] [PubMed] [Google Scholar]
- 33.Gudmundsson J, Sulem P, Thorlacius S. Genetic variants on chr 11q and 6q as markers for prostate and colorectal cancer predisposition. Patent number: CA2707350 A1. 2009 [Google Scholar]
- 34.Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.三木哲郎, et al. Identification of group of hypertension-susceptibility genes. Patent number: CN103667326 A. 2014 [Google Scholar]
- 36.Ingelsson E, Syvanen AC, Lind L. Endothelium-dependent vasodilation in conduit and resistance vessels in relation to the endothelial nitric oxide synthase gene. J Hum Hypertens. 2008;22:569–578. doi: 10.1038/jhh.2008.37. [DOI] [PubMed] [Google Scholar]
- 37.Chasman DI, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arndt AK, et al. Fine mapping of the 1p36 deletion syndrome identifies mutation of PRDM16 as a cause of cardiomyopathy. Am J Hum Genet. 2013;93:67–77. doi: 10.1016/j.ajhg.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen P, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castano Betancourt MC, et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci U S A. 2012;109:8218–8223. doi: 10.1073/pnas.1119899109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgenthaler S, Thilly WG. A strategy to discover genes that carry multi-allelic or mono-allelic risk for common diseases: a cohort allelic sums test (CAST) Mutat Res. 2007;615:28–56. doi: 10.1016/j.mrfmmm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83:311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu MC, et al. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji W, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Febbo PG, et al. Literature Lab: a method of automated literature interrogation to infer biology from microarray analysis. BMC Genomics. 2007;8:461. doi: 10.1186/1471-2164-8-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naiche LA, Harrelson Z, Kelly RG, Papaioannou VE. T-box genes in vertebrate development. Annu Rev Genet. 2005;39:219–239. doi: 10.1146/annurev.genet.39.073003.105925. [DOI] [PubMed] [Google Scholar]
- 49.Chapman DL, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 50.Leslie R, O’Donnell CJ, Johnson AD. GRASP: analysis of genotype-phenotype results from 1390 genome-wide association studies and corresponding open access database. Bioinformatics. 2014;30 doi: 10.1093/bioinformatics/btu273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westra HJ, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabakchiev B, Silverberg MS. Expression quantitative trait loci analysis identifies associations between genotype and gene expression in human intestine. Gastroenterology. 2013;144:1488–1496. doi: 10.1053/j.gastro.2013.03.001. 1496 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy A, et al. Mapping of numerous disease-associated expression polymorphisms in primary peripheral blood CD4+ lymphocytes. Hum Mol Genet. 2010;19:4745–4757. doi: 10.1093/hmg/ddq392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeller T, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heap GA, et al. Complex nature of SNP genotype effects on gene expression in primary human leucocytes. BMC Med Genomics. 2009;2:1. doi: 10.1186/1755-8794-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stranger BE, et al. Patterns of cis regulatory variation in diverse human populations. PLoS Genet. 2012;8:e1002639. doi: 10.1371/journal.pgen.1002639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou F, et al. Brain expression genome-wide association study (eGWAS) identifies human disease-associated variants. PLoS Genet. 2012;8:e1002707. doi: 10.1371/journal.pgen.1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schadt EE, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl J Med. 2016;374:1134–1144. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu DA, et al. Quantitative trait locus mapping of human blood pressure to a genetic region at or near the lipoprotein lipase gene locus on chromosome 8p22. J Clin Invest. 1996;97:2111–2118. doi: 10.1172/JCI118648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goodarzi MO, et al. Lipoprotein lipase is a gene for insulin resistance in Mexican Americans. Diabetes. 2004;53:214–220. doi: 10.2337/diabetes.53.1.214. [DOI] [PubMed] [Google Scholar]
- 62.Goodarzi MO, et al. The 3’ untranslated region of the lipoprotein lipase gene: haplotype structure and association with post-heparin plasma lipase activity. J Clin Endocrinol Metab. 2005;90:4816–4823. doi: 10.1210/jc.2005-0389. [DOI] [PubMed] [Google Scholar]
- 63.Goodarzi MO, et al. Haplotypes in the lipoprotein lipase gene influence fasting insulin and discovery of a new risk haplotype. J Clin Endocrinol Metab. 2007;92:293–296. doi: 10.1210/jc.2006-1195. [DOI] [PubMed] [Google Scholar]
- 64.Kraja AT, et al. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes. 2011;60:1329–1339. doi: 10.2337/db10-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kraja AT, et al. Pleiotropic genes for metabolic syndrome and inflammation. Mol Genet Metab. 2014;112:317–338. doi: 10.1016/j.ymgme.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet Chapter. 2013;7 doi: 10.1002/0471142905.hg0720s76. Unit7 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das S, Au E, Krazit ST, Pandey KN. Targeted disruption of guanylyl cyclase-A/natriuretic peptide receptor-A gene provokes renal fibrosis and remodeling in null mutant mice: role of proinflammatory cytokines. Endocrinology. 2010;151:5841–5850. doi: 10.1210/en.2010-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson D, et al. Isolated failure of autonomic noradrenergic neurotransmission. Evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- 69.Biaggioni I, Goldstein DS, Atkinson T, Robertson D. Dopamine-beta-hydroxylase deficiency in humans. Neurology. 1990;40:370–373. doi: 10.1212/wnl.40.2.370. [DOI] [PubMed] [Google Scholar]
- 70.Kim CH, et al. Mutations in the dopamine beta-hydroxylase gene are associated with human norepinephrine deficiency. Am J Med Genet. 2002;108:140–147. [PubMed] [Google Scholar]
- 71.Kapoor A, Shandilya M, Kundu S. Structural insight of dopamine beta-hydroxylase, a drug target for complex traits, and functional significance of exonic single nucleotide polymorphisms. PLoS One. 2011;6 doi: 10.1371/journal.pone.0026509. e26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Velasco M, Gilbert CA, Rutledge CO, McNay JL. Antihypertensive effect of a dopamine beta hydroxylase inhibitor, bupicomide: a comparison with hydralazine. Clinical Pharmacology & Therapeutics. 1975;18:145–153. doi: 10.1002/cpt1975182145. [DOI] [PubMed] [Google Scholar]
- 73.Dhalla NS, Adameova A, Kaur M. Role of catecholamine oxidation in sudden cardiac death. Fundam Clin Pharmacol. 2010;24:539–546. doi: 10.1111/j.1472-8206.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 74.Leon AS, Abrams WB. The role of catecholamines in producing arrhythmias. Am J Med Sci. 1971;262:9–13. doi: 10.1097/00000441-197107000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Pagliarini DJ, et al. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell. 2005;19:197–207. doi: 10.1016/j.molcel.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Grove ML, et al. Best Practices and Joint Calling of the HumanExome BeadChip: The CHARGE Consortium. PLoS One. 2013;8:e68095. doi: 10.1371/journal.pone.0068095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gauderman WJ. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol. 2002;155:478–484. doi: 10.1093/aje/155.5.478. [DOI] [PubMed] [Google Scholar]
- 78.Voorman A, Brody AJ, Chen H, Lumley T. seqMeta: an R Package for meta-analyzing region-based tests of rare DNA variants. 2014 [Google Scholar]
- 79.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 80.Liu DJ, et al. Meta-analysis of gene-level tests for rare variant association. Nat Genet. 2014;46:200–204. doi: 10.1038/ng.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zaykin DV. Optimally weighted Z-test is a powerful method for combining probabilities in meta-analysis. J Evol Biol. 2011;24:1836–1841. doi: 10.1111/j.1420-9101.2011.02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stergachis AB, et al. Exonic transcription factor binding directs codon choice and affects protein evolution. Science. 2013;342:1367–1372. doi: 10.1126/science.1243490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 84.Zhong H, Yang X, Kaplan LM, Molony C, Schadt EE. Integrating pathway analysis and genetics of gene expression for genome-wide association studies. Am J Hum Genet. 2010;86:581–591. doi: 10.1016/j.ajhg.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods-only References
- 5.Zhu X, et al. Combined admixture mapping and association analysis identifies a novel blood pressure genetic locus on 5p13: contributions from the CARe consortium. Hum Mol Genet. 2011;20:2285–2295. doi: 10.1093/hmg/ddr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tragante V, et al. Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am J Hum Genet. 2014;94:349–360. doi: 10.1016/j.ajhg.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wain LV, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padmanabhan S, Newton-Cheh C, Dominiczak AF. Genetic basis of blood pressure and hypertension. Trends Genet. 2012;28:397–408. doi: 10.1016/j.tig.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AD, et al. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson T, et al. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89:688–700. doi: 10.1016/j.ajhg.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganesh SK, et al. Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum Mol Genet. 2013;22:1663–1678. doi: 10.1093/hmg/dds555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini N, et al. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet. 2013;93:545–554. doi: 10.1016/j.ajhg.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton-Cheh C, et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy D, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehret GB, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgenthaler S, Thilly WG. A strategy to discover genes that carry multi-allelic or mono-allelic risk for common diseases: a cohort allelic sums test (CAST) Mutat Res. 2007;615:28–56. doi: 10.1016/j.mrfmmm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83:311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu MC, et al. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89:82–93. doi: 10.1016/j.ajhg.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Febbo PG, et al. Literature Lab: a method of automated literature interrogation to infer biology from microarray analysis. BMC Genomics. 2007;8:461. doi: 10.1186/1471-2164-8-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyle AP, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–1797. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westra HJ, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grove ML, et al. Best Practices and Joint Calling of the HumanExome BeadChip: The CHARGE Consortium. PLoS One. 2013;8:e68095. doi: 10.1371/journal.pone.0068095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gauderman WJ. Sample size requirements for association studies of gene-gene interaction. Am J Epidemiol. 2002;155:478–484. doi: 10.1093/aje/155.5.478. [DOI] [PubMed] [Google Scholar]
- 78.Voorman A, Brody AJ, Chen H, Lumley T. seqMeta: an R Package for meta-analyzing region-based tests of rare DNA variants. 2014 [Google Scholar]
- 79.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 80.Liu DJ, et al. Meta-analysis of gene-level tests for rare variant association. Nat Genet. 2014;46:200–204. doi: 10.1038/ng.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zaykin DV. Optimally weighted Z-test is a powerful method for combining probabilities in meta-analysis. J Evol Biol. 2011;24:1836–1841. doi: 10.1111/j.1420-9101.2011.02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stergachis AB, et al. Exonic transcription factor binding directs codon choice and affects protein evolution. Science. 2013;342:1367–1372. doi: 10.1126/science.1243490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 84.Zhong H, Yang X, Kaplan LM, Molony C, Schadt EE. Integrating pathway analysis and genetics of gene expression for genome-wide association studies. Am J Hum Genet. 2010;86:581–591. doi: 10.1016/j.ajhg.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.