Significance
Glycosyltransferase enzymes synthesize complex sugar-containing macromolecules that play pivotal roles in the biology of all cells. Bacteria produce a vast range of glycoconjugate structures that frequently dictate host–pathogen interactions. The composition and chain length of bacterial glycans are both important for biological function, yet the mechanisms for chain-length determination are generally poorly understood. Here, we describe a remarkable family of proteins in which each member contains multiple glycosyltransferase modules (often representing new glycosyltransferase families) for glycan polymerization and chain termination. The polymerase and terminator activities are separated by a molecular ruler that establishes product length. The modularity of these proteins provides an adaptable platform for generating antigenic diversity in nature and potential new strategies for glycoengineering.
Keywords: microbial glycobiology, lipopolysaccharides, glycosyltransferases, glycan biosynthesis, molecular ruler
Abstract
Lipopolysaccharides (LPS) are essential outer membrane glycolipids in most gram-negative bacteria. Biosynthesis of the O-antigenic polysaccharide (OPS) component of LPS follows one of three widely distributed strategies, and similar processes are used to assemble other bacterial surface glycoconjugates. This study focuses on the ATP-binding cassette (ABC) transporter-dependent pathway, where glycans are completed on undecaprenyl diphosphate carriers at the cytosol:membrane interface, before export by the ABC transporter. We describe Raoultella terrigena WbbB, a prototype for a family of proteins that, remarkably, integrates several key activities in polysaccharide biosynthesis into a single polypeptide. WbbB contains three glycosyltransferase (GT) modules. Each of the GT102 and GT103 modules characterized here represents a previously unrecognized GT family. They form a polymerase, generating a polysaccharide of [4)-α-Rhap-(1→3)-β-GlcpNAc-(1→] repeat units. The polymer chain is terminated by a β-linked Kdo (3-deoxy-d-manno-oct-2-ulosonic acid) residue added by a third GT module belonging to the recently discovered GT99 family. The polymerase GT modules are separated from the GT99 chain terminator by a coiled-coil structure that forms a molecular ruler to determine product length. Different GT modules in the polymerase domains of other family members produce diversified OPS structures. These findings offer insight into glycan assembly mechanisms and the generation of antigenic diversity as well as potential tools for glycoengineering.
Bacterial surfaces possess an array of complex glycoconjugates (sugar-containing macromolecules) that play varied and vital roles in the biology of these organisms. In pathogens, glycoconjugates participate in adhesion, biofilm formation, and interaction with innate and adaptive immune responses. For example, in gram-negative bacteria such as Escherichia coli, long glycan chains in capsular polysaccharides and the O-antigen polysaccharide (OPS) components of lipopolysaccharide (LPS) molecules typically confer resistance to opsonophagocytosis and complement-mediated killing (1, 2). Effective protection depends on the amount and surface distribution of glycan, as well as glycan chain lengths suited for a given purpose. As an example, shorter OPS chains offer no resistance to complement-mediated killing, whereas chains longer than an optimal size potentially represent an unnecessary energy cost while offering no additional advantage (3, 4). Despite its fundamental importance in colonization and virulence of bacterial pathogens, the molecular mechanisms of glycan chain-length regulation are often poorly understood (5). However, OPS biosynthesis provides influential prototypes for understanding the guiding principles underpinning chain-length regulatory mechanisms in bacterial glycoconjugates in general.
The LPS glycolipid is a major component of the outer membrane of gram-negative bacteria. Classical LPS molecules consist of three structural regions: lipid A, core oligosaccharide, and OPS (reviewed in refs. 6 and 7). All OPS are long-chain polysaccharides comprised of repeating oligosaccharide subunits with hypervariable structures. A range of different repeat-unit structures may be produced by a given species, with each OPS structure defining an O-antigen serotype. The LPS molecule is synthesized by two pathways, one for the lipid A-core and the other for the OPS, and these converge in the periplasm with a ligation step that joins the two parts of the molecule, before its translocation to the cell surface (6–8). There are two well-distributed assembly strategies for OPS biosynthesis and both generate undecaprenyl diphosphate (Und-PP)–linked OPS intermediates and both end with O-antigen ligase (WaaL) enzyme transferring the nascent OPS from Un-PP–linked donor to lipid A-core acceptor. The two OPS-biosynthesis strategies differ in their polymerization mechanisms, components, and membrane topology of the reaction steps (reviewed in ref. 6). In nature, OPS chain lengths typically fall into a limited (modal) size range. The two OPS assembly systems use different approaches to establish the modal distribution before its ligation to lipid A-core (5).
One strategy for OPS biosynthesis is provided by the ATP-binding cassette (ABC) transporter-dependent assembly pathway and it provides the focus of this investigation. Here, the OPS is polymerized in the cytoplasm and then exported to the periplasm, by the pathway defining ABC transporter, for ligation (6, 9). This pathway has two variants distinguished by whether (or not) the ABC transporter is specific for the carbohydrate structure of a particular OPS substrate (reviewed in ref. 9). In systems where the ABC transporter is not specific, the Und-PP–linked glycan is polymerized by a membrane-associated complex of glycosyltransferase (GT) enzymes. Polymerization and export are obligatorily coupled in this process and the resulting OPS products possess a broad range of chain lengths. Klebsiella pneumoniae O2a provides the prototype for this assembly strategy (10, 11). The polymannose OPSs shared by E. coli O8/O9/O9a and their K. pneumoniae O5/O3 counterparts offer the prototypes for the substrate-specific assembly pathway. A serotype-specific polymerase enzyme (designated WbdA), possessing two or more GDP-mannose-dependent GT catalytic sites, is responsible for building the repeat-unit structure of the Und-PP–linked polymannose OPS (12, 13). The polymerase activity is opposed by a chain terminator (WbdD) that adds a methyl or phosphomethyl residue (depending on the serotype) to the nonreducing ends, creating completed OPS chains with optimal lengths within a limited (modal) size range before the involvement of the ABC transporter (14, 15). Chain termination chemically defines OPS chain length by adding residues that prevent further chain extension. However, the system includes a mechanism for chain-length determination, which dictates when OPS chains reach the correct length for termination to take place. This is established by structural features of a membrane-associated complex composed of WbdA and WbdD (13, 16). Stoichiometry and geometry of the complex influences chain length control (5) but the distance between the polymerase and terminator catalytic sites provides the critical determinant of the OPS chain length. An extended coiled-coil structure in WbdD performs this function, acting as a molecular ruler (17). This cannot occur unless the polymerase operates with a distributive mechanism, where the growing nonreducing terminus of the glycan is released from the active site after each glycosyltransfer reaction. The in vitro properties of WbdA are consistent with this mechanism (12). Depending on the length of the glycan, it may then be available to the terminator (WbdD) active site, which is separated from the polymerase by the coiled-coil structure (17). To ensure that only optimal-sized (terminally capped) glycans are exported, the ABC transporter possesses a substrate-specific carbohydrate binding module (CBM) that recognizes the modified terminal structure (18, 19). This offers a quality-control step, so only mature OPS components are found in LPS molecules that reach the cell surface (18, 19). Although many of the details of this prototype system are now well established, it remains unclear how universally these key features apply to OPS synthetic pathways that produce dissimilar glycan structures.
Here, we report biosynthesis of an OPS produced by some isolates of Raoultella terrigena and K. pneumoniae (hereafter referred to as O12 after the K. pneumoniae serotype). This is an example of an OPS assembly pathway involving a substrate-specific ABC transporter. These bacteria share the same OPS repeat unit structure and gene cluster (Fig. 1), presumably reflecting lateral transfer (20). The α-Rhap-(1 →3)-β-GlcpNAc disaccharide repeat unit is capped with a nonreducing terminal residue of β-linked 3-deoxy-d-manno-octulosonic acid (β-Kdop) (21, 22), which is recognized by a CBM in the ABC transporter (23). Here we describe the biosynthetic pathway and the central role of a remarkable enzyme that combines a chain terminator and polymerase, as well as a coiled-coil molecular ruler within a single polypeptide.
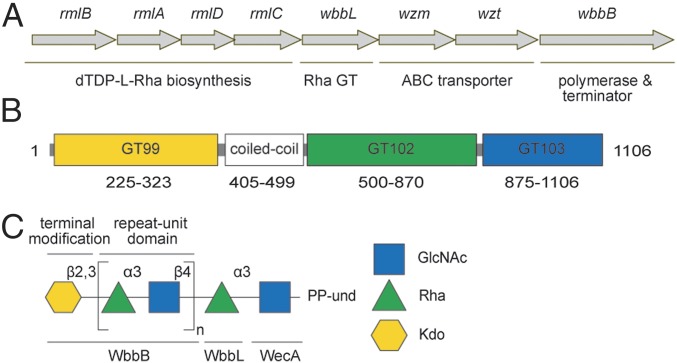
Fig. 1.
Structure and biosynthesis of K. pneumoniae O12 OPS. (A) Organization of the K. pneumoniae O12 OPS biosynthesis gene cluster. (B) Predicted location of WbbB domains. The N-terminal GT99 (yellow) identifies the area covered in a validated functional construct (22). (C) Structure of the K. pneumoniae O12 Und-PP–linked OPS intermediate predicted from structures of the authentic OPS glycans (20, 21). The standard colored symbol nomenclature (www.functionalglycomics.org/static/consortium/Nomenclature.shtml) is used to describe oligosaccharide structures. The basis for the functional assignments of the gene products is described in the text.
Results
The Biosynthesis Pathway for the O12 OPS.
The gene cluster responsible for OPS biosynthesis in the O12 system is composed of eight ORFs (Fig. 1A). Several of the gene products have been investigated or can be confidently assigned functions from sequence similarities. This, together with the data provided in the experiments described below, leads to the functional assignments for the O12 biosynthesis proteins in Fig. 1C.
ABC transporters possess two transmembrane domains forming the membrane channel and two nucleotide binding domains that hydrolyze ATP to drive transport (24). In most OPS ABC exporters, these domains are encoded by separate genes (wzm and wzt, respectively) and the function of the O12 ABC transporter has been validated (23). The rmlBADC genes encode the conserved pathway converting glucopyranose-1-phosphate to dTDP-l-rhamnopyranose (dTDP-Rhap) (25), whereas the UDP-N-acetyl-d-glucosamine (UDP-GlcpNAc) and CMP-3-deoxy-d-manno-octulosonic acid (CMP-Kdop) precursors are produced by “housekeeping enzymes,” because they are essential for production of peptidoglycan (26) and the lipid A-core component of LPS (7). Biosynthesis of K. pneumoniae OPS is initiated by WecA (23), a member of the UDP-d-N-acetylhexosamine:polyprenol phosphate N-acetylhexosamine-1-phosphate transferases, which initiate a variety of glycan assembly pathways (27). In OPS assembly, the WecA product (Und-PP-GlcpNAc) is modified by one or more NDP-glycose-dependent GT activities, creating an appropriate acceptor for OPS polymerization. In the O12 OPS pathway, the candidate for this transition step is WbbL, an enzyme also found in E. coli, where it is a dTDP-Rhap-dependent GT forming α-Rhap-(1→3)-β-GlcpNAc disaccharide (28); the same catalytic activity has been demonstrated for the K. pneumoniae O12 homolog (29). O12 OPS polymerization requires only two GTs (WbbB and WbbL) in addition to the initiating WecA (23). However, it was unknown before the current study whether WbbL action is confined to a single early step or whether it also forms the same linkage during polymerization of repeat units. A previous study proposed WbbB was a UDP-GlcpNAc-dependent GT, with WbbL providing the rhamnosyltransferase for polymerization (20, 29).
Using domain/motif search tools, WbbB is predicted to possess three GT modules (Fig. 1B). The N-terminal domain contains the recently characterized chain-terminating β-Kdop GT belonging to GT99 (22). BLAST conserved domain assignments (30) suggested two additional C-terminal putative GT domains annotated (in order from the N terminus) as “GT 1/RfaB-like” and “GT25.” However, neither shares any significant sequence similarity with bona fide GT1 or GT25 proteins in the Carbohydrate-Active enzymes (CAZy) database (31) and the putative C-terminal GT domains in WbbB were not formally assigned to a CAZy family in their own right, because there were no data to support their function(s). Based on their novel sequence features and the biochemical assignment of their activities identified here, the C-terminal GTs define two new families in the CAZy database, GT102 and GT103, respectively. The data supporting their functions are presented below.
WbbB540-1106 Is Sufficient for Polymerization of O12 OPS.
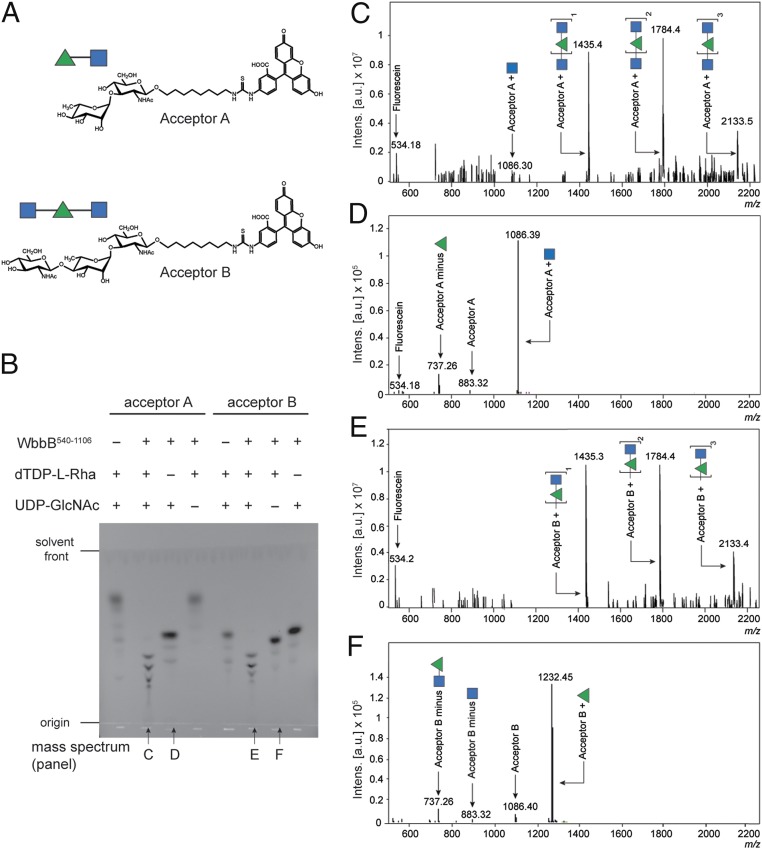
In vitro reactions with purified proteins and synthetic acceptors defined the minimal requirements for polymerization of the repeat-unit domain. The activity of WbbB540-1106, possessing both the GT102 and GT103 domains (Fig. 1B), was examined using synthetic acceptors A and B (Fig. 2A).
Fig. 2.
In vitro activity of the putative polymerase, WbbB540-1106, using single sugar donors. (A) Structures of the synthetic acceptors. (B) TLC analysis of the reaction mixtures obtained after incubating the enzyme with the identified acceptor (a synthetic di/trisaccharide attached via an aliphatic chain to fluorescein) and donor sugar(s). (C–F) Samples from the reactions indicated in B (identified under the relevant TLC lane) were analyzed by MS and the charge-deconvoluted electrospray ionization (ESI) mass spectra are presented.
Initial reactions were performed with a single donor substrate (UDP-GlcpNAc or dTDP-Rhap) to isolate specific activities. In these reactions, WbbB540-1106 generated reaction products with slower migration than acceptors A and B on TLC (Fig. 2B) and their compositions were verified by MS (Fig. 2 D and F). Typically, TLC also revealed a series of fluorescein-labeled compounds reflecting photodegradation products, which were verified in subsequent MS analysis. Consistent with the authentic O12 OPS structure, modification of each acceptor occurred with only one of the donors. WbbB540-1106 added a single GlcpNAc residue (Δm 203) to acceptor A (MW 884), resulting in a major product with molecular weight (MW) 1,087. No activity was evident with dTDP-Rhap donor. Conversely, the MS spectrum of the reaction of WbbB540-1106 with acceptor B and dTDP-l-Rhap revealed a major product with MW 1,233, consistent with the addition of a Rhap residue (Δm 146) to acceptor B (MW 1,087). No addition to acceptor B was evident with UDP-GlcpNAc. These results indicated that WbbB540-1106 possesses both Rhap and GlcpNAc GT activities, contrary to previous work (20, 29).
The ability of WbbB540-1106 to polymerize O12 antigen was then examined using each acceptor in reactions containing both donor substrates. These reactions generated a range of slower-moving products on TLC (Fig. 2B). The MS spectrum of the reaction with acceptor A (Fig. 2C) contained a range of products, beginning with MW 1,087, which represents a tagged trisaccharide formed by the addition of a single GlcpNAc residue to acceptor A. The larger products are consistent with the extension of the trisaccharide by one or two additional disaccharide repeat units (Δm 349), all with terminal GlcpNAc residues. The MS profile of reaction products using acceptor B revealed a series of products with additional repeat units (Δm 349) (Fig. 2E). Interestingly, there was no evidence of any product terminating in Rhap.
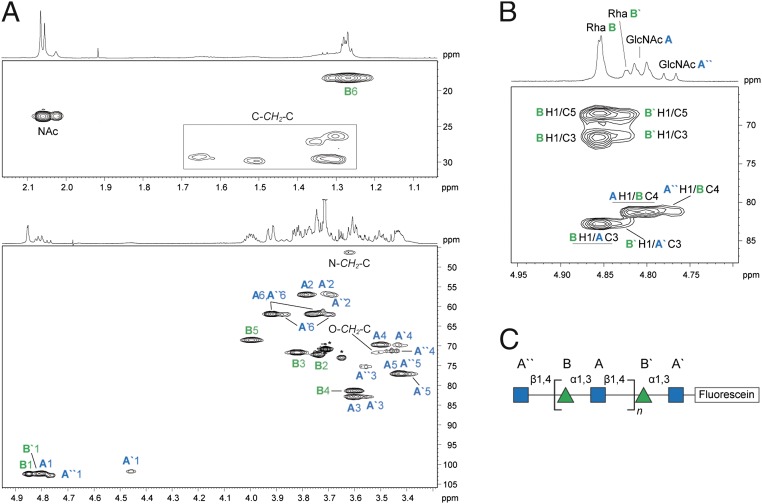
To confirm the authentic O12 structure in the in vitro products, the products generated by WbbB540-1106 using acceptor A and both donors were studied by NMR spectroscopy. The 1H and 13C NMR chemical shifts were assigned using a set of 2D experiments, including 1H,1H COSY, total correlation spectroscopy (TOCSY), rotating-frame nuclear Overhauser effect correlation spectroscopy (ROESY), 1H,13C heteronuclear single quantum coherence (HSQC), and heteronuclear multiple bond correlation (HMBC) (Table S1). The anomeric region of the 1H NMR spectrum contained four signals of different intensities in the region δ 4.77‒4.85, and a broad, low intensity signal at δ 4.46 (Fig. 3A). Analysis of the COSY and TOCSY spectra revealed spin systems for five sugar residues, including three β-GlcpNAc (labeled as A, A′, and A′′) and two α-Rhap residues (B and B′). The major series of signals belong to residues A and B, whereas the integral intensities of the signals belonging to residues A′, A′′, and B′ are approximately four to five times lower. The positions of glycosylation were defined by the significant downfield displacement of the signals for residue A C-3 and residue B C-4 by 8.0 and 8.2 ppm, respectively, compared with their positions in the nonsubstituted monosaccharides (32). The sequence of sugar residues was determined using HMBC and ROESY experiments. The HMBC spectrum demonstrated interresidue correlations A H-1/B C-4 and B H-1/A C-3 at δ 4.81/81.4 and 4.85/82.8, respectively (Fig. 3B). The major series of signals in the NMR spectra corresponds to the internal disaccharide repeat units [4)-α-Rhap-(1→3)-β-GlcpNAc-(1→], which are identical to the native O12 repeat unit (21). Consistent with the MS data, no minor signals were observed that would correspond to terminal Rhap residues. Collectively, these data establish that the WbbB fragment containing GT102 and GT103 is sufficient for polymerization and rule out WbbL as an obligatory participant in chain extension.
Table S1.
1H and 13C NMR chemical shifts (δ, parts per million) for the carbohydrate moiety of the product generated by WbbB540-1106 using acceptor A
| Sugar residue | Residue | C-1, H-1 | C-2, H-2 | C-3, H-3 | C-4, H-4 | C-5, H-5 | C-6, H-6 (6a, 6b) |
| →3)-β-d-GlcpNAc-(1→* | A | 102.3, 4.81 | 57.0, 3.78 | 82.8, 3.61 | 69.7, 3.50 | 77.0, 3.43 | 61.9, 3.75, 3.92 |
| →4)-α-l-Rhap-(1→† | B | 102.4, 4.85 | 72.1, 3.74 | 71.6, 3.82 | 81.4, 3.60 | 68.5, 4.00 | 18.2, 1.27 |
| →3)-β-d-GlcpNAc-(1→ | A′ | 101.7, 4.46 | 56.8, 3.71 | 82.8, 3.56 | 69.7, 3.43 | 77.1, 3.39 | 62.0, 3.70, 3.87 |
| β-d-GlcpNAc-(1→ | A′′ | 102.7, 4.77 | 57.1, 3.68 | 75.2, 3.56 | 71.3, 3.46 | 77.0, 3.43 | 61.9, 3.75, 3.92 |
Residues marked with a prime belong to the reducing-end repeat unit, and a residue marked with double primes is a terminal nonreducing residue.
Chemical shifts for NAc groups are at δH 2.03‒2.07, δC 23.6 (CH3) and 175.0‒175.8 (CO).
Rha B′ H-1 is at δH 4.82.
Fig. 3.
NMR spectroscopy analysis of the polymeric product generated by WbbB540-1106 using acceptor A. (A) Parts of the 1H,13C HSQC spectrum. The corresponding parts of the 1H NMR spectrum are shown along the axis. The numbers refer to H/C pairs in sugar residues designated by letters as shown in C. The O-CH2-C, C-CH2-N and C-CH2-C signals belong to an eight-carbon methylene linker. Stars indicate the signals from a polyethyleneglycol impurity whose source was not resolved. (B) Part of the 1H,13C HMBC spectrum. The underlined interresidue correlations between anomeric protons and the linkage carbon atoms demonstrate A(1→4)B and B(1→3)A linkages. (C) Structure of the enzymatic product.
In a two-site polymerase a distribution of glycan chains ending in either GlcpNAc or Rhap was anticipated, so the observation that all of the in vitro polymerization products terminated in GlcpNAc was surprising. The initial reactions included equimolar amounts of UDP-GlcpNAc and dTDP-Rhap. To determine whether this is a catalytic property of the polymerase, or a by-product of the reaction conditions, a series of reactions were performed using acceptor B, 5 mM dTDP-Rhap, and a titration series of UDP-GlcpNAc from 1 to 4 mM (Table S2). In these experiments, all of the higher mass peaks in the profile end in GlcpNAc, regardless of the dTDP-Rhap:UDP-GlcpNAc ratio. This observation could reflect different reaction rates in the two GT domains, where addition of Rhap is less efficient. The authentic O12 glycan is terminated by a β-(2→3)–linked Kdop added to a terminal Rhap and this reaction is potentially complicated by competing with a more rapid GlcpNAc addition and further chain elongation. Interestingly, these reactions did not generate products extended by more than three O12 repeat units. Longer-chain products are not observed in reactions with varied NDP-sugar:acceptor ratios. Furthermore, the same product spectrum is obtained in reactions with added UDP or dTDP, indicating that product inhibition is not involved. It is unclear whether these unexpected catalytic properties translate to the full-length WbbB protein in its natural (membrane-associated) environment, or whether it is an artifact of the in vitro system. The results may reflect use of truncated proteins, where the absence of other domains may prevent optimal folding and/or acceptor binding. Unfortunately, native WbbB is prone to aggregation and degradation, precluding analysis of the GTs in the context of the full protein.
Table S2.
In vitro product chain lengths are unaffected by varying UDP-GlcNAc concentration
| [GlcNAc], mM | Mexperimental | Mcalculated | Product identity |
| 1 | 534.23 | 534.18 | Fluorescein |
| 737.31 | 737.26 | Acceptor B minus Rha | |
| 1,232.42 | 1,232.46 | Acceptor B – Rha | |
| 1,435.57 | 1,435.54 | Acceptor B – Rha – GlcNAc | |
| 1,784.58 | 1,784.67 | Acceptor B – [Rha – GlcNAc]2 | |
| 2,133.69 | 2,133.81 | Acceptor B – [Rha – GlcNAc]3 | |
| 2 | 534.26 | 534.18 | Fluorescein |
| 737.33 | 737.26 | Acceptor B minus Rha | |
| 1,086.39 | 1,086.40 | Acceptor B | |
| 1,232.45 | 1,232.46 | Acceptor B + Rha | |
| 1,435.56 | 1,435.54 | Acceptor B + Rha - GlcNAc | |
| 1,784.62 | 1,784.67 | Acceptor B + [Rha – GlcNAc]2 | |
| 2,133.68 | 2,133.81 | Acceptor B + [Rha – GlcNAc]3 | |
| 3 | 534.24 | 534.18 | Fluorescein |
| 737.32 | 737.26 | Acceptor B minus Rha | |
| 1,086.35 | 1,086.40 | Acceptor B | |
| 1,232.49 | 1,232.46 | Acceptor B + Rha | |
| 1,435.56 | 1,435.54 | Acceptor B + Rha – GlcNAc | |
| 1,784.62 | 1,784.67 | Acceptor B + [Rha – GlcNAc]2 | |
| 2,133.67 | 2,133.81 | Acceptor B + [Rha – GlcNAc]3 | |
| 4 | 534.24 | 534.18 | Fluorescein |
| 737.31 | 737.26 | Acceptor B minus Rha | |
| 1,086.40 | 1,086.40 | Acceptor B | |
| 1,232.48 | 1,232.46 | Acceptor B + Rha | |
| 1,435.55 | 1,435.54 | Acceptor B + Rha – GlcNAc | |
| 1,784.62 | 1,784.67 | Acceptor B + [Rha – GlcNAc]2 | |
| 2,133.74 | 2,133.81 | Acceptor B + [Rha – GlcNAc]3 | |
| 5 | 534.20 | 534.18 | Fluorescein |
| 737.22 | 737.26 | Acceptor B minus Rha | |
| 1,086.30 | 1,086.40 | Acceptor B | |
| 1,435.38 | 1,435.54 | Acceptor B + Rha – GlcNAc | |
| 1,784.42 | 1,784.67 | Acceptor B + [Rha – GlcNAc]2 | |
| 2,133.50 | 2,133.81 | Acceptor B + [Rha – GlcNAc]3 |
MS analysis of reaction mixtures containing acceptor B, WbbB540-1106, 5 mM dTDP-l-Rha with varying amounts of UDP-GlcNAc.
Assignment of Catalytic Activities to the GT102 and GT103 Domains.
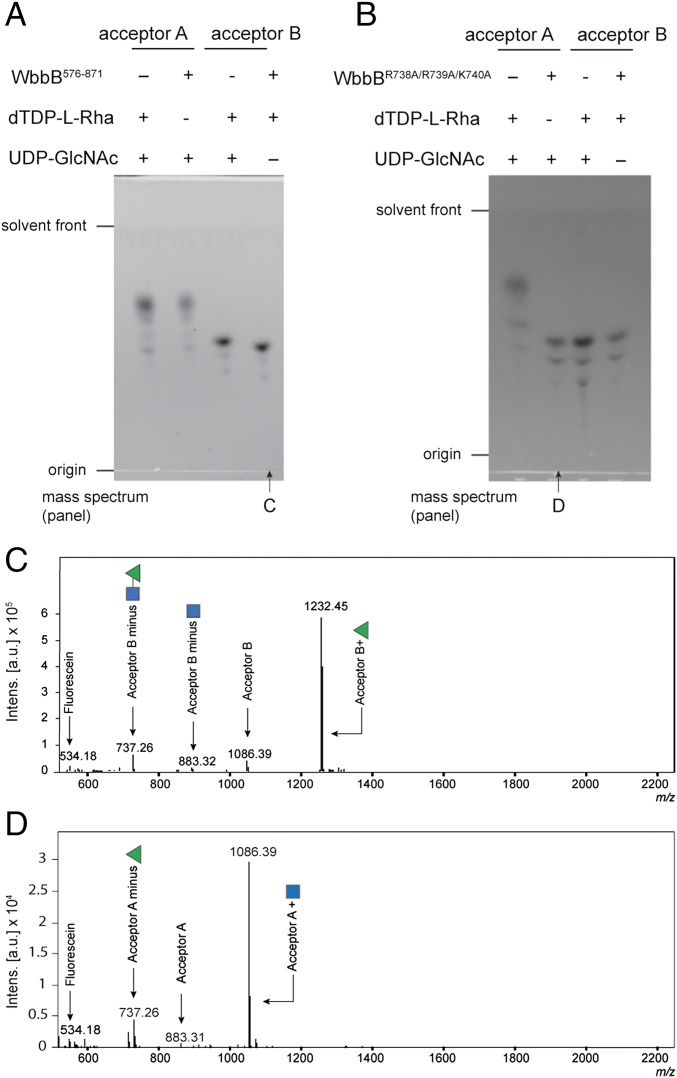
The data above establish WbbB540-1106 as the O12 polymerase but additional experiments were required to clarify the identities of the GlcpNAc and Rhap GTs. A domain containing GT102 was expressed alone (WbbB576-871) and shown to add a single Rhap residue to acceptor B, which was confirmed by TLC and MS (Fig. 4 A and C). This construct showed no activity with UDP-GlcpNAc and acceptor A, unequivocally identifying as a dTDP-l-Rhap-dependent GT. Attempts to generate active protein fragments containing only the GT103 domain were unsuccessful. To overcome this, site-directed mutagenesis was used to inactivate the GT102 domain to examine the activity of the GT103 domain in isolation. Three basic residues (R738, R739, and K740) were targeted for mutagenesis because they are strongly conserved among those (uncharacterized) proteins showing significant similarity to GT102 in BLAST searches. In addition, a role for basic residues in coordinating and stabilizing the phosphate leaving group is well-established in diverse GTs (e.g., refs. 33 and 34). In the absence of structural information to interpret possible functions, all three residues were mutated to alanine to maximize the strength of the phenotype; this variant eliminated the activity of the GT102 domain while retaining wild-type levels of N-acetylglucosaminyltransferase activity (Fig. 4 B and D). This result confirms the GT103 domain as the UDP-GlcpNAc-dependent GlcpNAc GT.
Fig. 4.
In vitro activities of GT102 and GT103. (A) TLC analysis of the reaction mixtures obtained after incubating the WbbB576-871 fragment with the identified acceptor and sugar donors. (B) The corresponding experiments with WbbBR738A/R739A/K740A, which inactivated the GT102 site and allowed evaluation of GT103. As expected, each enzyme showed activity with a single combination of acceptor and NDP-sugar donor. (C and D) Charge-deconvoluted ESI mass spectra of the reaction mixtures highlighted in A and B.
Chain-Length Regulation by WbbB.
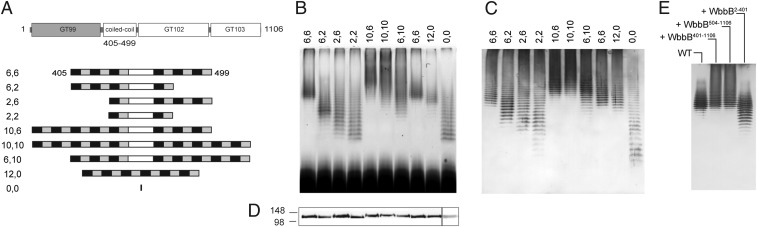
The chain-length-regulating molecular ruler provided by the coiled-coil structure in the E. coli O9a terminator component of the WbdD–WbdA complex has been established (17). Analysis of the WbbB sequence using the program COILS (35) predicted a coiled-coil region located between the GT99 and GT102 domains (Fig. 1B). Coiled-coils are widespread structural motifs and typically consist of two to five α-helices wrapped around one another forming a left-handed helical supercoil. The dimerization of the GT99 domain (22) suggests that two helices are involved in WbbB. Each helix is made of heptad repeats (36). COILS predicted that the WbbB coiled-coil consisted of two blocks of six heptad repeats flanking an 11-residue sequence that degenerates from the canonical heptad after six residues (Fig. 5A). The involvement of the WbbB coiled-coil domain in chain-length regulation was assessed by systematically varying the size of this region in WbbB. Silver-stained SDS/PAGE of whole-cell lysates identifies OPS species that are exported and linked to lipid A-core (23) (Fig. 5B). The OPS-carrying LPS species generated by wild-type WbbB (WbbB 6,6) formed a tight cluster of molecules, reflecting the native chain length. Reducing the number of heptads from either side of the nonheptad region resulted in an overall shift to shorter OPS chain lengths, compared with the wild-type construct. Increasing the number of heptad repeats resulted in an upward shift in the maximum chain length. Interestingly, the LPS species for the 2,2 construct were indistinguishable from those generated by WbbB with the entire coiled-coil domain removed. Most of these coiled-coil variants resulted in a broadening of the observed size distribution. Removal of the central region, which is not predicted to form a canonical heptad, led to a slight reduction in chain length, but, unlike the other variants, it did not result in any significant broadening of the range of chain lengths. To confirm that these results were not simply due to altered expression of the WbbB proteins their amounts were confirmed using the C-terminal FLAG tag (Fig. 5D). To focus only on the Und-PP–linked OPS the WbbB variants were examined in a background lacking the Wzt component of the ABC transporter, with detection performed by Western immunoblots with anti-O12 antibodies (Fig. 5C). The shifts in size distributions were consistent with those seen in completed LPS, but the distributions of Und-PP–OPS were generally broader with more molecules in the upper size ranges. The data are consistent with the WbbB coiled-coil domain acting as a molecular ruler, with a function analogous to the WbdD protein in E. coli O9a (17).
Fig. 5.
The coiled-coil domain of WbbB is a molecular ruler to determine OPS chain length. (A) The architecture of the coiled-coil region identified by COILS (35). The wild-type WbbB coiled-coil domain (designated 6,6) consists of two identical blocks of six heptads (black/gray boxes) flanking an 11-residue region that does not correspond to a canonical heptad structure. The series of engineered coiled-coil regions are shown with their designations. Each variant is named for the number of heptad repeats on either side of the nonheptad region. In 12,0, the central non-coiled-coil region was deleted, whereas the entire coiled-coil region was removed in 0,0. (B) Silver-stained SDS/PAGE gel of the LPS produced by the WbbB variants transformed in transport-proficient E. coli CWG1219 (pWQ673; wbbL-wzm-wzt). (C) Western immunoblot with anti-O12 antibodies showing Und-PP–linked OPS in an export-deficient background provided by CWG1219 (pWQ676; wbbL-wzm). (D) Anti-FLAG Western immunoblots to assess amounts of WbbB-variants. The amount of the 0,0 protein was lower, so it was necessary to increase the contrast to detect it (hence the separate box). (E) Western immunoblot with anti-O12 antibodies showing the effect of overexpressing the GT99 terminator (WbbB2-401) or polymerase (WbbB401-1106; WbbB504-1106) on the chain length distribution of Und-PP–OPS.
In E. coli O9a, the chain length of the OPS is affected by the stoichiometry of the interacting polymerase and terminator proteins, presumably within the context of the known complex (5). This can be manipulated experimentally (and potentially under natural conditions) because the two key activities are contributed by two separate polypeptides. To investigate whether the same principle exists in the O12 system, we investigated the size distribution of OPS assembled in vivo by cells overexpressing separate polymerase and catalytically active terminating β-Kdop GT99 [WbbB2-401 (22)] domains, in the presence of full-length WbbB. These experiments were performed in an export-deficient background. Two polymerase constructs, WbbB401-1106 and WbbB540-1106, were tested and these differed in the inclusion (or not) of the coiled-coil domain (Fig. 1A). Overexpression of the WbbB2-401 terminating GT99 domain caused an overall downward shift in the size distribution and a broadening of the modal distribution in the Und-PP–OPS (Fig. 5E). In contrast, overexpression of the polymerase constructs had the opposite effect, with a significant increase in the maximum chain length and a depletion of shorter chain lengths. The presence or absence of the coiled-coil domain did not affect the outcome.
Discussion
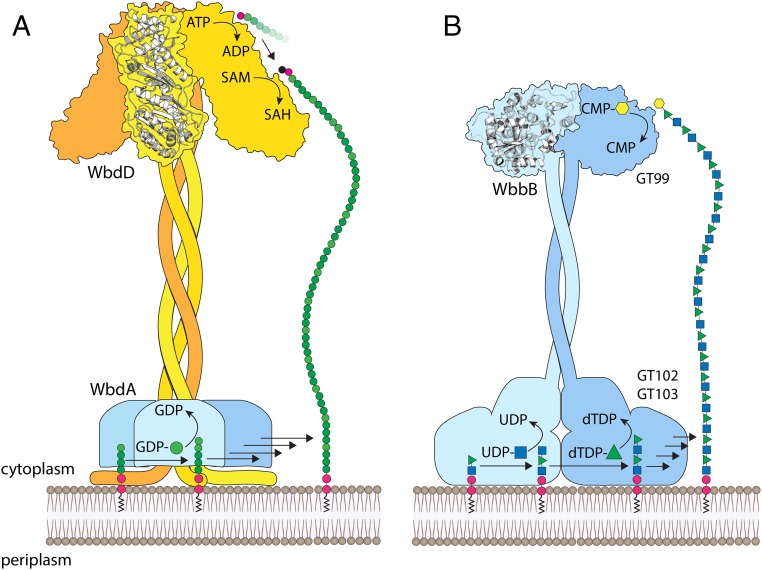
The biosynthesis of the O12 OPS requires a remarkable trifunctional protein WbbB that is responsible for all of the steps in assembly: polymerization, termination (i.e., the act of adding a nonreducing terminal residue to prevent further extension), and chain-length regulation (i.e., dictating the length of OPS that are candidates for termination). The essential components (polymerase, terminator, and molecular ruler) are required in both the O12 system and the E. coli O9a prototype, suggesting a shared biosynthesis strategy. However, the latter requires two proteins (WbdA and WbdD) to provide these key assembly steps (Fig. 6). The N terminus of WbbB contains the GT99 β-Kdop transferase (terminator) and its activity and structure have been reported (22). The C-terminal GTs show no significant similarity to any of the existing families in the CAZy database (31), and with the determination of their catalytic activities they now form founding members of the new GT102 and GT103 families. They operate in a two-site model of polymerization by inverting mechanisms (37), where the anomeric linkages in the activated donors (dTDP-β-l-Rhap and UDP-α-d-GlcpNAc) are inverted in the product [4)-α-Rhap-(1→3)-β-GlcpNAc-(1→]. Taken in aggregate, each of the three GT domains of WbbB represents a new GT family.
Fig. 6.
Cartoon representation of the polymerization and chain termination mechanisms from E. coli O9a and K. pneumoniae O12. (A) In E. coli O9a, WbdA is a polymerase containing two GDP-mannose-dependent mannosyltransferase catalytic sites and generates a polymannose glycan with a tetrasaccharide repeat unit defined by linkage sequences. The terminator is WbdD and possesses a membrane-associating amphipathic helix in close proximity to a region that interacts with the polymerase. Chain termination requires a dual kinase, methyltransferase domain whose structure has been solved. (B) K. pneumoniae O12 WbbB provides both polymerase (GT102 and GT103) and terminator (GT99) functions. The GT99 domain of WbbB forms dimers and this is therefore predicted for the full-length protein. The positioning of the polymerase is also predicted based on the model for E. coli O9a and the need to elongate an Und-PP–linked glycan anchored in the membrane. The function of the coiled-coil domains as molecular rulers requires polymerase reactions that are distributive (i.e., the growing glycan must be released from the active site after each step in elongation), to afford the opportunity for the growing glycan to interact with the spatially separated terminator active site once the glycan length is sufficient. The in vitro properties of WbdA and the O12 polymerase are consistent with a distributive mechanism.
The role of a coiled-coil structure as a molecular ruler for OPS chain-length determination was first demonstrated in E. coli O9a (17). The specificity of the ABC transporter for terminated O9a OPS chains precludes the premature export of shorter chains (19). These general principles are conserved in K. pneumoniae O12. In E. coli O9a, a coiled-coil with an estimated size of ∼200 Å gives rise to a modal chain-length distribution with an average of four tetrasaccharide repeat units. Although accurate O12 repeat-unit counts cannot be made because of the absence of low-MW species in the profile, dictated by the regulatory system, the expanded ranges of chain lengths seen in some coiled-coil variants allow an estimated wild-type average of ∼20 repeat units (Fig. 5B). In WbbB, the presence of a noncanonical heptad sequence flanked by canonical heptads makes it difficult to assess the length of the molecular ruler with certainty. However, units of six heptads have an estimated size of ∼60 Å, based on the parameters determined for WbdD in E. coli O9a. This would give rise to an average chain length of seven disaccharide repeats, each contributing ∼9 Å in chain length (calculated using the GLYCAM server; glycam.org/). A ∼120-Å spacer seems too small for the observed LPS products in PAGE, if the coiled-coil domain alone dictates spacing. Given that the coiled-coil structure is fused to the polymerase domain, it seems likely that the physical bulk of the polymerase domain acts as a substantive (∼60 Å) extension to the membrane-proximal end of the ruler; this idea is consistent with our finding that a minimal degree of polymerization is retained even in the shortest coiled-coil variants. In, E. coli O9a the stoichiometry and architecture of the complex also influences chain length distribution (5), and although the O12 system can be experimentally manipulated to alter chain length the deployment of the key activities in a single WbbB protein limit this in vivo.
In E. coli O9a, the coiled-coil region plays additional roles in interacting with the active site of the terminating kinase and ordering two loops needed for proper substrate binding, as well as stabilizing the WbdD trimer (17). The true oligomeric state of the full-length WbbB protein is unknown but the GT99 β-Kdop GT is a dimer in solution and in the solved crystal structures (22). For the coiled-coil domain of WbbB to be effective as a spacer, the C-terminal end of the molecule must presumably be anchored to the membrane. In addition, the polymerase domain requires proximity to the membrane to access its Und-PP–linked acceptor molecules. In the O9a system, the amphipathic helix of WbdD provides anchorage to the membrane with defined orientation. Analysis of the polymerase domain of WbbB indicates the presence of several candidate membrane-interacting helices that may play an analogous role.
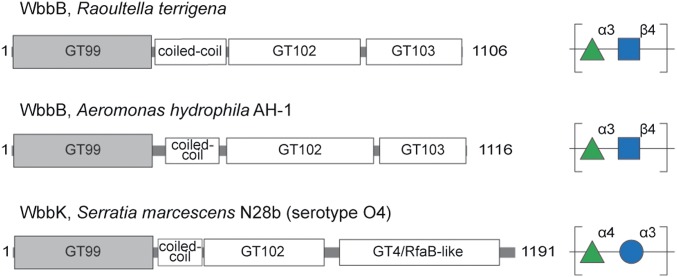
An important open question is how distributed these types of modular enzymes are in bacterial glycan biosynthesis. Sequence data identify several other members from the genera Klebsiella, Raoultella, Aeromonas, and Serratia among proteins containing GT99 family modules in the CAZy database (Table S3). Their GT99 sequences share ∼60% or greater similarity and share the same N-terminal location, upstream of a high-confidence predicted coiled-coil region. They all possess one or more C-terminal GT candidates. Although the structures of the polysaccharide repeat units are available for only two other examples for correlation with potential GT modules (Fig. 7), some conserved functional properties clearly emerge. The carbohydrate backbones of the OPS from K. pneumoniae O12 and Aeromonas hydrophila AH-1 are identical, although AH-1 OPS also possesses variable acetylation at the 2 and 3 positions of the Rhap residue (38). The OPS-biosynthesis genetic locus of AH-1 contains a putative acetyltransferase gene. Terminal residues were not reported for AH-1 OPS but the presence of a GT99 domain is a strong indicator that a terminal β-Kdop residue exists. Furthermore, the Wzt proteins from these bacteria possess conserved C-terminal sequences, which are predicted to encode a CBM similar to that recognizing the terminal β-Kdop in K. pneumoniae O12 (23). Both bacteria possess similar WbbL homologs, indicating the biosynthesis pathways and principles are conserved. The polymerase domains from A. hydrophila and R. terrigena share <60% overall similarity but, as expected from the conserved OPS repeat-unit structure, sequence and secondary structure alignments reveal strong candidates for GT102 (59% similarity/41% identity) and GT103 (61% similarity/45% identity) (Fig. S1).
Table S3.
Features of additional modular proteins related to WbbB from K. pneumoniae O12
| Bacterial species | Protein | Coiled-coil (residue nos.) | C-Terminal GTs* (residue nos.) | Accession no. |
| Aeromonas hydrophila AH-1 | WbbB | 443–504 | GT102 (520–864) | AKL88477 |
| GT103 (922–1010) | ||||
| Aeromonas hydrophila AL97-91 | ORF | 507–568 | GT102 (520–864) | AID70933 |
| GT103 (922–1010) | ||||
| Aeromonas hydrophila MN98-04 | ORF | 507–568 | GT102 (520–864) | AID71014 |
| GT103 (922–1010) | ||||
| Klebsiella pneumonia | ORF | 466–515 | GT102 (696–832) | AAN06494 |
| Klebsiella pneumoniae 262 | WbbB | 451–500 | GT2 (450–668) | CZQ25253 |
| RfaB (799–1040) | ||||
| Klebsiella pneumoniae AKPRH094188 | WbbB | 449–498 | GT102 (696–832) | CZQ25286 |
| GT103 (874–942) | ||||
| Klebsiella pneumoniae subspecies pneumoniae HKUPOLC | AKK42-08370 | 466–508 | GT102 (696–832) | ALD55318 |
| GT103 (874–942) | ||||
| Klebsiella pneumoniae subspecies pneumoniae MGH78578 ATCC 700721 | KPN-02484 | 468–517 | GT2 (450–558) | ABR77902 |
| RfaB (724–1040) | ||||
| Klebsiella quasipneumoniae subspecies similipneumoniae 708 | ORF | 491–540 | GT102 (785–921) | BAN08508 |
| GT103 (963–1031) | ||||
| Klebsiella variicola HKUPOLC | AK692-05245 | 448–490 | GT102 (742–878) | ALD04702 |
| GT103 (895–985) | ||||
| Raoultella ornithinolytica S12 | TE10_19175 | 454–549 | GT102 (732–856) | AJF74040 |
| GT103 (892–999) | ||||
| Serratia fonticola GS2 | AV650_12770 | 457–498 | GT102 (642–878) | ALX94372 |
| Serratia marcescens N28b | WbbK | 459–480 | GT2 (453–671) | AAC00183 |
| GT4/RfaB (727–1045) | ||||
| Serratia plymuthica 4Rx13 | SOD_C14770 | 460–551 | GT102 (772–907) | AGO54462 |
| GT103 (948–1033) |
The assignment of GT102 or GT103 was made based on alignments to the prototypes from K. pneumoniae O12 WbbB. All other designations are those from the conserved domains provided by BLAST. As indicated in the text, the latter is misleading but there is insufficient evidence to provide an appropriate alternative here.
Fig. 7.
Modular proteins related to WbbB in other bacterial genera. Several members of the GT99 family possess an organization resembling WbbB (Table S5) but only two are from organisms with known OPS structures. They are WbbB from A. hydrophila AH-1 (AKL88477) and WbbK [formerly WbbA (39)] from S. marcescens N28b (AAC00183).
Fig. S1.
Alignments of the polymerase GT domains from the WbbB protein from K. pneumoniae O12 with WbbB from Aeromonas hydrophila AH-1 (Left) and with WbbK from Serratia marcescens N28-b (Right).
The Serratia marcescens O4 OPS repeat unit differs from K. pneumoniae O12 by possessing a [3)-α-Rhap-(1→4)-α-Glcp-(1→] disaccharide backbone (39). This organism contains genes encoding WbbL and the modular protein, WbbK, containing a GT99 β-Kdop GT active site (Fig. 7). The Wzt protein from this OPS-assembly system also contains a putative β-Kdop–specific CBM, suggesting a biosynthetic pathway and terminal modification similar to K. pneumoniae O12. However, previous work has indicated that the ABC transporters were not exchangeable between S. marcescens O4 and K. pneumoniae O12 (29), which presumably reflects linkage differences in the terminal structure recognized by the CBMs. BLAST predictions identify two C-terminal GT modules identified as “GT2-like” and “GT4 or RfaB-like.” The former shares 57% similarity/29% identity with GT102, with the alignment being strongest in the predicted C-terminal part of the GT (Fig. S1). The “GT4 or RfaB-like” motif presumably identifies the retaining α-glucosyltransferase, which uses UDP-α-d-Glcp as its donor and this shares no significant similarity with GT103 but some sequence relatedness with GT102 (42% similarity/22% sequence identity) (Fig. S1).
In the other examples of these multidomain proteins there are different combinations of putative GT motifs that are identified in BLAST as “GT1,” “GT2,” “GT25,” and “GT4/RfaB-like” (Table S3). Most possess two separate GT motifs downstream of a coiled-coil region and the resulting structures are therefore predicted to contain disaccharide repeat units. The proteins from K. pneumoniae (AAN06494.1) and Serratia fonticola (ALX94372) provide exceptions, where only one GT motif is identified from sequence alone. In terms of the evolution of glycan diversity, (re)combining different GT modules behind a more conserved GT99 module and coiled-coil region allows the production of new structures within the same conceptual model. This general concept has been seen in other assembly systems, such as the production of Salmonella O antigens via the Wzy-dependent pathway (40), but in those cases diversity requires acquisition of new independent GT genes, rather than modules within a single polypeptide.
Aspects of this modular protein approach may also be used for the O-glycosylation of some S-layer proteins. In Geobacillus stearothermophilus, one protein (WsaE) is partially responsible for polymerization, as well as catalyzing chain termination (41). Sequence analysis of the Wzt homolog from this system reveals a CBM. The WsaE protein is predicted to possess a coiled-coil between the methyltransferase and rhamnosyltransferase domains, which suggests chain-length regulation in this system as well. In contrast, the polymerases for some gram-negative capsular polysaccharides synthesized by ABC transporter-dependent pathways often contain more than one GT module (e.g., ref. 42) but these processes operate without chain-terminating residues or coiled-coil molecular rulers and the ABC transporters lack CBMs (43).
In summary, the findings reported here provide insight into the assembly of bacterial polysaccharides via the ABC transporter-dependent pathway and a molecular strategy for producing glycan diversity. The inclusion of polymerization, termination, and chain-length regulation in a single protein significantly extends our understanding of the range of organizational possibilities for mechanisms that operate via a defined chain terminator mechanism. In addition, this work confirms the principle of coiled-coil rulers for establishing glycan chain lengths in these systems. Parallels between WbbB and the proteins involved in the biosynthesis of polyketides are obvious. These polyketide synthases are also single polypeptides containing multiple domains with distinct functions in the assembly of natural products (44). The fascinating integration of activities in a single protein also points to a potential avenue for glycoengineering to produce therapeutic proteins and vaccines. The inclusion of selected GT modules in a recombinant protein would afford development of a glycosylation cassette that modifies appropriate acceptors, such as glycosylated proteins and lipids, with a glycan of defined structure and chain length. In this regard we note that there has been considerable success in engineering polyketide synthases to produce novel structures with a host of biological functions (45). Such applications will be dependent on understanding the architectural principles from structures of prototypes such as WbbB.
Experimental Procedures
Bacterial Strains, Plasmids, and Growth Conditions.
The bacterial strains used in this study are E. coli BL21 [B F− dcm ompT hsdS(rB- mB-) gal [malB+]K-12 (λS)] (Novagen), Top10 [F−, mcrA Δ(mrr-hsdRMS-mcrBC) φ80, lacZΔM15, ΔlacX74, deoR, nupG, recA1, araD139, Δ(ara-leu)7697, galU, galK, rpsL(Strr), endA1] (Invitrogen), and CWG1219 (Top10 Δwzx-wbbK, ΔgtrA) (46). Bacteria were grown in lysogeny broth (LB) at 37 °C. When required, media contained one or more of the following supplements: d-glucose (0.2%, wt/vol), l-arabinose (0.02%, wt/vol), isopropyl-β-d-1-thiogalactoside (IPTG; 1 mM), ampicillin (100 µg/mL), anhydrotetracycline (2.5 µg/mL), chloramphenicol (34 µg/mL), or kanamycin (50 µg/mL). The plasmids are listed in Table S4.
Table S4.
Plasmids used in this study
| Plasmid | Description | Source |
| pWQ573 | Plasmid vector with l-arabinose-inducible promoter; Cmr | 52 |
| pWQ719 | pWQ573 derivative encoding WbbB-FLAG from Raoultella terrigena; Cmr | This study |
| pWQ673 | pWQ552 derivative encoding WbbL-Wzm-Wzt from R. terrigena; Apr | This study |
| pWQ672 | pWQ552 derivative encoding WbbL-Wzm-Wzt-WbbB from R. terrigena; Apr | This study |
| pWQ698 | pWQ573 derivative encoding His6-WbbB504-1106 ; Cmr | This study |
| pWQ701 | pWQ573 derivative encoding His6-WbbB402-1106; Cmr | This study |
| pWQ702 | pWQ573 derivative encoding His6-WbbB1-401; Cmr | This study |
| pWQ676 | pWQ552 derivative encoding WbbL-Wzm from R. terrigena, Apr | This study |
| pWQ677 | pWQ552 derivative encoding WbbL-Wzm-WbbB from R. terrigena, Apr | This Study |
| pET28a(+) | Plasmid vector with IPTG-inducible promoter; Kmr | Novagen |
| pET30a(+) | Plasmid vector with IPTGinducible promoter; Kmr | Novagen |
| pRmlB2 | pET30a(+) derivative encoding His6-RmlB from Salmonella enterica; Kmr | 47 |
| pRmlC3 | pET30a(+) derivative encoding His6-RmlC from S. enterica; Kmr | 47 |
| pRmlD2 | pET30a(+) derivative encoding His6-RmlD from S. enterica; Kmr | 47 |
| pWQ867 | pET28a(+) derivative encoding His6-WbbB540-1106; Kmr | This study |
| pWQ868 | pWQ719 derivative encoding WbbB-FLAG with native coiled-coil domain (6, 6) and introduced NotI and KpnI restriction sites; Cmr | This study |
| pWQ869 | pWQ868 derivative encoding WbbB-FLAG with altered coiled-coil domain (2, 6); Cmr | This study |
| pWQ870 | pWQ868 derivative encoding WbbB-FLAG with altered coiled-coil domain (6, 2); Cmr | This study |
| pWQ871 | pWQ868 derivative encoding WbbB-FLAG with altered coiled-coil domain (10, 6); Cmr | This study |
| pWQ872 | pWQ868 derivative encoding WbbB-FLAG with altered coiled-coil domain (6, 10); Cmr | This study |
| pWQ873 | pWQ868 derivative encoding WbbB-FLAG with altered coiled-coil domain (2, 2); Cmr | This study |
| pWQ874 | pWQ719 derivative encoding WbbB-FLAG with altered coiled-coil domain (12,0); Cmr | This study |
| pWQ875 | pWQ719 derivative encoding WbbB-FLAG (0,0)with the region encompassing the entire coiled-coil domain (residues 402–539) removed; Cmr | This study |
| pWQ677 | pWQ552 derivative encoding WbbL-Wzm-WbbB from R. terrigena; Apr | This study |
| pWQ876 | pWQ719 derivative encoding WbbB-FLAG with altered coiled-coil domain (10, 10); Cmr | This study |
| pWQ877 | pET28a(+) derivative encoding His6-WbbBΔR738AR739AK740A; Kmr | This study |
| pWQ878 | pET28a(+) derivative encoding His6-WbbB576-871; Kmr | This study |
Purification of the WbbB GT Domains.
Proteins containing both GT102 and GT103 GT modules (corresponding to WbbB540-1106), or GT102 alone (WbbB576-871), were purified from E. coli BL21 (DE3) transformed with either pWQ867 or pWQ878, respectively. Details of expression and purification conditions are provided in SI Experimental Procedures. Briefly, cells were resuspended in 25 mL Buffer A (0.1 M sodium phosphate buffer containing 250 mM NaCl, pH 7.4) with Complete Mini EDTA-free protease inhibitor tablets (Roche Applied Science) and lysed by sonication. Protein was purified from a cell and membrane-free supernatant obtained by sequential centrifugation culminating in a final step at 100,000 × g for 60 min. His6-tagged proteins were purified using a Ni2+-affinity column and the imidazole-containing eluant was exchanged with buffer A.
Synthesis of dTDP-l-Rhamnopyranose.
Synthesis of dTDP-l-rhamnopyranose was performed essentially as previously described (47). Briefly, a 5-mL reaction contained 20 μmol of dTDP-D-glucopyranose, 1 μmol of NAD+, 100 μmol of ammonium formate, 250 µg/mL each of purified RmlB, RmlC, and RmlD, and 3.5 units of formate dehydrogenase from Candida boidinii (Sigma) in buffer B (0.1 M Tris⋅HCl, pH 7.0). After reaction for 2 h at 37 °C, proteins were removed by ultrafiltration in a 3,000 molecular weight cutoff (MWCO) Vivaspin filtration unit (Sartorius Biolab Products). dTDP-L-Rhap product was confirmed using an Agilent LC-UHD Q-TOF instrument, operated in negative mode, in the Mass Spectrometry Facility at the University of Guelph Advanced Analysis Centre. The MS spectrum revealed a single major peak at m/z 547, which is the expected mass for dTDP-l-Rhap. Because the reaction resulted in essentially quantitative conversion of substrate to product, the protein-free reaction mixture was used without further purification in GT assays.
In Vitro GT Activities.
Two synthetic oligosaccharide acceptors carrying a fluorescein moiety for detection (Fig. 2A) were used as acceptor substrates to determine GT functions. Synthesis of acceptor A has been reported (22) and the synthetic scheme for acceptor B is provided in Fig. S2, with the experimental details given in SI Experimental Procedures. Acceptor A [α-Rhap-(1→3)-β-GlcpNAc-octyl-fluorescein] represents the disaccharide repeat unit of K. pneumoniae O12 OPS and is the presumed acceptor for the N-acetylglucosaminyltransferase activity required for polymerization, whereas acceptor B [β-GlcpNAc-(1→4)-α-Rhap-(1→3)-β-GlcpNAc-octyl-fluorescein] is the presumed acceptor for the rhamnosyltransferase. Unless indicated otherwise, reactions were performed in 20-µL reaction volumes of buffer C (50 mM Hepes, pH 7.5), containing 10 µM enzyme (purified WbbB576-871 or WbbB540-1106), 0.2 mM acceptor, and 5 mM UDP-GlcpNAc and/or dTDP-l-Rhap. Reactions were incubated at 37 °C for 30 min. A 1-µL aliquot from each reaction mixture was then spotted on an aluminum foil silica gel 60 F254 TLC plate (EDM Millipore). TLC plates were developed with ethyl acetate:water:1-butanol:acetic acid (5:4:4:2.5) and fluorescent reaction products were detected with a hand-held UV lamp.
Fig. S2.
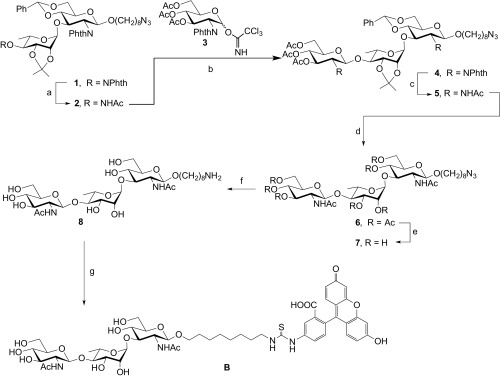
Scheme for synthesis of acceptor B. Reagents and conditions. (a) H2NNH2⋅H2O, AcOH, pyridine, room temperature (rt), 1 h, 95%. (b) TMSOTf, MS4Å, CH2Cl2, –10 °C, 1 h, 72%. (c) (i) Ethylenediamine, n-BuOH, 80 °C, overnight; (ii) Ac2O, pyridine, rt, overnight, 88% (two steps). (d) (i) Eighty percent AcOH aq., 60 °C, 7 h; (ii) Ac2O, pyridine, rt, overnight, 85% (two steps). (e) NaOMe, MeOH, rt, overnight, 88%. (f) Pd/C, H2, H2O, rt, overnight, 93%. (g) FITC, DIPEA, DMF, rt, overnight, 52%.
Structural Analysis of Reaction Products.
Mass spectra of the reaction products were obtained in the University of Guelph Advanced Analysis Centre, using either an Agilent LC-UHD Q-TOF instrument operated in positive mode or, for polymerized product, a Bruker AmaZon SL LC-MSn in positive mode, using UV detection at 437 nm. To generate sufficient product for NMR analysis, acceptor A was used and the reaction volume was scaled up to 500 µL. After 1 h, the reaction mixture was diluted in 10 mL of distilled H2O and loaded into Sep-Pak Cartridge (Waters). The cartridge was washed with water, and the products were eluted in 6 mL of 60% (vol/vol) aqueous acetonitrile and then concentrated using a SpeedVac. The polymerized product was dried twice in a SpeedVac from a 99.9% D2O solution and dissolved in 99.96% D2O. NMR spectra were recorded at 35 °C on a Bruker AvanceII 600-MHz spectrometer equipped with a cryoprobe in the NMR Facility at the University of Guelph Advanced Analysis Center. Internal sodium 3-trimethylsilylpropanoate-2,2,3,3-d4 (δH 0, δC –1.6) was used as a reference. Two-dimensional experiments were performed using standard Bruker software, and the Bruker TopSpin 2.1 program was used to acquire and process the NMR data. Mixing times of 100 and 200 ms were used in TOCSY and ROESY experiments, respectively. The HMBC spectroscopy experiment was optimized for the JH,C coupling constant 8 Hz.
Functional Analysis of WbbB Coiled-Coil Variants.
Eight WbbB coiled-coil variants (Fig. 5) were constructed, generating plasmids pWQ869-pWQ876. Details of the engineering strategy are given in SI Experimental Procedures. Each plasmid carrying a mutant wbbB gene was cotransformed with either pWQ673 (wbbL-wzm-wzt) or pWQ676 (wbbL-wzm) into E. coli CWG1219 (Δwzx-wbbK ΔgtrA). To assess effects on OPS chain lengths, cell samples were collected for detection of LPS by silver-nitrate staining and Und-PP–linked OPS by Western immunoblotting using anti-O12 antiserum. C-terminal FLAG tags were exploited for Western immunoblotting to detect expression of WbbB derivatives.
SI Experimental Procedures
General DNA Methods.
KOD DNA polymerase (EMD Millipore) was used for PCR amplification of DNA fragments used in cloning and for site-directed mutagenesis. Oligonucleotide primers (obtained from Sigma) were designed to introduce restriction sites and sequences encoding epitope tags; the specific features of the primers are described in the Table S5. DNA fragments and plasmids were purified using the PureLink PCR Purification and PureLink Quick Plasmid Miniprep kits, respectively (Invitrogen). Restriction endonucleases (Invitrogen and New England Biolabs) and T4 DNA ligase (New England Biolabs) were used as described by the manufacturers. Site-directed mutagenesis was performed using complimentary oligonucleotide primers containing the desired mutation, following the general strategy described by Makarova et al. (48). All plasmid constructs (Table S4) were verified through DNA sequencing by the Genomics Facility in the Advanced Analysis Center at the University of Guelph.
Table S5.
Sequences and applications of oligonucleotide primers used in this study
| Primer no. | Primer | Sequence (5′→3′) | Features |
| 1 | NMT | CTCAGCCACGGTCAATGCGGCCGCGTTTTACCTGTTCTCG | Forward primer used to introduce NotI restriction site |
| 2 | NMB | AACAGGTAAAACGCGGCCGCATTGACCGTGGCTGAGGCTC | Reverse primer used to introduce NotI restriction site |
| 3 | KMT | CAACCTCGGGTAACGGTACCCTATCGCGCTTCCTGCTTTCCGG | Forward primer used to introduce KpnI restriction site |
| 4 | KMB | AAGCAGGAAGCGCGATAGGGTACCGTTACCCGAGGTTGCATGATAGC | Reverse primer used to introduce KpnI restriction site |
| 5 | ILM264 | GAAGCTCGCGGCTACCCGTGAAAAACTCACCACCACCCTCG | Forward primer used to remove the non-coiled-coil region of WbbB |
| 6 | ILM265 | TGGTGGTGAGTTTTTCACGGGTAGCCGCGAGCTTCTCACGTTC | Reverse primer used to remove the non-coiled-coil region of WbbB |
| 7 | DW01 | TCCCAAGACAAAGCGATGCGTGAGAAGCTCGCGGCTACC | Forward primer used to remove four heptads from the WbbB coiled-coil (amino acids 401–434) |
| 8 | DW02 | CGCGAGCTTCTCACGCATCGCTTTGTCTTGGGAAATGGC | Reverse primer used to remove four heptads from the WbbB coiled-coil (amino acids 401–434) |
| 9 | DW10 | gatcgctagcATGCACCATCACCATCACCATGTGAAGATACTTATTACTGGCGGG | Forward primer for amplification of rmlB and cloning into pET28a(+); His-tag and NheI restriction site |
| 10 | DW11 | gatcaagcttTTACTGGCGTCCTTCATAGTTCTG | Reverse primer for the amplification of rmlB and cloning into pET28a(+); HindIII restriction site |
| 11 | DW12 | gatcgctagcATGCACCATCACCATCACCATGTGATGATTGTGATTAAAACAGCAATACC | Forward primer for amplification of rmlC and cloning into pET28a(+); His-tag and NheI restriction site |
| 12 | DW13 | gatcaagcttTTACTCTGTTAACAAGGCTTGATCCAG | Reverse primer for the amplification of rmlC and cloning into pET28a(+); HindIII restriction site |
| 13 | DW14 | gatcgctagcATGCACCATCACCATCACCATATGAATATCTTACTTTTTGGTAAGACAG | Forward primer for amplification of rmlD and cloning into pET28a(+); His-tag and NheI restriction site |
| 14 | DW15 | gatcgaattcTTAGATGGTTGTCGTCGTAAACATTTC | Reverse primer for the amplification of rmlD and cloning into pET28a(+); EcoRI restriction site |
| 15 | DW4 | gatcgctagcATGGATTCCGCGCCGGCAGCGGCG | Forward primer for the amplification of WbbB576-871 (isolation of GT1 domain) and cloning in pET28a(+); NheI restriction site |
| 16 | DW5 | gatcaagctttaagtgatgtgtatggtgatgCTGAAAATACAGGTTTTCATCAATCGA CAGCAAATAACGCTTCAG | Reverse primer for the amplification of WbbB576-871 (isolation of GT1 domain), introduction of a His6-tag and cloning in pET28a(+); HindIII restriction site |
| 17 | OL1028 | gatcccatggGCCTGCTGTCAAAATCGAAG | Forward primer for the amplification of WbbB540-1106 and cloning into pET28a(+); NcoI restriction site |
| 18 | OL1030 | gatcctcgaggCGGTTGCGCTTAAACTCCG | Reverse primer for the amplification of WbbB540-1106 and cloning into pET28a(+); XhoI restriction site |
| 19 | Bo/l-C-For | TAAccatggaggaggtatctcatATGTCGGGCCGAATTTGC | Forward primer used to introduce NcoI and NdeI restriction sites into the wbbB gene between amino acids 504 and 505 |
| 20 | Bo/l-C-Rev | catatgagatacctcctccatggttaGGTCAGCTTCCATGACAGGC | Reverse primer used to introduce NcoI and NdeI restriction sites into the wbbB gene between amino acids 504 and 505 |
| 21 | Bo/l-D-For | TAAccatggaggaggtacttcatATGCAAGACAAAGCGATGAGAAAGATTTTAG | Forward primer used to introduce NcoI and NdeI restriction sites into the wbbB gene between amino acids 401 and 402 |
| 22 | Bo/l-D-Rev | catatgaagtacctcctccatggTTAGGAAATGGCTTCATTTATCAATGATG | Reverse primer used to introduce NcoI and NdeI restriction sites into the wbbB gene between amino acids 401 and 402 |
| 23 | DW57 | GATCCCATGGATGCTGGCTGTATTTTTACCTCCC | Forward primer for the amplification of WbbBΔ402–539 (coiled-coil region), introduction a NcoI restriction site |
| 24 | DW58 | gatcaagcttttacttgtcatcgtcatccttataatcGCGGTTGCGCTTAAACTCCG | Reverse primer for the amplification of WbbBΔ402–539 (coiled-coil region), introduction of a FLAG tag and a HindIII restriction site |
| 25 | DW73 | ATTCTACGGCGATCCTAATTGCGAA GCAGCCGCGGCGTATCTGCAGGAGC | Forward primer used to mutation R738, R739, K740 to alanine. |
| 26 | DW74 | GCTTCTTCAGCTCCTGCAGATACGCCGC GGC TGCTTCGCAATTAGGATCGCCG | Reverse primer used to mutation R738, R739, K740 to alanine. |
Restriction sites are underlined in the sequences, regions of nonchromosomal sequence are identified by lowercase, and nucleotides changed for site-directed mutations are marked in bold.
Construction and Functional Analysis of WbbB Coiled-Coil Variants.
PCR primers (1–4) were designed with silent changes to introduce NotI and KpnI restriction sites flanking the DNA fragment encoding the coiled-coil region of WbbB-FLAG in pWQ719. This generated the plasmid pWQ868, which allowed replacement of the coiled-coil–encoding region in WbbB-FLAG with variants. Four custom synthesized DNA fragments encoding variations of the coiled-coil region were obtained from GeneArt (Life Technologies). These constructs along with PCR-based techniques allowed the generation of fragments encoding eight WbbB coiled-coil variants (Fig. 5) cloned in plasmids pWQ869–pWQ876. The (2, 6), (6, 2), (10, 6), and (10, 10) variants were provided by the synthesized sequences. The (12,0), (2,2) and (6,10) variants, as well as (0,0) lacking the entire coiled-coil domain, were generated by PCR-based methods. Each plasmid (pWQ869–pWQ876) carrying a mutant wbbB gene was cotransformed with either pWQ673 (wbbL-wzm-wzt) or pWQ676 (wbbL-wzm) into E. coli CWG1219 (Δwzx-wbbK ΔgtrA). To assess effects on OPS chain lengths, 5-mL cultures of each transformant were grown for 16 h at 37 °C in LB broth containing Cm and Amp. The cultures were then diluted 1:100 into 5 mL of fresh medium and grown at 37 °C until A600nm values of 0.6–0.7 were reached. Plasmid-encoded protein expression was then induced by adding l-arabinose (0.02% wt/vol) for plasmids pWQ869–pWQ876 and anhydrotetracycline (2.5 µg/mL) for pWQ673 and pWQ676. The cultures were then grown for a further 4 h at 37 °C and cell samples were collected for detection of WbbB-derivative expression and for LPS analysis.
Expression and Purification of the WbbB GT Domains.
Fragments of the wbbB gene encoding both GT102 and GT103 GT modules (corresponding to WbbB540-1106), or GT102 alone (WbbB576-871), were PCR-amplified from pWQ719. The primers incorporated HindIII and NheI restriction sites, in addition to the sequence required to add a C-terminal hexahistidine tag to the gene product, replacing the FLAG tag in the product encoded by pWQ719. These constructs were then cloned in pET28a(+) (generating pWQ867 and pWQ878) and transformed into E. coli BL21 (DE3) (Invitrogen). One-liter cultures of each transformant were grown at 37 °C in LB medium until A600 of 0.3 was reached. Cultures were then transferred to 20 °C and grown to A600 of 0.6, before expression of the recombinant proteins was induced by adding 1 mM IPTG. The cultures were then grown for a further 16 h and cells were collected by centrifugation (8, 000 × g for 15 min). The cell pellet was resuspended in 25 mL buffer A (0.1 M sodium phosphate buffer containing 250 mM NaCl, pH 7.4) with Complete Mini EDTA-free protease inhibitor tablets (Roche Applied Science). The cells were then lysed using sonication. Unbroken cells, large debris, and (any) inclusion bodies were removed by sequential centrifugation steps (8,000 × g for 15 min; 12,000 × g for 30 min). The resulting cell-free lysate was centrifuged at 100,000 × g for 60 min to remove membranes and the (soluble) proteins were purified from the supernatant using a Ni2+-affinity column. The column was washed sequentially with buffer A containing 0, 20, 50, and 75 mM imidazole and the desired His6-tagged proteins were eluted with buffer A containing 250 mM imidazole. Protein-containing fractions were pooled and the buffer was exchanged with buffer A using a PD10 desalting column (GE Healthcare). Where necessary, the samples were concentrated using a 3,000 MWCO Vivaspin filtration unit (Sartorius Biolab Products). The protein concentrations were determined using the A280, based on theoretical extinction coefficients of WbbB576-871 (35,827 M−1⋅cm−1) and WbbB540-1106 (66,340 M−1⋅cm−1), predicted by the ProtParam program (web.expasy.org/protparam/). The final concentrations of WbbB576-871 and WbbB540-1106 were 2.6 mg/mL and 1.8 mg/mL, respectively.
Determining the Influence of Altered Stoichiometry of the Polymerase and Terminator Domains on OPS Modal Chain Lengths.
DNA fragments encoding the GT99 terminator and polymerase domains of WbbB were cloned in separate plasmid constructs by removing selected portions of wbbB from pWQ719. NcoI and NdeI restriction sites were introduced by site-directed mutagenesis between the codons corresponding to amino acids 401 and 402. The introduced and vector-derived NcoI restriction sites allowed removal of the 3′ part of wbbB, generating pWQ702, encoding His6-WbbB2-401. A similar approach with NdeI digestion generated plasmid pWQ701, encoding His6-WbbB402-1106. Using the same strategy, an NdeI restriction site was introduced following the codon encoding amino acid 504, facilitating the removal of the 5′ part of wbbB in plasmid pWQ698 encoding His6-WbbB504-1106. Each construct was cotransformed with pWQ677 (wbbL-wzm-wbbB) into E. coli CWG1219 (Δwzx-wbbK ΔgtrA) and samples of each transformant were collected for Und-PP–OPS analysis by Western immunoblotting. In these genetic backgrounds, native chromosomal genes provide sources of NDP donors and WecA activity.
Polyacrylamide Gel Electrophoresis and Western Immunoblotting Methods.
Cells were solubilized in SDS/PAGE loading buffer and heated at 100 °C for 10 min, before separation by SDS/PAGE using a 10% acrylamide resolving gel in Tris-glycine buffer. To detect proteins, PAGE gels were stained with SimplyBlue SafeStain (Life Technologies) and separated proteins were transferred to nitrocellulose membranes for Western immunoblotting and probed with anti-FLAG (Qiagen) His6-WbbB or anti-His5 (Qiagen) antibodies. The secondary antibody was horseradish peroxidase-conjugated goat anti-mouse antibody (Cedarlane Laboratories). Protein immunoblots were developed using Luminata Crescendo chemiluminescent substrate (EDM Millipore).
OPS modal distribution in LPS and Und-PP–linked OPS populations were examined by SDS/PAGE and Western immunoblotting, respectively. Cells were collected from a volume equivalent to 1 A600nm unit of each culture by centrifugation (12,000 × g for 5 min) and proteinase K-digested whole-cell lysates were then prepared following a standard protocol (49). Samples were heated (100 °C for 10 min) before separation by PAGE on a 12% acrylamide resolving gel and LPS was visualized using silver staining (50). Western immunoblots on nitrocellulose membranes were probed with K. pneumoniae O12 OPS specific rabbit antiserum (23). Detection was performed using alkaline phosphatase-conjugated goat anti-rabbit secondary antibody (Cedarlane Laboratories) and nitrobluetetrazolium with 5-bromo-4-chloro-3-indolyl phosphate.
Synthesis of Acceptor B.
Trisaccharide B was synthesized as depicted in the scheme in Fig. S2. First, the levlinoyl group in 1 (22) was removed by treatment with hydrazine monohydrate in AcOH/pyridine to give a 95% yield of disaccharide acceptor 2. Glycosylation of 2 with 3 (51) upon treatment with trimethylsilyl trifluoromethanesulfonate afforded trisaccharide 4 in 72% yield. Full deprotection of sugar moiety was smoothly carried out by first ethylenediamine-mediated cleavage of the phthalimide protecting group and acetylation of the resulting amine to give trisaccharide 5 in 88% over the two steps. Next, acid hydrolysis of the acetal protecting groups in 5 and then acetylation of liberated hydroxyl groups produced, over the two steps, an 85% yield of compound 6. The acetylation step was done to facilitate purification of the compound. Removal of the O-acetate protecting groups in 6 afforded an 88% yield of compound 7. Hydrogenation of the azido group led to primary amine 8, which was obtained in 93% yield. Finally, 8 was treated with FITC to generate B in 52% yield.
SI Synthetic General Methods
All reagents were purchased from commercial sources and were used without further purification. TLC was performed on Silica Gel 60 F254 with detection under UV and/or charring with a solution of p-anisaldehyde in H2SO4, AcOH, and EtOH. Flash column chromatography was carried out on silica gel 60 (40–60 µm). The 1H and 13C NMR spectra were recorded with Varian 500-MHz NMR spectrometers and were referenced to residual proton signals in the deuterated solvents: CDCl3, 7.26 ppm (1H) and 77.16 ppm (13C); CD3OD, 3.31 ppm (1H) and 49.00 ppm (13C); D2O 4.79 (1H) ppm or external CH3CN for D2O, 1.49 ppm (13C). NMR peak assignments of 1H and 13C were based on COSY and HSQC. Optical rotations were measured at the sodium D line (589 nm) in a microcell (1 dm, 1 mL) on a Perkin-Elmer 241 polarimeter and are expressed in units of degrees⋅milliliter/(grams⋅decimeter). High-resolution ESI-MS spectra were recorded by Agilent Technologies 6220 oaTOF using samples dissolved in methanol, methanol–toluene 3:1, or dichloromethane.
8-Azidooctyl 2,3-O-Isopropylidene- α-l-Rhamnopyranosyl-(1→3)-4,6-O Benzylidene-2-Deoxy-2- Phthalimido-β-d-Glucopyranoside (2).
To a solution of 1 (20) (159 mg, 0.190 mmol) in pyridine (5.0 mL), 1M H2NNH2⋅H2O in pyridine–AcOH (3:2, 571 μL, 0.571 mmol) was added and the mixture was stirred for 1 h at room temperature. The reaction mixture was coconcentrated with toluene. The residue was diluted with EtOAc and washed with satd aq NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by column chromatography (2:1 n-hexane–EtOAc) to give 2 (133 mg, 95%) as a white amorphous solid. Rf 0.47 (1:1 n-hexane–EtOAc); [α]D = –35.7 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.87–7.84 (m, 2H, Ar), 7.76–7.72 (m, 2H, Ar), 7.49–7.47 (m, 2H, Ar), 7.35–7.31 (m, 3H, Ar), 5.53 (s, 1H, CHPh), 5.27 (d, 1H, J = 8.6 Hz, H-1), 4.69 (s, 1H, H-1′), 4.62 (dd, 1H, J = 8.9, 10.2 Hz, H-3), 4.39 (dd, 1H, J = 4.3, 10.4 Hz, H-6a), 4.26 (dd, 1H, J = 8.5, 10.2 Hz, H-2), 3.88 (dd, 1H, J = 5.8, 7.2 Hz, H-3′), 3.84–3.78 (m, 3H, H-6b, H-2′, OCHHCH2 a), 3.72–3.64 (m, 2H, H-4, H-5, H-5′), 3.41 (dt, 1H, J = 6.3, 9.8 Hz, OCHHCH2 b), 3.17 (t, 2H, J = 6.9 Hz, CH2N3), 3.11 (app t, 1H, J = 7.5 Hz, H-4′), 2.27 (d, 1H, J = 3.0 Hz, OH), 1.48–1.32 (m, 4H, CH2 x2), 1.25 (s, 3H, CH3C), 1.16 (quint, 2H, J = 7.4 Hz, CH2), 1.09–0.96 (m, 9H, CH2 x3, CH3C), 0.71 (d, 3H, J = 6.2 Hz, H-6′); 13C NMR (125 MHz, CDCl3, δC): 137.1, 134.4, 131.5, 129.2, 128.3, 126.5, 123.7, 109.2 (C(CH3)2), 102.2 (CHPh), 98.8 (C-1), 97.9 (C-1′), 80.7 (C-4), 78.2 (C-3′), 75.9 (C-2′), 74.4 (C-4′), 74.3 (C-3), 70.1 (OCH2CH2), 68.8 (C-6), 66.6 (C-5), 66.4 (C-5′), 57.0 (C-2), 51.4 (CH2N3), 29.2 (CH2), 29.0 (CH2), 28.9 (CH2), 28.8 (CH2), 27.8(C(CH3)2), 26.5 (CH2), 25.9 (CH2), 25.7 (C(CH3)2), 16.7 (C-6′). ESI-MS m/z calculated for C38H48N4O11Na 759.3212, found 759.3213 [M+Na]+.
8-Azidooctyl 3,4,6-triO-Acetyl-2-Deoxy-2-Phthalimido-β-d-Glucopyranosyl-(1→4)-2,3-O-Isopropylidene-α-l-Rhamnopyranosyl-(1→3)-4,6-O-Benzylidene-2-Deoxy-2-Phthalimido-β-d-Glucopyranoside (4).
A mixture of 3 (51) (247 mg, 0.426 mmol), 2 (262 mg, 0.355 mmol), and 4 Å MS (1.0 g) in CH2Cl2 (8 mL) was stirred under Ar atmosphere for 1 h at room temperature. The mixture was cooled to –10 °C, then TMSOTf (7.7 μL, 42.6 μmol) was added. The reaction mixture was stirred for 1h at –10 °C, then triethylamine was added. The mixture was filtered through Celite and washed with satd aq NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by column chromatography (2:1 n-hexane–EtOAc) to give 4 (96.5 mg, 72%) as a white amorphous solid. Rf 0.43 (1:1 n-hexane–EtOAc); [α]D = –2.5 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.79–7.78 (m, 4H, Ar), 7.71–7.69 (m, 4H, Ar), 7.42–7.40 (m, 2H, Ar), 7.37–7.31 (m, 3H, Ar), 5.87 (dd, 1H, J = 9.2, 10.5 Hz, H-3′′), 5.46 (s, 1H, CHPh), 5.31 (d, 1H, J = 8.5 Hz, H-1), 5.21 (d, 1H, J = 8.5 Hz, H-1′′), 5.05 (app t, 1H, J = 9.7 Hz, H-4′′), 4.53 (s, 1H, H-1′), 4.51 (dd, 1H, J = 8.9, 9.9 Hz, H-3), 4.36 (dd, 1H, J = 4.5, 10.6 Hz, H-6a), 4.22 (dd, 1H, J = 8.4, 10.7 Hz, H-2′′), 4.19 (dd, 1H, J = 5.6, 11.9 Hz, H-6′′a), 4.14 (dd, 1H, J = 8.6, 10.2 Hz, H-2), 4.05 (dd, 1H, J = 2.2, 12.0 Hz, H-6′′b), 3.80–3.74 (m, 3H, H-6b, H-5′′, OCHHCH2 a), 3.64–3.53 (m, 4H, H-4, H-5, H-3′, H-5′), 3.49 (d, 1H, J = 5.8 Hz, H-2′), 3.37 (dt, 1H, J = 6.4, 9.8 Hz, OCHHCH2 b), 3.15 (t, 2H, J = 6.9 Hz, CH2N3), 3.03 (dd, 1H, J = 7.5, 10.0 Hz, H-4′), 1.994 (s, 3H, CH3CO), 1.987 (s, 3H, CH3CO), 1.83 (s, 3H, CH3CO), 1.46–1.28 (m, 4H, CH2 x2), 1.16–1.11 (s, 5H, CH3C, CH2), 1.01–0.93 (m, 6H, CH2 x3), 0.63 (d, 3H, J = 6.1 Hz, H-6′), 0.54 (s, 3H, CH3C); 13C NMR (125 MHz, CDCl3, δC): 170.6, 170.1, 169.7, 136.9, 134.4, 134.0, 131.4, 129.2, 128.3, 126.4, 123.6, 108.8 (C(CH3)2), 102.1 (CHPh), 99.6 (C-1′′), 98.7 (C-1), 97.6 (C-1′), 83.8 (C-4′), 80.6 (C-4), 77.2 (C-3′), 75.7 (C-2′), 74.6 (C-3), 71.4 (C-5′′), 70.5 (C-3′′), 70.1 (OCH2CH2), 69.4 (C-4′′), 68.8 (C-6), 66.6 (C-5), 64.9 (C-5′), 57.0 (C-2), 55.1 (C-2′′), 51.4 (CH2N3), 29.2 (CH2), 28.94 (CH2), 28.91 (CH2), 28.8 (CH2), 27.4(C(CH3)2), 26.5 (CH2), 25.7 (CH2), 25.4 (C(CH3)2), 20.7 (CH3CO x2), 20.5 (CH3CO), 16.7 (C-6′). ESI-MS m/z calculated for C58H67N5O20Na 1,176.4272, found 1,176.4260 [M+Na]+.
8-Azidooctyl 2-Acetamido-3,4,6-triO-Aacetyl-2-Deoxy-β-d-Glucopyranosyl-(1→4)-2,3-O- Isopropylidene-α-l-Rhamnopyranosyl-(1→3)-2-Acetamido-4,6-O-Benzylidene-2-Deoxy-β-d-Glucopyranoside (5).
A mixture of 4 (201 mg, 0.174 mmol) and ethylenediamine (4.0 mL) in n-BuOH (4.0 mL) was stirred at 80 °C overnight. The reaction mixture was cooled and then coconcentrated with toluene. The residue was dissolved in pyridine (5.0 mL) and Ac2O (3.0 mL) was added to the solution. The mixture was stirred at room temperature overnight, then MeOH (3 mL) was added and the solution was coconcentrated with toluene. The residue was dissolved in CH2Cl2 and washed with satd aq NaHCO3 and brine. The organic layer was dried over Na2SO4 and concentrated. The residue was purified by column chromatography (3:2 toluene–acetone) to give 5 (150 mg, 88%) as a white amorphous solid. Rf 0.43 (1:1 toluene–acetone); [α]D = –58.3 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 7.43–7.41 (m, 2H, Ar), 7.37–7.31 (m, 3H, Ar), 5.74 (d, 1H, J = 9.7 Hz, NH), 5.62 (d, 1H, J = 9.3 Hz, NH), 5.48 (s, 1H, CHPh), 5.31 (d, 1H, J = 8.5 Hz, H-1), 5.05–5.03 (m, 3H, H-1′, H-3′′, H-4′′), 4.71–4.67 (m, 2H, H-1, H-1′), 4.36 (dd, 1H, J = 4.9, 10.5 Hz, H-6a), 4.19–4.14 (m, 2H, H-3, H-6′′a), 4.08–4.02 (m, 4H, H-2′, H-3′, H-2′′, H-6′′b), 3.85–3.73 (m, 3H, H-6b, H-5′, OCHHCH2 a), 3.67 (q, 1H, J = 8.9 Hz, H-2), 3.56–3.42 (m, 4H, H-4, H-5, H-5′′, OCHHCH2 b), 3.30–3.23 (m, 3H, H-4′, CH2N3), 2.019 (s, 3H, CH3CO), 2.016 (s, 3H, CH3CO), 2.00 (s, 3H, CH3CO), 1.98 (s, 3H, CH3CO), 1.93 (s, 3H, CH3CO), 1.61–1.55 (m, 4H, CH2 x2), 1.48 (s, 3H, CH3C), 1.35–1.25 (s, 11H, CH3C, CH2 x4), 0.71 (d, 3H, J = 6.2 Hz, H-6′); 13C NMR (125 MHz, CDCl3, δC): 171.2, 170.7, 170.4, 170.3, 169.5, 137.0, 134.3, 129.3, 128.4, 126.4, 123.6, 109.4 (C(CH3)2), 102.0 (CHPh), 101.8 (C-1′′), 100.8 (C-1), 97.7 (C-1′), 82.2 (C-4′), 80.1 (C-4), 78.2 (C-3′), 76.3 (C-2′), 76.1 (C-3), 73.5 (C-3′′), 72.0 (C-5′′), 70.0 (OCH2CH2), 68.8 (C-6, C-4′′), 66.6 (C-5), 64.5 (C-5′), 62.5 (C-6) 57.8 (C-2), 54.3 (C-2′′), 51.6 (CH2N3), 29.6 (CH2), 29.3 (CH2), 29.2 (CH2), 28.9 (CH2), 28.2 (CH2), 26.7 (C(CH3)2), 26.5 (CH2), 25.49(C(CH3)2), 23.5 (CH3CO), 23.4 (CH3CO), 20.82 (CH3CO), 20.77 (CH3CO), 20.7 (CH3CO), 16.8 (C-6′). ESI-MS m/z calculated for C46H67N5O18Na 1,000.4373, found 1,000.4362 [M+Na]+.
8-Azidooctyl 2-Acetamido-3,4,6-triO-Acetyl-2-Deoxy-β-d-Glucopyranosyl-(1→4)-2,3-di-O-Acetyl-α-l-Rhamnopyranosyl-(1→3)-2-Acetamido-4,6-O-Acetyl-2-Deoxy-β-d-Glucopyranoside (6).
A mixture of 5 (139 mg, 0.142 mmol) and 80% AcOH aq (10 mL) was stirred for 7 h at 60 °C. The reaction mixture was cooled and then coconcentrated with toluene. The residue was dissolved in pyridine (5 mL), then Ac2O (3.0 mL) was added. The reaction mixture was stirred at room temperature overnight, then MeOH (3 mL) was added and the solution was coconcentrated with toluene. The residue was purified by column chromatography (3:2 toluene–acetone) to give 6 (124 mg, 85%) as a white amorphous solid. Rf 0.50 (1:1 toluene–acetone); [α]D = –3.8 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3, δH): 6.13 (d, 1H, J = 6.8 Hz, NH), 5.60–5.56 (2H, H-3′′, NH), 5.13–5.10 (m, 2H, H-1, H-3′), 5.08 (s, 1H, H-2′), 5.05 (d, 1H, J = 8.1 Hz, H-1), 4.97 (app t, 1H, J = 9.7 Hz, H-4′′), 4.93 (app t, 1H, J = 9.5 Hz, H-4), 4.66 (s, 1H, H-1′), 4.50 (app t, 1H, J = 9.7 Hz, H-3), 4.21 (dd, 1H, J = 5.0, 12.3 Hz, H-6a), 4.17 (d, 2H, J = 3.1 Hz, H-6′′), 4.04 (d, 1H, J = 10.7 Hz, H-6b), 3.82 (app dq, 1H, 6.4, 9.8 Hz, OCHHCH2 a), 3.74–3.71 (m, 2H, H-5′, H-5′′), 3.63–3.59 (m, 2H, H-5, H-4′), 3.45 (dt, 1H, 6.8, 9.5 Hz, OCHHCH2 b), 3.28–3.21 (m, 3H, H-2′, CH2N3), 2.95 (dt, 1H, 7.9, 8.9 Hz, H-2), 2.08 (s, 3H, CH3CO), 2.052 (s, 3H, CH3CO), 2.047 (s, 3H, CH3CO), 2.04 (s, 6H, CH3CO x2), 1.99 (s, 6H, CH3CO x2), 1.96 (s, 3H, CH3CO), 1.83 (s, 3H, CH3CO), 1.59–1.53 (m, 4H, CH2 x2), 1.33–1.28 (s, 8H, CH2 x4), 1.20 (d, 3H, J = 6.0 Hz, H-6′); 13C NMR (125 MHz, CDCl3, δC): 171.7, 171.90. 171.88, 170.6, 170.5, 170.4, 170.2, 169.8, 169.7, 100.2 (C-1′), 99.0 (C-1′′), 98.9 (C-1), 80.0 (C-3), 75.3 (C-4′), 71.6 (C-5), 71.4 (C-3′), 71.3 (C-3′′), 71.2 (C-5′′), 70.5 (C-4), 70.2 (OCH2CH2), 69.6 (C-2′), 69.3 (C-4′′), 68.4 (C-5′), 62.5 (C-6), 62.2 (C-6′′) 59.2 (C-2), 56.7 (C-2′′), 51.5 (CH2N3), 29.5 (CH2), 29.24 (CH2), 29.15 (CH2), 28.9 (CH2), 26.7 (CH2), 25.8 (CH2), 23.5 (CH3CO), 23.2 (CH3CO), 21.24 (CH3CO), 21.19 (CH3CO),21.0 (CH3CO), 20.9 (CH3CO), 20.77 (CH3CO), 20.75 (CH3CO), 20.7 (CH3CO), 17.7 (C-6′). ESI-MS m/z calculated for C44H67N5O22Na 1,040.4170, found 1,040.4158 [M+Na]+.
8-Azidooctyl 2-Acetamido-2-Deoxy-β-d-Glucopyranosyl-(1→4)- α-l-Rhamnopyranosyl-(1→3)-2-Acetamido-2-Deoxy-β-d-Glucopyranoside (7).
To a solution of 6 (117 mg, 0.115 mmol) in MeOH (6.0 mL), 3.0 M NaOMe in MeOH (100 μL) was added. After stirring overnight at room temperature, the reaction mixture was neutralized with Amberlite IR-120 (H+ form), then filtered through cotton and concentrated to give 7 (73.4mg, 88%) as a white amorphous solid. 17: [α]D = –51.1 (c 1.0, MeOH); 1H NMR (500 MHz, CD3OD, δH): 4.84 (s, 1H, H-1′), 4.74 (d, 1H, J = 8.7 Hz, H-1), 4.40 (d, 1H, J = 8.7 Hz, H-1), 3.99 (app dq, 1H, J = 6.2, 9.6 Hz, H-5′), 3.90–3.85 (m, 3H, H-6a, H-6′′a, OCHHCH2 a), 3.79–3.67 (m, 5H, H-2, H-2′, H-3′, H-6b, H-6′′b), 3.61–3.54 (m, 3H, H-3, H-4′, H-2′′), 3.48–3.42 (m, 2H, H-3′′, OCHHCH2 b), 3.41–3.34 (m, 2H, H-4, H-4′′), 3.29–3.24 (m, 4H, H-5, H-5′′, CH2N3), 2.01 (s, 3H, CH3CO), 1.98 (s, 3H, CH3CO), 1.61–1.52 (m, 4H, CH2 x2), 1.38–1.29 (m, 8H, CH2 x4), 1.28 (d, 3H, J = 6.2 Hz, H-6′); C13 NMR (125 MHz, CD3OD, δC): 174.4, 173.3, 102.8 (C-1′), 102.7 (C-1′′), 102.2 (C-1), 83.3 (C-3), 80.9 (C-4′), 77.9 (C-5), 77.8 (C-5′′), 76.8 (C-3′′), 72.6 (C-2′), 72.4 (C-3′), 72.2 (C-4), 70.6 (C-4′′), 70.5 (OCH2CH2), 68.7 (C-5′), 62.9 (C-6), 62.7 (C-6′′), 58.2 (C-2′′), 56.9 (C-2), 52.4 (CH2N3), 30.6 (CH2), 30.3 (CH2), 30.2 (CH2), 29.9 (CH2), 27.8 (CH2), 27.0 (CH2), 23.1 (CH3CO), 23.0 (CH3CO), 18.1 (C-6′). ESI-MS m/z calculated for C30H53N5O15Na 746.3430, found 746.3424 [M+Na]+.
8-Aminooctyl 2-Aacetamido-2-Deoxy-β-d-Glucopyranosyl-(1→4)- α-l-Rhamnopyranosyl-(1→3)-2-Acetamido-2-Deoxy-β-d-Glucopyranoside (8).
To a solution of 7 (71.7 mg, 99.1 μmol) in H2O (4.0 mL) under Ar atmosphere, Pd/C (40 mg) was added. The flask was then flushed with H2 gas and the mixture was stirred at room temperature overnight under an H2 atmosphere. The reaction mixture was filtered through Celite to remove Pd/C and the filtrate was concentrated. The residue was purified by reverse phase chromatography (Sep-pak C18, 50% CH3CN) to afford 8 (64.3 mg, 93%) as a white amorphous solid. [α]D = –56.1 (c 1.0, H2O); 1H NMR (500 MHz, D2O, δH): 4.87 (s, 1H, H-1′), 4.40 (d, 1H, J = 8.0 Hz, H-1), 4.05–3.95 (m, 4H, H-6a, H-5′, H-6′′a, OCHHCH2 a), 3.85–3.70 (m, 6H, H-2, H-2′, H-2′′, H-3′, H-6b, H-6′′b), 3.62–3.57 (m, 4H, H-3, H-4′, H-3′′, OCHHCH2 b), 3.54–3.47 (m, 4H, H-4, H-5, H-4′′, H-5′′), 3.02–2.94 (m, 2H, CH2NH2) 2.10 (s, 6H, CH3CO ×2), 1.64–1.59 (m, 4H, CH2 x2), 1.36–1.31 (m, 11H, H-6′, CH2 x4); 13C NMR (125 MHz, CD3OD, δC): 175.4, 174.8, 102.3 (C-1′′), 101.7 (C-1′), 101.1 (C-1), 82.1 (C-3), 80.7 (C-4′), 76.6 (C-5), 76.3 (C-5′′), 74.5 (C-3′′), 71.5 (C-2′), 71.2 (OCH2CH2), 71.0 (C-3′), 70.5 (C-4), 69.0 (C-4′′), 67.9 (C-5′), 61.4 (C-6), 61.2 (C-6′′), 56.5 (C-2′′), 56.1 (C-2), 40.3 (CH2NH2), 29.2 (CH2), 28.9 (CH2), 28,8 (CH2), 28.3 (CH2), 26.2 (CH2), 25.6 (CH2), 22.9 (CH3CO), 22.8 (CH3CO), 17.5 (C-6′). ESI-MS m/z calculated for C30H55N3O15Na 720.3525, found 720.3533 [M+Na]+.
8-Aminooctyl 2-Acetamido-2-Deoxy-β-d-Glucopyranosyl-(1→4)- α-l-Rhamnopyranosyl-(1→3)-2-Acetamido-2-Deoxy-β-d-Glucopyranoside FITC Conjugate (B).
To a solution of 8 (61.3 mg, 87.9 µmol) in DMF (2.0 mL) containing 0.1% N,N-diisopropylethylamine, fluorescein isothiocyanate (41.0 mg, 0.105 mmol) was added. The reaction mixture was stirred overnight at room temperature protected from light and was then concentrated. The residue was purified by reverse-phase chromatography (Sep-pak C18, 50% MeOH in H2O) and size-exclusion chromatography (Sephadex LH-20, MeOH) to give B (50.0 mg, 52%) as an orange amorphous solid. [α]D = –31.3 (c 1.0, MeOH); 1H NMR (500 MHz, CD3OD, δH): 7.89 (s, 1H, Ar), 7.69 (d, 1H, J = 7.7 Hz, Ar), 7.21–7.17 (m, 3H, Ar), 6.62–6.60 (m, 4H, CH = CHC, Ar), 4.84 (s, 1H, H-1′), 4.75 (d, 1H, J = 8.4 Hz, H-1′′), 4.40 (d, 1H, J = 8.4 Hz, H-1), 3.98 (app dq, 1H, J = 6.3, 9.5 Hz, H-5′), 3.90–3.85 (m, 3H, H-6a, H-6′′a, OCHHCH2 a), 3.79–3.67 (m, 5H, H-2, H-2′, H-3′, H-6b, H-6′′b), 3.63–3.54 (m, 5H, H-3, H-4′, H-2′′, CH2N), 3.46 (dt, 1H, J = 6.4, 9.7 Hz, OCHHCH2 b), 3.41 (dd, 1H, J = 8.6, 10.1 Hz, H-3′′), 3.37–3.33 (m, 2H, H-4, H-4′′), 3.29–3.24 (m, 2H, H-5, H-5′′), 1.990 (s, 3H, CH3CO), 1.986 (s, 3H, CH3CO), 1.68–1.66 (m, 2H, CH2), 1.552–1.546 (m, 2H, CH2), 1.38–1.36 (m, 8H, CH2 x4), 1.27 (d, 3H, J = 6.2 Hz, H-6′); 13C NMR (125 MHz, CD3OD, δC): 174.4, 173.4, 159.4, 132.6, 122.4, 114.7, 104.1, 103.0 (C-1′), 102.7 (C-1′′), 102.3 (C-1), 83.5 (C-3), 81.0 (C-4′), 77.9 (C-5), 77.8 (C-5′′), 76.9 (C-3′′), 72.6 (C-2′), 72.3 (C-3′), 72.2 (C-4), 70.7 (C-4′′), 70.6 (OCH2CH2), 68.7 (C-5′), 62.9 (C-6), 62.7 (C-6′′), 58.2 (C-2′′), 56.9 (C-2), 46.0 (CH2N), 30.6 (CH2), 30.4 (CH2), 30.3 (CH2), 30.0 (CH2), 27.9 (CH2), 27.0 (CH2), 23.13 (CH3CO), 23.05 (CH3CO), 18.1 (C-6′). ESI-MS m/z calculated for C51H65N4O20S 1,085.3918, found 1,085.3940 [M–H]–.
Acknowledgments
We thank J. D. King and M. Leeson for their contributions during the early stages of this project. This work was supported by individual Natural Sciences and Engineering Research Council Discovery Grants (to M.S.K., T.L.L., and C.W.). These authors are also supported by GlycoNet (the Canadian Glycomics Network, National Centres of Excellence Program). A.K. was the recipient of a postdoctoral fellowship from the Naito Foundation (Japan). T.L.L. and C.W. are Canada Research Chairs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613609114/-/DCSupplemental.
References
- 1.Cress BF, et al. Masquerading microbial pathogens: Capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev. 2014;38(4):660–697. doi: 10.1111/1574-6976.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerouge I, Vanderleyden J. O-antigen structural variation: Mechanisms and possible roles in animal/plant-microbe interactions. FEMS Microbiol Rev. 2002;26(1):17–47. doi: 10.1111/j.1574-6976.2002.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 3.Grossman N, et al. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns SM, Hull SI. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect Immun. 1998;66(9):4244–4253. doi: 10.1128/iai.66.9.4244-4253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King JD, Berry S, Clarke BR, Morris RJ, Whitfield C. Lipopolysaccharide O antigen size distribution is determined by a chain extension complex of variable stoichiometry in Escherichia coli O9a. Proc Natl Acad Sci USA. 2014;111(17):6407–6412. doi: 10.1073/pnas.1400814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 8.Okuda S, Sherman DJ, Silhavy TJ, Ruiz N, Kahne D. Lipopolysaccharide transport and assembly at the outer membrane: the PEZ model. Nat Rev Microbiol. 2016;14(6):337–345. doi: 10.1038/nrmicro.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenfield LK, Whitfield C. Synthesis of lipopolysaccharide O-antigens by ABC transporter-dependent pathways. Carbohydr Res. 2012;356:12–24. doi: 10.1016/j.carres.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Kos V, Cuthbertson L, Whitfield C. The Klebsiella pneumoniae O2a antigen defines a second mechanism for O antigen ATP-binding cassette transporters. J Biol Chem. 2009;284(5):2947–2956. doi: 10.1074/jbc.M807213200. [DOI] [PubMed] [Google Scholar]
- 11.Kos V, Whitfield C. A membrane-located glycosyltransferase complex required for biosynthesis of the D-galactan I lipopolysaccharide O antigen in Klebsiella pneumoniae. J Biol Chem. 2010;285(25):19668–19687. doi: 10.1074/jbc.M110.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenfield LK, et al. Biosynthesis of the polymannose lipopolysaccharide O-antigens from Escherichia coli serotypes O8 and O9a requires a unique combination of single- and multiple-active site mannosyltransferases. J Biol Chem. 2012;287(42):35078–35091. doi: 10.1074/jbc.M112.401000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liston SD, et al. Domain interactions control complex formation and polymerase specificity in the biosynthesis of the Escherichia coli O9a antigen. J Biol Chem. 2015;290(2):1075–1085. doi: 10.1074/jbc.M114.622480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke BR, et al. In vitro reconstruction of the chain termination reaction in biosynthesis of the Escherichia coli O9a O-polysaccharide: The chain-length regulator, WbdD, catalyzes the addition of methyl phosphate to the non-reducing terminus of the growing glycan. J Biol Chem. 2011;286(48):41391–41401. doi: 10.1074/jbc.M111.295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagelueken G, et al. Structure of WbdD: A bifunctional kinase and methyltransferase that regulates the chain length of the O antigen in Escherichia coli O9a. Mol Microbiol. 2012;86(3):730–742. doi: 10.1111/mmi.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke BR, Greenfield LK, Bouwman C, Whitfield C. Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ATP-binding cassette transporter-dependent pathway. J Biol Chem. 2009;284(44):30662–30672. doi: 10.1074/jbc.M109.052878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagelueken G, et al. A coiled-coil domain acts as a molecular ruler to regulate O-antigen chain length in lipopolysaccharide. Nat Struct Mol Biol. 2015;22(1):50–56. doi: 10.1038/nsmb.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuthbertson L, Powers J, Whitfield C. The C-terminal domain of the nucleotide-binding domain protein Wzt determines substrate specificity in the ATP-binding cassette transporter for the lipopolysaccharide O-antigens in Escherichia coli serotypes O8 and O9a. J Biol Chem. 2005;280(34):30310–30319. doi: 10.1074/jbc.M504371200. [DOI] [PubMed] [Google Scholar]
- 19.Cuthbertson L, Kimber MS, Whitfield C. Substrate binding by a bacterial ABC transporter involved in polysaccharide export. Proc Natl Acad Sci USA. 2007;104(49):19529–19534. doi: 10.1073/pnas.0705709104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertens K, et al. Antiserum against Raoultella terrigena ATCC 33257 identifies a large number of Raoultella and Klebsiella clinical isolates as serotype O12. Innate Immun. 2010;16(6):366–380. doi: 10.1177/1753425909350057. [DOI] [PubMed] [Google Scholar]
- 21.Vinogradov E, et al. Structures of lipopolysaccharides from Klebsiella pneumoniae. Eluicidation of the structure of the linkage region between core and polysaccharide O chain and identification of the residues at the non-reducing termini of the O chains. J Biol Chem. 2002;277(28):25070–25081. doi: 10.1074/jbc.M202683200. [DOI] [PubMed] [Google Scholar]
- 22.Ovchinnikova OG, et al. Bacterial β-Kdo glycosyltransferases represent a new glycosyltransferase family (GT99) Proc Natl Acad Sci USA. 2016;113(22):E3120–E3129. doi: 10.1073/pnas.1603146113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann E, Mallette E, Clarke BR, Kimber MS, Whitfield C. The Klebsiella pneumoniae O12 ATP-binding cassette (ABC) transporter recognizes the terminal residue of its O-antigen polysaccharide substrate. J Biol Chem. 2016;291(18):9748–9761. doi: 10.1074/jbc.M116.719344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locher KP. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol. 2016;23(6):487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- 25.Dong C, et al. A structural perspective on the enzymes that convert dTDP-d-glucose into dTDP-l-rhamnose. Biochem Soc Trans. 2003;31(Pt 3):532–536. doi: 10.1042/bst0310532. [DOI] [PubMed] [Google Scholar]
- 26.Lovering AL, Safadi SS, Strynadka NCJ. Structural perspective of peptidoglycan biosynthesis and assembly. Annu Rev Biochem. 2012;81:451–478. doi: 10.1146/annurev-biochem-061809-112742. [DOI] [PubMed] [Google Scholar]
- 27.Price NP, Momany FA. Modeling bacterial UDP-HexNAc: Polyprenol-P HexNAc-1-P transferases. Glycobiology. 2005;15(9):29R–42R. doi: 10.1093/glycob/cwi065. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson G, et al. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol. 1994;176(13):4144–4156. doi: 10.1128/jb.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izquierdo L, Merino S, Regué M, Rodriguez F, Tomás JM. Synthesis of a Klebsiella pneumoniae O-antigen heteropolysaccharide (O12) requires an ABC 2 transporter. J Bacteriol. 2003;185(5):1634–1641. doi: 10.1128/JB.185.5.1634-1641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchler-Bauer A, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43(Database issue):D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue):D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansson PE, Kenne L, Widmalm G. CASPER: A computer program used for structural analysis of carbohydrates. J Chem Inf Comput Sci. 1991;31(4):508–516. doi: 10.1021/ci00004a013. [DOI] [PubMed] [Google Scholar]
- 33.Pak JE, Satkunarajah M, Seetharaman J, Rini JM. Structural and mechanistic characterization of leukocyte-type core 2 β1,6-N-acetylglucosaminyltransferase: A metal-ion-independent GT-A glycosyltransferase. J Mol Biol. 2011;414(5):798–811. doi: 10.1016/j.jmb.2011.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Fleites C, et al. Insights into the synthesis of lipopolysaccharide and antibiotics through the structures of two retaining glycosyltransferases from family GT4. Chem Biol. 2006;13(11):1143–1152. doi: 10.1016/j.chembiol.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 36.Mason JM, Arndt KM. Coiled coil domains: Stability, specificity, and biological implications. ChemBioChem. 2004;5(2):170–176. doi: 10.1002/cbic.200300781. [DOI] [PubMed] [Google Scholar]
- 37.Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: Structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–555. doi: 10.1146/annurev.biochem.76.061005.092322. [DOI] [PubMed] [Google Scholar]
- 38.Merino S, Canals R, Knirel YA, Tomás JM. Molecular and chemical analysis of the lipopolysaccharide from Aeromonas hydrophila strain AH-1 (Serotype O11) Mar Drugs. 2015;13(4):2233–2249. doi: 10.3390/md13042233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oxley D, Wilkinson SG. Structural studies of glucorhamnans isolated from the lipopolysaccharides of reference strains for Serratia marcescens serogroups O4 and O7, and of an O14 strain. Carbohydr Res. 1988;175(1):111–117. doi: 10.1016/0008-6215(88)80161-7. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, et al. Structure and genetics of Shigella O antigens. FEMS Microbiol Rev. 2008;32(4):627–653. doi: 10.1111/j.1574-6976.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 41.Steiner K, et al. Molecular basis of S-layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus. J Biol Chem. 2008;283(30):21120–21133. doi: 10.1074/jbc.M801833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romanow A, et al. Dissection of hexosyl- and sialyltransferase domains in the bifunctional capsule polymerases from Neisseria meningitidis W and Y defines a new sialyltransferase family. J Biol Chem. 2014;289(49):33945–33957. doi: 10.1074/jbc.M114.597773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev. 2010;74(3):341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weissman KJ. The structural biology of biosynthetic megaenzymes. Nat Chem Biol. 2015;11(9):660–670. doi: 10.1038/nchembio.1883. [DOI] [PubMed] [Google Scholar]
- 45.Williams GJ. Engineering polyketide synthases and nonribosomal peptide synthetases. Curr Opin Struct Biol. 2013;23(4):603–612. doi: 10.1016/j.sbi.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mann E, Ovchinnikova OG, King JD, Whitfield C. Bacteriophage-mediated glucosylation can modify lipopolysaccharide O-antigens synthesized by an ATP-binding cassette (ABC) transporter-dependent assembly mechanism. J Biol Chem. 2015;290(42):25561–25570. doi: 10.1074/jbc.M115.660803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graninger M, Nidetzky B, Heinrichs DE, Whitfield C, Messner P. Characterization of dTDP-4-dehydrorhamnose 3,5-epimerase and dTDP-4-dehydrorhamnose reductase, required for dTDP-L-rhamnose biosynthesis in Salmonella enterica serovar Typhimurium LT2. J Biol Chem. 1999;274(35):25069–25077. doi: 10.1074/jbc.274.35.25069. [DOI] [PubMed] [Google Scholar]
- 48.Makarova O, Kamberov E, Margolis B. Generation of deletion and point mutations with one primer in a single cloning step. Biotechniques. 2000;29(5):970–972. doi: 10.2144/00295bm08. [DOI] [PubMed] [Google Scholar]
- 49.Darveau RP, Hancock REW. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt RR, Hoffmann M. Synthesis of C-α- and C-β-D-glucopyranosyl derivatives from O-(α-D-glucopyranosyl) trichloroacetimidate. Angew Chemie Int Ed English. 1983;22(5):406–406. [Google Scholar]
- 52.Greenfield LK, et al. Domain organization of the polymerizing mannosyltransferases involved in synthesis of the Escherichia coli O8 and O9a lipopolysaccharide O-antigens. J Biol Chem. 2012;287(45):38135–38149. doi: 10.1074/jbc.M112.412577. [DOI] [PMC free article] [PubMed] [Google Scholar]