Significance
Calcific aortic valve disease (CAVD) is a chronic inflammatory and osteogenic condition with unknown underlying mechanism and unavailable pharmacological therapy. The present study shows that lower levels of IL-37 expression in aortic valve interstitial cells (AVICs) of diseased valves play a role in the elevated valvular osteogenic activity associated with CAVD. IL-37 inhibits NF-κB and ERK1/2 to suppress AVIC osteogenic responses, and recombinant IL-37 has a greater effect on AVICs of diseased valves. Moreover, expression of IL-37 in mice attenuates aortic valve thickening following a prolonged exposure to endotoxin or high fat diet. Thus, IL-37 is antiosteogenic in human AVICs and has the potential for limitation of CAVD progression.
Keywords: Toll-like receptors, inflammation, oxidized low-density lipoprotein, calcification, signal transduction
Abstract
Calcific aortic valve disease is a chronic inflammatory process, and aortic valve interstitial cells (AVICs) from diseased aortic valves express greater levels of osteogenic factors in response to proinflammatory stimulation. Here, we report that lower cellular levels of IL-37 in AVICs of diseased human aortic valves likely account for augmented expression of bone morphogenetic protein-2 (BMP-2) and alkaline phosphatase (ALP) following stimulation of Toll-like receptor (TLR) 2 or 4. Treatment of diseased AVICs with recombinant human IL-37 suppresses the levels of BMP-2 and ALP as well as calcium deposit formation. In mice, aortic valve thickening is observed when exposed to a TLR4 agonist or a high fat diet for a prolonged period; however, mice expressing human IL-37 exhibit significantly lower BMP-2 levels and less aortic valve thickening when subjected to the same regimens. A high fat diet in mice results in oxidized low-density lipoprotein (oxLDL) deposition in aortic valve leaflets. Moreover, the osteogenic responses in human AVICs induced by oxLDL are suppressed by recombinant IL-37. Mechanistically, reduced osteogenic responses to oxLDL in human AVICs are associated with the ability of IL-37 to inhibit NF-κB and ERK1/2. These findings suggest that augmented expression of osteogenic factors in AVICs of diseased aortic valves from humans is at least partly due to a relative IL-37 deficiency. Because recombinant IL-37 suppresses the osteogenic responses in human AVICs and alleviates aortic valve lesions in mice exposed to high fat diet or a proinflammatory stimulus, IL-37 has therapeutic potential for progressive calcific aortic valve disease.
Calcific aortic valve disease (CAVD) is a leading cardiovascular disease in people over the age of 65. Progressive aortic valve calcification associated with CAVD often leads to heart failure and results in valve replacement, the second most common cardiovascular surgery performed (1). With greater lifespan, CAVD is becoming an increasingly important healthcare issue. Unfortunately, pharmacological intervention for slowing the progression of this disease is unavailable.
CAVD is recognized as a chronic inflammatory and osteogenic process (2). Inflammatory mediators promote valvular osteogenic responses and are believed to contribute to the mechanism for the pathogenesis of CAVD (2, 3). Aortic valve interstitial cells (AVICs), the dominant cellular components of aortic valve leaflets, play a critical role in aortic valve inflammation and calcification (4, 5). In this regard, proinflammatory mediators, such as tumor necrosis factor-α, have been shown to up-regulate the expression of osteogenic factor bone morphogenetic protein-2 (BMP-2) and early osteoblastic differentiation biomarker alkaline phosphatase (ALP) in AVICs (6, 7). We and others have observed that stimulation of either Toll-like receptor (TLR) 2 or TLR4 induces the osteogenic responses characterized by the expression of BMP-2 and ALP, among several other osteogenic biomarkers, in human AVICs (8–12), leading to the formation of calcification deposits in vitro (11–13). Moreover, bacteria associated with chronic periodontal infection and bacterial agents have been detected in diseased human aortic valves (14, 15), and inoculation of rabbits with oral bacteria induces aortic valve lesions (16). These findings suggest a link between TLRs and CAVD.
AVICs of diseased aortic valves have augmented inflammatory and osteogenic responses to TLR2/4 agonists (11, 12, 17). It appears that an imbalance between proinflammatory and antiinflammatory mechanisms results in the disruption of valvular homeostasis, and such an imbalance may contribute to the mechanism underlying the development and progression of CAVD. Thus, investigation of the proinflammatory signaling pathway responsible for the osteogenic responses and antiinflammatory mechanisms in AVICs of diseased aortic valves may provide important information for development of therapeutic limitation of inflammatory and osteogenic changes in aortic valves.
Epidemiologic and histological studies have also suggested a link between proatherogenic factors and CAVD (18, 19). CAVD is a frequent disease in North America, and proatherogenic factors that have been linked with CAVD are closely related to the North American lifestyle. However, a prospective trial using a statin was not effective (20). It is likely that hypercholesterolemia is one of the multiple factors involved. Studies in laboratory animals have found that exposure to a “North American diet” consisting of high amounts of noncholesterol fats and carbohydrates could induce aortic valve abnormalities, including increased leaflet thickness and leaflet calcification if animals are fed with such a diet for a prolonged time (21, 22). Currently, the underlying mechanism of aortic valve lesions induced by high fat diet is incompletely understood. Low-density lipoprotein deposits in the diseased leaflets have been demonstrated (23–27). Interestingly, increased levels of oxidized low-density lipoprotein (oxLDL) in blood have been reported to correlate with aortic valve fibrosis and calcification (23). Further, oxLDL deposition in the vascular wall is known to provoke atherosclerotic calcification (28). We have reported that oxLDL induces BMP-2 expression in human coronary artery endothelial cells through a mechanism dependent on TLR2 and TLR4 (29).
Interleukin (IL)-37, previously known as IL-1 family member 7, is expressed in humans, but is absent in rodents (30, 31). IL-37 is an antiinflammatory member of the IL-1 family, which broadly inhibits innate and acquired immune responses in vitro and in vivo (31). It is unknown whether human AVICs express IL-37 and whether IL-37 plays a role in AVIC pathobiology associated with CAVD.
The objective of this study was to test the hypotheses that the proosteogenic phenotype of diseased AVICs is due to IL-37 deficiency and that IL-37 suppresses the osteogenic responses to prevent AVIC proosteogenic reprogramming. In this study, we examined the relationship of the augmented osteogenic responses of diseased human AVICs with cellular IL-37 levels, determined the role of endogenous IL-37 as a regulator of AVIC osteogenic responses, evaluated the effect of recombinant IL-37 on AVIC osteogenic responses, investigated the effect of expression of human IL-37 on aortic valve lesions in mice after prolonged exposure to a lipopolysaccharide (LPS) or high fat diet, and elucidated the molecular mechanism of IL-37 action in human AVICs.
Results
Endogenous IL-37 Negatively Modulates the Osteogenic Responses to Proinflammatory Stimuli in Human AVICs.
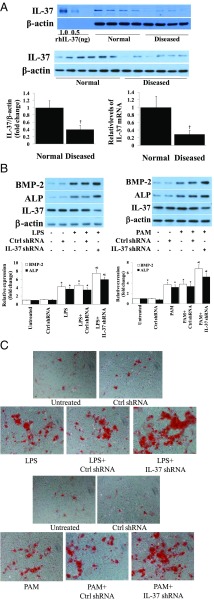
We analyzed IL-37 protein and mRNA levels in AVICs from 20 human aortic valves, 10 normal and 10 diseased. As shown in Fig. 1A, AVICs from diseased aortic valves had markedly lower levels of IL-37 protein and mRNA compared with cells of normal aortic valves. AVICs of diseased aortic valves produced higher levels of BMP-2 and ALP after stimulation with TLR4 agonist LPS or TLR2 agonist Pam3CSK4 (PAM) (Fig. S1A). These data indicate that IL-37 deficiency contributes to the mechanism underlying the augmented osteogenic responses to proinflammatory stimulation in diseased AVICs.
Fig. 1.
Relative IL-37 deficiency in diseased human AVICs results in augmented osteogenic responses to proinflammatory stimulation. (A) Immunoblots, densitometric data, and PCR analysis show that diseased AVICs express lower levels of IL-37 protein and mRNA. n = 10 isolates from different donors; †P < 0.05 vs. cells of normal valves. (B) Normal AVICs were treated with IL-37 shRNA or scrambled shRNA, and then stimulated with LPS (0.2 µg/mL) or Pam3CSK4 (PAM, 0.1 µg/mL) for 3 d. Representative immunoblots and densitometric data (n = 5 separate experiments using different isolates) show that IL-37 knockdown in normal AVICs enhances BMP-2 and ALP expression. *P < 0.05 vs. corresponding control; †P < 0.05 vs. stimulant alone and scrambled shRNA + stimulant. (C) Representative images (n = 5 separate experiments using distinct isolates) show that IL-37 knockdown augments calcification deposit formation after 21 d of stimulation in a conditioning medium. (Original magnification: 10×.)
Fig. S1.
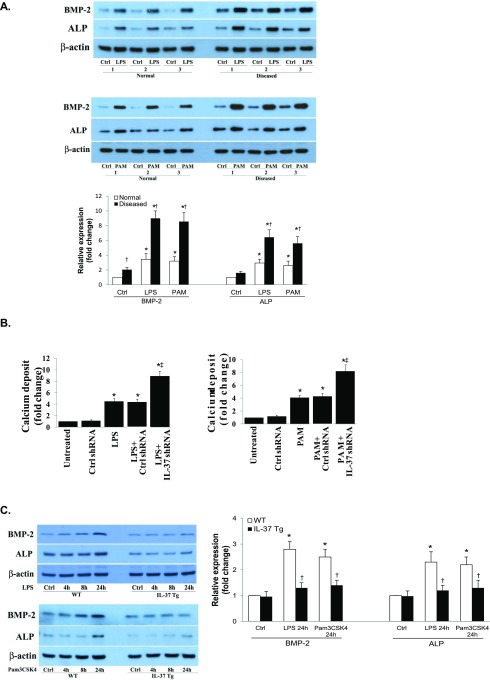
(A) AVICs of diseased human aortic valves have augmented osteogenic responses to proinflammatory stimulation. Normal and diseased AVICs were exposed to LPS (0.2 µg/mL) or Pam3CSK4 (PAM, 0.1 µg/mL) for 3 d. Representative immunoblots and densitometric data (n = 6 isolates from different donors) show that diseased AVICs produce higher levels of BMP-2 and ALP proteins in response to stimulation. *P < 0.05 vs. corresponding control (Ctrl; untreated); †P < 0.05 vs. normal cells receiving the same treatment. (B) IL-37 knockdown enhances calcium deposit formation in normal AVICs following in vitro stimulation with proinflammatory stimuli. Spectrophotometric data (n = 5 separate experiments using distinct cell isolates) show that IL-37 knockdown augments calcification deposit formation after 21 d of stimulation in a conditioning medium. *P < 0.05 vs. corresponding control; ‡P < 0.05 vs. stimulant alone and scrambled shRNA + stimulant. (C) AVICs of IL-37 Tg mice display attenuated osteogenic responses to proinflammatory stimulation. AVICs isolated from C57BL/6 (WT) mice and IL-37 transgenic (Tg) mice were treated with LPS (0.2 µg/mL) or Pam3CSK4 (0.1 µg/mL) for 4–24 h. Representative immunoblots and densitometric data (n = 5 separate experiments using different isolates) show that murine AVICs that express human IL-37 produce lower levels of BMP-2 and ALP following stimulation with LPS or Pam3CSK4. *P < 0.05 vs. corresponding control; †P < 0.05 vs. AVICs from WT mice receiving the same treatment.
To determine whether IL-37 modulates the osteogenic responses, we performed knockdown experiments in AVICs from normal aortic valves. Fig. 1B shows that IL-37 knockdown enhanced the BMP-2 and ALP responses to LPS and PAM in normal AVICs. Furthermore, IL-37 knockdown exaggerated calcification deposit formation induced by LPS and PAM (Fig. 1C and Fig. S1B). To confirm the role of IL-37 in modulation of the osteogenic responses, we examined BMP-2 and ALP production after stimulation with LPS and PAM in murine AVICs isolated from mice that constitutively express human IL-37 (IL-37 Tg mice). AVICs from IL-37 Tg mice displayed greatly reduced osteogenic responses to stimulation of TLR2 or TLR4 (Fig. S1C). Thus, IL-37 negatively modulates AVIC osteogenic responses to proinflammatory stimulation.
Recombinant IL-37 Suppresses the Osteogenic Responses in Diseased Human AVICs.
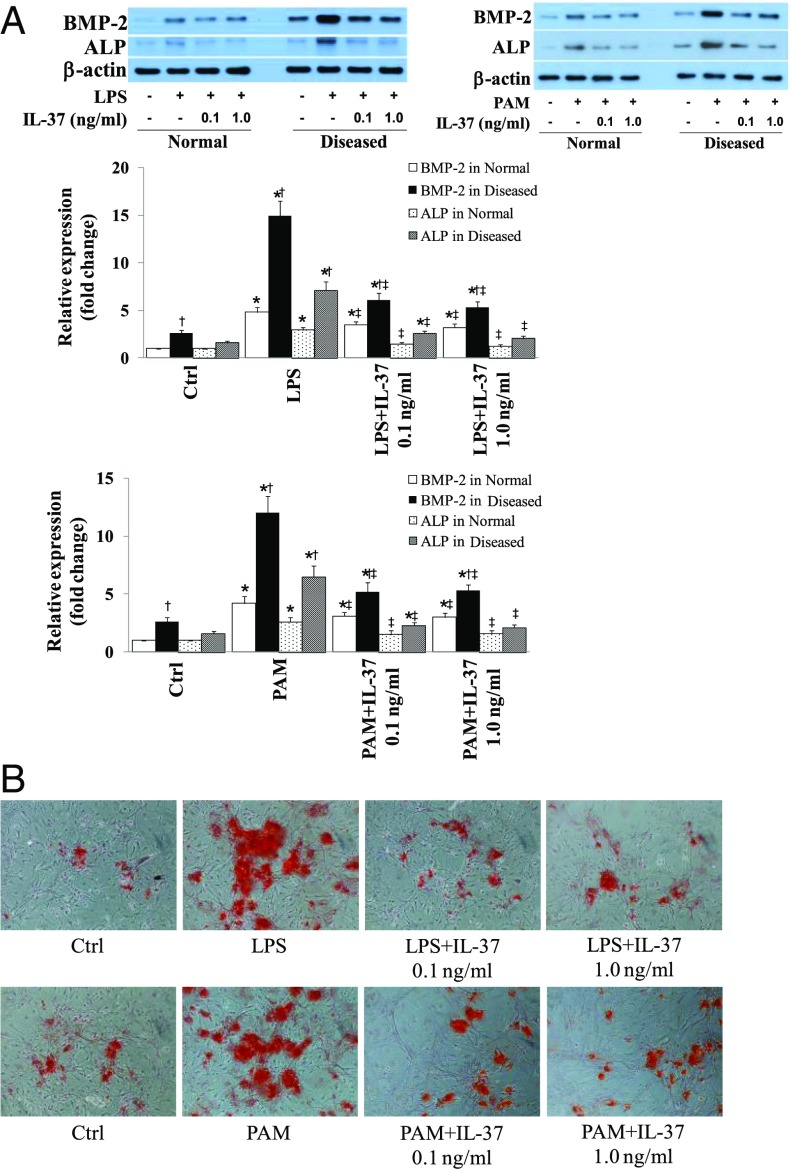
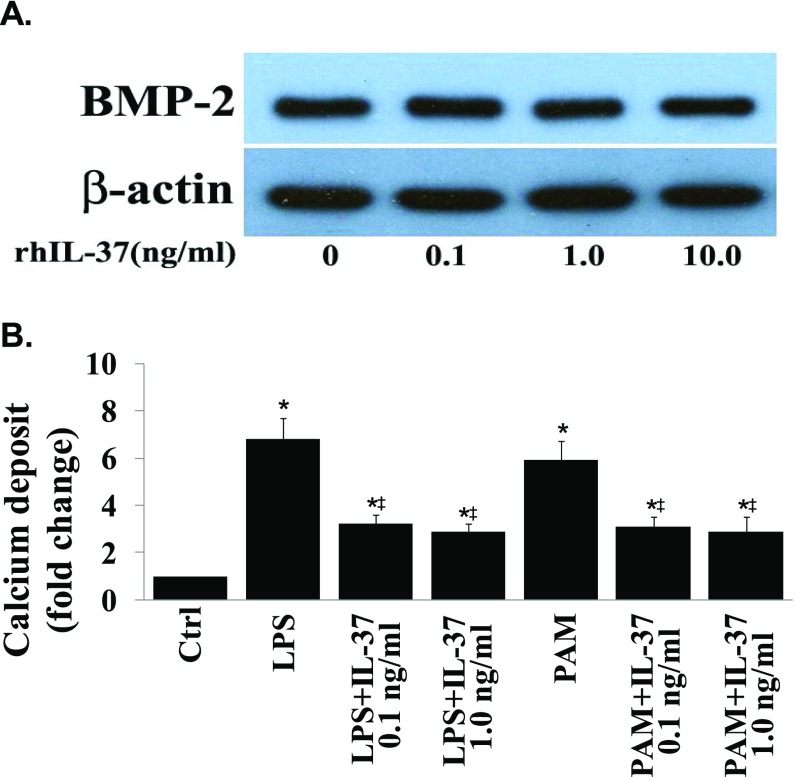
To determine whether IL-37 has an effect on the osteogenic responses in diseased human AVICs, we treated AVICs from normal and diseased human aortic valves with recombinant IL-37 (0.1 and 1.0 ng/mL) 1 h before exposure to LPS or PAM. As shown in Fig. 2A, recombinant IL-37 markedly reduced the levels of BMP-2 and ALP in AVICs from either normal or diseased valves. It is noteworthy that treatment with recombinant IL-37 resulted in a greater reduction in the levels of osteogenic factors in diseased AVICs. Whereas diseased AVICs displayed elevated levels of BMP-2 in the baseline, recombinant IL-37 had no effect on BMP-2 levels in the absence of stimulation (Fig. S2A). Therefore, recombinant IL-37 suppresses human AVIC osteogenic responses to proinflammatory stimulation, and it has a greater effect on diseased AVICs that are IL-37 deficient. Further, we stimulated diseased AVICs with LPS or PAM for 3 wk in the presence and absence of recombinant IL-37 to determine the effect of IL-37 on in vitro osteogenic activity. Interestingly, recombinant IL-37 reduced calcification deposit formation in diseased AVICs (Fig. 2B and Fig. S2B). Therefore, IL-37 negatively modulates AVIC osteogenic responses and suppresses in vitro osteogenic activity in AVICs of diseased human aortic valves.
Fig. 2.
Recombinant IL-37 suppresses the osteogenic responses in AVICs of diseased human aortic valves. AVICs of normal and diseased aortic valves were treated with recombinant IL-37 (0.1 and 1.0 ng/mL) 1 h before stimulation with LPS (0.2 µg/mL) or Pam3CSK4 (PAM, 0.1 µg/mL) for 3–21 d. (A) Representative immunoblots and densitometric data (n = 6 isolates from different donors) show that treatment with recombinant IL-37 results in greater reduction in BMP-2 and ALP levels at 3 d in AVICs of diseased aortic valves. (B) Representative images (n = 5 separate experiments using different isolates) show that recombinant IL-37 reduces calcium deposit formation in diseased cells after 21 d of stimulation in a conditioning medium. (Original magnification: 10×.) *P < 0.05 vs. corresponding control; †P < 0.05 vs. normal cells receiving the same treatment; ‡P < 0.05 vs. stimulant alone.
Fig. S2.
(A) IL-37 has no effect on BMP-2 levels in diseased human AVICs in the absence of stimulation. Diseased AVICs were treated with different concentrations of recombinant IL-37 (0.01–10.0 ng/mL) for 3 d. Levels of BMP-2 protein were assessed with immunoblotting. A representative immunoblot of three separate experiments show that recombinant IL-37 has no effect on cellular BMP-2 levels in the absence of proinflammatory stimulation. (B) Recombinant IL-37 reduces calcium deposit formation in AVICs of diseased aortic valves following in vitro stimulation with proinflammatory stimuli. Spectrophotometric data (n = 5 separate experiments using distinct cell isolates) show that recombinant IL-37 reduces calcium deposit formation in diseased cells after 21 d of stimulation in a conditioning medium. *P < 0.05 vs. corresponding control; ‡P < 0.05 vs. stimulant alone.
Expression of IL-37 in Mice Alleviates Aortic Valve Lesions in Vivo.
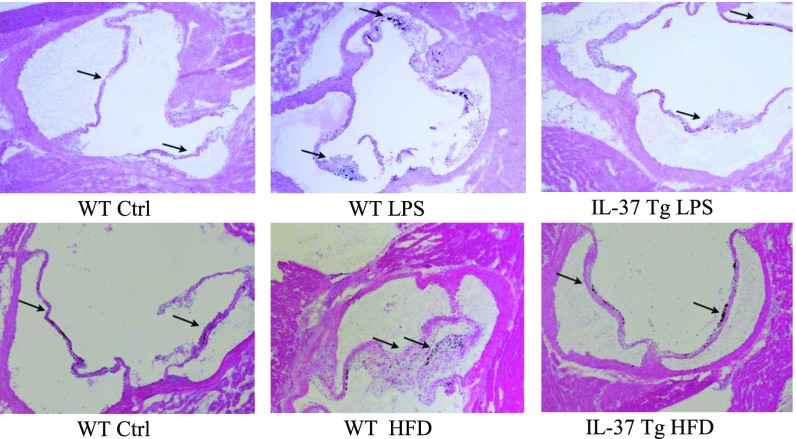
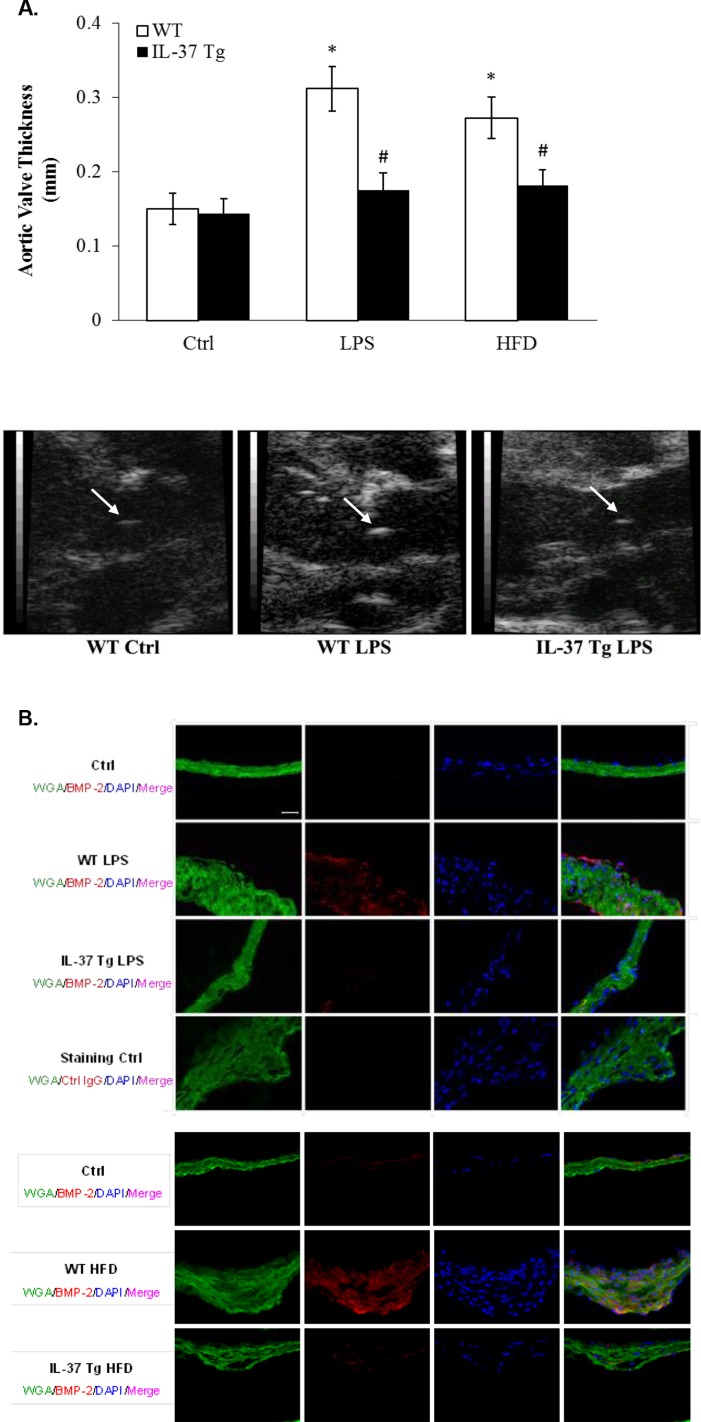
To evaluate the effect of IL-37 in vivo, we developed a mouse model of aortic valve lesions by using prolonged treatment with LPS. WT mice and IL-37 Tg mice were treated with LPS for 12 wk with the aid of osmotic pumps. Histological images in Fig. 3, and echocardiographs and quantification data presented in Fig. S3A revealed that aortic valve leaflet thickness was increased in WT mice, and that the thickening was accompanied by elevated levels of BMP-2 in the valvular tissue (Fig. S3B). However, essentially normal valve thickness and greatly reduced BMP-2 levels were observed in IL-37 Tg mice (Fig. 3 and Fig. S3 A and B).
Fig. 3.
IL-37 Tg mice display attenuated aortic valve thickening following a prolonged exposure to LPS or high fat diet. C57BL/6 (WT) mice and IL-37 transgenic (Tg) mice were treated with LPS (4.0 µg/d) for 12 wk by using osmotic pumps or fed with a high fat diet (HFD; 45% fat as in kcal%) for 16 wk. Representative histology images show that expression of IL-37 attenuates aortic valve thickening caused by LPS or HFD. n = 10 in each group. (Original magnification: 10×.)
Fig. S3.
(A) IL-37 Tg mice display attenuated aortic valve thickening following a prolonged exposure to LPS or high fat diet (HFD). C57BL/6 (WT) mice and IL-37 transgenic (Tg) mice were treated with LPS (4.0 µg/d) for 12 wk by using osmotic pumps or fed with a HFD (45% fat as in kcal%) for 16 wk. Quantitative data from ultrasound analysis in the bar graph show that expression of IL-37 attenuates aortic valve thickening caused by LPS or HFD. n = 10 in each group, *P < 0.05 vs. control, #P < 0.05 vs. WT LPS or WT HFD. Representative ultrasound images show that expression of IL-37 attenuates aortic valve (arrow) thickening in mice receiving prolonged treatment with LPS. (B) Expression of IL-37 suppresses BMP-2 production in aortic valve tissue caused by LPS or HFD. C57BL/6 (WT) mice and IL-37 transgenic (Tg) mice were treated with LPS (4.0 µg/d) for 12 wk by using osmotic pumps or fed with a HFD (45% fat as in kcal%) for 16 wk. Representative immunofluorescence images show that expression of IL-37 reduces BMP-2 levels in aortic valve tissue. n = 10 in each group. (Original magnification: 40×.)
We also examined aortic valve thickness in WT and IL-37 Tg mice fed with a high fat diet (containing 24% fat and 41% carbohydrate) for 16 wk. This high fat diet resulted in accelerated weight gain, hyperglycemia, and a mild increase in plasma oxLDL levels, but no difference in these parameters between WT mice and IL-37 Tg was observed. Although WT mice exhibited aortic valve leaflet thickening accompanied by elevated BMP-2 levels in valvular tissue (Fig. 3 and Fig. S3B), aortic valve leaflets in IL-37 Tg mice were essentially normal at the end of the experiment (Fig. 3). These in vivo experiments demonstrate that IL-37 reduces early aortic valve lesions caused by specific stimuli.
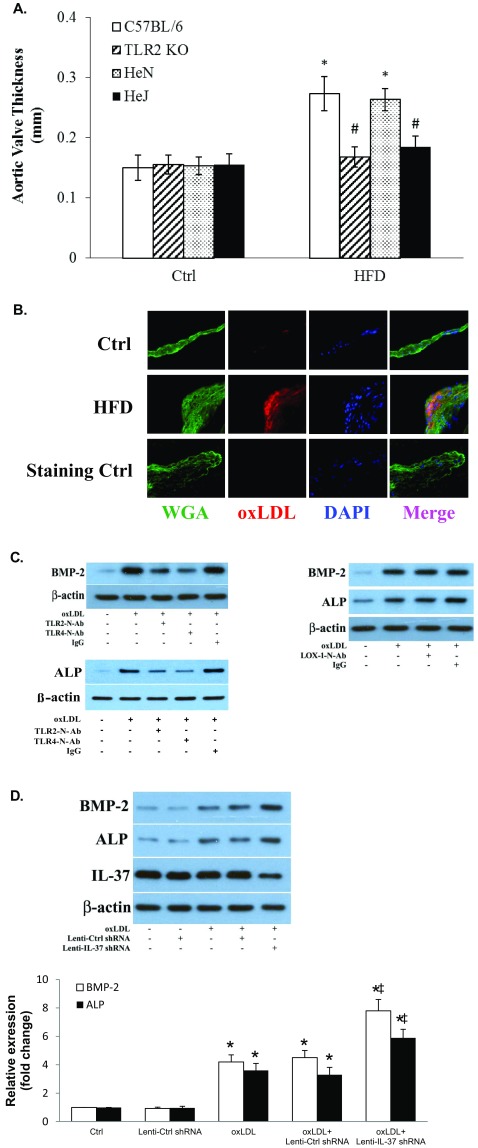
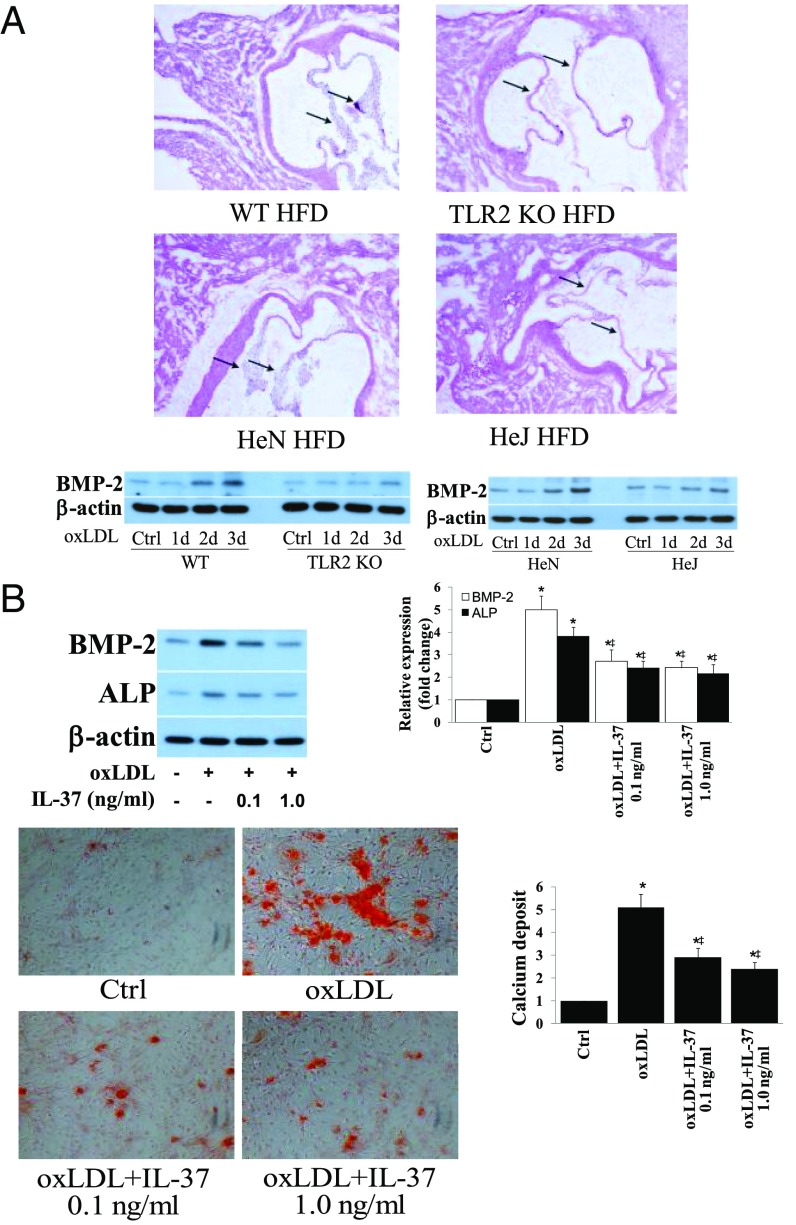
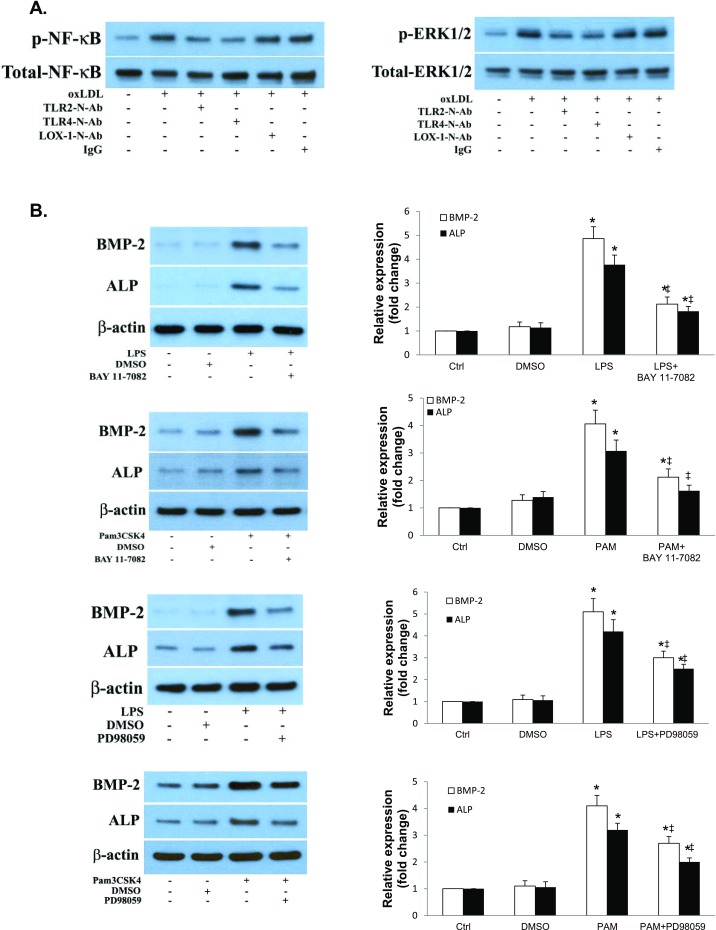
We observed oxLDL deposits in the aortic valve tissue of both WT and IL-37 Tg mice fed with high fat diet (images of WT mice shown in Fig. S4B). However, the levels of oxLDL in aortic valves of IL-37 Tg mice were significantly lower compared with WT mice. In vitro human AVIC cultures showed that exposure of cells to oxLDL (40 µg/mL for 72 h) increases BMP-2 and ALP levels. However, neutralization of either TLR2 or TLR4, but not LOX-1, reduces the proosteogenic effect of oxLDL (Fig. S4C). Further, aortic valve thickening was absent in TLR2 KO mice and TLR4 mutant mice fed a high fat diet (Fig. 4A and Fig. S4A). Importantly, recombinant IL-37 suppressed oxLDL-induced osteogenic responses and in vitro osteogenic activity in human AVICs (Fig. 4B). Conversely, IL-37 knockdown enhanced oxLDL-induced BMP-2 and ALP expression in human AVICs (Fig. S4D). Therefore, IL-37 regulates AVIC osteogenic responses induced by pathogen-associated molecular pattern (PAMP) as well as danger-associated molecular pattern (DAMP).
Fig. S4.
(A) Both TLR2 and TLR4 are involved in mediating aortic valve thickening caused by high fat diet (HFD). C57BL/6 (WT) mice, TLR2 knockout (TLR2 KO) mice, C3H/HeN (HeN, TLR4 competent) mice, and C3H/HeJ (HeJ, TLR4-defective mutant) mice were fed with a HFD (45% fat as in kcal%) for 16 wk. Quantification data from ultrasound analysis show that either TLR2 KO or TLR4 mutation attenuates aortic valve thickening caused by HFD. n = 10 in each group, *P < 0.05 vs. control, #P < 0.05 vs. C57BL/6 HFD or HeN HFD. (B) HFD causes oxLDL deposition in aortic valve leaflet. C57BL/6 (WT) mice fed with a HFD (45% fat as in kcal%) for 12 wk. A representative immunofluorescence image shows oxLDL accumulation in aortic valve tissue. (Original magnification: 40×.) (C) OxLDL induces the osteogenic responses through TLR2 and TLR4, not LOX-1. AVICs of normal aortic valves were treated with oxLDL (40 µg/mL) for 3 d in the absence or presence of a neutralizing antibody against TLR2, TLR4, or LOX1. Representative immunoblots show reduced levels of BMP-2 and ALP in AVICs treated with either anti-TLR2 or anti-TLR4, but not in cells treated with anti-LOX1. (D) IL-37 knockdown augments AVIC osteogenic responses to oxLDL. Human AVICs were treated with lentiviral IL-37 shRNA before being exposed to oxLDL (40 µg/mL) for 3 d. Representative immunoblots and densitometric data (n = 5) show that IL-37 KD augments BMP-2 and ALP expression in response to oxLDL. *P < 0.05 vs. corresponding control; ‡P < 0.05 vs. oxLDL or oxLDL+Ctrl shRNA.
Fig. 4.
Recombinant IL-37 suppresses AVIC osteogenic responses induced by oxLDL. (A) Representative histology images of 6 mice of each genotype show aortic valve thickening is essentially absent in TLR2 KO (C57BL/6 background) mice and TLR4 mutant (C3H/HeJ) mice fed with high fat diet for 12 wk. Representative immunoblots of four separate experiments show markedly reduced BMP-2 levels in murine AVICs from TLR2 KO mice and TLR4-mutant mice following treatment with oxLDL (40 µg/mL) for 1–3 d. (B) AVICs of normal human aortic valves were treated with oxLDL (40 µg/mL) for 3–21 d in the presence or absence of recombinant IL-37 (0.1 and 1.0 µg/mL). Representative immunoblots and densitometric data (n = 5 experiments using different isolates) show that recombinant IL-37 reduces BMP-2 and ALP levels in cells exposed to oxLDL for 3 d. Representative images and spectrophotometric data (n = 5 separate experiments using distinct cell isolates) show that reduced calcium deposit formation in IL-37–treated cells after 21 d of oxLDL stimulation in conditioning medium. (Original magnification: 10×.) *P < 0.05 vs. corresponding control; ‡P < 0.05 vs. oxLDL alone.
IL-37 Suppresses AVIC Osteogenic Responses Through Inhibition of NF-κB and ERK1/2.
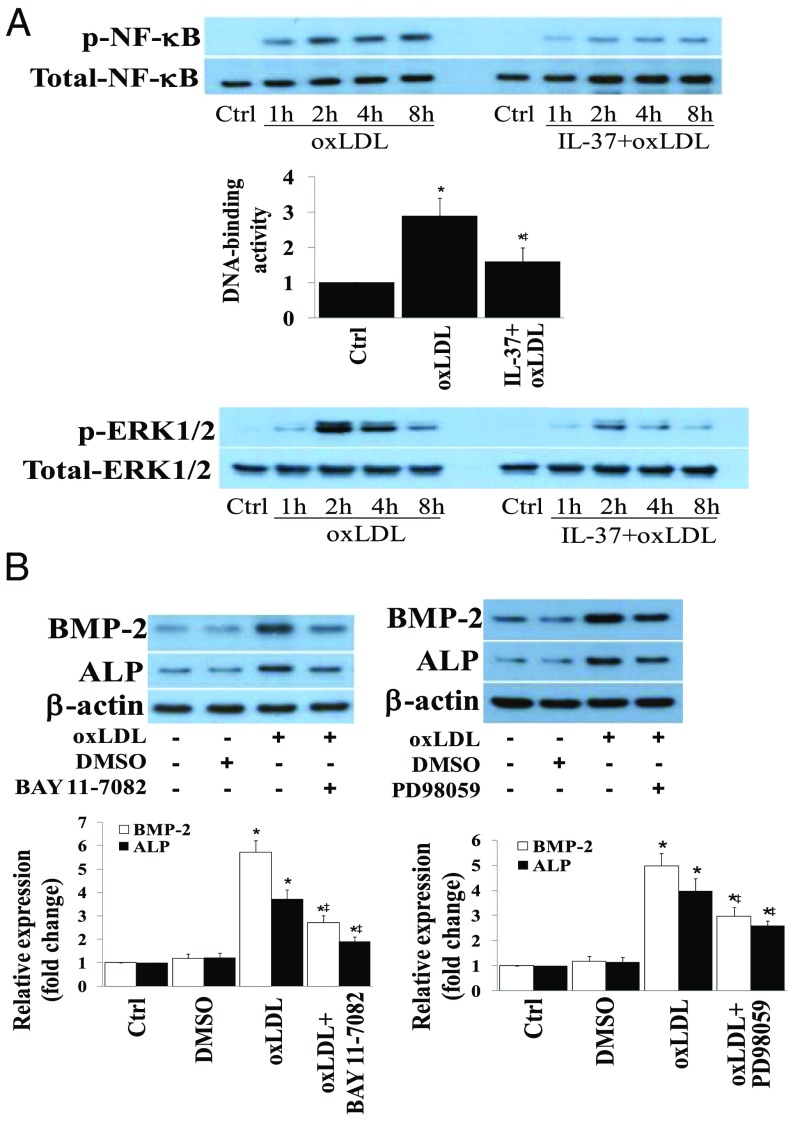
To elucidate the mechanism by which IL-37 suppresses the osteogenic response, we examined the activation of NF-κB and ERK1/2, two important signaling molecules that regulate AVIC osteogenic responses. Human AVICs were stimulated with oxLDL for 0–8 h in the presence or absence of recombinant IL-37. Recombinant IL-37 markedly reduced NF-κB phosphorylation, NF-κB DNA-binding activity, and ERK1/2 phosphorylation following an exposure to oxLDL (Fig. 5A). Interestingly, oxLDL activates NF-κB and ERK1/2 in human AVICs through a mechanism depending on TLR2 and TLR4 (Fig. S5A). To confirm the role of NF-κB and ERK1/2 in mediating the osteogenic responses to oxLDL, we exposed AVICs to specific inhibitors before the exposure to oxLDL. Inhibition of NF-κB by Bay11-7082 or ERK1/2 by PD98059 markedly reduced the proosteogenic effects of oxLDL (Fig. 5B) or TLR2/4 agonists (Fig. S5B). Together, these observations reveal that NF-κB and ERK1/2 mediate the osteogenic responses to oxLDL in human AVICs and suggest that IL-37 inhibits NF-κB and ERK1/2 to suppress the osteogenic responses.
Fig. 5.
The antiosteogenic effect of IL-37 is due to its impact on NF-κB and ERK1/2 activation. (A) AVICs of normal human aortic valves were treated with oxLDL (40 µg/mL) for 1–8 h in the presence or absence of recombinant IL-37 (0.1 ng/mL). Representative immunoblots and NF-κB DNA-binding data (n = 5 separate experiments using different isolates) show that recombinant IL-37 reduces NF-κB and ERK1/2 phosphorylation at each time point and suppresses NF-κB DNA-binding activity examined at 4 h. (B) AVICs of normal human aortic valves were treated with BAY 11-7082 (2.5 µM) or PD98059 (25 μM) 1 h before stimulation with oxLDL (40 µg/mL) for 3 d. Representative immunoblots and densitometric data (n = 5 separate experiments using different isolates) show that inhibition of NF-κB or ERK1/2 reduces BMP-2 and ALP expression after stimulation with oxLDL. *P < 0.05 vs. corresponding control; ‡P < 0.05 vs. oxLDL alone. DMSO, dimethyl sulfoxide.
Fig. S5.
(A) OxLDL activates NF-κB and ERK1/2 through TLR2 and TLR4. AVICs of normal aortic valves were treated with oxLDL (40 µg/mL) for 4 h in the absence or presence of a neutralizing antibody against TLR2, TLR4, or LOX1. Representative immunoblots show reduced NF-κB and ERK1/2 phosphorylation in cells treated with neutralizing antibody against TLR2 or TLR4. (B) NF-κB and ERK1/2 mediate AVIC osteogenic responses to proinflammatory stimulation. AVICs of normal human aortic valves were treated with BAY 11-7082 (2.5 μM) or PD98059 (25 μM) 1 h before stimulation with LPS (0.2 µg/mL) or Pam3CSK4 (PAM, 0.1 µg/mL) for 3 d. Representative immunoblots and densitometric data (n = 5 separate experiments using different isolates) show that inhibition of NF-κB or ERK1/2 reduces BMP-2 and ALP expression following stimulation with either of these two stimuli. *P < 0.05 vs. corresponding control; ‡P < 0.05 vs. stimulant alone.
Discussion
Chronic inflammatory and osteogenic activities in the aortic valve tissue promote CAVD progression (2, 32). The present study uncovered an antiosteogenic function of IL-37 in human AVICs. IL-37 is an antiinflammatory cytokine expressed in humans, but not in rodents. Expression of human IL-37 in mice protects against models of systemic endotoxemia (33, 34), chemical colitis (35), spinal cord injury (36), sleep deprivation (37), myocardial infarction (38), and contact dermatitis (39). Recombinant IL-37 administered to wild-type mice has resulted in reduced severity of inflammatory injuries (31).
We and others have observed that stimulation of either TLR2 or TLR4 induces osteogenic responses in human AVICs (9–12, 17); moreover, AVICs from diseased aortic valves exhibit augmented osteogenic responses to proinflammatory stimulation (10, 11, 17). These studies suggest that innate immune receptors play a role in the mechanisms underlying the pathogenesis of CAVD.
We show that the augmented osteogenic responses to TLR2 and TLR4 agonists in AVICs of diseased aortic valves correlate to constitutively lower levels of cellular IL-37. Reducing IL-37 levels in AVICs of normal human aortic valves by gene knockdown enhances their osteogenic responses. Two observations support the role of IL-37: recombinant IL-37 suppresses the osteogenic responses in AVICs of diseased aortic valves; AVICs from IL-37 Tg mice are less sensitive to TLR2 and TLR4 agonists for BMP-2 and ALP expression. Further, IL-37 Tg mice exhibit reduced aortic valve thickening and BMP-2 levels after prolonged exposure to LPS or feeding with a high fat diet. Mechanistic data show that IL-37 suppresses the activation of NF-κB and ERK1/2, two important pathways that regulate BMP-2 and ALP expression in human AVICs (12, 13). It should be noted that diseased aortic valve tissue is heterogeneous. To minimize variability, we collect tissue from the areas where no apparent calcification is observed. Thus, the diseased AVICs used in this study represent those of valvular tissue in the relatively early stage of CAVD.
IL-37 Has an Antiosteogenic Effect in Human AVICs.
Here, we demonstrated that AVICs of diseased human aortic valves have lower levels of IL-37 protein and mRNA and that this alteration in cellular IL-37 levels is associated with the augmented osteogenic response to TLR2/4 stimulation. IL-37 knockdown enhances the osteogenic responses and in vitro osteogenic activity (increased formation of calcification deposits) in normal AVICs. AVICs of IL-37 Tg mice exhibit markedly attenuated osteogenic responses to TLR2/4 stimulation. Recombinant IL-37 suppresses AVIC osteogenic responses to TLR2/4 agonists and oxLDL that up-regulates BMP-2 and ALP expression in human AVICs in a TLR2/4-dependent fashion. Our in vivo studies found that IL-37 Tg mice have markedly reduced BMP-2 levels in aortic valve tissue after treatment with LPS or high fat diet. Thus, these lines of evidence support the notion that endogenous IL-37 negatively regulates the osteogenic responses to proinflammatory stimulation in AVICs and suggest that relative IL-37 deficiency contributes to the mechanism underlying the augmented osteogenic responses in AVICs of diseased human aortic valves.
Low concentrations of recombinant IL-37 suppresses the osteogenic responses in human AVICs. Moreover, recombinant IL-37 has a greater effect on AVICs of diseased aortic valves and diminishes the differences in levels of BMP-2 and ALP between normal and diseased cells. In validating the effect of recombinant IL-37 on proosteogenic phenotypic changes in AVICs of diseased aortic valves, we observed that treatment with recombinant IL-37 reduces calcium deposit formation following prolonged stimulation with TLR2/4 agonists. These findings demonstrate that IL-37 suppresses in vitro osteogenic activity in diseased AVICs and further support our notion that relative IL-37 deficiency enhances the osteogenic responses in AVICs.
Our in vivo studies found that IL-37 Tg mice have markedly attenuated leaflet thickening after prolonged exposure to LPS or high fat diet. Similarly, aortic valve thickening is essentially absent in TLR2 KO mice and TLR4 mutant mice fed with a high fat diet, indicating an important role of TLR2/4 in mediating aortic valve lesions. It is interesting that oxLDL deposition is evident in the aortic valve tissue of mice fed with high fat diet because oxLDL has been reported to function as a DAMP (29, 40, 41). In this regard, we reported that oxLDL induces BMP-2 expression in human coronary artery endothelial cells through TLR2/4 (29). The in vitro murine AVIC culture experiments revealed that TLR2 KO or TLR4 mutation greatly reduces oxLDL-induced BMP-2 expression, providing a possible mechanism for the in vivo effect of high fat diet on aortic valves. It is particularly interesting that recombinant IL-37 markedly suppresses the osteogenic responses induced by oxLDL in human AVICs. This finding highlights the potential of IL-37 for the suppression of valvular osteogenic responses to both PAMP and DAMP. It should be noted that the attenuated aortic valve thickening in IL-37 Tg mice fed a high fat diet could be the combined effects of IL-37 on oxLDL accumulation and cellular osteogenic responses to oxLDL because oxLDL levels in the aortic valve leaflets of IL-37 Tg mice are lower than those in WT mice fed a high fat diet.
IL-37 Suppresses AVIC Osteogenic Responses Through Inhibition of NF-κB and ERK1/2.
Several studies show an important role of the NF-κB pathway in mediating vascular cell osteogenic responses (42). Although we have reported that NF-κB and ERK1/2 mediate induction of BMP-2 and ALP in human AVICs (12), we now note that recombinant IL-37 suppresses NF-κB phosphorylation and DNA-binding activity and inhibits ERK1/2 phosphorylation in human AVICs exposed to LPS, Pam3CSK4, or oxLDL. A specific inhibitor of NF-κB or ERK1/2 reduces the levels of BMP-2 and ALP in cells exposed to these stimuli.
IL-37 binds to IL-18Rα, but the affinity of IL-37 to IL-18Rα is relatively lower compared with the affinity of IL-18 to IL-18Rα (43, 44). However, once IL-37 binds to the IL-18Rα, IL-1R8 (formerly SIGIRR) is recruited and the complex signals in the cell to suppress NF-κB function (33, 34). More importantly, recombinant IL-37 does not suppress inflammation in mice deficient in IL-1R8 (31). The signaling mechanism of IL-37 includes marked decreases in the activity of several signaling pathways, for example mTOR, but increases in the activity of antiinflammatory pathways, such as PTEN and AMPK (31). In addition, there is an interaction of IL-37 with SMAD3 in LPS-stimulated macrophages (33). We assume that IL-37 may exert an influence on these signaling pathways in AVICs to modulate the osteogenic responses.
The present study shows that lower levels of IL-37 expression in AVICs of patients with CAVD are associated with augmented osteogenic responses to proinflammatory stimulation and that recombinant IL-37 suppresses the osteogenic responses of AVICs of diseased aortic valves. In mice, expression of IL-37 attenuates aortic valve thickening and reduces BMP-2 levels in valvular tissue following the exposure to LPS or high fat diet. Overall, these findings support the concept that IL-37 may have therapeutic potential for suppression of aortic valve osteogenic responses in an inflammatory milieu.
Methods
Isolation and Culture of Human AVICs.
Normal human aortic valve leaflets were collected from the explanted hearts of 10 patients (10 males, mean age 59 ± 8.1 y) undergoing heart transplantation, and diseased aortic valve leaflets were obtained from 10 patients (10 males, mean age 63 ± 11.1 y) with CAVD and undergoing aortic valve replacement (patient demographics is displayed as Table S1). All patients gave informed consent for the use of their valves for this study. This study was approved by the Institutional Review Board of the University of Colorado Denver.
Table S1.
Patient demographics
| Item | No aortic valve disease | With CAVD |
| No. of patients | 10 | 10 |
| Age, y | 59 ± 8.1 | 63 ± 11.1 |
| Male | 10 | 10 |
| Female | 0 | 0 |
| Bicuspid valve | 0 | 0 |
| Tricuspid aortic valve | 10 | 10 |
| IDCM | 10 | 0 |
| Diabetes mellitus | 0 | 0 |
| Atherosclerosis | 0 | 2 |
| VAD | 0 | 0 |
| COPD | 0 | 0 |
Values are mean ± SD. P > 0.05 is from Independent-Samples t Test. CAVD, calcific aortic valve disease; COPD, chronic obstructive pulmonary disease; IDMC, idiopathic dilated cardiomyopathy; VAD, ventricular assist device.
AVICs were isolated and cultured by using a described method (10). Briefly, valve leaflets were subjected to sequential digestions with collagenase. Cells were collected by centrifugation and cultured in M199 growth medium containing penicillin G, streptomycin, amphotericin B, and 10% (vol/vol) FBS. Cells from passages 3–6 were used for this study.
Cultures with ∼90% confluence were treated with LPS, Pam3CSK4, or oxLDL for 3 d. Cell lysate was used for the assessment of BMP-2 and ALP proteins. To examine the phosphorylation of ERK1/2 and NF-κB p65, and NF-κB DNA-binding activity, cells were treated for 1–8 h. To examine the formation of calcium deposits, cells were treated with LPS, Pam3CSK4, or oxLDL for 21 d in a conditioning medium (growth medium supplemented with 10 mmol/L β-glycerophosphate, 10 nmol/L vitamin D3, 10 nmol/L dexamethasone, and 8 mmol/L CaCl2).
Animal Models of Aortic Valve Lesions.
Twenty C57BL/6 male mice (WT, 3–5 mo old) and 20 IL-37 transgenic male mice (C57 BL/6 background, 3–4 mo old) were randomized, by drawing, to either vehicle (normal saline) or LPS group and were treated for 12 wk by using osmotic pumps. An additional 20 C57BL/6 mice and 20 IL-37 transgenic mice were randomized, by drawing, to regular diet or high fat diet (purchased from Research Diets; containing 24% fat, 41% carbohydrate, and 24% protein; 45% of kcal from fat) and observed for 16 wk. The sample size (10 animals in each group) was estimated with power analysis. Thicknesses of aortic valves were assessed by ultrasound, and then hematoxylin and eosin (H&E) staining at the end of the experiments. The tissue sections were examined by a blinded viewer. The experiments were approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver, and this investigation conforms to the Guide for the Care and Use of Laboratory Animals (National Research Council, revised 1996).
Statistical Analysis.
Data are presented as mean ± SE. Statistical analysis was performed by using StatView software (Abacus Concepts). ANOVA with the post hoc Bonferroni/Dunn test and t test were used to analyze differences between experimental groups, and differences were confirmed with Mann–Whitney U test. For time course data, two-way ANOVA was used to compare the difference between experimental groups at each time point. Statistical significance was defined as P < 0.05.
Materials and Additional Methods.
Information and associated references are available in SI Methods.
SI Methods
Materials.
Antibodies against BMP-2 (catalog no. ab14933 and ab6285) and bone ALP (catalog no. ab108337) were purchased from Abcam. Antibodies against β-actin (catalog no. 4967), phosphorylated ERK1/2 (catalog no. 9101), total ERK1/2 (catalog no. 9102), phosphorylated NF-κB p65 (Ser536; catalog no. 3033) and total NF-κB p65 (catalog no. 3034) were purchased from Cell Signaling. OxLDL (from human plasma, CuSO4-oxidized, endotoxin-free) was purchased from Biomedical Technologies. Antibody against mouse oxLDL (catalog no. ABIN1399812) was purchased from Antibody Online. Monoclonal neutralizing antibodies against human TLR2 (catalog no. IMG-416E, Clone: TL2.1) and TLR4 (catalog no. IMG-417E, Clone: HTA125) were purchased from Imgenex. Monoclonal neutralizing antibody against human lectin-like oxLDL receptor-1 (LOX-1) (catalog no. HM2138, Clone: amino acids 71–273) was purchased from HyCult Biotechnology. Recombinant human IL-37 (IL-37b) was produced by C.A.D.’s laboratory (45). IL-37 shRNA and scrambled shRNA were purchased from OriGene Technologies. Medium 199 was purchased from Lonza. ALZET miniosmotic pumps were purchased from DURECT. Specific NF-κB inhibitor BAY11-7082 was purchased from Enzo Life Sciences. Specific ERK1/2 inhibitor PD98059 was purchased from Cell Signaling. Pam3CSK4 was purchased from InvivoGen. LPS (Escherichia coli 0111:B4) and all other chemicals and reagents were purchased from Sigma-Aldrich Chemical.
Isolation of Murine AVICs.
We developed a method for isolation of the aortic valve interstitial cells from mice. After killing by cervical dislocation, aortic valve leaflets were collected under a microscope. Valve leaflets were pretreated with 1.0 mg/mL collagenase for 20 min to destroy endothelial cells. Small pieces of valve tissue were explanted on the surface of culture dishes to allow adherence. Then a small amount of M199 growth medium was added to cover the valve explants. The explants were cultured in an incubator. Migrated cells were grown to confluence and subcultured. We confirmed that the isolates are a mixture of fibroblasts and myofibroblasts by immunostaining of vimentin and α-smooth muscle actin. The phenotype of murine AVICs is comparable to that of human AVICs.
Cells were treated with oxLDL for 1–3 d. Cell lysate was used for the assessment of BMP-2 and ALP proteins.
Gene Knockdown.
Knockdown of IL-37 was carried out by lentiviral expression of IL-37 shRNA. IL-37 shRNA expression plasmids, scrambled shRNA control plasmids, and lentiviral packaging plasmids (pVSVG, pRSV-Rev, and pMDL) were amplified by using standard bacterial transformation technique and purified using HiSpeed Plasmid Midi Kit. Lentivirus that express IL-37 shRNA and scrambled shRNA were generated by Lipofectamine 2000 cotransfection of 293T-cells. After 48 h, lentiviral supernatants were collected and concentrated. Normal human AVICs were infected with lentivirus expressing IL37-shRNA or scrambled shRNA and cotransfected with TransDux transduction reagent. The expression of GFP was examined by a fluorescence microscope.
Alizarin Red S Staining.
Alizarin Red S staining for calcium deposits was performed as described (11). Briefly, cell monolayers were washed twice with PBS and fixed for 15 min in 4% paraformaldehyde, followed by incubation with 0.2% alizarin red solution (pH 4.2) for 30 min. Excessive dye was removed by washing with distilled water. Alizarin red staining was examined and photographed with a Nikon Eclipse TS100 microscope. To quantitatively analyze Alizarin Red stain, wells were rinsed with distilled water, and Alizarin red S stains were bleached with 10% acetic acid at 85 °C. Supernatant was spectrophotometrically analyzed at 450 nm (46).
Immunoblotting.
Immunoblotting was applied to analyze ICAM-1, BMP-2, ALP, IL-37, phosphorylated NF-κB p65, total NF-κB p65, SIGIRR, IL-18Rα, IRAK1, and β-actin. Cells were lysed in a sample buffer. Protein samples were separated on gradient (4–20%) minigels and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked with 5% nonfat dry milk solution for 1 h at room temperature. The blocked membranes were incubated with primary antibodies. After washing with TPBS (PBS containing 0.05% Tween 20), the membranes were incubated with a peroxidase-linked secondary antibody specific to the primary antibody. Following further washes, membranes were treated with enhanced chemiluminescence reagents. Then, the membrane was exposed on X-ray film. ImageJ (NIH) was used to measure the density of bands.
Real-Time RT-PCR.
Total RNA was extracted by using a Qiagen RNeasy Mini Kit. Reverse transcription (RT) and PCR were performed in triplicate by using iScriptTM cDNA Synthesis Kit (Bio-Rad) and iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s instructions. Amplification was for 40 cycles including denaturation at 94 °C for 10 s and annealing/extension at 59 °C for 30 s. The following primers were used to amplify specific cDNA fragments: human IL-37 (forward: 5′-CCC CAC CAT GAA TTT TGT TC-3′; reverse: 5′-CCT TTA GAG ACC CCC AGG AG-3′) (GenBank accession no. NM_0014439); GAPDH (forward: 5′-CAT GGC CTC CAA GGA GTA AG-3′; reverse: 5′-AGG GGT CTA CAT GGC AAC TG-3′). Human IL-37 mRNA levels were quantified by real-time PCR on the iQ 5 Multicolor Real-time PCR Detection system (Bio-Rad). Human IL-37 mRNA levels normalized to GAPDH mRAN were calculated by using the 2−ΔΔCT method.
Immunofluorescent Staining.
Immunofluorescent staining was applied to examine BMP-2 and oxLDL in mouse aortic valve. After permeabilization with a methanol/acetone mixture, tissue sections were fixed in 4% paraformaldehyde, incubated with a primary antibody (antibody against BMP-2 or oxLDL) overnight at 4 °C. After washing with PBS, sections were incubated with Cy3-tagged secondary antibody against corresponding primary antibody (imaged on the red channel). Nuclei were stained with bis-benzimide (DAPI, imaged on the blue channel), and glycoproteins on cell surfaces were stained with Alexa 488-tagged wheat germ agglutinin (imaged on the green channel). Microscopy was performed with a Leica DMRXA digital microscope (Leica Mikroskopie und Systeme) equipped with Slidebook software (I. I. I.).
Acknowledgments
This study was supported by National Institutes of Heart, Lung, and Blood Grants HL106582 (to X.M.) and HL121776 (to X.M.), National Institute of Allergy and Infectious Disease Grant AI15614 (to C.A.D.), and National Natural Sciences Foundation of China Grant 81570352 (to Q.Z.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619667114/-/DCSupplemental.
References
- 1.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111(24):3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 2.Rajamannan NM, et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124(16):1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathieu P, Bouchareb R, Boulanger M-C. Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res. 2015;2015:851945. doi: 10.1155/2015/851945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Choi J-H. Involvement of immune cell network in aortic valve Stenosis: Communication between valvular interstitial cells and immune cells. Immune Netw. 2016;16(1):26–32. doi: 10.4110/in.2016.16.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohler ER, 3rd, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8(3):254–260. [PubMed] [Google Scholar]
- 6.Kaden JJ, et al. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: A potential regulatory mechanism of valvular calcification. Int J Mol Med. 2005;16(5):869–872. [PubMed] [Google Scholar]
- 7.Yu Z, et al. Tumor necrosis factor-α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. 2011;337(1):16–23. doi: 10.1124/jpet.110.177915. [DOI] [PubMed] [Google Scholar]
- 8.Bertacco E, et al. Proteomic analysis of clonal interstitial aortic valve cells acquiring a pro-calcific profile. J Proteome Res. 2010;9(11):5913–5921. doi: 10.1021/pr100682g. [DOI] [PubMed] [Google Scholar]
- 9.López J, et al. Viral and bacterial patterns induce TLR-mediated sustained inflammation and calcification in aortic valve interstitial cells. Int J Cardiol. 2012;158(1):18–25. doi: 10.1016/j.ijcard.2010.12.089. [DOI] [PubMed] [Google Scholar]
- 10.Meng X, et al. Expression of functional Toll-like receptors 2 and 4 in human aortic valve interstitial cells: Potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294(1):C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, et al. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of Toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J Am Coll Cardiol. 2009;53(6):491–500. doi: 10.1016/j.jacc.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Q, et al. Notch1 promotes the pro-osteogenic response of human aortic valve interstitial cells via modulation of ERK1/2 and nuclear factor-κB activation. Arterioscler Thromb Vasc Biol. 2013;33(7):1580–1590. doi: 10.1161/ATVBAHA.112.300912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song R, et al. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via Toll-like receptor-2. Arterioscler Thromb Vasc Biol. 2012;32(11):2711–2720. doi: 10.1161/ATVBAHA.112.300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano K, et al. Detection of cariogenic Streptococcus mutans in extirpated heart valve and atheromatous plaque specimens. J Clin Microbiol. 2006;44(9):3313–3317. doi: 10.1128/JCM.00377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skowasch D, et al. Pathogen burden in degenerative aortic valves is associated with inflammatory and immune reactions. J Heart Valve Dis. 2009;18(4):411–417. [PubMed] [Google Scholar]
- 16.Cohen DJ, et al. Role of oral bacterial flora in calcific aortic stenosis: An animal model. Ann Thorac Surg. 2004;77(2):537–543. doi: 10.1016/S0003-4975(03)01454-1. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Q, et al. Cross-talk between the Toll-like receptor 4 and Notch1 pathways augments the inflammatory response in the interstitial cells of stenotic human aortic valves. Circulation. 2012;126(11) Suppl 1:S222–S230. doi: 10.1161/CIRCULATIONAHA.111.083675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capoulade R, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66(11):1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Novaro GM, Sachar R, Pearce GL, Sprecher DL, Griffin BP. Association between apolipoprotein E alleles and calcific valvular heart disease. Circulation. 2003;108(15):1804–1808. doi: 10.1161/01.CIR.0000097560.96431.3E. [DOI] [PubMed] [Google Scholar]
- 20.Cowell SJ, et al. Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 21.Drolet M-C, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol. 2006;47(4):850–855. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann B, et al. RAGE influences the development of aortic valve stenosis in mice on a high fat diet. Exp Gerontol. 2014;59:13–20. doi: 10.1016/j.exger.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Côté C, et al. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. 2008;94(9):1175–1180. doi: 10.1136/hrt.2007.125740. [DOI] [PubMed] [Google Scholar]
- 24.Mehrabi MR, et al. Accumulation of oxidized LDL in human semilunar valves correlates with coronary atherosclerosis. Cardiovasc Res. 2000;45(4):874–882. doi: 10.1016/s0008-6363(99)00389-2. [DOI] [PubMed] [Google Scholar]
- 25.Mohty D, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol. 2008;28(1):187–193. doi: 10.1161/ATVBAHA.107.154989. [DOI] [PubMed] [Google Scholar]
- 26.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19(5):1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 27.Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O’Brien KD. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90(2):844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 28.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185(2):219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Su X, et al. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem. 2011;286(14):12213–12220. doi: 10.1074/jbc.M110.214619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boraschi D, et al. IL-37: A new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22(3):127–147. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello CA, et al. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46(5):1067–1081. doi: 10.1002/eji.201545828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohler ER, 3rd, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103(11):1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 33.Nold MF, et al. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nold-Petry CA, et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16(4):354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- 35.McNamee EN, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coll-Miró M, et al. Beneficial effects of IL-37 after spinal cord injury in mice. Proc Natl Acad Sci USA. 2016;113(5):1411–1416. doi: 10.1073/pnas.1523212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis CJ, et al. Interleukin 37 expression in mice alters sleep responses to inflammatory agents and influenza virus infection. Neurobiol Sleep Circad Rhyth. 2017;3:1–9. doi: 10.1016/j.nbscr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasser G, et al. Expression of human interleukine-37 protects mouse heart against ischemic injury through suppression of monocyte chemoattractant protein-1-mediated mononuclear cell accumulation. Circulation. 2011;124:A8603. [Google Scholar]
- 39.Luo Y, et al. Suppression of antigen-specific adaptive immunity by IL-37 via induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA. 2014;111(42):15178–15183. doi: 10.1073/pnas.1416714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chávez-Sánchez L, et al. The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol. 2014;75(4):322–329. doi: 10.1016/j.humimm.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Miller YI. Toll-like receptors and atherosclerosis: Oxidized LDL as an endogenous Toll-like receptor ligand. Future Cardiol. 2005;1(6):785–792. doi: 10.2217/14796678.1.6.785. [DOI] [PubMed] [Google Scholar]
- 42.Csiszar A, et al. Regulation of bone morphogenetic protein-2 expression in endothelial cells: Role of nuclear factor-kappaB activation by tumor necrosis factor-α, H2O2, and high intravascular pressure. Circulation. 2005;111(18):2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-γ production. Cytokine. 2002;18(2):61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 44.Pan G, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13(1):1–7. doi: 10.1006/cyto.2000.0799. [DOI] [PubMed] [Google Scholar]
- 45.Li S, et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc Natl Acad Sci USA. 2015;112(8):2497–2502. doi: 10.1073/pnas.1424626112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowan CM, et al. NELL-1 increases pre-osteoblast mineralization using both phosphate transporter Pit1 and Pit2. Biochem Biophys Res Commun. 2012;422(3):351–357. doi: 10.1016/j.bbrc.2012.04.077. [DOI] [PubMed] [Google Scholar]