Significance
Neurons in the brain adapt to environmental challenges by rearranging their connections, or synapses, to other neurons. This process is called synaptic plasticity and it is unclear how synaptic plasticity is regulated. Here we show that the enzyme O-GlcNAc transferase (OGT) modulates the maturity of excitatory synapses. The function of OGT depends on the metabolic status of the cell and the body. Previously, OGT has been linked to appetitive behavior, learning and memory, and neurodegenerative diseases like Alzheimer’s disease. Our data suggest that OGT may underlie brain function by coordinating experience-driven synaptic plasticity with nutrient availability.
Keywords: O-GlcNAc, OGT, excitatory synapses, AMPA receptors
Abstract
Experience-driven synaptic plasticity is believed to underlie adaptive behavior by rearranging the way neuronal circuits process information. We have previously discovered that O-GlcNAc transferase (OGT), an enzyme that modifies protein function by attaching β–N-acetylglucosamine (GlcNAc) to serine and threonine residues of intracellular proteins (O-GlcNAc), regulates food intake by modulating excitatory synaptic function in neurons in the hypothalamus. However, how OGT regulates excitatory synapse function is largely unknown. Here we demonstrate that OGT is enriched in the postsynaptic density of excitatory synapses. In the postsynaptic density, O-GlcNAcylation on multiple proteins increased upon neuronal stimulation. Knockout of the OGT gene decreased the synaptic expression of the AMPA receptor GluA2 and GluA3 subunits, but not the GluA1 subunit. The number of opposed excitatory presynaptic terminals was sharply reduced upon postsynaptic knockout of OGT. There were also fewer and less mature dendritic spines on OGT knockout neurons. These data identify OGT as a molecular mechanism that regulates synapse maturity.
Neuronal synapses, the cell–cell junctions over which neurons communicate, are formed and eliminated throughout life, and their turnover has for decades been associated with the way neuronal circuits adapt to environmental challenges to optimize behavior (1, 2). In both humans and animals, early development is characterized by massive generation of new synapses. About half of all synapses are then lost during adolescence (2, 3). Most mature excitatory synapses occur on dendritic protrusions called spines and essentially all spines contain an excitatory synapse (4–6). In vivo imaging of individual spines for days to months has shown that adult spines are largely stable but a small subpopulation remains plastic (3, 7) and spine turnover is increased by novel experience (5, 8–10). Whereas most new spines are thin and withdraw rapidly, some enlarge and form stable synaptic contacts (2, 3, 7, 11–14). In fact, the stabilization of a subset of new spines correlates with behavioral performance in several different tasks in multiple animal species (13, 15–17). Rather than synapse formation or density, the selection of which spines are retained once formed has been suggested to match circuit architecture with behavior (18). Without affecting spine formation, deleting β-adducin, which regulates actin, perturbed the process by which nascent spines establish functional synapses and impaired long-term memory (19). Fragile X syndrome, a common form of mental retardation, exhibits a higher than normal density of spines but more of them exhibit an immature morphology and their turnover is not affected by sensory experience (9, 20). Conversely, the protein Telencephalin arrests the maturation of spines and its removal enhances several forms of learning (13, 21, 22).
Although many molecules have been identified that affect synapse number, it is unclear how newly formed spines mature into synapses (13, 23, 24). On a cellular level, learning and memory are associated with long-term potentiation (LTP) and long-term depression (LTD) of neurotransmission. It is widely believed that LTP and LTD involve synaptic insertion and removal, respectively, of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (25). AMPA receptors are glutamate-gated tetrameric ion channels composed of different combinations of subunits GluA1–4 and conduct the majority of the fast excitatory neurotransmission in the brain (26). Spines have been reported to incorporate AMPA receptors early in their development (13, 27, 28). Apart from additional roles in long-lasting synapses, AMPA receptor activity has been associated with the stabilization of immature spines, possibly, at least in part, via actin-dependent pathways (4, 14, 29–35).
In neurons, the O-GlcNAc pathway has emerged recently as critical for coupling cellular function to energy availability through nutrient-dependent flux via the hexosamine biosynthesis pathway (HBP), of which the OGT donor substrate uridine diphosphate (UDP)-GlcNAc is the end product (36–40). Unlike complex glycans present on the outside of cells and in the secretory pathway, O-GlcNAc is a highly dynamic sugar that is added and removed repeatedly over the lifespan of a single peptide chain. It is expressed mainly on the inside of cells in the nucleus, cytoplasm, and mitochondria. Only two enzymes regulate its cycling; O-GlcNAc transferase (OGT) attaches O-GlcNAc to proteins covalently, whereas O-GlcNAcase (OGA) hydrolyses O-GlcNAc from proteins. The brain is one of the organs where O-GlcNAc is the most abundant (39, 41–43). In the synapse, a myriad of proteins carry O-GlcNAc (44–46). Many of these are critical for synaptic plasticity, like alpha calcium/calmodulin-dependent kinase II (αCaMKII) and SynGAP (46, 47). O-GlcNAc signaling does affect learning and memory (48, 49). Acute inhibition of OGT or OGA pharmacologically, indicated that O-GlcNAc regulates LTP and LTD by affecting AMPA receptor trafficking. However, there have been contradictory reports of whether global elevation or depression of O-GlcNAc levels have a stimulatory or inhibitory effect, respectively, on excitatory synaptic function, possibly due to nonspecific effects of OGT inhibitors or differences in mode and length of application of drugs against OGA (49–51). We developed a mouse model where OGT was deleted genetically from mature brain neurons in vivo and demonstrated that OGT regulates normal food intake, at least in part, by modulating excitatory synaptic function in neurons in the hypothalamus (36). Using electrophysiology, we observed that knocking out OGT sharply reduced the frequency of miniature excitatory postsynaptic currents (mEPSCs). mEPSC amplitude was also decreased, but to a lesser degree. These findings indicate that OGT can underlie adaptive behavior partly by regulating normal excitatory synaptic function (36).
Here we investigate how OGT regulates excitatory synaptic function by taking advantage of primary neuronal cell culture where OGT is deleted genetically either broadly or sparsely in neurons. We discovered that OGT deletion selectively reduced the synaptic expression of the GluA2 and GluA3 subunits, two major AMPA receptor subunits. Mimicking our electrophysiological finding of lower mEPSC frequency in vivo, removal of OGT in vitro led to fewer mature morphological synapses and fewer dendritic spines. The spines that were present on OGT KO neurons were largely immature. Collectively, our observations suggest that OGT is important for the maturation of excitatory synapses, at least in part through modulating GluA2/3 expression. The regulation of synapse maturity by OGT represents a model for how neuronal circuits may respond to environmental challenges, such as nutrient fluctuations, to accommodate behavior.
Results
OGT Is Enriched in the Postsynaptic Density of Excitatory Synapses.
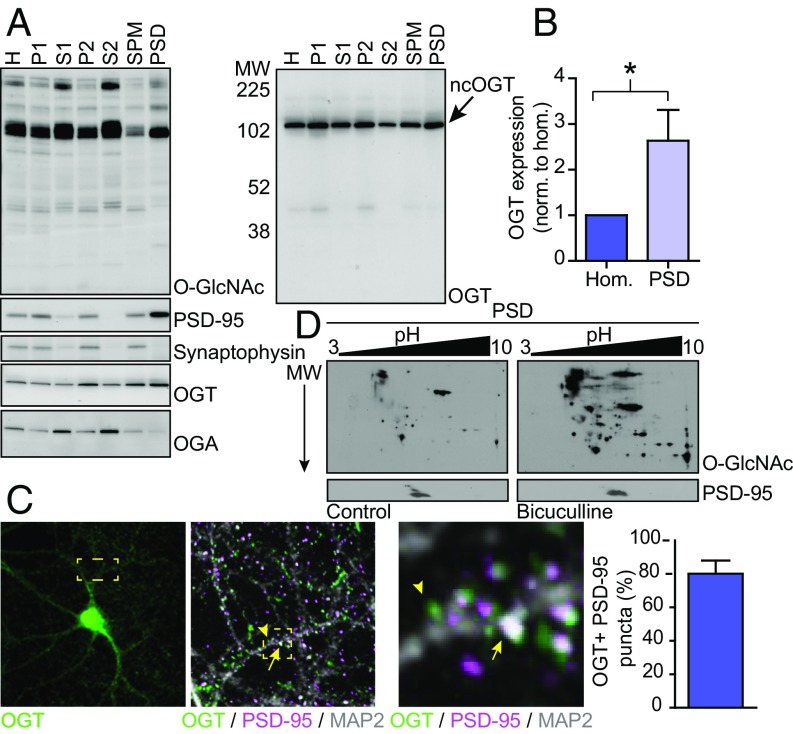
Based on biochemical fractionation, it has been shown previously that OGT is present in neuronal synapses (45, 51). Electron microscopy from the cerebellum indicated that OGT is more highly expressed in presynaptic, rather than postsynaptic, nerve terminals (44). To investigate the role of OGT in postsynaptic function, we isolated the postsynaptic density (PSD) fraction of excitatory synapses from rat forebrain homogenates. Equal amounts of all fractions were separated by SDS/PAGE and analyzed by Western blotting (Wb). Enrichment of the PSD marker PSD-95 and exclusion of the presynaptic marker synaptophysin ensured that the PSD fraction isolated PSDs specifically (Fig. 1A). Full-length nuclear cytoplasmic OGT (ncOGT) was present in all subcellular compartments, including in the PSD (Fig. 1A). Interestingly, compared with the whole-cell homogenate, OGT was more than twice as abundant in the PSD (Fig. 1B). In contrast to OGT, OGA was excluded from the PSD (Fig. 1A). We then used immunohistochemistry to examine the localization of OGT in primary cultured hippocampal neurons. Along the dendrite, endogenous OGT expression was punctate and many, but not all, puncta overlapped with PSD-95. Most PSD-95 puncta overlapped with OGT (Fig. 1C). It has been shown that depolarization of cultured neuroblastoma cells using KCl activates OGT via calcium-dependent phosphorylation by CaMKIV (52). Moreover, induced seizures in rodents have been reported to increase the O-GlcNAcylation of some proteins in the brain in vivo (53). However, whether, and to what extent, neuronal firing regulates O-GlcNAc incorporation in neuronal proteins is still unclear. Taking advantage of 2D gel electrophoresis and blotting for O-GlcNAc, we observed that stimulating neuronal network activity in cortical cultures by inhibiting GABAA receptors pharmacologically using bicuculline elevated O-GlcNAcylation in the PSD on multiple proteins (Fig. 1D). Together, our observations demonstrate that OGT is enriched in the PSD of excitatory synapses in forebrain neurons. Within the PSD, O-GlcNAcylation is a dynamic and activity-dependent posttranslational modification.
Fig. 1.
OGT is enriched in the postsynaptic density of excitatory synapses. (A) Wb of PSD fractions from brain. The Right blot shows the same sample run again and overexposed. (B) Quantification of OGT expression in the PSD relative to the whole-cell fraction [n = 7 for homogenate (Hom.) and PSD; two-tailed t test: *P < 0.05]. (C, Left) Immunohistochemistry of OGT (green), PSD-95 (magenta), and MAP2 (gray). Boxed areas show the same image in higher magnification. Arrow shows overlap between PSD-95 and OGT puncta. Arrowhead shows OGT puncta negative for PSD-95. (Right) Quantification of the percentage of PSD-95 puncta that overlap with OGT (n = 12 images). (D) Wb of 2D gel electrophoresis of the PSD fraction from control and stimulated neurons. Quantifications represent mean ± SEM. MW, molecular weight; SPM, synaptic plasma membrance.
OGT Regulates the Synaptic Expression of AMPA Receptors.
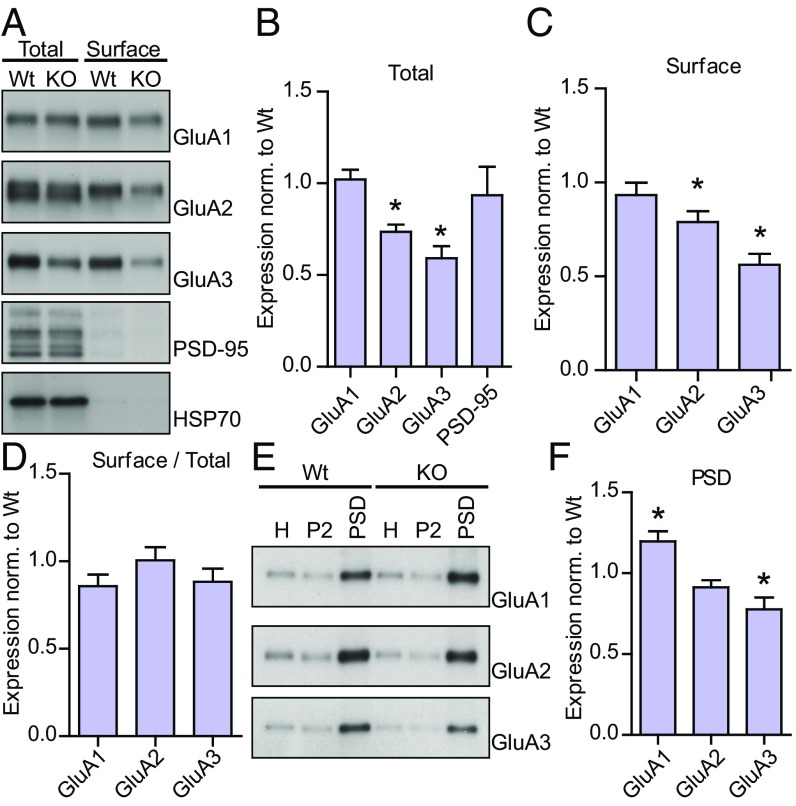
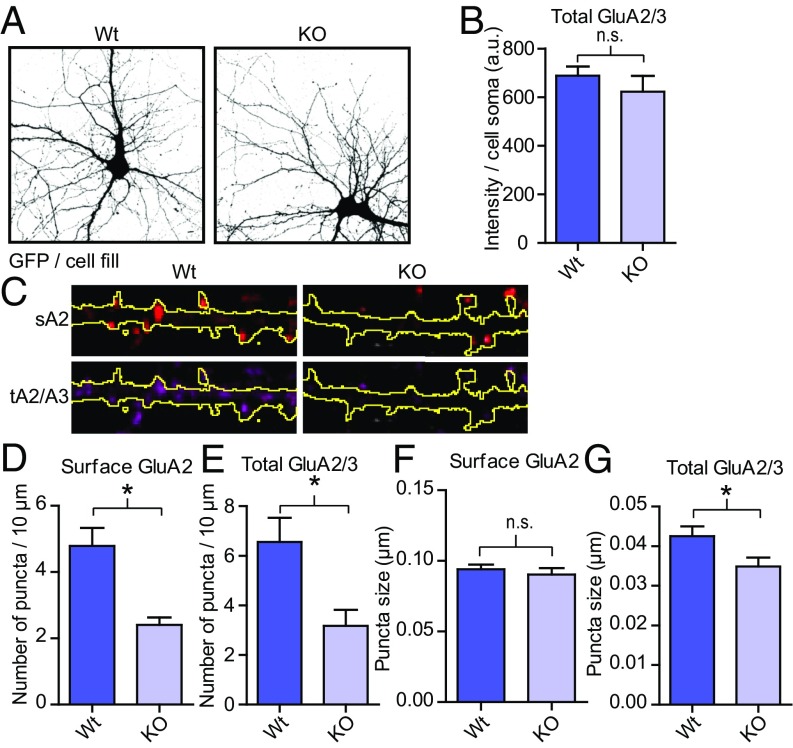
Recently, we knocked out OGT in adult animals and observed a marked attenuation of excitatory synaptic currents using electrophysiology (36). Other groups have shown that acute inhibition of OGT or OGA activity affects LTP and LTD in the CA1 region of the hippocampus, possibly through altered AMPA receptor trafficking (49–51). Two major AMPA receptor isoforms are GluA1/2 and GluA2/3 heteromers (26, 54). Here we deleted OGT in cortical neurons cultured from floxed OGT mice (OGTFl) by applying lentivirus expressing either GFP alone (WT) or GFP together with Cre recombinase (KO) at days in vitro (DIV)2. After the neurons had matured, we isolated the cell surface proteins using biotinylation techniques and blotted for the major AMPA receptor subunits. As shown in Fig. 2A, only proteins expressed on the surface of the cell were pulled down applying this method. There was no change in the total expression of PSD-95 upon deleting OGT (Fig. 2 A and B). In contrast, removal of OGT caused a sharp down-regulation of the surface expression of the GluA2 and the GluA3 AMPA receptor subunits but not the GluA1 subunit (Fig. 2 A and C). The total expression of GluA2 and GluA3 was also decreased (Fig. 2 A and B). There was no effect on the relative surface over total expression of either subunit, although there was a trend toward a small decrease for GluA1 (P = 0.05, n = 6 for WT and KO, Fig. 2D). We also isolated the PSD from OGT WT and KO cultures. In the PSD, there was a reduction in GluA3, whereas there was an increase in GluA1 (Fig. 2 E and F). The decrease in GluA2, again, was at a level between GluA1 and GluA3 (9% decrease, P = 0.08, Fig. 2 E and F). Next, we sparsely knocked out OGT in hippocampal cultures from OGTFl mice by transfecting a cell-fill (GFP) and an empty plasmid (WT) or a plasmid expressing Cre (KO) around DIV9. At DIV14, we used immunohistochemistry to stain the surface GluA2 population using an antibody directed against its N terminus and, after permeabilization, the total GluA2/3 population with a C terminus-targeted antibody. There was no overt difference in the overall morphology between WT and KO neurons (Fig. 3A). In the soma, the total GluA2/3 expression did not change upon OGT KO (Fig. 3B). In contrast, along dendrites, similar to what we showed biochemically, the number of surface GluA2 positive puncta decreased by 51% (P < 0.05, WT n = 27, 4.9 ± 0.5 puncta/10 μm; KO n = 28, 2.4 ± 0.2 puncta/10 μm, Fig. 3 C and D). Similarly, the number of total GluA2/3 puncta was 51% lower in OGT KO neurons (P < 0.05, WT n = 27, 5.4 ± 1.1 puncta/10 μm; KO n = 28, 2.7 ± 0.69 puncta/10 μm, Fig. 3 C and E). This change in expression was not reflected in the size of the surface GluA2 puncta and the size of the GluA2/3 puncta was only slightly smaller (Fig. 3 C, F, and G). Using biochemistry and imaging, our results indicate that OGT is necessary cell autonomously for normal synaptic expression of AMPA receptors, in particular the GluA2/3 heteromers.
Fig. 2.
OGT regulates the synaptic expression of AMPA receptors. (A) Wb of the total and surface expression of GluA1–3. (B) Quantifications of the total expression from A (GluA1–3: n = 5 for WT and KO; PSD-95: n = 3 for WT and KO; two-tailed t test: *P < 0.05). (C) Quantifications of the surface expression from A (GluA1–3: n = 6 for WT and KO; two-tailed t test: *P < 0.05). (D) Quantifications of the surface over total expression from A (GluA1–3: n = 6 for WT and KO; two-tailed t test: P < 0.05). (E) Wb of PSD preparation fractions from WT and KO cells. (F) Quantification of GluA1–3 expression in the PSD (n = 3 for WT and KO; two-tailed t test: *P < 0.05). Quantifications represent mean ± SEM.
Fig. 3.
Deletion of OGT leads to fewer surface GluA2 clusters. (A) Thresholded images of GFP expression in WT and KO hippocampal neurons. (B) Quantification of the total expression of GluA2/3 in the cell soma of individual hippocampal neurons (WT n = 18, KO n = 20; two-tailed t test: P < 0.05). (C) Immunohistochemistry of the surface expression of GluA2 and total expression of GluA2/3 in dendrites from transfected neurons. The outlines of the dendrites (GFP expression) are shown in yellow. (D–G) Quantifications from C (WT n = 27, KO n = 28, two-tailed t test: *P < 0.05). Quantifications represent mean ± SEM. n.s., not significant.
Postsynaptic Deletion of OGT Leads to Fewer Morphological Synapses.
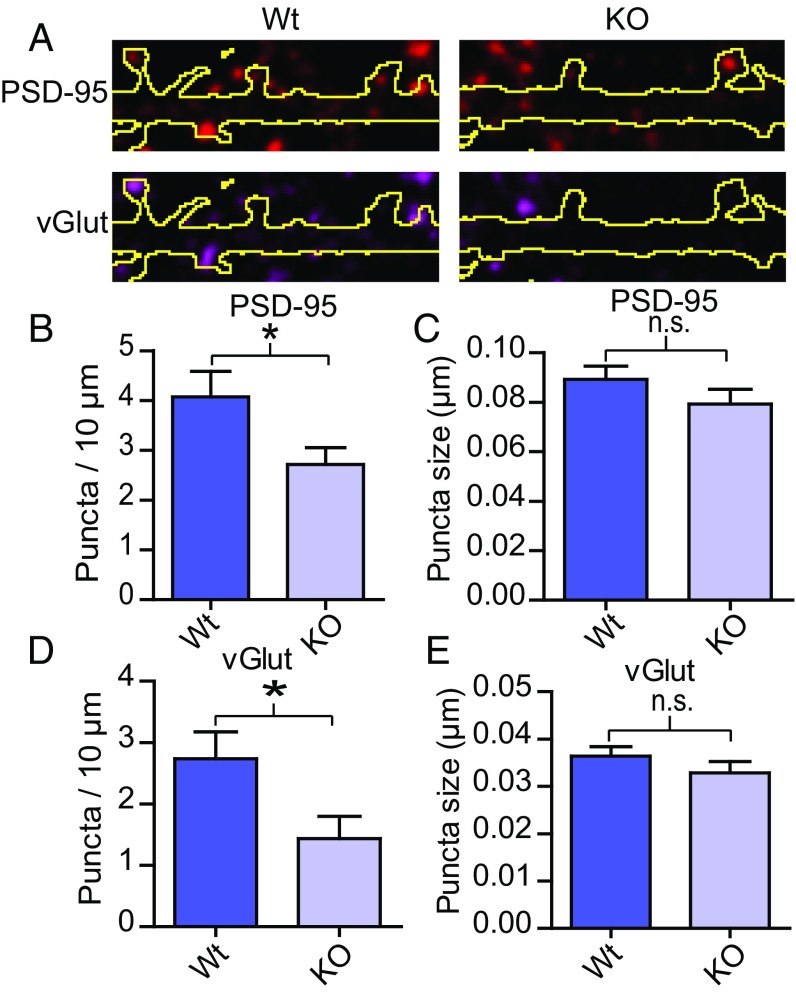
As deletion of OGT mainly affected the number of GluA2 puncta along dendrites rather than the somatic expression of GluA2/3 or the expression of surface GluA2 per puncta, we reasoned that OGT may regulate the number of synaptic contacts. This idea would be consistent with our previous electrophysiological data showing that OGT removal primarily leads to lower mEPSC frequency (36). After sparsely deleting OGT and labeling cells in hippocampal cultures as described above, immunohistochemistry was applied to stain for vGlut1, a marker for excitatory presynaptic terminals, and PSD-95 (Fig. 4A). The number of puncta and the size of each puncta overlapping with the cell fill was then quantified. Both the number PSD-95 and vGlut1 positive puncta decreased (Fig. 4 A, B, and D). In contrast, neither the size of the PSD-95 nor the vGlut1 puncta differed between WT and KOs (Fig. 4 C and E). Together, our data suggest that deleting OGT in the postsynaptic cell prevents the formation of morphologically mature synapses. These observations corroborate and expand the previous finding that the drop in mEPSC frequency upon OGT KO in vivo reflects a loss of functional synapses (36).
Fig. 4.
OGT regulates the number of mature synaptic contacts. (A) Immunohistochemistry of vGlut1 and PSD-95 overlapping with transfected neurons. The outlines of the dendrites (GFP expression) are shown in yellow. (B–E) Quantifications from A (WT n = 21, KO n = 20, except for KO vGlut puncta size where n = 17, two-tailed t test: *P < 0.05). Quantifications represent mean ± SEM. n.s., not significant.
OGT Removal Is Associated with Deficient Spine Maturation.
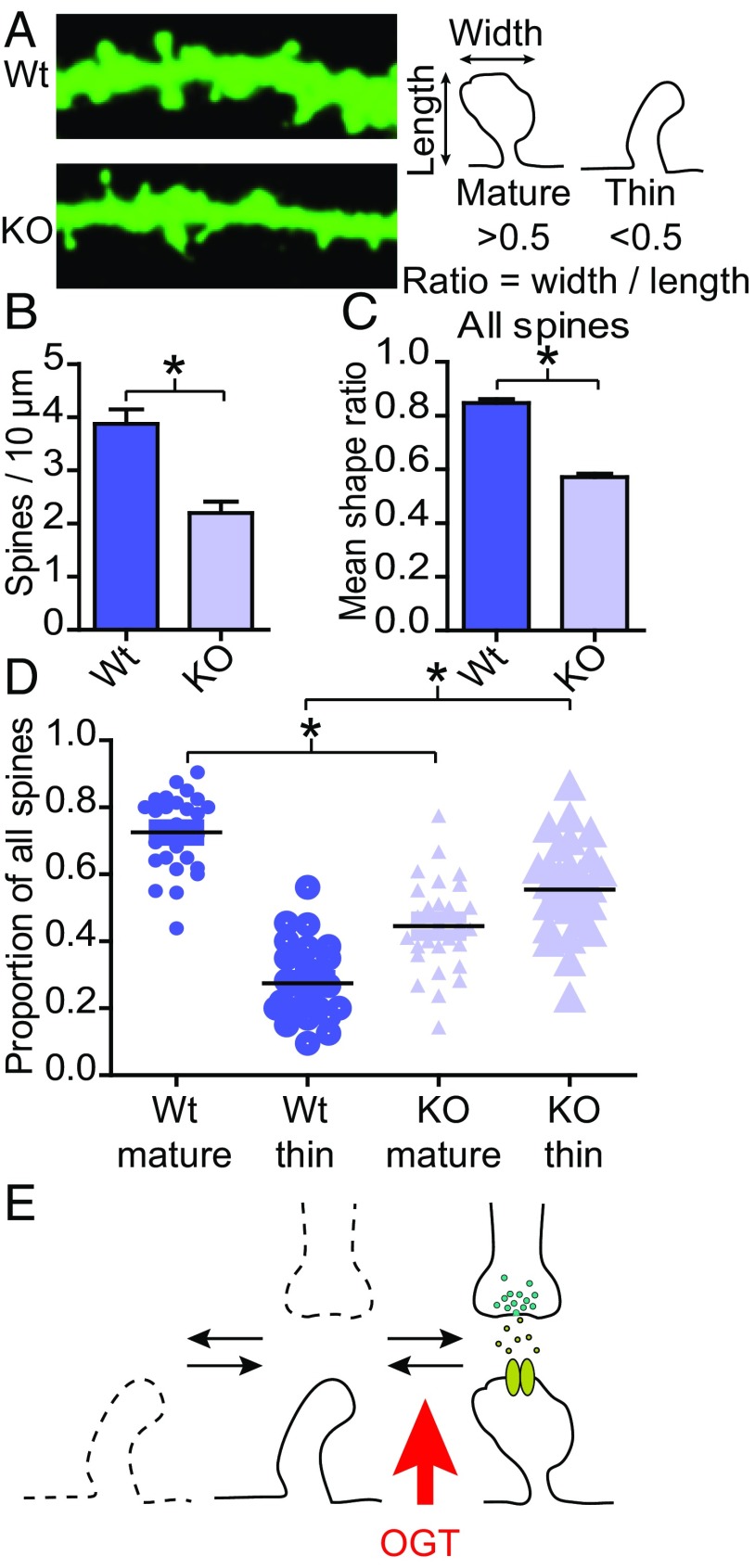
Most excitatory synapses occur on dendritic spines (2). Filling the neuron with a fluorescent marker, spines can be imaged and their number and shape measured. Whereas the number of spines is a function of the number of excitatory synapses, the shape of the spine indicates its maturity (4–6). Mature and stable spines typically exhibit a wider distal portion (“spine head”) (Fig. 5A) (2, 3, 11–14, 29). Here, again, hippocampal cultures from OGTFl mice were sparsely transfected to label cells and KO OGT at DIV9 and then fixed at DIV14. Upon deletion of OGT, spine number decreased by 43% (P < 0.05; WT n = 26, 3.9 ± 0.3 spines/10 μm; KO n = 28, 2.2 ± 0.2 spines/10 μm, Fig. 5 A and B). Spine shape was measured by quantifying the ratio between the width and the length of individual spines (Fig. 5A). Counting all spines, deleting OGT decreased their mean shape ratio by 33% (P < 0.05, WT n = 1108, 0.85 ± 0.02, KO n = 1074, 0.57 ± 0.01, Fig. 5C). To evaluate the proportion of mature versus immature spines, spines with a ratio above 0.5 were labeled “mature” and those with a ratio below 0.5 “thin” (Fig. 5A). In WT cells, the majority of all spines were mature (Fig. 5D). Of those spines that were present on OGT KO cells, in contrast, most were thin (Fig. 5D). To summarize, deleting OGT leads to a higher proportion of immature spines and fewer spines in total (Fig. 5E).
Fig. 5.
Fewer and more immature spines on OGT KO neurons. (A, Left) Expression of GFP in dendrites from WT and KO hippocampal neurons. (Right) Schematic of measurements taken to quantify spine shape. (B) Quantification of spine density (WT n = 26, KO n = 28, two-tailed t test: *P < 0.05). (C) Quantification of the mean spine shape ratio (WT n = 1108, KO n = 1074, two-tailed t test: *P < 0.05. (D) Quantification of the proportion of mature and thin spines along dendrites. (WT n = 27, KO n = 28, two-tailed t test: *P < 0.05). (E) Model of how OGT, at least in part, regulates excitatory synapse function; OGT inhibits the maturation of dendritic spines leading to fewer and more immature synapses. Quantifications represent mean ± SEM.
Discussion
Neuronal circuits need to constantly rearrange their connectivity pattern to match behavioral output to environmental influences. Here we find that OGT, a nutrient-dependent enzyme, is enriched in the postsynaptic density of excitatory synapses. In the PSD, the incorporation of O-GlcNAc was dynamic and dependent on neuronal activation. Deletion of OGT greatly depressed the synaptic abundance of the GluA2 and GluA3 subunits of the AMPA receptor. The reduction in GluA2 and GluA3 was largely due to fewer dendritic AMPA receptor clusters rather than a decrease in the amount of AMPA receptors in each cluster, indicating that the main effect of OGT removal is due to a down-regulation of the number of functional excitatory synapses. Indeed, removing OGT postsynaptically disrupted the formation of synapses, as determined by staining of the synaptic vesicle protein vGlut1 and the postsynaptic marker PSD-95. There were also fewer spines on OGT KO neurons. Those spines that did form were mostly immature and did not express morphological features reminiscent of stable synapses.
Total spine number is regulated by the formation of new spines and the termination of old spines. We deleted OGT genetically before or during the period of the most extensive synapse formation in culture (DIV7–14) and observed a marked reduction in spine density. Accordingly, although we have not applied methods such as live imaging of individual spine turnover to verify this idea, it is likely that the decrease in total spine number upon OGT KO mainly resulted from disrupted spine formation. In the PSD of OGT KO cells, there was a comparatively high abundance of GluA1-containing receptors, whereas GluA3-containing receptors were lower in abundance than in WT cells. Similarly, on the cell surface, there was a reduction in GluA2 and GluA3 but not GluA1. As GluA2 consistently expressed at levels between GluA1 and GluA3 upon removal of OGT, the main effect of deleting OGT presumably occurs on GluA2/3 heteromers as opposed to on GluA1/2 heteromers. Whether, and, if so, how the roles of GluA2/3 heteromers and GluA1/2 heteromers differ have been debated in the literature. Some evidence shows that synaptic insertion of GluA1 homomers and GluA1/2 heteromers require neuronal stimulation, whereas GluA2/3 heteromers have been proposed to be inserted under baseline conditions in exchange for preexisting receptors (55, 56). Early in development, GluA4 translocates into so-called silent synapses and are later replaced by GluA2-containing receptors (57–59). Unlike GluA2, GluA1 does appear to enter into silent synapses (55). Sensory experience and learning increase the abundance of GluA1 homomers in the synapse, but this increase is often transient (56, 60, 61). Although the trafficking of GluA2/3 can be regulated by neuronal activity, these and other data suggest that GluA2/3 heteromers, as opposed to GluA1 homomers or GluA1/2 heteromers, may be associated with the maintenance of synaptic strength in mature synapses (25, 26). Whereas total spine number was reduced, spines did develop on OGT KO cells. But of those spines that formed, most resembled immature spines and rarely evolved morphology similar to mature spines with a wider spine head as in WT cells. Spine formation is intimately linked to synapse formation (2, 3, 11–14). The formation of morphological synapses on dendrites where OGT had been deleted, as determined by staining of vGlut1 and PSD-95, was interrupted. Based on the selective effect on GluA2/3 heteromers, immature spine phenotype and reduction in the number of vGlut1 and PSD-95 puncta, it appears that OGT is necessary for late, rather than early, stages of postsynaptic development when unstable spines mature into functional synapses (Fig. 5E).
Although the experiments presented in this paper were performed in the context of synapse formation in culture, previously we have observed a similar decrease in excitatory synapse number in adult animals. After OGT was removed acutely in vivo, there was a sharp drop in mEPSC frequency (36). In addition, other groups have shown that short-term nutritional or pharmacological manipulation of global O-GlcNAc levels in hippocampal slices affects long-term potentiation and long-term depression of excitatory CA1 synapses (49–51). At least some of these effects may be related to altered trafficking of GluA2 (49, 50). Collectively, these observations suggest that OGT is important not only for the formation of new excitatory synapses during development but also for the stability of excitatory synapses in the adult. As discussed above, spines undergo constant remodeling where activity-dependent signaling through AMPA receptors, e.g., via actin, maintain their stability. Thus, it is possible that the synaptic depression of GluA2/3 heteromers upon OGT deletion prevented the development of thin spines into mature synapses. The idea that OGT underlies spine maturity via maintaining AMPA receptor expression would explain why removing OGT during development and in adult animals lead to the same phenotype; loss of excitatory synapse number. However, it cannot be excluded that OGT has a direct effect on spine assembly as well.
OGT may regulate AMPA receptor expression through several pathways. We show here that the O-GlcNAc content in the PSD is dynamic. We also observed that OGT is enriched in the PSD. Global and long-term deletion of OGT decreased the total expression of GluA2/3. In contrast, shorter and sparse OGT KO did not affect the somatic expression of GluA2/3. This observation indicates that OGT regulates AMPA receptor expression by a cell-autonomous mechanism, probably, at least in part, through degradation or trafficking of the receptor, which is supported by the fact that the effect of acute manipulation of global O-GlcNAc levels on excitatory neurotransmission is GluA2 dependent (49). Moreover, we and others have shown that O-GlcNAc regulates excitatory synapse function in hypothalamic, cortical, and hippocampal cells. Thus, the manipulation of synaptic AMPA receptor abundance may be a common and major endpoint for how OGT shapes neuronal circuits. Many O-GlcNAc–modified proteins known to affect AMPA receptor trafficking have been identified using mass spectrometry, e.g., SynGAP and αCaMKII (46, 47). Based on these observations, it is likely that OGT may regulate AMPA receptor expression via pathways local to the spine or the postsynaptic density, but other mechanisms cannot be excluded.
O-GlcNAc is increasingly being appreciated as an important modification of protein function. Its unique regulation where metabolic and other stimuli can affect O-GlcNAcylation on a global as well as a target-specific scale presents a mechanism for how cells respond to environmental challenges. Here we demonstrate that OGT is enriched in the postsynaptic density of excitatory synapses and is necessary for synapse maturation, possibly via regulating the synaptic expression and/or localization of GluA2/3 heteromers. O-GlcNAc cycling in the brain is linked to learning and memory, feeding behavior, and neurodegenerative diseases (39, 48, 49). Whereas many proteins in the excitatory postsynaptic density are known to be modified by O-GlcNAc, an important task will be to identify which O-GlcNAc sites are dynamically regulated to modify neuronal circuit function.
Methods
All animals were housed according to the Johns Hopkins University Animal Care and Use Committee guidelines. Primary neuronal cell cultures from WT animals or animals where an exon in the Ogt locus has been floxed (OGTFl) were derived according to standard procedures. To delete OGT, a vector expressing Cre recombinase was delivered virally (for biochemistry experiments) or by transfection (for imaging experiments). Surface proteins were pulled down by surface biotinylation. The PSD was isolated by a series of centrifugation steps. SI Methods for extended details.
SI Methods
Materials.
Mice where an exon in the Ogt locus has been floxed (OGTFl) to delete OGT has been described previously (36, 62). Antibodies used were: OGT (AL25 and AL28, produced in-house; 1:5,000), O-GlcNAc (110.6, produced in-house; 1:10,000), OGA (345, produced in-house; 1:5,000), HSP70 (Santa Cruz Biotechnology; 1:1,000–5,000), PSD-95 (NeuroMab, Wb; 1:5,000, IF; 1:2,000), GluA1 (4.9D, produced in-house; 1:10,000), GluA2 (Wb; Mab, produced in-house; 1:5,000, IF; 15F1, a kind gift from Eric Gouaux, Vollum Institute, Portland, OR; 1:500–1,000), GluA3 (JH4300, produced in-house; 1:5,000), GluA2/3 (JH4854, produced in-house; 1:250), MAP2 (nb300-213, Novus Biologicals; 1:10,000), Synaptophysin (SVP-38, Sigma; 1:10,000), vGlut1 (AB5905, Millipore; 1:2,500), and GFP (ab13970, Abcam; 1:2,500). All plasmid inserts used had been generated by standard cloning techniques previously in-house. All animals were housed according to the Johns Hopkins University Animal Care and Use Committee guidelines.
Primary Neuronal Cell Culture.
For biochemistry, rat and mice E18 cortical neurons were prepared as previously described (36). Briefly, after dissociation, cells were plated on poly-l-lysine–coated dishes in neuronal growth media with 5% (vol/vol) serum, NM5 [neurobasal growth medium (Gibco), 2% (vol/vol) B27 (Invitrogen), 2 mM glutamax (Gibco), 5% (vol/vol) FBS and penicillin/streptomycin (Gibco)]. Dividing cells were removed using 5 mM uridine and 5 mM (+)-5-fluor-2′-deoxyuridine in NM1 (1% serum) at days in vitro (DIV)3–5. Then, every 3–4 d, half of the culture media was exchanged for glia-conditioned NM1 until harvest. For OGT KO experiments, lentivirus was added on DIV2. SDS/PAGE and Western blotting were done according to standard procedures. For imaging, mice E16.5–17 and rat E18 hippocampal neurons were cultured in serum-free media (NM0). Upon transfection, half of the media was exchanged for fresh NM0. Transfections were done using Lipofectamine 2000 (Fisher).
Imaging.
Mature hippocampal neurons were fixed with 4% (vol/vol) paraformaldehyde and 4% (vol/vol) sucrose. When surface staining was performed, the fix was applied for 4.5 min in room temperature, which did not break the cell membrane. Thereafter, the GluA2 antibody raised against the N terminus of GluA2 was applied in room temperature for 2 h in a blocking buffer (0.1% gelatin, 250 mM NaCl, 15 mM phosphate buffer, pH 7.4) that did not contain any detergent. After washing, other primary antibodies were introduced in blocking buffer that did contain detergent (0.25% Triton X-100) for 2 h at room temperature. Secondary antibodies, diluted in blocking buffer plus 0.25% Triton X-100, were added for 1 h after washing. For other immunohistochemistry, the neurons were fixed using the same solution but for 15 min at 4 °C, permeabilized for 10 min at 4 °C (0.25% Triton X-100 in PBS) and blocked for at least 1 h [5–10% (vol/vol) normal goat serum in PBS]. All antibodies were diluted in the same blocking buffer and applied for 1–2 h.
Lentivirus.
To KO OGT in cultured neurons, pseudotyped VSV-G lentivirus expressing GFP alone (WT) or GFP together with Cre recombinase (KO) was produced according to standard procedures (36).
PSD Isolation.
From neuronal culture: cells were harvested and homogenized in homogenization buffer (0.32 M sucrose, 4 mM Hepes pH 7.4, including inhibitors for proteases, phosphatases, and O-GlcNAcases). After homogenization, in all subsequent steps including adding any solution, the same inhibitors as used for the homogenization buffer were added. The homogenate (H) was centrifuged (1,000 × g, 10 min, 4 °C). The supernatant was spun again (10,000 × g, 15 min, 4 °C). The pellet was resuspended in homogenization buffer and spun again (10,000 × g, 15 min, 4 °C). The pellet was lyzed by hypoosmotic shock in H2O (resuspended with P1000 pipette 20–30 times) and then adjusted to 4 mM Hepes, pH 7.4. This fraction was used as P2. The remaining material was rotated at 4 °C for 30 min before ultracentrifugation (25,000 × g, 20 min 4 °C). The pellet was resuspended in 50 mM Hepes, pH 7.4, 2 mM EDTA. After 0.5% Triton X-100 had been added, the solution was rotated at 4 °C for 15 min and then centrifuged again (32,000 × g, 20 min, 4 °C) to yield the PSD fraction. From brain (the cerebellum and most of the midbrain and brainstem were removed): the tissue was homogenized (fraction H, 0.32 M sucrose, 10 mM Hepes, pH 7.4, including inhibitors for proteases, phosphatases, and O-GlcNAcases) and then centrifuged (1,000 × g, 10 min, 4 °C) to yield the P1 fraction. After homogenization, in all subsequent steps including adding any solution, the same inhibitors as used for the homogenization buffer were added. The supernatant (S1) was spun again (13,800 × g, 20 min, 4 °C) where the supernatant was collected as S2. The pellet (P2) was resuspended in homogenization buffer and layered on a sucrose gradient (P2: 0.85 M, 1.0 M, 1.2 M sucrose in 1 mM Hepes, pH 7.4) and centrifuged (82,500 × g, 2 h, 4 °C). The material between the 1.0 M and 1.2 M layers was collected and diluted with 2.5 volumes of 10 mM Hepes, pH 7.4 before centrifugation (150,000 × g, 30 min, 4 °C). The pellet synaptic plasma membrane (SPM) was resuspended in 80 mM Tris⋅HCl, pH 8.0, and then diluted with 1.0% Triton X-100 to a final concentration of 0.5% Triton X-100. The solution was rotated for 15 min (4 °C) and then centrifuged (32,000 × g, 20 min, 4 °C) to yield the PSD fraction (the pellet).
Surface Biotinylation.
Cells were washed two to three times in aCSF (143 mM NaCl, 5 mM KCl, 2 mM CaCl2 1 mM MgCl2, 10 mM Hepes pH 7.4, 10 mM d-glucose) and then incubated on ice for 20 min in Sulfo-NHS-SS-Biotin (Fisher) diluted in aCSF. Remaining active biotin was quenched with TBS three times (4 °C, 5 min each wash) and then lyzed in RIPA buffer (50 mM Tris, 150 mM sodium chloride, 1% Nonidet P-40, 1% deoxycholate, 0.1% SDS, 1 mM EDTA, including inhibitors for proteases, phosphatases, and O-GlcNAcases). Biotinylated proteins were pulled down using neutravidin beads (Fisher).
Isoelectrofocusing.
Two-dimensional electrophoresis was done according to standard procedures (63). Briefly, from mature neuronal cell cultures (DIV14–20) treated with vehicle or bicuculline (40 μM) for 5 h, the PSD fraction was isolated and resuspended in 7 M urea, 2 M thiourea, 2% (wt/vol) dodecyl maltoside (D4641, Sigma), 50 mM DTT, 0.2% ampholytes (BioLyte 3–10 IEF buffer from Bio-Rad), and inhibitors for proteases, phosphatases, and O-GlcNAcases. The sample was then swelled overnight at room temperature onto Readystrip IPG Strips (pI 3–10) (Bio-Rad), isoelectrofocused and treated with DTT and iodoacetamide before separated using SDS/PAGE. Subsequent Western blotting was done according to standard procedures.
Image Quantification and Presentation.
All analysis was done on nonmanipulated, raw images in ImageJ. For quantification of puncta number and size, the cell-fill channel (GFP) was thresholded and then used to create a mask tracing the border of the cell. Only secondary and tertiary dendrites were included, and their total length was measured per image. Then the channel for each type of punctum was thresholded and counted within the cell-fill mask using the “analyze particles” function. The number and size of each punctum were recorded. The number of puncta was divided by the length of the corresponding dendrite, and both punctum density and punctum shape were quantified per image. Spines were counted manually. Only secondary and tertiary dendrites were included, and their total length was measured per image. The total spine number was divided by the length of the corresponding dendrite per image. The length between the tip of each spine and the junction between the spine and the dendrite and the width of the head of each spine were measured manually on about 50 randomly picked spines per image. The shape of each spine was calculated by dividing the width by the length of each spine. This ratio was compared between all WT and KO spines. For expression analysis comparing full-length and truncated OGT, the laser intensity varied slightly between images. The differences were minor and were not consistently lower or higher for either construct and did not affect the interpretation of the experiment. For quantifying PSD-95 and OGT overlap, both channels were first thresholded. Thereafter, a mask was created for every PSD-95 puncta by using the “analyze particles” function. Any positive OGT signal within the mask was counted as positive overlap. Whereas all analysis was done on raw, unmanipulated images, for image presentation purposes, most images shown in the figures were improved post hoc. Within the same experiment, all changes were done in the same way between WT and KO images. The manipulations were applied in Photoshop and MS Paint and included: levels and brightness/contrast and filtering (“despeckle” and “Gaussian blur”).
Statistical Analysis.
Student’s t tests were unpaired and two-tailed. *P < 0.05, error bars represent mean ± SEM.
Acknowledgments
We thank R. H. White for help with mating and genotyping. This work was supported by NIH Grants R01DK6167, N01-HV-00240, P01HL107153 (to G.W.H.), and R01NS036715 (to R.L.H.).
Footnotes
Conflict of interest statement: G.W.H. receives a share of royalties received by Johns Hopkins University (JHU) on sales of the CTD 110.6 antibody, which are managed by JHU.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621367114/-/DCSupplemental.
References
- 1.Bailey CH, Kandel ER. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DH, Zhang S, Gan WB. Dendritic spine dynamics. Annu Rev Physiol. 2009;71:261–282. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 3.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46(2):181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.McKinney RA. Excitatory amino acid involvement in dendritic spine formation, maintenance and remodelling. J Physiol. 2010;588(Pt 1):107–116. doi: 10.1113/jphysiol.2009.178905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420(6917):788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 6.Lai KO, Ip NY. Structural plasticity of dendritic spines: The underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta. 2013;1832(12):2257–2263. doi: 10.1016/j.bbadis.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Holtmaat AJ, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45(2):279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Zuo Y, Yang G, Kwon E, Gan WB. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436(7048):261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 9.Chen CC, Lu J, Zuo Y. Spatiotemporal dynamics of dendritic spines in the living brain. Front Neuroanat. 2014;8:28. doi: 10.3389/fnana.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders J, Cowansage K, Baumgärtel K, Mayford M. Elimination of dendritic spines with long-term memory is specific to active circuits. J Neurosci. 2012;32(36):12570–12578. doi: 10.1523/JNEUROSCI.1131-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knott GW, Holtmaat A, Wilbrecht L, Welker E, Svoboda K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat Neurosci. 2006;9(9):1117–1124. doi: 10.1038/nn1747. [DOI] [PubMed] [Google Scholar]
- 12.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59(2):253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19(2):146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 14.De Roo M, Klauser P, Mendez P, Poglia L, Muller D. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb Cortex. 2008;18(1):151–161. doi: 10.1093/cercor/bhm041. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462(7275):915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463(7283):948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jontes JD, Phillips GR. Selective stabilization and synaptic specificity: A new cell-biological model. Trends Neurosci. 2006;29(4):186–191. doi: 10.1016/j.tins.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Bednarek E, Caroni P. β-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69(6):1132–1146. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 20.Pan F, Aldridge GM, Greenough WT, Gan WB. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA. 2010;107(41):17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura K, et al. Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur J Neurosci. 2001;13(1):179–189. doi: 10.1046/j.0953-816x.2000.01366.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsuno H, et al. Telencephalin slows spine maturation. J Neurosci. 2006;26(6):1776–1786. doi: 10.1523/JNEUROSCI.2651-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma K, et al. High-throughput genetic screen for synaptogenic factors: Identification of LRP6 as critical for excitatory synapse development. Cell Reports. 2013;5(5):1330–1341. doi: 10.1016/j.celrep.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 27.Groc L, Gustafsson B, Hanse E. AMPA signalling in nascent glutamatergic synapses: There and not there! Trends Neurosci. 2006;29(3):132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61(2):247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008;6(9):e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill TC, Zito K. LTP-induced long-term stabilization of individual nascent dendritic spines. J Neurosci. 2013;33(2):678–686. doi: 10.1523/JNEUROSCI.1404-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinney RA, Capogna M, Dürr R, Gähwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2(1):44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mateos JM, et al. Synaptic modifications at the CA3-CA1 synapse after chronic AMPA receptor blockade in rat hippocampal slices. J Physiol. 2007;581(Pt 1):129–138. doi: 10.1113/jphysiol.2006.120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang MG, Guo Y, Huganir RL. AMPA receptor and GEF-H1/Lfc complex regulates dendritic spine development through RhoA signaling cascade. Proc Natl Acad Sci USA. 2009;106(9):3549–3554. doi: 10.1073/pnas.0812861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortin DA, et al. Long-term potentiation-dependent spine enlargement requires synaptic Ca2+-permeable AMPA receptors recruited by CaM-kinase I. J Neurosci. 2010;30(35):11565–11575. doi: 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagerlöf O, et al. The nutrient sensor OGT in PVN neurons regulates feeding. Science. 2016;351(6279):1293–1296. doi: 10.1126/science.aad5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158(1):54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruan HB, et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159(2):306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Mol Aspects Med. 2016;51:1–15. doi: 10.1016/j.mam.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272(14):9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 42.Prendergast J, et al. Ganglioside regulation of AMPA receptor trafficking. J Neurosci. 2014;34(39):13246–13258. doi: 10.1523/JNEUROSCI.1149-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagerlöf O, Hart GW. O-GlcNAcylation of neuronal proteins: Roles in neuronal functions and in neurodegeneration. Adv Neurobiol. 2014;9:343–366. doi: 10.1007/978-1-4939-1154-7_16. [DOI] [PubMed] [Google Scholar]
- 44.Akimoto Y, et al. Localization of the O-GlcNAc transferase and O-GlcNAc-modified proteins in rat cerebellar cortex. Brain Res. 2003;966(2):194–205. doi: 10.1016/s0006-8993(02)04158-6. [DOI] [PubMed] [Google Scholar]
- 45.Cole RN, Hart GW. Cytosolic O-glycosylation is abundant in nerve terminals. J Neurochem. 2001;79(5):1080–1089. doi: 10.1046/j.1471-4159.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- 46.Trinidad JC, et al. Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol Cell Proteomics. 2012;11(8):215–229. doi: 10.1074/mcp.O112.018366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vosseller K, et al. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5(5):923–934. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 48.Rexach JE, et al. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat Chem Biol. 2012;8(3):253–261. doi: 10.1038/nchembio.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor EW, et al. O-GlcNAcylation of AMPA receptor GluA2 is associated with a novel form of long-term depression at hippocampal synapses. J Neurosci. 2014;34(1):10–21. doi: 10.1523/JNEUROSCI.4761-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanno T, Yaguchi T, Nagata T, Mukasa T, Nishizaki T. Regulation of AMPA receptor trafficking by O-glycosylation. Neurochem Res. 2010;35(5):782–788. doi: 10.1007/s11064-010-0135-1. [DOI] [PubMed] [Google Scholar]
- 51.Tallent MK, et al. In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J Biol Chem. 2009;284(1):174–181. doi: 10.1074/jbc.M807431200. [DOI] [PubMed] [Google Scholar]
- 52.Song M, et al. o-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell Signal. 2008;20(1):94–104. doi: 10.1016/j.cellsig.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Khidekel N, et al. Probing the dynamics of O-GlcNAc glycosylation in the brain using quantitative proteomics. Nat Chem Biol. 2007;3(6):339–348. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]
- 54.Wenthold RJ, Petralia RS, Blahos J II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16(6):1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105(3):331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299(5612):1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- 57.Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: Delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3(11):1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- 58.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Pickard L, Noël J, Henley JM, Collingridge GL, Molnar E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci. 2000;20(21):7922–7931. doi: 10.1523/JNEUROSCI.20-21-07922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330(6007):1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49(5):663–670. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 62.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24(4):1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bullen JW, et al. Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK) J Biol Chem. 2014;289(15):10592–10606. doi: 10.1074/jbc.M113.523068. [DOI] [PMC free article] [PubMed] [Google Scholar]