Significance
A screen of Hordeum (barley) spp. genomes identified several instances of the presence of ribosomal DNA of panicoid origin. The Pooideae and Panicoideae lineages separated from one another around 60 Mya and are sexually incompatible. During the past 1–5 My, at least nine independent transfers of panicoid DNA into Hordeum seem to have occurred, confirming that the transfer of exotic DNA is not an isolated event, at least among the grasses. The supposed rarity of this event in plant genomes more likely reflects technical limitations in its detection rather than it being a genuine biological phenomenon.
Keywords: Hordeum, Triticeae, Panicoideae, transposable elements, horizontal gene transfer
Abstract
The movement of nuclear DNA from one vascular plant species to another in the absence of fertilization is thought to be rare. Here, nonnative rRNA gene [ribosomal DNA (rDNA)] copies were identified in a set of 16 diploid barley (Hordeum) species; their origin was traceable via their internal transcribed spacer (ITS) sequence to five distinct Panicoideae genera, a lineage that split from the Pooideae about 60 Mya. Phylogenetic, cytogenetic, and genomic analyses implied that the nonnative sequences were acquired between 1 and 5 Mya after a series of multiple events, with the result that some current Hordeum sp. individuals harbor up to five different panicoid rDNA units in addition to the native Hordeum rDNA copies. There was no evidence that any of the nonnative rDNA units were transcribed; some showed indications of having been silenced via pseudogenization. A single copy of a Panicum sp. rDNA unit present in H. bogdanii had been interrupted by a native transposable element and was surrounded by about 70 kbp of mostly noncoding sequence of panicoid origin. The data suggest that horizontal gene transfer between vascular plants is not a rare event, that it is not necessarily restricted to one or a few genes only, and that it can be selectively neutral.
The exchange and recombination of genetic material are major driving forces of evolution: in eukaryotes, the process operates via sexual fertilization, whereas in prokaryotes, horizontal gene transfer (HGT) is commonplace. The extent to which HGT has contributed to the evolution of multicellular eukaryotes is debatable (1–3), largely because of the supposed low frequency of HGT events. Plant to plant exchanges of nonnuclear DNA are relatively common (4–6), but the exchange of nuclear DNA has been recorded, at best, sporadically. Most of the established horizontal transfer events involving a plant genome result from interactions between a plant and a pathogen or parasite (7–11). Plant to plant transfers outside of the fertilization process are thought to be rare (12–17), presumably because they require a vector to move the DNA from one plant to the other.
Eukaryotic genomes harbor thousands of copies of ribosomal DNA (rDNA) arranged in tandem arrays. Thanks to the sequence diversity of their spacer sequences (ITS), this class of repetitive DNA has been highly informative concerning phylogenetic relationships. In an attempt to use ITS variation to identify the progenitors of hexaploid couch grass (Elymus repens, Triticeae, Pooideae), a nonnative ITS type was uncovered that was considered to have originated from a species of Panicum (18), a panicoid genus that separated from the pooids some 60 Mya (19). Elymus spp. are not known to intercross with Panicum spp. (20–22), presenting a puzzle of how the exotic rDNA was acquired. Because E. repens is an allopolyploid harboring genomes derived from both Pseudoroegneria and Hordeum (18, 23), it was of interest to determine whether the transfer pre- or postdated the allopolyploidization event. Using an assay that selectively amplified the panicoid ITS (18), it was possible to show the presence of panicoid rDNA in both progenitor species, although the sequence data favored the notion that E. repens had inherited its panicoid rDNA unit from Hordeum bogdanii (18).
Here, the aim was to characterize the occurrence and nature of panicoid-derived rDNA in Triticeae genomes, with a focus on Hordeum and Pseudoroegneria species. The sequence polymorphism characteristic of the ITS offered an opportunity to place a specific HGT in relation to the evolutionary history of the host. A detailed analysis of the structure of one HGT was carried out by sequencing a BAC clone of H. bogdanii, which harbored a panicoid rDNA unit. In conjunction with cytogenetic analyses, this information allowed for both the nature of the nonnative genetic material and its location in the barley genome to be identified.
Results and Discussion
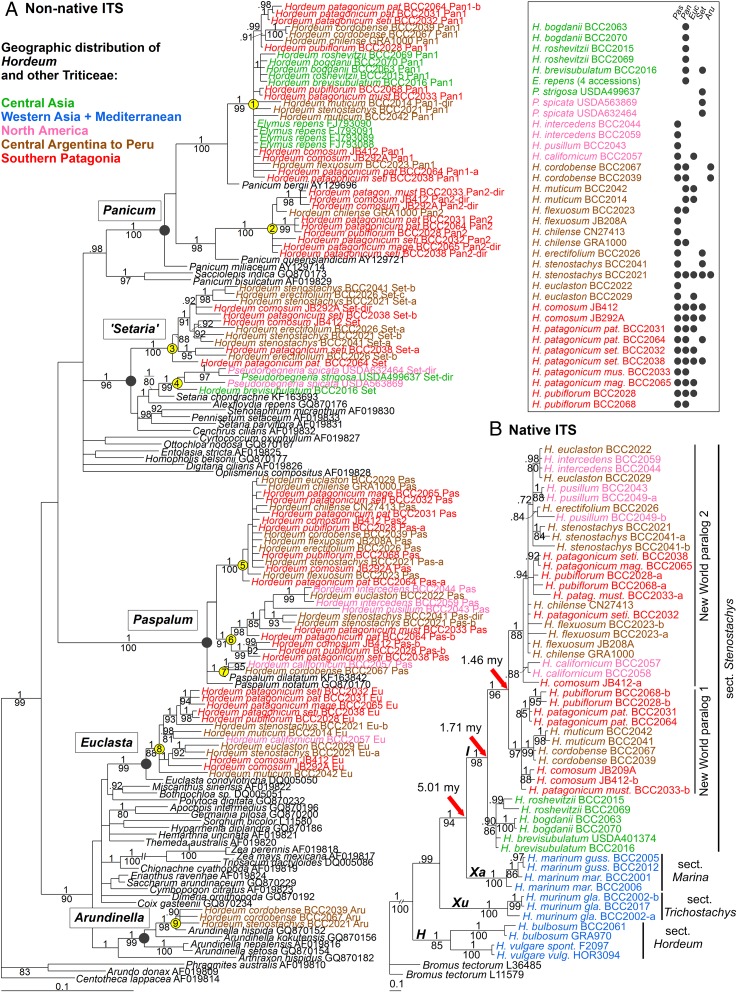
The initial PCR-based screen of a set of Triticeae species (a full list is in Table S1) revealed that nonnative panicoid rDNA was present only in species belonging to the two genera Hordeum and Pseudoroegneria (Table S2). It was found in all 16 diploid Hordeum species belonging to section Stenostachys, all of which harbor the I genome, but not found in any of the remaining Hordeum taxa (Table S2). Two of the five Pseudoroegneria species (Pseudoroegneria strigosa and Pseudoroegneria spicata) also tested positive. Sequence variation within the panicoid ITS was established by cloning and sequencing the relevant stretch of DNA (amplicon 2) (Fig. S1) based on a template of either Hordeum or Pseudoroegneria spp. DNA. The unexpected outcome was that the source of the rDNA was traceable to five distinct Panicoideae genera, namely Arundinella, Euclasta, Paspalum, Panicum, and Setaria (Fig. 1A). A PCR assay was then designed to amplify each of the five panicoid lineages separately (Table S3), and the screen was repeated to pick up any hitherto undetected nonnative ITS types. Between one and five panicoid ITS types were found in the various Hordeum and Pseudoroegneria individuals (Table S2). Although a single nonnative ITS derived from Setaria spp. was present in the Pseudoroegneria genomes, the Hordeum genomes harbored up to five distinct nonnative rDNA. Most of the Central Asian and North American taxa harbored a single nonnative ITS type, but the South American ones have accumulated multiple copies within individual plants (Fig. 1A and Table S2). The panicoid ITS sequences occurred in derived positions of the phylogenetic tree (Fig. 1A), suggesting a relatively recent acquisition. Some clustered with sequences of extant panicoid species (e.g., Paspalum dilatatum and Arundinella hispida), whereas others seemed to be derived from either nonsampled or extinct species belonging to one of the five panicoid genera. Some sequence variation was noted within each of the five lineages, resulting in distinct clusters of highly similar sequence shared by several Hordeum and Pseudoroegneria species; these patterns were most parsimoniously interpreted as being the outcome of a number of independent transfer events, in some cases involving more than one per panicoid genus (1–9 in Fig. 1A; a detailed evaluation is in Table S4).
Table S1.
List of Hordeum, Pseudoroegneria, and other Triticeae accessions used in the study
| Species | Accession | Genome | Origin | Collector/expedition |
| Hordeum | ||||
| Sect. Stenostachys | ||||
| 1. Hordeum bogdanii Wilensky | BCC2063 | I | Pakistan, Gilgit | R. von Bothmer 1983 |
| 2. Hordeum bogdanii Wilensky | BCC2070 | I | China, Xinjiang | C. Baden and B. Salomon 1987 |
| 3. Hordeum brevisubulatum (Trin.) Link | BCC2016 | I | Tajikistan, Gissar Mountains | B. Salomon and B. R. Lu 1991 |
| 4. Hordeum brevisubulatum Link ssp. violaceum (Boiss. & A. Huet) Tzvelev | USDA401374 | I | Iran | D. Dewey 1972 |
| 5. Hordeum californicum Covas & Stebbins | BCC2057 | I | United States, California | R. von Bothmer 1990 |
| 6. Hordeum californicum Covas & Stebbins | BCC2058 | I | United States, California | R. von Bothmer 1990 |
| 7. Hordeum chilense Roemer & Schultes | CN27413 | I | Argentina, Neuquen | B. R. Baum 1979 |
| 8. Hordeum chilense Roemer & Schultes | GRA1000 | I | Unknown | Dr. Schrader, Quedlinburg |
| 9. Hordeum comosum Presl | JB292A | I | Argentina, Santa Cruz | S. Jakob and F. Blattner 2005 |
| 10. Hordeum comosum Presl | JB412 | I | Argentina, Chubut | S. Jakob and F. Blattner 2006 |
| 11. Hordeum cordobense Bothmer, Jacobsen & Nicora | BCC2039 | I | Argentina, Cordoba | R. von Bothmer 1979 |
| 12. Hordeum cordobense Bothmer, Jacobsen & Nicora | BCC2067 | I | Argentina, Mendoza | O. Seberg, A. M. Molina, and R. Neumann 1989 |
| 13. Hordeum erectifolium Bothmer, Jacobsen & Jørgensen | BCC2026 | I | Argentina, Buenos Aires | N. Jacobsen 1978 |
| 14. Hordeum euclaston Steud. | BCC2022 | I | Argentina, Buenos Aires | N. Jacobsen 1978 |
| 15. Hordeum euclaston Steud. | BCC2029 | I | Argentina, Santa Cruz | N. Jacobsen 1979 |
| 16. Hordeum flexuosum Nees ex Steud. | BCC2023 | I | Argentina, Buenos Aires | N. Jacobsen 1978 |
| 17. Hordeum flexuosum Nees ex Steud. | JB208A | I | Argentina, Buenos Aires | S. Jakob and F. Blattner 2004 |
| 18. Hordeum intercedens Nevski | BCC2044 | I | United States, California | G. Bailey and N. Jacobsen 1980 |
| 19. Hordeum intercedens Nevski | BCC2059 | I | Mexico, San Quentin | B. R. Baum and G. Bailey 1985 |
| 20. Hordeum muticum J. Presl | BCC2014 | I | Bolivia, Oruro | L. van Soest 1980 |
| 21. Hordeum muticum J. Presl | BCC2042 | I | Argentina, Tucuman | R. von Bothmer 1979 |
| 22. Hordeum patagonicum (Haumann) Covas ssp. magellanicum (Parodi & Nicora) Bothmer, Giles & Jacobsen | BCC2065 | I | Argentina, Santa Cruz | O. Seberg 1987 |
| 23. Hordeum patagonicum (Haumann) Covas ssp. mustersii (Nicora) Bothmer, Giles & Jacobsen | BCC2033 | I | Argentina, Santa Cruz | N. Jacobsen 1979 |
| 24. Hordeum patagonicum (Haumann) Covas ssp. patagonicum | BCC2031 | I | Argentina, Santa Cruz | N. Jacobsen 1979 |
| 25. Hordeum patagonicum (Haumann) Covas ssp. patagonicum | BCC2064 | I | Argentina, Santa Cruz | O. Seberg and S. Diemar 1986 |
| 26. Hordeum patagonicum (Haumann) Covas ssp. setifolium (Parodi & Nicora) Bothmer, Giles & Jacobsen | BCC2032 | I | Argentina, Santa Cruz | N. Jacobsen 1979 |
| 27. Hordeum patagonicum (Haumann) Covas ssp. setifolium (Parodi & Nicora) Bothmer, Giles & Jacobsen | BCC2038 | I | Argentina, Chubut | B. R. Baum 1979 |
| 28. Hordeum pubiflorum Hook. f. | BCC2068 | I | Bolivia, Quijarro | P. Peterson, R. J. Soreng, and S. Laegaard |
| 29. Hordeum pubiflorum Hook. f. | BCC2028 | I | Argentina, Neuquen | N. Jacobsen 1979 |
| 30. Hordeum pusillum Nutt. | BCC2043 | I | United States, Colorado | G. Bailey and N. Jacobsen 1980 |
| 31. Hordeum pusillum Nutt. | BCC2049 | I | United States, New Mexico | R. von Bothmer and G. Bailey 1980 |
| 32. Hordeum roshevitzii Bowden | BCC2015 | I | Russia, Altai, Bashelakskij Mountains | R. von Bothmer and B. Salomon 1990 |
| 33. Hordeum roshevitzii Bowden | BCC2069 | I | China, Sichuan | Chinese–Swedish expedition no. 860150, 1986 |
| 34. Hordeum stenostachys Godr. | BCC2021 | I | Argentina, Buenos Aires | N. Jacobsen 1978 |
| 35. Hordeum stenostachys Godr. | BCC2041 | I | Argentina, La Rioja | R. von Bothmer 1979 |
| Sect. Marina | ||||
| 36. Hordeum marinum Huds. ssp. gussoneanum (Parl.) Thell. | BCC2005 | Xa | Spain, Lagrosan | N. Jacobsen 1977 |
| 37. Hordeum marinum Huds. ssp. gussoneanum (Parl.) Thell. | BCC2012 | Xa | Bulgaria, Dimitrovgrad | R. von Bothmer 1977 |
| 38. Hordeum marinum Huds. ssp. marinum | BCC2001 | Xa | Greece, Kikladhes Islands | S. Snogerup and R. von Bothmer 1968 |
| 39. Hordeum marinum Huds. ssp. marinum | BCC2006 | Xa | Spain, Toledo | N. Jacobsen 1977 |
| Sect. Trichostachys | ||||
| 40. Hordeum murinum L. glaucum (Steud.) Tzvelev | BCC2002 | Xu | Tunisia, Faid | H. Scholtz 1968 |
| 41. Hordeum murinum L. glaucum (Steud.) Tzvelev | BCC2017 | Xu | Tajikistan, Zeravshan Mountains | B. Salomon and B. R. Lu 1991 |
| Sect. Hordeum | ||||
| 42. Hordeum bulbosum (4x) L. | GRA970 | H | Greece | R. von Bothmer |
| 43. Hordeum bulbosum L. | BCC2061 | H | Italy | R. von Bothmer 1994 |
| 44. Hordeum vulgare L. ssp. spontaneum (K. Koch) Thell. | F2097 | H | Uzbekistan | R. Fritsch |
| 45. Hordeum vulgare L. ssp. vulgare | HOR3094 | H | United States | Beltsville: CI7574 |
| Pseudoroegneria | ||||
| 1. Pseudoroegneria gracillima (Nevski) Á. Löve | USDA440000 | St | Russia, Stavropol | D. Dewey 1977 |
| 2. Pseudoroegneria libanotica (Hack.) D.R.Dewey | USDA228389 | St | Iran, Sanandaj | H. Gentry 1955 |
| 3. Pseudoroegneria libanotica (Hack.) D.R.Dewey | USDA401274 | St | Iran, Sanandaj | D. Dewey 1972 |
| 4. Ps. spicata (Pursh) Á. Löve | USDA632464 | St | United States, Colorado | T. Jones |
| 5. Ps. spicata (Pursh) Á. Löve | USDA563869 | St | United States, Oregon | K. Asay 1980 |
| 6. Pseudoroegneria stipifolia (Czern. ex Nevski) Á. Löve | USDA325181 | St | Russia, Stavropol | W. H. Skrdla,1968 |
| 7. Ps. strigosa (M. Bieb.) Á. Löve | USDA499637 | St | China, Xinjiang | D. Dewey 1983 |
| Other Triticeae | ||||
| 1. Aegilops bicornis Jaub. & Spach | AE1079 | Sb | Jordan | Unknown |
| 2. Aegilops speltoides Tausch | AE747 | S | Turkey | J. Martens |
| 3. Aegilops speltoides Tausch | AE1085 | S | Syria | Unknown |
| 4. Aegilops tauschii Coss. | AE541 | D | Iran | H. Kuckuck 1952 |
| 5. Aegilops tauschii Coss. | AE1039 | D | Tajikistan | Expedition Mittelasien 1990: T 498 |
| 6. Aegilops uniaristata Steud. | AE680 | N | Italy | Expedition S-Italien 1980: 5354 |
| 7. Agropyron cristatum (L.) Gaertn. | GRA1009 | P | Russia | K. Hammer 1975 |
| 8. Dasypyrum villosum (L.) P. Candargy | GRA2991 | V | Greece | Unknown |
| 9. Eremopyrum bonaepartis (Spreng.) Nevski | GRA1120 | F or Xe | Armenia | Dr. Schmiedeknecht 1975 |
| 10. Henrardia persica (Boiss.) C. E. Hubb. | USDA577112 | O | Turkey | Unknown |
| 11. Heteranthelium piliferum Hochst. ex Jaub. & Spach | USDA401351 | Q | Iran, Shiraz | D. Dewey 1972 |
| 12. Psathyrostachys juncea (Fisch.) Nevski | USDA598615 | Ns | Kazakhstan | K. Asay, D. Johnson |
| 13. Secale strictum C. Presl ssp. kuprijanovii (Grossh.) K. Hammer | R1108 | R | Kazakhstan | Expedition Mittelasien 1990: K 607 |
| 14. Taeniatherum caput-medusae (L.) Nevski | GRA1126 | Ta | Tajikistan | Expedition Landsorten Tadshik. SSR 1983: 22 |
| 15. Taeniatherum caput-medusae (L.) Nevski | USDA598389 | Ta | Turkey, Sarigol | G. Kimber, R. Metzger 1984 |
| 16. Thinopyrum elongatum (Host) D. R. Dewey | USDA531718 | Ee | Tunisia | Unknown |
| 17. Triticum monococcum L. var. hohensteinii Flaksb. | TRI13612 | Am | Georgia | Expedition Landsorten Georg. SSR 1982: 288 |
| 18. Triticum urartu Tumanian ex Gandilyan var. turcicum K. Hammer & A. Filat. | TRI17159 | Au | Turkey | B. Johnson 1973 |
BCC, GRA, HOR, TRI, AE, F, and R refer to accessions from the Leibniz Institute of Plant Genetics and Crop Plant Research GenBank. USDA refers to accessions from the National Genetic Resources Program of the USDA (United States). CN is accession from Plant Genetic Resources Canada (PGR Canada) and JB are accessions from a private collection of F.R.B. Genome designations of Triticeae taxa follow those in ref. 26, and genomes and sectional classification in Hordeum are according to those in ref. 27. All accessions, except for tetraploid H. bulbosum GRA970, are diploids (2n = 14).
Table S2.
Panicoid rDNA types in Hordeum and Pseudoroegneria
| Accession no. | Amplicon 2: Total panicoid ITS rDNA amplified with a Panicoideae-specific primer | Genus-specific ITS rDNA types captured in cloned amplicon 2 (no. of clones sequenced) | Amplicon 3: PCR screening for individual ITS types (total no. of clones obtained for each type) | |||||
| Paspalum | Panicum 1 | Panicum 2 | Euclasta | Setaria | Arundinella | |||
| Hordeum comosum JB412 | + | − + − − − − (15) | + (3) | + (1) | + (1 Direct) | + (1) | + (1) | − |
| Hordeum comosum JB292A | + | − + − − − − (16) | + (4) | + (3) | + (1 Direct) | + (2) | + (1 Direct) | − |
| Hordeum patagonicum pat. BCC2031 | + | + − − + − − (15) | + (6) | + (3) | + (3) | + (4) | − | − |
| Hordeum patagonicum pat. BCC2064 | + | Not sequenced | + (3) | + (4) | + (1) | − | + (1) | − |
| Hordeum patagonicum set. BCC2032 | + | + − + + − − (16) | + (2) | + (2) | + (3) | + (2) | − | − |
| Hordeum patagonicum set. BCC2038 | + | + − − − + − (10) | + (1) | + (1) | + (1 Direct) | + (6) | + (14) | − |
| Hordeum patagonicum mus. BCC2033 | + | − + − − − − (10) | + (1) | + (3) | + (1 Direct) | − | − | − |
| Hordeum patagonicum mag. BCC2065 | + | + − − + − − (16) | + (5) | − | + (1 Direct) | + (4) | − | − |
| Hordeum pubiflorum BCC2028 | + | + − − + − − (21) | + (14) | + (2) | + (1) | + (2) | − | − |
| Hordeum pubiflorum BCC2068 | + | Not sequenced | + (3) | + (2) | − | − | − | − |
| Hordeum cordobense BCC2067 | + | − + − − − − (10) | + (1) | + (10) | − | − | − | + (1) |
| Hordeum cordobense BCC2039 | + | Not sequenced | + (3) | + (2) | − | − | − | + (3) |
| Hordeum muticum BCC2042 | + | − + − − − − (10) | − | + (7) | − | + (3) | − | − |
| Hordeum muticum BCC2014 | + | Not sequenced | − | + (1 Direct) | − | + (3) | − | − |
| Hordeum flexuosum BCC2023 | + | + − − − − − (16) | + (1) | + (1) | − | − | − | − |
| Hordeum flexuosum JB208A | + | Not sequenced | + (1) | − | − | − | − | − |
| Hordeum chilense CN27413 | + | + − − − − − (14) | + (1) | − | − | − | − | − |
| Hordeum chilense GRA1000 | + | Not sequenced | + (1) | + (4) | + (4) | − | − | − |
| Hordeum erectifolium BCC2026 | + | + − − − + − (22) | + (1) | − | − | − | + (15) | − |
| Hordeum stenostachys BCC2041 | + | − − − − + − (14) | + (1 Direct) | − | − | − | + (6) | − |
| Hordeum stenostachys BCC2021 | + | − + − + + + (16) | + (4) | + (2) | − | + (4) | + (4) | + (1) |
| Hordeum euclaston BCC2022 | + | + − − − − − (10) | + (5) | − | − | − | − | − |
| Hordeum euclastonBCC2029 | + | Not sequenced | + (2) | − | − | + (4) | − | − |
| Hordeum intercedensBCC2044 | + | + − − − − − (13) | + (4) | − | − | − | − | − |
| Hordeum intercedens BCC2059 | + | + − − − − − (9) | + (3) | − | − | − | − | − |
| Hordeum pusillum BCC2043 | + | + − − − − − (9) | + (3) | − | − | − | − | − |
| Hordeum pusillum BCC2049 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum californicum BCC2057 | + | + − − + − − (9) | + (1) | − | − | + (3) | − | − |
| Hordeum californicum BCC2058 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum bogdanii BCC2063 | + | − + − − − − (9) | − | + (4) | − | − | − | − |
| Hordeum bogdanii BCC2070 | + | Not sequenced | − | + (4) | − | − | − | − |
| Hordeum roshevitzii BCC2015 | + | − + − − − − (10) | − | + (2) | − | − | − | − |
| Hordeum roshevitzii BCC2069 | + | − + − − − − (10) | − | + (2) | − | − | − | − |
| Hordeum brevisubulatum PI401374 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum brevisubulatum BCC2016 | + | Not sequenced | − | + (1) | − | − | + (7) | − |
| Hordeum marinum BCC2001 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum marinum BCC2006 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum mar. gussoneanum BCC2005 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum mar. gussoneanum BCC2012 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum murinum glaucum BCC2002 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum murinum glaucum BCC2017 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum vulgare F2097 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum vulgare HOR3094 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum bulbosum BCC2061 | − | Not sequenced | − | − | − | − | − | − |
| Hordeum bulbosum (4x) GRA970 | − | Not sequenced | − | − | − | − | − | − |
| Total no. of clones (and direct sequences) | 73 (+1) | 60 (+1) | 12 (+5) | 38 | 48 (+1) | 5 | ||
| Pseudoroegneria gracillima USDA440000 | − | Not sequenced | − | − | − | − | − | − |
| Pseudoroegneria libanoticaUSDA228389 | − | Not sequenced | − | − | − | − | − | − |
| Pseudoroegneria libanoticaUSDA401274 | − | Not sequenced | − | − | − | − | − | − |
| Ps. spicata USDA632464 | + | Not sequenced | − | − | − | − | + (1 Direct) | − |
| Ps. spicata USDA563869 | + | − − − − + − (42) | − | − | − | − | + (42) | − |
| Pseudoroegneria stipifolia USDA325181 | − | Not sequenced | − | − | − | − | − | − |
| Ps. strigosa USDA499637 | + | Not sequenced | − | − | − | − | + (1 Direct) | − |
| Total no. of clones (and direct sequences) | 0 | 0 | 0 | 0 | 42 (+2) | 0 | ||
Initially, total (= native + nonnative) ITS rDNA was amplified in all Triticeae accessions (amplicon 1) (details in Materials and Methods and Fig. S1). Next, all accessions were screened with a Panicoideae-specific primer, amplifying total panicoid ITS types (amplicon 2); + and − characters denote successful and unsuccessful amplification, respectively. Cloning amplicon 2 and sequencing of clones (only in selected accessions; column 3) resulted in capture and identification of individual panicoid ITS types (+ and − denote the presence or absence, respectively, of individual ITS types corresponding to Paspalum, Panicum 1, Panicum 2, Euclasta, Setaria, and Arundinella, with the number of sequenced clones in parentheses). Next, all accessions were screened with the designed specific primers for the presence of individual panicoid ITS types (amplicon 3): presence, +; absence, −. The total number of clones obtained for that particular ITS type (including clones of amplicon 2) is given in parentheses. Direct indicates direct sequence of amplicon 3 for that accession. Additional details are in Materials and Methods and Fig. S1.
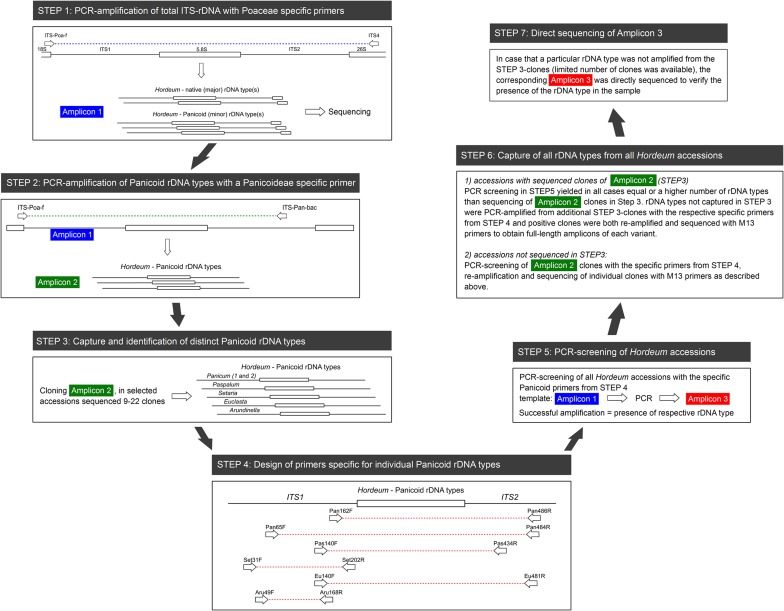
Fig. S1.
Scheme describing screening and amplification of panicoid rDNA in Hordeum (and other Triticeae). All Triticeae samples analyzed in this study were screened for the presence of panicoid rDNA using a Panicoideae-specific primer (steps 1 and 2). Panicoid rDNA was only found in Hordeum and Pseudoroegneria. The procedure is described for Hordeum; Pseudoroegneria samples were analyzed analogically.
Fig. 1.
Phylogenetic analysis of (A) nonnative (panicoid) and (B) native (pooid) rDNA ITS sequences. (A) The numbers inserted above and below the branches refer to the Bayesian posterior probabilities and the bootstrap values for maximum parsimony, respectively; –a and –b are variants of the same rDNA subtype, and –dir indicates a direct sequence of the respective rDNA type. Yellow circles including the numbers 1–9 indicate subclades referred to in the text and Table S4. Inset reports a summary of the distribution of panicoid rDNA (corresponding to five genera) across Hordeum, Pseudoroegneria, and Elymus species/accessions. (B) The New World species contain two rDNA loci (24). The dating of the nodes follows (25). H, Xu, Xa, and I are genome designators (26).
Table S3.
List of primers used in the study
| Amplicon and primer | Sequence (5′–3′) | Annealing temperature (°C) |
| Amplicon 1 | ||
| ITS-Poa-f | AAGGATCATTGTCGTGACC | 51 |
| ITS4 | TCCTCCGCTTATTGATATGC | |
| Amplicon 2 | ||
| ITS-Poa-f | AAGGATCATTGTCGTGACC | 51 |
| ITS-Pan-bac | ACACTGCACCGAGAACAAC | |
| Amplicon 3 | ||
| Pas_141F | ATATTGCCTTGCGAGGGTGA | 55 |
| Pas_435R | TGCACCATGGCACGAGAG | |
| Pan1_162F | GTTCGGCCTGTCGCGCAT | 55 |
| Pan1_486R | ACATGCAGGTGCCCGACA | |
| Pan2_65F | AAGGCCCCCAGTCAACATTT | 60 |
| Pan2_484R | CGTGCAAGTACCTAACACGG | |
| Euc_140F | TATTGACTTGCTCGGCGGAG | 60 |
| Euc_481R | TGCTGTGCCCGATACGATTC | |
| Set_31F | CGTGTCATCCATGCCGCAT | 55 |
| Set_202R | AGATAGCATCGTTACACGAGGT | |
| Aru_49F | GGGCTTCGGTCCGGTTTA | 54 |
| Aru_168R | GCCGACCGCTCCACCGT |
Table S4.
Patterns of panicoid rDNA distribution in relation to host phylogeny and speciation
| Genus and clade no. | No. of species*/accessions | Description | Interpretation/potential scenario |
| Panicum 1 | 11/21 (+ 4 Elymus repens) | All Central Asian and 8 of 10 South American Hordeum species share this ITS type. Sequence variation is very low. Nevertheless, Central Asian species and two Hordeum cordobense accessions form well-supported subclades. One accession (Hordeum patagonicum BCC2064) harbors two ITS subtypes. The South American species Panicum bergii is sister to this clade. | Because of low sequence variation and sharing of this ITS type across a large range of Hordeum species with distribution areas that do not overlap, the most parsimonious explanation is a single transfer from a species closely related to P. bergii predating the diversification of Hordeum section Stenostachys. The transfer presumably occurred in Central Asia before Hordeum colonized the New World in a timeframe between ∼5 and 1.7 Mya inferred from Hordeum phylogeny. In some host species (e.g., Central Asian taxa, H. cordobense), group- or species-specific sequence evolution has occurred after the transfer. |
| Panicum 2 | 4/10 | This ITS type is shared by four Hordeum species from southern South America. Two subtypes shared by three accessions each can be distinguished. The clade is sister to the Australian species Panicum queenslandicum. | Nine of 10 individuals showing this type also harbor the Panicum 1 ITS type suggesting a subsequent acquisition restricted to a few South American species from a Panicum donor related but not identical to P. queenslandicum. The event has likely happened in South America and either predated the speciation of the respective South American taxa (∼1 Mya) or spread among them later by introgression. |
| Setaria 3 | 4/7 | The monophyletic Setaria clade is composed of several panicoid genera, including two species of Setaria. None of the groups have the captured ITS type, which occurs only in South American Hordeum species and shows relatively high sequence diversity. Four of the accessions show two to three divergent subtypes (e.g., Set-a, Set-b), which are shared between species. | No closely related panicoid species is known/available; therefore, the donor species or even genus remains unclear in this case but likely has South American origin. Different ITS subtypes within several Hordeum individuals that are shared with other accessions/species suggest that they are paralogs that may have resulted from duplications of the ITS region after acquisition by their hosts. This scenario would argue for a transfer predating their speciation. However, the high sequence diversity within this ITS type may also suggest multiple independent transfers, even from different donor species. |
| Setaria 4 | 3/4 | One Central Asian Hordeum and two Pseudoroegneria species show this type. Hordeum brevisubulatum is basal to three Pseudoroegneria sequences, which show relatively high ITS sequence divergence. Setaria chondrachne is sister to this clade. | Only Central Asian and North American pooid species show this type, indicating acquisition by the Asian species S. chondrachne or a closely related taxon in Central Asia. Pseudoroegneria species may have obtained it by hybridization with H. brevisubulatum, after which it underwent additional sequence diversification and reached North America along with Ps. spicata, the only diploid New World taxon of this genus. |
| Paspalum 5 | 9/16 | Nine of 10 South American Hordeum species share this ITS type. All sequences within this clade are very similar. No particular species of Paspalum clusters with this type. | This is the second largest assemblage of species harboring a particular nonnative ITS type. Their high sequence similarity suggests a single transfer event that predated speciation of the South American Hordeum species (∼1 Mya). Multiple independent acquisitions or extensive hybridization are rather unlikely explanations in this case. |
| Paspalum 6 | 7/11 | This clade shows relatively high sequence diversity. Exclusively New World species harbor this type, among them two North American Hordeum species that occur in derived positions and do not possess any other panicoid ITS types. No particular species of Paspalum matches this type. | High sequence diversity implies high rates of molecular evolution after acquisition or reflects at least two independent events. Sequences of two North American Hordeum taxa are nested within South American species. This pattern is in accordance with their derived phylogenetic position and an inferred long-distance dispersal event from South America to North America. Here, the panicoid ITS, acquired from an unknown Paspalum species donor, seems to reflect the speciation patterns of the host. |
| Paspalum 7 | 2/2 | Panicoid ITS sequences of one North American and one South American Hordeum species cluster with Paspalum dilatatum, which is native to Brazil and Argentina but was introduced worldwide. | In this case, a particular panicoid species, Pa. dilatatum, can be identified as the likely donor of the exotic ITS type. The two species have nonoverlapping distribution, indicating either two independent transfers from the same donor species or one transfer to their common ancestor, with differential loss of this variant in all other species. |
| Euclasta 8 | 7/12 | Six South American and one North American Hordeum species show this ITS type. One accession (Hordeum stenostachys BCC2021) possesses two subtypes. Relatively high sequence divergence is observed in this clade, which is sister to E. condylotricha. | The geographically widespread occurrence of this type is in keeping with an acquisition that predated the speciation of the New World species (∼1.46 Mya). E. condylotricha has a wide distribution in the African and Asian tropics as well as southern North America and northern South America. Only two species of genus Euclasta are known (the second one does not occur in the New World); therefore, we assume that E. condylotricha was possibly the actual rDNA donor. |
| Arundinella 9 | 2/3 | Two Argentinian species have this rare ITS type with sequence that corresponds to A. hispida, a species occurring in tropical Asia, Central America, and South America. | This type occurs in only two species with overlapping distribution. All three accessions also contain Panicum and Paspalum types, which suggests a rather recent acquisition that has happened after the other events; this pattern is in keeping with ITS matching a particular donor species. |
Nine clades of panicoid rDNA sequences present in pooid grasses as depicted in Fig. 1A are described and interpreted in the context of host evolution and biogeographic patterns (Fig. 1B) (25, 28, 56, 57). Briefly summarized, three Western Asian and Mediterranean sections of Hordeum constitute the oldest lineages of the genus. The youngest section, Stenostachys, in which the panicoid rDNA is found, split from them ∼5 Mya. The barley genus then spread into Central Asia, and eventually, one lineage split from the Central Asian species ∼1.7 Mya and reached the New World via Beringia. The onset of speciation in the New World clade occurred ∼1.46 Mya. Long-distance dispersal brought the barleys to South America, where they underwent a rapid divergence (∼1 Mya) accompanied by incomplete lineage sorting, which results in allele and haplotype sharing across species. Two North American species, Hordeum pusillum and Hordeum intercedens, originate from a southern lineage that migrated back north again.
Different subspecies of H. patagonicum are not distinguished.
In an attempt to reveal the evolutionary origin of the transfers, the distribution of the nonnative ITS sequences was compared with a phylogeny of genus Hordeum based on native ITS sequences (Fig. 1B), incorporating preexisting information regarding its speciation and phylogeography (25, 27, 28) (Table S4). According to this analysis, the likely most ancient transfer event was Panicum 1, because this rDNA type was found in all of the Central Asian and most of the South American taxa (Fig. 1A). This event, therefore, likely occurred in their common ancestor, which predated the diversification of section Stenostachys, thereby establishing the timing of the transfer to 1.7–5.0 Mya and locating it to Central Asia. Of the other events, only Setaria 4 did not involve New World (American) species. Setaria 4 was present in the Central Asian species Hordeum brevisubulatum and two Pseudoroegneria species (Table S4). The acquisition of this nonnative rDNA by Pseudoroegneria spp. could have occurred independently of its acquisition by Hordeum, but it is more likely that the transfer was disseminated by wide hybridization given the number of established Elymus spp. allopolyploids, which have arisen from various Hordeum × Pseudoroegneria hybrids. The New World Hordeum species seem to have experienced a number of transfers, some of which (Paspalum 5 and 6 and Euclasta 8) were represented in a number of species, whereas others (Panicum 2 and Arundinella 9) were restricted to just a few species (Fig. 1A, Inset). The most parsimonious scenario for the former group posits that a small number of events transferred the panicoid rDNA into a New World Hordeum species before the major speciation period (about 1.46 Mya) and that these transfers were then carried vertically into the various new species. A more detailed evaluation of individual transfer events and their interpretation in relation to host phylogeny and speciation are provided in Table S4.
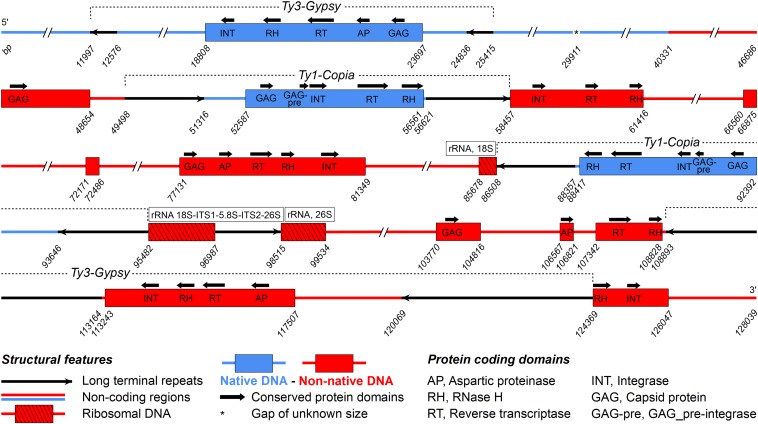
A characterization of the structural features of the panicoid rDNA was undertaken by constructing a BAC library of H. bogdanii, a Central Asian species harboring only the Panicum 1 ITS type. Sequencing of the single positive clone (BAC 46L9) recovered from the library revealed a retrotransposon-rich ∼70-kbp region of Panicum-derived DNA, which included parts of an rDNA unit. The nonnative sequence was interrupted by two Hordeum-specific Copia-like transposable elements (TEs), which implied that two transposition events must have followed the integration of the panicoid rDNA segment into the Hordeum genome (Fig. 2). One of the TEs has disrupted the 18S rRNA gene within the Panicum rDNA, thereby effectively disabling the unit. On the basis of sequence divergence in the 5′ and 3′ long-terminal repeats (LTRs) of TEs, the timing of the transposition events was estimated to be 0.25–0.29 Mya, which is substantially later than the date of the panicoid rDNA acquisition. The retention of such a large block of horizontally transferred nuclear DNA shows that even presumably selectively neutral material can be maintained over a significant (in evolutionary terms) time period.
Fig. 2.
A physical map of BAC clone 46L9 harboring a genomic fragment of panicoid origin.
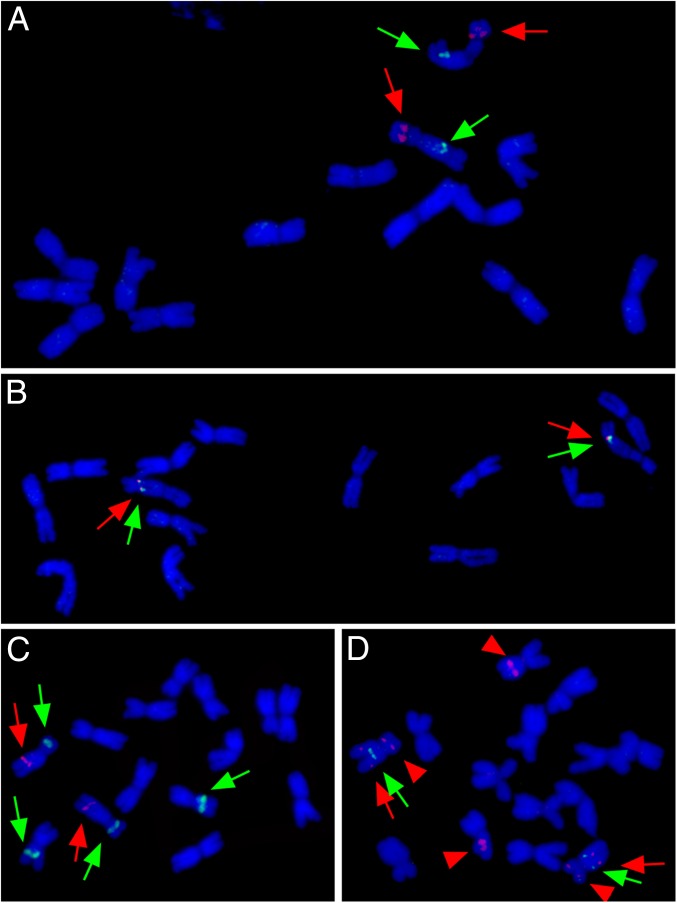
The FISH technique, using BAC 46L9 as the probe, was used to locate the insertion site of the Panicum-derived rDNA segment in H. bogdanii. The hybridization mix also included a labeled 45S rDNA probe comprising the 18S, 5.8S, and 26S rDNA genes and the ITS to locate the native rDNA sites of Hordeum. Both the Panicum and the native rDNA mapped to the same chromosome, but whereas the former corresponded to an interstitial site on the long arm, the latter located to a cluster on the short arm (Fig. 3A). When the experiment was repeated using BAC 46L9 and Panicum bergii genomic DNA as probes, the two hybridization sites fully overlapped with one another (Fig. 3B), showing that H. bogdanii harbored only a single segment derived from Panicum. A similar experiment was conducted on Hordeum pubiflorum, which harbored rDNA derived from both Panicum and Paspalum. When its mitotic chromosomes were hybridized with labeled genomic DNA of P. bergii and Paspalum dilatatum, the only Panicum rDNA site detected mapped to the identical site as in H. bogdanii (Fig. 3C), whereas the Paspalum rDNA mapped to a different site, although on the same chromosome (Fig. 3D). This outcome supports the phylogeny-based hypotheses concerning independent acquisitions from different Panicoideae sources as well as establishes the presence of the most ancient material at identical sites in two distantly related Hordeum species.
Fig. 3.
Molecular cytogenetic analysis of (A and B) H. bogdanii and (C and D) H. pubiflorum. (A) The BAC clone 46L9 signal (green arrows) localizes to a site on the long arm of the same chromosome that carries the 45S-rDNA cluster (red arrows). (B) The BAC clone 46L9 signal (green arrows) colocalizes with the signal obtained from the P. bergii genomic DNA probe (red arrows) to a site on a single chromosome pair. (C) The signal obtained from the P. bergii genomic DNA probe (red arrows) localizes to a site on the long arm of the same chromosome that carries one of two native rDNA clusters present (green arrows). (D) The signal obtained from the Pa. dilatatum genomic DNA probe (green arrows) highlights a site proximal to one hybridizing to P. bergii genomic DNA (red arrows). The red arrowheads show the native rDNA clusters.
To determine whether any of the panicoid rDNA segments had retained their functionality, RNA was isolated from the leaf of seven Hordeum and one Pseudoroegneria species, reverse-transcribed, and then, subjected to RT-PCR using Panicoideae-specific PCR primer pairs (Table S3). The experiment failed to show even one example of the transcription of panicoid rDNA. The mechanism underlying the silencing of these genes was explored by searching for base substitutions in the five most conserved motifs within both the 5.8S gene and the ITS2 region and also, alterations that may have compromised the ability of 5.8S RNA transcripts to form the necessary secondary structures (Dataset S1 and Table S5). Most of the nonnative rRNA present in both Hordeum and Pseudoroegneria spp. showed evidence of sequence alterations, which could have disrupted functionality. Thus, although it remains a possibility that some of the nonnative rDNA have retained functionality, the abundance of their transcript cannot be high enough in a background of a large excess of native rDNA transcript to offer any selective advantage to the host.
Table S5.
Inference of functionality of panicoid rDNA clones across Hordeum and Pseudoroegneria accessions
| rDNA type | No. of clones | Inferred no. of pseudogenes | |
| Conserved motifs | 5.8S secondary structure | ||
| Hordeum | |||
| Panicum 1 | 60 | 60 | 4 |
| Panicum 2 | 12 | 3 | 0 |
| Paspalum | 73 | 70 | 23 |
| Euclasta | 38 | 17 | 10 |
| Setaria | 48 | 30 | 15 |
| Arundinella | 5 | 0 | 5 |
| Pseudoroegneria | |||
| Setaria | 42 | 42 | 2 |
HGT may represent the most plausible explanation for the presence of the nonnative rDNA in these Triticeae species, although it is acknowledged that the evidence for it is only circumstantial. The alternative possibility requires a successful hybridization between Pooideae and Panicoideae grasses, which is hindered by crossing barriers (20–22). Even if the barriers to such wide crosses were overcome (which is possible when plants—particularly polyploid species—are raised under controlled conditions), the introduction via this route of segments derived from as many as five distinct Panicoideae genera into a diploid barley seems unlikely. Only few proven natural triple hybrids exist (ref. 29 and references therein), and to our knowledge, no natural intergeneric or interspecific hybrid with more than three parents has ever been reported. In contrast, both aphids and endophytic fungi represent potential vectors for HGT. A number of aphid species feed on a range of grass genera, whereas it has recently been shown that endophytic fungal symbionts of the genus Epichloë are capable of horizontally acquiring genes from their grass host (30). The proliferation of fungal endosymbionts in seeds ensures a prolonged physical contact with their host, which is necessary for HGT to occur.
The exchange of genetic material requires that the partners coincide with one another in time and space, which is no longer the case for all plant taxa involved here. It is known that the distribution of species is strongly influenced by climate, which has changed greatly over the past 5 My (31), resulting in historical opportunities for species to have overlapped that are no longer possible today (32). The inferred timing of the transfer events implies interactions between Central Asian and New World Pooideae species with subtropical or tropical Panicoideae. After established in a Hordeum host, the various panicoid DNA sequences could have been transferred from progenitor to a derived species and/or spread via hybridization between the mostly interfertile species of section Stenostachys.
The real frequency with which HGT occurs is highly uncertain (2, 3), which is also the case for the consequences (if any) of the presence of exotic DNA (14, 33). To date, the detection of HGT has been haphazard, and a systematic search for it in a large plant genome, such as that of Hordeum, is scarcely feasible. The evidence presented here is based on an approach that was specifically designed to target rDNA genes of Panicoideae after an initial chance discovery (18), and it is doubtful whether a single copy of exotic rDNA could be detected using a whole-genome approach given the large number of native copies present. These data suggest that HGT may not be an exceptional event in the grasses and by implication, similarly so for other angiosperm families. Although the capture of panicoid rDNA may have contributed to the rapid diversification experienced by section Stenostachys, the most species-rich Hordeum group (25), it seems more likely that the transfers were selectively neutral and hence, random.
Materials and Methods
Plant Materials.
Only diploid taxa (2n = 14) were considered here, with the exception of one autotetraploid accession of Hordeum bulbosum (GRA970; 2n = 28) (Table S1). All currently recognized diploid species belonging to the genus Hordeum were sampled (20 species along with nine subspecies); each was represented by two accessions, with the exception of Hordeum erectifolium, for which only a single population was available. The screen also included 7 Pseudoroegneria spp. accessions (spread across five species) and 18 accessions (15 species) belonging to other Triticeae genera (Table S1). The materials were sourced from the Leibniz Institute of Plant Genetics and Crop Plant Research GenBank, the US Department of Agriculture (USDA) National Small Grains Collection, the Plant Genetic Resources Canada (PGR Canada) and a private collection of F.R.B.
rDNA Amplification.
The total rDNA ITS was PCR-amplified from each accession using the Poaceae-specific primer pair ITS-Poa-f/ITS4 (34) (Fig. S1, step 1) in three independent reactions to reduce PCR bias. The resulting amplicons (amplicon 1) produced from a Hordeum sp. template were sequenced from both ends, and the acquired sequences were used to construct a native rDNA-based phylogeny (Fig. 1B). Where sequence reads were disturbed because of heterogeneity in the amplicon, the amplicon was first cloned using a TOPO TA Cloning Kit (Invitrogen); then, eight clones per amplicon were sequenced based on the M13 forward primer.
The presence of panicoid rDNA in the sample species genomes was detected in a PCR assay based on a Panicoideae-specific primer pair (18). Subsequently, a number of specific primer combinations were designed to selectively amplify particular ITS variants (Table S3), and these primers were applied to the full set of accessions. The steps involved in the screening procedure are described in detail in SI Materials and Methods.
Phylogenetic Analyses.
The nonnative rDNA present in the Hordeum and Pseudoroegneria genomes was placed in a phylogenetic context within the Panicoideae by carrying out a Bayesian inference and maximum parsimony analysis. Details regarding the necessary sequence alignments and the parameters chosen for the phylogenetic analysis are provided in SI Materials and Methods.
Construction and Analysis of an H. bogdanii BAC library.
A low-coverage genomic BAC library was constructed from H. bogdanii accession number BCC2063 (Table S1) based on high-molecular weight DNA prepared from flow-sorted nuclei. The procedure was modified from that in ref. 35. A 30-g sample of fresh leaf tissue was fixed in formaldehyde and homogenized. Nuclei were concentrated from the resulting suspension by passing it through an FACSAria device (Becton Dickinson), and they were embedded in agarose plugs (each plug harbored around 105 nuclei), which were then treated with HindIII. The partially digested DNA was separated by pulsed-field gel electrophoresis (two size selection steps), and fragments in the range 100–300 kbp were isolated from the gel and ligated into a dephosphorylated pCC1BAC plasmid (Epicentre). The recombinant plasmid was transformed into Escherichia coli strain MegaX DH10B T1 (Life Technologies/Invitrogen) via electroporation. In total, 18,432 clones were picked and ordered in a set of 384-well microtiter plates.
Screening of the BAC Library and Sequencing of Selected BAC Clones.
The BAC library was spotted onto 22.2 × 22.2-cm nylon filters (4 × 4 pattern; 18,432 clones on each filter in duplicate). To identify BAC clones possessing Panicum rDNA, a hybridization probe specific for each of the Panicum-like ITS1 and ITS2 sequences present in H. bogdanii was amplified, labeled with α32P using a Prime-It II Random Primer Labeling Kit (Agilent) according to the manufacturer’s protocol, and hybridized to the filters. DNA from 21 positive BAC clones was isolated using an Invitek Plant Mini DNA Kit (www.thistlescientific.co.uk) and prepared for sequencing using a TruSeq DNA PCR-Free Kit (Illumina). The BACs were sequenced using a MiSeq instrument and the MiSeq Reagent Kit v3 to achieve pair-end reads of length of 300 nt, giving an at least 100× coverage for each BAC.
Assembly and Analysis of the BAC Clone Sequences.
The BAC sequence reads were initially filtered to remove bacterial sequences using ERNE v.1.2 software (36). Adaptors were trimmed using the TRIMMOMATIC v.0.30 tool (37) with the following parameters: ILLUMINACLIP 2:30:10, LEADING:20, TRAILING:20, SLIDINGWINDOW:4:20, and MINLEN:85. The BAC assembly was performed using Ray v. 2.3.1 software (38), applying a range of K-mer values, and the best assembly was chosen based on a set of criteria (N50, BAC coverage, and N content). The sequences were subjected to a BLAST search to select which BAC clones contained a panicoid rDNA unit. The BAC sequences were scanned for the presence of the Panicum-like ITS sequence and the native ITS sequence of H. bogdanii (GenBank accession nos. FJ793096 and KU513503). The sequence of BAC 46L9 (two scaffolds comprising 128,039 bp) was analyzed using BLAST2GO v. 3.1.3 (39). BLASTN and BLASTX analyses were run to identify the highest similarity sequences and assess the function of the sequence, respectively. A conserved domain search was performed on the National Center for Biotechnology Information web server. The online version of LTR_FINDER (40) was used to identify retrotransposon LTRs. Subregions of the rDNA sequence were identified by reference to the sequence of Alloteropsis semialata rDNA [GenBank accession no. KT281159 (41)].
Inferring the Timing of TE Insertion.
The insertion times of the two Hordeum-like Copia retrotransposons nested within the panicoid DNA segment in H. bogdanii were estimated following the work in ref. 42. Paired LTR sequences were aligned, and the number of base substitutions was counted. The age of the insertion was estimated using the expression N/(2 × L × K), where N represented the number of substitutions between the two LTRs and L was the length of the LTR. A value of 6.5 × 10−9 was assumed for K (mutation rate per synonymous site per year) (43).
Cytogenetic Analyses.
FISH was used to localize native and nonnative rDNA sequences on mitotic chromosomes of selected Hordeum species as described earlier (18). DNA of BAC 46L9 was isolated and labeled with digoxigenin using a DIG-Nick Translation Kit (Roche Applied Science) according to the manufacturer’s protocol. The rDNA clone pTa71 (44), which contains the 18S-5.8S-26S cluster of rDNA genes and the ITS region, was labeled with biotin using a Biotin-Nick Translation Kit (Roche Applied Science) according to the manufacturer’s protocol. A second set of experiments used as probes genomic DNA of P. bergii (labeled with biotin) and BAC 46L9 (labeled with digoxigenin). In two additional experiments, genomic DNA of P. bergii (labeled with biotin) and pTa71 (labeled with digoxigenin) were hybridized to H. pubiflorum (BCC2028) chromosomes, and genomic DNA of Pa. dilatatum (labeled with digoxigenin) and genomic DNA of P. bergii (labeled with biotin) were hybridized to H. pubiflorum chromosomes. The latter preparations were reprobed with biotin-labeled pTa71. In each case, the site of probe hybridization was detected using a combination of anti–digoxigenin-FITC conjugate (Roche Applied Science) and streptavidin-Cy3 conjugate (Life Technologies/Invitrogen). The chromosomes were counterstained with 1.5 μg/mL DAPI in Vectashield Antifade Solution (Vector Laboratories).
Assay for the Transcription of Nonnative rDNA.
Real-time PCR was used to screen selected Hordeum accessions and Ps. spicata using primer pairs targeting the various panicoid rDNA types (Table S3). RNA was extracted from the leaf of H. bogdanii BCC2063, Hordeum roshevitzii BCC2015, Hordeum chilense GRA1000, Hordeum cordobense BCC2039, Hordeum patagonicum ssp. setifolium BCC2038, Hordeum patagonicum ssp. magellanicum BCC2065, H. pubiflorum BCC2028, and Ps. spicata USDA563869 using an RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. Contaminating genomic DNA was removed by treatment with a TURBO DNA-Free Kit (Life Technologies/Invitrogen), and cDNA was then synthesized using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche) based on random hexamer priming. The PCRs were identical to the DNA-based ones described above, except that cDNA rather than genomic DNA was used as the template. The concentration of the cDNA was varied from 0.125 to 12.5 ng/μL. Two control PCRs were carried out: one was primed with ITS-Poa-f/ITS4 to ensure that the cDNA preparation was satisfactory, and the other was supplied with DNase-treated RNA as the template to ensure that the DNase treatment had been effective. The PCR products were visualized by EtBr staining after electrophoretic separation through a 2.5% (wt/vol) agarose gel.
Nucleotide Substitutions in the Nonnative rDNA Sequences.
The functionality of the nonnative rDNA sequences across all accessions and each of the ITS types was assessed by querying the sequence of the highly conserved motifs of the 5.8S gene and the ITS2 (45); in addition, the ability of 5.8S RNA transcripts to form constrained secondary structures based on a prediction model was assessed. The analyses were performed as described in ref. 46, and only the latter was affected using the ViennaRNA Package 2.0 (47).
SI Materials and Methods
Amplification of Nonnative rDNA.
After amplification of the total ITS-rDNA region (step 1 in Fig. S1), all PCR products were subjected to a subsequent seminested PCR with a Panicoideae-specific primer ITS-Pan-bac used in combination with a Poaceae-specific ITS-Poa-f forward primer as described in our previous study (18) (step 2 in Fig. S1). PCR reagents and cycling conditions were the same as in step 1, with the exception that 1 µL 1:10 diluted amplicon 1 was used as a template. Panicoid rDNA was detected only in genera Hordeum and Pseudoroegneria (Table S2).
For each species with a positive amplification result (amplicon 2), at least one accession was selected (Table S2) in which amplicon 2 was cloned and Sanger sequenced to disentangle possible variation in panicoid rDNA. Between 9 and 22 clones of Hordeum accessions and 42 clones of Pseudoroegneria spicata were sequenced with the M13 forward primer (step 3 in Fig. S1). Using BLAST and preliminary phylogenetic analyses, rDNA variants corresponding to five genera belonging to the Panicoideae grass subfamily were identified: Panicum, Paspalum, Setaria, Euclasta, and Arundinella.
On the inspection of variation in panicoid rDNA, primers specific for each rDNA variant were designed (step 4 in Fig. S1). In the case of Panicum, two primer combinations were designed, amplifying two relatively diverse rDNA subtypes Panicum 1 and Panicum 2. Primer sequences are listed in Table S3.
The designed genus-specific primers were then used for PCR screening of all Hordeum and Pseudoroegneria accessions (step 5 in Fig. S1). PCRs with all primer combinations were carried out as nested PCRs with diluted amplicon 1 as a template. PCR reagents and cycling conditions, except for annealing temperatures, remained the same as in the step 1 PCR. Annealing temperatures are in Table S3. Successful amplification (amplicon 3 in Fig. S1) was interpreted as presence of the respective rDNA variant in the sample, which was confirmed by Sanger sequencing. In all cases, PCR screening with the genus-specific primers yielded equal or higher numbers of detected panicoid rDNA variants than sequencing of clones of amplicon 2 (step 3 in Fig. S1). Therefore, rDNA variants not captured in step 3 were PCR-amplified from additional clones of amplicon 2 (between 94 and 412 clones per sample were PCR-screened) with the respective specific panicoid primers from step 4. Because of the short lengths of these amplicons, positive clones were reamplified and sequenced with M13 primers to obtain full-length products of the respective variants.
If a particular rDNA variant was detected in step 5 but could not be amplified from the clones of amplicon 2 (probably because of its rarity coupled with the limited number of clones available), the corresponding amplicon 3 was directly sequenced from both strands to verify the identity of the rDNA variant in the sample. A complete list of presence/absence of all panicoid variants is provided in Table S2.
Sequence Alignments and Choice of Representative Sequences for Phylogenetic Analysis.
Native rDNA in Hordeum.
In each of eight cloned accessions, two different ITS types (paralogs or other allelic variation) (Fig. 1B) were encountered and included in the Hordeum alignment. In the other accessions, the chromatograms were clearly readable, with few rare polymorphisms, or completely lacking intraindividual variability of the multicopy marker. The initial alignment was done using Clustal_X (48) and adjusted manually in BioEdit v. 7.1.3.0 (49). Bromus tectorum L. and Bromus inermis Leyss. (GenBank accession nos. L36485 and L11579) were included as outgroup. The final alignment included 55 sequences and 601 characters.
Panicoid rDNA in Hordeum.
ITS sequences obtained in step 3 (300 sequences), step 6 (134 sequences), and step 7 (8 sequences) were pooled and aligned separately for each Hordeum accession using Clustal_X and BioEdit software as described above. To facilitate identification (and exclusion) of recombinant sequences, the alignment of each accession was augmented with (i) the sequence of respective native Hordeum ITS and (ii) sequences of taxa previously identified to be the most similar to individual panicoid rDNA types using BLAST [i.e., Panicum bergii Arechav. (GenBank accession no. AY129696), Paspalum dilatatum Trin. (GenBank accession no. KF163842), Setaria chondrachne Honda (GenBank accession no. KF163693), Euclasta condylotricha Stapf. (GenBank accession no. DQ005050), and Arundinella hispida Kuntze (GenBank accession no. GQ870152)]. The alignments were straightforward along their entire lengths, clearly differentiating sequences of individual variants. Mosaic sequences combining different parts of the sequences typical of individual ribotypes were considered as recombinant (198 sequences) and excluded from the analyses. The remaining 244 (236 cloned and 8 direct sequences) were used in additional analyses. To condense the dataset, only one of multiple identical sequences per individual accession (disregarding unique substitutions that were most likely caused by polymerase errors in cloned samples) was selected for phylogenetic analyses. In total, 90 ITS sequences (82 clones and 8 direct sequences) representing the panicoid rDNA types amplified from Hordeum accessions were included in the phylogenetic analysis.
Panicoid rDNA in Pseudoroegneria.
Forty-two ITS sequences of Ps. spicata obtained in step 3 and two sequences (from Ps. spicata and Pseudoroegneria strigosa) obtained in step 7 were pooled, aligned using Clustal_X and BioEdit, and inspected for recombinants (no recombinants were detected) as described for Hordeum. For each accession, a single sequence was selected for phylogenetic analyses.
Phylogenetic Analyses.
Hordeum phylogeny.
Bayesian inference was carried out in MrBayes v. 3.2.6 (50, 51) as follows. (i) SYM + G (symmetrical model with gamma-distributed rate variation among sites) was determined as the best-fitting model of molecular evolution by hierarchical likelihood ratio tests with MrModeltest, version 2.3 (52). (ii) Six substitution rates and gamma distribution were specified as settings. (iii) B. tectorum was used as outgroup. (iv) Two simultaneous Metropolis-coupled Markov chain Monte Carlo analyses (53) with four chains each were run, incrementally heated by a temperature of 0.1 for 1 million generations at a sampling frequency of 100. (v) SD of split frequencies (0.01) was used as a convergence diagnostic. (vi) After stationarity was reached, the first 25% of trees were discarded as burn-in, and a consensus tree with branch lengths and posterior probabilities was computed.
Maximum parsimony analysis was run in PAUP* 4b10 (54) using heuristic search; 10 random addition replicates, keeping no more than 100 trees of length greater than or equal to one in each replicate; tree bisection-reconnection branch swapping, and gaps treated as missing data. B. tectorum and B. inermis were used as outgroup. Bootstrapping as a measure of topological robustness was performed with 1,000 replicates using the same settings.
Panicoideae phylogeny.
Bayesian inference (BI) and maximum parsimony (MP) analyses were carried out with the same settings as described above for native Hordeum rDNA, with the following modifications. BI: (i) GTR + G (general time reversible model with gamma-distributed rate variation among sites) was determined as the best-fitting model of molecular evolution, (ii) 3 million generations were run, and (iii) Centhoteca lappacea was used as outgroup. MP: Phragmites australis, Arundo donax, and C. lappacea were used as outgroup.
Supplementary Material
Acknowledgments
We thank the Leibniz Institute of Plant Genetics and Crop Plant Research Gatersleben Ex Situ Gene Bank, the USDA National Small Grains Collection, and the Plant Genetic Resources Canada (PGR Canada) for providing the germplasm, and Veronika Bambasová and Jana Kadlecová for their contribution to the DNA analyses. Two anonymous reviewers are thanked for their suggestions to improve the manuscript. We also thank Robert Koebner for English editing of the manuscript. This research was financially supported by Czech Science Foundation Grant 13-04454S (to V.M. and J.Š.), Czech Academy of Sciences Long-Term Research Development Project RVO 67985939, and Ministry of Education, Youth and Sports of the Czech Republic (Award LO1204 from the National Program of Sustainability I).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KU513490–KU513542, KU551928–KU552018, KX344961, KX344962, and KX372731).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613375114/-/DCSupplemental.
References
- 1.Keeling PJ, Palmer JD. Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet. 2008;9(8):605–618. doi: 10.1038/nrg2386. [DOI] [PubMed] [Google Scholar]
- 2.Bock R. The give-and-take of DNA: Horizontal gene transfer in plants. Trends Plant Sci. 2010;15(1):11–22. doi: 10.1016/j.tplants.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Soucy SM, Huang J, Gogarten JP. Horizontal gene transfer: Building the web of life. Nat Rev Genet. 2015;16(8):472–482. doi: 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]
- 4.Won H, Renner SS. Horizontal gene transfer from flowering plants to Gnetum. Proc Natl Acad Sci USA. 2003;100(19):10824–10829. doi: 10.1073/pnas.1833775100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stegemann S, Keuthe M, Greiner S, Bock R. Horizontal transfer of chloroplast genomes between plant species. Proc Natl Acad Sci USA. 2012;109(7):2434–2438. doi: 10.1073/pnas.1114076109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice DW, et al. Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science. 2013;342(6165):1468–1473. doi: 10.1126/science.1246275. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Maruyama S, Nozaki H, Shirasu K. Horizontal gene transfer by the parasitic plant Striga hermonthica. Science. 2010;328(5982):1128. doi: 10.1126/science.1187145. [DOI] [PubMed] [Google Scholar]
- 8.Xi Z, et al. Horizontal transfer of expressed genes in a parasitic flowering plant. BMC Genomics. 2012;13:227. doi: 10.1186/1471-2164-13-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, et al. Evolution of a horizontally acquired legume gene, albumin 1, in the parasitic plant Phelipanche aegyptiaca and related species. BMC Evol Biol. 2013;13:48. doi: 10.1186/1471-2148-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, et al. Root parasitic plant Orobanche aegyptiaca and shoot parasitic plant Cuscuta australis obtained Brassicaceae-specific strictosidine synthase-like genes by horizontal gene transfer. BMC Plant Biol. 2014;14:19. doi: 10.1186/1471-2229-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim G, LeBlanc ML, Wafula EK, dePamphilis CW, Westwood JH. Plant science. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science. 2014;345(6198):808–811. doi: 10.1126/science.1253122. [DOI] [PubMed] [Google Scholar]
- 12.Diao X, Freeling M, Lisch D. Horizontal transfer of a plant transposon. PLoS Biol. 2006;4(1):e5. doi: 10.1371/journal.pbio.0040005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallenback P, Jaarola M, Ghatnekar L, Bengtsson BO. Origin and timing of the horizontal transfer of a PgiC gene from Poa to Festuca ovina. Mol Phylogenet Evol. 2008;46(3):890–896. doi: 10.1016/j.ympev.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Christin PA, et al. Adaptive evolution of C(4) photosynthesis through recurrent lateral gene transfer. Curr Biol. 2012;22(5):445–449. doi: 10.1016/j.cub.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 15.El Baidouri M, et al. Widespread and frequent horizontal transfers of transposable elements in plants. Genome Res. 2014;24(5):831–838. doi: 10.1101/gr.164400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markova DN, Mason-Gamer RJ. The role of vertical and horizontal transfer in the evolutionary dynamics of PIF-like transposable elements in Triticeae. PLoS One. 2015;10(9):e0137648. doi: 10.1371/journal.pone.0137648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markova DN, Mason-Gamer RJ. Diversity, abundance, and evolutionary dynamics of Pong-like transposable elements in Triticeae. Mol Phylogenet Evol. 2015;93:318–330. doi: 10.1016/j.ympev.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Mahelka V, Kopecký D. Gene capture from across the grass family in the allohexaploid Elymus repens (L.) Gould (Poaceae, Triticeae) as evidenced by ITS, GBSSI, and molecular cytogenetics. Mol Biol Evol. 2010;27(6):1370–1390. doi: 10.1093/molbev/msq021. [DOI] [PubMed] [Google Scholar]
- 19.Chalupska D, et al. Acc homoeoloci and the evolution of wheat genomes. Proc Natl Acad Sci USA. 2008;105(28):9691–9696. doi: 10.1073/pnas.0803981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riera-Lizarazu O, Rines HW, Phillips RL. Cytological and molecular characterization of oat x maize partial hybrids. Theor Appl Genet. 1996;93(1-2):123–135. doi: 10.1007/BF00225737. [DOI] [PubMed] [Google Scholar]
- 21.Gernand D, et al. Uniparental chromosome elimination at mitosis and interphase in wheat and pearl millet crosses involves micronucleus formation, progressive heterochromatinization, and DNA fragmentation. Plant Cell. 2005;17(9):2431–2438. doi: 10.1105/tpc.105.034249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii T, Ueda T, Tanaka H, Tsujimoto H. Chromosome elimination by wide hybridization between Triticeae or oat plant and pearl millet: Pearl millet chromosome dynamics in hybrid embryo cells. Chromosome Res. 2010;18(7):821–831. doi: 10.1007/s10577-010-9158-3. [DOI] [PubMed] [Google Scholar]
- 23.Mason-Gamer RJ. Reticulate evolution, introgression, and intertribal gene capture in an allohexaploid grass. Syst Biol. 2004;53(1):25–37. doi: 10.1080/10635150490424402. [DOI] [PubMed] [Google Scholar]
- 24.Blattner FR. Phylogenetic analysis of Hordeum (Poaceae) as inferred by nuclear rDNA ITS sequences. Mol Phylogenet Evol. 2004;33(2):289–299. doi: 10.1016/j.ympev.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Brassac J, Blattner FR. Species-level phylogeny and polyploid relationships in Hordeum (Poaceae) inferred by next-generation sequencing and in silico cloning of multiple nuclear loci. Syst Biol. 2015;64(5):792–808. doi: 10.1093/sysbio/syv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang RRC, et al. Genomic symbols in the Triticeae (Poaceae) In: Wang RRC, Jensen KB, Jaussi C, editors. Proceedings of the 2nd International Triticeae Symposium. Utah State Univ Press; Logan, UT: 1996. pp. 29–34. [Google Scholar]
- 27.Blattner FR. Progress in phylogenetic analysis and a new infrageneric classification of the barley genus Hordeum (Poaceae: Triticeae) Breed Sci. 2009;59:471–480. [Google Scholar]
- 28.Jakob SS, Blattner FR. A chloroplast genealogy of Hordeum (Poaceae): Long-term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequences for phylogenetic inference. Mol Biol Evol. 2006;23(8):1602–1612. doi: 10.1093/molbev/msl018. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan Z, Fehrer J. Molecular evidence for a natural primary triple hybrid in plants revealed from direct sequencing. Ann Bot (Lond) 2007;99(6):1213–1222. doi: 10.1093/aob/mcm072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ambrose KV, Koppenhöfer AM, Belanger FC. Horizontal gene transfer of a bacterial insect toxin gene into the Epichloë fungal symbionts of grasses. Sci Rep. 2014;4:5562. doi: 10.1038/srep05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewitt GM. Speciation, hybrid zones and phylogeography - or seeing genes in space and time. Mol Ecol. 2001;10(3):537–549. doi: 10.1046/j.1365-294x.2001.01202.x. [DOI] [PubMed] [Google Scholar]
- 32.Kadereit JW. The geography of hybrid speciation in plants. Taxon. 2015;64:673–687. [Google Scholar]
- 33.Li FW, et al. Horizontal transfer of an adaptive chimeric photoreceptor from bryophytes to ferns. Proc Natl Acad Sci USA. 2014;111(18):6672–6677. doi: 10.1073/pnas.1319929111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahelka V, Fehrer J, Krahulec F, Jarolímová V. Recent natural hybridization between two allopolyploid wheatgrasses (Elytrigia, Poaceae): Ecological and evolutionary implications. Ann Bot (Lond) 2007;100(2):249–260. doi: 10.1093/aob/mcm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Šafář J, et al. Creation of a BAC resource to study the structure and evolution of the banana (Musa balbisiana) genome. Genome. 2004;47(6):1182–1191. doi: 10.1139/g04-062. [DOI] [PubMed] [Google Scholar]
- 36.Del Fabbro C, Scalabrin S, Morgante M, Giorgi FM. An extensive evaluation of read trimming effects on Illumina NGS data analysis. PLoS One. 2013;8(12):e85024. doi: 10.1371/journal.pone.0085024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boisvert S, Laviolette F, Corbeil J. Ray: Simultaneous assembly of reads from a mix of high-throughput sequencing technologies. J Comput Biol. 2010;17(11):1519–1533. doi: 10.1089/cmb.2009.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conesa A, et al. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z, Wang H. LTR_FINDER: An efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res. 2007;35(Web Server issue):W265-8. doi: 10.1093/nar/gkm286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundgren MR, et al. Photosynthetic innovation broadens the niche within a single species. Ecol Lett. 2015;18(10):1021–1029. doi: 10.1111/ele.12484. [DOI] [PubMed] [Google Scholar]
- 42.Blavet N, et al. Comparative analysis of a plant pseudoautosomal region (PAR) in Silene latifolia with the corresponding S. vulgaris autosome. BMC Genomics. 2012;13:226. doi: 10.1186/1471-2164-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaut BS, Morton BR, McCaig BC, Clegg MT. Substitution rate comparisons between grasses and palms: Synonymous rate differences at the nuclear gene Adh parallel rate differences at the plastid gene rbcL. Proc Natl Acad Sci USA. 1996;93(19):10274–10279. doi: 10.1073/pnas.93.19.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerlach WL, Bedbrook JR. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harpke D, Peterson A. 5.8S motifs for the identification of pseudogenic ITS regions. Botany. 2008;86:300–305. doi: 10.1007/s10265-008-0156-x. [DOI] [PubMed] [Google Scholar]
- 46.Mahelka V, Kopecký D, Baum BR. Contrasting patterns of evolution of 45S and 5S rDNA families uncover new aspects in the genome constitution of the agronomically important grass Thinopyrum intermedium (Triticeae) Mol Biol Evol. 2013;30(9):2065–2086. doi: 10.1093/molbev/mst106. [DOI] [PubMed] [Google Scholar]
- 47.Lorenz R, et al. ViennaRNA Package 2.0. Algorithms Mol Biol. 2011;6:26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall TA. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 50.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 51.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 52.Nylander JAA. 2004. MrModeltest v2 (Evolutionary Biology Centre, Uppsala Univ, Uppsala, Sweden)
- 53.Geyer CJ. Markov chain Monte Carlo maximum likelihood. In: Keramidas EM, editor. Computing Science and Statistics: Proceedings of the 23rd Symposium on the Interface. Interface Foundation; Fairfax Station, VA: 2001. pp. 156–163. [Google Scholar]
- 54.Swofford DL. 2003. PAUP*, Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer, Sunderland, MA), Version 4.
- 55.White TJ, Burns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sninsky J, White T, editors. PCR Protocols: A Guide to Methods and Applications. Academic; San Diego: 1990. pp. 315–322. [Google Scholar]
- 56.Yu HQ, et al. Phylogenetic relationships of species in Pseudoroegneria (Poaceae: Triticeae) and related genera inferred from nuclear rDNA ITS (internal transcribed spacer) sequences. Biologia. 2008;63:498–505. [Google Scholar]
- 57.Blattner FR, Pleines T, Jakob SS. Rapid radiation in the barley genus Hordeum (Poaceae) during the Pleistocene in the Americas. In: Glaubrecht M, editor. Evolution in Action. Springer; Berlin: 2010. pp. 17–33. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.