The rise of allergy and autoimmune diseases is due to much more than rampant cleanliness. Is it time to throw out the hygiene hypothesis?
Early exposure to microbes has important health effects, leading many researchers to question the value of the “hygiene hypothesis” label. Image courtesy of Shutterstock/Purino.
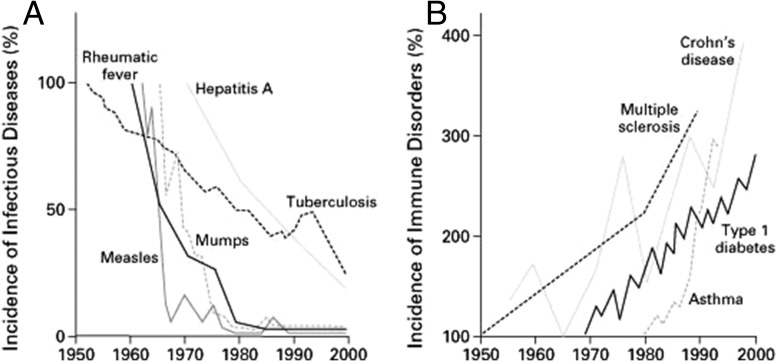
Graph the data points, and the trend is unmistakable. Since the 1950s, rates of multiple sclerosis, Crohn’s disease, type 1 diabetes, and asthma have soared by 300% or more (1). Similar graphs depict concurrent spikes in hay fever and food allergies (2).
Mirroring this alarming surge in autoimmune and allergic disorders are simultaneous sharp declines in the incidence of mumps, measles, tuberculosis, and other infectious diseases in developed countries, thanks to the advent of vaccines and antibiotics, and to improved hygiene. In the 1990s, scientists began to suspect that two trends were connected: Perhaps the reduction in infections was causing human immune systems to malfunction in some way.
That “hygiene hypothesis,” first proposed in 1989 (3), has become enshrined in popular culture: We’re too clean for our own good. It’s a straightforward, compelling idea. And many scientists are eager to see it thrown out.
“We know an awful lot now about why our immune system’s regulation is not in terribly good shape, and it’s got absolutely nothing to do with hygiene,” says Graham Rook, an emeritus professor of medical microbiology at University College London. Today, epidemiological, experimental, and molecular evidence support a different hypothesis: Early exposure to a diverse range of “friendly” microbes—not infectious pathogens—is necessary to train the human immune system to react appropriately to stimuli.
If this new hypothesis is true, then cutting back on personal hygiene will not have an impact on rates of chronic inflammatory and allergic disorders; it will, however, increase infections. The hygiene hypothesis is a “dangerous misnomer which is misleading people away from finding the true causes of these rises in allergic disease,” says Sally Bloomfield, chair of the International Scientific Forum on Home Hygiene and an honorary professor at the London School of Hygiene and Tropical Medicine. “I’ve even seen things in the media saying we shouldn’t wash our hands. What the hell are they talking about?”
Still, the catchy hygiene hypothesis continues to be widely embraced by the public, the media, and even scientists: Uses of the term in the scientific literature rose threefold over the past 10 years compared with the decade prior, according to a search on Thomson Reuters Web of Science. “In science, when something has been propagated for so long, it can be hard to change,” says Marsha Wills-Karp, chair of environmental health and engineering at Johns Hopkins Bloomberg School of Public Health. Even worse, because various changes in Western lifestyle are disrupting our exposure to microbes, it’s not easy to come up with an equally simple and appealing replacement theory. “The problem is, because it is so complicated, you can’t point to one particular thing and coin a phrase,” says Wills-Karp.
Nothing to Sneeze At
Prevalence of food allergy in preschool children is now as high as 10% in Western countries, but remains just 2% in areas like mainland China (4). The number of new cases of type 1 diabetes (T1D) in Finland per year is 62.3 per every 100,000 children, compared with just 6.2 in Mexico and 0.5 in Pakistan (5). Ulcerative colitis, a form of inflammatory bowel disease (IBD), is twofold higher in Western Europe than in Eastern Europe—6.5 per 100,000 people versus 3.1 per 100,000 (6).
In each of these disorders, either the immune system is overreacting to a trigger, such as pollen, peanuts, or pollution, or it’s attacking tissues it shouldn’t, such as beta cells in the pancreas in the case of T1D and in the intestines in IBD.
These various disorders have one other thing in common: Their increasing incidence has occurred almost exclusively in developed and rapidly developing countries. A small part of that increasing incidence may have to do with cases going undiagnosed in less developed areas, but that cannot account for most of the gap.
In 1989, David Strachan, an epidemiologist at the London School of Hygiene and Tropical Medicine, made an observation: In a survey of more than 17,000 British children, he noted that infants born into a household with many siblings were less susceptible to eczema in the first year of life, and to hay fever later in life (3). Assuming that more children in a house means more germs shared, Strachan proposed that early childhood infections protect against allergic disease. He used the word “hygiene” in the title of his paper—nowhere else—but it was enough. The hygiene hypothesis was born.
The media and scientific community loved the idea, and it was soon extrapolated beyond family size to include other modern changes in personal hygiene. Based on the known immunological underpinnings of allergy and asthma, the hypothesis soon also had a molecular mechanism: Bacteria and protozoa infections activate T helper 1 (Th1) cells of the immune system, which release signaling molecules called cytokines. Reduced contact with infectious agents therefore reduces Th1 activity in the body, resulting in a compensatory increase in the activity of T helper 2 (Th2) cells, a hallmark of allergic disorders (7). So a lack of infections during childhood decreases one’s Th1 activity, causing an increase in Th2 activity and an increased risk of allergic disease.
However, once researchers began looking past allergies, the hypothesis began to show cracks. First were the parasites. Helminth infections—common in developing countries but not in the industrialized world—are associated with decreased allergic disease, and are even protective against it in some animal models (8). However, these parasitic worm infections are also characterized by high levels of Th2 activity.
Maria Yazdanbakhsh at Leiden University Medical Center was studying helminth infections in developing nations at the time the hygiene hypothesis mechanism was proposed. “We thought, ‘How is that possible?’ In Africa, there was plenty of Th2 but no allergic disorders in any of the villages where we were working,” she says. In a series of experiments, Yazdanbakhsh’s team discovered that long-term helminth infection causes a rise in antiinflammatory molecules, such as interleukin-10, which is inversely correlated with allergy (9). In this case, a persistent immune challenge by an infectious agent leads to a robust immune system that doesn’t overreact.
Another apparent flaw in the hypothesis stemmed from studies of autoimmune disorders and IBD, showing that both are mediated by an increase in Th1 activity, rather than by a decrease. Then, epidemiological studies began to break down the link between disease-causing germs and reduced risk of allergy: Measles and many respiratory diseases proved not to be protective against allergic disease, and, in many cases, even increased the risk (10).
The inverse relationship between (A) infectious disease incidence and (B) the rates of immune disorders suggested that a reduction in infections might be causing the human immune system to malfunction. But the idea, popularized in the 1990s, has fallen out of favor. Reprinted with permission from ref. 1.
Baby’s First Bugs
In 2003, Graham Rook and colleagues proposed a new explanation for the rise of immune disorders, which Rook called the “old friends” hypothesis (11). “We realized human beings coevolved with a whole host of organisms, and it was far more likely what was going on was that we were being deprived of organisms on which we are dependent,” says Rook.
The hypothesis suggests that early and regular exposure to harmless microorganisms—“old friends” present throughout human evolution and recognized by the human immune system—train the immune system to react appropriately to threats. It’s not that children in developed countries aren’t subject to enough infections when they are young, but that their exposure to the microbial world is far more circumscribed than it once was.
In the decades since the hygiene hypothesis was formed, it has become increasingly clear that the personal microbiome plays an active role in human health, starting before we’re born and continuing throughout life. Maternal microbes colonize the human gut while babies are in utero (12), and again as they pass through the birth canal and start breastfeeding. Young children continue amassing microbiota in every contact with family members, while playing outside in dirt, getting licked by dogs, and sharing toys with friends. The developing immune system takes cues from all of these encounters.
Around the same time as Rook’s theory was taking shape, scientists discovered regulatory T cells (Treg), which dampen immune responses. Rook proposed that exposure to nonpathogenic microbes activates a variety of immune processes, including Treg cells, to regulate the immune system appropriately. So, with fewer old friends to learn from, our immune systems grow up to be trigger-happy.
Rook likens the immune system to a computer: It has software, but it needs data—in the form of exposure to a diverse set of microbes—to train it to identify threats appropriately. “It’s not about just learning what to attack, but learning what to tolerate,” says Bloomfield. “The problem comes when our immune system meets an allergen like pollen or peanuts and doesn’t know that is harmless.”
Hygiene did not stop playing a role in Rook’s hypothesis: It is likely that radical improvements in sanitation, food, and water in the late 20th century were involved in reducing our exposure to microbes. However, simultaneous changes in other factors most likely had an even larger influence, says Rook, especially in early life. Caesarean sections have been linked to increased risk of allergy and asthma; owning a pet or growing up on a farm is protective against them; and antibiotic use (which kills off both good and bad microbes) in youth has been linked to asthma, cow’s milk allergy, IBD, and eczema.
“We’re talking about a number of factors, not just one. It’s the diet, sanitation, antibiotic use, parasites, and more,” says Wills-Karp. “We’ve altered all of those simultaneously and overwhelmed the host's ability to modulate the immune system.”
Impoverished Gut
One of the largest studies to test the link between microbes and immune-regulatory disorders is the multicountry DIABIMMUNE study, which, beginning in 2008, followed families from three countries with close genetic backgrounds but clear differences in rates of asthma and T1D: industrialized Finland, with the highest global incidence of T1D; rapidly modernizing Estonia, with ever-increasing rates of T1D and asthma; and Russia, where both disorders are, comparatively, still rare.
By analyzing monthly stool samples from over 200 children from birth to age 3 years, the DIABIMMUNE team recently found that Finnish and Estonian infants have a distinct early gut microbiome compared with Russians. The intestines of the two former were chock full of Bacteroides species, whereas the latter hosted primarily commensal Escherichia coli. The outer membranes of both types of bacteria contain large molecules called lipopolysaccharides, or LPS, but, whereas E. coli LPS activates a potent response from the human immune system, Bacteroides LPS actually inhibits the immune system. So, compared with that of Russian infants, the gut immune system in Finnish and Estonian infants is silent, potentially making these children prone
“It's not about just learning what to attack, but learning what to tolerate.”
—Sally Bloomfield
to strong, unregulated immune reactions and disorders like T1D, the authors suggest (13).
“The gut microbiome has changed considerably between folks who live in underdeveloped countries and developed countries, and we’re beginning to hone in on some specific bacteria,” says Wills-Karp, who has led numerous studies identifying asthma susceptibility factors in the gut. “It still fits with the concept that there is some microbial exposure that used to protect us, and that we’ve lost.” For example, Western diets, lacking in plant fiber and other diverse foods that nourish commensal species, appear to disrupt the healthy microbiota in our gut (14).
From diet to antibiotics, each new factor contributing to our altered immunological state raises new questions. Individual genetics likely play a role, because it is still unclear why some people in modern, urban environments get allergic or autoimmune diseases, and others do not. The relationship between the timing of exposure and the onset of disease also remains a mystery. “By not being exposed to immune regulatory forces in childhood, then you’re more apt to develop an inflammatory process later in life,” says gastroenterologist Eran Israeli of Hebrew University, who has studied the role of hygiene in IBD. “But why later in life and why not in childhood? There are many more questions than answers.”
Rebranding
In February 2016, Rook, Bloomfield, and four other infectious and allergic disease experts gathered to come up with a consensus view on the shift in thinking since the hygiene hypothesis was proposed 27 years ago. They decided the name has to go (15).
“The trouble is, as soon as you use the words ‘hygiene hypothesis,’ the word hygiene prejudges what the cause is,” says Bloomfield. To the public, “hygiene” is interpreted as personal cleanliness: washing hands, keeping food clean and fresh, sanitizing the home. However, because the hypothesis has been largely uncoupled from infections, the idea that we need to be less hygienic is wrong. Relaxing hygiene standards would not reverse the trend but only serve to increase the risks of infectious disease, says Bloomfield. The term “hygiene hypothesis” also fails to incorporate all of the other factors now linked to the increase in immunoregulatory diseases.
But the call to abandon the original simplistic theory has fallen on deaf ears. Several researchers interviewed for this article said the issue was semantics, and they didn’t care what name is used. That makes Bloomfield crazy. “I don’t know what to do about it. I’ve tried and tried and tried,” she says. Bloomfield has taken to calling it the “hygiene hypothesis misnomer” or the “so-called hygiene hypothesis.”
Various research teams have proposed alternate names: microbiome depletion hypothesis, the microbial diversity hypothesis, and, of course, the old friends hypothesis. None have caught on. In an effort to at least change public behaviors, some experts now speak of “targeted hygiene”—eliminating the spread of pathogens while promoting steps to restore a diverse microbiome. For example, one can teach children to wash their hands after handling raw chicken but also encourage them to play outside in the dirt. “If your child has been out in the garden and comes in with slightly grubby hands, I, personally, would let them come in and munch a sandwich without washing,” says Rook.
Unfortunately, nuanced messages—such as “wash your hands sometimes, but not others,” or “use antibiotics, but only when needed”—can be difficult to communicate to the public. However, that communication will be key to reversing the rise in autoimmune and allergic disorders.
The second major concern among researchers is a lack of evidence demonstrating how to reduce rates of allergic and autoimmune diseases. Although there are hundreds of observational and epidemiological studies supporting a more nuanced theory that moves beyond the hygiene hypothesis, there are only a few randomized, controlled prospective studies testing interventions to reregulate the immune system. These include an experimental infection with helminths to treat IBD, which met with mixed results (16), and probiotics treatments for illnesses ranging from severe acute pancreatitis to eczema. In some cases, probiotics alleviated symptoms, but, in others, they had no effect (17).
Interventions with young children have been rarer. One pilot study published in 2016 swabbed babies delivered by C-section with gauze soaked in the microbe-rich fluid of their mother’s birth canal. During the first month of life, those infants had mouth, gut, and skin microbial populations that were more similar to babies delivered vaginally than to C-section babies who were not swabbed (18). But the study was small—only four C-section babies were swabbed—and the long-term effect on their immune regulation remains unknown. What's more, a recent study of 162 mother–infant pairs suggests that C-sections have no discernible effect on the infant microbiome by 6 weeks of age (19).
Instead of targeting environmental factors, researchers hope that, at some point, they will be able to identify which regulatory pathways train the immune system. “If we could find common pathways, we could adopt drugs or probiotics to activate [those pathways] to condition the immune system properly in early life,” says Wills-Karp. Early in life is the key: It is likely any intervention will need to be done by 3 or 4 years of age, by which time a child’s microbiome is established and the immune system has completed much of its training.
From an immunological standpoint, any therapeutic intervention also needs to be specific, Yazdanbakhsh emphasizes. “You don’t want to completely dampen the immune system in a nonspecific way,” she says, citing steroids as a case in point. Instead, individuals could be treated with a personalized microbial mixture that induces immune regulatory cells. “It’s a big challenge,” she adds, “but we need to start thinking about it.”
References
- 1.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills TA. The allergy epidemics: 1870-2010. J Allergy Clin Immunol. 2015;136(1):3–13. doi: 10.1016/j.jaci.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prescott SL, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1):21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetes Federation 2015 IDF Diabetes Atlas, 7th Ed, www.diabetesatlas.org/across-the-globe.html. Accessed January 20, 2017.
- 6.Burisch J, et al. EpiCom-group East-West gradient in the incidence of inflammatory bowel disease in Europe: The ECCO-EpiCom inception cohort. Gut. 2014;63(4):588–597. doi: 10.1136/gutjnl-2013-304636. [DOI] [PubMed] [Google Scholar]
- 7.Matricardi PM, Bonini S. High microbial turnover rate preventing atopy: A solution to inconsistencies impinging on the Hygiene hypothesis? Clin Exp Allergy. 2000;30(11):1506–1510. doi: 10.1046/j.1365-2222.2000.00994.x. [DOI] [PubMed] [Google Scholar]
- 8.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 9.van den Biggelaar AH, et al. Decreased atopy in children infected with Schistosoma haematobium: A role for parasite-induced interleukin-10. Lancet. 2000;356(9243):1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 10.Benn CS, Melbye M, Wohlfahrt J, Björkstén B, Aaby P. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. BMJ. 2004;328(7450):1223–1230. doi: 10.1136/bmj.38069.512245.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rook GAW, Martinelli R, Brunet LR. Innate immune responses to mycobacteria and the downregulation of atopic responses. Curr Opin Allergy Clin Immunol. 2003;3(5):337–342. doi: 10.1097/00130832-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Romano-Keeler J, Weitkamp JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77(1-2):189–195. doi: 10.1038/pr.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vatanen T, et al. DIABIMMUNE Study Group Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165(4):842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsnelson A. Core Concept: Prebiotics gain prominence but remain poorly defined. Proc Natl Acad Sci USA. 2016;113(50):14168–14169. doi: 10.1073/pnas.1618366113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloomfield SF, et al. Time to abandon the hygiene hypothesis: New perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health. 2016;136(4):213–224. doi: 10.1177/1757913916650225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmby H. Human helminth therapy to treat inflammatory disorders - where do we stand? BMC Immunol. 2015;16:12. doi: 10.1186/s12865-015-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease--follow-up at 8-9 years of age. Allergy. 2013;68(8):1015–1020. doi: 10.1111/all.12191. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez-Bello MG, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253. doi: 10.1038/nm.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu DM, et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017 doi: 10.1038/nm.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]