Significance
The recent success of immune checkpoint blockades, such as the use of antibodies against CTLA4, PD-1, and PD-L1 in cancer therapies has reinvigorated the concept of intrinsic antitumor immunity, but how the immune system detects tumors and generates antitumor immunity is still not well understood. Here we showed that the PD-L1 blockade lost its antitumor effects in mice lacking the cytosolic DNA sensor cGAS, suggesting that cGAS is essential for intrinsic antitumor immunity. Further, we showed that the cGAS product cGAMP has strong antitumor effects, especially when it is combined with the PD-L1 antibody. These results demonstrate that cGAS plays a pivotal role in cancer immunity and that cGAMP and its derivatives may be used directly for cancer immunotherapy.
Keywords: cGAS, cGAMP, PD-L1, STING, cancer
Abstract
cGMP-AMP (cGAMP) synthase (cGAS) is a cytosolic DNA sensor that activates innate immune responses. cGAS catalyzes the synthesis of cGAMP, which functions as a second messenger that binds and activates the adaptor protein STING to induce type I interferons (IFNs) and other immune modulatory molecules. Here we show that cGAS is indispensable for the antitumor effect of immune checkpoint blockade in mice. Wild-type, but not cGAS-deficient, mice exhibited slower growth of B16 melanomas in response to a PD-L1 antibody treatment. Consistently, intramuscular delivery of cGAMP inhibited melanoma growth and prolonged the survival of the tumor-bearing mice. The combination of cGAMP and PD-L1 antibody exerted stronger antitumor effects than did either treatment alone. cGAMP treatment activated dendritic cells and enhanced cross-presentation of tumor-associated antigens to CD8 T cells. These results indicate that activation of the cGAS pathway is important for intrinsic antitumor immunity and that cGAMP may be used directly for cancer immunotherapy.
Recognition of DNA as a pathogen-associated molecular pattern (PAMP) by the immune system provides a versatile mechanism to detect a large variety of microbial pathogens that contain DNA or require DNA in their life cycles. DNA is also a danger-associated molecular pattern (DAMP) when self-DNA, which is normally confined to the nucleus and mitochondria in an eukaryotic cell, is inadvertently present in the cytosol (1, 2). Cytosolic DNA in immune and nonimmune cells can trigger strong innate immune responses, including the production of type I interferons (IFNs) and other inflammatory cytokines. A major sensor for cytosolic DNA is cGMP-AMP (cGAMP) synthase (cGAS), which binds to double-stranded DNA irrespective of the DNA sequence (3, 4). The DNA binding causes a conformational change of cGAS, leading to its activation (5–9). The activated cGAS catalyzes the conversion of GTP and ATP into 2′3′-cGAMP, which contains two phosphodiester bonds, one between the 2′-OH of GMP and 5′-phosphate of AMP, and the other between the 3′-OH of AMP and 5′-phosphate of GMP (6, 10–12). 2′3′-cGAMP (hereinafter referred to as cGAMP) then functions as a second messenger that binds and activates the adaptor protein STING, which is localized on the endoplasmic reticulum membrane (4, 13, 14). STING in turn activates the protein kinases IKK and TBK1, leading to activation of the transcription factors NF-κB and IRF3, respectively (1, 2, 15). NF-κB and IRF3 enter the nucleus where they function together to induce a battery of immune and inflammatory gene products, including type I IFNs and TNFα.
Genetic and biochemical studies have demonstrated that cGAS is an innate immune sensor that detects a wide spectrum of pathogens, including DNA viruses such as herpes simplex virus, vaccinia virus, adenovirus, retroviruses such as HIV, and bacteria such as Mycobacterium tuberculosis (1). As a general sensor of cytosolic DNA, cGAS activation has also been shown to cause autoimmune diseases resulting from accumulation of self-DNA in the cytoplasm in several mouse models, such as those deficient in the DNase Trex1 or DNase II (16, 17). Another potential source of self-DNA that can activate cGAS is tumor cell DNA. When tumor cells are taken up by phagocytes such as dendritic cells (DCs), a fraction of tumor DNA may enter the cytoplasm to activate the cGAS–STING pathway (18, 19). Indeed, recent studies suggest that STING-deficient mice are less responsive to radiation and immunotherapies, such as blockade of immune suppressive molecules, including PD-1, PD-L1, CTLA4, and CD47 (20–23). Consistent with this model, stimulation of STING with cGAMP or its analogs by intratumorial injection inhibits tumor growth in immune competent mice. However, some other studies suggest that STING activation may contribute to tumor growth and metastasis by inducing a suppressive tumor microenvironment (24, 25). Thus, the role of STING in tumor immunity remains complex and is not well understood.
Immune checkpoint blockade through inhibition of negative regulators of T cells, such as PD-1, PD-L1, and CTLA4, has emerged as one of the most successful therapies of cancers in humans (26, 27). The effectiveness of such therapies depends on the intrinsic antitumor immunity, most notably the recognition of tumor antigens and generation of tumor-specific cytotoxic T cells (CTLs). However, the majority of cancer patients remain unresponsive to immune checkpoint inhibitor therapies, in large part because they do not generate adequate antitumor immunity. Thus, there is a pressing need to understand innate and adaptive immune responses to tumors and to harness the body’s immune system to develop more effective strategies to fight cancers.
Here, we show that cGAS-deficient mice are refractory to the antitumor effects of a PD-L1 antibody in a mouse model of melanoma. Moreover, intramuscular delivery of cGAMP strongly enhanced the ability of the PD-L1 antibody to inhibit tumor growth and prolong mouse survival. cGAMP treatment stimulated the activation of dendritic cells and enhanced cross-presentation of tumor-associated antigens to CD8 T cells. These results demonstrate that cGAS–cGAMP signaling plays a pivotal role in the intrinsic antitumor immunity and that this pathway may be harnessed to improve cancer immunotherapy in human patients.
Results
cGAS Is Essential for the Therapeutic Effect of PD-L1 Blockade.
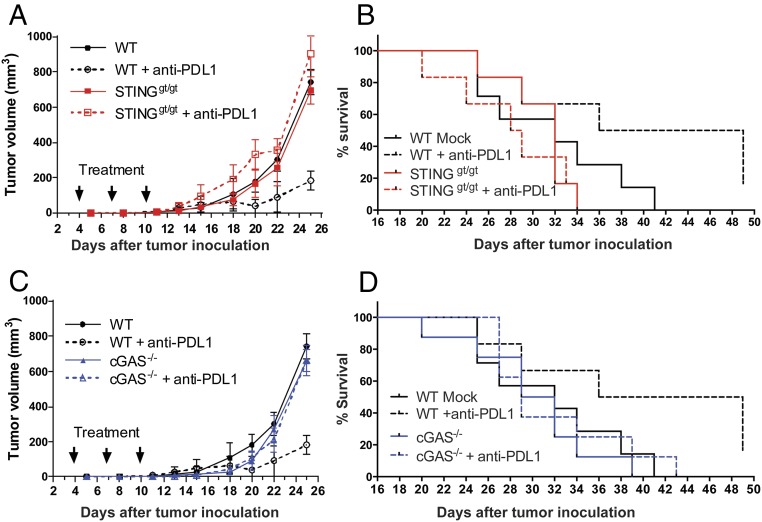
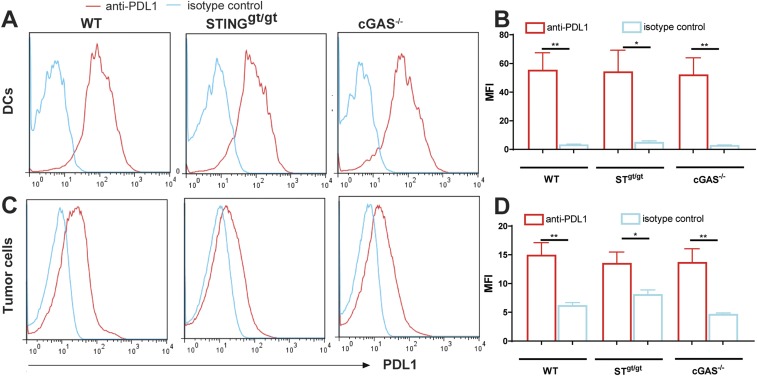
We used the highly aggressive B16F10 melanoma model to investigate the role of cGAS in antitumor immunity. B16F10 tumor cells were transplanted s.c. into the wild-type (WT) and cGas−/− mice, as well as the Sting golden-ticket (Stinggt/gt) mice, which do not express STING (28). Mice were subsequently treated with a PD-L1 antibody by i.p. injection, followed by measurements of tumor volumes and monitoring of mouse survival. Without treatment, no marked difference in the B16 tumor growth was observed among WT, cGas−/−, and Stinggt/gt mice. However, in response to PD-L1 antibody treatment, WT, but not cGas−/− or Stinggt/gt, mice had significant decrease of tumor volumes (Fig. 1 A and C). PD-L1 antibody treatment also significantly extended the survival of the tumor-bearing WT mice, but not those deficient in cGAS or STING (Fig. 1 B and D). Analyses of PD-L1 expression showed that dendritic cells and tumor cells isolated from the established B16 melanoma expressed similar levels of PD-L1 among WT, cGas−/−, and Stinggt/gt mice (Fig. S1), suggesting that the impaired antitumor response to the PD-L1 antibody in the cGas−/− and Stinggt/gt mice was not due to reduced expression of PD-L1 in these mice. These results demonstrate that cGAS and STING are required for the antitumor effects of PD-L1 blockade through a mechanism that is independent of the PD-L1 expression level.
Fig. 1.
cGAS and STING are essential for the antitumor effect of PD-L1 blockade. WT, cGAS−/−, and STINGgt/gt mice (n = 6–8 per group) were injected s.c. with 1 × 105 B16F10 melanoma cells, followed by three treatments with 200 μg of PD-L1 antibody at indicated time points. Tumor volumes were measured on the indicated dates and calculated according to the following formula: π/6 × length × width × height. Data are shown as mean ± SEM (A and C). Loss of survival was defined as death or when tumor diameter reached or exceeded 2 cm in any dimension (B and D).
Fig. S1.
Normal expression of PD-L1 on DCs and tumor cells in cGAS−/− and STINGgt/gt mice. WT, cGAS−/−, and STINGgt/gt mice (n = 3–4 per group) were injected s.c. with 1 × 106 B16F10 melanoma cells, and tumors were harvested on day 14. Homogenous tumor suspension was prepared and analyzed by FACS using antibodies against CD45, MHCII, CD11c, and PD-L1. Dendritic cells are defined as MHCII+ CD11c+ (A and B) and tumor cells as CD45− population (C and D). Representative FACS plots are shown in A and C, and mean fluorescence intensity (MFI) of PD-L1–expressing cells in indicated experimental groups is shown in B and D. Data are shown as mean ± SEM. Statistical analysis was performed with a one-tailed, unpaired Student’s t test. *P < 0.05 and **P < 0.01.
cGAS and STING Promote the Generation of Tumor-Infiltrating Cytotoxic T Cells.
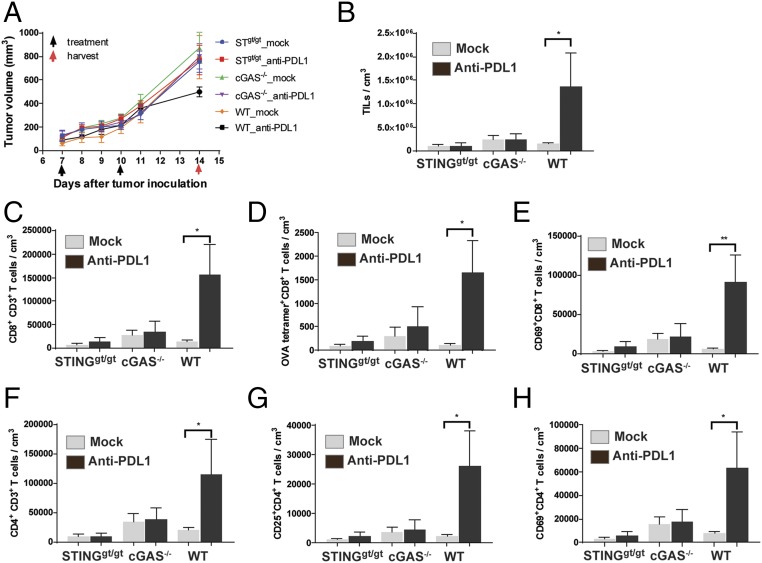
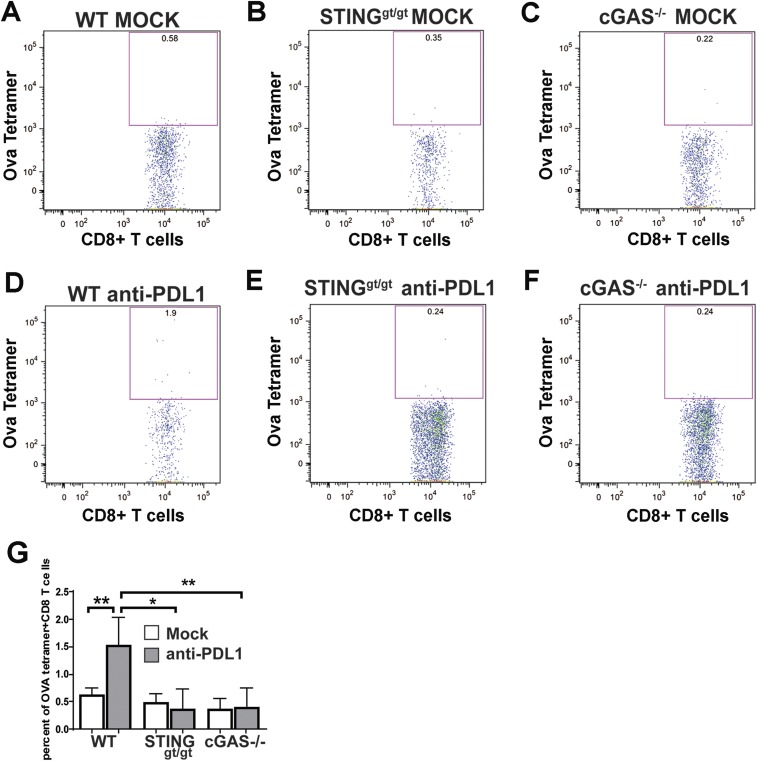
To investigate how the cGAS–STING pathway might enhance the antitumor effects of PD-L1 blockade, we inoculated WT, cGas−/−, and Stinggt/gt mice with B16 melanoma cells that stably expressed chicken ovalbumin (B16-Ova). Seven days after the tumor injection, the mice were treated with the PD-L1 antibody followed by another treatment on day 10. Tumors were harvested on day 14 to isolate leukocytes, which were stained with the H2-Kb MHC-class I tetramer bound to the ovalbumin peptide SIINFEKL, as well as an antibody against CD8 to identify tumor-specific CD8 T cells (Fig. 2D and Fig. S2). The leukocytes were also stained with antibodies against other cell surface markers, including CD45 (for leukocytes), CD3 (T cells), CD4, CD25 (regulatory T cells), and CD69 (activated T cells). In WT mice, PD-L1 antibody treatment decreased tumor volumes (Fig. 2A) and increased the total number of leukocytes in the tumors (Fig. 2B). Further analyses of these tumor-infiltrating leukocytes revealed that PD-L1 antibody treatment also increased the number of CD8 and CD4 T cells in the tumors (Fig. 2 C and F), including Ova-specific CD8 T cells (Fig. 2D), CD69 positive CD8 and CD4 T cells (Fig. 2 E and H), and regulatory CD4 T cells (Fig. 2G). Such effects were not observed in cGas−/− or Stinggt/gt mice, suggesting that both cGAS and STING are required for the generation or infiltration of leukocytes, including antigen-specific cytotoxic T cells, in the tumors.
Fig. 2.
cGAS and STING are required for generation of tumor-infiltrating CD8 T cells. (A) WT, cGAS−/−, and STINGgt/gt mice (n = 5 each group) were injected s.c. with 1 × 106 B16F10-OVA cells. PD-L1 antibody was administered on days 7 and 10 after tumor inoculation. Tumor volume was measured on indicated dates (A). Tumors were harvested on day 14 to isolate and analyze tumor-infiltrating leukocytes (TILs). TILs are defined as CD45+ cells and normalized with tumor volume (B). CD8+ T cells are defined as CD8+CD3+ CD45+ TILs (C). CD4+ T cells are defined as CD4+CD3+ CD45+ TILs (F). Ova-specific CD8+ T cells are those bearing the T-cell receptor specific for an Ova-H2Kb tetramer (D). Regulatory T cells are defined as CD25+ CD4+ T cells (G). Activated CD8 (E) and CD4 (H) T cells are defined as CD69+ CD8+ and CD69+ CD4+ T cells. Data are shown as mean ± SEM. Statistical analysis was performed with a one-tailed, unpaired Student’s t test. *P < 0.05 and **P < 0.01.
Fig. S2.
cGAS and STING are required for tumor-specific CD8 T-cell response to PD-L1 antibody treatment. WT, cGAS−/−, and STINGgt/gt mice (n = 5 per group) were injected s.c. with 1 × 106 B16F10-Ova melanoma cells, followed by two treatments with 200 μg of PD-L1 antibody as described in Fig. 2. Tumors were harvested on day 14, and tumor-infiltrating leukocytes were prepared and analyzed by FACS using an Ova-H2Kb tetramer and antibodies against CD8 and CD3. Representative FACS plots are shown in A–F, and data from all experimental groups are shown in G as mean ± SEM. *P < 0.05 and **P < 0.01.
Intramuscular Delivery of cGAMP Strongly Enhances the Antitumor Effects of PD-L1 Blockade.
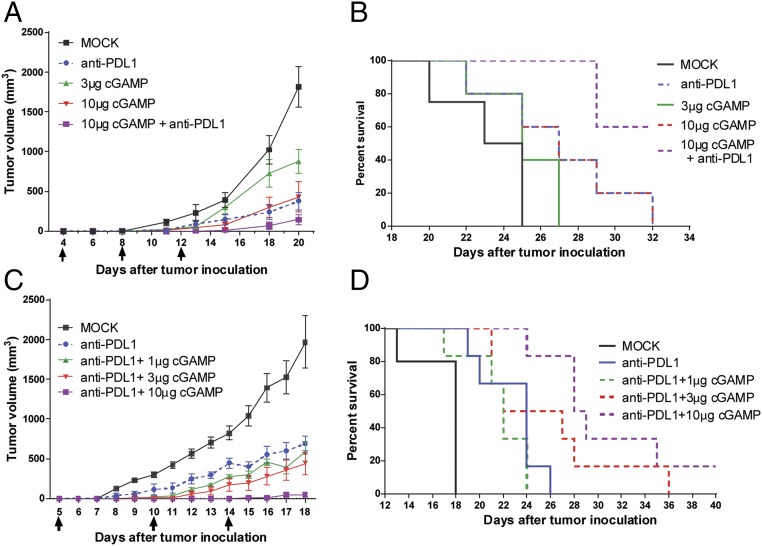
The requirement of cGAS and STING for tumor inhibition suggests that the cGAS product cGAMP can stimulate the antitumor immunity. Indeed, we have previously shown that intramuscular delivery of cGAMP in mice strongly boosts antibody production and both CD4 and CD8 T-cell activation (29). To test whether cGAMP has antitumor activity, we delivered cGAMP into the muscle in the hind legs of mice, which are distant from the flank where B16 melanoma cells were implanted. Different doses of cGAMP were injected 4 d after the tumors were implanted and the treatment was repeated two more times with 4-d intervals. Notably, 10 μg of cGAMP was as effective as 200 μg of the PD-L1 antibody, and the combination treatment was even more effective (Fig. 3 A and B). Titration experiment showed that cGAMP enhanced the antitumor effects of PD-L1 antibody in a dose-dependent manner (Fig. 3 C and D). No weight loss or other apparent side effects were observed in the mice treated with cGAMP alone or in combination with PD-L1 antibody. These results demonstrate that intramuscular injection of cGAMP at a site distant from tumors can strongly enhance the therapeutic effects of immune checkpoint blockade.
Fig. 3.
Antitumor effects of cGAMP and PD-L1 antibody. C57BL/6J mice (n = 4–5 per group) were injected with 1 × 105 B16F10 melanoma cells, followed by treatments with cGAMP at indicated doses or 200 μg of PD-L1 antibody on days 4, 8, and 12 after tumor inoculation. Tumor volumes were measured on indicated dates (A). Survival of the tumor-bearing mice is shown in B. (C and D) Similar to A and B, except that mice (n = 5–6 per group) were treated with 200 μg of PD-L1 antibody in combination with different amounts of cGAMP as indicated on days 5, 10, and 14 after tumor inoculation. Data are shown as mean ± SEM in A and C.
cGAMP Stimulates Dendritic Cell Activation.
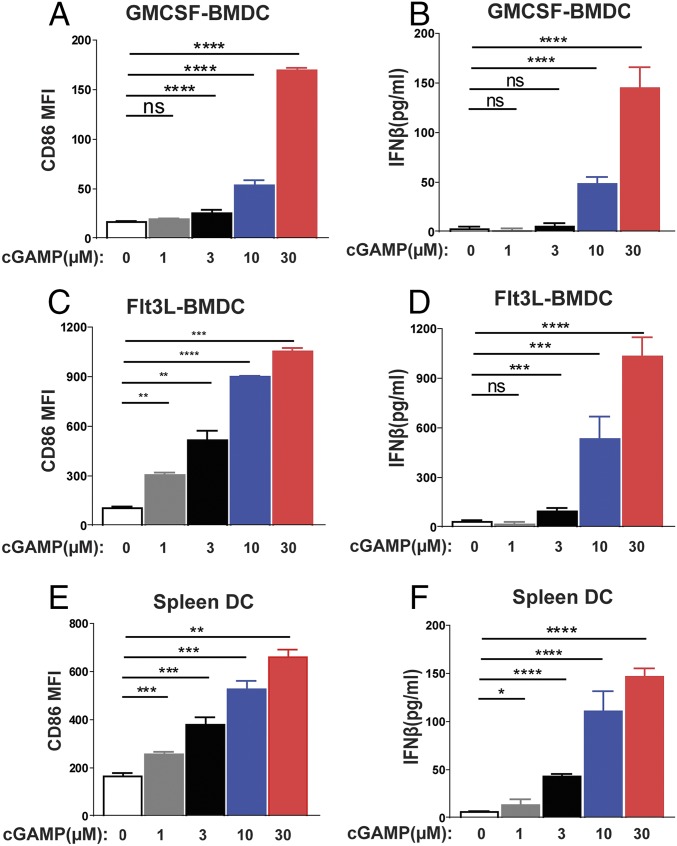
cGAMP contains two phosphodiester bonds, which may hinder its ability to enter cells. Thus, it is surprising that delivery of cGAMP alone, without any transfection reagent, could have potent antitumor effects in vivo. One possibility is that cGAMP can be taken up by phagocytes, such as dendritic cells, through pinocytosis or endocytosis. To test this possibility, we obtained bone-marrow–derived dendritic cells (BMDCs) culturing in the presence of GM-CSF, which stimulates conventional DC differentiation, or Flt-3 ligand, which stimulates the differentiation of plasmacytoid DCs. These DCs, as well as DCs isolated directly from spleens, were cultured in the presence of different concentrations of cGAMP without transfection. cGAMP stimulated the production of IFNβ, as well as surface expression of CD86, a costimulatory ligand of T cells (Fig. 4). These results suggest that DCs can take up cGAMP from extracellular space, which may explain its adjuvant effects in vivo when it was directly injected into mice without a delivery vehicle.
Fig. 4.
cGAMP activates dendritic cells. GM-CSF DCs (A and B), Flt3 DCs (C and D), and splenic DCs (E and F) were cultured in the presence of cGAMP at different concentrations (1, 3, 10, and 30 µM). Eighteen hours after incubation, CD86 expression on MHCII+CD11c+ DCs was analyzed by FACS (A, C, and E). IFNβ in the cell culture media was analyzed by ELISA (B, D, and F). Data are shown as mean ± SEM. Statistical analysis was performed with a one-tailed, unpaired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
cGAMP Stimulates Cross-Presentation of Tumor-Associated Antigens.
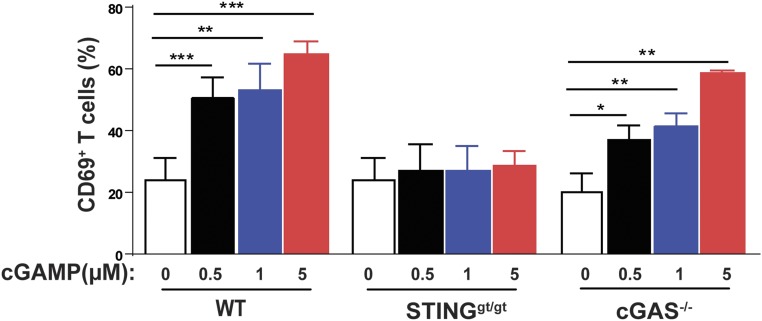
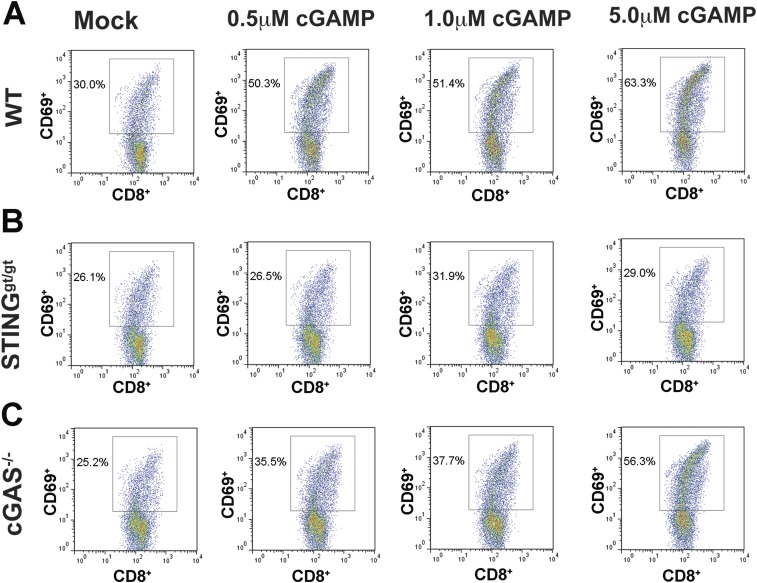
To further investigate the mechanism by which cGAMP enhances antitumor immunity, we isolated BMDCs from WT, cGas−/−, and Stinggt/gt mice, then incubated the DCs with irradiated B16-Ova cells in the presence of different concentrations of cGAMP. CD11c+ DCs were purified and incubated with CD8 T cells isolated from the transgenic mice expressing the T-cell receptor specific for the ovalbumin peptide SIINFEKL (OT-I). Activation of CD8 T cells was measured by the surface expression of the activation marker CD69, which showed that cGAMP strongly stimulated the activation of CD8 T cells by the tumor-associated antigen in WT and cGas−/− mice, but not Stinggt/gt mice (Fig. 5 and Fig. S3). These results demonstrate that cGAMP stimulates cross-presentation of tumor antigens to CD8 T cells by DCs in a STING-dependent manner.
Fig. 5.
cGAMP stimulates cross-presentation of a tumor-associated antigen. BMDCs from WT, cGas−/−, and Stinggt/gt mice were incubated with irradiated B16F10-OVA cells in the presence of indicated concentrations of cGAMP. CD11c+ DC cells were then purified and cocultured with CD8+ T cells isolated from OT-I T-cell receptor transgenic mice. Cross-presentation efficiency of DCs was analyzed by CD69 expression of OT-I T cells and the average of four independent experiments is shown (representative FACS plots are shown in Fig. S3). Statistical analysis was performed with a one-tailed, unpaired Student’s t test. Data are shown as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Fig. S3.
cGAMP enhances tumor antigen cross-presentation in dendritic cells in a STING-dependent manner. BMDCs from WT (A), STINGgt/gt (B), and cGAS−/− (C) mice were incubated with irradiated B16F10-Ova cells and indicated concentrations of cGAMP. CD11c+ DCs were then isolated and cocultured with CD8 T cells from transgenic mice expressing the Ova-specific T-cell receptor (OT-I). Activated T cells were analyzed by FACS using antibodies specific for CD69 and CD8. Representative FACS plots from four independent experiments are shown, and data from all experimental mouse groups are shown in Fig. 5.
Discussion
How the immune system detects tumor cells and mounts an appropriate antitumor response is a central question in tumor immunology and immunotherapy. In this report, we provide evidence that the cGAS–STING pathway plays an important role in intrinsic antitumor immunity and that this pathway is required for the therapeutic effects of immune checkpoint blockade. We showed that PD-L1 antibody exerted antitumor effects in wild-type mice but not in those deficient in cGAS or STING. In response to PD-L1 antibody treatment, there is a large increase of tumor-infiltrating leukocytes, including tumor antigen-specific CD8 T cells, in wild-type mice, but not cGAS- or STING-deficient mice. A possible explanation for this phenotype is that PD-L1 antibody treatment leads to the killing of some tumor cells, which are taken up by dendritic cells. The tumor cell DNA somehow gains access to the cytoplasm of the dendritic cells, leading to activation of the cGAS–STING pathway, which enhances T-cell priming and produces chemokines that recruit leukocytes to the tumors. It is also possible that PD-L1 antibody acts directly on dendritic cells. Indeed, a previous study showed that a blockade of PD-L1 expression in human myeloid dendritic cells enhanced antitumor T-cell activation (30), suggesting that PD-L1 blockade not only permits killing of PD-L1–expressing tumor cells by activated T cells, but also promoted T-cell priming by dendritic cells. Our data suggest that the promotion of T-cell priming by PD-L1 blockade requires the cGAS pathway. Further research is needed to determine in what cell types the cGAS pathway plays a role in activating the antitumor responses and how tumor DNA is delivered to the cytosol to trigger the cGAS pathway.
Whereas our study focused on the role of the cGAS pathway in the antitumor effects of a PD-L1 antibody, it is likely that this pathway is also important for antitumor responses triggered by the PD-1 antibody, although this remains to be formally tested. Whether or not the cGAS pathway plays a role in other immune checkpoint blockades, such as CTLA4 and CD47, also requires further investigation. Although we chose B16F10 melanoma, one of the most aggressive tumors, as our mouse model, future studies should determine the role of the cGAS pathway in other tumor models, including transplanted tumors in syngeneic mice and endogenous tumors in genetically engineered mice.
Our previous studies showed that intramuscular injection of cGAMP into mice strongly stimulates antibody production and T-cell activation, suggesting that cGAMP can be used directly as an adjuvant despite its negative charge (29). Here we showed that cGAMP at high concentrations could activate dendritic cells to express IFNβ and the costimulatory molecule CD86, suggesting that phagocytes such as dendritic cells might take up cGAMP through pinocytosis or endocytosis. Importantly, cGAMP strongly enhanced the cross-presentation of a tumor-associated antigen (ovalbumin) from dendritic cells to CD8 T cells, and this effect is dependent on STING in the dendritic cells. Although enhancement of cytokine production and expression of costimulatory molecules is clearly a mechanism by which cGAMP stimulates cross-presentation, whether cGAMP also modulates the processing and presentation of tumor-associated antigens in dendritic cells requires further investigation.
Using the B16 melanoma model, we have demonstrated that intramuscular injection of cGAMP led to inhibition of tumor growth and improved the survival of tumor-bearing mice. Combination of cGAMP and PD-L1 antibody had stronger antitumor effects than either treatment alone. Previous studies have shown that intratumor injection of cGAMP and its analogs also had strong antitumor effects (19–21). Whereas intratumor injection can be effective for tumors that are detectable and accessible, it may not be applicable to many tumors that are not easily accessible. Our finding that intramuscular delivery of cGAMP at a site distant from the tumor site suggests that cGAMP and its analogs may be used to treat a variety of tumors, including those that are not accessible for intratumor injections.
Materials and Methods
Mice.
C57BL/6J wild-type mice and Sting golden-ticket (Stinggt/gt) mice were purchased from The Jackson Laboratory. cGas−/− mice were generated in our laboratory and had been reported previously (29). OT-I T-cell receptor transgenic mice on Rag1-deficient background were from Taconic (cat. no. 4175). All mice were maintained in the animal care facility of the University of Texas Southwestern Medical Center at Dallas. Experimental protocols were approved by the Institutional Animal Care and Use Committee.
Preparation of Dendritic Cells.
To obtain bone-marrow–derived dendritic cells, bone marrow cells were aspirated from femurs and tibiae of mice and cultured with RPMI 1640, 10% FCS, 1% GM-CSF, or Flt3L conditioned media. GM-CSF–induced BMDCs (GM-CSF DC) were collected on days 5–7, and Flt3L-induced BMDCs (Flt3L DC) were collected on day 9.
Spleens were minced and incubated with digestion buffer containing collagenase A (Sigma; 1.5 mg/mL), DNase (Sigma; 150 μg/mL), 5% FCS, and 11 mM d-glucose in 1.5 mL volume for 30 min at 37 °C with shaking. Tissues were crushed through a 70-µm cell strainer into a 50-mL tube containing PBS, 5% FCS, 11 mM d-glucose, and 2 mM EDTA. Splenic DCs were isolated with Dynabead Mouse DC Enrichment Kit (Invitrogen, 11429D).
Stimulation and Analyses of DCs.
Dendritic cells were seeded in round-bottom 96-well plates at 3 × 105 cells per well and stimulated with indicated concentrations of cGAMP in 300 µL of RPMI 1640 containing 10% FCS for 18 h. Cells were blocked with anti-CD16/32 (BioLegend) and stained with anti-CD86 (BioLegend), anti–I-A/I-E (BioLegend) and anti-CD11c (BioLegend). Stained cells were analyzed with a FACS Calibur instrument (BD Biosciences) and the FACS data were analyzed using FlowJo software. Secretion of IFNβ by the DCs was measured using an ELISA kit (R&D Systems).
Tumor Inoculation, Treatment, and Measurement.
B16F10 melanoma tumor cells were grown in DMEM containing 10% FCS. A total of 105 B16 cells in 100 μL PBS were injected into the flank of a mouse s.c. to establish tumors. Four days after tumor inoculation, mice were treated with cGAMP or PD-L1 antibody or both. 2′3′-cGAMP was synthesized as described previously (10). A total of 50 μL of 2′3′-cGAMP in PBS at indicated concentration was injected into the muscle of the hind legs. A total of 200 μg of PD-L1 antibody in 100 μL PBS was injected intraperitoneally into mice alone or immediately after cGAMP injection. These treatments were repeated three times with 4-d intervals. Tumors were measured with a digital caliper (Fisher) and the tumor sizes were calculated using the following formula: π/6 × length × width × height.
Tumor-Infiltrating Leukocyte Separation and Staining.
For analyses of tumor-infiltrating leukocytes, a B16F10 melanoma cell line stably expressing ovalbumin was used to establish tumors. A total of 1 × 106 B16F10-Ova cells were injected into the flank of a mouse s.c. At 7 and 10 days after tumor inoculation, PD-L1 antibody was injected intraperitoneally into the mice, followed by harvesting of the tumors on day 14. Tumors were minced and filtered through a 100-μm strainer to obtain single-cell suspensions. Red blood cells were further lysed with RBC lysis buffer (Sigma, cat. no. R7757). After pelleting, cells were resuspended in 11 mL of 40% Percoll (GE Healthcare) in RPMI and overlaid onto 3.5 mL of 70% Percoll in a 15-mL conical tube. After centrifugation at 800 × g for 20 min without break, leukocytes were collected from the gradient interface and washed with 10 mL of cold RPMI. The leukocytes were stained with a mixture of antibodies, including anti-mouse CD25-FITC (BioLegend), anti-mouse CD45.2-PE (BioLegend), anti-mouse CD3-Percp Cy5.5 (BioLegend), anti-mouse CD4-PE Cy7 (BioLegend), anti-mouse CD8-APC Cy7 (BioLegend), anti-mouse CD69-pacific blue (BioLegend), and APC-conjugated H-2Kb/OVA (SIINFEKL) tetramer reagent (NIH). Stained cells were analyzed with an LSRII instrument (BD Biosciences) and the FACS data were analyzed using FlowJo software.
Cross-Presentation of Tumor-Associated Antigens.
BMDCs were generated by culturing bone marrow progenitors in RPMI medium 1640 supplemented with 20 ng/mL GM-CSF (Peprotech) and 10% FCS (Sigma), plus 100 IU/mL penicillin and 100 µg/mL streptomycin, 2 mM Glutamax, and 55 μM β-mercaptoethanol (all from Life Technologies). BMDCs were harvested for stimulation assay on day 7. B16F10-OVA cells were treated with 40 Gy irradiation and then cocultured with BMDCs at a ratio of 1:1 in the presence of fresh GM-CSF with or without cGAMP overnight. Subsequently CD11c+ DCs were purified using CD11c Microbeads (Miltenyi Biotec) or by FACS sorting using APC-conjugated CD11c (BioLegend) antibody. CD8+ T cells from OT-I T-cell receptor transgenic mice were isolated using Dynabeads Untouched Mouse CD8 Cell Isolation kits (Life Technologies) according to the manufacturer’s instructions. Purified CD11c+ DCs were incubated with CD8+ T cells for 24 h and CD69 expression on the CD8 T cells was measured by flow cytometry.
Statistical Analysis.
Statistical analysis was performed by one-tailed Student’s t tests using the software GraphPad Prism 7. Mouse survival curves and statistics were analyzed using the Mantel–Cox test.
Acknowledgments
We thank Dr. Xiao-dong Li for generating the B16F10-Ova cell line and Zhiqun Zeng for assistance with mouse experiments. This work was supported by the Cancer Prevention and Research Institute of Texas (Grants RP120718 and RP150498) and the Welch Foundation (Grant I-1389). Z.J.C. is an Howard Hughes Medical Institute Investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621363114/-/DCSupplemental.
References
- 1.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat Immunol. 2016;17(10):1142–1149. doi: 10.1038/ni.3558. [DOI] [PubMed] [Google Scholar]
- 2.Ablasser A, Gulen MF. The role of cGAS in innate immunity and beyond. J Mol Med (Berl) 2016;94(10):1085–1093. doi: 10.1007/s00109-016-1423-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339(6121):826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Civril F, et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature. 2013;498(7454):332–337. doi: 10.1038/nature12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao P, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153(5):1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kranzusch PJ, Lee AS, Berger JM, Doudna JA. Structure of human cGAS reveals a conserved family of second-messenger enzymes in innate immunity. Cell Reports. 2013;3(5):1362–1368. doi: 10.1016/j.celrep.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, et al. Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity. 2013;39(6):1019–1031. doi: 10.1016/j.immuni.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, et al. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. Cell Reports. 2014;6(3):421–430. doi: 10.1016/j.celrep.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, et al. Cyclic GMP-AMP containing mixed phosphodiester linkages is an endogenous high-affinity ligand for STING. Mol Cell. 2013;51(2):226–235. doi: 10.1016/j.molcel.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diner EJ, et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. Cell Reports. 2013;3(5):1355–1361. doi: 10.1016/j.celrep.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ablasser A, et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Xiao TS, Fitzgerald KA. The cGAS-STING pathway for DNA sensing. Mol Cell. 2013;51(2):135–139. doi: 10.1016/j.molcel.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao D, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc Natl Acad Sci USA. 2015;112(42):E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray EE, Treuting PM, Woodward JJ, Stetson DB. Cutting Edge: cGAS is required for lethal autoimmune disease in the Trex1-deficient mouse model of Aicardi-Goutières syndrome. J Immunol. 2015;195(5):1939–1943. doi: 10.4049/jimmunol.1500969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrales L, Gajewski TF. Endogenous and pharmacologic targeting of the STING pathway in cancer immunotherapy. Cytokine. 2016;77:245–247. doi: 10.1016/j.cyto.2015.08.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrales L, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Reports. 2015;11(7):1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demaria O, et al. STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci USA. 2015;112(50):15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng L, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemos H, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res. 2016;76(8):2076–2081. doi: 10.1158/0008-5472.CAN-15-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer JD, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79(2):688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li XD, et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341(6152):1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]