Significance
Recent epidemiological studies report that lifelong bilingualism may delay dementia onset. However, the underlying neural mechanism of these protective effects is largely unknown. Using fluorodeoxyglucose and PET to investigate brain metabolism and neural connectivity in individuals with Alzheimer’s dementia, we unravel the neural mechanism responsible for the bilingual individuals’ ability to cope better with Alzheimer’s dementia. These findings foster the view that lifelong bilingualism contributes to brain cognitive reserve.
Keywords: bilingualism, Alzheimer’s dementia, fluorine-18-fluorodeoxyglucose PET, brain reserve, brain metabolic connectivity
Abstract
Cognitive reserve (CR) prevents cognitive decline and delays neurodegeneration. Recent epidemiological evidence suggests that lifelong bilingualism may act as CR delaying the onset of dementia by ∼4.5 y. Much controversy surrounds the issue of bilingualism and its putative neuroprotective effects. We studied brain metabolism, a direct index of synaptic function and density, and neural connectivity to shed light on the effects of bilingualism in vivo in Alzheimer’s dementia (AD). Eighty-five patients with probable AD and matched for disease duration (45 German-Italian bilingual speakers and 40 monolingual speakers) were included. Notably, bilingual individuals were on average 5 y older than their monolingual peers. In agreement with our predictions and with models of CR, cerebral hypometabolism was more severe in the group of bilingual individuals with AD. The metabolic connectivity analyses crucially supported the neuroprotective effect of bilingualism by showing an increased connectivity in the executive control and the default mode networks in the bilingual, compared with the monolingual, AD patients. Furthermore, the degree of lifelong bilingualism (i.e., high, moderate, or low use) was significantly correlated to functional modulations in crucial neural networks, suggesting both neural reserve and compensatory mechanisms. These findings indicate that lifelong bilingualism acts as a powerful CR proxy in dementia and exerts neuroprotective effects against neurodegeneration. Delaying the onset of dementia is a top priority of modern societies, and the present in vivo neurobiological evidence should stimulate social programs and interventions to support bilingual or multilingual education and the maintenance of the second language among senior citizens.
Many studies reported that cognitive activities and environmental factors, such as lifelong exposure to stimulating cognitive, social, and physical activities, as well as high socioeconomic status and educational and occupational attainments, provide a cognitive reserve (CR) potentially delaying dementia onset (1, 2). In vivo neuroimaging has provided important insights into the neural correlates of CR. For example, structural MRI studies in healthy aging consistently reported positive associations between CR and increased gray and white matter volumes in associative frontal and temporoparietal cortices (3), as well as reduced mean diffusivity (i.e., better integrity) in the bilateral hippocampi (4). Limited evidence exists on the protective role of CR in neurodegenerative diseases, such as Alzheimer’s dementia (AD), as shown by measures of cerebral metabolism with fluorodeoxyglucose and PET (FDG-PET), used as a measure of neuronal activity and viability (5, 6). FDG-PET offers the unique capability to measure resting-state brain metabolism, which is a direct index of synaptic function and density (7, 8).
In individuals with AD and mild cognitive impairment, higher education, and occupation (as proxies of reserve) correlated with more severe hypometabolism in temporoparietal areas and in the precuneus (9, 10) and, in addition, with increased metabolism in the dorsolateral prefrontal cortex, suggesting a compensatory mechanism against AD-related cerebral neurodegeneration (11).
To date, strong epidemiological evidence suggests that bilingualism may also contribute to CR (12). Crucially, older bilingual individuals manifest symptoms of AD significantly later than comparable monolinguals (13–15), with an approximate delay of 4.5 y. Furthermore, bilingual speakers also show significantly better cognitive recovery following stroke than monolinguals (16). As to causal mechanism, these protective effects may be a direct consequence of how the human brain has adapted to the “extra effort” provided by handling two or more languages (17, 18). The most important cognitive mechanism has been referred to as the “language control” mechanism (19). This language control device is considered part of the more general executive control system, and the extra use of this system in bilingual speakers may induce brain plasticity within the related cognitive control brain network (20). Structural neuroimaging studies have consistently reported increased gray and/or white matter densities for bilingual individuals in brain structures linked to executive control, such as the anterior cingulate cortex (ACC), the left prefrontal cortex, the left inferior parietal lobule, and the left caudate (for a review, see ref. 17). Of note, also in older healthy bilingual subjects, with many years of second language experience, recent structural neuroimaging studies reported increased white matter integrity (21) and gray matter volume in the anterior temporal lobes, orbitofrontal cortex (22), and inferior parietal lobules (23). Specifically for aging populations, this neural reserve may eventually protect against cognitive decline (24). An enhanced neural efficiency was also shown in bilingual seniors, with an increased functional connectivity in the frontoparietal network for executive control (ECN) and in the default mode network (DMN) (25), and increased neural efficiency in prefrontal and ACC regions (26).
Notwithstanding the epidemiological evidence of the effect of bilingualism in delaying dementia, its specific effects on the brain of patients with neurodegenerative dementia, such as AD, has not yet been investigated. The notion of bilingualism as a protective factor contributing to the CR is relatively new and not universally accepted (27, 28).
This research aimed at crucially contributing to the issue, by assessing the cerebral resting-state metabolic activity combined with connectivity analyses (29) in bilingual and monolingual individuals with AD. Our hypothesis is that bilingualism, acting as a protective factor, should contribute to the neural reserve through relevant neurobiological effects.
Results
Demographic Characteristics and Neuropsychology.
Descriptive statistics of demographic variables (means and SDs) and their comparison are reported in Table 1. Significant differences were found for age (P = 2.75 × e−7) and for education (P = 0.019) in bilingual compared with monolingual subjects. The Mini-Mental State Examination (MMSE) and Clinical Dementia Rating (CDR) scores did not differ between the two groups.
Table 1.
Means and SDs of the demographic characteristics and neuropsychological scores in bilingual and monolingual Alzheimer's disease patients and significance of t test in the between-groups comparisons
| Variables | Bilinguals (n = 45) | Monolinguals (n = 40) | P |
| Age, y | 77.13 ± 4.52 | 71.42 ± 4.88 | 0.00000027* |
| Male/female | 13/32 | 19/21 | — |
| Disease duration, y | <3 | <3 | — |
| MMSE | 22.40 ± 4.19 | 21.10 ± 4.84 | 0.19 |
| CDR | 0.89 ± 0.39 | 1.06 ± 0.48 | 0.20 |
| Education, y | 8.26 ± 4.55 | 10.5 ± 4.07 | 0.019* |
| BI | 0.74 ± 0.30 | — | — |
| Language production | 2.06 ± 1.12 | 2.03 ± 1.51 | 0.94 |
| Visuospatial short-term memory | 1.60 ± 1.48 | 0.58 ± 1.09 | 0.0035* |
| Verbal short-term memory | 1.46 ± 1.28 | 0.39 ± 0.62 | 0.000044* |
| Verbal long-term memory | 0.71 ± 0.89 | 0.08 ± 0.35 | 0.00041* |
| Attention | 1.20 ± 1.98 | 1.48 ± 1.43 | 0.33 |
See Table S3 for details on neuropsychological tests. —, not applicable.
Language production and attentional functions did not differ between the two groups, whereas visuospatial short-term memory, verbal short-term, and long-term memory were significantly more impaired in monolingual than in bilingual patients (Table 1 for details).
FDG-PET Comparisons.
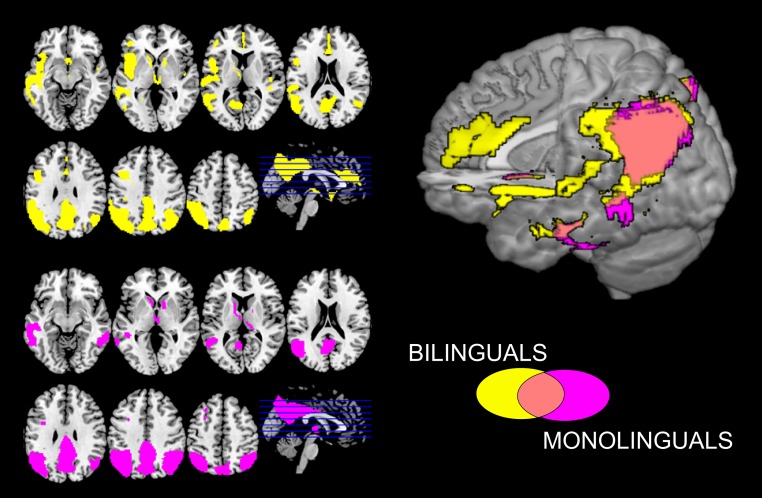
Analyses of brain hypometabolic structures revealed both similarities and differences between bilingual and monolingual individuals with AD. Specifically, both groups showed severe and extensive hypometabolism in temporoparietal associative cortices, as well as in the posterior cingulum and precuneus. In addition, hypometabolism affected in the left hemisphere also the middle and superior temporal cortex, the inferior frontal gyrus, the insula, and the ACC in the bilingual group only [all results are reported at P < 0.05 familywise error (FWE) correction for multiple comparisons; cluster extent (k) > 100]. Results from the statistical parametrical mapping (SPM) analyses were overlaid on the MRI standard template to illustrate commonalities and differences of cerebral hypometabolic patterns between the two groups (Fig. 1).
Fig. 1.
Brain hypometabolism in bilingual and monolingual patients with probable Alzheimer’s dementia. second level analysis depicting the commonalities and the differences in brain hypometabolism in bilingual and monolingual patients with Alzheimer’s dementia [P < 0.05 FWE; k = 100]. Images are displayed in neurological convention (the left side of the brain at left in the figure).
The second-level direct comparison between groups confirmed the more severe left hemispheric hypometabolism in bilingual, compared with monolingual, subjects in the inferior frontal gyrus and the operculum, the orbitofrontal cortex, the superior temporal gyrus, the inferior parietal lobule and operculum, the parahippocampal gyrus, the insula, the putamen, and the cerebellum. On the right hemisphere, there was a metabolic difference in the putamen and cerebellum [P < 0.05 false-discovery rate (FDR)] (Table S1).
Table S1.
Anatomical regions and standard MNI coordinates for the direct comparison between bilingual and monolingual subjects
| Decreased metabolism (bilinguals < monolinguals) | |
| Region | Coordinates |
| L frontal inferior operculum | [−52;4;6] |
| L inferior frontal gyrus | [−48;12;12] |
| L orbitofrontal cortex | [−6;14;−26] |
| L superior temporal gyrus | [−49;−11;−3] |
| L parietal operculum | [−56;−14;26] |
| L inferior parietal lobule | [−48;−32;30] |
| L insula | [−38;2;0] |
| L parahippocampal gyrus | [−16;−26;−16] |
| L putamen | [−23;−9;15] |
| L cerebellum | [−34;−42;−44] |
| R putamen | [26;−8;15] |
| R cerebellum | [32;−76;−26] |
L, left; R, right.
Correlations.
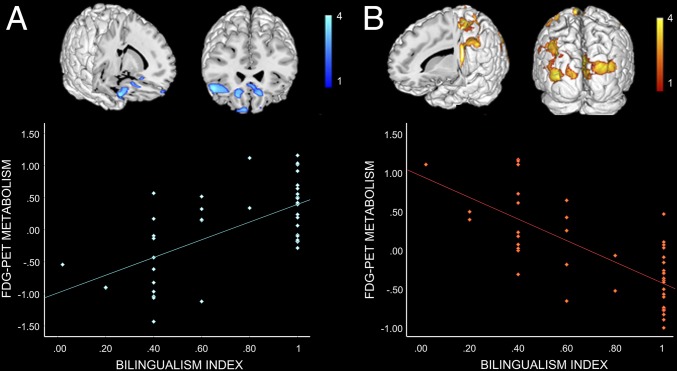
The bilingual index (BI) was associated to statistically significant positive and negative correlations with brain glucose metabolism (P < 0.005 uncorrected). Positive correlations were found in the right inferior frontal gyrus and bilaterally in the orbitofrontal cortex and left ACC (Fig. 2). Negative correlations were identified bilaterally in the precuneus and cuneus and in the left sensorimotor cortex and middle temporal gyrus (Fig. 2 and Table S2 for details).
Fig. 2.
Correlations between bilingualism index and brain metabolism. Positive (A) and negative (B) correlations between BI and FDG-PET glucose metabolism in the bilingual group (n = 45). All of the correlations are shown at P < 0.005 uncorrected for multiple comparison and k = 100. See also Table S2.
Table S2.
Anatomical regions and standard MNI coordinates for the correlation analysis between BI and brain glucose metabolism
| Positive correlation | Negative correlation | ||
| Region | Coordinates | Region | Coordinates |
| L orbitofrontal cortex | [−12;30;−18] | L precentral gyrus | [−40;−16;66] |
| L anterior cingulum | [−4;26;−10] | L middle temporal gyrus | [−40;−58;−4] |
| L precuneus | [−6;−48;80] | ||
| R inferior frontal gyrus | [50;24;−12] | L cuneus | [−6;−88;−33] |
| R middle orbitofrontal cortex | [6;66;−14] | ||
| R superior orbitofrontal cortex | [10;58;−24] | R postcentral gyrus | [50;−32;56] |
L, left; R, right.
FDG-PET Metabolic Connectivity.
ECN.
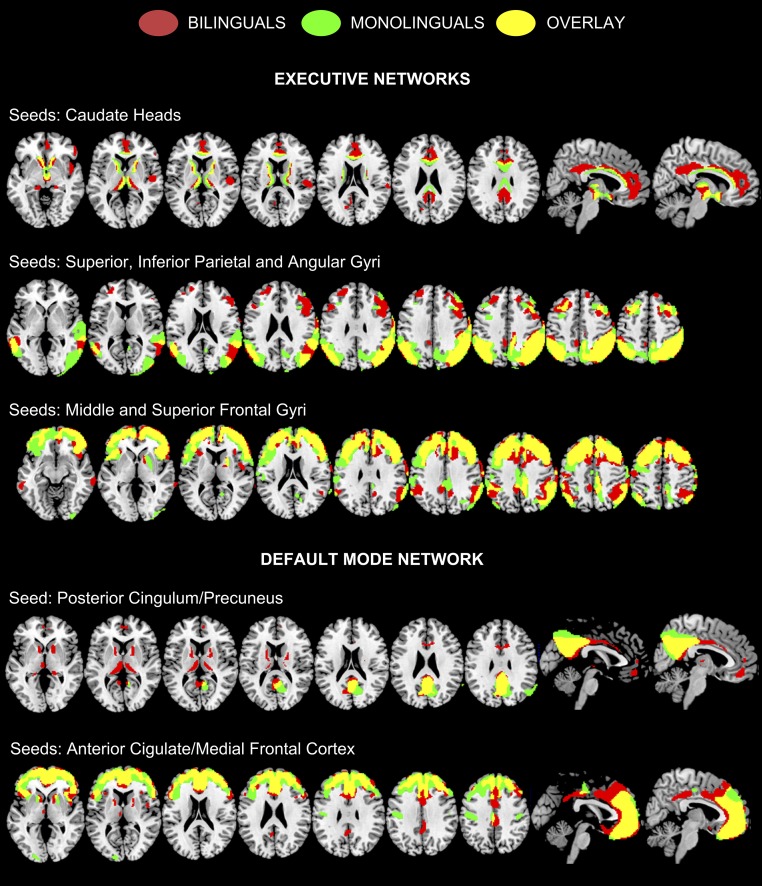
Consistent connectivity results were found for both AD groups in the seed regions. An increased anterior–posterior metabolic connectivity was found in the bilingual group. Using the bilateral posterior seeds (encompassing both the inferior and superior lobules as well as the angular gyrus), we found increased long-distance metabolic connectivity between these parietal areas and the dorsolateral prefrontal cortex in bilingual compared with monolingual individuals. A similar finding was evident when the seeds were located in the bilateral middle and superior frontal gyri, resulting in increased connectivity with the superior parietal cortices only in the bilingual group. Along similar lines, using bilateral caudate nucleus as seeds, increased connectivity was associated to an extensive network encompassing the anterior, middle, and posterior cingulate cortex, the right insula, the right inferior frontal gyrus, and the right parietal operculum, again only for bilinguals (Fig. 3).
Fig. 3.
Results of the metabolic connectivity analysis in the ECN and dorsal and anterior DMN. The seeds are indicated (Materials and Methods). All of the results are shown at P < 0.01 with False Discovery Rate for multiple comparisons and k = 100. Images are displayed in neurological convention (the left side of the brain is shown at left in the figure).
DMNs.
The resting-state whole-brain metabolic connectivity of the dorsal DMN showed major differences between bilingual and monolingual subjects. Specifically, significant metabolic correlations were found within the seed region (i.e., autocorrelation within the posterior cingulum/precuneus itself) in both bilingual and monolingual patients, whereas additional metabolic correlations encompassing the cingulate cortex, the orbitofrontal cortex, the caudate nucleus, and the thalamus, bilaterally were identified only in the bilingual group (P < 0.01, FDR). For the anterior DMN, significant metabolic connectivity was identified between the frontal seed regions (i.e., ACC and medial frontal cortex) and the posterior cingulum, again only in the bilingual group (Fig. 3).
Discussion
CR is a protective factor against age-associated cognitive decline and dementia. Recent epidemiological evidence suggests that lifelong bilingualism may act as a CR factor, delaying the onset of dementia by ∼4–5 y. No evidence exists, however, on the possible neural protective effects of bilingualism in AD. In the present study, we used FDG-PET to measure brain metabolism and connectivity to shed light on the neuroprotective effects of bilingualism. In agreement with theories of CR and with our predictions, cerebral hypometabolism was much more extended in bilingual individuals with AD in comparison with the monolinguals (Fig. 1 and Table S1). Despite the more severe pattern of brain hypometabolism, bilingual individuals with AD actually outperformed monolinguals on short- and long-term verbal memory and visuospatial tasks but not on language tasks (Table 1). The findings of better performance on memory tasks fit well with the general notion that healthy bilingual subjects may have an advantage over monolingual individuals in memory tasks and, in particular, in visuospatial memory tasks (30–32). The observed lack of differences on language tasks should be interpreted with caution. In language tasks, healthy bilingual subjects usually have more difficulties than monolingual subjects (33, 34), particularly with respect to lexical production, as reported in previous studies (e.g., ref. 35). Hence, the lack of differences for language tasks reported here could actually reflect the overcoming of a known disadvantage.
Overall, these findings strongly suggest that bilingual individuals with AD compensate better for the loss of brain structure and function. Of note, the BI [i.e., the relative use and exposure to a second language (L2)] correlated not only with more severe hypometabolism in several posterior brain regions but also with increased metabolism in the orbitofrontal, inferior frontal, and cingulate cortex, perhaps as a compensation mechanism (i.e., increased efficiency) for the severe brain hypometabolism observed in bilingual individuals with AD (Fig. 2 and Table S2). Further support for our assumption derives from the connectivity analysis, indicating compensation in the anterior frontal network underlying cognitive control and the presence of stronger connections in the ECN and DMN in bilingual individuals with AD.
Notably, in our patient cohort, there was a significant difference for age, with bilingual subjects being on average 5 y older, which is in line with recent findings (14) in larger cohorts of dementia cases. Overall, this protective bilingual effect was shown independently of other potential confounding factors, such as education, sex, occupation, and urban vs. rural dwelling of subjects (for a review, see ref. 12). It is also unlikely that the differences between bilingual and monolingual AD groups may be due to some demographic variables. All subjects belong to the same geographical area, namely Northern Italy, and the bilingual subjects in this series were older and had lower education than their monolingual peers (respectively, mean 77.13 y of age vs. 71.42 and mean 8.26 y of education vs. 10.5 y). Education and occupation are indeed among the main sources and proxies of CR (1), and our bilingual subjects could be expected to be at a disadvantage in terms of CR (1). Many studies reported that brain hypometabolism in AD is more severe in subjects with higher education because they can compensate longer with brain neurodegeneration (1, 10). Nevertheless, we observed a more severe hypometabolism and comparable or better cognitive performance in the bilingual group despite the significantly inferior years of education compared with the monolinguals. Our findings suggest that the effects of speaking two languages are more powerful than both age and education in providing a protection against cognitive decline.
We have recently advocated two distinct neural mechanisms to explain how bilingualism protects the aging brain, respectively, “neural reserve” and “neural compensation” (12). Following the former mechanism, lifelong use of two languages would result in structural changes in the brain such as increased gray and white matter densities in specific networks [i.e., those related to domain general executive functions (24) and language learning (36)]. Studies comparing older healthy bilingual subjects to matched monolinguals do, indeed, report that bilingual speakers have increased white matter density in the frontal lobes (21), in the ACC (37), the inferior parietal lobules (23), and the temporal pole areas (22, 38). The causative explanation for these structural modifications is that lifelong overuse of executive functions (i.e., to control two language systems to speak in one language without interference from the other) can induce plastic changes in the brain, resulting in a neural reserve that eventually renders the bilingual brain more resistant against brain aging effects.
The second mechanism is neural compensation, acting as the mechanism to overcome the loss of brain structure such as brain atrophy in aging or neurodegeneration. The suggested mechanism for compensation is that bilingualism is associated with stronger functional connectivity induced by the increased cognitive load on executive functions entailed by bilingualism (see ref. 25 for functional MR connectivity in older healthy bilingual subjects reporting stronger intrinsic functional connectivity in the frontoparietal control network). This stronger functional connectivity, in turn, renders the brain capable of coping better also with neurodegeneration and the loss of neurons such as in dementia (i.e., the bilingual brain better compensates) (12). To corroborate this hypothesis, we performed a metabolic connectivity analysis on the FDG-PET data on two distinct networks: the ECN and the DMN. Notably, there was increased metabolic connectivity both in the frontoparietal ECN and in DMN, restricted only to bilingual subjects. The DMN showed increased connectivity between the posterior cingulum and subcortical structures (i.e., the thalamus and the caudate nucleus bilaterally) and the anterior cingulum, all pivotal brain structures for language control in bilingual subjects (19, 39). As for the ECN, we revealed for bilingual individuals connectivity increases both in frontoparietal networks, bilaterally, and in a specific network encompassing several regions for cognitive and language control, such as the cingulate cortex, the inferior frontal gyrus, the parietal operculum, the insula, and the caudate nucleus, more evident on the right hemisphere (18). This asymmetry may reflect a compensatory mechanism for the dysfunctional involvement of the language dominant hemisphere.
The selective connectivity patterns found for both the DMN and the ECN in bilingual subjects suggests a strong functional integration between these structures (40). In particular, the results supporting an increased anterior–posterior connectivity for bilingual compared with monolingual individuals with AD are in line with theories of brain compensation during healthy aging, such as the posterior to anterior shift in aging (41).
Previous evidence reported decreased connectivity in the DMN paralleled by enhanced resting-state functional connectivity in frontal regions in AD, likely in an attempt to maintain cognitive efficiency (42, 43). Our present resting-state metabolic connectivity findings are also compatible with the suggestion by Grady et al. (44) that recruitment of additional prefrontal regions in AD patients may reflect a compensative strategy to maintain cognitive functions. Our results highlight an increased connectivity in the anterior DMN in the bilingual AD group, suggesting not only that functional compensatory mechanisms in AD patients involve a posterior to anterior shift but also that this shift is more pronounced in bilinguals.
Increased functional connectivity was also recently reported by means of fMRI investigations in healthy older bilingual subjects, indicating a protective effect of bilingualism on white matter integrity (21, 25). Because bilingualism heavily relies on the constant control of two languages, the cerebral regions and connections responsible for such control become more tuned (12, 25). Here, we show that the strengthening of connections is still present in AD dementia as the direct result of lifelong bilingualism. The enhanced connectivity found in the ECN and DMN may represent a strong compensation mechanism, allowing bilingual individuals with AD to cope with dementia more efficiently compared with monolinguals. To further strengthen this hypothesis, our correlational analysis indicates that the BI correlated positively with glucose metabolism in frontal structures. In other words, those individuals who were more exposed to both languages had increased metabolism in frontal regions, which, in turn, may compensate for the neurodegeneration.
We suggest that both mechanisms (i.e., neural reserve and compensation) may explain the present and previous findings from retrospective studies with monolingual and bilingual patients with dementia compared for age of symptom onset. The 4- to 5-y delay of dementia onset for bilinguals from different populations, such as Canada (13), India (14), and Belgium (15) is approximately the difference of age found between the bilinguals and monolinguals in the present study.
Considering that bilingualism is a global phenomenon, and that half of the world is actually bilingual (45), it is highly unlikely that half of the world is protected against dementia. Crucially, what actually protects the brain may be specific types of bilingualism. We have elsewhere suggested that only those bilinguals with lifelong exposure to and use of both languages will have the maximum benefits (12, 22). The differences in variables related to bilingualism (e.g., low vs. high exposure and extent of use of a second language) may have crucial repercussions on building up a CR. Specifically for this purpose, in this study, we have created a BI, measuring the daily and long-life use of languages in our patient cohort. Consider a typical scenario in the world of globalization: many native speakers generally acquire a L2 for schooling and professional purposes (such as English worldwide or Mandarin in China). Usually, both languages are used regularly during professional lives, but once these individuals retire, they use the L2 less than their native language. We previously reported that in these aging populations, only those individuals who maintained high use of a second language showed the most significant neuroprotective effects (22). In the present study, we add further evidence in AD, showing that patients with a higher BI show better neural compensation.
In conclusion, the present FDG-PET brain metabolic study provides unique evidence of how lifelong bilingualism can protect the AD brain. Delaying the onset of dementia is a top priority of modern societies, and the notion that bilingualism acts as a powerful CR should stimulate governments and health systems to activate social programs and interventions to support bilingual or multilingual education.
Materials and Methods
Participants.
Eighty-five patients were selected from two centers: the San Raffaele Hospital in Milan (n = 40; 19 male and 21 female) and the Bozen Central Hospital (n = 45; 13 male and 32 female). All of the patients were diagnosed as probable AD according to the validated National Institute on Aging–Alzheimer’s Association consensus criteria (46) and were in the early disease stages (disease duration, <3 y). The clinical diagnosis was supported by extensive neuropsychological testing and by the presence of a cerebral hypometabolism pattern suggestive of AD, as semiquantitatively assessed at the single-subject level. It is of note that, despite both the participating centers being located in Northern Italy, bilingualism is rooted only in the city of Bozen for geographical and historical reasons. All bilingual individuals with AD permanently resided in Bozen and were German-Italian bilingual speakers. The native language (L1) was German in 30 cases and Italian in 15 patients.
Notably, a language background questionnaire derived from the Bilingual Aphasia Test (47) assessed the percentage of daily use and life exposure to each language (respectively, %L1 and %L2) in the bilingual group. Specifically, the following information was obtained by interviews with patients and their relatives: place of birth (country, rural district, or city); language spoken in the original family; the main language of education; language certification or language degree; language spoken with the partner; language in the environment of residence during lifetime or for many years; type of occupational attainment and its language demand; and amount of use of each language in various context in the daily living. Based on these data the BI was computed as follows:
Thus, the BI ranges from 0 (i.e., completely monolingual) to 1 (i.e., perfect bilingual who uses L1 and L2 for the same amount of time daily).
This study was approved by the San Raffaele Hospital scientific ethical committee. All patients provided written informed consent, following detailed explanation of the FDG-PET experimental procedure, and the study was performed in compliance with the Declaration of Helsinki.
Neuropsychology.
All patients were fully evaluated for cognitive impairments in the diagnostic workup for dementia assessment. Because different neuropsychological test batteries were applied, we used the equivalent scores (range: 0–4) for the tests assessing the same cognitive domain [i.e., language production, visuospatial short-term memory, verbal short-term and long-term memory, and attention functions: a standard procedure allowing the statistical comparisons for different tests developed by Capitani and Laiacona (48)] (Table 1, SI Text, Neuropsychology, and Table S3 for details). To test for differences in cognitive impairment, we compared the neuropsychological equivalent scores between bilingual and monolingual patients. The comparison was performed by means of a two independent sample t tests.
Table S3.
Neuropsychological tests used for the comparison between bilingual and monolingual AD patients in the considered cognitive domains
| Cognitive domain | Bilingual patients | Monolingual patients |
| Language production | RWT: formallexikalische Wortflüssigkeit (54) (phonemic verbal fluency test) | Test di fluenza verbale per lettere (55) (phonemic verbal fluency test) |
| Test di fluenza verbale fonemica (56) (phonemic verbal fluency test) | ||
| Short-term visuospatial memory | Test di Corsi/Test zur nonverbalen Merkspanne (56) (Corsi test) | Test di Corsi (56) (Corsi test) |
| Figura di Rey copia (57) (Rey figure copy) | ||
| Short- and long-term verbal memory | VLMT: Gesamtlernleistung (58) (verbal learning test) | Lista di parole di Rey (59) (Rey auditory verbal learning test) |
| Test di ripetizione seriale di parole (56) (verbal learning test) | Lista di parole di Mauri (60) (word list learning) | |
| Memoria di prosa/Wiedergabe eines Prosatextes (56) (short story) | Breve racconto (55) (short story) | |
| Attention | Matrizen test (61) (attentional matrices) | Matrici attentive (61) (attentional matrices) |
All patients were fully evaluated for cognitive impairments in the diagnostic workup for dementia assessment. To perform group comparison of cognitive impairment, we selected those neuropsychological tests that were comparable for the assessed domain (either in Italian or German language). For bilinguals, the choice of language for cognitive assessment and consequently of the test was done by the neurologists and neuropsychologists on the basis of language dominance and patients’ compliance. RWT, Regensburger Wortflüssigkeitstest (verbal fluency test); VLMT, Verbaler Lern- und Merkfähigkeitstest (verbal learning test).
FDG-PET.
Details regarding FDG-PET acquisition and preprocessing are reported in SI Text.
At the first level, each of the single-subject FDG images was analyzed by means of an optimized single-subject procedure (49, 50), ending in a comparison of each AD case to a large dataset of healthy controls (SI Text). The comparison generated single SPM t-maps showing regions of hypometabolism with a strong level of significance (P < 0.05 FWE correction for multiple comparisons). Each individual SPM t-Map was evaluated by neuroimaging experts (D.P., T.B., and M.M.) to check for the presence of AD-related brain hypometabolism. A second-level, voxel-wise, one-sample t test was applied to identify the brain hypometabolism patterns in the bilingual and monolingual groups. The analysis was run separately for the two groups. Subsequently, a second-level, whole-brain two-independent-sample t test was applied to directly compare contrast images of monolingual and bilingual cases to identify the between-groups differences. (See SI Text for analysis details.)
Correlation Analysis.
Whole-brain positive and negative correlations between brain glucose metabolism and the BI were tested by means of a voxel-level multiple regression on the entire bilingual group (n = 45). Single-subject contrast images were entered in the model setting the bilingualism index as a covariate of interest. In addition, also nuisance covariates were included into the statistical model: education, global cognitive status (i.e., MMSE scores), and equivalent scores of neuropsychological tests assessing four cognitive domains (i.e., verbal memory, visuospatial memory, language and attention functions). To allow an easier interpretation of results, the signs of the values in contrast images were reversed, so that a positive value represents an increase in glucose metabolism and a negative one a decrease. Hence, positive and negative correlations mean that increasing BI correlate respectively with an increase or a decrease in glucose consumption. The significance level was set at P < 0.005 uncorrected for multiple comparisons with a minimum cluster extent of ≥100 voxels.
FDG-PET Metabolic Connectivity Analysis.
We further carried out a brain metabolic connectivity analysis with the specific aim to investigate resting-state metabolic networks in both groups. The core assumption of this analysis was that brain regions whose glucose metabolism is correlated at rest are functionally associated (52). In this study, we applied the seed-based interregional correlation analysis using voxel-wise SPM procedure as described in Lee et al. (29), to investigate metabolic connectivity of the anterior and dorsal DMN and the bilateral ECN. First, seed regions were defined either from a functional atlas of resting state networks [as defined by Shirer et al. (53) (findlab.stanford.edu/functional_ROIs.html] or based on a priori hypotheses for the caudate nuclei (18, 19). Namely, the posterior cingulum and precuneus were considered as seeds for the dorsal DMN, and the anterior cingulum and medial frontal cortex for the anterior DMN. For the ECN, three seeds were considered bilaterally: the caudate nuclei, the superior and inferior parietal cortex, and the superior and middle frontal gyri. Then, intensity normalization to the global mean was applied on the warped and smoothed FDG-PET images, and mean FDG uptake was extracted from the seeds, separately for the monolingual and bilingual individuals. The extracted mean seed counts were set as variables of interest in a multiple regression model in SPM5, testing for voxel-level correlations with the whole brain metabolic activity in the two groups (P < 0.01, FDR correction for multiple comparisons; k > 100).
SI Text
Neuropsychology.
Not all neuropsychological tests are identical between the two groups because of regional differences in clinical assessment (i.e., Milan and South Tyrol use different testing batteries for standard dementia assessment). However, to perform group comparison of cognitive impairment, we selected those neuropsychological tests that were comparable for the assessed domain (either in Italian or German language) (Table 1 and Table S3). For bilinguals, the choice of language for cognitive assessment and consequently of the test was done by the neurologists and neuropsychologists on the basis of language dominance and patients’ compliance.
FDG-PET Acquisition and Preprocessing.
All of the included patients underwent a FDG-PET imaging session using 3D PET/CT scans (Philips Gemini Time-of-Flight in Bozen and multiring GE Discovery STE in Milan). All subjects were fasted for at least 6 h and their measured blood glucose level before radiopharmaceutical injection of [18F]FDG was <120 mg/dL. Scan static acquisition started 45 min after the injection and lasted for 15 min. Image reconstruction was performed by means of an ordered subset expectation maximization algorithm. The coregistered CT was used for attenuation correction of the signal and dedicated software integrated in the PET scans was used for scatter correction.
FDG-PET SPM Analysis.
Image preprocessing and first- and second-level analyses were run by means of the SPM software (SPM5; www.fil.ion.ucl.ac.uk/spm), implemented in MATLAB (MathWorks). At the first level, all of the AD patients were analyzed by means of an optimized single-subject procedure (49, 50). Briefly, FDG images were first spatially normalized to a dementia-specific FDG-PET template (50) and smoothed by means of an isotropic 3D Gaussian Kernel (full-width-half-maximum: 8 mm). Then, the warped and smoothed images entered a voxel-level statistical comparison with a large dataset of neurologically intact controls selected from the Nuclear Medicine San Raffaele Hospital and the European Alzheimer’s Disease Consortium (www.eadc.info) databases (49). Age was entered as nuisance variable in this analysis to exclude its effect.
To identify the specific areas of brain hypometabolism in bilingual and monolingual individuals with AD, a second level voxel-wise one-sample t test was applied on contrast images obtained from the first level single-subject assessment. The analysis was run separately for the two groups. The P value was set at P < 0.05 with FWE correction for multiple comparisons with a minimum cluster size of 100 voxels. Education was added as a nuisance variable in the model to account for the between-groups differences.
Subsequently, a second level whole-brain, two-independent-sample t test was applied to directly compare contrast images of monolingual and bilingual cases to identify the between-groups differences. Given that each first-level analysis uses a covariate estimated in healthy controls to correct for age-related differences, all of the resulting contrast images have this effect factored out (51). Education was also controlled for. Contrast weights in SPM were assigned to identify significant metabolic decreases in bilingual compared with monolingual individuals. To allow a reliable estimate of these differences, the significance level was set at P < 0.05 with FDR correction for multiple comparisons and minimal cluster extent was equal to 100 voxels.
Acknowledgments
We thank Prof. Stefano Cappa for the useful suggestions. This research was funded by the European Union Seventh Framework Programme (FP7) Imaging of Neuroinflammation in Neurodegenerative Diseases Project (FP7-HEALTH-201; Grant Agreement 278850) and the Italian Ministry of Health (Ricerca Finalizzata 2008 Conv 12: European Union Drug Regulating Authorities Clinical Trials 2011-004415-24 Clinical Trial “Molecular imaging for the early diagnosis and monitoring of Alzheimer’s disease in old individuals with cognitive disturbances”; Sponsor Protocol 09/2011 Molecular Imaging).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610909114/-/DCSupplemental.
References
- 1.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. 2013;17(10):502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenaza-Urquijo EM, et al. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage. 2013;83:450–457. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 4.Piras F, Cherubini A, Caltagirone C, Spalletta G. Education mediates microstructural changes in bilateral hippocampus. Hum Brain Mapp. 2011;32(2):282–289. doi: 10.1002/hbm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977;29(1):13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- 6.Perani D. FDG-PET and amyloid-PET imaging: the diverging paths. Curr Opin Neurol. 2014;27(4):405–413. doi: 10.1097/WCO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 7.Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283(5401):496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- 8.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25(12):621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 9.Perneczky R, et al. Schooling mediates brain reserve in Alzheimer’s disease: findings of fluoro-deoxy-glucose-positron emission tomography. J Neurol Neurosurg Psychiatry. 2006;77(9):1060–1063. doi: 10.1136/jnnp.2006.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garibotto V, et al. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71(17):1342–1349. doi: 10.1212/01.wnl.0000327670.62378.c0. [DOI] [PubMed] [Google Scholar]
- 11.Morbelli S, et al. Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: a European Alzheimer disease consortium project. J Nucl Med. 2013;54(6):894–902. doi: 10.2967/jnumed.112.113928. [DOI] [PubMed] [Google Scholar]
- 12.Perani D, Abutalebi J. Bilingualism, dementia, cognitive and neural reserve. Curr Opin Neurol. 2015;28(6):618–625. doi: 10.1097/WCO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 13.Bialystok E, Craik FIM, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45(2):459–464. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Alladi S, et al. Bilingualism delays age at onset of dementia, independent of education and immigration status. Neurology. 2013;81(22):1938–1944. doi: 10.1212/01.wnl.0000436620.33155.a4. [DOI] [PubMed] [Google Scholar]
- 15.Woumans E, et al. Bilingualism delays clinical manifestation of Alzheimer’s disease. Biling Lang Cogn. 2015;18(3):568–574. [Google Scholar]
- 16.Alladi S, et al. Impact of bilingualism on cognitive outcome after stroke. Stroke. 2016;47(1):258–261. doi: 10.1161/STROKEAHA.115.010418. [DOI] [PubMed] [Google Scholar]
- 17.Green DW, Abutalebi J. Language control in bilinguals: the adaptive control hypothesis. J Cogn Psychol (Hove) 2013;25(5):515–530. doi: 10.1080/20445911.2013.796377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abutalebi J, Green DW. Neuroimaging of language control in bilinguals: Neural adaptation and reserve. Biling Lang Cogn. 2016;19(4):689–698. [Google Scholar]
- 19.Abutalebi J, Green D. Bilingual language production: the neurocognition of language representation and control. J Neurolinguist. 2007;20(3):242–275. [Google Scholar]
- 20.Abutalebi J, Weekes BS. The cognitive neurology of bilingualism in the age of globalization. Behav Neurol. 2014;2014:536727. doi: 10.1155/2014/536727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luk G, Bialystok E, Craik FIM, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abutalebi J, et al. Bilingualism protects anterior temporal lobe integrity in aging. Neurobiol Aging. 2014;35(9):2126–2133. doi: 10.1016/j.neurobiolaging.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Abutalebi J, Canini M, Della Rosa PA, Green DW, Weekes BS. The neuroprotective effects of bilingualism upon the inferior parietal lobule : a structural neuroimaging study in aging Chinese bilinguals. J Neurolinguist. 2015;33:3–13. [Google Scholar]
- 24.Bialystok E, Abutalebi J, Bak TH, Burke DM, Kroll JF. Aging in two languages: implications for public health. Ageing Res Rev. 2016;27:56–60. doi: 10.1016/j.arr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grady CL, Luk G, Craik FIM, Bialystok E. Brain network activity in monolingual and bilingual older adults. Neuropsychologia. 2015;66:170–181. doi: 10.1016/j.neuropsychologia.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold BT. Lifelong bilingualism and neural reserve against Alzheimer’s disease: a review of findings and potential mechanisms. Behav Brain Res. 2015;281:9–15. doi: 10.1016/j.bbr.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawton DM, Gasquoine PG, Weimer AA. Age of dementia diagnosis in community dwelling bilingual and monolingual Hispanic Americans. Cortex. 2015;66:141–145. doi: 10.1016/j.cortex.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bak TH, Alladi S. Bilingualism, dementia and the tale of many variables: why we need to move beyond the Western World. Commentary on Lawton et al. (2015) and Fuller-Thomson (2015) Cortex. 2016;74:315–317. doi: 10.1016/j.cortex.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Lee DS, et al. Metabolic connectivity by interregional correlation analysis using statistical parametric mapping (SPM) and FDG brain PET; methodological development and patterns of metabolic connectivity in adults. Eur J Nucl Med Mol Imaging. 2008;35(9):1681–1691. doi: 10.1007/s00259-008-0808-z. [DOI] [PubMed] [Google Scholar]
- 30.Calvo N, Ibáñez A, García AM. The impact of bilingualism on working memory: a null effect on the whole may not be so on the parts. Front Psychol. 2016;7:265. doi: 10.3389/fpsyg.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerrigan L, Thomas MSC, Bright P, Filippi R. Evidence of an advantage in visuo-spatial memory for bilingual compared to monolingual speakers. Biling Lang Cogn. February 1, 2016 doi: 10.1017/S1366728915000917. [DOI] [Google Scholar]
- 32.Linck JA, Osthus P, Koeth JT, Bunting MF. Working memory and second language comprehension and production: a meta-analysis. Psychon Bull Rev. 2014;21(4):861–883. doi: 10.3758/s13423-013-0565-2. [DOI] [PubMed] [Google Scholar]
- 33.Bialystok E, Craik FIM, Green DW, Gollan TH. Bilingual minds. Psychol Sci Public Interest. 2009;10(3):89–129. doi: 10.1177/1529100610387084. [DOI] [PubMed] [Google Scholar]
- 34.Bialystok E, Craik F, Luk G. Cognitive control and lexical access in younger and older bilinguals. J Exp Psychol Learn Mem Cogn. 2008;34(4):859–873. doi: 10.1037/0278-7393.34.4.859. [DOI] [PubMed] [Google Scholar]
- 35.Gollan TH, Montoya RI, Fennema-Notestine C, Morris SK. Bilingualism affects picture naming but not picture classification. Mem Cognit. 2005;33(7):1220–1234. doi: 10.3758/bf03193224. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex. 2014;58:301–324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Abutalebi J, et al. Bilingualism provides a neural reserve for aging populations. Neuropsychologia. 2015;69:201–210. doi: 10.1016/j.neuropsychologia.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 38.Olsen RK, et al. The effect of lifelong bilingualism on regional grey and white matter volume. Brain Res. 2015;1612:128–139. doi: 10.1016/j.brainres.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 39.Abutalebi J, et al. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb Cortex. 2012;22(9):2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- 40.Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23(2):162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(Pt 5):1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, et al. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28(10):967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grady CL, et al. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. J Neurosci. 2003;23(3):986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grosjean F. Bilingual: Life and Reality. Harvard University Press; Cambridge, MA: 2010. [Google Scholar]
- 46.McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paradis M, Libben G. The Assessment of Bilingual Aphasia. Lawrence Erlbaum Associates; Mahwah, NJ: 1987. [Google Scholar]
- 48.Capitani E, Laiacona M. The Italian Group for the Neuropsychological Study of Ageing Composite neuropsychological batteries and demographic correction: Standardization based on equivalent scores, with a review of published data. J Clin Exp Neuropsychol. 1997;19(6):795–809. doi: 10.1080/01688639708403761. [DOI] [PubMed] [Google Scholar]
- 49.Perani D, et al. EADC-PET Consortium Validation of an optimized SPM procedure for FDG-PET in dementia diagnosis in a clinical setting. Neuroimage Clin. 2014;6:445–454. doi: 10.1016/j.nicl.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Della Rosa PA, et al. EADC-PET Consortium A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics. 2014;12(4):575–593. doi: 10.1007/s12021-014-9235-4. [DOI] [PubMed] [Google Scholar]
- 51.Friston KJ, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2(4):189–210. [Google Scholar]
- 52.Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab. 1984;4(4):484–499. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- 53.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aschenbrenner S, Tucha O, Lange KW. 2000. Regensburg Word Fluency Test [Regensburger Wortflüssigkeits-Test (RWT)]. (Hogrefe, Goettingen, Germany). German. [DOI] [PubMed]
- 55.Novelli P, Capitani L, Vallar C, Cappa S. Test di fluenza verbale. Archivio di Psicologia, Neurologia e Psichiatria. 1986;47:278–296. [Google Scholar]
- 56.Spinnler H, Tognoni G. Taratura e standardizzazione italiana di test neuropsicologici. Italian J Neurol Sci. 1987;8(Suppl 6):8–120. [PubMed] [Google Scholar]
- 57.Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A. Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol Sci. 2002;22(6):443–447. doi: 10.1007/s100720200003. [DOI] [PubMed] [Google Scholar]
- 58.Helmstädter C, Lendt M, Lux S. 2001 Verbaler Lern- und Merkfähigkeitstest: VLMT (Beltz Test GmbH, Goettingen). Available at https://www.psychologie.uni-freiburg.de/studium.lehre/klin-master/skripte/Vergangene_Semester/psychologische-diagnostik-m2-baumeister-SS2012/Test/VLMT. Accessed January 15, 2016.
- 59.Carlesimo GA, et al. The mental deterioration battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol. 1996;36(6):378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 60.Mauri M, et al. Standardizzazione di due nuovi test di memoria: Apprendimento di liste di parole correlate e non correlate semanticamente. Archivio di Psicologia Neurologia e Psichiatria. 1997;58:621–645. [Google Scholar]
- 61.Della Sala S, Laiacona M, Spinnler H, Ubezio C. A cancellation test: Its reliability in assessing attentional deficits in Alzheimer's disease. Psychol Med. 1992;22(4):885–901. doi: 10.1017/s0033291700038460. [DOI] [PubMed] [Google Scholar]