Significance
The nucleosome, the unit of coiling DNA in chromatin, has long been known to interfere with the initiation of transcription in vitro. Nevertheless, we find that chromatin isolated from yeast is a better template for transcription than the corresponding naked DNA in vitro. Transcription of chromatin requires an additional 20 proteins beyond those required for the transcription of naked DNA.
Keywords: RNA polymerase II, PHO5, Saccharomyces cerevisiae
Abstract
Chromatin isolated from the chromosomal locus of the PHO5 gene of yeast in a transcriptionally repressed state was transcribed with 12 pure proteins (80 polypeptides): RNA polymerase II, six general transcription factors, TFIIS, the Pho4 gene activator protein, and the SAGA, SWI/SNF, and Mediator complexes. Contrary to expectation, a nucleosome occluding the TATA box and transcription start sites did not impede transcription but rather, enhanced it: the level of chromatin transcription was at least sevenfold greater than that of naked DNA, and chromatin gave patterns of transcription start sites closely similar to those occurring in vivo, whereas naked DNA gave many aberrant transcripts. Both histone acetylation and trimethylation of H3K4 (H3K4me3) were important for chromatin transcription. The nucleosome, long known to serve as a general gene repressor, thus also performs an important positive role in transcription.
Assembly of purified histones on promoter DNA interferes with the initiation of transcription by RNA polymerase II and general transcription factors (GTFs) in vitro (1) and in vivo (2). Factors that relieve inhibition by histones have been identified by transcribing chromatin reconstituted with purified histones and genetic analysis. These factors include ATP-dependent chromatin remodelers (3, 4), histone-modifying enzymes (5–7), FACT (8), and TFIIS (9). Although informative, these studies are incomplete, because chromatin reconstituted with purified histones differs from chromatin assembled in vivo. Reconstituted chromatin lacks the patterns of histone modification, histone variants, and nonhistone proteins shown to play important roles in transcription in vivo. Nucleosome positioning, also important for transcription in vivo, cannot be accurately reconstituted in vitro. We have, therefore, investigated chromatin assembled in vivo as a template for transcription in vitro.
Results
PHO5 chromatin in the transcriptionally repressed state was excised from yeast chromosomes in circular form by recombination and purified by affinity chromatography as described (Fig. S1 A and B) (10, 11). PHO5 chromatin isolated in this way was indistinguishable from chromatin at the chromosomal locus on the basis of digestion with specific and nonspecific endonucleases (Fig. S1 C and D) (10, 12). Because the chromatin was derived from a single copy gene, very small quantities were obtained: on the order of 10 fmol/L cell culture. At this low level, transcription cannot be detected directly by radioisotope incorporation or fluorescence, and therefore, we turned to RT-PCR. Two sets of primers were used: one to amplify the region downstream of the transcription start sites (TSSs) and detect all transcripts (“downstream” primer pair) and one to amplify any signal from cryptic transcripts originating upstream and reading through the promoter (“upstream” primer pair) (Fig. 1A and Fig. S2A). Subtraction of the upstream signal from the downstream signal revealed the level of promoter-specific transcription. This procedure was validated by controls: upstream and downstream primer pairs amplified their target sequences at the same rate (Fig. S2 B and C), and primer pairs were specific as shown by RT-PCR with mRNA extracted from cells grown under conditions of PHO5 activation or repression (Fig. S2D) and RT-PCR with synthetic PHO5 RNA (Fig. S2E). As an internal control against variation of reverse transcription efficiency and variable recovery of RNA after purification, lacI RNA was added to all samples as an internal standard, reverse-transcribed, and used for normalization (Materials and Methods and Fig. S3 A and B). To further reduce any aberrant signal from read-through transcription of the circular template, the template was linearized by cleavage at an NcoI site in the nucleosome-free 3′ UTR (Figs. S1A and S3A) (cutting efficiency was 94.0 ± 1.2% after 1 h at room temperature).
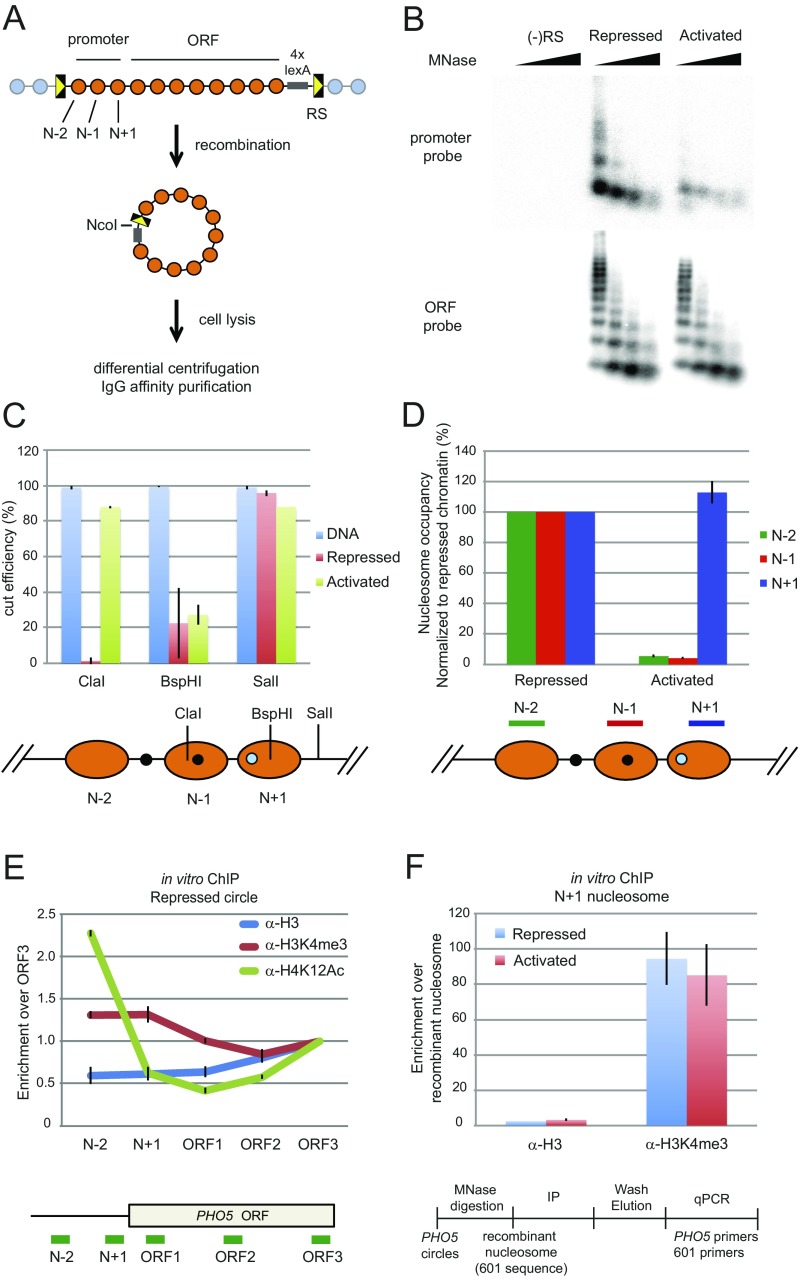
Fig. S1.
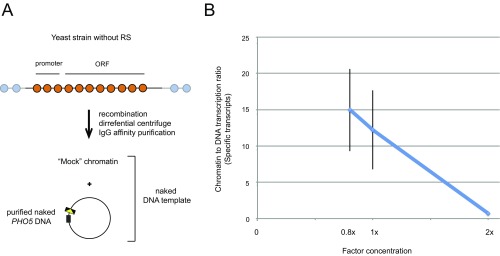
Purification of PHO5 chromatin circles. (A) The genomic PHO5 gene is flanked by two recognition sites (RSs) for R recombinase. Excision of the PHO5 locus occurs after expression of the recombinase is induced with galactose. The resulting chromatin circles were purified by differential centrifugation and affinity purification with the use of an LexA binding site within the chromatin circle and a TAP-tagged LexA adaptor protein constitutively expressed from a chromosomal locus (10, 11). (B) Micrococcal nuclease (MNase) digestion of purified gene circles. Chromatin circles purified from pho4Δ (repressed), pho80Δ (activated), and (−)RS (mock strain) cells were digested with 4, 12, 36, and 108 units MNase (NEB). DNA was extracted, subjected to 1.5% agarose gel electrophoresis, and analyzed by blot hybridization with a probe spanning PHO5 nucleosomes N + 2 to N + 4 (ORF probe) or nucleosomes N − 2 to N + 1 (promoter probe). Hybridization signal from the activated promoter is about one-third of that from the repressed promoter, showing that an average of one promoter nucleosome remains in the transcriptionally activated state (12). (C) Restriction endonuclease accessibility of the purified PHO5 circles. Restriction enzymes were used to examine the positioning and occupancy of the promoter nucleosomes. Chromatin circles (repressed or activated; 1 fmol) or equivalent amounts of naked DNA were digested with 0 (no digest control) or 20 units restriction endonuclease indicated for 1 h at 30 °C. The DNA was extracted, and the amount of DNA before or after digestion was determined by qPCR using PCR primer sets flanking each restriction site. The cut efficiency was subsequently calculated. (D) MNase digestion of repressed or activated PHO5 circles. Chromatin circles (1 fmol) purified from WT (repressed) and pho80Δ (activated) cells were digested with 0 (no digest control) or 72 (generating mononucleosomes; B) units MNase (NEB). DNA was extracted, and the amount of each nucleosomal DNA before or after digestion was determined by qPCR using the PCR primer pairs shown below. To calculate the occupancy of each nucleosome, the amount of DNA after digestion was first divided by the amount of DNA before digestion. These values in the repressed chromatin were set as 1 [N + 1, N − 1, and N − 2 nucleosomes are always present in the repressed promoter (24, 45)], and the occupancy of each nucleosome in the activated promoter circles was subsequently calculated. (E) Repressed PHO5 gene circles showed characteristic distribution of H3K4me3 and acetylated H4K12 (H4K12Ac) as shown in vivo (23, 26). PHO5 chromatin circles were digested to mononucleosomes by MNase, and IP was performed with α-H3, α-H3K4me3, or α-H4K12Ac antibodies. Fold increase reflects the ratio of signals at different PHO5 locations divided by that of PHO5 ORF3 after normalization to the ratio obtained in the input fraction. The mean of three experiments is shown with SEM. (F) H3K4me3 is associated with the promoter of both repressed and activated PHO5 gene circles as in vivo (26). PHO5 chromatin circles were digested to mononucleosomes by MNase followed by the addition of recombinant nucleosomes (with no modifications) assembled on the 601 positioning sequence serving as a normalization reference. Independent IP reactions were performed with α-H3 and α-H3K4me3 antibodies. Enrichment of H3 or H3K4me3 was calculated as the ratio of qPCR signals at the PHO5 promoter (N + 1) divided by that of the 601 sequence after normalization to the ratio obtained in the input fraction. A mean of several experiments is shown with SEM.
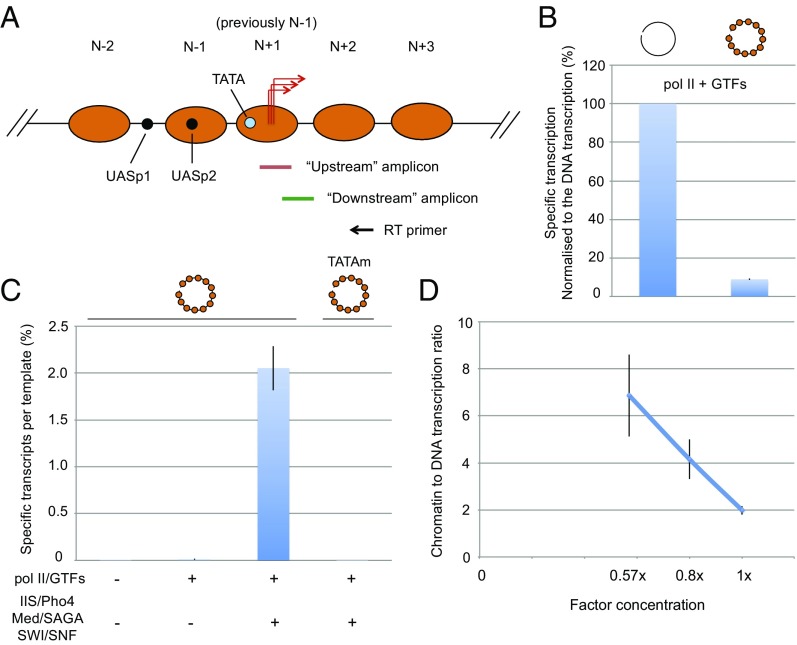
Fig. 1.
Chromatin transcription with purified proteins. (A) Diagram of PHO5 gene and promoter chromatin in the transcriptionally repressed state (17). Nucleosomes (orange ovals) previously numbered N − 3, N − 2, and N − 1 are renumbered N − 2, N − 1, and N + 1 for consistency with the literature for all eukaryotic genes. UASp1 and UASp2, binding sites for the Pho4 activator protein, are indicated by black circles, and the TATA box is indicated by a blue circle. TSSs are indicated by red arrows. Quantitation of PHO5 transcripts was performed by reverse transcription (RT primer shown by the black arrow) followed by qPCR with the use of two primer sets (Fig. S2): a downstream primer pair to amplify the region downstream of the TSSs in vivo (18) (amplicon 85 bp) and an upstream primer pair to amplify the region that includes TSSs (amplicon 81 bp). As explained in the text, the result for the upstream primer pair was subtracted from that for the downstream pair to obtain the level of promoter-dependent (“specific”) transcription. (B) Repressed PHO5 chromatin (right bar) or an equivalent amount of naked DNA (left bar) purified from repressed chromatin was transcribed with pol II and six GTFs at 1× concentration (Materials and Methods). Transcription levels were normalized to the value obtained from the naked DNA reaction (percentage; n = 3). (C) Repressed PHO5 chromatin was transcribed with pol II, GTFs, TFIIS, Pho4, Mediator, SAGA, and SWI/SNF in the combinations indicated below the bars at 1× concentration. TATAm denotes a mutant chromatin template, in which a 24-bp core promoter sequence containing the TATA box was replaced by an unrelated sequence (12). Absolute amounts of specific transcripts were determined with the use of synthetic PHO5 RNA as a reference. The activity is presented as the mean number of transcripts per template (percentage) ± SE. (D) Transcription reactions were performed with equal amounts of chromatin or naked DNA templates and the complete set of transcription proteins at the levels indicated on the abscissa. Chromatin to DNA transcription ratio (± SE) is plotted against the level of transcription proteins (n = 8).
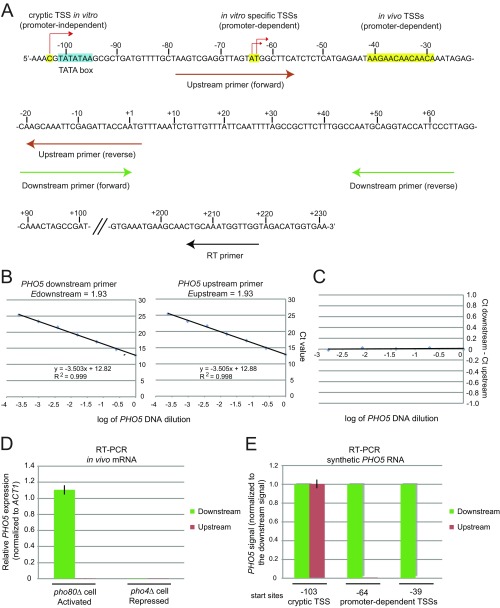
Fig. S2.
Validation of RT-PCR method. (A) 5′ RACE of in vitro PHO5 transcripts revealed three major initiation regions (Fig. 2): in vivo TSSs (positions from −41 to −29), TATA-dependent in vitro-specific TSSs [positions −63 and −64 corresponding to TSSs in metazoans (46)], and a TATA-independent cryptic TSS (position −103). Two sets of PHO5 primers were used to detect TATA-dependent specific transcripts. The downstream primer pair detects all transcripts, whereas the upstream primer pair only detects cryptic transcripts (E). Because the upstream and downstream primer pairs amplified their target sequences at the same rate (B and C), promoter-dependent transcription signal was calculated by subtracting the upstream signal from the downstream signal. (B) Amplification efficiency (E) of each prime pair was determined by replicate analyses of dilution series prepared from linear reference PHO5 DNA of known amount containing the entire promoter and ORF sequence (Edownstream = 1.93; Eupstream = 1.93; ElacI = 1.93). (C) When the difference in Ct value (ΔCt) between the upstream and downstream primer pair was recorded with dilution series of reference PHO5 DNA of known amount, ΔCt values were nearly zero at all template concentrations tested. This control suggested that the PHO5 upstream and downstream primer pairs amplified their target sequences exactly at the same rate, enabling us to directly calculate and compare ΔCt values between the upstream and downstream primer pairs. (D) Validation of the specificity of each primer pair. RT-PCR was performed with in vivo mRNA extracted from the indicated strains. PHO5 expression is constitutively activated in the pho80Δ strain, whereas PHO5 expression is completely repressed in the pho4Δ strain. The downstream primer pair detected in vivo PHO5 transcripts extracted from the pho80Δ strain, whereas the upstream primer pair did not. No signal was detected from the RNA extracted from the pho4Δ strain, attesting to the specificity of the primer pairs. (E) As an additional test of specificity of each primer pair, RT-PCR was performed with synthetic PHO5 RNA transcribed in vitro with T7 RNA polymerase. Synthetic PHO5 RNA molecules were transcribed from three different sites: a cryptic TSS (position −103), an in vitro-specific TSS (TATA-dependent; position −64), and an in vivo TSS (position −38). Each synthetic PHO5 RNA molecule was reverse-transcribed with the PHO5 gene-specific RT primer (RT2) (Table S2), and cDNA was analyzed by qPCR using the PHO5 upstream and downstream primer pairs. For each PHO5 RNA, the signal detected by the downstream primer pair was set as one, and the signal detected by the upstream primer pair was calculated using the equation: 1.93ΔCTPHO5, where ΔCtPHO5 = Ctdownstream − Ctupstream. The downstream primer pair detects all RNA molecules, whereas the upstream primer pair only detects the RNA molecule transcribed from a cryptic TSS. In addition, the downstream and upstream primer pairs detect the same amount of transcription arising from the cryptic TSS.
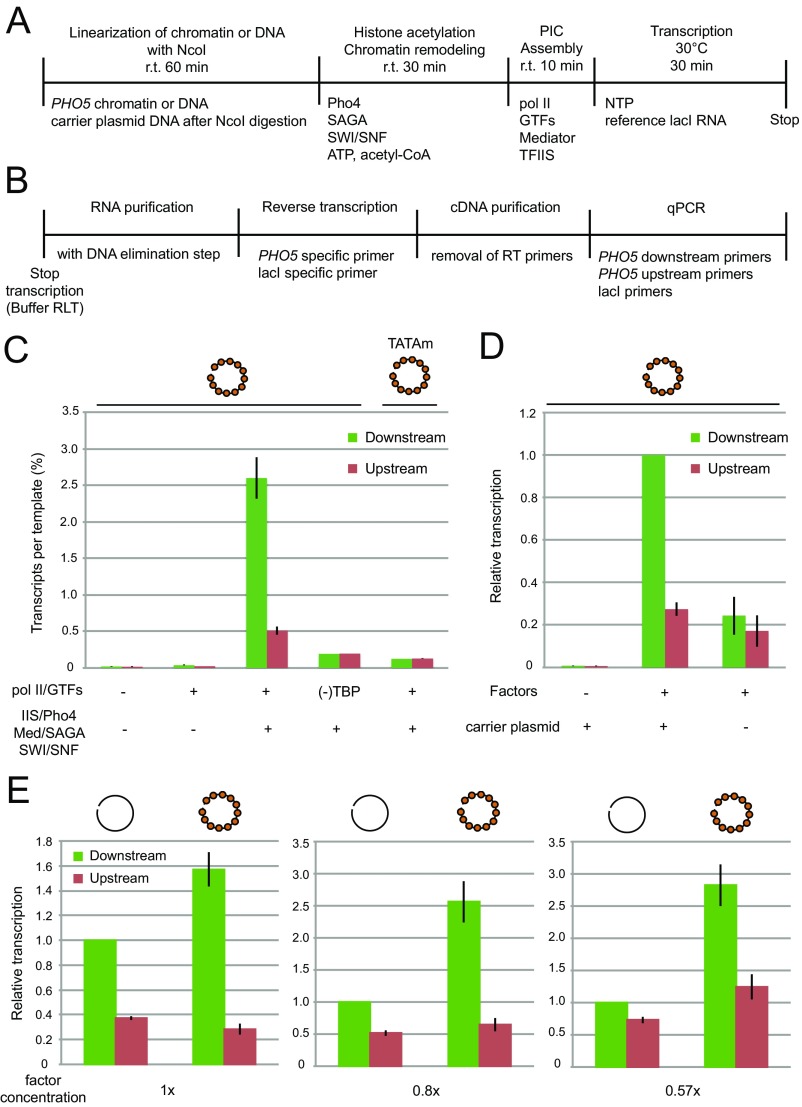
Fig. S3.
Reconstitution of chromatin transcription with purified proteins. (A) Schematic representation of chromatin transcription assay. Template chromatin or DNA circles were first linearized by cleavage at an NcoI site in the 3′ UTR for 60 min at room temperature (r.t.) followed by the addition of carrier plasmid DNA. To allow for histone acetylation and chromatin remodeling, linearized templates were incubated with Pho4 activator, SAGA, and SWI/SNF in the presence of ATP and acetyl-CoA at r.t. for 30 min. Pol II, GTFs, Mediator, and TFIIS were then added to the reaction, and PIC formation was allowed to proceed for 10 min at r.t. After PIC assembly, NTP and the reference lacI RNA were added to the reaction, and transcription was allowed to proceed for 30 min at 30 °C. (B) Schematic representation of the assay to quantify in vitro transcription reactions. Quantitation of in vitro transcripts was performed with RT-PCR using spike in lacI RNA as a normalization reference. Addition of lacI RNA controls for systematic variables, such as RNA integrity, RNA purification, and reverse transcription efficiencies. After transcription termination, transcripts were purified (including DNA elimination step; QIAGEN RNeasy Plus Micro) and reverse-transcribed with the PHO5 and lacI gene-specific RT primers. Quantitation of PHO5 transcripts was performed by qPCR using three sets of primers: the PHO5 upstream, the PHO5 downstream, and lacI primer pairs. Details are in Materials and Methods. (C) Repressed PHO5 chromatin circles were transcribed with 1× concentration (Materials and Methods) of pol II, GTFs, TFIIS, Pho4, Mediator, SAGA, and SWI/SNF. TATAm denotes a mutant chromatin template, in which a 24-bp core promoter sequence containing the TATA box was replaced by an unrelated sequence (12). Absolute amounts of transcripts detected by the downstream and upstream primer pairs were determined with dilution series of synthetic PHO5 RNA (transcribed with T7 RNA polymerase) as a reference. The amount of PHO5 transcripts divided by the amount of templates gave transcription efficiency. The activity is presented as the mean number of transcripts ± SEM per template (percentage). (D) Carrier plasmid DNA is required for chromatin transcription. The complete reaction was performed in the presence or absence of carrier plasmid DNA (100 ng per reaction). Factors denote the complete set of transcription proteins. Carrier plasmid may facilitate eviction of promoter nucleosomes by serving as histone acceptor as shown previously (15, 16, 47). (E) Chromatin potentiates transcription when transcription proteins are limiting. Transcription reactions were performed with equal amounts of chromatin or naked DNA templates and the complete set of transcription proteins at the levels indicated along the bottom. Transcription signals were normalized to the value obtained by the downstream primer pair on naked DNA template. These results show that the level of all transcripts (detected by the downstream primer pair) from chromatin is much higher than that from naked DNA, whereas the level of cryptic initiation (detected by the upstream primer pair) is more or less the same for both templates. This result suggests that chromatin enhances promoter-dependent transcription initiation.
Transcription of Repressed Chromatin.
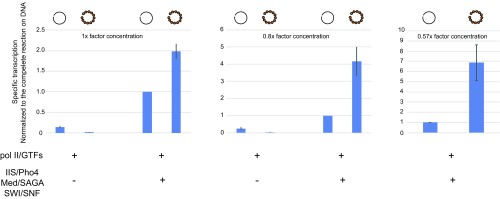
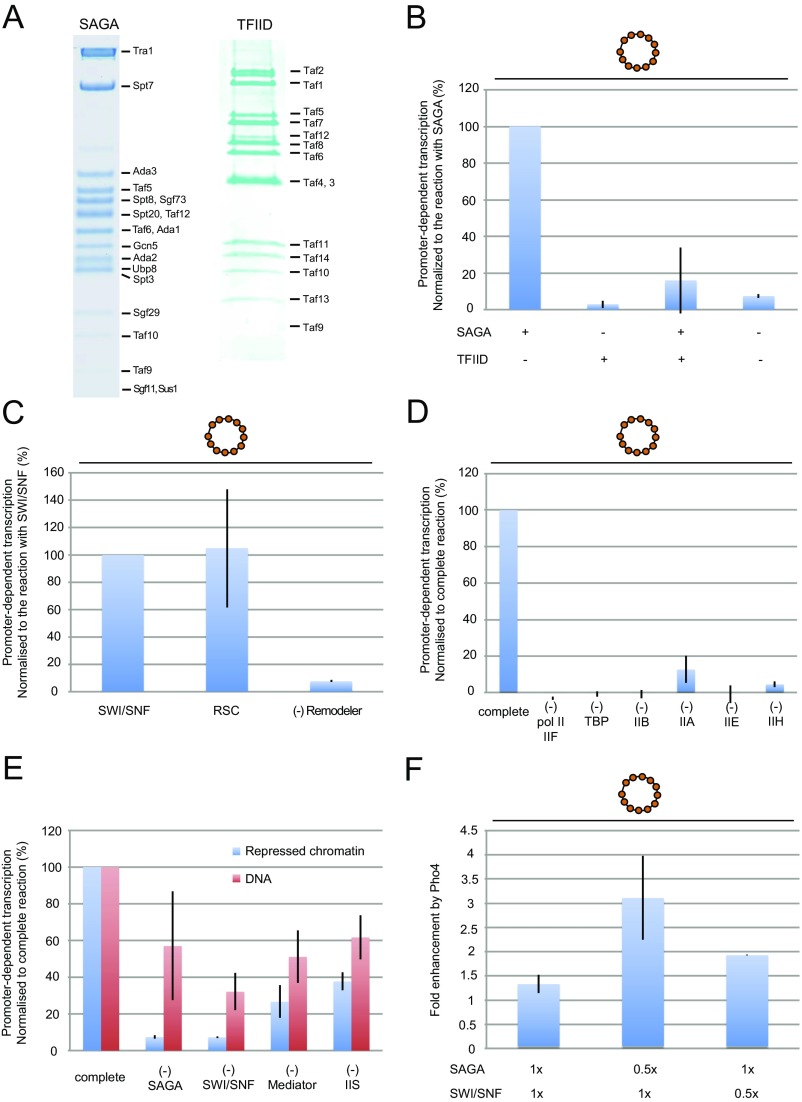
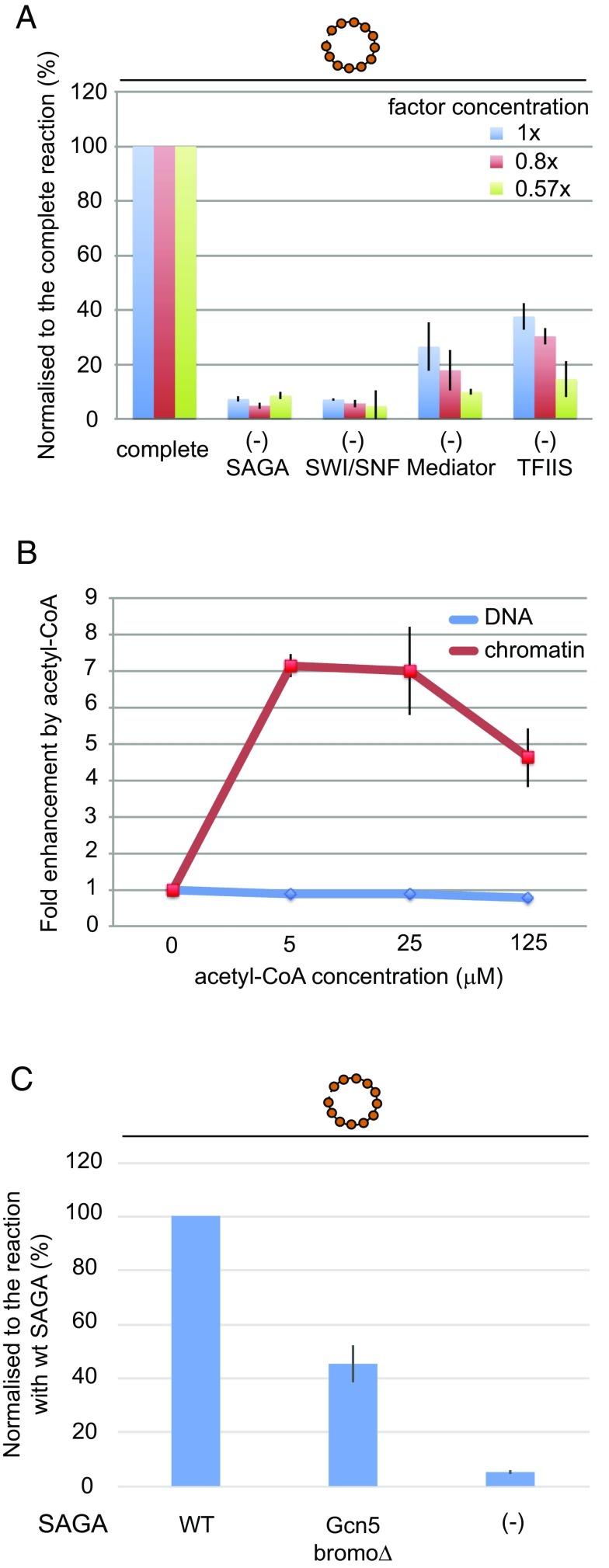
Promoter-specific transcription of naked DNA extracted from PHO5 chromatin circles by pol II and GTFs (Fig. S4) was detectable by the RT-PCR procedure (Fig. 1B and Fig. S5). In contrast, virtually no transcripts were produced from the native, repressed chromatin circles (Fig. 1C and Figs. S3C and S5). Addition of five proteins—Pho4, Mediator, TFIIS, SAGA, and SWI/SNF (Fig. S4)—elicited transcription from the chromatin circles (2.05 ± 0.24% transcripts per templates) (Fig. 1C and Figs. S3C and S5). Remarkably, the level of transcription from chromatin circles was greater than that from naked DNA under the same conditions (Fig. 1D and Figs. S3E and S5). SAGA could not be replaced by TFIID, consistent with SAGA-dependent transcription of PHO5 in vivo (Fig. S6 A and B) (13). SWI/SNF could be replaced by RSC (Fig. S6C) (14), whereas addition of the histone chaperones Nap1 and Asf1 was without effect. The promoter specificity of transcription was confirmed by the use of a mutant chromatin template, in which the 24-bp PHO5 core promoter sequence containing the TATA box was replaced by an unrelated sequence (12); virtually no specific transcripts were obtained (Fig. 1C and Fig. S3C).
Fig. S4.
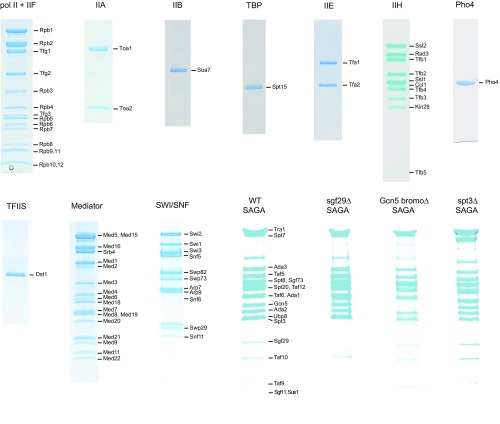
SDS/PAGE of proteins for in vitro transcription. Coomassie staining of each factor for in vitro transcription subjected to SDS/PAGE. The labels on the right side denote the factor or its subunits. Mutant SAGA complexes were purified from the sgf29Δ, gcn5 bromoΔ-13Myc, and spt3Δ yeast strains (Table S2). SDS/PAGE and MS (Table S1) confirmed that the mutant SAGA complexes contained all of the subunits, except for the deleted subunit, as shown previously (22, 25, 37).
Fig. S5.
Chromatin potentiates transcription. Equal amounts of repressed PHO5 chromatin or naked DNA were transcribed with pol II, GTFs, TFIIS, Pho4, Mediator, SAGA, and SWI/SNF in the combinations indicated below the bars at the levels indicated above the bars. Promoter-dependent transcription levels were normalized to the value obtained from the naked DNA reaction with the complete set of transcription proteins.
Fig. S6.
Requirement of each factor in transcription. (A) Coomassie staining of SDS/PAGE containing purified SAGA and TFIID. The labels on the right side denote the factor or its subunits. (B) SAGA cannot be replaced by the related TFIID complex. The complete reaction in the presence of SAGA and/or TFIID was performed at 1× concentration of factors. Promoter-dependent transcription signals were normalized to the value obtained from the SAGA-containing reaction (percentage). (C) Addition of RSC or SWI/SNF results in PHO5 chromatin transcription. The complete reaction in the presence of SWI/SNF or RSC was performed at 1× concentration of factors. Promoter-dependent transcription signals were normalized to the value obtained from the SWI/SNF-containing reaction (percentage). (D) Requirement for pol II and GTFs in chromatin transcription. The complete reaction was performed at 1× concentration of factors. Other reactions contained all factors, except the component indicated. Transcription signals were normalized to the value obtained from the complete reaction (percentage). (E) Requirement for each factor in DNA and chromatin transcription. The complete reaction was performed at 1× concentration of factors. Other reactions contained all factors, except the component indicated. Transcription signals were normalized to the value obtained from the complete reaction (percentage). (F) Requirement of Pho4 in chromatin transcription. The reaction was performed with the complete set of transcription proteins at the levels of SAGA and SWI/SNF indicated on the abscissa; y axis shows the ratio of transcripts levels with and without Pho4.
Notably, transcription of chromatin required addition of carrier plasmid DNA (Fig. S3D) or carrier rat liver chromatin. Carrier nucleic acid might serve as a histone acceptor (15, 16) or a trap for adventitiously bound proteins contaminating the PHO5 promoter chromatin and interfering with transcription.
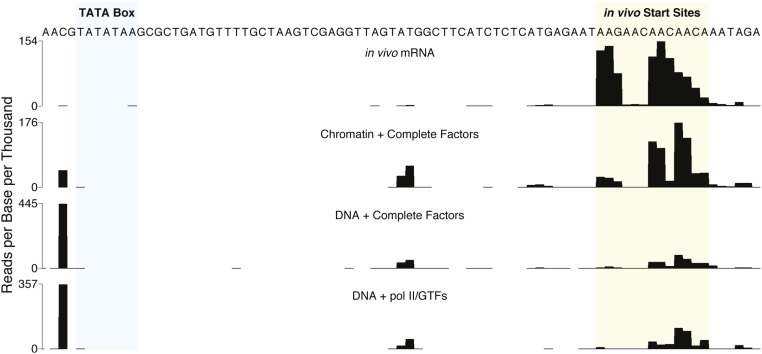
As an additional test of significance, the TSSs of the transcripts from naked DNA and chromatin were mapped by 5′ RACE followed by deep sequencing. For comparison with TSSs of PHO5 transcripts in vivo, the same procedure was applied to total RNA extracted from a pho80Δ strain, in which PHO5 transcription is constitutively activated (17). Most TSSs in vivo were located within a 20-bp initiation region 50–70 bp downstream of the TATA box (Fig. 2), consistent with a previous report (18). Our transcripts from chromatin in vitro exhibited a closely similar pattern, whereas the TSSs of naked DNA transcripts differed, especially by the occurrence of a prominent initiation site upstream of the TATA box (Fig. 2 and Fig. S2A).
Fig. 2.
Determination of TSSs by 5′ RACE. PHO5 TSSs in vivo were determined with total RNA extracted from a pho80Δ strain, in which PHO5 is constitutively activated (17). PHO5 TSSs in vitro were determined with products of transcription reactions performed with repressed (pho4Δ) PHO5 chromatin or naked DNA and transcription proteins at 1× concentration (Materials and Methods). The data are normalized to the total number of mapped reads per experiment and displayed as reads per thousand. The region of the promoter containing the TATA box is shaded light blue, and the region within which initiation occurs in vivo is shaded yellow.
The greater efficiency of chromatin than naked DNA as a template for transcription became apparent when transcription proteins were limiting. At a high concentration of all proteins, the transcription of chromatin was twofold greater than that of naked DNA (Fig. 1D and Figs. S3E and S5), whereas transcription of chromatin was about sevenfold greater than that of naked DNA when the level of transcription proteins was reduced about 1.75-fold (Fig. 1D and Figs. S3E and S5). Similar results were obtained when transcription from chromatin was compared with that from naked DNA in the presence of “mock chromatin” (the fraction resulting from the same chromatin purification procedure performed on the yeast strain lacking sites for the R recombinase) (Fig. S7). The possibility that the enhanced transcription of chromatin was caused by contaminants in the solution was thereby excluded. These observations raise the possibility of near-total dependence of specific transcription on chromatin structure at lower levels of transcription proteins in vivo (5 molecules pol II per PHO5 gene in vivo compared with 500 molecules per gene in vitro).
Fig. S7.
Chromatin potentiates transcription. (A) To exclude the possibility that the enhanced transcription of chromatin was because of contaminants in the solution, purified PHO5 naked DNA was mixed with mock chromatin [the solution resulting from the same procedure as that used for the purification of chromatin, except starting from the yeast strain without sites for the R recombinase (ySH139)] (Table S2). RS, recognition site. (B) Transcription reactions were performed with equal amounts of chromatin or naked DNA templates (supplemented with mock chromatin) and the complete set of transcription proteins at the levels indicated on the abscissa. Chromatin to DNA transcription ratio (±SE) is plotted against the level of transcription proteins (n = 3).
Pol II, GTFs, SAGA, and SWI/SNF were required at all concentrations of the transcription proteins (Fig. 3A and Fig. S6D). The dependence on SAGA and SWI/SNF reproduces the requirement for these factors for PHO5 transcription in vivo (13, 19). SWI/SNF also stimulated transcription of naked DNA (Fig. S6E), perhaps through its Snf6 subunit, shown to stimulate transcription in vivo independently of the rest of the SWI/SNF complex (20). The effect of Mediator was concentration-dependent, increasing from 5- to 10-fold on 2-fold dilution of the other proteins; the effect of TFIIS increased from about three- to sixfold on twofold dilution (Fig. 3A). TFIIS has also been implicated in the initiation of transcription in vivo (21). A stimulatory effect of Pho4 on transcription was observed when the concentration of SAGA was decreased (Fig. S6F), suggesting a Pho4–SAGA interaction.
Fig. 3.
Dependence of chromatin transcription on protein factors and acetyl-CoA. (A) Repressed PHO5 chromatin was transcribed as in Fig. 1C with transcription proteins at 1×, 0.8×, and 0.57× concentrations, except for the omission of SAGA, SWI/SNF, Mediator, and TFIIS as indicated. Transcription levels were normalized to the value obtained from the complete reaction (percentage). (B) As in A with acetyl-CoA at the concentrations indicated. The ratios of transcription levels with and without acetyl-CoA are plotted on the ordinate. (C) Effect of gcn5 bromoΔ SAGA on chromatin transcription. Repressed (pho4Δ) PHO5 chromatin was transcribed as in Fig. 1C with transcription proteins at 1× concentration, except that gcn5 bromoΔ SAGA was substituted for WT SAGA where indicated. Transcription levels were normalized to the value obtained from the reaction with WT SAGA (percentage).
The importance of SAGA for chromatin transcription could be, at least in part, because of its histone acetyltransferase activity. Indeed, chromatin transcription was strongly dependent on the concentration of acetyl-CoA in the reaction, with an optimum at about 5 μM (Fig. 3B). In contrast, there was no effect of acetyl-CoA on transcription of naked DNA. The requirement of acetyl-CoA for chromatin transcription may reflect an interaction with nucleosomes, because bromodomains in the Gcn5 and Spt7 subunits of SAGA bind acetylated H3/H4 lysine tails (22). PHO5 promoter chromatin is acetylated on H4 in the repressed state (Fig. S1E) and further acetylated on H3 by SAGA on activation in vivo (23). Support for a role of acetylated histone tails in the recruitment of transcription proteins came from the use of a mutant SAGA complex lacking the Gcn5 bromodomain (Fig. S4 and Table S1). Replacement of WT SAGA with the mutant diminished transcription of PHO5 chromatin (Fig. 3C).
Table S1.
MS analysis of mutant SAGA complexes
| Subunit | Yeast strain | |||
| WT | sgf29Δ | gcn5 bromoΔ | spt3Δ | |
| Tra1 | 383 | 460 | 473 | 597 |
| Spt7 | 205 | 231 | 240 | 317 |
| Ada3 | 122 | 115 | 144 | 175 |
| Spt20 | 109 | 134 | 146 | 178 |
| Taf5 | 84 | 98 | 100 | 144 |
| Ada1 | 73 | 75 | 83 | 104 |
| Taf12 | 104 | 87 | 94 | 111 |
| Spt8 | 58 | 76 | 80 | 106 |
| Taf6 | 52 | 62 | 64 | 79 |
| Sgf73 | 80 | 92 | 98 | 125 |
| Ubp8 | 53 | 59 | 63 | 79 |
| Ada2 | 52 | 55 | 62 | 85 |
| Gcn5 | 56 | 67 | 55 | 100 |
| Sgf29 | 34 | 0 | 42 | 52 |
| Taf10 | 32 | 36 | 39 | 37 |
| Spt3 | 32 | 37 | 50 | 6* |
| Taf9 | 23 | 24 | 23 | 31 |
| Sgf11 | 18 | 15 | 13 | 10 |
| Sus1 | 22 | 21 | 20 | 20 |
The numbers represent total peptides obtained for each component.
Note that the spt3Δ strain has partial Spt3 deletion (spt3Δ203) (Table S2). Therefore, trace amounts of Spt3 were detected.
Transcription of Activated Chromatin.
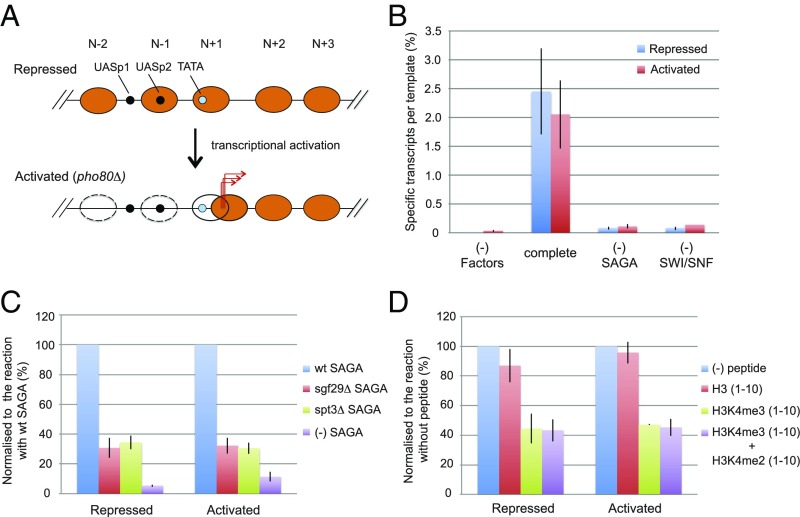
Previous studies have shown the remodeling of PHO5 promoter chromatin on transcriptional activation (17). A nucleosome covering a Pho4 binding site and one farther upstream (N − 1 and N − 2 in Fig. 4A) are largely removed (12) (Fig. 4A and Fig. S1 C and D), whereas the nucleosome covering the TSSs (N + 1) is removed from only 40% of promoters and shifted downstream in the remainder, exposing the TATA box but still covering the TSSs (Fig. 4A) (12, 24). Chromatin circles isolated from the PHO5 gene in the activated state retain this remodeled chromatin structure (Fig. S1 C and D) (12). We expected that activated PHO5 circles would be transcribed even more efficiently than the repressed circles, with a loss of requirement for the histone-modifying and remodeling factors SAGA, acetyl-CoA, and SWI/SNF complex. We were, therefore, surprised to find transcription of activated chromatin at about the same level as or even slightly less than that of repressed chromatin (Fig. 4B). Transcription of activated PHO5 chromatin remained dependent on the SWI/SNF and SAGA complexes (Fig. 4B).
Fig. 4.
Involvement of H3K4me3 in chromatin transcription. (A) Diagram of PHO5 gene and promoter chromatin in the transcriptionally repressed and activated states (12), with labeling as in Fig. 1A. (B) Repressed (pho4Δ) or activated (pho80Δ) PHO5 chromatin was transcribed as in Fig. 1C with transcription proteins at 1× concentration, except for the omission of SAGA, SWI/SNF, or all transcription proteins as indicated. Activity is presented as in Fig. 1C (n = 7). (C) Effect of sgf29Δ and spt3Δ SAGA on chromatin transcription. Repressed (pho4Δ) or activated (pho80Δ) PHO5 chromatin was transcribed as in Fig. 1C with transcription proteins at 1× concentration, except that sgf29Δ or spt3Δ SAGA was substituted for WT SAGA where indicated. Transcription levels were normalized to the value obtained from the reaction with WT SAGA (percentage). (D) Effect of histone H3 peptides on chromatin transcription. Repressed (pho4Δ) or activated (pho80Δ) PHO5 chromatin was transcribed as in Fig. 1C with transcription proteins at 1× concentration with the addition of histone H3 peptides (1–10) with or without methylation at K4 as indicated (40 μM final concentration). Transcription levels were normalized to the value obtained from the reaction without H3 peptide (percentage).
The increase in transcription of chromatin relative to that of DNA, observed on reduction in concentration of transcription proteins (Fig. 1D and Figs. S3E and S5), points to an interaction of the proteins with chromatin. The Sgf29 subunit of SAGA binds di- or trimethylated H3K4 (25), and trimethylation of H3K4 (H3K4me3) is found at the promoters of both repressed and activated PHO5 circles in vitro (Fig. S1 E and F) as previously reported in vivo (26). Support for a role of H3K4me3 in chromatin transcription came from the use of a mutant SAGA complex lacking Sgf29 (Fig. S4 and Table S1). Replacement of WT SAGA with the mutant diminished transcription of PHO5 chromatin in both repressed and activated states (Fig. 4C).
Additional support for a role of H3K4me3 in chromatin transcription came from competition with H3 peptides (H3 residues 1–10). Addition of the H3 peptide containing K4me3 diminished transcription, whereas addition of peptides with no modification had no significant effect (Fig. 4D). Addition of a dimethylated H3K4 peptide produced no greater effect (Fig. 4D). The inhibition of transcription by H3K4me3 peptide increased with increasing concentration of peptide, reaching a plateau at about 20 μM. As mentioned, the enhancement of transcription by chromatin points to transcription protein–chromatin interaction, and the involvement of H3K4me3 suggests that a target of this interaction is the nucleosome.
The essential role of SAGA after chromatin remodeling (Fig. 4B) may reflect a role as a component of the transcription preinitiation complex (PIC). The Spt3 and Spt8 subunits of SAGA have been shown to interact with TBP, and these interactions are important for transcription in vivo (13, 27). Support for a role of SAGA in the PIC came from the use of a mutant SAGA complex lacking Spt3 (Fig. S4 and Table S1). Replacement of WT SAGA with the mutant diminished transcription of PHO5 chromatin in both repressed and activated states (Fig. 4C). The requirement for both Spt3 and Sgf29, even after chromatin remodeling (Fig. 4C), points to the persistence of SAGA in the PIC.
Discussion
As long known (1, 2) and recapitulated here (Fig. 1B and Fig. S5), nucleosomes inhibit the initiation of transcription by RNA polymerase II and GTFs. It is commonly assumed that chromatin remodeling relieves this inhibition by the removal of nucleosomes and exposure of naked DNA for transcription. On this basis, the most that we could hope for in the transcription of chromatin in vitro would be a level of transcription comparable with that obtained with naked DNA. It was, therefore, surprising that a level almost an order of magnitude greater was achieved. Because transcription from chromatin relative to naked DNA increases as the concentration of transcription proteins is reduced and because the levels of transcription proteins are much lower in vivo (see above), it is likely that chromatin is not only stimulatory but required for transcription in vivo.
Several lines of evidence suggest that interaction of transcription proteins with nucleosomes is responsible for the stimulation of transcription: an inverse relationship of stimulation with transcription protein concentration points to direct transcription protein–nucleosome interaction, and the H3K4me3 and histone tail acetylation, maximal at the promoter region (Fig. S1 E and F), play important roles in the recruitment (Figs. 3C and 4 C and D). It remains to be determined whether nonhistone components of promoter chromatin contribute to the potentiation of transcription. MS of purified PHO5 chromatin circles has thus far revealed only histones (11), but proteins present in one copy or a small number of copies on the circles may have escaped detection by this analysis.
Our finding of the stimulation of transcription by chromatin should not be conflated with the relief of repression by histone modifications, such as acetylation and ubiquitylation (5–7, 28). These modifications relieve repression by recruiting remodelers (5, 7) and histone chaperones (6) or by chromatin decondensation (28). Relief of repression represents the removal of a negative effect, not the presence of a positive one. Only the comparison reported here, of naturally assembled chromatin with the corresponding naked DNA, could reveal a positive role of the nucleosome.
Our findings are in keeping with other evidence for interaction of transcription proteins with nucleosomes. The YEATS domain of Tfg3 (Taf14), a subunit of TFIIF (Fig. S4), binds to acetylated H3K9 (29). Mediator binds to nucleosomes (30) and the histone H4 tail (31). Bromodomains in SWI/SNF and RSC contribute to the binding of these complexes to acetylated nucleosomes in vitro (7, 22). RSC is persistently associated with the +1 nucleosome of transcriptionally active genes in vivo (32). Deletion of RSC resulted in a genome-wide decrease in occupancy of a nucleosome at the +1 position and a decrease in gene expression (32), pointing to the importance of the nucleosome for transcription.
As mentioned above, activation of the PHO5 gene is accompanied by movement of the +1 nucleosome downstream, exposing the TATA box but still covering the TSSs (Fig. 4A). Mutation of the TATA box makes PHO5 expression dependent on acetylatable lysine residues in the histone H4 N-terminal region and on the bromodomain factor Bdf1, a TFIID-associated protein (33). Apparently, in the absence of a key promoter element, promoter nucleosomes may even play a predominant role in the assembly of the PIC. Many yeast genes, including so-called “housekeeping” genes, resemble the activated PHO5 gene, with a nucleosome-free region followed by a +1 nucleosome covering the TSSs. Despite the absence of well-defined TATA and initiator elements, the TSSs of these genes are almost always about 10–15 bp inside the upstream border of the +1 nucleosome (34). The findings reported here for PHO5 may, therefore, apply generally: promoter chromatin potentiates transcription.
Materials and Methods
Transcription Assay.
Naked DNA templates were purified from chromatin circles (yeast strains listed in Table S2) by phenol–chloroform extraction or the QIAquick PCR Purification Kit (QIAGEN) and resuspended in the same buffer as chromatin circles [40 mM Hepes-KOH, pH 7.4, 50 mM potassium acetate, 1 mM EDTA, 10% (vol/vol) glycerol, 5 mM DTT, 1× protease inhibitors]. Template chromatin or DNA circles were first linearized by cleavage at an NcoI site in the 3′ UTR for 60 min at room temperature [cut efficiency on chromatin checked by quantitative PCR (qPCR); 94.0 ± 1.2%] followed by the addition of carrier plasmid DNA (100 ng per reaction). To allow for histone acetylation and chromatin remodeling, 20-μL linearized templates were incubated with Pho4 activator (11.76 nM), SAGA (20 nM), and SWI/SNF (20 nM) in the presence of 1 mM ATP, 10 μM acetyl-CoA, 5 mM sodium butyrate, 8 mg/mL creatine kinase, and 10 mM phosphocreatine for 30 min at room temperature in a reaction volume of 25 μL. After histone acetylation and chromatin remodeling, purified pol II, GTFs, TFIIS, and Mediator were added to the reaction, and PIC formation was allowed to proceed for 10 min at room temperature. After PIC assembly, NTP, RNaseOUT (Invitrogen), and the reference lacI RNA were added to the reaction, and transcription was allowed to proceed for 30 min at 30 °C. Final reaction mixtures (50-μL reaction) contained 1 fmol chromatin or DNA templates (20 pM); 100 ng carrier plasmid (0.5 nM); Pho4 (5.88 nM); SAGA (10 nM); SWI/SNF (10 nM); pol II (8.3 nM); TBP (10.6 nM); IIB (12.5 nM); IIE (15 nM); IIF (8.3 nM); IIH (6.3 nM); IIS (12 nM); Mediator (10 nM); 40 mM Hepes-KOH, pH 7.5; 80 mM potassium acetate; 5 mM DTT; 7.5 mM magnesium acetate; 5% (vol/vol) glycerol; 2 mM ATP; 0.8 mM UTP, TTP, and GTP; 40 units RNaseOUT; 5 μM acetyl-CoA; 2.5 mM sodium butyrate; 4 mg/mL creatine kinase; 5 mM phosphocreatine; and 12.2 pg reference lacI RNA (2 pM). These concentrations of purified proteins were considered as 1× concentration. Reactions were terminated with the addition of buffer RLT containing guanidine (RNeasy Plus Micro; QIAGEN), and samples were spun through gDNA Eliminator Spin Columns to remove DNA. RNA was subsequently purified according to the manufacturer’s instructions.
Table S2.
Yeast strains used in this study
| Name | Genotype | Source |
| FY2031 | MATa; HA-SPT7-TAP::TRP1; ura3Δ0; leu2Δ1; trp1Δ63; his4-917d; lys2-173R2 (S288C) | Ref. 37 |
| FY2040 | MATa; spt3Δ203::trp1; HA-SPT7-TAP::TRP1; ura3Δ0; leu2Δ1; trp1Δ63; his4-917d; lys2-173R2 (S288C) | Ref. 37 |
| ySH139 | MATα; his3-11 his3-15 leu2-3 leu2-112 ura3∆ URA3::LEU2 pCYC1 LEXA-TAP pGAL RecR | Ref. 11 |
| ySH140 | MATα; his3-11 his3-15 leu2-3 leu2-112 ura3∆ PHO5:RS-3xLEXA-RS URA3::LEU2 pCYC1 LEXA-TAP pGAL RecR | Ref. 11 |
| ySH141 | ySH140 pho80::HIS3 | Ref. 11 |
| YS3 | MATa; SNF6-TAP::HIS3; ura3Δ0; leu2Δ0; his3Δ1; met15Δ0 (S288C) | Ref. 38 |
| YS7 | ySH140 PHO5-GC (TATAm) | This study |
| YS13 | ySH140 pho4::HIS3 | This study |
| YS27 | FY2031 sgf29::KanMX6 | This study |
| YS35 | FY2031 gcn5 bromoΔ-13Myc::KanMX6 | This study |
Reverse Transcription.
Quantitation of in vitro PHO5 transcripts was performed with RT-PCR using lacI RNA as a normalization reference. Because lacI RNA was spiked into the transcription reaction at the time of initiation, it was subjected to almost all of the experimental error introduced during transcription and the multistage process required to purify and process the RNA. Purified PHO5 transcripts and lacI reference RNA were reverse-transcribed to cDNA in the same tube (QuantiTect Reverse Transcription Kit; QIAGEN) using the following gene-specific RT (reverse transcription) primers: PHO5 RT primer (RT2: 5′-CCAACCATTTGCAG-3′) and lacI RT primer (RT3: 5′-AGCTTCCACAGC-3′). RT reaction was performed for 15 min at 42 °C followed by inactivation of reverse transcriptase for 3 min at 95 °C. First-strand cDNA was purified (Econospin Column; Epoch Life Science) and analyzed by qPCR as described below. All primers used for qPCR are listed in Table S3.
Table S3.
Primers used in this study
| Name | Sequence | Purpose |
| Reverse transcription primer | ||
| RT2 | 5′-CCAACCATTTGCAG-3′ | Used for reverse transcription of PHO5 gene circle transcripts |
| RT3 | 5′-AGCTTCCACAGC-3′ | Used for reverse transcription of lacI reference RNA |
| In vitro transcription | ||
| p5 | 5′-TGGTGGTGTCGATGGTAGAA-3′ | Forward primer for lacI; used with p6; amplicon 112 bp. |
| p6 | 5′-TGGTCATCCAGCGGATAGT-3′ | Reverse primer for lacI; used with p5; amplicon 112 bp. |
| p14 | 5′-CAAGCAAATTCGAGATTACCAA-3′ | Downstream primer for PHO5 gene circle transcripts; used with p16. Amplicon 85 bp. |
| p16 | 5′-AGGGAATGGTACCTGCATTG-3′ | Downstream primer for PHO5 gene circle transcripts; used with p14. Amplicon 85 bp. |
| p21 | 5′-AAGTCGAGGTTAGTATGGCTTCA-3′ | Upstream primer for PHO5 gene circle transcripts; used with p22. Amplicon 81 bp. |
| p22 | 5′-CATTGGTAATCTCGAATTTGCTT-3′ | Upstream primer for PHO5 gene circle transcripts; used with p21. Amplicon 81 bp. |
| 5′ RACE | ||
| p43 | 5′-TAGCCAGACTGACAGTAGGGTATC-3′ | PHO5-specific outer primer (for gene circle); used with 5′ RACE outer primer (Ambion) |
| p47 | 5′-ATTCTGCAGCATTTCACAACCTTCAGGCAAATC-3′ | PHO5-specific inner primer (for gene circle); used with 5′ RACE inner primer (Ambion) |
| In vitro ChIP | ||
| p19 | 5′-GGTAATCTCGAATTTGCTTGC-3′ | N + 1 forward primer; used with p20 |
| p20 | 5′-GATGTTTTGCTAAGTCGAGGT-3′ | N + 1 reverse primer; used with p19 |
| p127 | 5′-TCATCTTATGTGCGCTGCTT-3′ | N − 2 forward primer; used with p128 |
| p128 | 5′-GCGCTATGAACCTTTTACCTTC-3′ | N − 2 reverse primer; used with p127 |
| p135 | 5′-CTTGGATGACATTGCCAAGA-3′ | ORF2 forward primer; used with p136 |
| p136 | 5′-CAAATGCACACCACGAGAAT-3′ | ORF2 reverse primer; used with p135 |
| p137 | 5′-CAACTCGGACGATGTTTTGA-3′ | ORF1 forward primer; used with p138 |
| p138 | 5′-CTGGTTTGGTTTTCGACCAT-3′ | ORF1 reverse primer; used with p137 |
| p189 | 5′-aactgccgaatacgttccat-3′ | ORF3 forward primer; used with p190 |
| p190 | 5′-ttcggtgtagacacgagcac-3′ | ORF3 reverse primer; used with p189 |
Quantitation of in Vitro Transcripts.
After reverse transcription, PHO5 transcripts were quantified with qPCR (ABI POWER SYBR Green PCR Master Mix) using an ABI 7900HT Fast Real-Time PCR System. For quantitation of PHO5 in vitro transcripts, the following primers were used (300 nM final concentration): PHO5 downstream primer pair (p14: 5′-CAAGCAAATTCGAGATTACCAA-3′, p16: 5′-AGGGAATGGTACCTGCATTG-3′), PHO5 upstream primer pair (p21: 5′-AAGTCGAGGTTAGTATGGCTTCA-3′, p22: 5′-CATTGGTAATCTCGAATTTGCTT-3′), and lacI primer pair (p5: 5′-TGGTGGTGTCGATGGTAGAA-3′, p6: 5′-TGGTCATCCAGCGGATAGT-3′). Amplification efficiency (E) of each primer pair was determined by replicate qPCR analysis of a dilution series prepared from reference PHO5 and lacI DNA of known amounts using the equation E = 10(−1/slope) (Fig. S2B) (Edownstream = 1.93; Eupstream = 1.93; ElacI = 1.93). Importantly, when the difference in Ct (cycle threshold) value (ΔCt) between the upstream and downstream PHO5 primer pairs was recorded with a dilution series of reference PHO5 DNA of known amount, ΔCt values were nearly zero at all template concentrations tested (Fig. S2C). This control shows that the PHO5 upstream and downstream primer pairs amplify their target sequences at exactly the same rate, enabling us to accurately calculate ΔCt between the upstream and downstream Ct values and thereby, accurately determine the relative amount of bona fide vs. cryptic transcription.
About 12 reactions (samples 1–12) were performed in each transcription assay. We first quantified relative PHO5 expression levels between different samples using lacI as a normalization reference [comparative quantitation (35)]. The amount of PHO5 transcripts in sample 1 (usually chromatin transcribed with a complete set of factors) detected by the downstream primer pair was set as one. Then, the relative amount of PHO5 transcripts (R) detected by the upstream or downstream primer pair in a given sample was calculated using the efficiency corrected method (35):
After comparative quantitation, the absolute amount of PHO5 transcripts was determined as follows. For each transcription assay, the absolute amount of PHO5 transcripts in sample 1 was determined using synthetic PHO5 RNA as a reference. Synthetic PHO5 RNA was transcribed in vitro with T7 RNA polymerase using a T7 promoter–PHO5 hybrid DNA as a template (transcribed from a known PHO5 in vivo TSS), with the concentration measured by NanoDrop (Thermo Scientific). Serial dilutions of synthetic PHO5 RNA in parallel with the PHO5 transcripts from sample 1 were reverse-transcribed, and purified cDNA was analyzed by qPCR with the PHO5 downstream primer pair. A standard curve of synthetic PHO5 RNA dilution series was generated and used to determine the absolute amount of PHO5 transcripts in sample 1 detected by the downstream primer pair. The absolute amount of PHO5 transcripts in each sample (detected by the upstream or downstream primer pair) was subsequently calculated using the relative PHO5 expression (R) determined by comparative quantitation described above. The amount of PHO5 transcripts divided by the amount of DNA template gave the transcription efficiency [transcripts per template (percentage)]. Promoter-dependent transcription signal in each sample was calculated by subtracting the upstream signal from the downstream signal. RT-PCR results [transcripts per template (percentage)] are presented as the mean of several transcription experiments (n = 3–36) ± SEM.
5′ RACE.
In vitro transcripts that contained γ and β phosphates at the 5′ end were first converted to 5′-monophosphorylated RNA using 5′ RNA polyphosphatase (Epicentre). This treatment allows TSSs from in vitro transcripts to be assayed by the T4 RNA ligase-mediated RNA adaptor tagging 5′ RACE strategy (FirstChoice RLM-RACE Kit, Ambion). In vivo total RNA was extracted from pho80Δ strains (ySH141), in which PHO5 is constitutively activated (17); 5′ RACE reactions were performed according to the manufacturer’s instructions (FirstChoice RLM-RACE Kit; Ambion). The following primers were used: 5′ RACE PHO5-specific outer primer (5′-TAGCCAGACTGACAGTAGGGTATC-3′) and 5′ RACE PHO5-specific inner primer (5′-ATTCTGCAGCATTTCACAACCTTCAGGCAAATC-3′).
Deep Sequencing.
After nested PCR of 5′ RACE samples, DNA was prepared for next generation sequencing using an NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs), except that the end prep step was omitted; in the final purification step, 17 μL 0.1× TE buffer (10 mM Tris pH 8, 1 mM EDTA) was used to elute the DNA from the AMPure XP Beads, and 15 μL was reclaimed as the purified RACE library. DNA concentration was determined using a Qubit dsDNA HS Assay Kit (Life Technologies), and DNA integrity was determined by running an aliquot on a Bioanalyzer High Sensitivity DNA Chip (Agilent). The library was accurately quantified using a KAPA Library Quantification Kit (KAPA) and then, subjected to paired end sequencing on an Illumina MiSeq instrument. For analysis, paired ends were separated, and each end was treated as a single-end read. Reads that began precisely at the 5′ end with the RLM (RNA ligase mediated)-RACE adaptor (5′-CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATGAAA-3′) were identified, the adaptor was removed, and after trimming, only reads 25 bp or longer were retained. Reads were then mapped to the Saccharomyces cerevisiae genome (April 2011/sacCer3 assembly) with bowtie. The number of reads with identical 5′ ends was determined, and the 5′-most base was identified as the TSS. Data were normalized to the number of reads per base per thousands of aligned reads per sample and displayed using the UCSC Genome Browser.
SI Materials and Methods
Plasmids.
Carrier plasmid pSH47 for transcription assays is described (36). Hexahistidine-tagged Pho4 was expressed from the plasmid EB067, which was provided by Erin O’Shea, Harvard University, Cambridge, MA.
Yeast Strains and Methods.
All strains used in this study are listed in Table S2. Spt7-TAP strains (FY2031 and FY2040) are provided by Fred Winston, Harvard University, Cambridge, MA (37). Snf6-TAP strain is derived from yeast ORF TAP-tagged library (Thermo Scientific) (38).
Yeast strains for purification of PHO5 gene circles were provided by Joachim Griesenbeck, University of Regensburg, Regensburg, Germany (39). These strains contain a chromosomally integrated cassette for constitutive expression of LexA-TAP fusion protein under the control of the moderately expressed yeast CYC1 promoter. Use of the CYC1 promoter reduces LexA-TAP expression levels, thereby greatly reducing retention of chromatin fragments unrelated to the PHO5 locus during purification of chromatin circles (39). The TATA mutant (TATAm) PHO5 strain was constructed by replacing the 24-bp PHO5 core promoter sequence (including the TATA box) AAATGAAACGTATATAAGCGCTGA with an unrelated sequence TCATCGATCCCCCGGGGACGAAGT.
For yeast strain constructions, complete null alleles were obtained by the PCR-based gene disruption technique using primers that were within 100 bp of the beginning and the end of each gene (40). All insertions or deletions were verified by PCR and phenotypic assays.
Purification of pol II, GTFs, TFIIS, Mediator, and RSC.
TFIIA, TFIIB, TBP, TFIIE, and TFIIS were available in recombinant form (41). Pol II-TFIIF complex, TFIIH, and Mediator were isolated from Saccharomyces cerevisiae as previously described (41, 42). RSC was purified from Rsc2-TAP yeast strain as described (43). The purity of the proteins was verified by SDS/PAGE and staining with Coomassie brilliant blue (Fig. S4).
Purification of Pho4.
Escherichia coli BL21 cells were transformed with plasmids EB067 (from E. O’Shea). Cells were cultured in 2 L LB containing 100 μg ampicillin per 1 mL at 37 °C to midlog phase. Expression of recombinant proteins was induced by addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mM. Cells were cultured for an additional 3 h at 30 °C and then, sedimented by centrifugation. Cells were resuspended in 80 mL lysis buffer [20 mM Tris⋅HCl, pH 8.0, 500 mM sodium chloride, 10 mM imidazole, 0.2% Triton X-100, 5 mM DTT, 1× protease inhibitors (0.6 μM leupeptin hemisulfate, 2 μM pepstatin A, 1 mM PMSF, 2.1 mM benzamidine hydrochloride)]. Bacterial lysates were prepared using an MISONIX Sonicator (20 s on/off; 5 min process time). The lysate was cleared by centrifugation and loaded onto a 5-mL Ni2+-NTA Column (GE Healthcare). The column was washed with 30 column vol wash buffer (20 mM Tris⋅HCl, pH 8.0, 500 mM sodium chloride, 5 mM imidazole, 0.02% Triton X-100, 1× protease inhibitors) followed by 50 column vol wash buffer without Triton X-100. Bound proteins were eluted in a 25–500 mM imidazole gradient. Peak fractions were pooled and dialyzed against gel filtration buffer (40 mM Hepes-KOH, pH 7.4, 200 mM potassium acetate, 1 mM EDTA). Samples were size-fractionated using a Superdex 200 GL Column (GE Healthcare), which was equilibrated with gel filtration buffer. Peak fractions were pooled and concentrated, and glycerol was added to a final concentration of 10% (vol/vol). The purity of the proteins was verified by SDS/PAGE and staining with Coomassie brilliant blue (Fig. S4).
Purification of SAGA and SWI/SNF.
Purification of SAGA and SWI/SNF was performed as follows: S. cerevisiae harboring Spt7-TAP (SAGA) or Snf6-TAP (SWI/SNF) was grown at 30 °C in 24 L YPAD (yeast extract, peptone, adenine, dextrose) to A600 ∼ 8.0; 400 g cells were lysed by continuous flow bead beating in lysis buffer [50 mM Hepes-KOH, pH 7.8, 25 mM ammonium sulfate, 0.5 mM EDTA, 100 μM zinc sulfate, 5% (vol/vol) glycerol, 5 mM DTT, 1× protease inhibitors]. The cell debris was pelleted by centrifugation at 11,300 × g for 20 min at 4 °C, and the supernatant was brought to 200 mM ammonium sulfate with the addition of cold ammonium sulfate solution. Nucleic acids were precipitated from the lysate by adding 0.2% (wt/vol; final concentration) polyethylenimine. After 10 min of gentle stirring, nucleic acids were pelleted by centrifugation (17,664 × g for 40 min at 4 °C), and proteins in the supernatant were precipitated by adding ammonium sulfate to 2.2 M final concentration followed by centrifugation (17,664 × g for 40 min at 4 °C). Pellets were resuspended in buffer AS-0 [50 mM Hepes-KOH, pH 7.6, 0.5 mM EDTA, 100 μM zinc sulfate, 5% (vol/vol) glycerol, 2 mM DTT, 1× protease inhibitors], and the ammonium sulfate concentration was adjusted to 300–500 mM. The suspended pellet was loaded onto a 5-mL IgG-Sepharose column and washed with 50 column vol buffer AS-500 (25 mM Hepes-KOH, pH 7.6, 500 mM ammonium sulfate, 0.1 mM EDTA, 50 μM zinc sulfate, 5% glycerol, 2 mM DTT, 1× protease inhibitors) followed by 10 column vol buffer AS-100 [25 mM Hepes-KOH, pH 7.6, 100 mM ammonium sulfate, 0.1 mM EDTA, 50 μM zinc sulfate, 5% (vol/vol) glycerol, 2 mM DTT, 1× protease inhibitors]. Proteins were released by proteolytic cleavage overnight at 4 °C with 20 μg Tobacco Etch Virus (TEV) protease per 1 mL beads in buffer AS-100. Peak fractions were pooled and concentrated, and glycerol was added to a final concentration of 10% (vol/vol). The purity of the proteins was verified by SDS/PAGE and staining with Coomassie brilliant blue (Fig. S4).
Purification of Chromatin Circles.
Yeast strains for purification of PHO5 gene circles were grown at 30 °C in 12 L YP (yeast extract, peptone) media containing 2% (wt/vol) raffinose. Recombination was induced at a cell density of 5 × 107 cells per 1 mL by adding galactose to a final concentration of 2% (wt/vol). Cells were grown for an additional 1.5 h at 30 °C before harvesting.
Chromatin circles were purified essentially as described (10) with some modifications; 50 g frozen cells were ground in a liquid nitrogen–dry ice mixture by using a commercial coffee grinder (TEFAL) for 4 min. After evaporation of liquid nitrogen and most of the dry ice, 180 mL lysis buffer [25 mM Hepes-KOH, pH 7.4, 200 mM potassium acetate, 2 mM EDTA, 0.01% (wt/vol) Nonidet P-40, 10% (vol/vol) glycerol, 5 mM DTT, 1× protease inhibitors] was added. The crude extract was stirred for 30 min at 4 °C and cleared by centrifugation at 72,700 × g for 1 h at 4 °C. Chromatin circles in the supernatant were sedimented by centrifugation at 371,000 × g for 2 h at 4 °C. The pellet was suspended in 16 mL buffer A-200 [25 mM Hepes-KOH, pH 7.4, 200 mM potassium acetate, 1 mM EDTA, 0.01% (wt/vol) Nonidet P-40, 1 mM DTT, 1× protease inhibitors]. The suspended pellet was loaded onto 1 mL human IgG-Sepharose resin (GE Healthcare) that had been equilibrated with buffer A-200. Beads were incubated for 2 h at 4 °C and washed with 250 column vol buffer A-200 followed by 50 column vol buffer A-200 without Nonidet P-40. Chromatin circles were released by proteolytic cleavage overnight at 4 °C with 20 μg TEV protease per 1 mL beads in buffer A-200 without Nonidet P-40. After elution, purified chromatin circles were dialyzed against buffer containing 40 mM Hepes-KOH, pH 7.4, 50 mM potassium acetate, 1 mM EDTA, 10% (vol/vol) glycerol, 5 mM DTT, and 1× protease inhibitors. To check the integrity of chromatin after purification, chromatin circles were digested with 4, 12, 36, and 72 units micrococcal nuclease (NEB) in a reaction volume of 100 μL for 5 min at 37 °C. DNA was extracted, subjected to 1.5% agarose gel electrophoresis, and analyzed by blot hybridization with a probe spanning PHO5 nucleosomes N + 2 to N + 4 (ORF probe) or nucleosomes N − 2 to N + 1 (promoter probe) (Fig. 1 A and B).
Measurement of Template Concentration.
Naked DNA templates were purified from chromatin circles by phenol–chloroform extraction or the QIAquick PCR Purification Kit (QIAGEN). Template concentration was precisely measured by qPCR as follows. PHO5 chromatin templates, naked DNA templates, and PHO5 DNA of known amount (reference PHO5 DNA) were each mixed with an equal amount of lacI DNA, which serves as a normalization reference. DNA was subsequently purified (QIAquick PCR Purification Kit; QIAGEN) and analyzed by qPCR using two sets of primers: PHO5-specific primers (p14 and p16 in Table S3) and lacI-specific primers (p5 and p6). Using a dilution series of PHO5 and lacI DNA, a standard curve of each primer pair was generated for each qPCR reaction. The amount of PHO5 signal divided by that of lacI signal gave relative amounts of PHO5 DNA in each sample. Absolute amount of PHO5 chromatin or naked DNA templates was determined based on the relative amount of reference PHO5 DNA.
Transcription Assay.
Naked DNA templates were purified from chromatin circles by phenol–chloroform extraction or the QIAquick PCR Purification Kit (QIAGEN), and resuspended in the same buffer as chromatin circles [40 mM Hepes-KOH, pH 7.4, 50 mM potassium acetate, 1 mM EDTA, 10% (vol/vol) glycerol, 5 mM DTT, 1× protease inhibitors]. Where indicated (Fig. S7), naked DNA templates were mixed with “mock” chromatin prepared from (−)RS strain (ySH139) to ensure that reactions with naked DNA contained any contaminants present in the chromatin. Template concentration was precisely measured by qPCR as described above.
Template chromatin or DNA circles were first linearized by cleavage at an NcoI site in the 3′ UTR for 60 min at room temperature (cut efficiency on chromatin checked by qPCR; 94.0 ± 1.2%) followed by the addition of carrier plasmid DNA (100 ng per reaction). To allow for histone acetylation and chromatin remodeling, 20 μL linearized templates were incubated with Pho4 activator (11.76 nM), SAGA (20 nM), and SWI/SNF (20 nM) in the presence of 1 mM ATP, 10 μM acetyl-CoA, 5 mM sodium butyrate, 8 mg/mL creatine kinase, and 10 mM phosphocreatine for 30 min at room temperature in a reaction volume of 25 μL. After histone acetylation and chromatin remodeling, purified pol II, GTFs, TFIIS, and Mediator were added to the reaction, and PIC formation was allowed to proceed for 10 min at room temperature. After PIC assembly, NTP, RNaseOUT (Invitrogen), and the reference lacI RNA were added to the reaction, and transcription was allowed to proceed for 30 min at 30 °C. Final reaction mixtures (50-μL reaction) contained 1 fmol chromatin or DNA templates (20 pM); 100 ng carrier plasmid (0.5 nM); Pho4 (5.88 nM); SAGA (10 nM); SWI/SNF (10 nM); pol II (8.3 nM); TBP (10.6 nM); IIB (12.5 nM); IIE (15 nM); IIF (8.3 nM); IIH (6.3 nM); IIS (12 nM); Mediator (10 nM); 40 mM Hepes-KOH, pH 7.5; 80 mM potassium acetate; 5 mM DTT; 7.5 mM magnesium acetate; 5% (vol/vol) glycerol; 2 mM ATP; 0.8 mM UTP, TTP, and GTP; 40 units RNaseOUT; 5 μM acetyl-CoA; 2.5 mM sodium butyrate; 4 mg/mL creatine kinase; 5 mM phosphocreatine; and 12.2 pg reference lacI RNA (2 pM). These concentrations of purified proteins were considered as 1× concentration. Reactions were terminated with the addition of buffer RLT containing guanidine (RNeasy Plus Micro; QIAGEN) and samples were spun through gDNA Eliminator Spin Columns to remove DNA. RNA was subsequently purified according to the manufacturer’s instructions.
Reverse Transcription.
Quantitation of in vitro PHO5 transcripts was performed with RT-PCR using lacI RNA as a normalization reference. Because lacI RNA was spiked into the transcription reaction at the time of initiation, it was subjected to almost all of the experimental error introduced during transcription and the multistage process required to purify and process the RNA.
Purified PHO5 transcripts and lacI reference RNA were reverse-transcribed to cDNA in the same tube (QuantiTect Reverse Transcription Kit; QIAGEN) using the following gene-specific RT primers: PHO5 RT primer (RT2: 5′-CCAACCATTTGCAG-3′) and lacI RT primer (RT3: 5′-AGCTTCCACAGC-3′). RT reaction was performed for 15 min at 42 °C followed by inactivation of reverse transcriptase for 3 min at 95 °C. First-strand cDNA was purified (Econospin Column; Epoch Life Science) and analyzed by qPCR as described below. All primers used for qPCR are listed in Table S3.
Quantitation of in Vitro Transcripts.
After reverse transcription, PHO5 transcripts were quantified with qPCR (ABI POWER SYBR Green PCR Master Mix) using an ABI 7900HT Fast Real-Time PCR System. For each sample, qPCR reactions were performed in triplicate, and the cycle at which a fluorescence threshold was attained was recorded (Ct value). The mean of the replicate sample Ct values was used for quantitation of PHO5 expression as described below.
For quantitation of PHO5 in vitro transcripts, the following primers were used (300 nM final concentration): PHO5 downstream primer pair (p14: 5′-CAAGCAAATTCGAGATTACCAA-3′, p16: 5′-AGGGAATGGTACCTGCATTG-3′), PHO5 upstream primer pair (p21: 5′-AAGTCGAGGTTAGTATGGCTTCA-3′, p22: 5′-CATTGGTAATCTCGAATTTGCTT-3′), and lacI primer pair (p5: 5′-TGGTGGTGTCGATGGTAGAA-3′, p6: 5′-TGGTCATCCAGCGGATAGT-3′). Amplification efficiency (E) of each primer pair was determined by replicate qPCR analysis of a dilution series prepared from reference PHO5 and lacI DNA of known amounts using the equation E = 10(−1/slope) (Fig. S2B) (Edownstream = 1.93; Eupstream = 1.93; ElacI = 1.93). Importantly, when the difference in Ct value (ΔCt) between the upstream and downstream PHO5 primer pairs was recorded with a dilution series of reference PHO5 DNA of known amount, ΔCt values were nearly zero at all template concentrations tested (Fig. S2C). This control shows that the PHO5 upstream and downstream primer pairs amplify their target sequences at exactly the same rate, enabling us to accurately calculate ΔCt between the upstream and downstream Ct values and thereby, accurately determine the relative amount of bona fide vs. cryptic transcription.
About 12 reactions (samples 1–12) were performed in each transcription assay. We first quantified relative PHO5 expression levels between different samples using lacI as a normalization reference [comparative quantitation (35)]. The amount of PHO5 transcripts in sample 1 (usually chromatin-transcribed with a complete set of factors) detected by the downstream primer pair was set as one. Then, the relative amount of PHO5 transcripts (R) detected by the upstream or downstream primer pair in a given sample (sample X; X = 1–12) was calculated using the efficiency corrected ΔΔCt method (35):
After comparative quantitation, the absolute amount of PHO5 transcripts was determined as follows. For each transcription assay, the absolute amount of PHO5 transcripts in sample 1 was determined using synthetic PHO5 RNA as a reference. Synthetic PHO5 RNA was transcribed in vitro with T7 RNA polymerase using a T7 promoter–PHO5 hybrid DNA as a template (transcribed from a known PHO5 in vivo TSS) with the concentration measured by NanoDrop (Thermo Scientific). Serial dilutions of synthetic PHO5 RNA in parallel with the PHO5 transcripts from sample 1 were reverse-transcribed, and purified cDNA was analyzed by qPCR with the PHO5 downstream primer pair. A standard curve of synthetic PHO5 RNA dilution series was generated and used to determine the absolute amount of PHO5 transcripts in sample 1 detected by the downstream primer pair. The absolute amount of PHO5 transcripts in each sample (detected by the upstream or downstream primer pair) was subsequently calculated using the relative PHO5 expression (R) determined by comparative quantitation described above. The amount of PHO5 transcripts divided by the amount of DNA template gave the transcription efficiency [transcripts per template (percentage)]. Promoter-dependent transcription signal in each sample was calculated by subtracting the upstream signal from the downstream signal. RT-PCR results [transcripts per template (percentage)] are presented as the mean of several transcription experiments (n = 3–36) ± SEM.
5′ RACE.
In vitro transcripts that contained γ and β phosphates at the 5′ end were first converted to 5′-monophosphorylated RNA using 5′ RNA polyphosphatase (Epicentre). This treatment allows TSSs from in vitro transcripts to be assayed by the T4 RNA ligase-mediated RNA adaptor-tagging 5′ RACE strategy (FirstChoice RLM-RACE Kit; Ambion). In vivo total RNA was extracted from pho80Δ strains (ySH141), in which PHO5 is constitutively activated (44); 5′ RACE reactions were performed according to the manufacturer’s instructions (FirstChoice RLM-RACE Kit; Ambion). The following primers were used: 5′ RACE PHO5-specific outer primer (5′-TAGCCAGACTGACAGTAGGGTATC-3′) and 5′ RACE PHO5-specific inner primer (5′-ATTCTGCAGCATTTCACAACCTTCAGGCAAATC-3′).
Deep Sequencing.
After nested PCR of 5′ RACE samples, DNA was prepared for next generation sequencing using an NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs), except that the end prep step was omitted; in the final purification step, 17 μL 0.1× TE buffer was used to elute the DNA from the AMPure XP Beads, and 15 μL was reclaimed as the purified RACE library. DNA concentration was determined using a Qubit dsDNA HS Assay Kit (Life Technologies), and DNA integrity was determined by running an aliquot on a Bioanalyzer High Sensitivity DNA Chip (Agilent). The library was accurately quantified using a KAPA Library Quantification Kit (KAPA) and then, subjected to paired end sequencing on an Illumina MiSeq instrument. For analysis, paired ends were separated, and each end was treated as a single-end read. Reads that began precisely at the 5′ end with the RLM-RACE adaptor (5′-CGCGGATCCGAACACTGCGTTTGCTGGCTTTGATGAAA-3′) were identified, the adaptor was removed, and after trimming, only reads 25 bp or longer were retained. Reads were then mapped to the S. cerevisiae genome (April 2011/sacCer3 assembly) with bowtie. The number of reads with identical 5′ ends was determined, and the 5′-most base was identified as the TSS. Data were normalized to the number of reads per base per thousands of aligned reads per sample and displayed using the UCSC Genome Browser.
In Vitro ChIP.
Chromatin circles were digested to mononucleosomes with 108 units micrococcal nuclease (NEB) for 5 min at 37 °C in the presence of 10 mM Tris, pH 7.5, 50 mM NaCl, and 5 mM calcium chloride. The reaction was quenched by the equal amount of 2× stop buffer (20 mM Tris, pH 7.5, 250 mM NaCl, 1% Triton X-100, 0.2% Tween-20, 20 mM EGTA). Where indicated (Fig. S1F), reference recombinant mononucleosomes (recombinant histones assembled on the 601 positioning sequence) were added.; 20 μL input samples were kept before immunoprecipitation (IP). IP was performed with magnetic beads (Dynabeads; M-280 sheep anti-rabbit IgG) bound to the following antibodies: antihistone H3 (ab46765; abcam), anti-H3K4me3 (trimethyl K4; ab8580), and antiacetyl H4K12 (ab46983). After incubation at 4 °C for 2 h, the beads were washed once with IP buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 0.5% Triton X-100, 0.1% Tween-20), three times with wash buffer (20 mM Tris, pH 8.0, 250 mM NaCl, 0.5% Triton X-100, 0.1% Tween-20), and once with TE (10 mM Tris, pH 8.0, 1 mM EDTA). Protein–DNA complexes were eluted in the elution buffer (10 mM Tris, pH 8.0, 1 mM EDTA, 1% SDS) for 10 min at 65 °C. Input and IP DNA were subsequently purified (Econospin Column; Epoch Life Science) and analyzed by qPCR using the ABI 7900HT Fast Real-Time PCR System. For each ChIP, qPCR was performed in triplicate. Relative enrichment for histones and histone modifications were calculated as follows: for each PHO5 location, the signal from a PHO5 site was normalized to that from 601 sequences (Fig. S1F) or PHO5 ORF3 (Fig. S1E) in IP and input DNA samples. For each site, the normalized IP signals were then normalized to input DNA signals. ChIP results are presented as the mean of two to four experiments ± the SEM.
Acknowledgments
We thank Drs. J. Griesenbeck and S. Hamperl for yeast strains and advice on chromatin purification. We also thank Drs. H. Boeger, P. Geiduschek, and J. Griesenbeck for discussions. We thank the Parham laboratory for assistance with DNA sequencing and access to their Illumina MiSeq instrument (supported by NIH Grant U01AI090905 to Peter Parham). S.N. was supported by the Human Frontier Science Program, the Swiss National Science Foundation, and the Kanae Foundation. This research was supported by NIH Grant GM36659 (to R.D.K.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The 5′ RLM (RNA ligase mediated)-RACE sequence data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE93669).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620312114/-/DCSupplemental.
References
- 1.Lorch Y, LaPointe JW, Kornberg RD. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell. 1987;49(2):203–210. doi: 10.1016/0092-8674(87)90561-7. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Grunstein M. Nucleosome loss activates yeast downstream promoters in vivo. Cell. 1988;55(6):1137–1145. doi: 10.1016/0092-8674(88)90258-9. [DOI] [PubMed] [Google Scholar]
- 3.Lemon B, Inouye C, King DS, Tjian R. Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature. 2001;414(6866):924–928. doi: 10.1038/414924a. [DOI] [PubMed] [Google Scholar]
- 4.LeRoy G, Orphanides G, Lane WS, Reinberg D. Requirement of RSF and FACT for transcription of chromatin templates in vitro. Science. 1998;282(5395):1900–1904. doi: 10.1126/science.282.5395.1900. [DOI] [PubMed] [Google Scholar]
- 5.Mizuguchi G, Vassilev A, Tsukiyama T, Nakatani Y, Wu C. ATP-dependent nucleosome remodeling and histone hyperacetylation synergistically facilitate transcription of chromatin. J Biol Chem. 2001;276(18):14773–14783. doi: 10.1074/jbc.M100125200. [DOI] [PubMed] [Google Scholar]
- 6.Pavri R, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125(4):703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Carey M, Li B, Workman JL. RSC exploits histone acetylation to abrogate the nucleosomal block to RNA polymerase II elongation. Mol Cell. 2006;24(3):481–487. doi: 10.1016/j.molcel.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92(1):105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 9.Guermah M, Palhan VB, Tackett AJ, Chait BT, Roeder RG. Synergistic functions of SII and p300 in productive activator-dependent transcription of chromatin templates. Cell. 2006;125(2):275–286. doi: 10.1016/j.cell.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Griesenbeck J, Boeger H, Strattan JS, Kornberg RD. Affinity purification of specific chromatin segments from chromosomal loci in yeast. Mol Cell Biol. 2003;23(24):9275–9282. doi: 10.1128/MCB.23.24.9275-9282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamperl S, et al. Compositional and structural analysis of selected chromosomal domains from Saccharomyces cerevisiae. Nucleic Acids Res. 2014;42(1):e2. doi: 10.1093/nar/gkt891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeger H, Griesenbeck J, Strattan JS, Kornberg RD. Nucleosomes unfold completely at a transcriptionally active promoter. Mol Cell. 2003;11(6):1587–1598. doi: 10.1016/s1097-2765(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 13.Barbaric S, Reinke H, Hörz W. Multiple mechanistically distinct functions of SAGA at the PHO5 promoter. Mol Cell Biol. 2003;23(10):3468–3476. doi: 10.1128/MCB.23.10.3468-3476.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musladin S, Krietenstein N, Korber P, Barbaric S. The RSC chromatin remodeling complex has a crucial role in the complete remodeler set for yeast PHO5 promoter opening. Nucleic Acids Res. 2014;42(7):4270–4282. doi: 10.1093/nar/gkt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuryan BG, et al. Histone density is maintained during transcription mediated by the chromatin remodeler RSC and histone chaperone NAP1 in vitro. Proc Natl Acad Sci USA. 2012;109(6):1931–1936. doi: 10.1073/pnas.1109994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelan ML, Schnitzler GR, Kingston RE. Octamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPases. Mol Cell Biol. 2000;20(17):6380–6389. doi: 10.1128/mcb.20.17.6380-6389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korber P, Barbaric S. The yeast PHO5 promoter: From single locus to systems biology of a paradigm for gene regulation through chromatin. Nucleic Acids Res. 2014;42(17):10888–10902. doi: 10.1093/nar/gku784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudolph H, Hinnen A. The yeast PHO5 promoter: Phosphate-control elements and sequences mediating mRNA start-site selection. Proc Natl Acad Sci USA. 1987;84(5):1340–1344. doi: 10.1073/pnas.84.5.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CR, Mao C, Falkovskaia E, Law JK, Boeger H. In vivo role for the chromatin-remodeling enzyme SWI/SNF in the removal of promoter nucleosomes by disassembly rather than sliding. J Biol Chem. 2011;286(47):40556–40565. doi: 10.1074/jbc.M111.289918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurent BC, Carlson M. Yeast SNF2/SWI2, SNF5, and SNF6 proteins function coordinately with the gene-specific transcriptional activators GAL4 and Bicoid. Genes Dev. 1992;6(9):1707–1715. doi: 10.1101/gad.6.9.1707. [DOI] [PubMed] [Google Scholar]
- 21.Prather DM, Larschan E, Winston F. Evidence that the elongation factor TFIIS plays a role in transcription initiation at GAL1 in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25(7):2650–2659. doi: 10.1128/MCB.25.7.2650-2659.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassan AH, et al. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell. 2002;111(3):369–379. doi: 10.1016/s0092-8674(02)01005-x. [DOI] [PubMed] [Google Scholar]
- 23.Reinke H, Hörz W. Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol Cell. 2003;11(6):1599–1607. doi: 10.1016/s1097-2765(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 24.Small EC, Xi L, Wang JP, Widom J, Licht JD. Single-cell nucleosome mapping reveals the molecular basis of gene expression heterogeneity. Proc Natl Acad Sci USA. 2014;111(24):E2462–E2471. doi: 10.1073/pnas.1400517111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian C, et al. Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. EMBO J. 2011;30(14):2829–2842. doi: 10.1038/emboj.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SS, Zhou BO, Zhou JQ. Histone H3 lysine 4 hypermethylation prevents aberrant nucleosome remodeling at the PHO5 promoter. Mol Cell Biol. 2011;31(15):3171–3181. doi: 10.1128/MCB.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudley AM, Rougeulle C, Winston F. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 1999;13(22):2940–2945. doi: 10.1101/gad.13.22.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tse C, Sera T, Wolffe AP, Hansen JC. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol Cell Biol. 1998;18(8):4629–4638. doi: 10.1128/mcb.18.8.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanle EK, et al. Association of Taf14 with acetylated histone H3 directs gene transcription and the DNA damage response. Genes Dev. 2015;29(17):1795–1800. doi: 10.1101/gad.269977.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorch Y, Beve J, Gustafsson CM, Myers LC, Kornberg RD. Mediator-nucleosome interaction. Mol Cell. 2000;6(1):197–201. doi: 10.1016/s1097-2765(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z, Myers LC. Med5(Nut1) and Med17(Srb4) are direct targets of mediator histone H4 tail interactions. PLoS One. 2012;7(6):e38416. doi: 10.1371/journal.pone.0038416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramachandran S, Zentner GE, Henikoff S. Asymmetric nucleosomes flank promoters in the budding yeast genome. Genome Res. 2015;25(3):381–390. doi: 10.1101/gr.182618.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Campa C, et al. Precise nucleosome positioning and the TATA box dictate requirements for the histone H4 tail and the bromodomain factor Bdf1. Mol Cell. 2004;15(1):69–81. doi: 10.1016/j.molcel.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Albert I, et al. Translational and rotational settings of H2A.Z nucleosomes across the Saccharomyces cerevisiae genome. Nature. 2007;446(7135):572–576. doi: 10.1038/nature05632. [DOI] [PubMed] [Google Scholar]
- 35.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Güldener U, Heck S, Fielder T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 1996;24(13):2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu PY, Winston F. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol Cell Biol. 2002;22(15):5367–5379. doi: 10.1128/MCB.22.15.5367-5379.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 39.Hamperl S, et al. Purification of specific chromatin domains from single-copy gene loci in Saccharomyces cerevisiae. Methods Mol Biol. 2014;1094:329–341. doi: 10.1007/978-1-62703-706-8_26. [DOI] [PubMed] [Google Scholar]
- 40.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 41.Murakami K, et al. Formation and fate of a complete 31-protein RNA polymerase II transcription preinitiation complex. J Biol Chem. 2013;288(9):6325–6332. doi: 10.1074/jbc.M112.433623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robinson PJ, Bushnell DA, Trnka MJ, Burlingame AL, Kornberg RD. Structure of the mediator head module bound to the carboxy-terminal domain of RNA polymerase II. Proc Natl Acad Sci USA. 2012;109(44):17931–17935. doi: 10.1073/pnas.1215241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorch Y, Kornberg RD. Isolation and assay of the RSC chromatin-remodeling complex from Saccharomyces cerevisiae. Methods Enzymol. 2004;377:316–322. doi: 10.1016/S0076-6879(03)77019-0. [DOI] [PubMed] [Google Scholar]
- 44.O’Neill EM, Kaffman A, Jolly ER, O’Shea EK. Regulation of PHO4 nuclear localization by the PHO80-PHO85 cyclin-CDK complex. Science. 1996;271(5246):209–212. doi: 10.1126/science.271.5246.209. [DOI] [PubMed] [Google Scholar]
- 45.Brown CR, Mao C, Falkovskaia E, Jurica MS, Boeger H. Linking stochastic fluctuations in chromatin structure and gene expression. PLoS Biol. 2013;11(8):e1001621. doi: 10.1371/journal.pbio.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami K, et al. Uncoupling promoter opening from start-site scanning. Mol Cell. 2015;59(1):133–138. doi: 10.1016/j.molcel.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorch Y, Zhang M, Kornberg RD. Histone octamer transfer by a chromatin-remodeling complex. Cell. 1999;96(3):389–392. doi: 10.1016/s0092-8674(00)80551-6. [DOI] [PubMed] [Google Scholar]