Significance
Taz (transcriptional coactivator with PDZ-binding motif) and Yap (yes-associated protein), the Hippo pathway effector proteins regulating cell fate, organ size, and homeostasis, are targeted by the potent viral oncogene polyomavirus middle T antigen (PyMT). A key process of PyMT is the activation of Src, a cellular proto-oncogene. Remarkably, active Src prevents the Hippo pathway from enhancing Taz decay mediated by its E3-ubiquitin ligase SCF(β-TrCP). We resolved the underlying mechanism by demonstrating that Src inhibits β-TrCP. β-TrCP is a key cellular component regulating a wide range of cellular signaling in cell fate determination. Our discovery of a function of the well-studied Src tyrosine kinase in abrogating β-TrCP function is expected to have wide ramifications for cell fate determination, including in oncogenesis.
Keywords: virus host interaction, middle T antigen, viral oncogene, Hippo pathway, SCF(β-TrCP)
Abstract
The polyomavirus middle T antigen (PyMT) oncogene activates the cellular nonreceptor tyrosine kinase c-Src and recruits the Hippo pathway effectors, Yap (yes-associated protein) and Taz (transcriptional coactivator with PDZ-binding motif), as key steps in oncogenesis. Yap and Taz are transcription coactivators shuttling from the cytoplasm to the nucleus. The Hippo pathway kinase Lats1/2 (large tumor suppressor homolog) reduces Yap/Taz nuclear localization and minimizes their cytoplasmic levels by facilitating their ubiquitination by the E3 ligase SCF(β-TrCP). In contrast, PyMT increases the cytoplasmic Taz level. Here we show that this unique PyMT behavior is mediated by Src. We demonstrate that PyMT-induced Src activation inhibits degradation of both wild-type and tyrosine-less Taz, ruling out Taz modification as a mechanism of escaping degradation. Instead, we found that Src attenuates the SCF(β-TrCP) E3-ligase activity in blunting Taz proteasomal degradation. The role of Src in rescuing Taz from TrCP-mediated degradation gives rise to higher cell proliferation under dense cell culture. Finally, IkB (NF-kappa-B inhibitor), a known substrate of β-TrCP, was rescued by Src, suggesting a wider effect of Src on β-TrCP substrates. These findings introduce the Src tyrosine kinase as a regulator of SCF(β-TrCP).
Viral oncogenes have evolved to subjugate cellular components, attending to viral needs. The study of viral strategies to overcome cell restrictions has yielded profound insights into understanding cell biology. The polyomavirus middle T antigen (PyMT) is necessary, and in some cases sufficient, for transformation of established fibroblasts (1). PyMT expression in mice mammary tissue, under the mouse mammary tumor virus (MMTV) promoter, consistently results in tumor formation, serving as a model for breast cancer and metastasis (2). Middle T antigen interacts with Src family kinases and activates them (3). This serves as a strict requirement for transformation (4, 5). The Src kinase phosphorylates middle T on several tyrosine residues, forming binding sites for key cellular signaling moieties, recruitment of which transforms cells (1). Taz (transcriptional coactivator with PDZ-binding motif), a Hippo pathway effector, binds to PyMT (6) and supports Src activation and cellular transformation by PyMT (7).
The mammalian core Hippo pathway kinases Lats1/2 (large tumor suppressor, homolog 1/2) phosphorylate the downstream effectors of the Hippo pathway, the paralog proteins Yap (yes-associated protein) and Taz (8, 9). These proteins coactivate transcription and positively regulate proliferation and tissue homeostasis, mainly by association with the TEAD (TEA domain transcription factor) family of transcription factors (10, 11). The kinase Lats phosphorylates Yap and Taz on five and four serine residues, respectively, conforming to an HxRxxS motif (12, 13). Phosphorylation creates recognition sites for 14-3-3 proteins, which, on binding, inhibit Yap/Taz nuclear entry and functions (14). Cytoplasmic retention constitutes a major regulation of the Hippo pathway tumor suppressor function. PyMT dramatically increases the cytoplasmic localization of Yap/Taz by inducing their phosphorylation on Lats1/2 sites. Taz/Yap cytoplasmic retention culminates in recruitment of Shp2 [PTPN11 (protein tyrosine phosphatase nonreceptor, type 11)], a tyrosine phosphatase, to the PyMT signaling complex, supporting Src activation, in a positive feedback loop (7).
Recent evidence suggests that Yap and Taz protein levels are critical in determining cell fate (15). Lats increases the cytoplasmic Yap and Taz susceptibility to proteasomal degradation (9, 16). Phosphorylation by Lats within a noncanonical DSGxS sequence, known as a phosphodegron (16, 17), recruits the F-box protein β-TrCP for Yap/Taz ubiquitination via the SCF(β-TrCP) complex (9, 16). The SCF (Skip1, Cullin1, F-box) is a four-subunit E3 ubiquitin ligase (18). An elongated scaffold protein, Cullin1, is bound at the C-terminal end to the RING domain protein, Rbx1, which putatively recruits ubiquitin conjugating enzymes (E2s). The N terminus of Cullin1 is bound to the scaffold protein Skp1. Skp1 binds one of many F-box proteins, such as β-TrCP, which determine substrate specificity of the complex (18).
In this study, we investigated the role of Src in regulating Taz protein levels. We found that PyMT-mediated Src activation down-regulates Taz degradation via β-TrCP, a process that gave rise to cell proliferation of dense culture. The negative effect of Src on β-TrCP was not restricted to Taz and could also be observed with IkB (NF-kappa-B inhibitor), another established substrate of β-TrCP. These findings reveal another facet of the interplay among PyMT, the tyrosine kinase Src, and the Hippo pathway effector Taz in the process of viral oncogenesis.
Results
Src Induces Taz Accumulation and Stabilization.
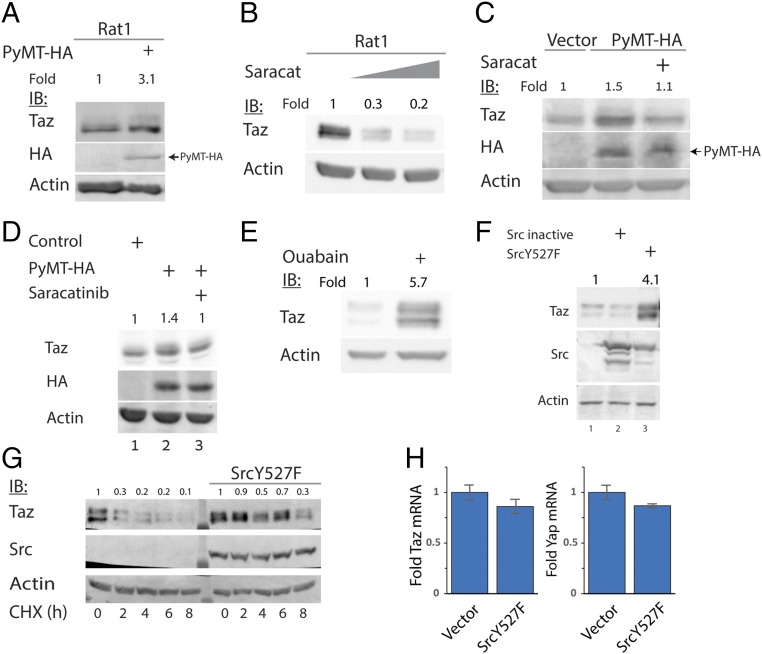
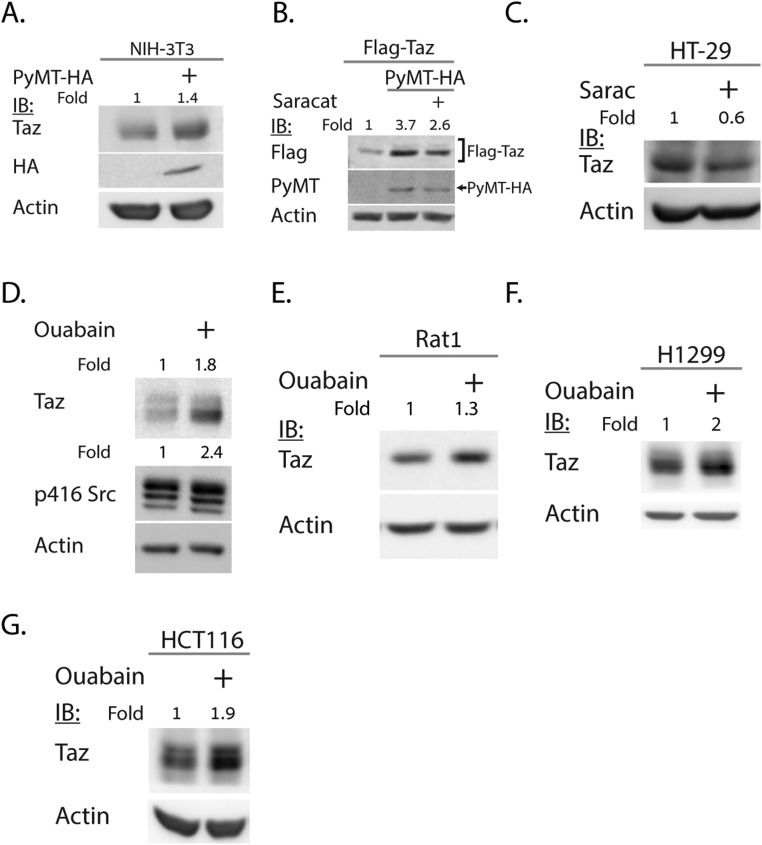
Lats1/2 phosphorylation of cytoplasmic Taz is expected to support Taz degradation via phosphorylation-dependent recruitment of its E3-ubiquitin ligase, SCF(β-TrCP) (β-TrCP below) (9). Previously, we demonstrated that PyMT induces Lats-dependent cytoplasmic localization of Taz (7). Here we show that PyMT induced the accumulation of endogenous (Fig. 1A and Fig. S1A) and overexpressed Taz (Fig. S1B). Because PyMT is known to activate the tyrosine kinase Src, we asked whether Src was involved in Taz accumulation. Rat1 cells were treated with saracatinib, a Src kinase inhibitor, and the endogenous Taz level was markedly decreased (Fig. 1B). Similar results were obtained with HT-29 cells (Fig. S1C), in which Src was reported to be highly active (19). In addition, the Taz accumulation by PyMT was decreased by the Src inhibitor in both HEK293 (Fig. 1C and Fig. S1B) and Rat1 cells (Fig. 1D). We next treated cells with ouabain, a reported activator of Src (ref. 20 and Fig. S1D). Ouabain increased Taz level in HEK293 cells (Fig. 1E) and in Rat1, H1299, and HCT116 cells (Fig. S1 E–G).
Fig. 1.
Src induces Taz accumulation and stabilization. (A) Rat1 cells were transduced with PyMT-expressing or control retroviral vectors, as indicated. Endogenous Taz level was detected by immunoblot, using indicated antibodies. (B) Rat1 cells were treated with 1–2 μM saracatinib for 24 h, and endogenous Taz level was detected. (C) HEK293 cells were cotransfected with PyMT and treated with 250 nM saracatinib for 24 h where indicated. (D) PyMT-positive Rat1 cells were treated with 1 μM saracatinib for 24 h, and endogenous Taz level was detected. (E) HEK293 cells were treated with 30 nM ouabain for 24 h, and endogenous Taz protein level was observed. (F) HEK293 cells were transfected with active (Y527F) and inactive Src, and endogenous Taz accumulation was followed. (G) Degradation kinetics of endogenous Taz are slowed down by Src. HEK293 cells were transfected with active Src and treated with 50 μg/mL cycloheximide (CHX) for indicated times, to follow protein degradation. Endogenous Taz level was detected. (H) Taz and Yap mRNA is not increased by Src cotransfection. HEK293 cells were transfected with Src, and after 24 h, total mRNA was extracted. Quantitative PCR was conducted to evaluate the levels of Taz and Yap mRNA relative to TBP and 18S mRNA level (n = 3). In all panels, band quantification was calculated relative to loading control.
Fig. S1.
PyMT and Src lead to Taz accumulation in additional cell lines. (A) PyMT-positive NIH 3T3 cells were subjected to Western blotting to detect endogenous Taz. (B) HEK293 cells were transfected with PyMT and treated with 250 nM saracatinib and analyzed as in A. (C) HT-29 cells were treated with 1 μM saracatinib and analyzed as in A. (D) Rat1 cells were treated with 100 nM ouabain. Endogenous Taz as well as level of endogenous active Src (pSrc-416) were detected by immunoblot. (E–G) Indicated cell line was treated with 30 nM ouabain, and endogenous Taz level was detected. In all panels, band quantification was calculated relative to loading control.
We next asked whether a constitutively active Src (Src Y527F) was sufficient to induce Taz accumulation. Interestingly, Src Y527F led to an increase in the endogenous Taz levels (Fig. 1F). Furthermore, the half-life of endogenous Taz was markedly increased by Src Y527F (Fig. 1G). Under these conditions, Taz and Yap mRNA levels were not significantly affected (Fig. 1H). These data suggest Src induces Taz protein accumulation by increasing Taz protein stability.
Taz Tyrosine Phosphorylation Does Not Explain Taz Stabilization.
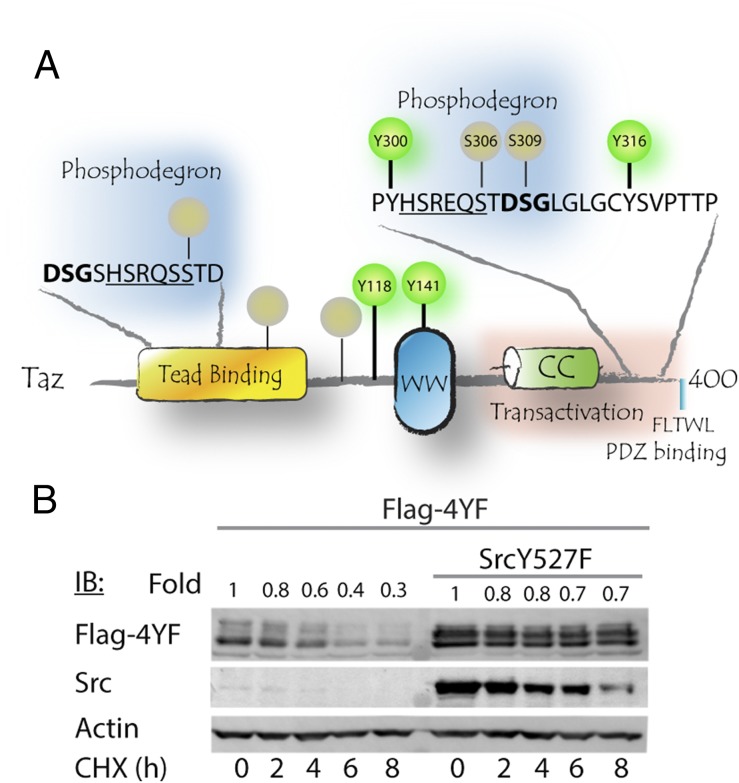
Tyrosine phosphorylation of Yap, the Taz paralog, by nonreceptor tyrosine kinase ABL1 (c-Abl) leads to its accumulation and stabilization (21). Taz contains four tyrosine residues (Fig. 2A). To test the possibility of Taz tyrosine phosphorylation as a mechanism of escaping degradation, we generated a Y-less Taz mutant (4YF) by mutating all four Taz tyrosine residues to phenylalanine. This mutant is expected to be completely refractory to tyrosine phosphorylation. We then evaluated Taz protein stability by conducting cycloheximide chase experiments. We found that the half-life of the phosphorylation-refractory Taz 4YF mutant was substantially increased by cotransfection with active Src (Fig. 2B). These data rule out the possibility that Taz phosphorylation by Src increases Taz accumulation, and therefore we looked for an alternative mechanism.
Fig. 2.
Taz phosphorylation fails to explain Taz stabilization. (A) Protein interaction domain architecture scheme, depicting Taz domains and phosphorylation sites. Tyrosine phosphorylation sites are denoted in green, whereas Serine phosphorylation is in gray. N- and C-terminal phosphodegrons are depicted, as well as Lats phosphorylation motifs directly adjacent to them (underlined). Numbering refers to mouse Taz. The human numbering correlates as follows: S309 (m)/S314 (h), Y316 (m)/Y321 (h). (B) HEK293 cells were cotransfected with Taz tyrosine phosphorylation refractory mutant (4YF) and active Src. Cells were treated with 50 μg/mL CHX for indicated times and then subjected to SDS/PAGE and Western blot, using indicated antibodies.
Src Attenuates β-TrCP–Mediated Taz Degradation.
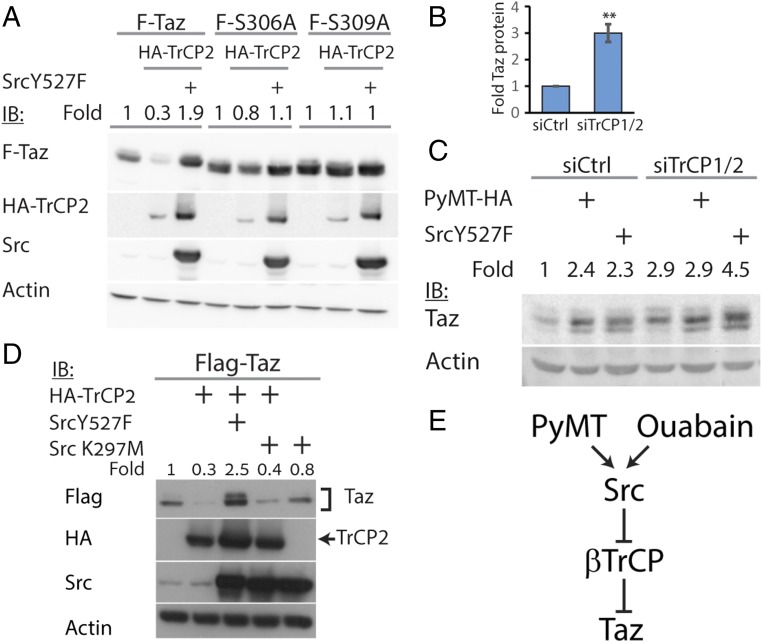
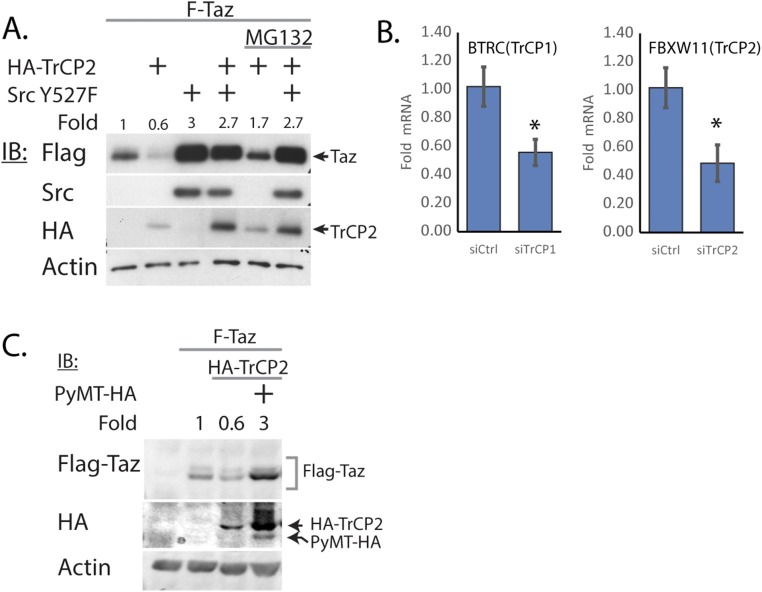
To uncover the alternative mechanism, we investigated Taz degradation by SCF-β-TrCP. In agreement with published report (9), cotransfection of Taz and β-TrCP led to Taz degradation (Fig. 3A), a process that was abrogated by a proteasomal inhibitor (Fig. S2A). The Taz phosphodegron motif undergoes phosphorylation to render it susceptible to β-TrCP–mediated degradation. We used the S306A and S309A phosphodegron mutants (9). These mutants accumulated to high levels and were found to be β-TrCP refractory (Fig. 3A). Interestingly, Src did not lead to further accumulation of the phosphodegron mutants, suggesting that the effect of Src was coupled to β-TrCP-mediated degradation. Depletion of β-TrCP1/2 led to a threefold increase in Taz protein level (Fig. 3B and Fig. S2B). Under these conditions, the effect of Src on endogenous Taz level was minor, supporting the coupling of Src effect with β-TrCP-mediated degradation (Fig. 3C). Furthermore, PyMT-mediated endogenous Taz accumulation was also β-TrCP–dependent (Fig. 3C and Fig. S2C). Next, we asked whether the attenuation of β-TrCP was Src kinase-dependent. To this end, we used the Src kinase dead mutant (Src K297M) and found it incapable of inducing Taz accumulation or of rescuing Taz from β-TrCP-dependent degradation (Fig. 3D). These data suggest that PyMT and Src attenuate β-TrCP activity (Fig. 3E).
Fig. 3.
Src rescues Taz from β-TrCP–mediated degradation. (A) HEK293 cells were transfected with Taz or Taz mutants, β-TrCP2, and active Src, as indicated, and then subjected to Western blotting using indicated antibodies. (B) Cells were transfected with RNAi toward TrCP1/2 or control siRNA (siCtrl), and Taz protein level was observed (n = 4). **P < 0.01. (C) Cells were transfected with RNAi toward TrCP1/2 or siCtrl. After 24 h, cells were transfected with PyMT and active Src. Endogenous Taz level was observed the next day. (D) Cells were transfected with Taz, β-TrCP, active Src, or Src kinase dead mutant (K297M) and subjected to Western blotting, using the indicated antibodies. (E) Model for Taz accumulation on Src activation. PyMT or ouabain activate Src, which in turn attenuate β-TrCP1/2 activity toward Taz, leading to an increase in Taz level. In all panels, band quantification was calculated relative to loading control.
Fig. S2.
Additional controls for Src rescue of Taz from β-TrCP degradation. (A) HEK293 cells were transfected with Taz, β-TrCP2, and active Src, as indicated. MG-132, an inhibitor of proteasomal degradation, was added 24 h after transfection, at 25 μM, for 4 h. Cells were harvested and analyzed by Western blot, using the indicated antibodies. (B) mRNA levels for TrCP1 and TrCP2 in siTrCP1/2 transfected cells (n = 3). (C) HEK293 cells were transfected with Taz, β-TrCP2, and PyMT, as indicated, and then subjected to Western blotting, using indicated antibodies. *P < 0.05.
Src Attenuates β-TrCP E3 Ubiquitin Ligase Activity.
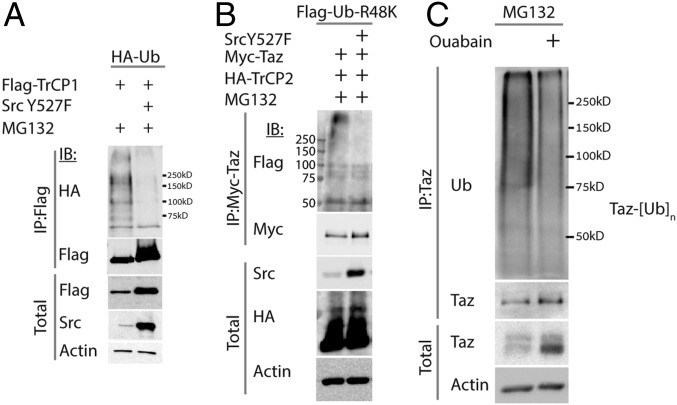
Next we asked whether Src inhibited the E3-ligase activity of β-TrCP. β-TrCP2 is an unstable protein subject to proteasomal degradation (17). To study the activity of β-TrCP in the presence of Src, we overexpressed β-TrCP along with tagged ubiquitin and followed the ubiquitination pattern of β-TrCP itself. Cotransfection of β-TrCP and ubiquitin yielded the expected ubiquitination pattern. (Fig. 4A and Fig. S3A). In the presence of active Src, but not the kinase dead mutant (Fig. S3A), a marked decrease in the ubiquitination pattern was observed.
Fig. 4.
Src attenuates ubiquitination by β-TrCP. (A) Autoubiquitination of TrCP is attenuated by Src. Ubiquitination of β-TrCP was determined using cotransfection of HEK293 cells with tagged-Ubiquitin (HA-Ub), active Src, and β-TrCP. Cells cotransfected with β-TrCP and Src show attenuated ubiquitination pattern. Cells were treated with 25 μM MG132 for 2 h to prevent proteasomal degradation of ubiquitinated substrates. (B) Taz ubiquitination by β-TrCP is attenuated in the presence of Src. HEK293 cells were cotransfected with Flag-R48K-Ub mutant, Flag-Taz, β-TrCP, and active Src (Y527F). Taz was immunoprecipitated, and its ubiquitination was observed using anti-ubiquitin antibody (P4D1). Cells were treated with 25 μM MG132 for 2 h. (C) Endogenous Taz ubiquitination is decreased by ouabain treatment. HEK293 cells were treated with 30 nM ouabain for 24 h. The next day, cells were treated with 25 μM MG132 for 2 h. Taz was immunoprecipitated, and its ubiquitination pattern was analyzed by Western blotting, as indicated.
Fig. S3.
Additional controls for ubiquitination assays. (A) Autoubiquitination of TrCP is attenuated by Src. Ubiquitination of β-TrCP was determined using cotransfection of HEK293 cells with F-R48K-Ub, active Src, kinase dead Src (KD), and β-TrCP. Cells cotransfected with β-TrCP and Src but not with Src KD, show attenuated ubiquitination pattern. Cells were treated with 25 μM MG132 for 2 h to prevent proteasomal degradation of ubiquitinated substrates. (B) Taz ubiquitination by β-TrCP is attenuated in the presence of Src. HEK293 cells were cotransfected with Flag-R48K-Ub mutant, Flag-Taz, β-TrCP, active Src (Y527F), and kinase dead Src (KD). Cells were treated with 25 μM MG132 for 2 h, Taz was immunoprecipitated, and its ubiquitination was observed.
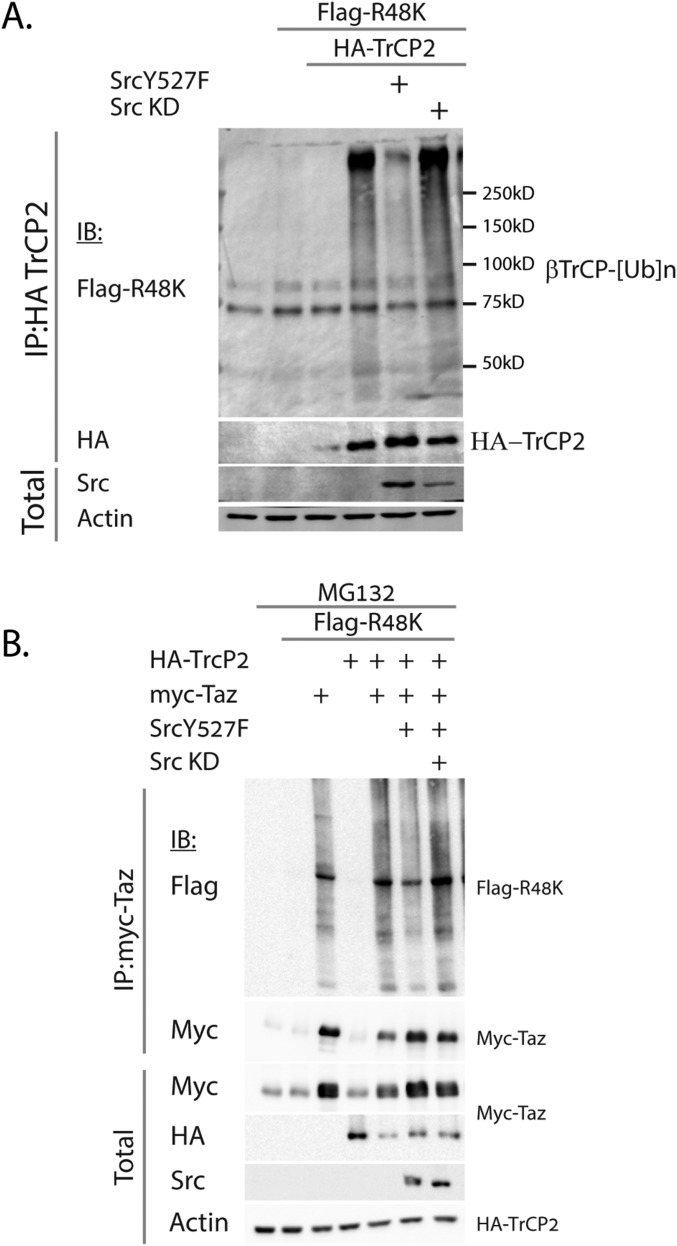
Next, we evaluated the ubiquitination of Taz by β-TrCP using a ubiquitin mutant in which all of the K residues were mutated to R except at the position K48 (R48K). Polyubiquitination with linkages at this residue is involved in proteasomal degradation (22). Cotransfection and immunoprecipitation of Taz with β-TrCP yielded a polyubiquitination pattern, which was diminished in the presence of active Src (Fig. 4B), but not the kinase dead mutant Src (Fig. S3B). To evaluate endogenous Taz ubiquitination under endogenous active Src, we treated cells with ouabain and immunoprecipitated Taz. Ubiquitination of Taz was attenuated under ouabain treatment (Fig. 4C). These data suggest that Src attenuated SCF (β-TrCP) ubiquitin ligase activity.
Src Supports CTGF and ANKRD1 Expression.
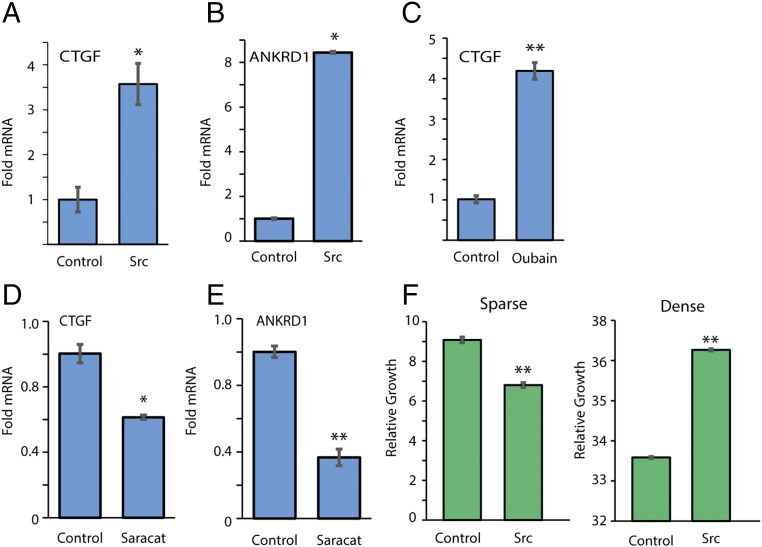
CTGF and ANKRD1 genes are diagnostic Taz and Yap nuclear targets. Interestingly, Src supported CTGF and ANKRD1 up-regulation (Fig. 5 A and B). Similar results were obtained with ouabain treatment (Fig. 5C). The induced gene expression of CTGF and ANKRD1 was sensitive to saracatinib, the Src inhibitor (Fig. 5 D and E). Lats1/2 kinases, the core element of the Hippo pathway, are inactive in sparse cells, and therefore Taz and Yap accumulate and induce target genes involved in cell growth (12). In dense culture Lats1/2 are active, blocking Yap/Taz nuclear entry and supporting their TrCP-mediated degradation. We therefore expected to obtain an effect by Src mainly in dense cells. Indeed, we found that Src preferentially promoted growth of dense cells (Fig. 5F). These data suggest that Src-mediated Taz accumulation supports cell growth in dense culture.
Fig. 5.
Src supports CTGF and ANKRD1 expression. (A) Cells were transfected with active Src and mRNA expression level of CTGF was detected using quantitative PCR, relative to TBP and 18S mRNA (n = 3). (B) The same as A, with detection of ANKRD1. (C) Cells were treated with 30 nM ouabain for 24 h. CTGF expression was detected using quantitative PCR, relative to TBP and 18S mRNA (n = 3). (D) Cells were treated with 100 nM saracatinib for 24 h, and expression of CTGF was detected, relative to TBP and 18S mRNA (n = 3). (E) The same as in D for ANKRD1 detection. (F) Cell growth as measured by the XTT cell proliferation assay of the stable line Rat1-SrcY527F was observed in sparse and dense conditions (n > 3). *P < 0.05; **P < 0.01.
Src Partially Attenuates IkB Degradation by β-TrCP.
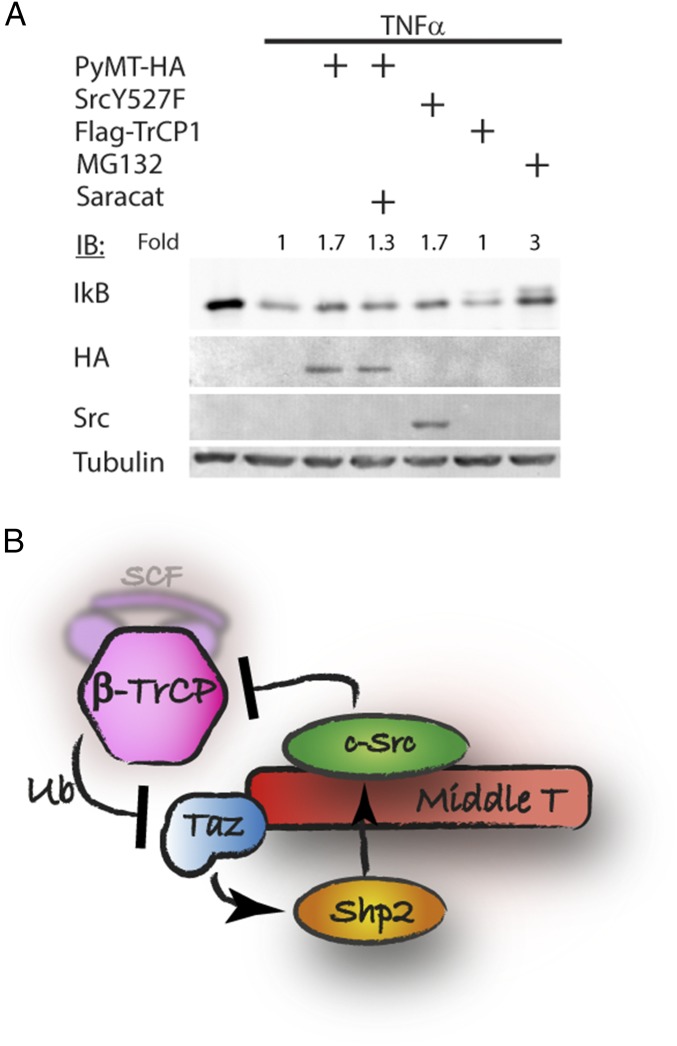
Finally, we asked whether Src inhibition of β-TrCP activity is Taz-specific or a general phenomenon. IkB is a well-known substrate of β-TrCP, subject to phosphorylation-dependent degradation, stimulated by TNFα (23). We therefore examined IkB degradation by β-TrCP after TNFα induction. As reported previously (23), IkB was degraded on TNF treatment (Fig. 6A). Interestingly, PyMT and Src both prevented IkB degradation under TNF treatment. Saracatininb partially inhibited the effect of PyMT, suggesting that the process requires Src activity. These data suggest that Src-mediated TrCP attenuation is not Taz-specific.
Fig. 6.
Src rescues IkB from β-TrCP–mediated degradation. (A) HEK293 cells were transfected with PyMT, active Src, or β-TrCP and treated with 250 nM saracatinib, as indicated. The next day, cells were treated with TNFα (20 ng/mL) for 15 min. Cells were harvested, and IkB was detected by Western blotting. (B) Suggested model for Taz accumulation by PyMT and long-term Src activation. PyMT activates Src, which inhibits SCF(β-TrCP) activity, leading to Taz accumulation. Taz localizes to the cytoplasm and accumulates and recruits Shp2, which serves in Src activation, creating a positive feedback loop supporting sustained Src activation.
Discussion
The proto-oncogene Src, a nonreceptor tyrosine kinase, has been widely investigated under various contexts. We attribute to Src a function that was thus far overlooked. We found that Src regulates protein degradation by attenuating SCF(β-TrCP), one of the key E3 ligases, which mediates degradation of main proteins imperative for several cellular signal pathways (17).
The following observations support our model of Src attenuating β-TrCP activity. We activated the endogenous Src by PyMT and by ouabain, and both gave rise to Taz accumulation. Furthermore, Taz, a TrCP substrate, was accumulated and its half-life extended by active Src. Moreover, the effect of Src on Taz accumulation was not compromised while using a tyrosine-less Taz mutant, ruling out a direct effect of Src on Taz in increasing its accumulation. Strikingly, Taz degradation, mediated by overexpressed β-TrCP, was blunted by an active Src. Furthermore, the level of β-TrCP–mediated substrate ubiquitination is inefficient in the presence of active Src. Finally, PyMT behaves similar to overexpressed active Src in abrogating the degradation of IkB, another well-studied β-TrCP substrate. The similarity between the behavior of overexpressed active Src and the physiologically induced Src activation by PyMT, in regulating β-TrCP activity, lends strong support to the proposed model.
Regulation of Taz/Yap localization by the tumor suppressor Hippo pathway has generally occupied the central stage in the Hippo pathway field. Recently, however, growing recognition is being attributed to Taz/Yap protein levels in determining activity (15). We demonstrate the importance of Src attenuating TrCP in two distinct physiological systems. The first model demonstrates the importance of Src-mediated Taz accumulation by PyMT in the process of cell oncogenic transformation. We previously demonstrated that the PyMT oncogene counter intuitively requires cytoplasmic Taz and Yap for efficient cellular transformation. PyMT induces their nuclear exclusion, which is associated with hyperphosphorylation at positions attributed to Lats phosphorylation. Because Lats also regulates Taz degradation via β-TrCP, Taz was expected to undergo increased degradation under these conditions. However, in the presence of PyMT, Taz was stabilized. We resolved this enigma by demonstrating that Src attenuates β-TrCP activity toward Taz (Fig. 6B). We further demonstrate that the PyMT-mediated inhibition of β-TrCP and Taz accumulation improves Shp2 recruitment by PyMT. Shp2 is a tyrosine protein phosphatase that activates Src (7). Activated Src, in turn, attenuates TrCP to further increase Taz level, establishing a positive feedback loop, critical for sustaining long-lasting Src activation required to transform cells (see model Fig. 6B).
In the second system, we demonstrated the importance of Taz accumulation by Src for cell growth. Hippo pathway functions to accelerate proliferation of sparse cells and to attenuate the proliferation of dense cells. In dense cells, Lat1/2 phosphorylates Taz to render it susceptible to TrCP, and therefore decreases cell growth. Therefore, attenuation of TrCP is expected to support cell growth under dense conditions, as we have indeed found. The fact that the role of Src is minor and even repressive on cell proliferation under sparse conditions, but significantly positive for dense culture, is of much interest and requires further investigation.
An important question is how Src compromises β-TrCP-mediated protein degradation of Taz and IkB. The SCF E3 ubiquitin ligase is composed of Cullin1, a scaffold protein, which binds Skp1 and Rbx1 on opposite ends. Skp1 recruits an F-box protein, which confers substrate specificity to the complex, whereas Rbx1 recruits E2 via the RING domain. The F-box protein, β-TrCP, interacts with a substrate protein to increase the local concentration of substrate lysine residues in the vicinity of E2 enzymes for ubiquitin conjugation (24, 25). On the basis of Phosphosite Plus (26), a repository of protein modification, β-TrCP is tyrosine phosphorylated. Whether Src is the responsible kinase is an open question. However, it is also possible that Src either directly or indirectly phosphorylates other components of the SCF complex. For example, Rbx1 was also found to be tyrosine phosphorylated, according to Phosphosite Plus.
Tyrosine phosphorylation is a major regulatory mechanism determining protein function. We previously demonstrated that tyrosine phosphorylation of Yap affects its stability and activity. In that case, Yap is phosphorylated by c-Abl in response to DNA damage (21, 27). Abl leads to stabilization of the Yap protein, a Taz paralog, which is dependent on Yap phosphorylation by Abl at Y357, corresponding to Taz phosphorylation by Src at Y316. However, we found that Taz stabilization was largely not dependent on Taz tyrosine phosphorylation, as the phosphorylation refractory mutant (4YF) was readily stabilized by Src. It seems that Taz and Yap levels are under the regulation of tyrosine kinases, but by two distinctive mechanisms: Whereas Abl phosphorylation of Yap directly stabilizes Yap, Src-mediated accumulation relies on inhibition of the E3-ligase.
In addition, our findings expand the cross-talk between PyMT and host signaling to include the NFkB pathway. Targeting of NFkB for inactivation is expected to blunt the innate immune response (28), a process that restricts viral propagation. Whether inhibition of the NFkB pathway serves PyMT-mediated transformation remains an open possibility.
Materials and Methods
Cell Culture.
HEK293, HEK293T, NIH 3T3, Rat1, and HCT116 were cultured in DMEM (GIBCO). HT-29 and H1299 cells were grown in RPMI 1640. Growth media was supplemented with 8% (vol/vol) FBS (GIBCO), 100 units/mL penicillin, 100 µg/mL streptomycin and cultured at 37 °C in a humidified incubator with 5% CO2. MG132 proteasomal inhibitor (ApexBio) was used at 25 μM for 2 h. Cycloheximide (Sigma) was used to inhibit translation at 50 μg/mL, as described previously (21). Transfections were performed by the calcium phosphate method, as previously described (29). TNFα (Peprotech) was added to cells at a concentration of 20 ng/mL for 15 min. Ouabain was used at 30 nM for HEK293 cells and 100 nM for Rat1 cells. Stable cell lines expressing PyMT or Src were created by retroviral transduction to create Rat1 pBabe puro (pBP), Rat1 pBP-PyMT-HA, Rat1 pBP-SrcY527F, and NIH 3T3 pBP-PyMT-HA, as described previously (7). Cell lines were selected with 2 μg/mL puromycin for several days.
Plasmids.
pCDNA3-Flag-mTaz and pCDNA3 myc-Taz were obtained from R. Bassel-Duby, University of Texas Southwestern, Dallas. pCDNA3-Flag-4YF mutant was cloned from pCDNA3-Flag-Taz by site-directed mutagenesis. pBabe-hygro PyMT (pBH-PyMT) was a gift from F. Giancotti, University of Texas MD Anderson Cancer Center, Houston (Addgene plasmid #22305) (30). PyMT was recloned from pBHPyMT into pCDNA with a C-terminal HA-tag, resulting in pCDNA-PyMT-HA. Src sequence was mutated by PCR to produce the constitutively active Src-Y527F (constitutively active) or double-mutant Y527F and K297M (inactive) and cloned into pCDNA3. Flag-β-TrCP1 construct was a gift from P. Howley, Harvard Medical School, Boston (Addgene plasmid #10865) (31). HA-TrCP2, Flag-Taz-S306A, and Flag-Taz- S309A constructs were obtained from X. J. Yang, McGill University, Montreal. pCMV-Yap1 was supplied by M. Sudol, Institute of Mechanobiology, Singapore. Plasmid pCDNA3-Flag-Ub-R48K was a gift from Y. Yarden, Weizmann Institute of Science. Plasmid pIRES-HA-Ub was a gift from C. Kahana, Weizmann Institute of Science.
Immunoblot and Coimmunoprecipitation Studies.
Immunoblots and immunoprecipitations were performed as previously described (29). The antibodies used were: anti-HA (Babco), anti-actin (Sigma), anti-Flag M2 and M5 (Sigma), anti-Yap antibody (H-125, Santa-Cruz), anti-Yap/Taz antibody (D24E4; Cell Signaling Technology), anti–v-Src (Calbiochem, Ab-1), anti-mSrc (Santa Cruz; sc-5266), anti-ubiquitin (P4D1; Santa Cruz), and anti-IkB antibody (BD transduction laboratories). For immunoprecipitation of Flag-tagged or HA-tagged proteins, anti-Flag M2 conjugated agarose beads (Sigma) or anti-HA conjugated agarose beads (Sigma) were loaded for 2 h. Horseradish peroxidase-conjugated secondary antibodies were from Jackson Laboratories. Enhanced chemiluminescence was performed with the EZ-ECL kit (Biological Industries), and signals were detected by the ImageQuant LAS 4000 (GE Healthcare). Band quantification was performed using ImageJ software.
Cell Proliferation Assay.
Cells were seeded at 1–5 × 103 cells/well in 96-well plates and then monitored using cell proliferation XTT (sodium 2,3,bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium)-based cell proliferation kit (Bet Haemek) at days 3 and 6, according to the manufacturer's instructions.
Quantitative PCR.
Quantitative RT-PCR was performed with SYBR Green PCR Master Mix (Kapa Biosystems), using the LightCycler 480 Instrument (Roche Diagnostics). Oligo sequences used were: CTGF TTGGCCCAGACCCAACTA/GCAGGAGGCGTTGTCATT and CCCTAGCTGCCTACCGACT/TGGCTCGCATCATAGTTGGG, ANKRD1 ACAGAGAAGGAGACACCCCT/TCCCAGCACAGTTCTTGACA and CACTTCTAGCCCACCCTGTGA/CCACAGGTTCCGTAATGATTT, Taz ATCCCAGCCAAATCTCGTGA/GCCCTGCGGGTGGGT, Yap CCAAGGCTTGACCCTCGTTTTG/TCGCATCTGTTGCTGCTGGTTG, TrCP1 ACCAACATGGGCACATAAACTC/TGGCATCCAGGTATGACAGAAT, TrCP2 AAGCTGATTGAACGAATGGTACG/CCACACCGCCAGTTAGATTCTAT, 18S TCGGAACTGAGGCCATGATTAAG/CGGAACTACGACGGTATCTGATC, TBP GAGTCGCCCTCCGACAAAG/GTTTCCTCTGGGATTCCATCG and CCCTATCACTCCTGCCACACCAGC/GTGCAATGGTCTTTAGGTCAAGTTTACAGCC Relative mRNA levels were normalized to TATA binding protein or 18S mRNA levels.
RNA Interference.
RNAi targeting TrCP1/2 was from Sigma, directed against the sequence GUGGAAUUUGUGGAACAUC, previously reported (32). siGENOME Control siRNA (nontargeting siRNA #5) was used as nontargeting control.
Acknowledgments
This research was supported by the Israel Science Foundation (Grant 1951/15) and by a project grant from the Israel Cancer Research Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610223114/-/DCSupplemental.
References
- 1.Fluck MM, Schaffhausen BS. Lessons in signaling and tumorigenesis from polyomavirus middle T antigen. Microbiol Mol Biol Rev. 2009;73(3):542–563. doi: 10.1128/MMBR.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12(3):954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtneidge SA. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8(1):23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Amini S, DeSeau V, Reddy S, Shalloway D, Bolen JB. Regulation of pp60c-src synthesis by inducible RNA complementary to c-src mRNA in polyomavirus-transformed rat cells. Mol Cell Biol. 1986;6(7):2305–2316. doi: 10.1128/mcb.6.7.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian Y, Li D, Dahl J, You J, Benjamin T. Identification of TAZ as a binding partner of the polyomavirus T antigens. J Virol. 2004;78(22):12657–12664. doi: 10.1128/JVI.78.22.12657-12664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanzer M, Ricardo-Lax I, Keshet R, Reuven N, Shaul Y. The polyomavirus middle T-antigen oncogene activates the Hippo pathway tumor suppressor Lats in a Src-dependent manner. Oncogene. 2015;34(32):4190–4198. doi: 10.1038/onc.2014.347. [DOI] [PubMed] [Google Scholar]
- 8.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 9.Liu C-Y, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J Biol Chem. 2010;285(48):37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahoney WMJ, Jr, Hong J-H, Yaffe MB, Farrance IKG. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem J. 2005;388(Pt 1):217–225. doi: 10.1042/BJ20041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lei Q-Y, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai F, et al. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19(24):6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015;29(12):1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs SY, Spiegelman VS, Kumar KGS. The many faces of beta-TrCP E3 ubiquitin ligases: Reflections in the magic mirror of cancer. Oncogene. 2004;23(11):2028–2036. doi: 10.1038/sj.onc.1207389. [DOI] [PubMed] [Google Scholar]
- 18.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 19.Rosen N, et al. Analysis of pp60c-src protein kinase activity in human tumor cell lines and tissues. J Biol Chem. 1986;261(29):13754–13759. [PubMed] [Google Scholar]
- 20.Tian J, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17(1):317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29(3):350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Ramakrishna S, et al. Lys-63-specific deubiquitination of SDS3 by USP17 regulates HDAC activity. J Biol Chem. 2011;286(12):10505–10514. doi: 10.1074/jbc.M110.162321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaron A, et al. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature. 1998;396(6711):590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 24.Cardozo T, Pagano M. The SCF ubiquitin ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5(9):739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 25.Zheng N, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416(6882):703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 26.Hornbeck PV, et al. PhosphoSitePlus: A comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40(Database issue) D1:D261–D270. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keshet R, et al. c-Abl antagonizes the YAP oncogenic function. Cell Death Differ. 2015;22(6):935–945. doi: 10.1038/cdd.2014.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randall RE, Goodbourn S. Interferons and viruses: An interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 29.Levy D, Adamovich Y, Reuven N, Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ. 2007;14(4):743–751. doi: 10.1038/sj.cdd.4402063. [DOI] [PubMed] [Google Scholar]
- 30.Pylayeva Y, et al. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J Clin Invest. 2009;119(2):252–266. doi: 10.1172/JCI37160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou P, Bogacki R, McReynolds L, Howley PM. Harnessing the ubiquitination machinery to target the degradation of specific cellular proteins. Mol Cell. 2000;6(3):751–756. doi: 10.1016/s1097-2765(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 32.Kim TY, et al. Substrate trapping proteomics reveals targets of the βTrCP2/FBXW11 ubiquitin ligase. Mol Cell Biol. 2015;35(1):167–181. doi: 10.1128/MCB.00857-14. [DOI] [PMC free article] [PubMed] [Google Scholar]