Significance
Blood–brain barrier (BBB) breakdown is a catastrophic event in the pathogenesis of various neurological disorders, including stroke. Here we report that overexpressing heat shock protein 27 (HSP27) specifically within microvascular endothelial cells protects the BBB in models of ischemic stroke. HSP27 achieves this protection through a previously unexplored mechanism—the inhibition of actin polymerization—that suppresses early structural changes within endothelial cells that appear to initiate the BBB breach. Furthermore, intravenous delivery of a cell membrane-permeable TAT-HSP27 protein after postischemic reperfusion boosts endothelial HSP27 and improves BBB integrity and long-term functional outcomes. Thus, HSP27 has translational potential as a therapeutic agent to ameliorate BBB disruption, reduce the progression of brain injury, and improve long-term neurological outcomes in stroke victims.
Keywords: endothelial cell, stress fiber, tight junction, neuroinflammation, HSP27
Abstract
The damage borne by the endothelial cells (ECs) forming the blood–brain barrier (BBB) during ischemic stroke and other neurological conditions disrupts the structure and function of the neurovascular unit and contributes to poor patient outcomes. We recently reported that structural aberrations in brain microvascular ECs—namely, uncontrolled actin polymerization and subsequent disassembly of junctional proteins, are a possible cause of the early onset BBB breach that arises within 30–60 min of reperfusion after transient focal ischemia. Here, we investigated the role of heat shock protein 27 (HSP27) as a direct inhibitor of actin polymerization and protectant against BBB disruption after ischemia/reperfusion (I/R). Using in vivo and in vitro models, we found that targeted overexpression of HSP27 specifically within ECs—but not within neurons—ameliorated BBB impairment 1–24 h after I/R. Mechanistically, HSP27 suppressed I/R-induced aberrant actin polymerization, stress fiber formation, and junctional protein translocation in brain microvascular ECs, independent of its protective actions against cell death. By preserving BBB integrity after I/R, EC-targeted HSP27 overexpression attenuated the infiltration of potentially destructive neutrophils and macrophages into brain parenchyma, thereby improving long-term stroke outcome. Notably, early poststroke administration of HSP27 attached to a cell-penetrating transduction domain (TAT-HSP27) rapidly elevated HSP27 levels in brain microvessels and ameliorated I/R-induced BBB disruption and subsequent neurological deficits. Thus, the present study demonstrates that HSP27 can function at the EC level to preserve BBB integrity after I/R brain injury. HSP27 may be a therapeutic agent for ischemic stroke and other neurological conditions involving BBB breakdown.
The blood–brain barrier (BBB) is a unique microvascular structure in the brain that helps to control the passage of material between blood and brain and maintain the homeostatic microenvironment for normal neuronal functions (1). When BBB integrity is compromised in disease or injury states, proinflammatory factors produced within the injured brain may cross the BBB to attract circulating immune cells, and immune cells, pathogens, and toxins in the blood may penetrate the brain parenchyma and elicit a secondary injury cascade (1). Following cerebral ischemia/reperfusion (I/R), BBB breakdown may produce severe clinical consequences, such as vasogenic brain edema and hemorrhagic transformation, leading to poor neurological outcomes and limiting the use of tissue plasminogen activator (tPA) thrombolysis (2, 3). Therefore, it is necessary to develop effective therapeutic strategies that protect BBB integrity and prevent permanent neurovascular injury after I/R. Treatments that preserve the structure of the BBB would mitigate diffusion of deleterious mediators from blood to brain and from brain to blood.
Brain capillary endothelial cells (ECs) are tightly connected by apical tight junctions (TJs) that form a barrier to the penetration of hydrophilic molecules between plasma and brain and also by adherens junctions (AJs) that connect the cytoskeletons of adjacent cells (1). In many earlier studies, matrix metalloproteinase (MMP)-mediated degradation of EC junctions and basal lamina was thought to be primarily responsible for all types of BBB breakdown after I/R injury (4–6). However, recent reports have revealed additional, MMP-independent mechanisms at the EC level that act before the frank degradation of junctional proteins after I/R (7–10). For example, increased transcellular vesicular trafficking mediated by caveolin-1 and exocytotic machinery may account for BBB hyperpermeability as early as 6 h after cerebral I/R, well before profound TJ structural deficits are observed at 48 h after I/R (8). Moreover, our recent study (9) identified cytoskeletal alterations in brain ECs as an initiator of BBB rupture at even earlier stages (30–60 min after I/R). Specifically, I/R rapidly triggers robust actin polymerization in ECs through activation of the Rho-associated protein kinase (ROCK)/myosin light chain (MLC) signal transduction cascade, resulting in the formation of force-generating, contractile stress fibers consisting of F-actin, myosin, and other proteins (9, 11). Possibly as a consequence of tension transmission, at least in part, TJ and AJ proteins that are normally anchored to the actin cytoskeleton are disassembled at cell–cell contacts and internalized (12, 13), thereby widening the paracellular space between ECs. This mild damage renders the BBB more vulnerable to subsequent attack by MMPs and facilitates peripheral leukocyte and macrophage infiltration, eventually leading to permanent neurovascular injury (9). Thus, aberrant actin polymerization in ECs enables this vicious feedforward process and represents a rational therapeutic target for BBB protection after I/R.
Heat shock proteins (HSPs) are molecular chaperones that are highly evolutionarily conserved due to their critical role in the response to stressors. HSP27 is a member of the small HSP family with a monomeric molecular mass of 25 and 27 kDa in mouse and human, respectively (14). Under physiological conditions, the level of HSP27 is extremely low in the brain; however, HSP27 expression is transiently induced in brain glial cells and/or ECs at 24 h or later following cerebral I/R (14). Previous studies by our and other groups demonstrated robust neuroprotective effects of HSP27 overexpression against I/R brain injury (15–18), which were largely credited to its antiapoptotic actions. However, HSP27 exerts additional actions on ECs (14). For example, HSP27 can directly inhibit actin polymerization by capping the barbed end of filamentous actin (F-actin) (19, 20) and by sequestering globular actin (G-actin) (21). Thus, the present study sought to test the hypothesis that HSP27 suppresses I/R-induced early onset BBB leakage through inhibiting actin polymerization and the disassembly of junctional proteins in cerebral ECs. The results show that endothelium-targeted overexpression of HSP27 provides long-lasting protection against I/R-induced BBB disruption and neurological deficits.
Results
EC-Specific Overexpression of HSP27 Ameliorates BBB Disruption After I/R Brain Injury.
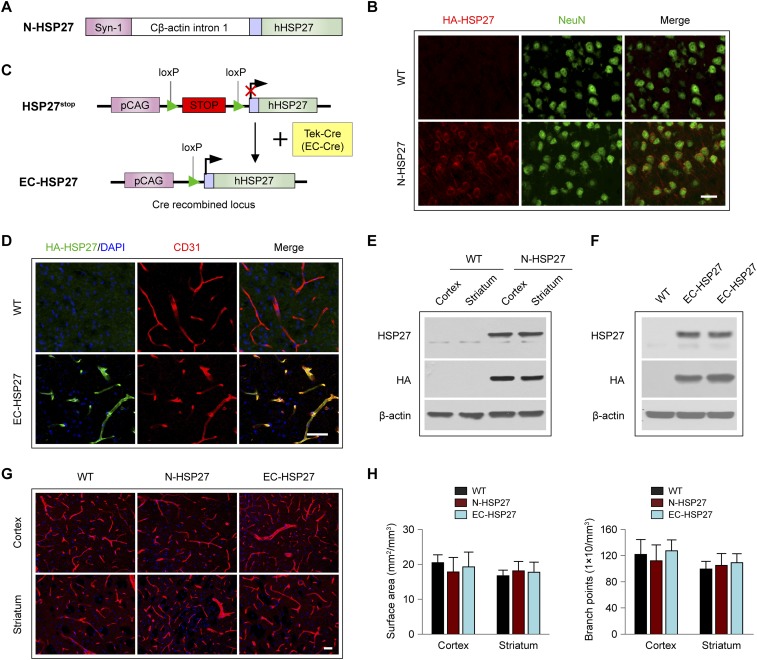
We previously reported that transgenic mice with ubiquitous overexpression of HSP27 developed significantly less brain damage after cerebral I/R, including less BBB disruption (17, 18, 22). These beneficial effects of HSP27 were attributed mainly to its well-characterized antiapoptotic functions in neurons (17, 18) and ECs (22). However, neuronal, glial, or endothelial HSP27 may have distinct effects on the pathogenesis of I/R brain injury, which would not be distinguishable in global HSP27-overexpressing mice. Although HSP27 has been shown to attenuate EC apoptosis and TJ degradation at 24 h after I/R (22), whether HSP27 affects the integrity of the BBB at earlier stages is unknown. To elucidate the specific role of HSP27 in various cell types after I/R, we generated transgenic mice overexpressing HSP27 specifically in neurons (N-HSP27; Fig. S1A) or in ECs (EC-HSP27; Fig. S1C). HA-tagged human HSP27 protein was robustly expressed in brain neurons of N-HSP27 mice (Fig. S1 B and E) and in cerebral microvascular ECs of EC-HSP27 mice (Fig. S1 D and F), respectively. In nonstroke brains, no significant difference was observed in the microvasculature of N-HSP27 and EC-HSP27 mice compared with WT mice, with respect to surface area and branch points (Fig. S1 G and H). When subjected to transient focal cerebral ischemia (tFCI), N-HSP27 and EC-HSP27 transgenic mice showed comparable reductions in cortical cerebral blood flow (CBF) as WT mice (Fig. 1 A and B), suggesting that HSP27 overexpression in either neurons or ECs does not affect the severity of the original ischemic insult.
Fig. S1.
Generation and characterization of HSP27-overexpressing transgenic mice. (A) Illustration of the construct used to generate neuron-specific HSP27-overexpressing mice (N-HSP27). The chimeric constructs contain full-length human HSP27 cDNA under the control of the neuron-specific synapsin I promoter and the first intron of chicken β-actin. An HA tag was added before the HSP27 coding region (blue box). (B) Representative images showing that HA-HSP27 was expressed in NeuN+ neurons in N-HSP27 mouse brains. No HA-HSP27 immunosignal was observed in WT brains. (Scale bar: 40 µm.) (C) Illustration of the targeting strategy used for generating EC-specific HSP27-overexpressing mice (EC-HSP27). cDNA of full-length human HSP27 was targeted to the Rosa26 locus downstream of a stop codon flanked by two loxP sites, driven by the CAG promoter (HSP27stop). Homozygous HSP27stop mice were crossed with hemizygous Tek-Cre mice, in which the Cre recombinase expression is driven by the Tek promoter and thus restricted to ECs. In the presence of Cre recombinase, the stop codon is excised and a 5′ HA-tagged HSP27 protein is expressed specifically in ECs. (D) Double-label immunostaining for the endothelial marker CD31 and HA-HSP27 in the cerebral cortex of WT or EC-HSP27 mice. HA-HSP27 was expressed in EC-HSP27 but not in WT mouse brains. In EC-HSP27 brains, expression of HA-HSP27 was predominantly in microvessels, as shown by the colocalization of HA and CD31 in yellow. (Scale bar: 200 µm.) (E and F) Overexpression of HA-tagged HSP27 was confirmed by Western blotting for HSP27 and HA in (E) the total protein extracts from cortex and striatum of WT and N-HSP27 mice, and (F) the protein extracts from cerebral microvessels of WT and EC-HSP27 mice. (G) Cerebral microvasculature was examined in the cortex and striatum of WT, N-HSP27, and EC-HSP27 mouse brains by immunohistochemistry for CD31 (red). Brains were stained with DAPI (blue) for nuclear labeling. (Scale bar: 200 µm.) (H) The vascular surface area and branch points were quantified on CD31 immunofluorescence images. n = 3 mice per group. No significant difference was observed in microvessel distribution and anatomy between WT and transgenic animals.
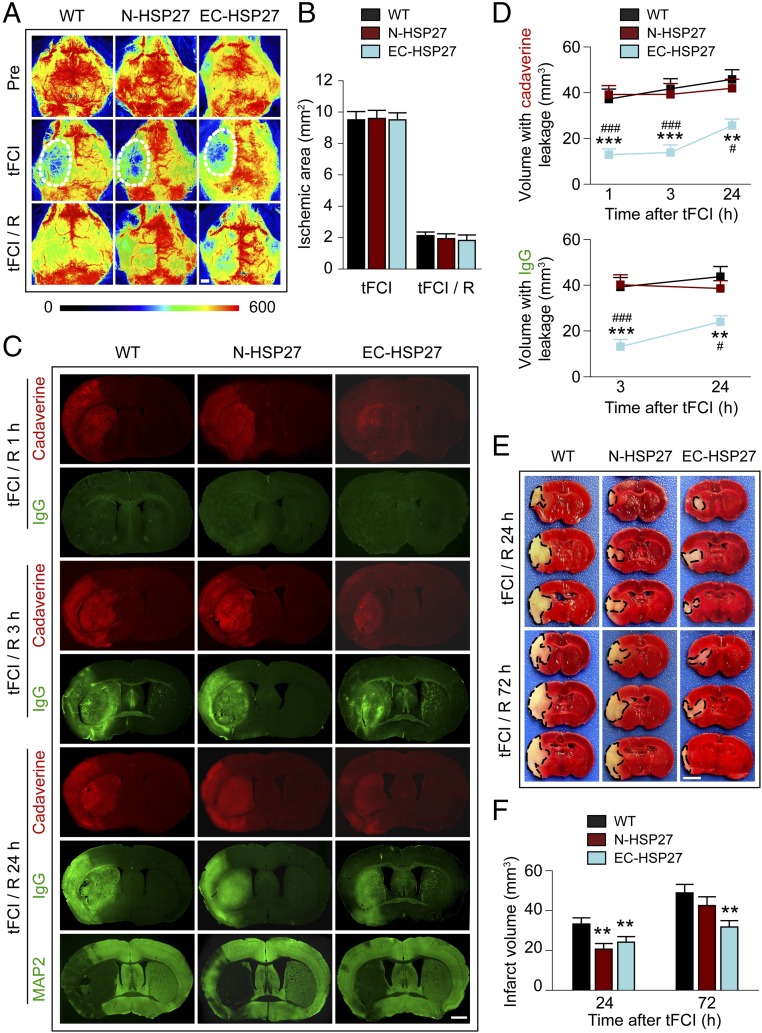
Fig. 1.
Targeted overexpression of HSP27 within ECs but not within neurons ameliorates both early and late-onset BBB impairments and I/R brain injury. tFCI was induced for 1 h in WT mice, transgenic mice with neuron-specific HSP27 overexpression (N-HSP27), or transgenic mice with endothelial cell-targeted HSP27 overexpression (EC-HSP27). (A) Representative 2D laser speckle images show cortical CBF at 15 min before tFCI (Pre), 15 min after the onset of tFCI, and after 15 min of reperfusion (R). White oval, ischemic area. (Scale bar: 1 mm.) (B) Quantitative measurement of ischemic cortical areas by laser speckle imaging after 15 min tFCI and after 15 min poststroke reperfusion (tFCI/R) in WT, N-HSP27, and EC-HSP27 mice. n = 5–6 mice per group. Ischemic areas are defined as cortical CBF reduction greater than 65% compared with pre-tFCI CBF levels in the same animals. (C) Representative images demonstrate the extravasation of Alexa 555 cadaverine (0.95 kDa, red) or endogenous plasma IgG (∼150 kDa, green) into the brain parenchyma 1, 3, or 24 h after 1-h tFCI. At 24 h after tFCI, the area with loss of microtubule-associated protein 2 (MAP2) immunofluorescence illustrates the infarct zone on adjacent sections from the same brains. (Scale bar: 1 mm.) (D) Volume of the brain with leakage of cadaverine and IgG at indicated time of reperfusion after tFCI. n = 6 mice per group. (E and F) Brain infarct volume at 24 and 72 h after tFCI was measured on TTC-stained coronal sections. Dashed line, infarct. (Scale bar: 2 mm.) n = 6–8 mice per group. **P ≤ 0.01, ***P ≤ 0.001 vs. WT. #P ≤ 0.05, ###P ≤ 0.001 vs. N-HSP27.
To assess BBB permeability after tFCI, we analyzed the extravasation of blood components into the brain parenchyma, including i.v.-injected fluorescent tracer Alexa 555 cadaverine (0.95 kDa) and endogenous plasma IgG (∼150 kDa). Consistent with our recent findings (9), tFCI induced progressive deterioration of BBB integrity in WT mice, manifested as the staggered leakage of small macromolecules followed by large macromolecules. Specifically, the extravasation of cadaverine was detected in both the ipsilateral cortex and striatum at 1 h after tFCI, whereas IgGs were seen in the same regions at 3 h after tFCI, the first time point examined after 1 h (Fig. 1C and Fig. S2). N-HSP27 and WT mice showed comparable volumes with visible leakage of cadaverine at 1–24 h after tFCI and of IgG at 3–24 h after tFCI (Fig. 1D). These data suggest that neuron-specific HSP27 overexpression does not protect against I/R-induced BBB disruption. In contrast, EC-HSP27 mice showed significantly reduced extravasation of both cadaverine (1–24 h after tFCI) and IgG (3–24 h after tFCI). Furthermore, brain regions with robust BBB breakdown at acute stages (1–3 h) after tFCI evolved into infarct zones at 24 h after tFCI (Fig. 1C, MAP2-negative areas). Although N-HSP27 mice had reduced infarct volumes at 24 h after tFCI compared with WT mice, this difference disappeared by 72 h after tFCI (Fig. 1 E and F). In contrast, EC-HSP27 mice showed significantly reduced infarct volumes at both 24 h (P ≤ 0.01 vs. WT) and 72 h (P ≤ 0.01 vs. WT; P = 0.077 vs. N-HSP27) after tFCI. In summary, these results suggest that EC-targeted overexpression of HSP27 not only ameliorates tFCI-induced BBB damage but also confers greater protection against ischemic infarction than neuron-specific overexpression.
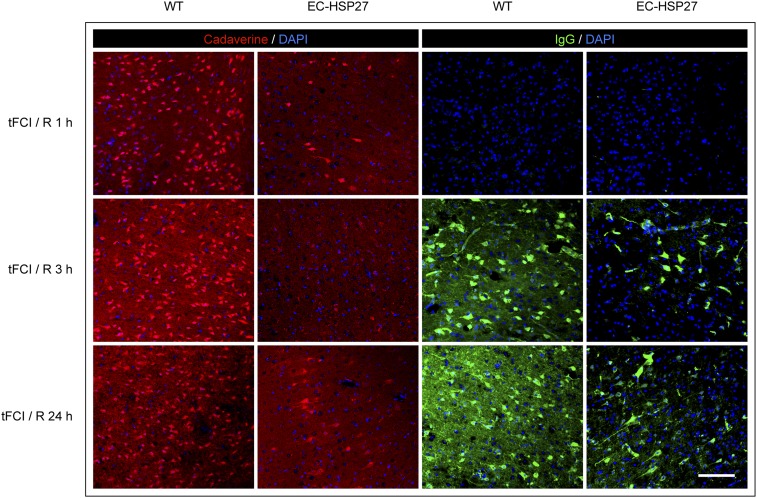
Fig. S2.
EC-targeted HSP27 overexpression preserves BBB integrity after tFCI. WT or EC-HSP27 mice were subjected to 1 h of tFCI followed by 1–24 h of reperfusion. Representative high-power microscopic images demonstrate the extravasation of Alexa 555 cadaverine (1, 3, and 24 h after tFCI) and endogenous IgG (3 and 24 h after tFCI) into ipsilateral cortical parenchyma, which resulted in positive staining of nonvascular cells. These cells were perhaps cells with damaged cell membrane. (Scale bar: 100 µm.) EC-targeted overexpression of HSP27 markedly reduced the extravasation of both cadaverine (1, 3, and 24 h after tFCI) and IgG (3 and 24 h after tFCI).
EC-Targeted HSP27 Overexpression Improves Long-Term Stroke Outcomes.
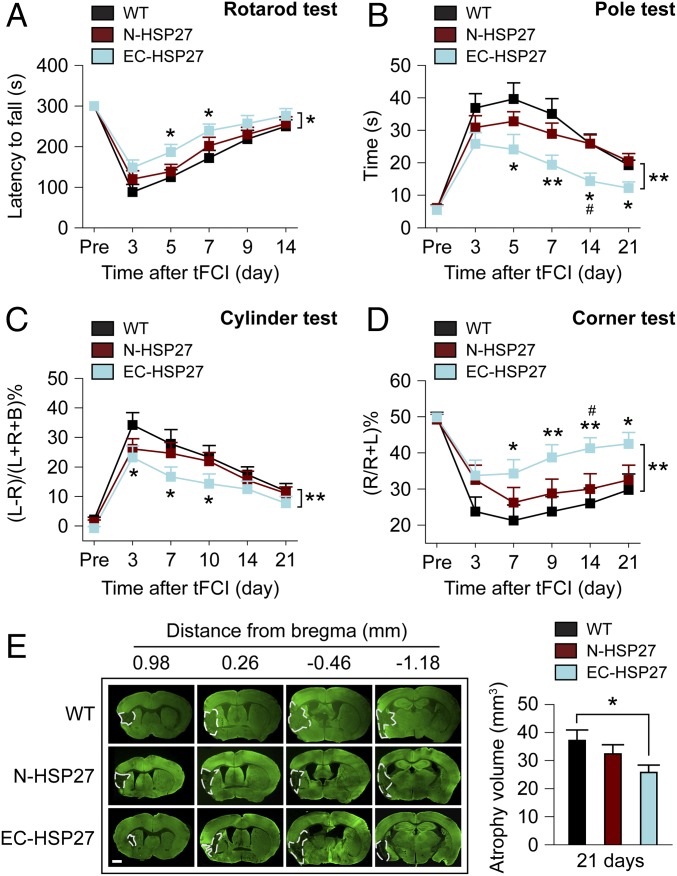
Next we investigated whether EC-targeted HSP27 overexpression leads to long-term functional improvements in stroke mice. Commonly used neurobehavioral tests for assessing neurological deficits of mice show different patterns and time course of spontaneous recovery in stroke animals (23–25). To comprehensively evaluate sensorimotor functions after stroke, a battery of four different behavioral tests was performed on WT, N-HSP27, and EC-HSP27 mice before and up to 21 d after tFCI (Fig. 2 A–D). In all groups of mice, tFCI induced motor deficiencies (rotarod and pole tests) and sensorimotor and postural asymmetries (cylinder and corner tests), and these deficits were the most prominent in the first week after tFCI. Over a 2- to 3-wk period after tFCI, all stroke groups experienced at least partial recovery in all four tests, and EC-HSP27, but not N-HSP27 mice, demonstrated superior functional recovery after tFCI compared with WT mice (Fig. 2 A–D; P ≤ 0.05 or P ≤ 0.01 by two-way ANOVA). Specifically, EC-HSP27 but not N-HSP27 mice performed significantly better than WT mice at earlier time points after stroke in the rotarod (day 5 and 7) and cylinder (day 3, 7, and 10) tests (P ≤ 0.05 vs. WT by one-way ANOVA), whereas all three stroke groups (WT, N-HSP27, and EC-HSP27) recovered to comparable levels within 14–21 d after tFCI (Fig. 2 A and C; P > 0.05 between any two groups by one-way ANOVA). These results suggest that EC-targeted HSP27 overexpression facilitated spontaneous sensorimotor recovery after stroke, as demonstrated by the rotarod and cylinder tests. In contrast, WT mice showed relatively poorer long-term recovery (21 d after tFCI; Fig. 2 B and D) in the pole test and corner test; in both tests, EC-HSP27 but not N-HSP27 mice exhibited significant improvements in performance not only at earlier time points (day 5 and 7 in the pole test; day 7 and 9 in the corner test; P ≤ 0.05 or P ≤ 0.01 vs. WT by one-way ANOVA), but also at later time points (day 14 and 21, P ≤ 0.05 or P ≤ 0.01 vs. WT by one-way ANOVA) compared with WT mice, indicating that EC-targeted HSP27 overexpression enhanced overall sensorimotor recovery after stroke.
Fig. 2.
EC-targeted overexpression of HSP27 improves long-term histological and functional outcomes after I/R brain injury. tFCI was induced for 1 h in WT mice, and in neuron- or EC-specific HSP27-overexpressing mice. (A–D) Sensorimotor deficits were assessed before (Pre) and up to 21 d after tFCI by a battery of behavioral tests. (A) Rotarod test. (B) Pole test. (C) Cylinder test. L, left; R, right; B, both forepaws. (D) Corner test. R, number of right turns (impaired side after occlusion of the left middle cerebral artery); L, number of left turns (nonimpaired side). (E) Tissue atrophy was measured at 21 d after tFCI on MAP2 (green)-stained coronal sections. Dashed line, brain infarct. n = 8 mice per group. (Scale bar: 1 mm.) *P ≤ 0.05, **P ≤ 0.01 EC-HSP27 vs. WT by one-way ANOVA (individual time point) or two-way ANOVA (bracket). #P ≤ 0.05 EC-HSP27 vs. N-HSP27 by one-way ANOVA.
At 21 d after tFCI, all postischemic mice developed brain atrophy in the ipsilateral hemisphere (Fig. 2E). Consistent with the observed improvements in neurological functions, brain atrophy was significantly reduced in EC-HSP27 mice (P ≤ 0.05), but not in N-HSP27 mice (P = 0.12), compared with WT mice. In summary, these results demonstrate that HSP27 overexpression targeted to ECs, but not to neurons, results in long-term functional and histological improvements in mice after tFCI. Although neuronal HSP27 overexpression offered acute protection against ischemic infarct (at 24 h after tFCI; Fig. 1E), its beneficial effects dissipated beyond the 24-h time window.
HSP27 Mitigates I/R-Induced BBB Permeability in an in Vitro Model.
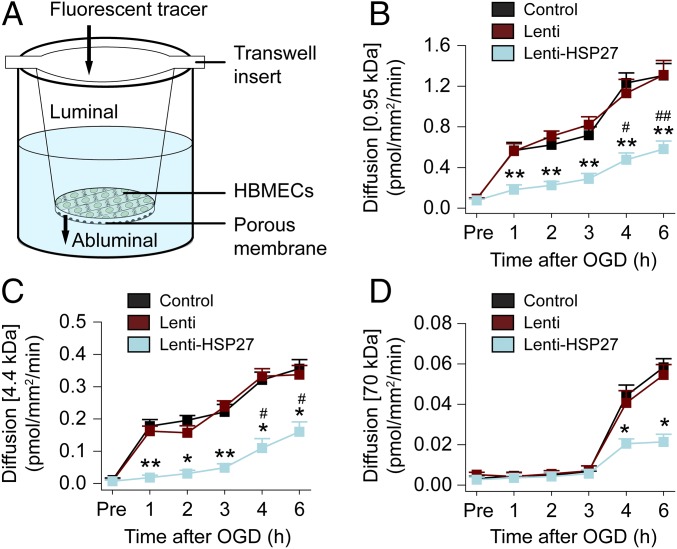
To investigate the mechanisms underlying BBB protection against I/R injury in endothelial HSP27-overexpressing mice, we established an in vitro BBB model consisting of a monolayer of human brain microvessel endothelial cells (HBMECs) in a Transwell culture insert (Fig. 3A and SI Materials and Methods). The system was subjected to the ischemia-like insult of oxygen–glucose deprivation (OGD) for 1 h, and the luminal-to-abluminal barrier permeability to fluorescent tracers of three different sizes was evaluated after 1–6 h of post-OGD reperfusion. Compared with non-OGD cultures, OGD and reperfusion induced progressive increases in barrier permeability, manifested as increased diffusion rate. In particular, the two small-size tracers (0.95 kDa cadaverine and 4.4 kDa dextran) crossed the EC barrier already at 1 h after OGD (Fig. S3 and Fig. 3 B and C), but the larger test molecule (70 kDa dextran) did not cross the EC layer during the first 3 h after OGD (Fig. 3D). At 4 and 6 h after OGD, the larger macromolecules also crossed the EC barrier; meanwhile, there was further increased leakage of the two small-size tracers across the barrier compared with the 3-h time point after OGD (Fig. S3 and Fig. 3 B–D). When HSP27 was overexpressed in HBMECs by lentivirus-mediated transfection before OGD, the leakage of the small-size tracers was significantly reduced, but not prevented, at 1–6 h after OGD and of the large tracer at 4–6 h after OGD (Fig. 3 B–D). Thus, we confirmed that endothelial HSP27 significantly attenuated OGD-induced BBB leakage to both small- and large-size macromolecules in vitro, and used this HBMEC culture model for subsequent mechanistic studies.
Fig. 3.
Overexpression of HSP27 within ECs reduces BBB permeability after OGD in vitro. (A) Illustration of the in vitro BBB model. Cultured HBMECs were seeded on top of a membrane in the cell culture insert and grown for 4 d to form a confluent monolayer. HBMECs were infected for 72 h with empty lentivirus (Lenti), or lentiviral vectors carrying HA-tagged human HSP27 (Lenti-HSP27), and then subjected to an ischemia-like insult OGD for 1 h. Paracellular permeability was quantified at 1–6 h after OGD by the luminal to abluminal diffusion rate of three fluorescent tracers with different molecular weights. (B–D) Shown are the diffusion rates of the 0.95-kDa Alexa 555 cadaverine (B), the 4.4-kDa TRITC–dextran (C), or the 70-kDa FITC–dextran (D) before OGD (Pre) and at 1–6 h after OGD. Data represent the mean and SEM of four independent experiments. *P ≤ 0.05, **P ≤ 0.01 Lenti-HSP27 vs. Lenti. #P ≤ 0.05, ##P ≤ 0.01 vs. Pre-OGD for Lenti-HSP27.
Fig. S3.
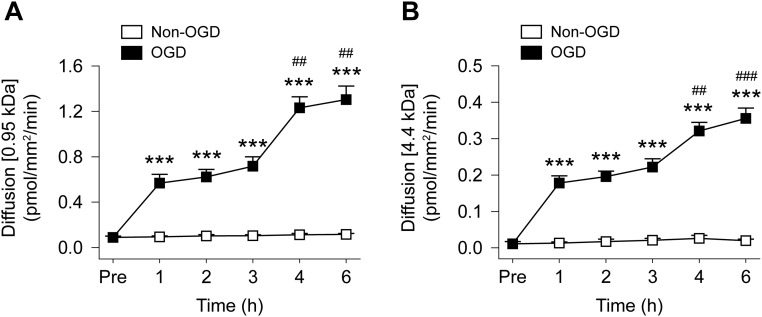
Progressive endothelial barrier leakage after OGD. Cultured HBMECs were exposed to 1 h of OGD or control non-OGD condition. Shown are the diffusion rates of the 0.95-kDa Alexa 555 cadaverine (A) and the 4.4-kDa TRITC–dextran (B) before (Pre) and at 1–6 h after OGD or non-OGD. Data represent the mean and SEM of four independent experiments. ***P ≤ 0.001 OGD vs. non-OGD. ##P ≤ 0.01, ###P ≤ 0.001 vs. 3 h. Non-OGD HBMECs did not permit the passage of either the 0.95-kDa or 4.4-kDa tracer after 1–6 h. In contrast, OGD induced progressive leakage of both tracers, starting from 1 h after OGD. This leakage was significantly augmented at 4 and 6 h compared with that at 3 h after OGD.
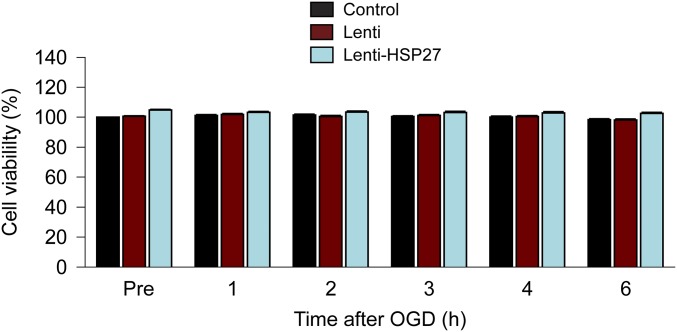
One hour of OGD did not cause overt cell death in cultured HBMECs within the first 6 h of reperfusion (Fig. S4), suggesting that the HSP27-afforded attenuation of BBB leakage in vitro does not depend on its antiapoptotic properties.
Fig. S4.
Absence of overt endothelial cell death at early stages after OGD. Cultured HBMECs were infected with empty lentivirus (Lenti) or lentiviral vectors carrying HA-tagged human HSP27 (Lenti-HSP27). Seventy-two hours later, noninfected (Control) or infected HBMECs were exposed to 1 h of OGD. Cell viability was assessed using the MTT assay 1–6 h after OGD and expressed as percentage of noninfected controls before OGD (Pre). Data represent the mean and SEM from four independent experiments. Within the first 6 h after OGD, no significant cell death was observed, consistent with our previous report (9).
HSP27 Inhibits OGD-Induced Actin Polymerization and Junctional Protein Redistribution in Cultured ECs.
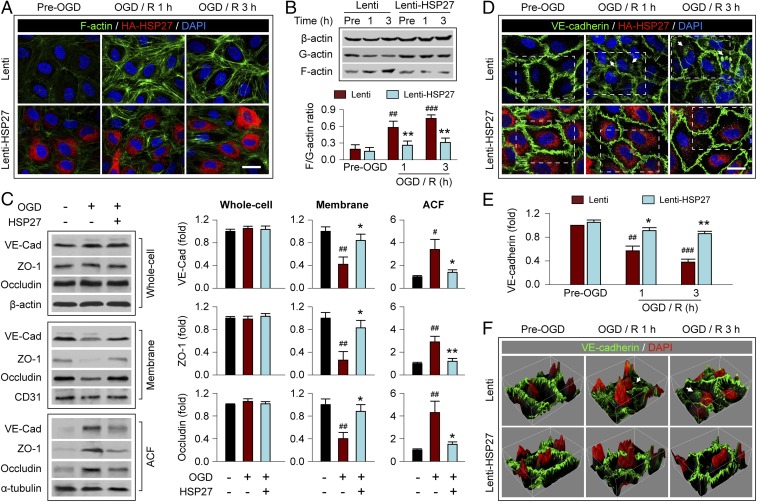
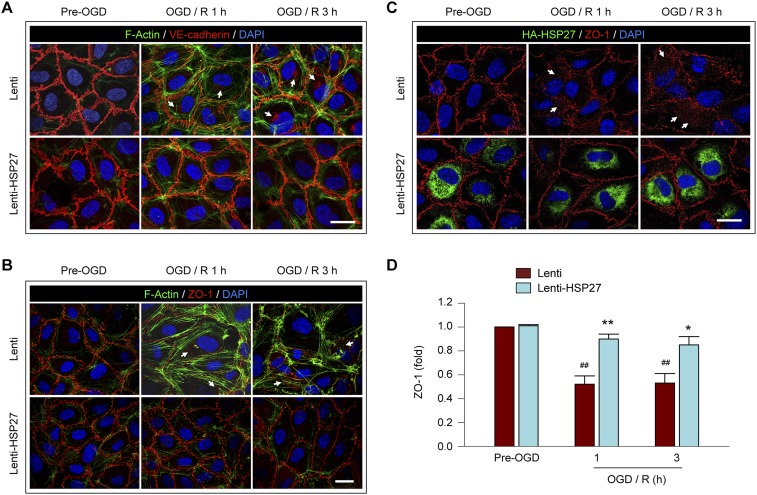
We recently reported that aberrant actin polymerization and the translocation of endothelial junctional proteins from the extracellular space to the cytosol are responsible for the increase in BBB permeability during the first 3 h after OGD, the evidence being that this loss of barrier function could be prevented by overexpression of actin depolymerizing factor (ADF), which inhibits actin polymerization, or knockdown of MLC (9). Previous studies demonstrated that HSP27 directly interacts with actin and is a potent inhibitor of actin polymerization (19, 20). Thus, we hypothesized that HSP27 may, like ADF, ameliorate BBB disruption by inhibiting the aberrant actin polymerization in ECs after OGD. Indeed, OGD rapidly enhanced actin polymerization in cultured HBMECs, as manifested by the robust formation of F-actin+ stress fibers (Fig. 4A and Fig. S5) and by an increase in the ratio of F-actin to G-actin at 1 and 3 h after OGD (Fig. 4B). Lentivirus-mediated HSP27 overexpression significantly inhibited the F-actin+ stress fiber formation and reduced the F/G-actin ratio to near control levels at 1 and 3 h after OGD (Fig. 4 A and B), thus confirming the role of HSP27 as an inhibitor of actin polymerization in post-OGD ECs.
Fig. 4.
Overexpression of HSP27 inhibits OGD-induced formation of stress fibers and translocation of junctional proteins in cultured ECs. Cultured HBMECs were transfected with Lenti or Lenti-HSP27. Seventy-two hours later, cells were subjected to 1 h of OGD, followed by 1 or 3 h of reperfusion (R). (A) Cells were triple-labeled for F-actin+ stress fibers, HA-tagged HSP27, and the nuclear marker DAPI. OGD-induced stress fiber formation was markedly attenuated by HSP27 overexpression at both 1 and 3 h of reperfusion. (Scale bar: 30 µm.) (B) The expression of total β-actin, soluble G-actin, and polymerized F-actin in HBMECs was examined by Western blotting before OGD or at 1 and 3 h after OGD. The ratio of F-actin to G-actin was quantified as a measure of stress fiber formation; the ratio was increased by OGD/R but significantly less in Lenti-HSP27–transfected cells. (C) Whole-cell extract, the membrane fraction, or the ACF were prepared at 1 h after post-OGD reperfusion and immunoblotted for VE-cadherin (VE-Cad), ZO-1, occludin, and subfraction markers β-actin, CD31, or α-tubulin. Representative images and quantification of blots are presented. (D) HBMECs were stained for VE-cadherin, HA-tagged HSP27, and DAPI. HA-HSP27 distributes uniformly in the cytosol, and VE-cadherin is characteristically located at cell–cell appositions under physiological, uninjured conditions. OGD resulted in a loss of VE-cadherin from the appositions, and label was increased in the cytosol (arrow). This effect was inhibited by lentiviral HSP27 overexpression. Rectangles: regions that were used to construct the 3D plots in F. (Scale bar: 30 μm.) (E) The immunofluorescence of VE-cadherin at the cell–cell contacts was semiquantitatively measured, and data are expressed relative to pre-OGD controls. (F) 3D plots generated from the fluorescence of VE-cadherin and DAPI in D. The luminance of the fluorescence image was represented by height of the plot. Arrow: the cytosolic staining of VE-cadherin. Data in B, C, and E represent the mean and SEM of four independent experiments. *P ≤ 0.05, **P ≤ 0.01 Lenti-HSP27 vs. Lenti. #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 OGD/R vs. pre-OGD.
Fig. S5.
HSP27 inhibits OGD-induced formation of stress fibers and redistribution of junctional proteins in HBMECs. Cultured HBMECs were infected with Lenti or Lenti-HSP27. Seventy-two hours later, cells were subjected to 1 h of OGD, followed by 1 or 3 h of reperfusion (R). Cells were triple-labeled for F-actin+ stress fibers, junctional proteins VE-cadherin (A) or ZO-1 (B) and DAPI. (Scale bars: 30 µm.) OGD induced robust formation of stress fibers within HBMECs, with a concomitant redistribution of VE-cadherin and ZO-1 from cell interfaces to cytosol. These changes were blunted in HBMECs overexpressing HSP27. Arrow: the cytosolic staining of VE-cadherin or ZO-1. (C) HBMECs were stained for ZO-1, HA-tagged HSP27, and DAPI. OGD resulted in reduction of ZO-1 immunofluorescence at the cell–cell junctions (arrow). In many post-OGD cells, the presence of ZO-1 immunofluorescence was increased in the perinuclear zone, suggesting that ZO-1 was redistributed from cell–cell junctions to the cytosol after OGD. Lentiviral HSP27 overexpression attenuated ZO-1 redistribution after OGD. (Scale bar: 30 µm.) (D) The immunofluorescence of ZO-1 at the cell–cell junctions was semiquantitatively measured, and data are expressed relative to pre-OGD controls. Data represent the mean and SEM from four independent experiments. *P ≤ 0.05, **P ≤ 0.01 Lenti-HSP27 vs. Lenti. ##P ≤ 0.01 OGD/R vs. pre-OGD.
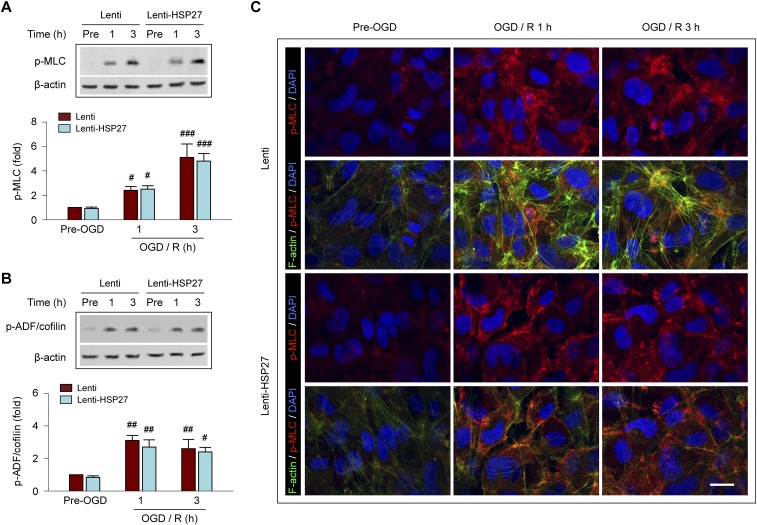
We recently identified two signaling pathways that underlie actin polymerization and stress fiber formation in post-OGD ECs, i.e., phosphorylation/inactivation of ADF/cofilin and phosphorylation/activation of MLC, which promote actin polymerization and formation of stress fibers from short F-actins, respectively (9). To determine whether HSP27 affects either ADF/cofilin or MLC phosphorylation in ECs, HBMECs transfected with HSP27 or control vectors were subjected to OGD followed by 1 or 3 h of reperfusion. OGD markedly elevated the levels of both p-ADF/cofilin and p-MLC in HBMECs, whereas HSP27 overexpression did not modify the level of either phosphoprotein (Fig. S6). These results are in line with previous findings that HSP27 is a direct actin depolymerizing protein (19, 20).
Fig. S6.
HSP27 inhibits I/R-induced stress fiber formation in ECs without affecting the phosphorylation of MLC or ADF/cofilin. Cultured HBMECs were infected with Lenti or Lenti-HSP27. Seventy-two hours later, cells were subjected to 1 h of OGD followed by 1 or 3 h of reperfusion (R). (A and B) The levels of phosphorylated myosin light chain (p-MLC) and phosphorylated actin depolymerizing factor/cofilin (p-ADF/cofilin) were measured by Western blotting. β-actin was used as an internal loading control. OGD induced robust phosphorylation of both MLC and ADF/cofilin after 1 and 3 h of reperfusion. Lentivirus-mediated overexpression of HSP27 did not change the level of either p-MLC or p-ADF/cofilin, compared with cells infected by empty lentiviral vectors. Data represent the mean and SEM of three independent experiments. #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 vs. Pre-OGD for Lenti and for Lenti-HSP27. (C) Representative images showing triple-labeling of F-actin+ stress fibers, p-MLC, and DAPI in HBMECs. HSP27 overexpression inhibited OGD-induced stress fiber formation but did not change p-MLC immunofluorescence in HBMECs. (Scale bar: 20 µm.) These results support that HSP27 directly inhibits actin polymerization independent of MLC and ADF/cofilin.
Contractile stress fibers transmit tension to cell junctions that are anchored to the actin cytoskeleton and disassemble the junctional complexes (9, 11). At 1 h after OGD, the distribution of TJ proteins occludin and zona occludens (ZO)-1, as well as the AJ protein vascular endothelial (VE)-cadherin partially shifted from the membrane fraction to the actin cytoskeleton fraction (ACF), whereas total protein levels in whole-cell lysates remained unchanged (Fig. 4C). This protein translocation was greatly reduced when HSP27 was overexpressed in HBMECs. Immunocytochemical studies confirm the decreased levels of VE-cadherin and ZO-1 at their normal expression sites—the cell–cell contact zones—at 1 and 3 h after OGD compared with non-OGD conditions, and perinuclear immunofluorescence of VE-cadherin and ZO-1 was observed in some cells (Fig. 4 D and E and Fig. S5 C and D), consistent with the redistribution of these two junctional proteins after OGD. This effect was markedly attenuated in HBMECs overexpressing HSP27. On 3D plots showing fluorescence intensity on the z axis (Fig. 4F), we observed abundant VE-cadherin immunostaining surrounding the nucleus in some OGD-treated HBMECs. In contrast, VE-cadherin and nuclear signals were topographically separated under non-OGD conditions or with HSP27 overexpression (Fig. 4F).
Endothelial HSP27 Overexpression Attenuates Stress Fiber Formation and Junctional Protein Redistribution in Brain Microvessels After tFCI.
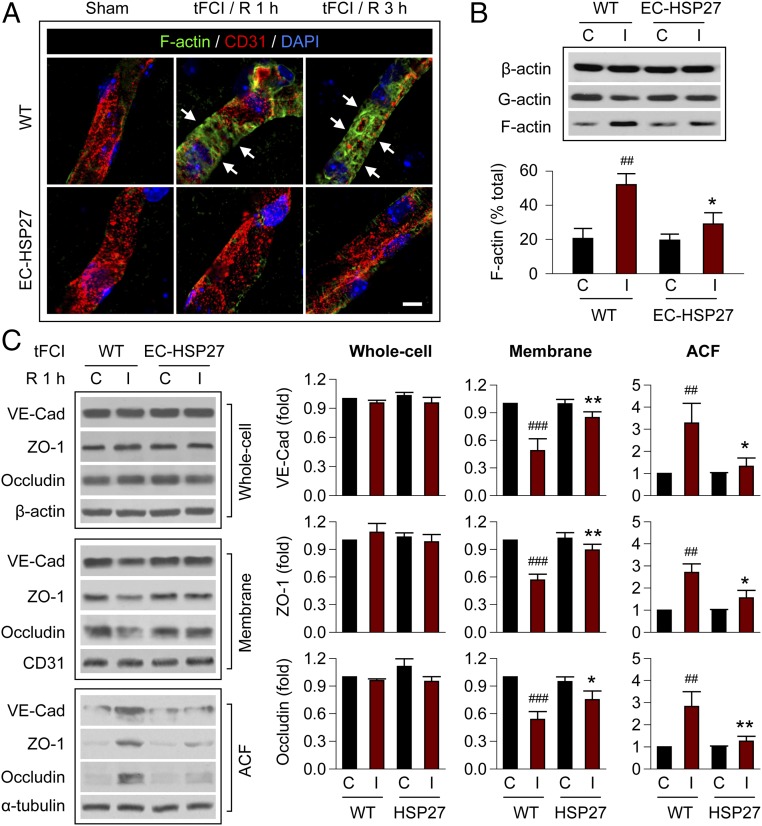
Thus far, we have demonstrated that endothelium-targeted HSP27 overexpression mitigated actin polymerization and redistribution of junctional proteins after OGD exposure in vitro. Therefore, we further examined the mechanism underlying BBB protection by HSP27 in vivo, using endothelial HSP27-overexpressing mice and WT mice subjected to tFCI and 1 or 3 h of reperfusion. Using confocal microscopy, F-actin+ dense stress fibers, a hallmark of increased actin polymerization, were frequently detected in cortical microvessels of WT mice after tFCI, but only rarely observed in postischemic EC-HSP27 mice (Fig. 5A). Immunoblot analysis of protein extracts from purified cortical microvessels revealed that the percentage of F-actin over total actin was significantly elevated in the postischemic hemisphere at 1 h after tFCI compared with the contralateral nonischemic hemisphere in WT mice, and that this I/R-induced increase was attenuated in EC-HSP27 mice (Fig. 5B).
Fig. 5.
EC-targeted HSP27 overexpression reduces stress fiber formation and translocation of junctional proteins in cerebral microvessels after tFCI. WT or EC-HSP27 mice were subjected to 1 h of tFCI or sham operation. (A) Representative images showing the formation of F-actin+ stress fibers in CD31+ microvessels (arrows) in the cortex of the ischemic hemisphere at 1 and 3 h after tFCI. (Scale bar: 10 µm.) Endothelial HSP27 overexpression inhibited tFCI-induced formation of stress fibers. (B) Brain microvessel extracts were collected from the contralateral (C) and ipsilateral (I) cortex at 1 h reperfusion after 1 h tFCI for Western blot analysis of total β-actin, G-actin, and F-actin. The content of F-actin was quantified as percentage of total β-actin. n = 4 mice per group. (C) Whole-cell extract, the membrane fraction, or the ACF was prepared from cortical microvessels at 1 h reperfusion after 1 h tFCI and subsequently immunoblotted. Representative images and quantification of blots (normalized to WT contralateral) are presented. Endothelial HSP27 overexpression suppressed tFCI-induced redistribution of junctional proteins VE-cadherin, ZO-1, and occludin from the membrane fraction to the ACF. n = 4–5 mice per group. *P ≤ 0.05, **P ≤ 0.01 EC-HSP27 vs. WT (ipsilateral hemisphere). ##P ≤ 0.01, ###P ≤ 0.001 ipsilateral vs. contralateral hemisphere.
Results of subcellular fractionation of cortical microvessel extracts followed by immunoblotting revealed that the junctional proteins occludin, ZO-1, and VE-cadherin were partially but significantly redistributed from the membrane fraction to ACF at 1 h after tFCI (Fig. 5C), similar to that observed in HBMEC cultures after OGD (Fig. 4C). This tFCI-induced protein translocation was reduced in EC-HSP27 mice. Together, these data from in vitro and in vivo models support our hypothesis that HSP27 preserves BBB integrity after I/R by inhibiting actin polymerization and the barrier-destructive redistribution of junctional proteins in postischemic ECs.
EC-Targeted Overexpression of HSP27 Impedes the Infiltration of Peripheral Immune Cells After tFCI.
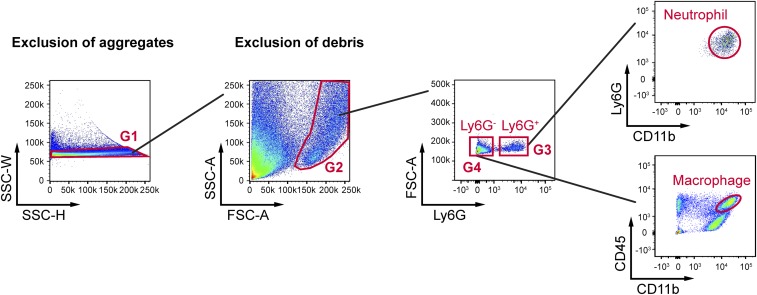
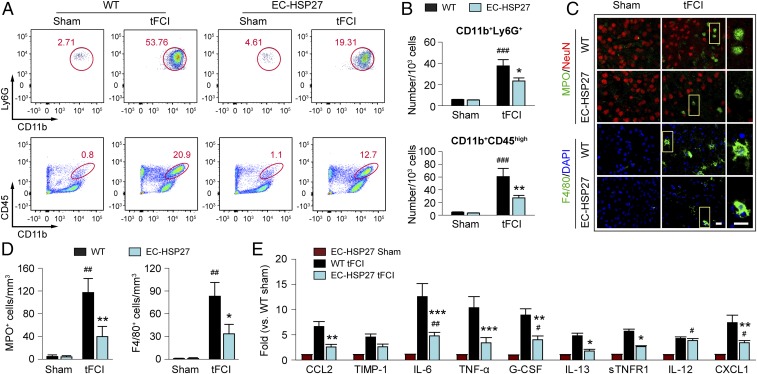
Leukocytes that infiltrate through the impaired BBB play an important role in the development of permanent neurovascular injury and neurological deficits after I/R (26). Blood neutrophils and macrophages carry proinflammatory mediators into the brain, such as gelatinase B/MMP-9, causing further BBB damage and secondary expansion of ischemic injury (9, 27). We hypothesized that the preservation of BBB integrity by HSP27 blocks post-tFCI infiltration of immune cells, thereby improving long-term stroke outcomes. A flow cytometry-based strategy was used to quantitatively analyze the brain infiltration of leukocytes at 48 h after tFCI (Fig. S7). In WT mice, CD11b+Ly6G+ cells were dramatically increased in the post-tFCI brain hemisphere compared with sham-operated mice (Fig. 6 A and B), reflecting the robust infiltration of blood neutrophils (28) into the brain. In the Ly6G− cell population, tFCI also induced an increase in the number of CD11b+CD45high cells (representing infiltrated macrophages and activated microglia; Fig. 6 A and B). In contrast to WT mice, tFCI-induced brain infiltration of neutrophils and macrophages was significantly reduced in EC-HSP27 mice. These results were confirmed by immunohistochemistry in another cohort of mice, in which neutrophils and macrophages were immunolabeled for myeloperoxidase (MPO) and F4/80 (29, 30), respectively (Fig. 6C). Quantitatively, tFCI-induced increases in brain infiltration of MPO+ and F4/80+ cells were significantly smaller in EC-HSP27 mice compared with WT mice (Fig. 6D). To test whether the attenuation of neutrophil and macrophage infiltration alleviates the proinflammatory reactions in the postischemic brain, we measured a panel of inflammatory mediators in the ipsilateral brain hemisphere at 48 h after tFCI using the ChemiArray Mouse Antibody Array Kit. Indeed, of the nine inflammation markers that were elevated by tFCI in WT mice compared with sham controls, seven showed reduced expression in EC-HSP27 mice compared with WT mice (Fig. 6E).
Fig. S7.
Gating strategy for flow cytometry on brain cells after tFCI. Mice were subjected to 1 h of tFCI followed by 48 h of reperfusion. Cell suspensions were prepared from the ipsilateral hemisphere of each mouse as described in SI Materials and Methods. Cells were first gated (G1) on a side scatter (SSC-H/SSC-W) dot plot to exclude aggregates. The G1 events (single cells) were visualized using a forward scatter/side scatter (FSC-A/SSC-A) dot plot and gated (G2) to exclude debris and dead cells. The G2 events were next gated on a Ly6G/FSC-A dot plot for Ly6G-positive (G3) and Ly6G-negative (G4) cells. Ly6G-positive cells (infiltrated neutrophils, circle) were displayed on a CD11b/Ly6G dot plot and quantified. Ly6G-negative cells were displayed on a CD11b/CD45 dot plot, and CD11b+CD45high cells (macrophages and activated microglia, oval) were gated and quantified.
Fig. 6.
EC-targeted HSP27 overexpression reduces the infiltration of peripheral immune cells after tFCI. WT or EC-HSP27 mice were subjected to sham operation or 1 h of tFCI followed by 48 h of reperfusion. (A and B) Cell suspensions were prepared from the ipsilateral hemispheres and flow cytometry was performed to quantify Ly6G+ (infiltrated neutrophils) and CD45high (macrophages and activated microglia) cells among the CD11b+ cell populations. (A) Representative density plots. (Upper) CD11b+Ly6G+ cells (circle) gated from total single cells. Numbers shown are the percentage of circled cells to total single cells (Fig. S7, G1 events). (Lower) CD11b+CD45high cells (ovals) gated from the Ly6G− cell population. Numbers shown are the percentage of cells in each oval to total Ly6G− cells (Fig. S7, G4 events). (B) Cells were quantified and presented as the number of cells per 103 single cells. n = 4 mice for WT sham, n = 9 mice for EC-HSP27 sham, n = 6 mice for WT and EC-HSP27 tFCI. *P ≤ 0.05, **P ≤ 0.01 vs. WT. ###P ≤ 0.001 vs. sham. (C) Representative images taken from the ipsilateral periinfarct cortex after tFCI or the corresponding region after sham operation, showing immunofluorescence of the following markers: MPO (neutrophil), F4/80 (macrophage), and NeuN (neuron). Rectangle: the region enlarged in high-power images (third column). (Scale bar: 20 µm.) (D) MPO+ and F4/80+ cells were counted in the areas described in C and data expressed as the number of cells per mm3. n = 4 mice per group for sham. n = 7–8 mice per group for tFCI. MPO+ and /F4/80+ cells were increased more in WT than in EC-HSP27 mice. *P ≤ 0.05, **P ≤ 0.01 vs. WT. ##P ≤ 0.01 vs. sham. (E) A panel of inflammation markers was measured in the ipsilateral hemisphere at 48 h after tFCI (SI Materials and Methods). Shown are nine markers that were significantly increased (P ≤ 0.05) in the WT brains after tFCI compared with WT sham controls among the total 32 markers tested. n = 4 mice per group for sham. n = 5 mice per group for tFCI. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 EC-HSP27 tFCI vs. WT tFCI. #P ≤ 0.05, ##P ≤ 0.01 EC-HSP27 tFCI vs. EC-HSP27 sham.
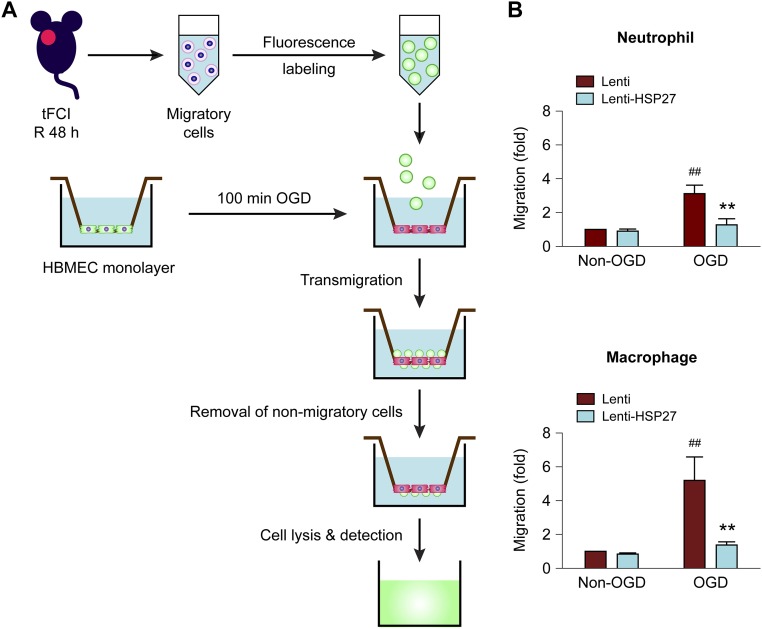
Using the in vitro BBB model, we tested the effect of HSP27 overexpression on leukocyte transmigration across the endothelial barrier after OGD (Fig. S8A). Preexposure of HBMECs to OGD markedly enhanced the transmigration of neutrophil and macrophage across the barrier, whereas these changes were significantly attenuated in HBMEC cultures transfected with HSP27 (Fig. S8B). Taken together, these results support our hypothesis that HSP27 inhibits the brain infiltration of leukocytes and inflammatory responses after I/R by strengthening the endothelial barrier. Nevertheless, these experiments do not prove the mechanism by which HSP27 reduces the transmigration of neutrophils and macrophages across the barrier, because both of the paracellular and transcellular pathways have been hypothesized for such transmigration (31, 32). Because both pathways involve actin dynamics (32), whether HSP27 acts through either or both pathways warrants further investigation.
Fig. S8.
HSP27 overexpression reduces transmigration of neutrophils and macrophages across the ECs after OGD. (A) Illustration of the in vitro migration assay. HBMECs were infected by empty lentiviral vectors or Lenti-HSP27, and seeded on top of a membrane (with 3-μm pores) in the cell culture insert for 48 h to form a monolayer. Migratory cells (neutrophils and macrophages) were isolated from the blood of WT mice after 1 h of tFCI and 48 h of reperfusion, as described in SI Materials and Methods. Neutrophils or macrophages were labeled with fluorochrome and plated on top of the HBMEC monolayer that was preexposed to 100 min of OGD or normal condition (non-OGD) in the migration inserts. Cells were allowed to migrate for 6 h and then nonmigratory cells were removed. Cells in the bottom chamber were lysed and fluorescence intensity was measured as a quantification of neutrophil/macrophage transmigration across the EC barrier. (B) Summarized data of neutrophil and macrophage migration, expressed relative to empty vector-infected, non-OGD controls. OGD facilitated the transmigration of both neutrophils and macrophages. When HSP27 was overexpressed in HBMECs, OGD-induced migration of neutrophils and macrophages was significantly reduced. Data represent the mean and SEM of three independent experiments. **P ≤ 0.01 Lenti-HSP27 vs. Lenti. ##P ≤ 0.01 OGD vs. non-OGD.
Post-tFCI Administration of TAT-HSP27 Alleviates BBB Disruption and Improves Long-Term Stroke Outcomes.
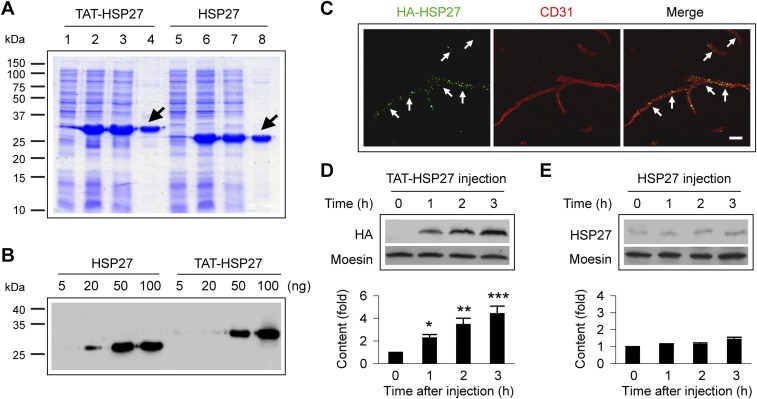
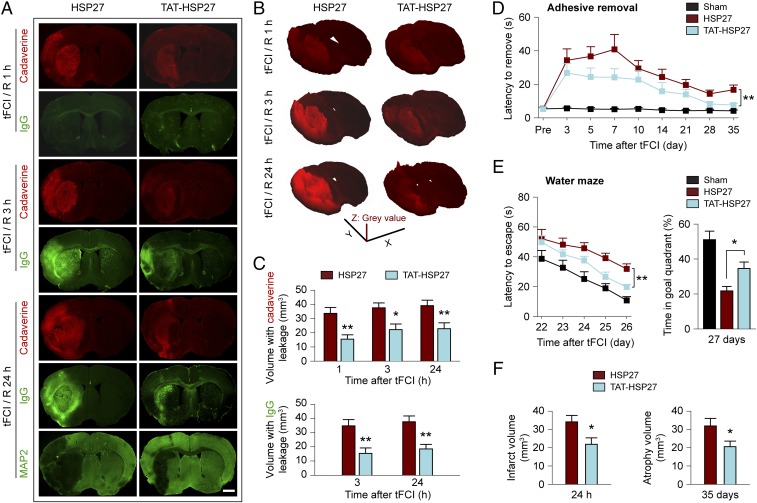
Thus far, we had demonstrated that transgenic overexpression of human HSP27 in ECs is sufficient to attenuate I/R-induced BBB disruption and improve long-term stroke outcome. Next, we determined whether administration of a cell-penetrating recombinant HSP27 protein after the onset of reperfusion elevates HSP27 levels in brain ECs and protects against I/R-induced BBB disruption. To this end, a cell-penetrating HSP27 fusion protein containing the 11-aa protein transduction domain (PTD) derived from the HIV TAT protein was generated and purified (Fig. S9 A and B). Intravenous injection of TAT-HSP27, but not the HSP27 protein without the PTD, resulted in widespread delivery of HSP27 into brain microvascular endothelium within 1 h after injection (Fig. S9 C–E). When administered at 10 min, 2 h, and 24 h after tFCI, TAT-HSP27 effectively reduced the blood-to-brain leakage of Alexa 555 cadaverine and plasma IgG at 1, 3, and 24 h after tFCI, compared with mice treated with the control HSP27 protein (Fig. 7A). This amelioration of BBB disruption was manifested by a reduction in the amount of tracers found in the regions with BBB leakage (shown by decreased fluorescent intensity of cadaverine on surface plot images; Fig. 7B), as well as a reduction in the volume of brain showing leakage of cadaverine and of IgG (Fig. 7C). In separate cohorts of mice, post-tFCI administration of TAT-HSP27 significantly improved long-term sensorimotor (adhesive removal test; Fig. 7D) and spatial learning and memory (Morris water maze; Fig. 7E) functions of poststroke mice. Together with these improvements in neurological function, TAT-HSP27 treatment also reduced the acute ischemic infarct formed at 24 h after tFCI and the development of brain atrophy determined at 35 d after tFCI (Fig. 7F). Collectively, these data demonstrate that the cell-penetrating TAT-HSP27, when delivered at 10 min, 2 h, and 24 h after onset of reperfusion, is effective not only in preserving BBB integrity but also conferring prolonged protection against neurovascular injury and impairment of brain functions.
Fig. S9.
Purification and delivery of the TAT-HSP27 protein. (A) Coomassie blue-stained SDS/PAGE gel showing the bands for TAT-HSP27 (lanes 1–4) and unconjugated HSP27 (lanes 5–8) after serial purifications. Lanes 4 and 8: proteins (arrows) after the final purification. (B) Western blots for purified TAT-HSP27 and unconjugated HSP27 proteins. HSP27 protein without the TAT sequence generates bands with a lower molecular weight than TAT-HSP27. (C) Double-label immunostaining shows that i.v. injection of TAT-HSP27 (3 mg/kg) resulted in transduction of the protein into brain microvascular endothelium (arrows) at 1 h after injection. (Scale bar: 100 µm.) (D and E) Western blots on brain microvessel extracts collected at 0–3 h after i.v. injection of TAT-HSP27 (D, using the anti-HA antibody) or HSP27 (E, using the anti-HSP27 antibody, because immunoblotting with the anti-HA antibody failed to detect any bands at the predicted sizes in HSP27-treated animals). The cell membrane protein moesin was used as an internal loading control. Representative images and quantification of blots are presented. n = 3–4 mice per group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. 0 h. i.v. injection of the cell-penetrating TAT-HSP27, but not the unconjugated HSP27, resulted in rapid (<1 h) and time-dependent uptake of TAT-HSP27 into brain microvessels that continued to increase from 1 to 3 h after injection.
Fig. 7.
Delivery of cell-penetrating TAT-HSP27 after tFCI ameliorates BBB impairment and improves functional outcomes. tFCI was induced in WT mice for 1 h followed by reperfusion. For each animal, TAT-HSP27 or control HSP27 protein (without the TAT domain) was administered at 10 min, 2 h, and 24 h after reperfusion was begun. (A) Representative images demonstrate the extravasation of Alexa 555 cadaverine or endogenous plasma IgG into the brain parenchyma 1, 3, or 24 h after tFCI. At 24 h after tFCI, MAP2 immunofluorescence on adjacent sections illustrates infarcts in the same brains. (Scale bar: 1 mm.) (B) 3D plots of brain coronal sections generated from the fluorescence of cadaverine in A, using luminance as height of the plot. (C) Brain volume showing leakage of cadaverine and IgG at indicated times of reperfusion after tFCI. n = 5–7 mice per group. (D) Sensorimotor functions were assessed up to 35 d after tFCI using the adhesive removal test. (E) Spatial cognitive functions were assessed at 22–27 d after tFCI by the Morris water maze. n = 8 mice per group. (F) Brain infarct volume at 24 h after tFCI, and chronic tissue atrophy at 35 d after tFCI were measured on MAP2-stained coronal sections. n = 6–7 mice per group for the 24-h measurement; n = 8 mice per group for the 35-d measurement. *P ≤ 0.05, **P ≤ 0.01 TAT-HSP27 vs. HSP27.
SI Materials and Methods
Animals.
C57BL/6J and Tek-Cre mice were purchased from the Jackson Laboratory. Transgenic mice with neuron-specific HSP27 overexpression (N-HSP27) were generated using a chimeric construct containing full-length human HSP27 cDNA (HA-tagged) under the control of the neuron-specific synapsin I promoter and the chicken β-actin first intron (Fig. S1A).
For EC-specific HSP27 overexpression, the HSP27stop knock-in mice (Fig. S1C) were generated (Shanghai Research Center for Model Organisms). Briefly, a targeting vector was constructed to contain human full-length HSP27 cDNA downstream of CAG promoter and a loxP-flanked stop sequence (including a neomycin resistance gene and a trimer of the SV40 polyadenylation sequence). In the presence of Cre recombinase, the stop codon is excised and a 5′ HA-tagged HSP27 protein is expressed. The construct was inserted into the Gt(ROSA)26Sor locus on the C57BL/6J background via CRISPR/Cas9 technology. Mutant mice progeny were continuously backcrossed to the C57BL/6J background. To obtain EC-HSP27, the homozygous HSP27stop knock-in mice were crossed with hemizygous Tek-Cre mice (43), in which the expression of Cre recombinase is driven by the Tek promoter. Thus, the HSP27 overexpression is restricted to ECs. Fifty percent of the offspring were hemizygous for both the Tek-Cre and HSP27stop transgenes, in which the HA-tagged HSP27 protein was expressed specifically in ECs. Overexpression of HSP27 was validated by Western blotting (Fig. S1 E and F) and double-label immunohistochemistry with markers for specific cell types (Fig. S1 B and D). C57BL/6J mice were used as matched WT control.
Mice were housed in a temperature- and humidity-controlled animal facility with a 12-h light/dark cycle. Food and water were available ad libitum.
Choice of Sample Size.
The number of animals required for the in vivo studies was determined by a power analysis based on our previous experience with variance in outcomes and effect sizes in the murine MCAO model. To detect a 30% decrease in infarct volume or neurological deficits with 80% power at an α value of 0.05 (two-tailed), ∼6–8 mice were needed per group. For immunohistochemistry, 4–5 samples were required to detect a 30% change after tFCI with 80% power (β = 0.8, α = 0.05). For Western blotting analyses, 4–5 samples were required to detect a 30% change with 80% power (β = 0.8, α = 0.05). For flow cytometry analysis, each sample of single-cell suspensions was prepared from one mouse brain. Four samples (four mouse brains) were required to detect a 30% change after tFCI with 80% power (β = 0.8, α = 0.05).
tFCI Model.
tFCI was induced in adult male mice (8–10 wk old, 25–30 g) by intraluminal occlusion of the left middle cerebral artery for 60 min (9). Briefly, mice were anesthetized with 3% (vol/vol) isoflurane in 67:30% (vol/vol) N2O/O2 until they were unresponsive to the tail pinch test. Mice were then fitted with a nose cone blowing 1.5% (vol/vol) isoflurane for anesthesia maintenance. An 8-0 monofilament with silicon-coated tip was introduced into the common carotid artery, advanced to the origin of the MCA, and left in place for 60 min. Rectal temperature was maintained at 37.0 ± 0.5 °C during surgery through a temperature-controlled heating pad. Mean arterial blood pressure was monitored during surgery by a tail cuff. Regional CBF was measured using laser-Doppler flowmetry over intact skull. Alternatively, cortical CBF was monitored using 2D laser speckle techniques as described below. Failure to reduce CBF to 25% or less of baseline level, or death during surgery or prior to outcome evaluation, led to subject exclusion (less than 10%). Sham-operated animals underwent the same anesthesia and surgical procedures with the exception of the MCA occlusion.
Generation and Delivery of the Cell-Penetrating TAT-HSP27 Protein.
The HSP27 fusion protein containing the 11-amino acid TAT PTD was produced from human HSP27 cDNA. The expression vector of TAT-HSP27 was constructed as described previously (42). The final DNA construct contained sequences in the following order: His-6 tag (MHHHHHH), thrombin cleavage site (SGLVPRGS), PTD (YGRKKRRQRRR), HA tag (YPYDVPDVA), and the human HSP27 ORF (206 amino acids). The purified proteins were verified by Coomassie blue staining and Western blot analysis and were then stored in 10% (vol/vol) glycerol/PBS at −80 °C until used. The control HSP27 contained the HA tag but not the PTD.
Immediately after the MCAO surgery, mice were randomly assigned to receive TAT-HSP27, or control HSP27 protein without the TAT PTD with the use of a lottery-drawing box. TAT-HSP27 or HSP27 (in PBS) was administrated through the tail vein (9 mg/kg) at 10 min, 2 h, and 24 h of reperfusion.
Compliance with Stroke Therapy Academic Industry Roundtable Guidelines.
Experimental procedures were performed following criteria derived from Stroke Therapy Academic Industry Roundtable (STAIR) group guidelines for preclinical evaluation of stroke therapeutics (45). For example, experimental group assignments—such as for tFCI or sham operation, for treatment of TAT-HSP27 or HSP27, or for neurobehavioral testing or sacrifice for histological assessments—were randomly performed with the use of a lottery-drawing box. We verified that blood pH, gases, glucose levels, and CBF (in randomly selected mice for each group) were not altered by transgene expression. Furthermore, surgeries and all stroke outcome assessments were performed by investigators blinded to mouse genotype and experimental group assignments. Animals that died during surgery or prior to outcome evaluation were excluded from the studies. In all mice subjected to tFCI, neurological deficits were scored by a blinded investigator right after the mice recovered from anesthesia and again at 24 h after tFCI as described previously (22): 0, no visible neurological deficits; 1, forelimb flexion; 2, contralateral forelimb grips weakly (the rater pulls the tail gently while the animal is on a padded surface); 3, circling to the paretic side only when pulled by the tail (the animal is allowed to move freely); and 4, spontaneous circling. Mice receiving a score less than 1 were excluded from further studies. Animals were closely observed for the first 2 h after surgery, and then observed twice daily for the first 3 d and three times per week until euthanized. Animals that showed one or more of the following signs were also excluded: (i) Weight loss greater than 20% of baseline weight. Animals were weighed before surgery and every day for the first 5 d after surgery; (ii) moribund and not responsive to external stimuli; (iii) unable to attend to normal physiological needs such as eating, drinking, and grooming; or (iv) unable to ambulate.
2D Laser Speckle Imaging.
Cortical CBF was monitored using the laser speckle technique according to the manufacturer’s instructions as we described previously (9). Briefly, a charge-coupled device camera (PeriCam PSI System; Perimed Inc.) was placed above the head, and the intact skull surface was illuminated by a laser diode (785 nm) to allow laser penetration into the brain. 2D microcirculation images were obtained starting 15 min before tFCI and continued throughout the ischemic period until 15 min after the onset of reperfusion. Ischemic areas were defined as areas with CBF reduced to less than 35% of pre-tFCI CBF levels in the same animals, and quantitatively measured on laser speckle images at 15 min after the onset of tFCI and after 15 min of post-tFCI reperfusion (Fig. 1B).
Neurobehavioral Tests.
Neurobehavioral tests were carried out before and up to 35 d after tFCI. To minimize the confounding impacts from the intrinsic variabilities of different tests (24, 25) and potential functional compensation (23), we used a complementary array of tests to evaluate the functional recovery of mice after tFCI. Sensorimotor deficits were evaluated by the rotarod test, pole test, cylinder test, corner test, and adhesive removal test. Long-term cognitive deficits were evaluated by the Morris water maze test.
Rotarod test.
The rotarod test was performed to assess poststroke motor functions (46). Briefly, mice were placed on a rotating drum (model 47650; Ugo Basile) with a speed accelerating from 5 to 40 rpm within 5 min. The time at which the mouse fell off the drum (latency to fall) was recorded. The test began 1 d before surgery and consisted of two trials. On the day of surgery, five trials were performed on each mouse and the mean of trials 3, 4, and 5 was used as the presurgery baseline value. After surgery, mice were tested for five trials on each testing day with intervals of 15 min, and the data for trials 3–5 were used to calculate the mean latency to fall on that day (Fig. 2A).
Pole test.
The pole test was performed as described previously (24). Briefly, a vertical steel pole (height: 50 cm; diameter: 0.9 cm) was placed into the home cage. The pole was covered with tape to create a rough surface. The mouse was placed with the head facing upward on the top of the pole. The total time that it takes the mouse to turn downward and reach the bottom of the cage with the front paws were recorded. The test consists of 3–4 trials on each testing day with intervals of 5 min, and the data were expressed as the mean value of 3–4 trials. Some poststroke mice (less than 15% overall) fell off the pole at the first test; these mice were given a second test immediately; if the mouse fell off again, the longer time it stayed on the pole in the two tests was recorded and included in data analysis. Presurgery training was performed for 3 d, and the mean value of the three trials on day 3 was used as presurgery baseline value. After surgery, mice were tested for up to 21 d (Fig. 2B).
Cylinder test.
The cylinder test was performed to assess forepaw use asymmetry after tFCI (46). The mouse was placed in a transparent cylinder (diameter: 9 cm; height: 15 cm), and videotaped from the top for 5 min. Videotapes were analyzed in slow motion by a rater, and forepaw (left/right/both) use during the first contact against the cylinder wall after rearing and during lateral exploration was recorded. Preference for the nonimpaired forepaw (left) was calculated as follows: (left − right)/(left + right + both) × 100% (Fig. 2C). Uninjured mice typically show no preference for either forepaw, whereas injured mice have increased left forepaw preference depending on the severity of the injury.
Corner test.
The corner test was performed as described previously (47). Briefly, two boards (30 × 20 × 1 cm each) were placed into the home cage of the mouse with their edges attached at a 30° angle. A small opening was present along the joint between the two boards to encourage entry into the corner. The mouse was placed between the boards facing the corner and halfway to the corner. Upon entering the corner, the vibrissae on both sides were stimulated simultaneously. The mouse then reared forward and upward, and turned back to face the open end. The turns in one vs. the other direction were recorded. Turning movements that were not part of a rearing movement were not counted. Uninjured mice typically show no preference for left vs. right turns. The injured animal turns preferentially toward the nonimpaired, ipsilateral side (the left side after left MCA occlusion). On each testing day, 10 trials were performed. Data were expressed as the percentage of right turns of the total 10 trials (Fig. 2D).
Adhesive removal test.
The adhesive removal test was performed as described previously (25) to assess the forepaw sensitivity and motor impairments. Briefly, an adhesive tape (0.3 × 0.4 cm) was applied to the right forepaw to cover the glabrous region. The time the mouse required to completely remove the adhesive tape (latency to remove) was recorded, with a maximum of 60 s (Fig. 7D). Mice were trained once daily for 5 d before surgery. After surgery, this test was performed daily for 35 d.
Morris water maze test.
The Morris water maze test was carried out 22–27 d after tFCI to evaluate long-term cognitive functions (Fig. 7E) (9). Briefly, a square platform (11 × 11 cm) was submerged in a pool (diameter: 109 cm) of opaque water. To examine the spatial learning ability, the mouse was placed into the pool from one of the four locations and allowed to swim for 60 s to locate the hidden platform. The time at which the animal found the platform (latency to escape) was recorded for each trial. At the end of each trial, the mouse was placed on the platform or allowed to stay on the platform for 30 s with prominent spatial cues displayed around the room. Mice were pretrained for three consecutive days before MCAO (three trials on each day). Four trials were performed on each testing day for five consecutive days (22–26 d after tFCI). To evaluate spatial memory, a single 60-s probe trial was performed at 27 d after tFCI in which the platform was removed. The time the mouse spent in the goal quadrant where the platform was previously located was recorded and expressed as percentage of the total testing time of 60 s.
Immunohistochemistry and Image Analysis.
At multiple reperfusion time points after tFCI, mice were deeply anesthetized and transcardially perfused with 0.9% NaCl followed by 4% (wt/vol) paraformaldehyde in PBS. Brains were harvested and cryoprotected in 30% (wt/vol) sucrose in PBS, and frozen serial coronal brain sections (30 μm thick) were prepared on a cryostat (CM1900; Leica). Sections were blocked with 10% (vol/vol) donkey serum in PBS for 1 h, followed by overnight incubation (4 °C) with the following primary antibodies: rabbit anti-MAP2 (1:200; Santa Cruz Biotechnology), rabbit anti-NeuN (1:500; EMD Millipore), rat anti-CD31 (1:200; BD Biosciences), rat anti-F4/80 (Clone: BM8; 1:200; BioLegend), rabbit anti-MPO (1:50; Abcam), and rabbit anti-HA (1:1,000; Cell Signaling). After washing, sections were incubated for 1 h at room temperature with donkey secondary antibodies conjugated with DyLight 488 or Cy3 (1:1,000; Jackson ImmunoResearch Laboratories, Inc.). Alternate sections from each experimental condition were incubated in all solutions except the primary antibodies to assess nonspecific staining. F-actin was stained using Alexa Flour 488-conjugated phalloidin (1:2,000; Invitrogen). Nuclei were then counterstained with DAPI (Thermo Scientific) for 2 min at room temperature, mounted, and coverslipped with Fluoromount-G (Southern Biotech). Fluorescence images were captured with an inverted Nikon Diaphot-300 fluorescence microscope equipped with a SPOT RT slider camera and Meta Series Software 5.0 (Molecular Devices) or with an Olympus Fluoview FV1000 confocal microscope and FV10-ASW 2.0 software (Olympus America).
Infiltration of neutrophils and macrophages after tFCI was quantified by counting the numbers of cells immunolabeled for MPO and F4/80, respectively. Cells were counted in the periinfarct cortex at two coronal levels (bregma 0.2 and −0.5 mm). Images were processed by a blinded investigator with ImageJ for cell-based counting of automatically recognized cells. Cells were counted from three randomly selected fields in each brain section and expressed as mean number of cells per square millimeter.
3D analysis of cerebral microvasculature was done on CD31-stained brain sections as described previously (48). Six sections (30 µm thick) were analyzed for each brain, and six regions of interest (ROIs; 233 × 233 μm2) in the cerebral cortex or striatum were selected from each section. The ROIs were scanned at 512 × 512 pixels in the x–y direction, and 1-μm step-size optical sections along the z axis were acquired with a 40× objective lens. 3D reconstruction was performed using an image analysis software package (3D Doctor 3.5; Able Software). The vascular surface area (mm2) was calculated by the software, and the number of vascular branch points was counted in the 3D images by a blinded investigator.
Measurement of Brain Infarct.
In some mice, 2,3,5-triphenyltetrazolium (TTC) staining was performed to measure brain infarct after tFCI (17). Mice were killed and brains were harvested. The forebrain was sliced into seven coronal sections, each 1 mm thick. Sections were stained with 3% (wt/vol) TTC in 0.9% NaCl for 20 min, followed by fixation with 4% (wt/vol) paraformaldehyde in PBS. Infarct volume was determined with ImageJ as the volume of the contralateral hemisphere minus the noninfarcted volume of the ipsilateral hemisphere, by an observer blinded to experimental group assignment.
Alternatively, infarct volume or tissue atrophy was measured on seven equally spaced MAP2-stained sections encompassing the MCA territory using ImageJ. The actual infarct volumes with corrections for edema were calculated as the volume of the contralateral hemisphere minus the noninfarcted volume of the ipsilateral hemisphere. Tissue atrophy was calculated as the MAP2-positive volume of the contralateral hemisphere minus that of the ipsilateral hemisphere.
Assessment of BBB Permeability After tFCI.
The fluorescent tracer Alexa Fluor 555-conjugated cadaverine (0.95 kDa; Invitrogen) was injected through the femoral vein at a dose of 200 μg per mouse 60 min before sacrifice. To visualize the leakage of tracers through the impaired BBB, mice were killed and coronal brain sections (30 μm thick) were prepared as described above. To measure the extravasation of endogenous IgG, sections were blocked in avidin and biotin solution (two drops of avidin/biotin solution into 10 mL of PBS; Vector Laboratories) for 15 min each, followed by 5% (wt/vol) BSA for 1 h. Sections were then incubated with biotinylated anti-mouse IgG antibody (1:500; Vector Laboratories) at 4 °C overnight. Sections were then incubated with Alexa 488 Streptavidin (1:1,000; Jackson ImmunoResearch Laboratories). Sections were processed for detection of Alexa 555 fluorescence or IgG immunofluorescence. Whole-section images were acquired using an inverted Nikon fluorescence microscope. Six equally spaced sections encompassing the MCA territory were quantified for cross-sectional area of fluorescence. These areas were summed and multiplied by the distance between sections (1 mm) to yield a volume of leakage (in mm3). 3D plots were generated using the built-in Surface Plot function of ImageJ using the fluorescence luminance as height. Alternatively, high-power images were captured with an Olympus confocal microscope as described above.
Brain Microvessel Isolation, Subcellular Fractionation, and Western Blotting.
Protein isolation from brain tissues or cultured HBMECs was performed as described previously (49). Microvessels were extracted from mouse brains as described previously (50). Briefly, brain tissues were homogenized and centrifuged at 5,800 × g at 4 °C for 30 min. The pellet was resuspended and filtered by an 85-µm nylon mesh. The filtrate was then passed over a 3 × 4 cm glass bead column (0.45 mm glass beads) with a 44-µm nylon mesh at the bottom, and then washed. Microvessels, which adhered to the glass beads during washing, were recovered by repeated gentle agitation of the glass beads. The supernatant with microvessels was decanted and spun at 500 × g for 5 min, and the final pellet was stored at −80 °C until various biochemical assays were performed. To examine the distribution of TJ and AJ proteins in OGD-treated HBMECs or postischemic brain microvessels, the membrane fraction and ACF were prepared using the ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem) according to the manufacturer’s instructions. Fractions were then processed for Western blotting using standard SDS/PAGE and enhanced chemiluminescence detection reagents (GE Healthcare Biosciences) (49). Immunoreactivity was semiquantitatively measured by gel densitometric scanning and analyzed with ImageJ. Antibodies against the following proteins were used: rabbit anti-occludin (1:1,000; Invitrogen), rabbit anti–VE-cadherin (1:1,000; Abcam), rabbit anti–ZO-1 (1:250; Abcam), rabbit anti-HSP27 (1:1,000; Abcam), rabbit anti-moesin (1:250; Abcam), rabbit anti–phospho-ADF/cofilin (Ser3; 1:1,000; Cell Signaling), mouse anti–phospho-MLC (Ser19; 1:1,000; Cell Signaling), rabbit anti-CD31 (1:1,000; Abcam), rabbit anti–α-tubulin (1:1,000; Abcam), and mouse anti–β-actin antibody (1:2,000; Sigma-Aldrich).
In Vitro BBB Model.
Primary HBMECs were purchased from Cell Systems (ACBRI 376). HBMECs were grown in Clonetics EGM-2 MV medium (CC-3202; Lonza), and only up to eight passages were used for experiments. The in vitro BBB model was established in cell culture inserts as described previously (9). The Transwell PET membranes (0.4 µm pore, 11 mm diameter; Corning) were coated with collagen (15 μg/mL) and fibronectin (30 μg/mL). HBMECs were seeded onto the membrane at a density of 2.5 × 105 cells per membrane (Fig. 3A). Cultures were maintained in medium at 37 °C in humidified 95% (vol/vol) air and 5% (vol/vol) CO2 for 4 d to reach confluence. To assess paracellular permeability, Alexa 555 cadaverine (0.95 kDa), TRITC–dextran (4.4 kDa; Sigma-Aldrich), or FITC–dextran (70 kDa; Sigma-Aldrich) was added into the luminal chamber at a concentration of 1 µM in 300 µL media. Fluorescence intensity was measured with a fluorescence reader at 1, 2, 3, 4, and 6 h after OGD using 30 µL medium from the lower (abluminal) chamber where medium was replaced with fresh medium (700 µL) 30 min before each measurement. The concentrations of tracers in samples were calculated from a standard curve fitted using known concentrations of tracers. Next, 30 µL fresh media was added after each reading. The diffusion rate of tracers from the luminal to the abluminal chamber was determined and expressed as pmol⋅mm2⋅min−1.
Lentiviral Vectors for Gene Overexpression or Knockdown.
Lentiviral vectors were constructed overexpressing full-length human HSP27 (Lenti-HSP27) as described (17). Briefly, the HA-tagged cDNA was inserted into the lentiviral vector pFPWS under the control of the cytomegalovirus promoter. The constructed transfer vectors were transformed into Stbl3 Escherichia coli and then isolated using the EndoFree Plasmid Maxi Kit (Qiagen). Large-scale production of the virus was achieved as described previously (17). The HBMEC cultures were infected with Lenti-HSP27 or the control empty vector. The overexpression of HSP27 was confirmed by Western blot analyses. Experiments were performed 72 h after vector infection.
Oxygen–Glucose Deprivation.
To model ischemia in vitro, cultured HBMECs were exposed to transient OGD for 60 min as described previously (17). Briefly, culture medium was replaced with glucose-free medium, and cultures were placed in a Billups–Rothenberg modular incubator chamber (Del Mar), which was flushed for 5 min with 95% (vol/vol) argon and 5% (vol/vol) CO2 and then sealed. The chamber was placed in a water-jacketed incubator (Forma; Thermo Fisher Scientific) at 37 °C for 60 min and then returned to 95% (vol/vol) air, 5% (vol/vol) CO2, and glucose-containing medium (reperfusion) for a period indicated in each experiment. Control glucose-containing cultures were incubated for the same period at 37 °C in humidified 95% (vol/vol) air and 5% (vol/vol) CO2.
Cell Viability Assay.
Cultured HBMECs were assessed for metabolic viability after OGD using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were incubated with 0.5 mg/mL of MTT at 37 °C for 2 h. Medium was then carefully removed and 100 µL of dimethylformamide was added into each well for 1 h to dissolve the resulting dark blue crystals. Absorbance was measured at 570 nm (OD570) with a Universal Microplate Reader (Elx800; BioTek Instruments).
Immunocytochemistry.
HBMECs grown on collagen-coated coverslips in 24-well culture plates were fixed with 4% (wt/vol) paraformaldehyde followed by blocking with donkey serum in 0.3 M glycine in PBS for 1 h at room temperature. The cells were then incubated with the following primary antibodies overnight at 4 °C: mouse anti–phospho-MLC (Ser19, 1:200; Cell Signaling), rabbit anti–VE-cadherin (1:200; Abcam), rabbit anti-HA (1:500; Abcam), or rabbit anti–ZO-1 (1:100; Cell Signaling). After rinses in PBS, cells were incubated with donkey secondary antibodies conjugated with DyLight 488 or Cy3 (1:1,000). F-actin was stained using Alexa Fluor 488-conjugated phalloidin. Cells were then counterstained with DAPI for nuclear labeling. After PBS rinses, coverslips were mounted on glass slides with antifade VectaShield solution (Vector Laboratories). Fluorescence images were captured with a confocal microscope. Immunofluorescence intensities of VE-cadherin and ZO-1 at the cell–cell interfaces were quantified by ImageJ. Three ROIs were randomly selected for each coverslip (one coverslip per well), and two or three coverslips were quantified for each experimental condition in each independent culture. 3D surface plots were created from fluorescence images using the Interactive 3D Surface Plot plugin in ImageJ, where the luminance of an image is represented by height of the plot (Fig. 4E).
Flow Cytometry.
At 48 h after tFCI, mice were deeply anesthetized and transcardially perfused with 0.9% NaCl. Brains were harvested and hemispheres separated. Cell suspensions were prepared from each hemisphere using the Neural Tissue Dissociation Kit (Miltenyi Biotec) and gentle MACS Octo Dissociator with Heaters (Miltenyi Biotec) according to manufacturer’s instructions. The suspension was passed through a 70-μm cell strainer, resuspended in 40 mL of complete RPMI 1640, and centrifuged at 2,000 × g for 10 min at 4 °C. Cells were fractionated on a 30% (vol/vol) and 70% (vol/vol) Percoll gradient (GE Healthcare Bio-Sciences) at 500 × g for 30 min. The mononuclear cells at the interface were washed before staining. Isolated cells were resuspended at 1 × 106 per mL and stained with anti-mouse CD11b, CD45, Ly6G, and the appropriate isotype controls following the manufacturer’s instructions (eBioscience). Briefly, cells were stained with antibody mixtures for 30 min at 4 °C and washed with HBSS (Sigma-Aldrich) containing 1% FBS (Sigma-Aldrich) before further analysis. Flow cytometry was performed using a fluorescence-activated cell sorter (BD Biosciences). Data analysis was performed using FlowJo software (FlowJo, LLC).
Proteomic Array Analysis.
Brain extracts were prepared from the ipsilateral hemisphere at 48 h after tFCI or sham operation as described above. The contents of 32 cytokines in the microvessel extracts were measured using a ChemiArray Mouse Antibody Array Kit (Chemicon) according to the manufacturer’s instructions: IL-5, chemokine ligand 12 (CCL12), IL-6, CCL3, IL-9, chemokine ligand 2, IL-10, CCL19, CCL21, IL-12, CCL5, CCL27, IL-12 p70, stem cell factor, eotaxin, IL-13, granulocyte-colony stimulating factor, IL-17, CCL17, GM-CSF, IFN-γ, tissue inhibitor of metalloproteinases-1, IL-2, CXCL1, TNF-α, soluble TNF receptor 1, IL-3, leptin, thrombopoietin, IL-4, CCL2, and VEGF. The concentrations of various cytokines were normalized to levels of sham brains and data expressed as fold changes.
F-Actin/G-Actin Assay.
Actin polymerization in HBMEC cultures and mouse brain microvessels was evaluated using the G-actin/F-actin In Vivo Assay Biochem Kit (Cytoskeleton, Inc.) according to manufacturer’s instructions. Briefly, lysate was collected from cultured HBMECs or isolated brain microvessels using the Lysis and F-actin Stabilization Buffer provided in the kit. F-actin and G-actin were separated by centrifugation at 100,000 × g for 1 h at 37 °C. The supernatant containing G-actin was collected, and the F-actin in the pellets was depolymerized to the globular form by the F-actin Depolymerization Buffer. Samples from both the G- and F-actin components were examined by Western blotting with anti-actin antibodies as described above.
In Vitro Migration Assay.
Transmigration of immune cells across the BBB was measured in vitro (Fig. S8) using the CytoSelect Leukocyte Transmigration Assay Kit (Cell BioLabs) according to the manufacturer’s instructions. Briefly, HBMECs were seeded into the migration insert provided in the kit at a density of 6 × 105 cells per insert and allowed 48 h to form a confluent monolayer. Neutrophils were extracted from the blood of post-tFCI mice using the Neutrophil Isolation Kit (Miltenyi Biotec), and macrophages were extracted from blood and spleen using CD11b MicroBeads and magnetic cell sorting (Miltenyi Biotec) according to manufacturer’s instructions. HBMECs were subjected to 100 min of OGD, a predetermined optimal duration that induces robust transmigration of neutrophils and macrophages across the HBMEC barrier within 6 h of cell incubation. Migratory cells (neutrophils or macrophages) were labeled with the fluorochrome LeukoTracker (Cell BioLabs) and plated in the upper chamber of the migration insert containing HBMECs at a density of 3 × 105 neutrophils per insert or 5 × 105 macrophages per insert. Neutrophils and macrophages were allowed to migrate for 6 h, and nonmigratory cells were removed. The migration of labeled neutrophils or macrophages from the upper to the lower chamber was quantified by measuring the fluorescence intensity of cell lysates collected from the lower chamber. Fluorescence was measured with a spectrofluorometer at 480/520 nm and expressed as relative fluorescence units.
Discussion
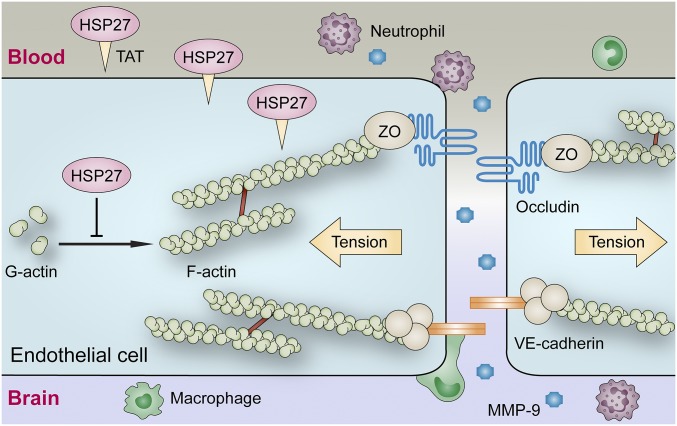
The present study investigated the protective role of endothelium-targeted overexpression of HSP27 against BBB damage in a rodent model of brain I/R (Fig. 8). The results demonstrate that HSP27 overexpression in ECs attenuates I/R-induced early onset BBB disruption, presumably by inhibiting actin polymerization and the formation of force-generating, contractile stress fibers that lead to translocation of TJ and AJ proteins. By preserving BBB integrity after I/R, HSP27 reduces the recruitment of tissue destructive circulating neutrophils and macrophages into the brain parenchyma, thereby eliciting long-term protection against neurological deficits. The results also provide preclinical evidence that post-I/R administration of a cell membrane-permeable HSP27 fusion protein is effective in ameliorating BBB damage and improving long-term stroke outcome.
Fig. 8.
Proposed mechanisms underlying HSP27-afforded protection of the BBB after cerebral I/R injury. HSP27 robustly inhibits I/R-induced actin polymerization and stress fiber formation in brain microvascular ECs, which reduces cellular tension from stress fibers that may cause disassembly and internalization of junctional proteins. After I/R, translocation of TJ and AJ proteins from cell–cell contacts to the cytosol opens the BBB and increases paracellular permeability to small macromolecules (less than 3 kDa). Subsequently, permeability to larger molecules increases. By fortifying the BBB, HSP27 blocks the infiltration of peripheral immune cells (not shown to scale) and reduces secondary brain injury, leading to sustained improvements in histological and neurological outcomes. Intravenous administration of a cell-penetrating TAT-HSP27 fusion protein results in rapid uptake of HSP27 into ECs and exerts similar protection as transgenic HSP27 overexpression against I/R-induced neurovascular injury.
HSP27 is a unique stress-responsive protein that can serve as a dynamic signaling molecule under various insults (14). For example, HSP27 can inhibit apoptosis by reducing caspase activity (33), decrease cytochrome c release from mitochondria (34), and/or inactivate the apoptosis signal-regulating kinase 1/JNK prodeath signaling cascade (17). A number of previous studies reported the neuroprotective effects of HSP27 against cerebral I/R injury when globally expressed in the brain (15–17, 22). However, the anti-cell death properties of HSP27 are unlikely to directly contribute to protection against BBB leakage 1–3 h after I/R, because cell death is not apparent until some hours after this early stage in postischemic ECs (Fig. S4). Moreover, the expression of endogenous HSP27 is induced in postischemic brain mainly in nonneuronal cells, glia (18) as well as ECs (22), raising the possibility that HSP27 works through additional mechanisms. Our results show that HSP27 overexpression specifically targeted to ECs not only reduces I/R-induced BBB disruption but also leads to long-term parenchymal protection and superior neurological outcomes. In contrast, neuronal overexpression of HSP27 fails to reduce I/R-induced BBB disruption or prevent parenchymal damage beyond the 24-h time point after tFCI (Figs. 1 and 2). These observations suggest that the nonneuronal actions of HSP27 in ECs contribute to its overall protective effects against I/R injury.
Two important features of brain ECs, including the high sealing efficiency via paracellular junctions and relatively low transcytosis rate, help maintain the low permeability of the BBB to cerebrospinal fluid and blood components (35). Soon after I/R, there are increases in transcellular trafficking and paracellular permeability via MMP-independent mechanisms (8, 9). Transcellular efflux is increased as early as 6 h after I/R (8) and promotes caveolin-1–dependent transcytosis of blood albumin across the endothelial barrier. However, in the paracellular pathway of BBB disruption, which begins much earlier (at 0.5–1 h) after I/R (ref. 9 and present study), the aberrant subcellular redistribution of TJ proteins markedly reduces the EC sealing efficacy (and movement of AJ proteins may contribute), thus allowing the passage of proinflammatory macromolecules of small sizes (≤3 kDa) from blood to brain and, likely, from brain to blood across the barrier as well. HSP27 may inhibit the paracellular pathway of BBB impairment by preventing the endothelial subcellular redistribution of TJ and, possibly, AJ proteins after I/R injury. It remains to be determined whether HSP27 also affects the transcellular pathway of BBB disruption, although a recent study does not support an important role for this pathway in the I/R model used here (9).
The present findings show that HSP27 blocks aberrant actin polymerization and stress fiber formation in ECs, the putative driving force behind opening of the paracellular pathway for early BBB disruption after I/R. A cortical actin rim is formed in ECs under physiological conditions, serving as an important anchor for cell–cell and cell–matrix adhesions (36). Fluid shear stress experienced by quiescent ECs provides chronic mild stimulation toward the formation of stress fibers, which may interrupt the cortical actin network and break intercellular junctions through centripetal tension when homeostasis is disrupted (11, 37). It is well established that HSP27 directly inhibits actin polymerization by capping the barbed end of actin (20), where rapid addition of actin monomers occurs. Additionally, HSP27 can sequester G-actin monomers (21). As a consequence of either action, G-actin cannot bind to the plus end of F-actin and be further aggregated by myosin to form contractile stress fibers. Endogenous actin regulatory proteins, such as ADF/cofilin, play an important role in balancing actin polymerization and depolymerization processes, thus preventing excessive formation of stress fibers (38). Soon after cerebral I/R, endothelial ADF/cofilin is phosphorylated at serine 3, becoming disabled (9). Meanwhile, the RhoA/ROCK signaling pathway is activated, which may further promote the aggregation and cross-linking of F-actin into force-generating linear stress fibers through the phosphorylation/activation of MLC (9, 11, 36, 39). Our data presented here show that HSP27 attenuates I/R-induced actin polymerization and stress fiber formation, but without influencing the phosphorylation of either ADF/cofilin or MLC (Fig. S6). These results are consistent with previous findings that HSP27 is a direct inhibitor of actin polymerization (20, 21).
Why does the attenuation of early BBB disruption by HSP27 lead to long-term brain protection against I/R injury? Our results demonstrate that EC-HSP27 reduces the infiltration of neutrophils and macrophages into the postischemic brain parenchyma after tFCI (Fig. 6). The fortified BBB may directly block the transmigration of these cells from blood to brain, or reduce the efflux of inflammatory signals from brain to blood that recruit the blood cells. These infiltrating reactive immune cells may contribute to the development of permanent neurovascular injury via at least three distinctive yet interrelated mechanisms. First, leukocytes are the main source of MMP-9 production in the brain after I/R (40), although several components of the postischemic neurovascular unit (ECs, neurons, and microglia) can also release MMP-9 (41). Whereas I/R-induced early BBB disruption (less than 3 h of postischemic reperfusion) does not require MMPs per se, MMP-9 contributes to the secondary BBB breakdown 3–24 h after I/R by degrading the junctional complexes and basal lamina (9). The widening of EC paracellular space appears to provide a breakthrough point that allows leukocyte-derived MMPs to gain access to their proteolytic cleavage sites on TJ and AJ protein (9). Second, the infiltrating peripheral immune cells may aggravate the proinflammatory responses of the postischemic brain, because many of the overproduced cytokines and reactive oxygen or nitrogen species are tissue destructive. Indeed, our results revealed that endothelial HSP27 overexpression not only decreases leukocyte infiltration but also attenuates the otherwise marked elevations in multiple cytokines after I/R (Fig. 6). Third, the excessive infiltration of peripheral immune cells into the postischemic brain may produce an unfavorable microenvironment that hinders brain repair processes. However, it is widely accepted that neurovascular remodeling may be facilitated by infiltrated leukocytes during the poststroke recovery phase (26). Thus, a complete blockage of immune cell infiltration could be harmful for brain recovery. In the present study, post-I/R immune cell infiltration was attenuated, but not completely blocked, in EC-HSP27 mice (Fig. 6); this may balance the harmful vs. beneficial actions of leukocytes. Together, the early microarchitectural changes in ECs that disrupt the BBB serve as a starting point for a vicious feedforward cycle in which infiltration of tissue-destructive peripheral immune cells recruits further cells, eventually culminating in progressive and irreversible neurovascular injury. By protecting against early BBB disruption, HSP27 prevents initiation of such a vicious cycle, thereby preventing secondary injury.
Proof-of-concept experiments were performed to test the efficacy of administration of a cell-permeable HSP27 protein against I/R injury. Intravenous injection of TAT-HSP27 resulted in rapid delivery of the protein into cerebral ECs, and led to not only early BBB protection but also long-term improvement of histological and functional stroke outcomes. These results hold translational potential from the perspective of developing an effective therapy for I/R brain injury. However, the TAT protein transduction domain does not show cell type specificity in facilitating protein delivery. It has been shown that TAT fusion protein can be delivered into the brain parenchyma within 4 h after systemic injection of the protein (42). Accordingly, our data cannot exclude the potential non-BBB effects of TAT-HSP27 that could contribute to its long-term efficacy against I/R injury (or lead to side effects in brain or other tissues). Further, it is unknown whether this treatment is effective after relatively prolonged cerebral ischemia, because a reperfusion event usually occurs 2–4 h after ischemic stroke in the clinic, such as in patients who receive tPA thrombolytic therapy. Nevertheless, future research is warranted to test the effect of TAT-HSP27 in stroke models that encompass tPA-induced reperfusion and also to determine the time window of efficacy of this therapeutic strategy.
In conclusion, the present study demonstrates profound BBB protection by endothelium-targeted overexpression of HSP27 after I/R brain injury. TAT-HSP27 should be further explored as a treatment for ischemic stroke, especially in conjunction with recanalization therapy.
Materials and Methods
Animals.
Transgenic mice that overexpress human HSP27 specifically in neurons were generated at the University of Pittsburgh Transgenic Core Facility. HSP27stop transgenic mice for conditional overexpression of HSP27 were generated at the Shanghai Research Center for Model Organisms (Shanghai, China) through a service contract. To obtain mice with EC-targeted overexpression of HSP27, HSP27stop mice were crossed with Tek-Cre mice (43), in which the Cre-mediated recombination is restricted to ECs. C57BL/6J mice were used as matched WT controls. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals (44). All efforts were made to minimize animal suffering and the number of animals used.
Transient Focal Cerebral Ischemia Model.
tFCI was induced for 1 h in adult male mice (8–10 wk old, 25–30 g) by intraluminal occlusion of the left middle cerebral artery (MCAO) (9). Surgeries and all outcome assessments were performed by investigators blinded to mouse genotype and experimental group assignments.
Assessment of BBB Impairment After tFCI.
BBB permeability was assessed by measuring the extravasation of an i.v.-injected fluorescent tracer (Alexa 555 cadaverine) and endogenous plasma IgG into the brain parenchyma. Coronal brain sections were processed for direct fluorescence detection of Alexa 555, or subjected to immunofluorescent labeling of IgG and detection. The brain volume with cadaverine or IgG leakage was calculated on six equally spaced brain sections encompassing the MCA territory (9).
In Vitro BBB Model.
Primary HBMECs were purchased from Cell Systems (ACBRI 376). The in vitro BBB model was established in cell culture inserts (9). To model ischemia in vitro, HBMECs were exposed to 1 h of OGD (17). Fluorescent tracers were added into the luminal chamber, and the diffusion rate of tracers from the luminal to the abluminal chamber was measured (9).
Statistical Analyses.
Data are presented as mean ± SEM. Statistical comparison of the means between two groups was accomplished by Student’s t test (two-tailed). Differences in means among multiple groups were analyzed using one- or two-way ANOVA followed by a Bonferroni/Dunn post hoc correction. A P value less than 0.05 was deemed statistically significant.
Acknowledgments
This project was supported by US Department of Veterans Affairs (VA) Merit Review BX002495 (to J.C.); NIH Grants NS089534, NS045048, and NS056118 (to J.C.) and NS036736 and NS095029 (to M.V.L.B. and J.C.); Chinese Natural Science Foundation Grant 81571285 (to Y.G.); the Shanghai Municipal Science and Technology Commission Support Program 14431907002 (to Y.G.); and American Heart Association Grant 15POST22260011 (to Y.S.). J.C. is the Richard King Mellon Professor of Neurology and a recipient of the VA Senior Research Career Scientist Award. M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and a recipient of the High-End Distinguished Professorship GDW20133100069 from the State Administration of Foreign Experts Affairs, China.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621174114/-/DCSupplemental.
References
- 1.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13) Suppl 1:S52–S57. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahi M, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21(19):7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32(9):3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27(4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 7.Krueger M, et al. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 2015;35(2):292–303. doi: 10.1038/jcbfm.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowland D, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger M, Härtig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One. 2013;8(2):e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burridge K, Wittchen ES. The tension mounts: Stress fibers as force-generating mechanotransducers. J Cell Biol. 2013;200(1):9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284(28):19053–19066. doi: 10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 14.Stetler RA, et al. Heat shock proteins: Cellular and molecular mechanisms in the central nervous system. Prog Neurobiol. 2010;92(2):184–211. doi: 10.1016/j.pneurobio.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An JJ, et al. Transduced human PEP-1-heat shock protein 27 efficiently protects against brain ischemic insult. FEBS J. 2008;275(6):1296–1308. doi: 10.1111/j.1742-4658.2008.06291.x. [DOI] [PubMed] [Google Scholar]
- 16.Badin RA, et al. Neuroprotective effects of virally delivered HSPs in experimental stroke. J Cereb Blood Flow Metab. 2006;26(3):371–381. doi: 10.1038/sj.jcbfm.9600190. [DOI] [PubMed] [Google Scholar]
- 17.Stetler RA, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. J Neurosci. 2008;28(49):13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stetler RA, et al. Phosphorylation of HSP27 by protein kinase D is essential for mediating neuroprotection against ischemic neuronal injury. J Neurosci. 2012;32(8):2667–2682. doi: 10.1523/JNEUROSCI.5169-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miron T, Wilchek M, Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur J Biochem. 1988;178(2):543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- 20.Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114(2):255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.During RL, et al. Anthrax lethal toxin paralyzes actin-based motility by blocking Hsp27 phosphorylation. EMBO J. 2007;26(9):2240–2250. doi: 10.1038/sj.emboj.7601687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leak RK, et al. HSP27 protects the blood-brain barrier against ischemia-induced loss of integrity. CNS Neurol Disord Drug Targets. 2013;12(3):325–337. doi: 10.2174/1871527311312030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boltze J, Lukomska B, Jolkkonen J. MEMS–IRBI Consortium Mesenchymal stromal cells in stroke: Improvement of motor recovery or functional compensation? J Cereb Blood Flow Metab. 2014;34(8):1420–1421. doi: 10.1038/jcbfm.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balkaya M, Kröber J, Gertz K, Peruzzaro S, Endres M. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Methods. 2013;213(2):179–187. doi: 10.1016/j.jneumeth.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Bouët V, et al. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203(2):555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 26.An C, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: Dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justicia C, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23(12):1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- 28.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 29.van Leeuwen M, et al. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28(1):84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 30.Greter M, Lelios I, Croxford AL. Microglia versus myeloid cell nomenclature during brain inflammation. Front Immunol. 2015;6:249. doi: 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11(5):366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 32.Schnoor M. Endothelial actin-binding proteins and actin dynamics in leukocyte transendothelial migration. J Immunol. 2015;194(8):3535–3541. doi: 10.4049/jimmunol.1403250. [DOI] [PubMed] [Google Scholar]
- 33.Voss OH, et al. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J Biol Chem. 2007;282(34):25088–25099. doi: 10.1074/jbc.M701740200. [DOI] [PubMed] [Google Scholar]
- 34.Bruey JM, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2(9):645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Zvi A, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77(1):53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franke RP, et al. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 38.Bamburg JR. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 39.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 40.Li P, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol. 2013;74(3):458–471. doi: 10.1002/ana.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosell A, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37(6):1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 42.Cao G, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J Neurosci. 2002;22(13):5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koni PA, et al. Conditional vascular cell adhesion molecule 1 deletion in mice: Impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193(6):741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 45.Fisher M, et al. STAIR Group Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gan Y, et al. Transgenic overexpression of peroxiredoxin-2 attenuates ischemic neuronal injury via suppression of a redox-sensitive pro-death signaling pathway. Antioxid Redox Signal. 2012;17(5):719–732. doi: 10.1089/ars.2011.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117(2):207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103. doi: 10.1016/j.nbd.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao G, et al. Intracellular Bax translocation after transient cerebral ischemia: Implications for a role of the mitochondrial apoptotic signaling pathway in ischemic neuronal death. J Cereb Blood Flow Metab. 2001;21(4):321–333. doi: 10.1097/00004647-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Yin KJ, et al. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30(18):6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]