We would like to thank Johnson et al. (1) for their interest in our paper. They contest our view (2) that simultaneous expression of cell membrane, organellar, and cytoplasmic protein genes of Teleaulax amphioxeia in Mesodinium rubrum indicate a complete endosymbiont.
First, Johnson et al. compared their microscopic and transcriptomic data (figure 1 and table 1 of ref. 1) to argue that a stolen nucleus can express cryptophyte nuclear genes as abundantly as a complete cryptophyte cell. However, their figure 1 only depicts an ultrathin section, which could have missed parts of a complete endosymbiotic cell such as a nucleus that must be present to have any cryptophyte nuclear genes expressed. On the contrary, their figure 1 shows distinct cytoplasm surrounding the cryptophyte plastids and thin cell membranes separating different “organelle complex” entities, essentially similar to our micrographs (2).
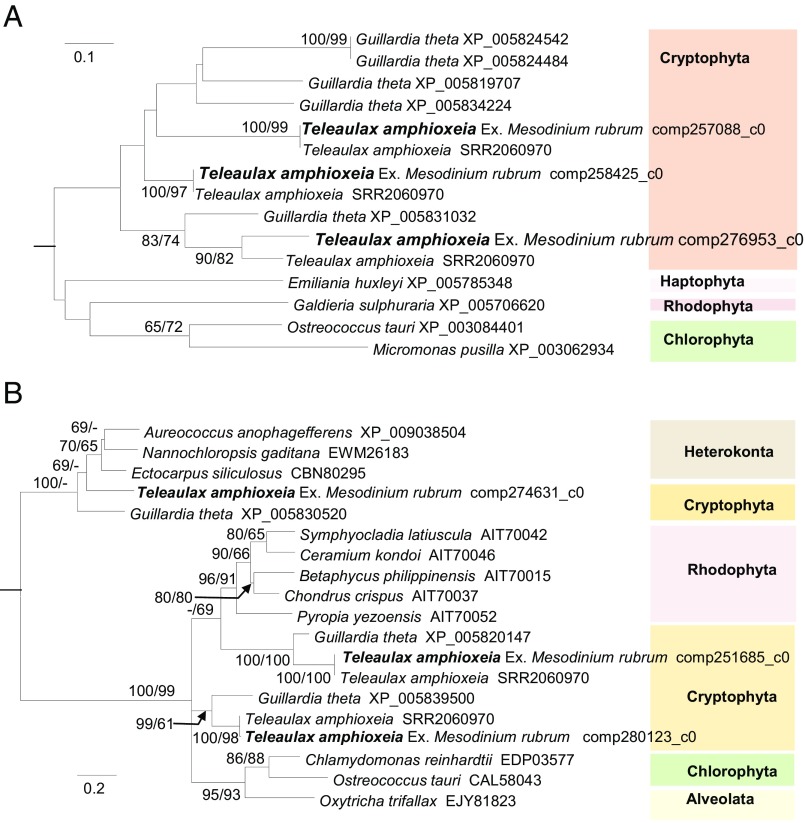
Second, they questioned whether expressed cell membrane and cytoplasmic protein genes we reported were really from T. amphioxeia. Without available genomic or transcriptomic data of Teleaulax we had made sure they were cryptophyte genes based on the Guillardia theta genome. Now, with the T. amphioxea transcriptome (Sequence Read Archive Study SRP059399) available (3), we conducted BLAST analysis for cell membrane ammonium transporter and cytoplasmic pyruvate kinase genes. The high (93–100%) sequence identity and tight phylogenetic affiliation (Fig. 1) with T. amphioxeia reinforced the T. amphioxeia origin of these genes.
Fig. 1.
Maximum likelihood trees of ammonium transporter (A) and pyruvate kinase (B) genes. Amino acid sequences obtained in our study (in bold type) are tightly clustered with those found in T. amphioxeia monospecific culture transcriptome (various sequences under SRR2060970) with strong support (bootstrap values of neighbor joining/maximum likelihood methods with 1,000 resamplings are shown at nodes; only values greater than 60 are shown).
Third, Johnson et al. (1) suggest that the cell membrane and cytoplasmic protein genes might have been a “contamination” by undigested cryptophyte cells in Mesodinium food vacuole. We examined this by comparing the expression levels of these genes relative to chloroplast protein ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco) in our Mesodinium-symbiotic versus in free-living T. amphioxeia. We found that the ammonium transporter [reads per kilobase of transcript per million mapped reads (RPKM) = 321] and pyruvate kinase (RPKM = 290) to Rubisco (RPKM = 11.73) ratios in our sample, 27 and 25, respectively, were remarkably higher than the ratios (69.19/195.67 = 0.35 and 55.73/195.67 = 0.28) in the free-living culture (3). A lower ratio would be expected for the scenario of contamination, especially given the high ratio of chloroplast to vacuole-contained cell in figure 1 of Johnson et al. (1).
We agree that our Mesodinium-farming-Teleaulax postulation would have been strengthened had we obtained time series samples. This should be pursued in the future. However, although this one sample would be inadequate for proving the absence of any cellular structure or gene expression, the detection of any cellular structure and gene expression in this sample is still proof of their presence.
These new assessments have bolstered our view (2) that the T. amphioxeia in the bloom Mesodinium we investigated were whole-cell endosymbionts. Findings by Johnson et al. (ref. 1 and references therein) of gene expression of cryptophyte nuclei and chloroplasts in Mesodinium are consistent with this postulation. We differ only in the interpretation of the altered cellular structure of the cryptophyte in Mesodinium. We believe that the alteration, including cytoplasm shrinking and cell membrane thinning, might have been rendered by the host to facilitate symbiosis. Nevertheless, ultimate proof will require further scrutiny, including mechanistic understanding of how the host ciliate regulates the function of the endosymbiont.
Footnotes
The authors declare no conflict of interest.
References
- 1.Johnson MD, et al. Mesodinium rubrum: The symbiosis that wasn’t. Proc Natl Acad Sci USA. 2017;114:E1040–E1042. doi: 10.1073/pnas.1619247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu D, Huang L, Lin S. Cryptophyte farming by symbiotic ciliate host detected in situ. Proc Natl Acad Sci USA. 2016;113(43):12208–12213. doi: 10.1073/pnas.1612483113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim GH, et al. Cryptophyte gene regulation in the kleptoplastidic, karyokleptic ciliate Mesodinium rubrum. Harmful Algae. 2016;52:23–33. doi: 10.1016/j.hal.2015.12.004. [DOI] [PubMed] [Google Scholar]