Significance
In contrast to other T helper cell populations, the manner in which type 2 immune responses are induced within the female genital tract remains unknown. For these reasons, we show that IL-33 derived from the vaginal epithelium plays a critical role in the generation of type 2 immunity, likely through the activation of type 2 innate lymphoid cells via the myeloid differentiation primary response gene 88 (MyD88) signaling pathway. In addition, intravaginal papain-induced type 2 immunity is dependent on a specific dendritic cell subset that is controlled by interferon regulatory factor 4 (IRF4). These findings provide insight into the mechanisms by which the secretion of proteases from opportunistic pathogens in susceptibility to various sexually transmitted pathogens may induce type 2 immunity within the female genital tract.
Keywords: protease allergen, papain, type 2 immunity, genital tract, IL-33
Abstract
The genital mucosa is a barrier that is constantly exposed to a variety of pathogens, allergens, and external stimuli. Although both allergen exposure and parasite infections frequently occur in the genital area, the mechanism by which immune responses—particularly type 2 immunity—are induced has rarely been studied in the genital mucosa. Here, we demonstrate the induction of T helper type 2 (Th2) immunity in the genital mucosa in response to a model allergen, the protease papain. Intravaginal papain immunization induced type 2 immunity in a manner that was dependent on protease activity and the estrous phase of the mice. In addition, IL-33 was released from the vaginal epithelia after intravaginal papain immunization, leading to the activation of type 2 innate lymphoid cells (ILC2s). Moreover, the IL-33–MyD88 (myeloid differentiation primary response gene 88) signaling pathway was critical for the induction of type 2 immunity. We also found that Th2 differentiation in response to intravaginal papain treatment requires a specific dendritic cell (DC) subset that is controlled by interferon regulatory factor 4 (IRF4). These findings suggest that type 2 immunity is induced by a unique mechanism in the genital tract, which is an important, but often overlooked, barrier surface.
Allergic disorders, such as asthma, atopy, and helminth infections, are major concerns across the globe because their prevalence is increasing (1, 2). These inflammatory and infectious diseases are commonly involved in “type 2 immunity.” Type 2 immunity is characterized by the induction of T helper type 2 (Th2) cells, which secrete cytokines such as IL-4, -5, and -13, as well as by IgE production. Th1 and Th17 responses, which are induced by viral, bacterial, and fungal infections, are well studied. Pattern recognition receptors recognize pathogen-specific molecules, and dendritic cells (DCs) control Th cell differentiation by acting as antigen-presenting cells and producing cytokines required for Th cell differentiation. However, the mechanisms underlying the recognition of allergens and helminth infections and the subsequent induction of Th2 and IgE responses are much more complex. Although recent studies have demonstrated many aspects of innate and adaptive type 2 immunity (3–7), these results were derived from mouse models by using s.c. needle injections or allergen sensitization and helminth infections of the lungs and intestines.
The vaginal tract is a mucosal barrier that is constantly exposed to a variety of pathogens, allergens, and external stimuli. In addition, this mucosal barrier has the unique feature of being under the control of the hormonal cycle. During the estrous cycle, the thickness of the epithelial layers, the immune cell populations within the vagina, and the receptors expressed on the vaginal epithelial cells change. Thus, the host responses to external stimuli within the vaginal tract are also dependent on the estrous cycle. In this context, the vaginal mucosa should be considered distinct from other mucosal barriers, such as the lungs and intestines.
Although often underestimated, genital allergies are a common problem in females. Various materials contacting the genital area may be allergens or irritants, including hygienic soap, topical preparations, sanitary pads, and even seminal fluid (8). These irritants not only induce genital swelling and itching, but may also give rise to systemic symptoms. Candida is a well-recognized allergen that can induce asthma or “tea tasters’ cough” in the lungs (8). Accordingly, vulvovaginal candidiasis is also correlated with genital allergy (9, 10). In addition, trichomoniasis caused by the protozoan parasite Trichomonas vaginalis is a common cause of vaginitis in women. One of the virulence factors of T. vaginalis is a cysteine protease that degrades the extracellular matrix, which facilitates the binding of this parasite to the vaginal epithelial cells. Based on the observation that proteases secreted from helminth parasites trigger Th2 responses (11), infection of the vaginal tract by this common parasite could be associated with type 2 immunity. Nevertheless, it is unknown which components of allergens and parasites are sensed by the host immune system and how host immune responses, particularly Th2 and IgE responses, are induced in response to allergens and parasitic infections of the genital tract. Therefore, in this study, we used a well-known model allergen, the protease papain, to assess the generation of type 2 immunity in the female genital tract. We found that papain challenge through the vaginal mucosa induces type 2 immune responses in a manner that is dependent on its enzymatic activity and the host’s estrous cycle. Intravaginal papain immunization induces IL-33 secretion from the vaginal epithelia, which can activate type 2 innate lymphoid cells (ILC2s) to produce type 2 cytokines. Accordingly, papain-induced type 2 immunity in the female genital tract is dependent on IL-33. Moreover, we show that interferon regulatory factor 4 (IRF4)-expressing CD301b+PDL2+ DCs are a specialized DC subset that induce Th2 differentiation after intravaginal papain immunization. Finally, we found that intravaginal papain-induced Th2 responses and IgE production are mediated by myeloid differentiation primary response gene 88 (MyD88).
Results
Intravaginal Papain Immunization Induces a Type 2 Immune Response.
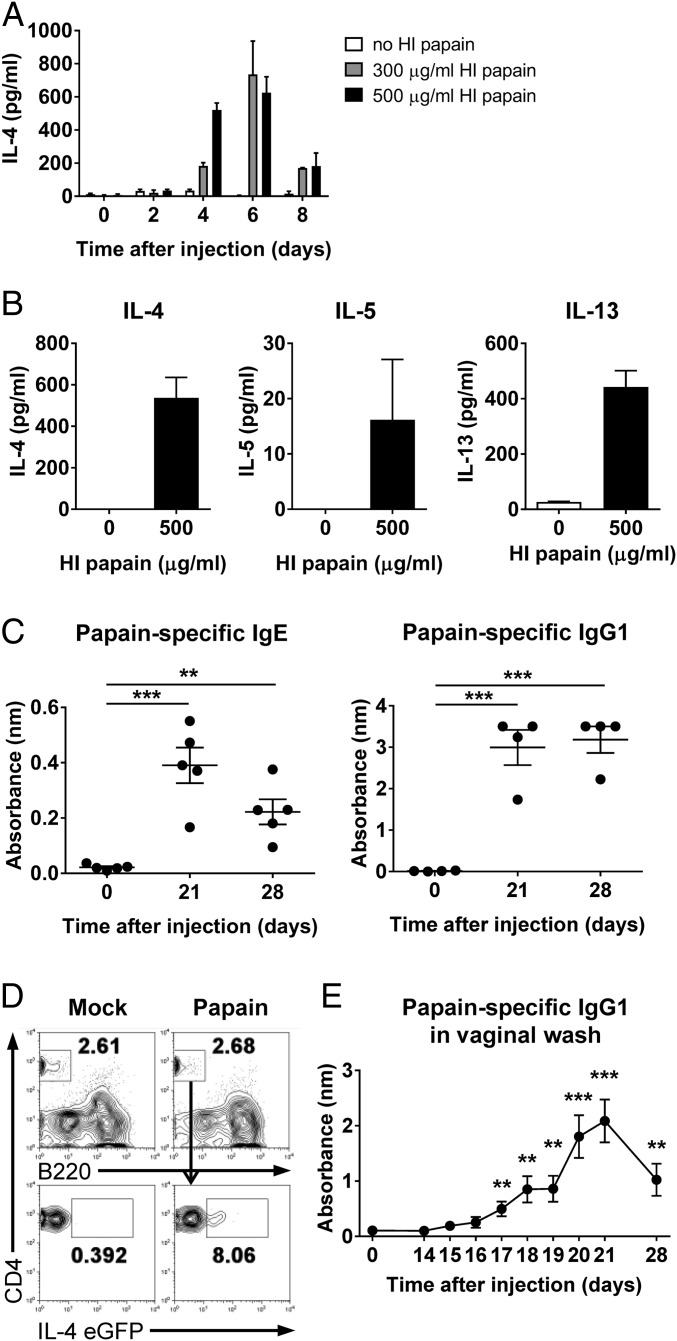
To investigate whether intravaginal papain injection induces Th2 immunity, we first examined IL-4 production at various time points after the ex vivo restimulation of CD4+ T cells (Fig. 1A). IL-4 production by CD4+ T cells peaked 6 d after intravaginal immunization with 200 μg of papain. Furthermore, Th2 cytokines, including IL-4, -5, and -13, were produced by CD4+ T cells after intravaginal papain immunization (Fig. 1B).
Fig. 1.
Papain treatment of the genital mucosa induces Th2 differentiation and the production of antigen-specific IgE and IgG1. (A and B) BALB/c mice were intravaginally injected with papain after MPA (medroxyprogesterone acetate) treatment. (A) On the indicated days after the injection, the production of IL-4 was measured by using ELISA after the restimulation of CD4+ T cells isolated from the iliac LNs with heat-inactivated (HI) papain (n = a pool of five mice per group). (B) The levels of Th2 cytokines, including IL-4 (Left), -5 (Center), and -13 (Right), were measured after ex vivo restimulation of CD4+ T cells isolated from iliac LNs of BALB/c mice 6 d after intravaginal papain injection (n = 5 mice pooled). (C) BALB/c mice were intravaginally immunized with papain on days 0 and 14. The serum levels of papain-specific IgE (Left) and IgG1 (Right) were measured by using ELISA (n = 4 or 5 mice). (D) The 4get mice were intravaginally injected with papain after MPA treatment. On day 6 after injection, IL-4 EGFP expression by the CD4+ T cells of the vaginal tissues was assessed by using flow cytometry. The numbers indicate the percentage of gated cells. (E) BALB/c mice were intravaginally immunized with papain on days 0 and 14. The level of papain-specific IgG1 in the vaginal washes was measured by using ELISA (n = 10 mice). The data are representative of two or three independent experiments. **P < 0.01; ***P < 0.001. Error bars: SEM.
We next examined intravaginal papain-induced IgE and IgG1 production, which is dependent on help from IL-4–producing T cells. Papain-specific IgE and IgG1 were detected in the serum on days 21 and 28 after intravaginal immunization with papain on days 0 and 14 (Fig. 1C). We also found that CD4+ T cells in the vagina of IL-4–IRES–EGFP (4get) reporter mice (12) expressed IL-4 EGFP after intravaginal papain immunization (Fig. 1D). In addition, papain-specific IgG1 was secreted from the vagina after secondary immunization with papain (Fig. 1E). These results indicate that intravaginal papain immunization can induce a type 2 immune response.
Induction of Type 2 Immunity by Intravaginal Papain Immunization Is Dependent on Protease Activity.
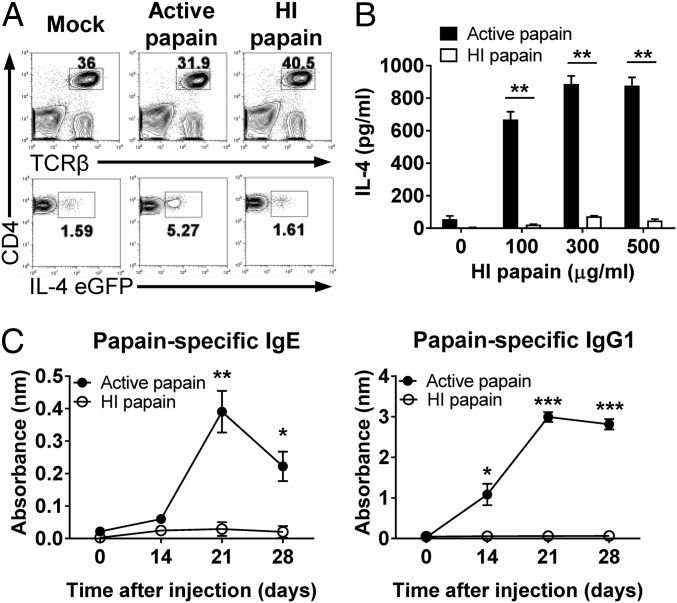
Many studies have shown that the induction of a Th2 immune response by papain requires its enzymatic activity (13, 14). Therefore, we tested whether the protease activity of papain was also critical for the induction of the Th2 response after intravaginal immunization. First, we measured the induction of the Th2 response after intravaginal injection with active papain or heat-inactivated papain using 4get mice. Intravaginal immunization with active papain increased the expression of IL-4 EGFP by CD4+ T cells in the draining lymph nodes (LNs), whereas heat-inactivated papain did not induce IL-4 EGFP expression (Fig. 2A). Moreover, we found that IL-4 production was impaired in the mice immunized with heat-inactivated papain compared with the mice immunized with active papain after the ex vivo restimulation of CD4+ T cells (Fig. 2B). Papain-specific IgE and IgG1 production was also dependent on the protease activity of papain (Fig. 2C). Collectively, we concluded that the induction of Th2 immunity by intravaginal papain immunization was dependent on the protease activity of papain.
Fig. 2.
Papain-induced type 2 immunity is dependent on protease activity. (A) The 4get mice were intravaginally immunized with active or HI papain. On day 6 after injection, IL-4 EGFP expression by the CD4+ T cells of the iliac LNs was assessed by using flow cytometry. The numbers indicate the percentage of gated cells. The data are representative of three independent experiments. (B) BALB/c mice were intravaginally injected with active or HI papain. On day 6 after injection, the amount of IL-4 produced by restimulated CD4+ T cells isolated from the iliac LNs was measured by using ELISA (n = 5 mice pooled per group). (C) BALB/c mice were intravaginally immunized with active or HI papain on days 0 and 14. The serum levels of papain-specific IgE (Left) and IgG1 (Right) were measured by using ELISA (active papain, n = 5 mice; HI papain, n = 3 mice). The data are representative of two or three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars: SEM.
Intravaginal Papain Induces IL-33 Release and ILC2 Activation.
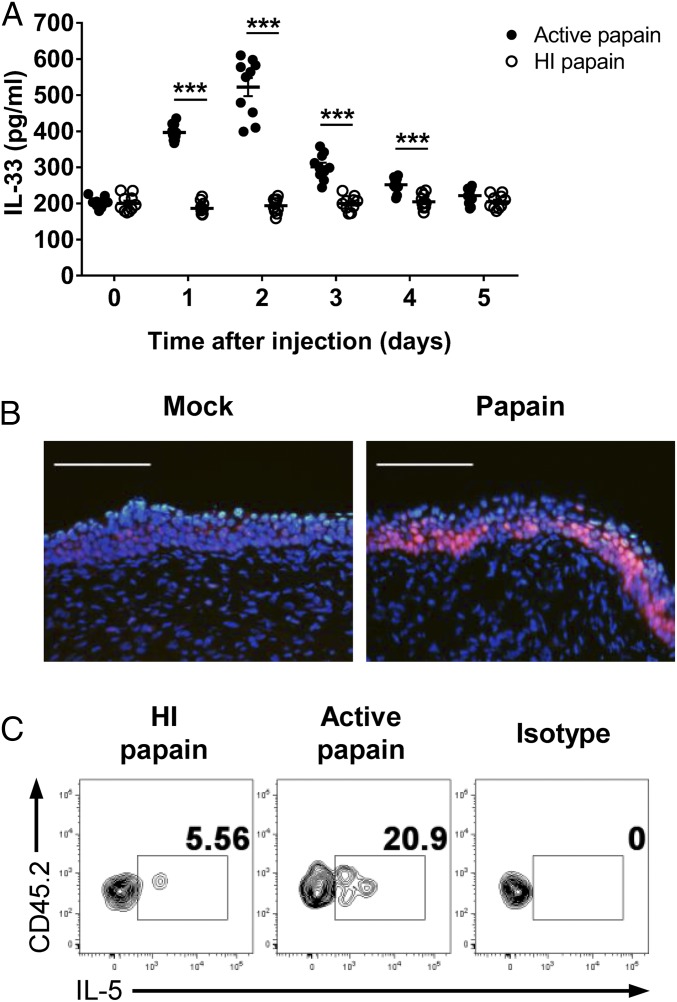
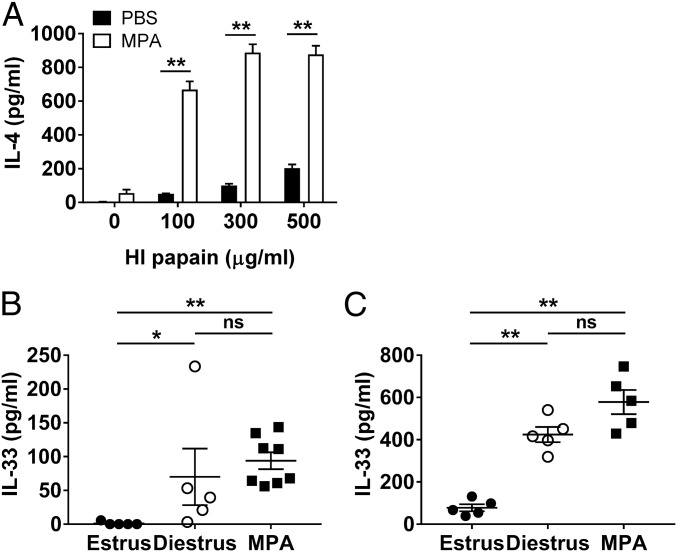
Several studies have shown that IL-33 plays an important role in inducing Th2 immunity in response to allergens or helminth infections (15, 16). Because IL-33 is known to be released in response to tissue damage or necrotic cell death, we assumed that intravaginal papain could induce IL-33 production in the vaginal tissue via its protease activity. Indeed, we detected increased IL-33 levels in the vaginal washes after the intravaginal injection of active papain (Fig. 3A). We also demonstrated that vaginal epithelial cells produced IL-33 after intravaginal papain injection (Fig. 3B).
Fig. 3.
Intravaginal papain immunization induces IL-33 secretion, which activates ILC2s. (A) The IL-33 levels in vaginal washes of BALB/c mice collected after the intravaginal injection of active or HI papain were measured by ELISA (n = 10 mice). (B) Frozen sections of the vaginal tissues on day 2 after intravaginal papain immunization were stained with antibody against IL-33 (red) and DAPI. The images were captured by using a 20× objective lens. (Scale bars: 100 μm.) (C) BALB/c mice were intravaginally immunized with papain for three consecutive days. The amount of IL-5 produced by ILC2s, defined as CD45.2+Lin−CD90.2+CD25+, isolated from the vaginal tissues on day 4 was measured by intracellular cytokine staining after stimulation with PMA and ionomycin (n = 5 mice pooled per group). The data are representative of two or three independent experiments. ***P < 0.001. Error bars: SEM.
Recently, IL-33 has become well known for its activation of ILC2s, which produce type 2 cytokines, such as IL-5 and -13 (17). Based on our results showing that intravaginal papain immunization induced IL-33 release in the vagina, we examined the IL-33–induced activation of ILC2s by measuring IL-5 production. We found that ILC2s isolated from the vaginas of active papain-immunized mice produced IL-5, whereas ILC2s from heat-inactivated papain-immunized mice did not (Fig. 3C). Collectively, the results show that intravaginal immunization with active papain induces IL-33 release from the vaginal epithelia, which stimulates ILC2s to produce IL-5.
Intravaginal Papain-Induced Th2 Immune Response Is Dependent on IL-33.
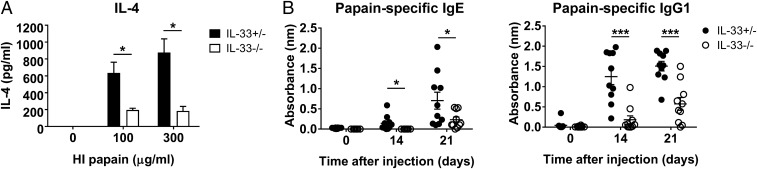
Based on the increase in the IL-33 levels after intravaginal papain injection, we sought to determine whether IL-33 was required for the induction of Th2 immunity. IL-33–deficient mice showed reduced IL-4 production in response to intravaginal papain immunization (Fig. 4A). Furthermore, papain-specific IgE and IgG1 production was also dependent on IL-33 (Fig. 4B). These results indicate that IL-33 can control the induction of the Th2 immune response to intravaginal papain injection.
Fig. 4.
Induction of type 2 immunity in the genital mucosa by papain is dependent on IL-33. (A) IL-4 levels were measured after ex vivo restimulation of CD4+ T cells isolated from the iliac LNs of IL-33 heterozygous or knockout mice 6 d after the intravaginal injection of papain (n = 5 mice pooled per group). (B) IL-33 heterozygous or knockout mice were intravaginally immunized with papain on days 0 and 14. The serum levels of papain-specific IgE (Left) and IgG1 (Right) were measured by using ELISA (n = 10 mice). The data are representative of two or three independent experiments. *P < 0.05; ***P < 0.001. Error bars: SEM.
Basophils and Eosinophils Are Dispensable for the Intravaginal Papain-Induced Th2 Immune Response.
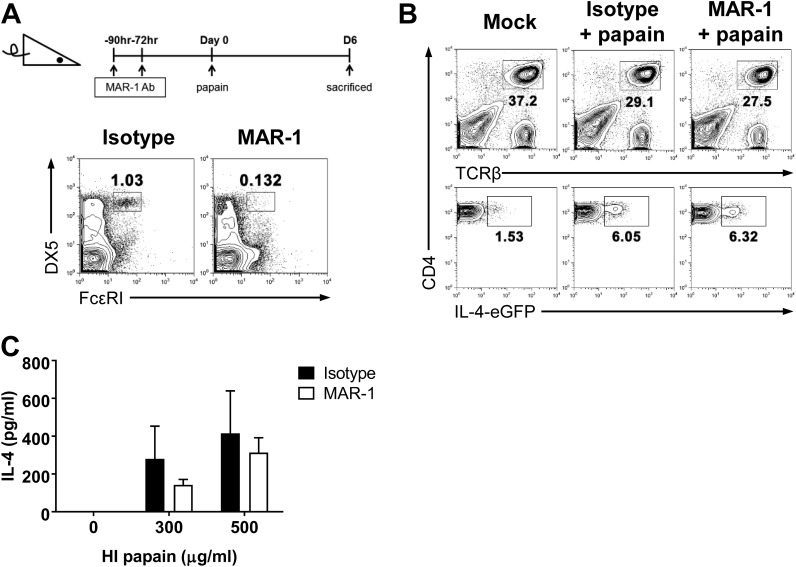
The identity of the initial cytokine source that induces Th2 differentiation has been elusive; however, many studies have suggested possible sources of IL-4 (4, 18–20). One study using s.c. papain immunization revealed that it directly activated basophils, which produced IL-4 and contributed to Th2 differentiation in vivo (4). To examine whether basophils are also critical for Th2 induction after intravaginal papain immunization, we treated 4get or BALB/c mice with MAR-1 antibody to deplete basophils (4). MAR-1 injection induced a reduction of ∼90% in the blood basophil counts by the third day after the final MAR-1 treatment (Fig. S1A). When intravaginal papain was used to treat isotype-treated or MAR-1–treated 4get mice, IL-4–EGFP expression was comparable between the two groups (Fig. S1B). Furthermore, the MAR-1–treated mice did not display defects in IL-4 production by CD4+ T cells after ex vivo restimulation (Fig. S1C).
Fig. S1.
Basophils are dispensable for the papain-induced Th2 response in the genital mucosa. (A) Mice were treated with hamster IgG isotype control or MAR-1 antibodies at the indicated time points before intravaginal papain injection to deplete basophils from the peripheral blood. The FACS plots show the number of basophils in the blood, defined as FcεRI+DX5+, after the isotype or MAR-1 antibody treatments. (B) IL-4 EGFP expression in CD4+ T cells from the iliac LNs of 4get mice was assessed 6 d after intravaginal papain injection and isotype or MAR-1 pretreatment, as shown in A. (C) The IL-4 levels were measured after the in vitro restimulation of CD4+ T cells isolated from the iliac LNs of isotype- or MAR-1-treated BALB/c mice 6 d after intravaginal papain injection (n = 5 mice pooled per group). The data are representative of three independent experiments. Error bars: SEM.
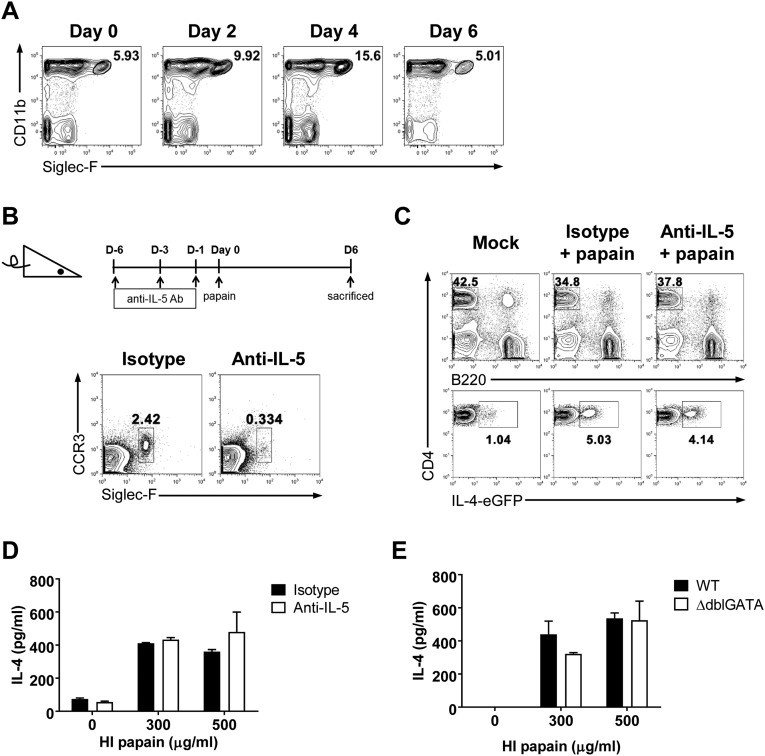
Eosinophils are also known to contribute to the generation of Th2 immunity. In fact, many studies have shown that eosinophil deficiency inhibits the efficient generation of the Th2 immune response (21, 22). In our model, we found that eosinophils were recruited to the vaginal tissues after intravaginal papain immunization (Fig. S2A). Therefore, we tested whether eosinophils were required for the induction of Th2 immunity after intravaginal papain immunization. Injection of an anti–IL-5 antibody depleted >90% of the blood eosinophils (Fig. S2B). After intravaginal papain injection, the anti–IL-5 antibody- and isotype-treated 4get mice showed similar levels of IL-4–EGFP expression by CD4+ T cells isolated from the draining LNs (Fig. S2C). In addition, IL-4 production by CD4+ T cells after ex vivo restimulation was not impaired in the anti–IL-5 antibody-treated mice after intravaginal papain immunization (Fig. S2D). To corroborate these findings, we used genetically modified eosinophil-deficient mice, ∆dblGATA mice, which have a unique loss of cells of the eosinophil lineage (23). After intravaginal papain immunization, the ∆dblGATA and control mice produced comparable amounts of IL-4 upon the ex vivo restimulation of CD4+ T cells (Fig. S2E). Together, these results suggest that basophils and eosinophils are dispensable for the generation of Th2 immunity in response to intravaginal papain challenge.
Fig. S2.
Eosinophils are dispensable for the papain-induced Th2 response in the genital mucosa. (A) Eosinophils, defined as Siglec-F+CD11b+ cells, within the vaginal tissues were examined by flow cytometry on the indicated days after intravaginal papain injection. The plots were gated on PI−CD45.2+ cells, and the numbers indicate the percentage of gated cells. (B) Mice were treated with an isotype control or anti–IL-5 antibody on the indicated days before intravaginal papain injection to deplete eosinophils from the peripheral blood. The FACS plots show the number of eosinophils in the blood, defined as Siglec-F+CCR3+, after isotype control or anti–IL-5 antibody treatment. (C) IL-4 EGFP expression in CD4+ T cells from the iliac LNs in 4get mice was assessed 6 d after intravaginal papain injection and pretreatment with the isotype control or anti–IL-5 antibodies, as shown in B. (D) The IL-4 levels were measured after the in vitro restimulation of CD4+ T cells isolated from the iliac LNs of isotype or anti–IL-5 antibody-treated BALB/c mice 6 d after intravaginal papain injection (n = 5 mice pooled per group). (E) The IL-4 levels were measured after in vitro restimulation of CD4+ T cells isolated from the iliac LNs of WT or ∆dblGATA mice 6 d after intravaginal papain injection (n = 5 mice pooled per group). The data are representative of two or three independent experiments. Error bars: SEM.
Intravaginal Papain-Induced Type 2 Immunity Is Dependent on IRF4 Expression in DCs.
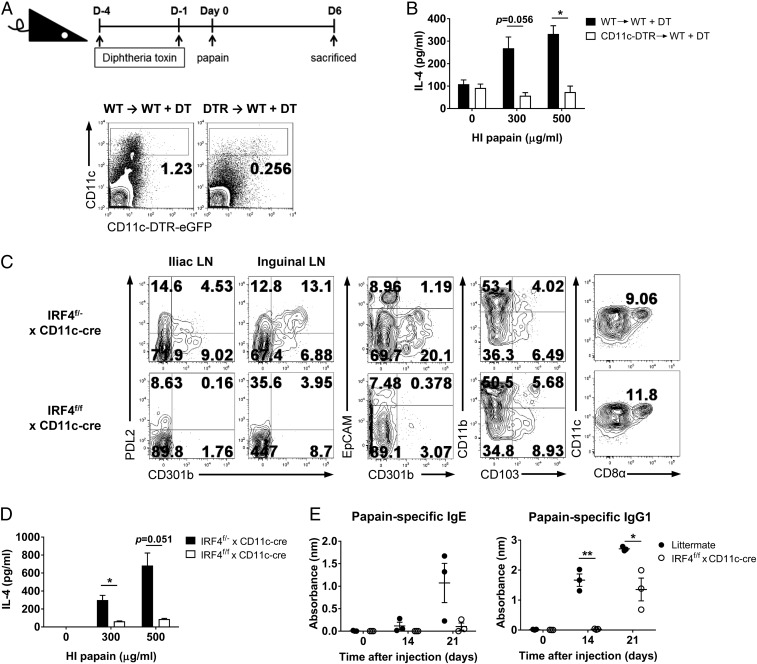
The differentiation of Th cells is regulated by specialized antigen-presenting cells. Although DC-mediated Th1 and Th17 cell differentiation has been thoroughly researched, the relevant DC subsets involved in the initiation of Th2 immunity have only recently been studied (6, 24). We used the CD11c-diphtheria toxin receptor (DTR) –EGFP system to determine whether CD11c+ DCs were required for the intravaginal papain-induced Th2 response. In this system, CD11c+ cells express DTR and can be selectively depleted by diphtheria toxin (DT) treatment (25). However, because CD11c–DTR–EGFP mice tend to die after repeated treatment with DT (26), we generated chimeric CD11c–DTR–EGFP mice by transferring CD11c–DTR–EGFP bone marrow cells into WT B6 recipients and assessed the depletion of CD11c+ DCs in these mice after DT treatment (Fig. 5A). Restimulation of CD4+ T cells with heat-inactivated papain in vitro showed that intravaginal papain-induced Th2 differentiation was impaired by DT treatment (Fig. 5B).
Fig. 5.
IRF4-dependent DCs play indispensable roles in papain-induced Th2 responses in the genital mucosa. (A) CD11c-DTR → WT chimeric mice were treated with DT on the indicated days before intravaginal papain injection to deplete DCs. The FACS plots show the number of CD11c+ DCs after DT treatment. (B) IL-4 levels were measured after ex vivo restimulation of CD4+ T cells isolated from the iliac LNs of DT-pretreated WT → WT or CD11c-DTR → WT chimeric mice 6 d after intravaginal papain injection (n = 6 mice pooled per group). (C) PDL2 and CD301b expression and other DC subsets such as EpCAM+ Langerhans cells (CD301b−EpCAM+), CD103+ DCs (CD103+CD11b−), and CD8α+ DCs (CD8α+CD11chigh) in MHCIIhighCD11c+B220−CD3ε−NK1.1− DCs from the iliac or inguinal LNs of IRF4f/− × CD11c-cre or IRF4f/f × CD11c-cre mice were examined by flow cytometry. The numbers indicate the percentage of cells within each quadrant. (D) IL-4 levels were measured after ex vivo restimulation of CD4+ T cells isolated from the iliac LNs of IRF4f/− × CD11c-cre or IRF4f/f × CD11c-cre mice 6 d after intravaginal papain injection (n = 5 mice pooled per group). (E) IRF4f/f × CD11c-cre or littermate mice were intravaginally immunized with papain on days 0 and 14. The serum levels of papain-specific IgE (Left) and IgG1 (Right) were measured by using ELISA (n = 3 mice). The data are representative of two or three independent experiments. *P < 0.05; **P < 0.01. Error bars: SEM.
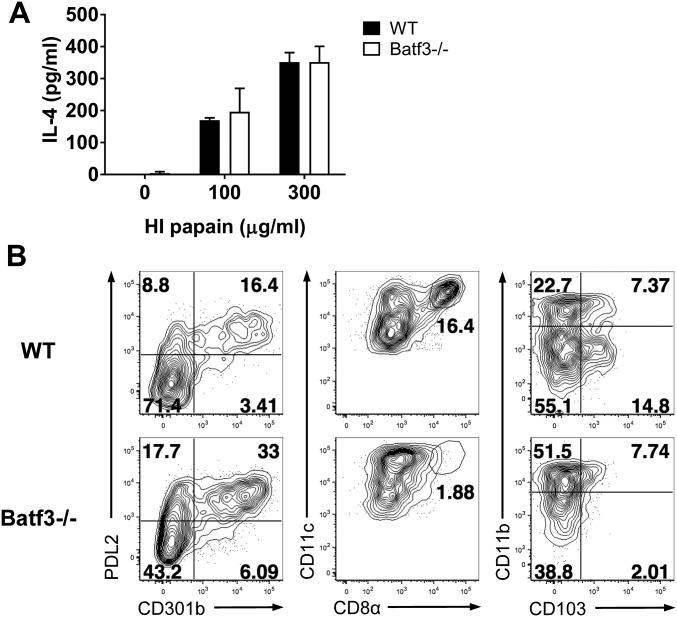
A recent study showed that IRF4 expression, which is required for the differentiation of PDL2+ DCs, drives Th2 responses after s.c. immunization with OVA plus papain (6). In this study, IRF4flox/flox mice were crossed with CD11c-cre mice, which exhibit IRF4 deficiency specifically in CD11c+ DCs, and these mice had severely impaired papain-induced Th2 responses. Similarly, CD301b+ DC depletion resulted in significantly impaired Th2 cell development after infection with Nippostrongylus brasiliensis (24). Therefore, we investigated whether IRF4 expression by DCs was critical for intravaginal papain-induced Th2 immunity. IRF4flox/flox × CD11c-cre mice showed an almost complete lack of the PDL2+CD301b+ DC subset in the iliac and inguinal LNs (draining LNs after intravaginal injection), whereas other DC subsets, including EpCAM+ Langerhans cells, CD103+ DCs, and CD8α+ DCs, were not affected (Fig. 5C). Furthermore, these mice could not generate Th2 immune responses or produce IgE and IgG1 after intravaginal papain injection (Fig. 5 D and E). Because IRF4 works cooperatively with the Batf3 transcription factor to regulate transcription (27), and Batf3 is highly expressed in PDL2+ DCs (6), we examined the role of Batf3 in the induction of Th2 immunity by intravaginal papain immunization. Batf3-deficient mice did not display defects in their Th2 cell responses (Fig. S3A) or in the number of PDL2+ DCs (Fig. S3B). Collectively, these findings show that a specific DC subset controlled by IRF4 is essential for the generation of type 2 immunity after intravaginal papain immunization.
Fig. S3.
Th2 immune responses after intravaginal papain immunization are not impaired in Batf3-deficient mice. (A) IL-4 levels were measured after in vitro restimulation of CD4+ T cells isolated from the iliac LNs of WT or Batf3−/− mice 6 d after intravaginal papain injection (n = 5 mice pooled per group). (B) PDL2 and CD301b expression and other DC subsets including CD8α+ DCs (CD8α+CD11chigh) and CD103+ DCs (CD103+CD11b−) in MHCIIhighCD11c+B220−CD3ε−NK1.1− DCs from the inguinal LNs of WT or Batf3−/− mice were examined by flow cytometry. The numbers indicate the percentage of cells within each quadrant. The data are representative of three independent experiments. Error bars: SEM.
Type 2 Immunity Induced by Intravaginal Papain Is Dependent on the Estrous Phases Showing the Differential Level of IL-33 in the Vagina.
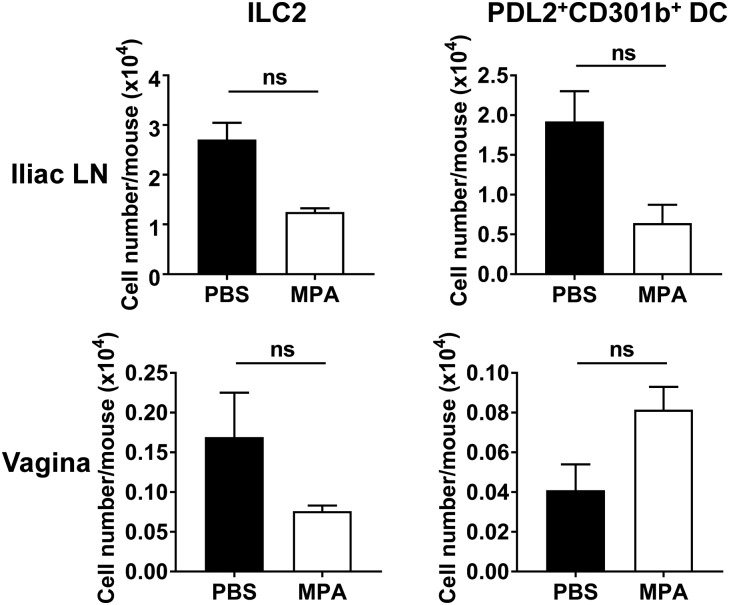
Unlike other mucosal systems, the vaginal mucosa is under the control of the hormonal cycle. Studies using sexually transmitted viruses, such as herpes simplex virus, have shown that susceptibility to these viruses is dependent on the estrous cycle (28, 29). Therefore, we examined whether the induction of the Th2 immune response after intravaginal papain immunization was also dependent on the estrous phase of the mice. When the mice were treated with medroxyprogesterone acetate (MPA), which induces the diestrus and proestrus phases of the estrous cycle, IL-4 production by CD4+ T cells was markedly increased after intravaginal papain immunization compared with that produced by CD4+ T cells from untreated mice (Fig. 6A). To investigate the mechanism by which estrous phases affect the Th2 immune responses, we measured IL-33 levels in vaginal washes at different phases of estrous cycles. Interestingly, the levels of IL-33 were higher in vaginal washes of diestrus-phase and MPA-treated mice than those of estrus phase (Fig. 6B). In addition, after intravaginal papain immunization at the different estrous phases, the levels of IL-33 at day 2 after injection were higher in vaginal washes of diestrus phase and MPA-treated mice than those of estrus phase (Fig. 6C). However, the number of cell populations related to the Th2 induction, such as ILC2 and PDL2+CD301b+ DCs, was not significantly changed after MPA treatment (Fig. S4). Therefore, we concluded that the induction of Th2 immunity by intravaginal papain immunization is enhanced after MPA treatment (at diestrus phase), likely through the increased production of IL-33 in the vagina.
Fig. 6.
Type 2 immunity induced by intravaginal papain is dependent on the estrous phases showing the differential level of IL-33 in the vagina. (A) BALB/c mice were intravaginally injected with papain after pretreatment with or without MPA. On day 6 after papain injection, the amount of IL-4 produced by restimulated CD4+ T cells isolated from the iliac LNs was measured by using ELISA (n = 5 mice pooled per group). (B) The IL-33 levels in the vaginal washes of BALB/c mice at the indicated estrous phases or treated with MPA were measured by ELISA (n = 5–8 mice). (C) On day 2 after intravaginal papain injection to BALB/c mice at the indicated estrous phases or pretreated with MPA, the IL-33 levels in the vaginal washes were measured by ELISA (n = 5 mice). The data are representative of two independent experiments. *P < 0.05; **P < 0.01; ns, not significant. Error bars: SEM.
Fig. S4.
The number of ILC2 or PDL2+CD301b+ DCs is not significantly changed after MPA treatment. The number of ILC2 or PDL2+CD301b+ DCs with or without MPA treatment was assessed by using flow cytometry (n = 5 mice pooled per group). ILC2 were defined as CD45.2+Lin−IL-7Rα+CD25+ cells, and PDL2+CD301b+ DCs as PDL2+CD301b+MHCIIhighCD11c+B220−CD3ε−NK1.1− cells. Data are a compilation of two experiments. ns, not significant. Error bars: SEM.
Induction of Type 2 Immunity by Intravaginal Papain Is Dependent on MyD88, but Not TLR4 or IL-1R.
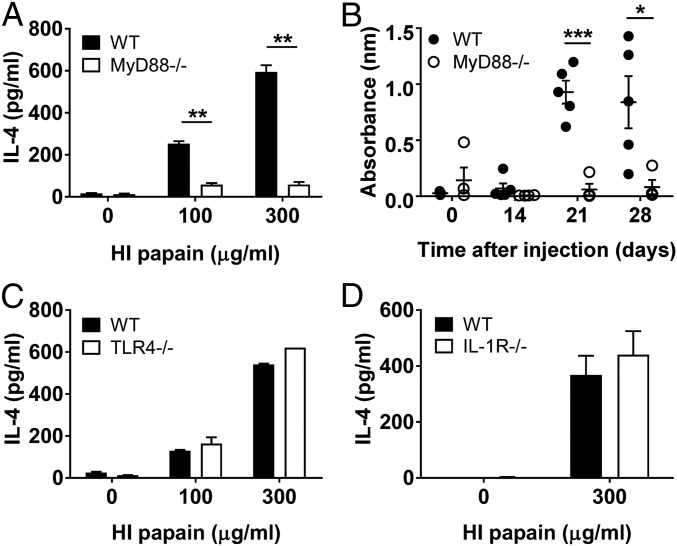
Next, we examined the immune-signaling pathways involved in the intravaginal papain-induced Th2 response. Because MyD88 is an important adaptor molecule that is downstream of many different receptors, we were interested in the role of MyD88 in the intravaginal papain-induced Th2 response. MyD88-deficient mice displayed substantially impaired Th2 responses, including IL-4 production by CD4+ T cells and antigen-specific IgE antibody production, in response to intravaginal papain immunization (Figs. 7 A and B).
Fig. 7.
Papain-induced type 2 immunity in the genital mucosa is dependent on MyD88 signaling, but not on TLR4 or IL-1R. (A) MyD88−/− mice were intravaginally injected with papain. On day 6 after injection, the amount of IL-4 produced by CD4+ T cells isolated from the iliac LNs was measured by using ELISA after restimulation with HI papain for 5 d (n = 5 mice pooled per group). (B) MyD88−/− mice were intravaginally immunized with papain on days 0 and 14. The serum levels of papain-specific IgE were measured by using ELISA (WT, n = 5 mice; MyD88−/−, n = 4 mice). (C and D) IL-4 levels were measured after in vitro restimulation of CD4+ T cells isolated from the iliac LNs of WT or TLR4−/− (C) and IL-1R−/− (D) mice 6 d after intravaginal active papain injection (n = 5 mice pooled per group). The data are representative of two or three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars: SEM.
MyD88 is not only important for signals from TLRs but is also an adaptor molecule in the signaling pathways downstream of IL-1 family cytokine receptors, including the IL-1, -18, and -33 receptors. We found that the intravaginal papain-induced Th2 response was independent of TLR4 and IL-1R, which indicated that the effects of endotoxin and IL-1 could be ruled out (Figs. 7 C and D). Together with the data showing that IL-33 has important roles in our model, we concluded that the Th2 immune response might be induced by signals from IL-33 through MyD88 after intravaginal papain immunization.
Discussion
In this study, we show that papain immunization through the vaginal mucosa induces type 2 immune responses in mice, including Th2 differentiation and IgE and IgG1 production. Moreover, these effects are dependent on the protease activity of papain and the estrous cycle of the mice. In addition, intravaginal papain immunization induces IL-33 secretion from the vaginal epithelia, which activates ILC2s to produce type 2 cytokines such as IL-5. However, basophils and eosinophils, well-known IL-4 producers, are dispensable for the intravaginal papain-induced Th2 response. In addition, IRF4 expression by DCs is critical for Th2 differentiation after intravaginal papain immunization. Furthermore, the type 2 immune responses induced by intravaginal papain are mediated by MyD88-dependent signaling pathways.
The vaginal mucosa is distinct from other mucosal systems in that it is under the control of the estrous cycle. In this context, a sufficient amount of IL-4 was only produced by CD4+ T cells from MPA-treated mice after intravaginal papain immunization in our study. MPA treatment induces the diestrus and proestrus phases of the estrous cycle, which affect the thickness of the epithelium and the presence of many leukocytes within the vaginal cavity (30). One possible mechanism is, as shown in our data, that the increased production of IL-33 in the vagina of MPA-treated mice (or at diestrus phase) could promote the induction of Th2 immunity. Another mechanism by which the Th2 response was induced in MPA-treated mice might relate to the thickness and permeability of the vaginal epithelium. Specifically, a sufficient amount of protease antigen might penetrate through the thinned and permeable epithelium and stimulate Th2 induction pathways. The other possibility is the differential expression of receptors on the vaginal surface. Because the vaginal epithelium undergoes continuous regenerative changes, receptor expression may change depending on the phases of the estrous cycle. However, because the exact sensors of papain remain unknown, future studies will be required to determine which factors involved in hormonal control affect the papain-induced Th2 response in the vaginal mucosa.
Recent studies have demonstrated the critical roles of IL-33 in type 2 immunity. IL-33, -25, and thymic stromal lymphopoietin are epithelial-derived cytokines that are known to be induced after tissue damage or necrotic cell death. In our study, IL-33 was secreted from the vaginal epithelia after intravaginal papain injection. Although IL-33 could be produced by some populations of immune cells, such as DCs and macrophages, the level of IL-33 secreted by epithelial cells is much higher than that secreted by immune cells (31, 32). Although the exact mechanisms by which IL-33 is released from damaged tissues or necrotic cells remain unclear, we assume that IL-33 may be produced as a result of the hydrolysis of proteins in the vaginal epithelium by the protease activity of papain. This hypothesis is also supported by the data showing that heat-inactivated papain could not induce IL-33 secretion. IL-33 activates a variety of cell populations that express the ST2/IL–1RAcP complex, including ILC2s, mast cells, basophils, eosinophils, Th2 cells, natural killer (NK) cells, and NKT cells (15, 16). These cell populations produce various cytokines and chemokines, including those involved in Th2 polarization, in particular, such as IL-5 and -13. Our study also showed that ILC2s isolated from intravaginal papain-immunized mice could produce IL-5. These results indicate that papain-induced IL-33 secretion in the vagina contributes to the initiation of type 2 immunity by activating ILC2s. Moreover, based on the results showing that IL-33–deficient mice could not sufficiently induce type 2 immunity, IL-33 directly or indirectly regulates the generation of type 2 immunity.
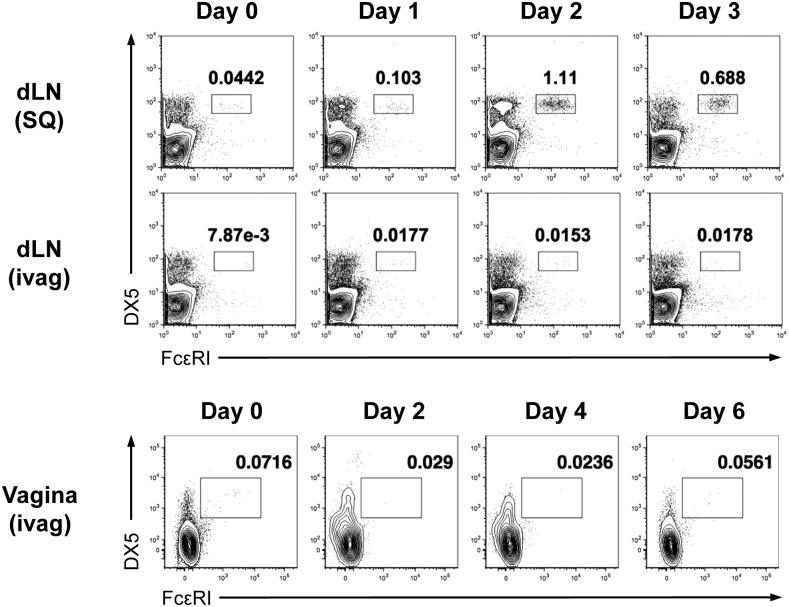
The mechanism involved in promoting Th2 immune responses remains largely unknown. Unlike the Th1 responses induced by DCs, which act as both antigen-presenting cells and the source of IL-12, a critical cytokine required for Th1 differentiation, the mechanism of Th2 differentiation is more complex. A variety of cells, such as basophils, eosinophils, mast cells, and ILC2s, have been suggested as the initial source of IL-4 during Th2 differentiation. A study using a s.c. papain immunization model showed that IL-4–producing basophils were recruited to the draining LNs and that depletion of basophils attenuated the papain-induced Th2 response (4). However, our study showed that basophil depletion did not affect the induction of Th2 responses after intravaginal papain immunization. Moreover, basophil recruitment was not detected in either the draining LNs or the vaginal tissues after intravaginal papain injection (Fig. S5). Based on these findings, we assumed that the mechanism of papain-induced Th2 differentiation is largely dependent on the route of immunization, although the precise mechanism responsible for these differences remains to be elucidated.
Fig. S5.
Basophils are not recruited into the draining LNs or the vaginal tissues after intravaginal papain injection. FcεRI+DX5+ basophils in CD45.2+DAPI− cells from the draining LNs and the vaginal tissues at the indicated time points after s.c. (SQ) or intravaginal (ivag) papain injection were examined by flow cytometry. The numbers indicate the percentage of cells within each quadrant. The data are representative of two independent experiments.
Th2 differentiation and IgE production through Th2 cell cognate help are representative immune responses of type 2 immunity. In our study, both the induction of Th2 responses and IgE production in response to intravaginal papain immunization were dependent on MyD88-mediated signaling, whereas IgE production after s.c. papain immunization was independent of MyD88 signaling (4). Because primary immunization with papain could not induce detectable levels of IgE, IgE production could only be measured after secondary immunization. In other words, both primary and secondary immunization with intravaginal papain requires signaling through MyD88, whereas secondary s.c. immunization with papain does not require MyD88 signaling to activate memory responses. One feature of intravaginal immunization distinguishing it from s.c. immunization is the involvement of the mucosal epithelium. Unlike s.c. injection with a needle, the mucosal epithelium participates in the induction of type 2 immunity after an intravaginal injection. Together with the data showing that IL-33 is also critical for the induction of the Th2 response and antibody production, it might be suggested that both IL-33, which is produced by protease-damaged epithelia, and MyD88 are components of critical signaling pathways required for the induction of the Th2 response and antibody production after intravaginal papain immunization.
The present study suggests a mechanism by which type 2 immunity is induced in response to intravaginal papain immunization. Despite the high prevalence of genital allergies and parasite infections, the generation of type 2 immunity in the female genital tract has not been studied. Because the genital tract is under the control of the hormonal cycle and is continuously changing, it is not surprising that the induction of type 2 immunity in the vaginal tract is dependent on the estrous phase of the mice. The epithelial-derived cytokine IL-33 signals through MyD88 and activates ILC2, which might induce polarization toward type 2 immunity. In support of this hypothesis, IRF4-expressing PDL2+CD301b+ DCs promote the differentiation of Th2 cells. Although we used a model allergen, papain, our findings suggest a mechanism by which allergens or parasite infections induce type 2 immune responses in the female genital tract.
Materials and Methods
Mice.
BALB/c and C57BL/6 mice were bred in our colony. The 4get (12), CD11c–DTR–EGFP (25), MyD88−/− (33), and TLR4−/− (34) mice were provided by S. J. Kang, Korea Advanced Institute of Science and Technology (KAIST), Daejeon. IL-33−/− mice (35) were provided by S. Nakae, RIKEN Center for Developmental Biology, Kobe, Japan. IRF4f/f mice and Batf3−/− mice were purchased from Jackson Laboratories. CD11c-cre and IL-1R−/− mice were provided by A. Iwasaki, Yale University, New Haven, CT. ∆dblGATA mice were provided by M. H. Jang, Pohang University of Science and Technology, Pohang, Korea.
IRF4f/f x CD11c-cre mice were bred in-house. All mice were housed in a specific pathogen-free facility at KAIST. All procedures involving animals were performed in accordance with the guidelines and protocols (KA2013-55) for rodent experimentation provided by the Institutional Animal Care and Use Committee of KAIST.
T-Cell Assay.
For the in vitro restimulation of T cells, mice were intravaginally immunized with 200 μg of papain (Calbiochem) in 10 μL of PBS 5–7 d after the s.c. injection of 2 mg of MPA (Tokyo Chemical Industry) as described (36). CD4+ T cells were isolated from the iliac LNs by using anti-CD4–conjugated microbeads (Miltenyi Biotech) or a MagniSort CD4-positive selection kit (eBioscience), according to the manufacturer’s instructions. Purified CD4+ T cells (2 × 105 cells) were cocultured with irradiated (1,500 rad) splenocytes (2 × 105 cells) in the presence of various amounts of heat-inactivated papain for 5 d at 37 °C. IL-4 (eBioscience), IL-5 (BD Biosciences), and IL-13 (eBioscience) production was measured in the supernatants by ELISA, according to the manufacturer’s instructions.
Flow Cytometry.
Single-cell suspensions were prepared from LNs, vaginal tissues, or blood by using described methods (37). Single cells were pretreated with an anti-CD16/32 antibody (2.4G) to block the Fc receptors and were then stained with the following antibodies: TCRβ (H57-597), CD4 (GK1.5), FcεRI (MAR-1), CD103 (2E7), and CD49b (DX5) (eBioscience); CD3ε (145-2C11), CD11c (HL3), CD273 (PDL2, TY25), Siglec-F (E50-2440), CD11b (M1/70), CCR3 (83103), and MHCII (M5/114.15.2) (BD Biosciences); B220 (RA3-6B2), CD8α (53-6.7), CD301b (MGL2, URA-1), CD127 (IL-7Rα, A7R34), and CD326 (Ep-CAM, G8.8) (BioLegend); or NK1.1 (PK136) (TONBO Biosciences). Leukocytes were gated based on their forward and side scatter properties, and live cells were gated based on DAPI (Invitrogen) or propidium iodide (BioLegend) exclusion. All samples were acquired on FACSCalibur or LSR Fortessa cell analyzers (BD Biosciences). All data were analyzed by using FlowJo (TreeStar).
Antibody Production.
Mice were intravaginally immunized with 200 μg of papain on days 0 and 14 as described above to analyze papain-specific antibody production. Sera and vaginal washes were collected on the indicated days after the first immunization. A Nunc Maxisorp 96-well plate was coated with papain (50 μg/mL) and E64 (100× molar excess; Sigma-Aldrich), and diluted samples were added to detect the levels of papain-specific IgE and IgG1 antibodies (4). The presence of papain-specific antibodies was detected with biotinylated anti-IgE (R35-118; BD Biosciences) or biotinylated anti-IgG1 (RMG1-1; BioLegend) antibodies. Antibody binding was detected with streptavidin–HRP (BioLegend), and color was developed in the plates with 3,3′,5,5′-tetramethylbenzidine (eBioscience).
Measurement of Vaginal Cytokine Levels.
Vaginal fluids were collected on days 0–5 after immunization with papain by pipetting PBS (50 μL) into and out of the vagina 20 times. The IL-33 levels in the vaginal fluids were measured by using an ELISA kit (eBioscience), according to the manufacturer’s instructions.
Immunofluorescence Staining.
Tissues were embedded in Neg-50 Frozen Section Medium (ThermoFisher Scientific) in cryomold (Sakura Finetek) and cut into 6-µm sections. Before staining, tissues were fixed with 4% (wt/vol) paraformaldehyde for 30 min at room temperature (RT) and blocked in blocking buffer [10% donkey serum and 0.05% Tween 20 in 1× Dulbecco’s PBS (DPBS)] overnight at 4 °C. Polyclonal goat anti-mouse IL-33 antibody (AF3626; R&D Systems) was diluted in blocking buffer and incubated with tissue sections for 1 h at RT. Sections were washed in 0.05% Tween 20 containing 1× DPBS three times for 5 min and incubated with Cy3-conjugated donkey anti-goat IgG antibody (Jackson ImmunoResearch Laboratories) for 40 min at RT. After washing, sections were mounted in DAPI containing mounting buffer. A Leica DM2500 microscope was used to visualize fluorescent images (20×). Images were captured with a MicroPublisher 3.3 RTV digital camera (Q-Imaging) and processed by using Motic Images Advanced (Version 3.2; Motic).
Papain-Induced Activation of ILC2s.
Mice were intravaginally immunized with 200 μg of papain for three consecutive days. Cells were harvested from the vaginal tissue on day 4 by using described methods (36). Leukocytes were cultured in the presence of 50 ng/mL phorbol myristate acetate (PMA) (Sigma-Aldrich) and 500 ng/mL ionomycin (Sigma-Aldrich) for 18 h, and 2 μM GolgiStop (BD Biosciences) was added for the final 12 h. Single cells were stained with Ghost Dye (TONBO Biosciences) to exclude dead cells and were surface-stained with the following antibodies: lineage markers [CD3ε, CD19 (1D3; BD Biosciences), CD11c, CD11b, Gr1 (RB6-8C5; BD Biosciences), NK1.1, FcεRI], CD90.2 (Thy-1.2; 53-2.1), c-kit (2B8; eBioscience), CD45.2 (104; BD Biosciences), ST2 (DIH9), and CD25 (PC61; BioLegend). The cells were then fixed and permeabilized by using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. APC-labeled anti-mouse IL-5 Ab (TRFK5; BD Biosciences) was used for intracellular cytokine staining. Cell acquisition was performed on an LSR Fortessa (BD Biosciences), and the data were analyzed with FlowJo software (TreeStar).
Cell Depletion.
For basophil depletion, the mice were i.v. injected with 100 μg of purified FcεRI antibody (MAR-1; eBioscience) 90 and 72 h before intravaginal immunization with papain (4). For eosinophil depletion, the mice were intraperitoneally injected with a total of 100 μg of purified IL-5 antibody (TRFK5; eBioscience) on days −6, −3, and −1 before intravaginal immunization with papain. Blood leukocytes were stained and analyzed by using flow cytometry as described above to confirm the depletion of basophils and eosinophils. For DC depletion, CD11c–DTR–EGFP chimeras were generated as described (37). Briefly, bone marrow cells (5 × 106 cells) from WT or CD11c–DTR–EGFP mice were i.v. injected into lethally irradiated C57BL/6 WT mice. The chimeric mice were used for experiments 8 wk after reconstitution. The chimeras were intraperitoneally injected with 4 ng/g (mouse weight) DT (Sigma) on days −4 and −1 before intravaginal immunization with papain. Single cells isolated from the iliac LNs were stained and analyzed by flow cytometry as described above to confirm the depletion of DCs.
Identification of Estrous Phases.
Mouse estrous phases were determined by vaginal cytology as described (30, 38). Vaginal washes were collected, transferred to a dry glass slide, and overlaid with a coverslip. The slides were viewed immediately at 200× magnification under brightfield illumination. Estrus phase was defined as consisting of anucleated cornified cells, and diestrus as predominantly consisting of leukocytes.
Statistical Analysis.
The data are expressed as the means ± SEM. Differences between groups at individual time points were analyzed by using an unpaired, two-tailed Student’s t test. Differences were considered statistically significant when P < 0.05 and are indicated as follows: *P <0.05; **P < 0.01; and ***P < 0.001.
Acknowledgments
We thank the members of the Laboratory of Host Defenses and the members of the Hyehwa Forum for helpful advice on experiments and data discussions. This work was supported by National Research Foundation Grants NRF-2016R1A2B2015028, NRF-2015R1A4A1042416, NRF-2014M3A9A5044964, NRF-2012M3A9B4028274, and NRF-2010-0012891; and the KAIST Future Systems Healthcare project funded by the Ministry of Science, ICT, and Future Planning of Korea (MSIP). This study was also supported by Korean Health Technology R&D Project HI15C3516, which is funded by the MSIP and the Ministry of Health & Welfare, Republic of Korea.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612997114/-/DCSupplemental.
References
- 1.Pawankar R, Canonica GW, Holgate ST, Lockey RF. Allergic diseases and asthma: A major global health concern. Curr Opin Allergy Clin Immunol. 2012;12(1):39–41. doi: 10.1097/ACI.0b013e32834ec13b. [DOI] [PubMed] [Google Scholar]
- 2.Huppatz C, Durrheim DN. Control of neglected tropical diseases. N Engl J Med. 2007;357(23):2407, author reply 2407–2408. doi: 10.1056/NEJMc072881. [DOI] [PubMed] [Google Scholar]
- 3.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10(4):225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9(3):310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43(1):29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39(4):722–732. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halim TY, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40(3):425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonnex C. Genital allergy. Sex Transm Infect. 2004;80(1):4–7. doi: 10.1136/sti.2003.005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkin SS, Jeremias J, Ledger WJ. A localized vaginal allergic response in women with recurrent vaginitis. J Allergy Clin Immunol. 1988;81(2):412–416. doi: 10.1016/0091-6749(88)90909-8. [DOI] [PubMed] [Google Scholar]
- 10.Witkin SS, Jeremias J, Ledger WJ. Vaginal eosinophils and IgE antibodies to Candida albicans in women with recurrent vaginitis. J Med Vet Mycol. 1989;27(1):57–58. [PubMed] [Google Scholar]
- 11.Finkelman FD, Urban JF., Jr Cytokines: Making the right choice. Parasitol Today. 1992;8(9):311–314. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 12.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15(2):303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 13.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers L, et al. Enzymatically active papain preferentially induces an allergic response in mice. Biochem Biophys Res Commun. 1998;253(3):837–840. doi: 10.1006/bbrc.1998.9862. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 17.Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270(5243):1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 19.Noben-Trauth N, Hu-Li J, Paul WE. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J Immunol. 2000;165(7):3620–3625. doi: 10.4049/jimmunol.165.7.3620. [DOI] [PubMed] [Google Scholar]
- 20.Zuany-Amorim C, et al. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280(5367):1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 21.Spencer LA, Weller PF. Eosinophils and Th2 immunity: Contemporary insights. Immunol Cell Biol. 2010;88(3):250–256. doi: 10.1038/icb.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JJ, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305(5691):1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 23.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195(11):1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumamoto Y, et al. CD301b+ dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39(4):733–743. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17(2):211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett CL, Clausen BE. DC ablation in mice: Promises, pitfalls, and challenges. Trends Immunol. 2007;28(12):525–531. doi: 10.1016/j.it.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Tussiwand R, et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature. 2012;490(7421):502–507. doi: 10.1038/nature11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallichan WS, Rosenthal KL. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996;224(2):487–497. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- 29.Parr MB, et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70(3):369–380. [PubMed] [Google Scholar]
- 30.Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7(4):e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43(2):488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pichery M, et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel Il-33-LacZ gene trap reporter strain. J Immunol. 2012;188(7):3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 34.Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–3752. [PubMed] [Google Scholar]
- 35.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107(43):18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh JE, et al. Dysbiosis-induced IL-33 contributes to impaired antiviral immunity in the genital mucosa. Proc Natl Acad Sci USA. 2016;113(6):E762–E771. doi: 10.1073/pnas.1518589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh JE, Lee MS, Kim YJ, Lee HK. OASL1 deficiency promotes antiviral protection against genital herpes simplex virus type 2 infection by enhancing type I interferon production. Sci Rep. 2016;6:19089. doi: 10.1038/srep19089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;4(Appendix):4I. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]