Significance
Rett syndrome is a neurodevelopmental disorder in girls who are heterozygous for a mutation in the X-linked gene MeCP2. Because cells in these individuals will be missing MeCP2 function only when the wild-type copy of the gene is on the inactive X, reactivation of the silenced copy of MeCP2 presents a potential therapeutic strategy. To identify genes that silence MeCP2 on the inactive X and that could therefore prove valuable as therapeutic targets, we carried out a screen for genes whose down-regulation reactivated a MeCP2 reporter on the inactive X. The 30 genes we have identified reveal a genetic circuitry required for maintenance of X-chromosome inactivation in differentiated cells and a large number of targets suitable for pharmacologic intervention.
Keywords: XIST, X inactivation, MeCP2, Rett syndrome, BMP/TGF-β
Abstract
Rett syndrome (RS) is a debilitating neurological disorder affecting mostly girls with heterozygous mutations in the gene encoding the methyl-CpG–binding protein MeCP2 on the X chromosome. Because restoration of MeCP2 expression in a mouse model reverses neurologic deficits in adult animals, reactivation of the wild-type copy of MeCP2 on the inactive X chromosome (Xi) presents a therapeutic opportunity in RS. To identify genes involved in MeCP2 silencing, we screened a library of 60,000 shRNAs using a cell line with a MeCP2 reporter on the Xi and found 30 genes clustered in seven functional groups. More than half encoded proteins with known enzymatic activity, and six were members of the bone morphogenetic protein (BMP)/TGF-β pathway. shRNAs directed against each of these six genes down-regulated X-inactive specific transcript (XIST), a key player in X-chromosome inactivation that encodes an RNA that coats the silent X chromosome, and modulation of regulators of this pathway both in cell culture and in mice demonstrated robust regulation of XIST. Moreover, we show that Rnf12, an X-encoded ubiquitin ligase important for initiation of X-chromosome inactivation and XIST transcription in ES cells, also plays a role in maintenance of the inactive state through regulation of BMP/TGF-β signaling. Our results identify pharmacologically suitable targets for reactivation of MeCP2 on the Xi and a genetic circuitry that maintains XIST expression and X-chromosome inactivation in differentiated cells.
X-chromosome inactivation (XCI) is a dosage-compensation phenomenon in which one of the two X chromosomes in female cells becomes transcriptionally silent. This process assures that the ratio of X to autosome expression in females mimics that in males, who have only a single X chromosome. One of the two X chromosomes is inactivated during the random XCI stage after implantation, and that X chromosome remains stably inactivated (1, 2). One of the initiating events in XCI is random expression of XIST (X-inactive specific transcript) from the X chromosome to be inactivated. XIST is a noncoding RNA that coats that chromosome in cis, recruits additional epigenetic modifiers, and renders it transcriptionally inactive. As a result, in any female cell, only one of two alleles on the X chromosome is transcriptionally active.
When an X-linked disease gene is heterozygous, it can be critically important whether the wild-type allele is on the active or inactive X: When the mutant allele is on the active X, transcriptional inactivation of the remaining wild-type allele can cause a complete loss of gene function. However, the resulting disease state could potentially be ameliorated by reactivation of the wild-type allele on the inactive X chromosome (Xi). Several neurodevelopmental disorders in females are caused by heterozygous mutations of X-linked genes including MeCP2 (3), DDX3X (4), KIAA2022 (5), USP9X (6), CDKL5 (7), HDAC8 (8), and SMC1A (9). Rett syndrome, one of the most common hereditary forms of severe cognitive impairment in girls, is caused by a heterozygous mutation in the MeCP2 gene (3), which encodes a methyl-DNA–binding protein essential for normal development and function of the neurons (10). Because restoration of MeCP2 expression in a mouse model for Rett syndrome reverses neurologic deficits, both in young and adult animals (11), reactivation of the wild-type MeCP2 allele from the inactive X presents a potential therapeutic strategy.
Here we report results of a large-scale genetic screen for regulators of MeCP2 on the Xi. We generated a cell line harboring a MeCP2-linked reporter on the Xi, using a transgenic mouse we described previously (12), and exposed it to a library of 60,000 different small hairpin RNAs (shRNAs). We identified 30 genes whose down-regulation led to reactivation of the silent MeCP2 on the Xi. These genes fell into several functional groups, including mitotic kinases, PI3K/AKT signaling, chromatin components, sister chromatid cohesion, acyl-pool biosynthesis, and bone morphogenetic protein (BMP)/TGF-β signaling pathway. We found that the BMP signaling pathway is required for maintenance of XIST expression. Furthermore, we show that Rnf12, a known regulator of BMP signaling (13), modulates XIST expression in adult cells, suggesting that the role of Rnf12 as a regulator of XIST during initiation of X-chromosome inactivation in ES cells (14, 15) is retained in differentiated cells through control of BMP signaling.
Results
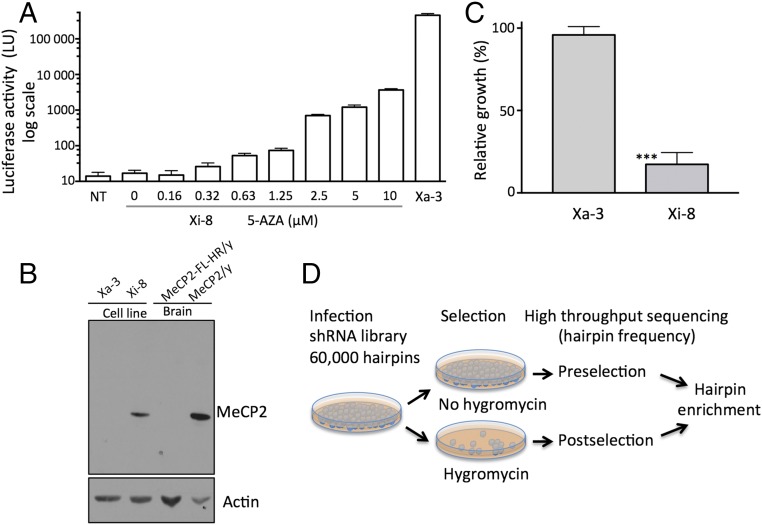
We previously described a transgenic mouse model for monitoring MeCP2 expression with a fusion of the MeCP2 gene with firefly luciferase and hygromycin resistance genes (MeCP2-FL-HR) (12). Whereas the fusion protein proved unstable, resulting in a MeCP2 loss-of-function phenotype in mice, the reporter expression pattern paralleled the expression of the native MeCP2 gene (12). To develop clonal cell lines that have the transgene on either the active (Xa) or inactive X chromosome, we immortalized tail tendon fibroblasts from heterozygous MeCP2-FL-HR/MeCP2 female mice. One cell line with the reporter gene on the Xi (Xi-8) and one with the reporter gene on the Xa (Xa-3) were chosen for further studies. Xi-8 cells had no detectable luciferase activity (Fig. 1A) but expressed the wild-type MeCP2 (Fig. 1B), whereas Xa-3 exhibited the opposite properties. Based on comparison of the luciferase activity in the two cell lines and the known sensitivity of our luciferase assay, we estimated that the expression of the reporter gene on the Xi was ∼100,000-fold lower than the expression on the Xa. Treatment with the DNA-hypomethylating agent 5-azacytidine (5-AZA) (16, 17) led to a dose-dependent reactivation of the MeCP2-FL-HR reporter in Xi-8 cells, indicating that this cell line would be suitable for identifying other regulators of MeCP2 silencing. Of note, the highest level of luciferase reactivation achieved in Xi-8 cells treated with 5-AZA was ∼1% of the activity of Xa-3 cells (Fig. 1A).
Fig. 1.
Characterization of the cell lines with the FL-HR reporter on the inactive and active X chromosome. (A) Luciferase activity of cells with the reporter on the inactive X (Xi-8) with varying concentrations of 5-AZA, and the reporter on the active X (Xa-3). All measurements were performed in triplicate; error bars indicate SD. LU, light unit; NT, vehicle treated. (B) Western blot showing the presence or absence of the WT copy of MeCP2 in Xi-8 and Xa-3 cells. Brain lysates from 4-wk-old MeCP2/Y and MeCP2-FL-HR/y animals were included as controls. (C) Hygromycin B resistance of cells with the reporter on the active (Xa-3) and inactive (Xi-8) X chromosome. Cells were subjected to hygromycin B at a similar dose and duration (20 µg/mL for 6 d) as was used in the screen, and growth was normalized to untreated controls. Error bars indicate SD; n = 3, P < 0.001. (D) Screen design.
Consistent with the pattern of luciferase expression in Xa-3 cells, the HR gene expression from the same cassette conferred resistance to hygromycin compared with Xi-8 cells (Fig. 1C). Reasoning that reactivation of the silent HR in Xi-8 cells would promote hygromycin resistance, we used hygromycin selection in our screening approach, as outlined in Fig. 1D. We infected Xi-8 cells with a library containing 60,000 shRNAs targeting 25,000 different genes. We immediately harvested DNA from half of the infected cell pool, and subjected the other half to hygromycin selection. After the latter was completed and the respective DNA was extracted, we amplified hairpin DNA from both pools and subjected it to high-throughput sequencing. The frequency of hairpins in the pre- and postselection specimens was compared with the calculated enrichment for each hairpin (Dataset S1). To reduce the false positive rate, we performed the screen in quadruplicate. The most enriched hairpins in all of the screens were four hairpins targeting XIST, with enrichment varying from 2.4- to 220-fold in different screens (Table S1). Identification of the four hairpins targeting XIST as the most enriched hairpins validated our screening strategy for identifying genes involved in silencing of MeCP2 on the Xi.
Table S1.
Enrichment for XIST-specific hairpins
| XIST hairpin, 5′–3′ | Screen 1 | Screen 2 | Screen 3 | Screen 4 |
| CTGCTAGTTTCCCAATGAT | 33.7 | 5.7 | 57.2 | 185.0 |
| CCAACTGTCTGCTTAAGAA | 20.2 | 2.2 | 77.6 | 254.2 |
| CCAGAACTCTGGAAGGATA | 11.4 | 0.4 | 20.4 | 70.0 |
| CCGTTCCATTCCTTTGTAT | 10.9 | 0.3 | 63.4 | 227.5 |
Boldfacing indicates greater than twofold enrichment.
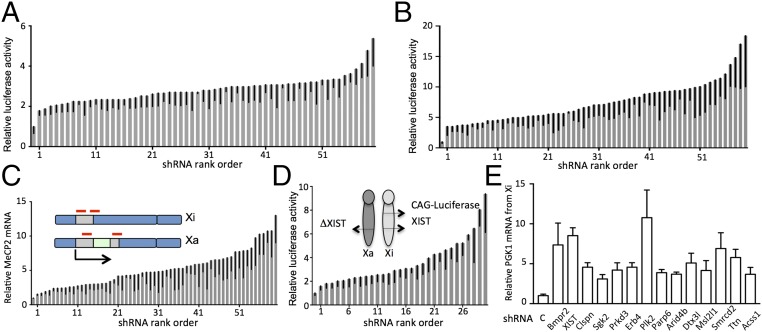
We next tested the selected hairpins for down-regulation of their intended target genes and activation of the luciferase reporter. We used several criteria for choosing enriched hairpins for further analysis, including enrichment in at least two of the four screens, the magnitude of the enrichment, and the presence of multiple hairpins targeting the same gene. In instances where a gene was targeted with a single hairpin, we separately tested additional hairpins targeting the same gene. This approach confirmed reactivation of the luciferase reporter for the 30 target genes listed in Table 1. These could be classified in several functional categories, including the TGF-β family, PI3K/AKT signaling pathway, chromatin-modifying enzymes and components, sister chromatid cohesion, mitotic kinases, and acyl donor synthesis enzymes (Table 1). The magnitude of activation with individual hairpins ranged from 1.8- to 5.4-fold, with a median of 2.8-fold (Fig. 2A and Dataset S2). The modest level of reactivation with down-regulation of a single target was consistent with results in other screens for X-chromosome reactivation (18–21), indicating that several separate mechanisms are necessary for the tight transcriptional repression of the MeCP2 locus. To determine whether we could increase the level of reactivation by inactivating multiple targets, we combined shRNAs with 5-AZA in a dose that by itself did not cause appreciable reporter reactivation. The level of luciferase activity using this combination was 3.2- to 18.4-fold higher than untreated, with a median of 7.1-fold, which was significantly increased in comparison with activation obtained with hairpins alone [Fig. 2B (note a different scale in Fig. 2A) and Dataset S2].
Table 1.
Validated hits
| Functional group | Gene | Functional group | Gene |
| TGF-ß superfamily | Acvr1 | Chromatin components | Dnmt1 |
| Acvr1b | Hdac3 | ||
| Bmpr2 | Parp6 | ||
| Smad2 | Arid4b | ||
| Snip1 | Dtx3l | ||
| Zfyve9 | Msl2l1 | ||
| Smarcd2 | |||
| Titin | |||
| Signaling kinases | Pi3kcb | DNA replication | Gins3 |
| Pdpk1 | Claspin | ||
| Sgk2 | Mcm8 | ||
| Prkd3 | Brca2 | ||
| Erb4 | |||
| Acyl donor synthesis | Acss1 | Mitotic kinases | Aurka |
| Acaca | Plk2 | ||
| Cohesion | Rad21 | Other | XIST |
| Nipbl1 |
Boldfacing indicates genes that have not previously been associated with XCI.
Fig. 2.
Reactivation of the Xi induced by shRNAs. (A) Firefly luciferase activity induced by shRNAs in Xi-8 cells normalized to shControl. For each graph in A–D a control is plotted followed by an average of three measurements for each shRNA in ascending order, with SD indicated by error bars. P < 0.05 for all hairpins; individual measurements for each shRNA are provided in Dataset S2. Rank order of shRNAs is provided on the x axis to facilitate identification of individual hairpins from Dataset S2 in the graphs. (B) Firefly luciferase activity induced by shRNAs in Xi-8 cells in combination with 0.2 µM 5-AZA normalized to the control shRNA in combination with 0.2 µM 5-AZA. At this concentration, the treatment of 5-AZA did not induce a measurable increase in luciferase activity in shControls. (C) Reactivation of the native MeCP2 gene on the inactive X in Xa-3 cells by shRNAs in combination with 0.2 µM 5-AZA. RT-qPCR of MeCP2 mRNA in Xa-3 cells containing different shRNAs in combination with 0.2 µM 5-AZA normalized to shControls in combination with 0.2 µM 5-AZA, measured using the primers indicated by red lines. (D) Reactivation of the CMV-luciferase reporter at the Hprt locus on the inactive X by shRNAs in combination with 0.2 µM 5-AZA. Firefly luciferase activity induced by shRNAs in CMV-FL cells in combination with 0.2 µM 5-AZA normalized to control shRNA in combination with 0.2 µM 5-AZA. (E) Allele-specific RT-PCR for the PGK1 gene on the Xi in M. spretus/M. musculus hybrid Patski cell line demonstrates reactivation using a combination of shRNA and 0.2 µM 5-AZA. The knockout efficiency expressed as the fraction remaining was as follows: Bmpr2, 0.33; XIST, 0.24; Clspn, 0.26; Sgk2, 0.19; Prkd3, 0.28; Erb4, 0.32; Plk2, 0.23; Parp6, 0.19; Arid4b, 0.50; Dtx3l, 0.37; Msl2l1, 0.25; Smrcd2, 0.24; Ttn, 0.42; and Acss1, 0.27. Expression levels in shRNA-containing cells treated with 0.2 µM 5-AZA are compared with shControl cells treated with 0.2 µM 5-AZA (P < 0.05 for all hairpins), error bars indicate SD.
To determine whether the same hairpins could reactivate the wild-type MeCP2 allele and not just the reporter, we used Xa-3 cells, where the wild-type copy of MeCP2 was on the Xi, and applied quantitative (q)PCR primers designed to span the MeCP2 stop codon and allow only amplification of wild-type MeCP2 but not the fused gene (Fig. 2C). We confirmed that hairpins for each of the genes combined with a low dose of 5-AZA reactivated the native MeCP2 allele, with the magnitude of reactivation similar to the one in the luciferase assay (Fig. 2C and Dataset S2).
Four of the 30 genes identified in our screen, Acvr1, Aurka, Dnmt1, and Pdpk1, were previously identified in a screen using a CMV-driven GFP reporter on the Xi (22). Moreover, these four genes were targeted with the same hairpins in the two screens, as both screens used the same shRNA library. Given that the methods for detection of reactivation in the two screens were vastly different (hygromycin selection vs. GFP fluorescence), identification of the same hairpins in these two independent screens provides strong cross-validation of these targets and of our screening strategy. However, this finding suggested that inactivation of at least these four genes not only reactivated MeCP2 but also other genes on the Xi. To determine whether the genes in our screen reactivated other genes on the X chromosome, we used a mouse cell line carrying the CMV-luciferase reporter inserted on the Xi (18). We observed that, when combined with low-dose 5-AZA, each of the hairpins that reactivated the MeCP2 reporter also reactivated the CMV-luciferase reporter locus on the Xi (18) (Fig. 2D and Dataset S2), indicating that the hits we have identified are not selective for reactivation of the MeCP2 locus but can instead derepress transcription at other loci on the Xi. We further tested reactivation of a PGK1 gene on the Xi using allele-specific PCR in clonal Mus musculus/Mus spretus hybrid Patski cells (23) focusing on 14 genes that were first linked to XCI in this study. We found that a combination of 5-AZA and shRNA targeting each of these genes led to reactivation of the PGK1 allele on the Xi (Fig. 2E). We conclude that the genes we have identified in our screen repress transcription of other endogenous genes on the Xi.
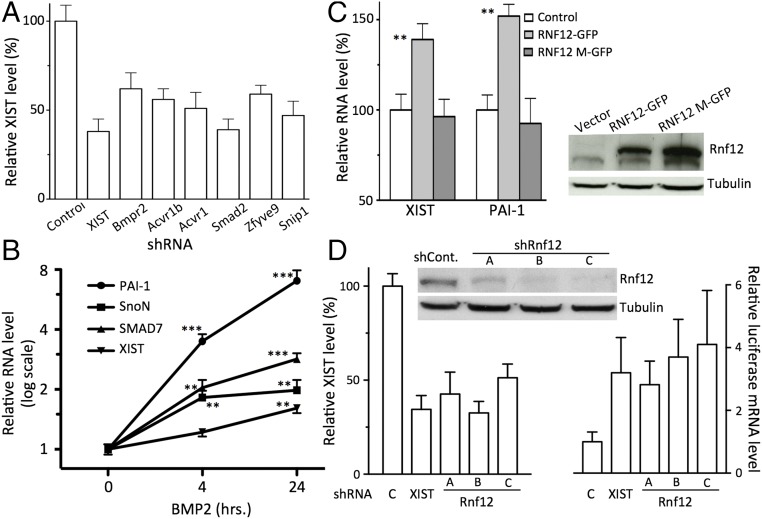
Given that XIST was a prominent hit in our screen, we next tested whether other genes we identified might repress transcription on the Xi by activating XIST. Such XIST modulators [e.g., protein kinase PDPK1 and activin receptor A, type I (ACVR1)] have recently been found among factors that are required for XCI by Bhatnagar et al. (22). When we measured XIST levels in Xi-8 cells expressing shRNAs from our screen (Dataset S2), we found that the knockdown of BMP/TGF-β superfamily members induces a robust and consistent down-regulation of XIST (Fig. 3A). Whereas knockdown of several members of the PI3K/AKT group exhibited modestly reduced XIST levels (Dataset S2), as was previously observed for PDPK1 (22), the magnitude of the effect was smaller relative to the TGF-β pathway. Given the robust effect on XIST levels among all members of the TGF-β superfamily, we focused our attention on this pathway.
Fig. 3.
Effect of shRNAs for genes in the BMP/TGF-β group and Rnf12 on XIST levels. (A) shRNAs targeting members of the BMP/TGF-β pathway down-regulate XIST levels. RT-qPCR measurements for XIST normalized to the XIST level in shControl; n = 3; error bars indicate SD. P < 0.05 for each shRNA. (B) BMP2 treatment induces XIST and three known BMP target genes. Cells were treated with BMT2 (50 ng/mL) for the indicated time, and levels of XIST and mRNA for PAI-1, SnoN, and Smad7 were measured using RT-qPCR and normalized to cells treated with vehicle (n = 3, error bars indicate SD, **P < 0.01, ***P < 0.001). (C, Left) Overexpression of WT, but not catalytically inactive Rnf12 (Rnf12-M), induces XIST (n = 3, error bars indicate SD, **P < 0.01). (C, Right) Western blot with Rnf12 and Rnf12-GFP in Xi-8 cells. (D) shRNAs targeting Rnf12 (Left) decrease XIST levels (n = 3, error bars indicate SD, P < 0.01) and (Right) activate the luciferase reporter in Xi8 cells. (n = 3, error bars indicate SD, P < 0.01 for each comparison). (D, Top) Western blot for Rnf12.
The TGF-β superfamily signaling pathway was the largest class of targets after chromatin-modifying enzymes that we identified in our screen. The TGF-β superfamily is composed of cell signaling proteins, including BMPs and TGF-β, which play important roles in development and morphogenesis (24). Upon binding to their respective receptors on the cell surface, members of the TGF-β superfamily activate receptors’ tyrosine kinase activity, leading to phosphorylation and activation of receptor-regulated SMADs (R-SMADs), intracellular signaling proteins that form trimeric complexes and act as transcription factors in the nucleus (24). Our screen identified different components of the TGF-β signaling pathways, including receptors (e.g., Bmpr2) and downstream signaling molecules (e.g., Smad2). We found that down-regulation of each of the targets in the TGF-β superfamily functional group down-regulates XIST expression (Fig. 3A). For example, inactivation of Acvr1 and Bmpr2, which both serve as receptors for the BMP ligand family (25), inhibited XIST expression, suggesting that BMPs up-regulate XIST. To confirm this observation, we treated Xi-8 cells with exogenous BMP2 and examined transcript levels of XIST and several known BMP2 target genes, including PAI-1, Smad7, and SnoN. Both Smad7 and SnoN are known inhibitors of TGF-β/BMP signaling, and their activation by BMP/TGF-β acts to prevent excessive activation of these pathways. BMP2 caused a time-dependent increase in the mRNA level of all of the aforementioned genes—PAI-1, Smad7, SnoN, and XIST (Fig. 3B). Taken together, these results demonstrate that BMP signaling indeed promotes XIST expression.
Among the key regulators of TGF-β and BMP pathways is Smad7, whose binding to the BMP receptors prevents access of R-SMAD and inhibits their phosphorylation and activation. Smad7 itself is subjected to proteolysis-based regulation by the ubiquitin ligase Rnf12, which acts as an indirect activator of BMP and TGF-β signaling (13, 26). Given the known function of Rnf12 as a regulator of XIST expression during embryonic development (14, 27), we assessed whether Rnf12 might also be involved in maintenance of the Xi in adult cells by regulating BMP/TGF-β signaling. We observed that Rnf12 overexpression in Xi-8 cells increased the expression of PAI-1 (Fig. 3C), a known BMP transcriptional target, similar to the effect of exogenous BMP2 on Xi-8 cells (Fig. 3B). The level of XIST was also increased in cells overexpressing wild-type Rnf12 but not the mutant without catalytic activity (Fig. 3C). Conversely, we found that Rnf12 down-regulation reduced XIST expression and led to activation of the luciferase reporter gene in Xi-8 cells (Fig. 3D). These results suggest that the role of Rnf12 as a regulator of XIST expression during XCI in ES cells might be retained in adult cells through modulation of BMP signaling and maintenance of XIST expression.
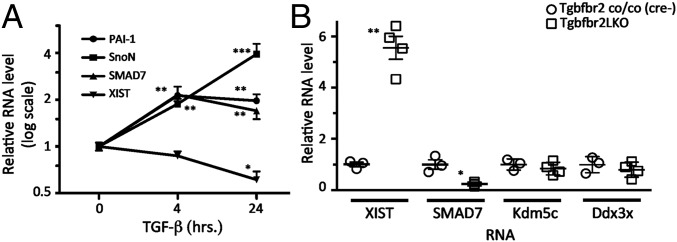
After demonstrating that BMP2 promotes XIST expression, we tested whether a different member of the superfamily, TGF-β, also has a similar role. BMP and TGF-β signaling pathways have been previously shown to act antagonistically in several biological contexts (28) and cell types (29). In contrast, several of the transcriptional targets, particularly those related to negative feedback control such as Smad7 or SnoN, are activated by both TGF-β and BMP signaling. Upon exposure of Xi-8 cells to TGF-β1, we observed a dose-dependent activation of PAI-1, Smad7, and SnoN, similar to cells treated with BMP2. However, in contrast to the up-regulation of XIST observed with BMP2, we found that TGF-β1 down-regulated XIST (Fig. 4A), suggesting that the two pathways are antagonistic with respect to XIST regulation.
Fig. 4.
Effect of TGF-β signaling on XIST in Xi-8 cells and in mice. (A) TGF-β down-regulates XIST, whereas inducing three known TGF-β targets in Xi-8 cells. Cells were treated with TGF-β1 (5 ng/mL) for the indicated times, and levels of XIST and mRNA for PAI-1, SnoN, and Smad7 were measured by RT-qPCR. Error bars indicate SD. All measurements were carried out in quadruplicate. *P < 0.05, **P < 0.01, ***P < 0.001. (B) Albumin-Cre–mediated excision of Tgfbr2 in female mouse livers induces XIST and represses Smad7. Mice with liver-specific knockout of Tgfbr2 (Tgfbr2LKO) were generated by albumin-Cre–mediated deletion of the floxed Tgfbr2 gene (Tgfbr2 co/co). Cre-Tgfbr2 (co/co) littermate controls have an intact Tbfbr2. XIST RNA, Smad7, Kdmc5, and Ddx3x mRNA were measured by RT-qPCR. The levels of XIST, Smad7, Kdmc5, and Ddx3x mRNA in Cre-Tgfbr2 (co/co) animals were set to 1. *P < 0.05, **P < 0.01. Error bars indicate SD.
Because the effects of the TGF-β superfamily are often cell type- and context-specific, we next tested whether dysregulation of TGF-β signaling might have consequences for XIST expression in vivo. We used previously described animals carrying a liver-specific knockout of TGF-β receptor 2 (Tgfbr2LKO) (30). Tgfbr2 is an obligatory component of the TGF-β receptor complex essential for signaling by TGF-β but not by BMP ligands (31). We compared the expression of XIST in mice carrying a TGFBR2LKO (30) with the expression level of XIST in their littermates with the intact Tgfbr2. Livers from animals lacking Tgfbr2 exhibited 4.2- to 6.3-fold up-regulation of XIST compared with livers from animals with the intact Tgfbr2 (Fig. 4B), demonstrating that modulation of TGF-β signaling regulates expression of XIST in vivo. Whereas the majority of genes on the Xi are transcriptionally inactive, a fraction of genes on the Xi are not subjected to XIST-mediated silencing and remain transcriptionally active (23). To determine whether increased expression of XIST induced by Tgfbr2 KO might induce their repression, we compared the expression level of two of the “escaper” genes, Kdm5c and Ddx3x, in livers from animals with and without Tgfbr2 (Fig. 4B). Because we did not find a significant difference in the Kdm5c or Ddx3x expression level in livers with or without Tgfbr2 KO, we conclude that an increased level of XIST induced by Tgfbr2 KO does not repress genes that are normally active on the Xi.
Discussion
The observation that restoration of MeCP2 expression reverses neurologic deficits in the mouse model of Rett syndrome (11) creates a compelling case for identifying therapeutic agents that can reactivate wild-type MeCP2 on the inactive X chromosome. To this end, we performed a large shRNA screen and found 30 genes, from seven different functional categories, whose down-regulation reactivated the MeCP2 reporter on the Xi. One of these groups represented members of the BMP/TGF-β signaling pathway, which were not previously linked to XCI. We demonstrate that these genes regulate XIST in tissue culture and that modulation of the TGF-β pathway regulates XIST levels in adult mice, providing a novel mechanism for maintenance of X-chromosome inactivation in differentiated cells.
Four observations served to validate the results of our screen. First, the most prominent hit was XIST, with all four shRNAs in our library appearing among the most enriched shRNAs in all four replicates of our screen. Second, the largest functional group we identified consisted of eight genes involved in chromatin structure and regulation, including Dnmt1 and HDAC3, which had previously been linked to XCI (19, 32). Third, our screen identified 4 of the 13 genes found by Bhatnagar et al. (22) in a screen that used a fundamentally different reporter to identify Xi reactivators, namely GFP driven by the CMV promoter. Finally, our functional screen identified factors involved in sister chromatid cohesion (SCC), whose components were independently identified in a biochemical screen as XIST-interacting factors (20). Taken together, these observations gave us high confidence that the 30 genes identified in our screen are indeed involved in XCI.
The second largest functional group identified in our screen, after genes involved in chromatin regulation, was composed of six genes in the BMP/TGF-β signaling pathway. This result suggested an entirely novel function for this pathway, namely maintenance of the repressed state of the inactive X. Because XIST was the most prominent hit in our screen, we reasoned that if the BMP/TGF-β pathway functioned in the maintenance of XCI, it might do so by regulating levels of XIST. Consistent with this expectation, shRNA-mediated down-regulation of six genes in this group down-regulated XIST. Furthermore, modulating levels of Rnf12, an X-encoded ubiquitin ligase that promotes BMP/TGF-β signaling by degrading Smad7 (13), a repressor of this pathway, had the predicted effects: Overexpression of Rnf12 increased whereas down-regulation of Rnf12 using shRNA decreased levels of XIST.
The involvement of Rnf12 in maintenance of XCI via the BMP/TGF-β pathway is particularly intriguing, because Rnf12 has a known role in initiation of XCI that is independent of TGF-β: During ES cell differentiation, Rnf12 induces XIST expression by promoting ubiquitylation and degradation of REX1, a pluripotency factor that represses XIST transcription in ES cells (14). Whereas REX1 is no longer expressed in differentiated cells, our results indicate that Rnf12 has retained its role as an XIST activator in differentiated cells through augmentation of BMP signaling. Thus, even though the targets differ in the two contexts, REX1 and Smad7 in ES cells and differentiated cells, respectively, in both situations Rnf12 serves as an X-encoded activator of XIST expression and of XCI by promoting degradation of an autosome-encoded negative regulator of XIST, which might balance autosome-to-X chromosome expression. Together, these results implicate Rnf12 and the BMP signaling pathway in balancing the ratio of autosomes to active the X chromosome in already-differentiated cells.
Even though further work is required to elucidate the exact mechanism by which BMP increases XIST expression, our analysis of published chromatin immunoprecipitation data (33) suggests that the effect of BMP signaling on XIST transcription might be direct, as indicated by the presence of SMAD1, a transcriptional effector of BMP signaling at the XIST-regulatory regions, adjacent to the binding sites of YY1. YY1 serves as an activator of XIST expression during initiation, establishment, and the maintenance phase of XCI (34). Overlapping binding sites for SMAD1 and YY1 have also been identified in the regulatory regions of histone genes, specifically in differentiated cells (33). Furthermore, YY1 and SMAD1 jointly regulate expression of genes during cardiac development (35). Based on these considerations, we suggest that BMP activates XIST expression by promoting YY1-mediated activation of XIST.
Although DNA replication factors and acyl-pool biosynthesis enzymes have not been previously associated with XCI, studies in model organisms implicate both functional groups in faithful propagation of chromatin states. Mutations in DNA replication factors lead to defects in position-effect variegation in flies (36–38) and silencing defects at telomeres and silent mating loci in yeast (39–41). Likewise, perturbations of cellular acyl pools in yeast by genetic inactivation orthologs of Acaca (42) and Acss1 (43) cause global alterations in histone acetylation and genome-wide expression. Identification of DNA replication factors and acyl-pool biosynthesis enzymes in our screen suggests that maintenance of XCI, similar to maintenance of heterochromatin states in other organisms, can be perturbed by defects in DNA replication and changes in availability of acyl donors.
Two major findings of our study raise the prospects of MeCP2 reactivation as a therapeutic strategy. First, approximately two-thirds of the genes (19/30) identified in our screen encode for proteins with known enzymatic activity, highly suitable targets for pharmacologic intervention. Second, we have identified BMP as an inducer of XIST expression, suggesting a pharmacologic strategy for down-regulation of XIST through inhibition of BMP receptor signaling. The BMP receptors, including those identified in our screens, Bmpr2 and Acvr1, have associated tyrosine kinase activity, and small-molecule inhibitors targeting these kinases are available (44, 45). Other kinases have also been identified in the screens: (i) PI3-kinase signaling network, including receptor-associated tyrosine kinase (Erb4), proximal kinases (e.g., PI3K and PDPK1), and a distal member (SGK2); (ii) protein kinase C member PRKD3; and (iii) mitotic kinases PLK2 and Aurka. Consistent with the idea that pharmacologic inhibition of kinase signaling networks identified in our screen can reactivate the Xi, work by other groups has demonstrated reactivation of Xi genes by small-molecule inhibitors of PDPK1 and PI3K (22). Furthermore, Aurora kinase inhibitors have been identified in small-molecule screens for Xi reactivation (22, 46). Given the success of tyrosine kinase inhibitors in different pathologic conditions, this class of agents could be used for therapeutic reactivation of genes on the Xi.
Our results also highlight potential therapeutic challenges. First, despite identification of the large numbers of genes that are required for maintenance of the silent state of MeCP2 on the Xi, the level of reactivation upon inactivation any single target was low. It is unclear what level of reactivation is required to provide a therapeutic benefit, and it is possible that even low rates would suffice. Furthermore, our observation that combining 5-AZA with inactivation of specific genes led to more potent reactivation than either strategy alone suggests that combination therapies might be effective. Second, even though the use of a reporter gene driven by the native MeCP2 promoter in our screen provided an opportunity to identify targets that reactivate only MeCP2, leaving expression of other genes on the Xi unaltered, all of the 30 genes identified in the screen reactivated a CMV-driven reporter gene located at a different locus. It is likely that a strategy that effectively reactivates MeCP2 will, at least to some extent, reactivate other loci on the Xi. Even though undesirable side effects resulting from such a nonselective reactivation of the Xi might hinder use of MeCP2 reactivation for therapeutic purposes, a recent study demonstrating grossly normal organogenesis of XIST-deficient female mice suggests that partial deficiency in dosage compensation is surprisingly well tolerated and compatible with both cellular and organismal viability (22, 47).
Materials and Methods
Generation of cell lines, Western blotting, firefly luciferase assays, and allele-specific PCR were carried out using standard techniques. Mice were maintained and cared for using protocols approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. Complete experimental details and the sequences of the hairpins (Table S2) and the primers used in the study (Table S3) are in SI Materials and Methods.
Table S2.
shRNA information
| Gene | shRNA no. | Open Biosystems or pLKOl vector | Targeting sequence, 5′–3′ |
| Acaca | 1 | V2LMM_159091 | CAGCACTCCCGATTCATAA |
| Acaca | 2 | pLKO | CCACAGGATCTGTATGAGAAA |
| Acss1 | 1 | V2LMM_22738 | CTCATGATATCAAAGGAGA |
| Acss1 | 2 | PLKO | GCCAATACACTGAAGAGACAT |
| Acvr1 | 1 | V2LMM_75565 | CCCATTTGCACATAGAGAT |
| Acvr1 | 2 | V2LMM_76215 | CGAACATTAGGAATTGTTT |
| Acvr1b | 1 | V2LMM_171666 | CCAATTTACTGGCCATTGA |
| Acvr1b | 2 | pLKO | GTGACCATTGAGGGAATGATT |
| Arid4B | 1 | V2LMM_191884 | GAAGCCTGATGCTGTATTA |
| Arid4B | 2 | pLKO | CGCCTTCTTCAGTCACTATAA |
| Aurka | 1 | V2LMM_71909 | CTCATTTCAAGACTGTTAA |
| Aurka | 2 | V2LMM_188488 | GAGTTTATCTGATTCTAGA |
| Bmpr2 | 1 | V2LMM_16207 | CTTGCTTTATCCACTCATA |
| Bmpr2 | 2 | pLKO | TTCAAGCTTATGGAGTGAAAT |
| Brca2 | 1 | V2LMM_81503 | GTCATTATCAGAACTGAAA |
| Brca2 | 2 | V2LMM_73750 | GTCAGTTTAACAAAGATTT |
| Claspin | 1 | V2LMM_89053 | GCAGCAAAGGACTCATCTA |
| Claspin | 2 | pLKO | GCAGACCAGTAGAATTTAATA |
| Dnmt1 | 1 | V2LMM_46797 | CCTTTACTTTCAACATCAA |
| Dnmt1 | 2 | pLKO | ATCTATGGAAGGTGGTATTAA |
| Dtx3l | 1 | V2LMM_132377 | CAAGTTTGCCGACAACTTT |
| Dtx3l | 2 | V2LMM_211086 | GCACCATTGTGATTAATTA |
| Erbb4 | 1 | V2LMM_37720 | GAGCATTCTGTGTTAAGAA |
| Erbb4 | 2 | pLKO | GTACCGAGCCTTGCGCAAATA |
| Gins3 | 1 | V2LMM_70812 | CAGATAACACAGTTGTTTA |
| Gins3 | 2 | pLKO | AGAGGGATGGAGGACTGTATT |
| Hdac3 | 1 | V2LMM_71142 | CATATGTGGTTCTAGAATT |
| Hdac3 | 2 | pLKO | GTGTTGAATATGTCAAGAGTT |
| Mcm8 | 1 | V2LMM_66650 | CATATCGACTTATACGATA |
| Mcm8 | 2 | pLKO | CGGACGATAGAATGTGAACTT |
| Msl2l1 | 1 | V2LMM_135026 | GTAGCAAAGTTGAATCGAA |
| Msl2l1 | 2 | pLKO | CTGATCCTCAAGCTAGCTTAT |
| Nipbl1 | 1 | V2LMM_205185 | CTAATAAGCTGACTAATAA |
| Nipbl1 | 2 | V2LMM_159802 | GATATATGATGAAGTCGAA |
| Parp6 | 1 | V2LMM_110605 | CAGAGGATTCCAACGTTAA |
| Parp6 | 2 | pLKO | GGATCCATCTGCCCTTGTAAA |
| Pdpk1 | 1 | V2LMM_75859 | CCGGGAATGAATATCTTAT |
| Pdpk1 | 2 | pLKO | GCTGAGATTGTGTCTGCTTTA |
| Pik3cb | 1 | V2LMM_53092 | GGGAAGCTACCATTTCTTA |
| Pik3cb | 2 | pLKO | GCCTCTTATGTCCTCGGCATT |
| Plk2 | 1 | V2LMM_35599 | CTAGGGAACTTTATTATTA |
| Plk2 | 2 | pLKO | CCCAGCCAAGAATTTCTTTAA |
| Prkd3 | 1 | V2LMM_59682 | GCTATTAAAGTGATTGATA |
| Prkd3 | 2 | pLKO | GCATTGGAGAACGTTACATTA |
| Rad21 | 1 | V2LMM_14758 | CGATTATTCTGATATTGTT |
| Rad21 | 2 | pLKO | GATGAGTTCCTCAAAGAGTTT |
| Sgk2 | 1 | V2LMM_64098 | CTCTTGGTATCTGAATTTA |
| Sgk2 | 2 | V2LMM_75651 | CCCTCTAGGGCCAATGGGA |
| Smad2 | 1 | V2LMM_19589 | GGTTGCCACATGTTATATA |
| Smad2 | 2 | pLKO | TGGTGTTCAATCGCATACTAT |
| Smarcd2 | 1 | V2LMM_48431 | GATCTATATTTCCAATACA |
| Smarcd2 | 2 | pLKO | CTTCGGATCTATATTTCCAAT |
| Snip1 | 1 | V2LMM_87786 | CTCCCACGGGTGGAAAGTA |
| Snip1 | 2 | pLKO | AGTCACCACGGACCAAGAGAA |
| Titin | 1 | V2LMM_161069 | GATTTCTCCTCTTAGCTAA |
| Titin | 2 | pLKO | GCGCAGTGGGATAGGATTAAT |
| XIST | 1 | V2LMM_95767 | CTGCTAGTTTCCCAATGAT |
| XIST | 2 | V2LMM_95769 | CCAACTGTCTGCTTAAGAA |
| Zfyve9 | 1 | V2LMM_106892 | CTGATGGGATCTTACCTAA |
| Zfyve9 | 2 | V2LMM_106890 | CAATGAACCTTATTCCTGA |
| Rnf12 | A | pLKO | CGAGAGTAGCTCAGAGATGTT |
| Rnf12 | B | V2LMM_70309 | CAACCTAGAGGACTCACTA |
| Rnf12 | C | V2LHS_97664 | GTGATTTCAGATTCAGTTT |
Table S3.
Oligonucleotides used for qPCR
| Gene | RT-qPCR oligonucleotides | |
| Forward sequence, 5′–3′ | Reverse sequence, 5′–3′ | |
| Acaca | CTCCGTCAGCTCAGATACACT | TGCTGGATCTCATGTGAAAGGC |
| AcSS1 | AGCCTATCAACCACGAAGCC | AGATGCCTCCAGTTTCCGTT |
| Acvr1 | TGTTGGAGTGTGTCGGGAAG | CCATGACTTCTCGTCTCGGG |
| Acvr1b | CCTCCGACCCTTCCATTGAG | CATCACTCGCAAGGCCTCAT |
| Arid4B | TTCCTCTGGAGGGCTGTGAG | AGCTTCACCTGCACCTTTGT |
| Aurka | Bio-Rad primer, qMmuCIP0029736 | |
| Bmpr2 | GTAAAGAATGACGGCGCGTG | AGCGAATTGTGCCAACCTCA |
| Brca2 | Bio-Rad primer, qMmuCED0048279 | |
| Claspin | ACAGCTTGACGAGACCGAAG | TTCCCCTGTTGAGCCTGTTC |
| Dnmt1 | CCTGGAGAGCAGAAATGGCA | GTGTCACTGTCCGACTTGCT |
| Dtx3l | AGGTGGGGAGTGCTCTGTC | TTTTCAACACTTTCTCCTTATCTGC |
| Erbb4 | Bio-Rad primer, qMmuCIP0032666 | |
| Gins3 | Bio-Rad primer, qMmuCID0008031 | |
| Hdac3 | GCATTCGAGGACATGGGGAA | TTTCGGACAGTGTAGCCACC |
| Mcm8 | CCTCTTTGCACCAACATGGC | GGCACAGGACACTTTGTAGGA |
| Msl2l1 | GAGACCCCAAGGCGTTTACA | AATGTCCGCAAACACAGCAC |
| Nipbl1 | Bio-Rad primer, qMmuCID0022121 | |
| Parp6 | CGGACTTCTCCTTTGGTCAGT | ACGTTGGAATCCTCTGCTCTG |
| Pdpk1 | AGAGAGCAAACAAGCCAGGG | AAGGGCCCAAAGGTCTGAAC |
| Pik3cb | Bio-Rad primer, qMmuCID0026635 | |
| Plk2 | ACGTCCGCAGTGGAAAACAA | TCTTCAAGGCATTCGCTGCT |
| Prkd3 | GCAAGGGAAGGACCACAATCT | TCTGGTAAACGCTGCTGATGT |
| Rad21 | GATGACAATGGCTCACTGGGT | CTTCTTCCTCGTTTGGGACGA |
| Sgk2 | GCTGCATAGAGCCTACCTGA | GATGGCCCCAGGTTGATGTT |
| Smad2 | GATCCCACCAGGCTGTAACC | GCCTCCGATATTCTGCTCCC |
| Smarcd2 | Bio-Rad primer, qMmuCED0005003 | |
| Snip1 | CAGTACCGGCTTGTGGAGTA | GCCTGAGCCAAGGTCGATAAT |
| Titin | Bio-Rad primer qMmuCED0037550 | |
| Xist | GGTTCTCTCTCCAGAAGCTAGGAAAG | TGGTAGATGGCATTGTGTATTATATGG |
| Zfyve9 | GAAATGGGAAACTCCTTGGGC | AGTGGACAGGGGTAAAGTCG |
| Rnf12 | GGTCCACCACCACAGAGC | TGACCACTTCTTGTTGTATTTCC |
| ACTB | ACTATTGGCAACGAGCGGTTC | AGAGGTCTTTACGGATGTCAACG |
| Smad7 | GGCCGGATCTCAGGCATTC | TTGGGTATCTGGAGTAAGGAGG |
| PAI-‐1 | TTCAGCCCTTGCTTGCCTC | ACACTTTTACTCCGAAGTCGGT |
| SNON | AGGCAGAGACAAGTAAGTCCA | CGTCTGGGTAAGACACTGTTTTT |
| F.LUCIFERASE | CAACTGCATAAGGCTATGAAGAGA | ATTTGTATTCAGCCCATATCGTTT |
| DDX3X | CAGAGTGGAGGAAGTACAGCA | TCACCCCGTGATCCAAAACTG |
| Kdm5c | GAGGCCCAGACAAGAGTGAAA | TTGGGAATCTTTAAGGATGAGCC |
| PGK1_PCR1 | CAAGGCTTTGGAGAGTCCAG | GCTTTCACCACCTCATCCAT |
| PGK1-‐qPCR | TGGAATGGCCTTTACCTTCCTTA | TTTCTCAGCTTTGGACATGAGAT |
SI Materials and Methods
Generation of Cell Lines.
Tail tendon fibroblasts from female MeCP2-FL-HR/MeCP2 animals (12) were immortalized using the E6/E7 expression vector and subjected to single-cell cloning via limiting dilution. A cell line carrying the CMV-luciferase reporter at the HPRT locus on the Xi was generated from mouse embryonic fibroblasts derived from female CMV-luciferase/ΔXIST animals (18), a gift from Kathrin Plath, University of California, Los Angeles, CA, by immortalization with E6/E7 and single-cell cloning. All cell lines were maintained in DMEM supplemented with 10% (vol/vol) FBS.
Western Blots.
The following antibodies were used: anti-MeCP2 (Sigma; M7443), anti-Rnf12 (Abnova; H000051132-B01P), anti-actin (I-19; Santa Cruz Biotechnology), and anti–alpha-tubulin (Calbiochem; CP06).
TGF-β1 and BMP2 Source.
Recombinant TGF-β1 was obtained from PeproTech (100-21C). BMP2 was obtained from R&D Systems (335-BM-010).
shRNA Screen.
The shRNA library consisted of 64,159 shRNAs targeting the mouse genome (provided by Open Biosystems) cloned into the MSCV-shRNA-pgk-Puro-ires-GFP vector (kindly provided by Ross Dickins, University of Melbourne, Parkville, Victoria, Australia) (48). shRNA amplification and sequencing were performed as previously described (49, 50). Xi-8 cells were infected with a retroviral library pool at a multiplicity of infection of 0.35. Forty-eight hours after infection, cells were split into two pools, one for DNA extraction after an additional 48-h recovery (preselection DNA) and the other for hygromycin B selection (15 µg/mL for 6 d, followed by a 48-h recovery; postselection) before DNA extraction. Half-hairpins were amplified from the pre- and postselection DNA, and the PCR product was subjected to high-throughput sequencing. Fold enrichment for each hairpin after hygromycin B selection was calculated by dividing the proportion of each hairpin in the postselection pool with the proportion of the same hairpin in the preselection pool (Dataset S1).
Data Analysis.
Sequencing data are available under Gene Expression Omnibus (GEO) accession no. GSE90685. shRNAs were enumerated using a Perl script that first counted sequences with perfect matches and then from the remaining sequences accepted sequences with a single mismatch. Only shRNAs that had at least 10 reads in the preselection pool were considered further. Enrichments were then calculated as ratios of post- to preselection counts, and false discovery rates (FDRs) were calculated on the basis of 1,000 permutations. Genes targeted by shRNAs with <5% FDR were tested further, and 30 of the 37 genes tested were confirmed as true hits.
Differences in expression levels for data presented in Figs. 1–4 were analyzed using Student t test, with the corresponding P values and number of replicates indicated in the figure legends.
Firefly Luciferase Assay.
To measure reactivation of the FL reporter in the Xi in the Xi-8 cells, 2 × 105 cells were plated in six-well plates, transduced with individual shRNAs (Table S2), and subjected to puromycin selection for 4 d. Cells were allowed to recover for 2 d, removed from the plates using trypsin, and lysed for 20 min in 100 µL of Cell Culture Lysis Reagent (Promega). Overexpression studies with Rnf12 or the catalytically inactive mutant (Rnf12M) were carried out by infecting Xi-8 cells with lentiviral vectors expressing Rnf12-GFP or Rnf12M-GFP fusion proteins derived from the expression plasmids kindly provided by Joost Gribnau (15). In experiments using 5-AZA, following selection and recovery, cells were subjected to 0.2 µM 5-AZA for 48 h before harvesting. After clearing by centrifugation at 15,000 × g for 5 min, the lysate was transferred to 96-well white plates and 100 µL of luciferase assay reagent (Luciferase Assay System; E1500; Promega) was added to the lysate. To increase the sensitivity of the assay, all luminescence measurements were carried out in white plates, with special attention to avoiding any exposure of the plates to fluorescent ceiling light to lower background readings. Luminescence was measured using the TopCount NXT counter (PerkinElmer).
Allele-Specific RT-qPCR.
Allele-specific RT-qPCR in a Patski M. musculus/M. spretus hybrid cell line, a gift from Christine Disteche, University of Washington, Seattle, was used to measure reactivation of the silenced M. spretus PDK1 allele, as described (23). Briefly, after reverse transcription, a PDK1 cDNA fragment containing the polymorphism was amplified for 10 PCR cycles using the primers shown in Table S3. One-half of the resulting cDNA was digested with BglII, which leaves the M. spretus PDK1 cDNA fragment intact and digests the M. musculus fragment. An internal set of primers was then used for qPCR of digested and undigested DNA to determine the expression of the M. spretus PDK1 relative to total PDK1. The expression level in cells containing control shRNAs treated with 0.2 µM 5-AZA was compared with the expression levels in cells containing specific shRNAs and treated with 0.2 µM 5-AZA to calculate shRNA-induced reactivation levels.
Mice.
Alb-Cre transgenic mice [B6.Cg-Tg(Alb-cre)21Mgn] (51) were crossed with Tgfbr2flx/flx mice (B6.129S6-Tgfbr2tm1Hlm) (52) to generate Alb-Cre;Tgfbr2flx/flx (Tgfbr2LKO) and Tgfbr2flx/flx (control) mice. Animals carrying liver-specific deletion of Tgfbr2 (Tgfbr2LKO) used in our study have been previously characterized (30). Mice were from a mixed genetic background of C57BL6/129. Genotypes were determined by PCR following published protocols (30). Mice were maintained and cared for using protocols approved by the institutional animal care and use committee.
Supplementary Material
Acknowledgments
We thank Kathrin Plath for providing us with CMV-luciferase/ΔXIST mouse embryonic fibroblasts, Joost Gribnau for the Rnf12 expression plasmids, Ross Dickins for shRNA library, and Christine Disteche for Patski cells. This work was supported by grants from the Rett Syndrome Research Trust (to A.B., M.S.B., and J.T.L.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequencing data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE90685).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621356114/-/DCSupplemental.
References
- 1.Maduro C, de Hoon B, Gribnau J. Fitting the puzzle pieces: The bigger picture of XCI. Trends Biochem Sci. 2016;41(2):138–147. doi: 10.1016/j.tibs.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Disteche CM. Dosage compensation of the sex chromosomes and autosomes. Semin Cell Dev Biol. 2016;56:9–18. doi: 10.1016/j.semcdb.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.Snijders Blok L, et al. DDD Study Mutations in DDX3X are a common cause of unexplained intellectual disability with gender-specific effects on Wnt signaling. Am J Hum Genet. 2015;97(2):343–352. doi: 10.1016/j.ajhg.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lange IM, et al. EuroEPINOMICS-RES MAE Working Group De novo mutations of KIAA2022 in females cause intellectual disability and intractable epilepsy. J Med Genet. 2016;53(12):850–858. doi: 10.1136/jmedgenet-2016-103909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reijnders MR, et al. DDD Study De novo loss-of-function mutations in USP9X cause a female-specific recognizable syndrome with developmental delay and congenital malformations. Am J Hum Genet. 2016;98(2):373–381. doi: 10.1016/j.ajhg.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T, et al. Recurrent occurrences of CDKL5 mutations in patients with epileptic encephalopathy. Hum Genome Var. 2015;2:15042. doi: 10.1038/hgv.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deardorff MA, et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature. 2012;489(7415):313–317. doi: 10.1038/nature11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pié J, et al. Mutations and variants in the cohesion factor genes NIPBL, SMC1A, and SMC3 in a cohort of 30 unrelated patients with Cornelia de Lange syndrome. Am J Med Genet A. 2010;152A(4):924–929. doi: 10.1002/ajmg.a.33348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JD, et al. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69(6):905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- 11.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315(5815):1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, et al. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature. 2015;521(7552):E1–E4. doi: 10.1038/nature14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, et al. RNF12 controls embryonic stem cell fate and morphogenesis in zebrafish embryos by targeting Smad7 for degradation. Mol Cell. 2012;46(5):650–661. doi: 10.1016/j.molcel.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Jonkers I, et al. RNF12 is an X-encoded dose-dependent activator of X chromosome inactivation. Cell. 2009;139(5):999–1011. doi: 10.1016/j.cell.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Gontan C, et al. RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature. 2012;485(7398):386–390. doi: 10.1038/nature11070. [DOI] [PubMed] [Google Scholar]
- 16.Jüttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA. 1994;91(25):11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka M, et al. De novo DNA methyltransferases Dnmt3a and Dnmt3b primarily mediate the cytotoxic effect of 5-aza-2′-deoxycytidine. Oncogene. 2005;24(19):3091–3099. doi: 10.1038/sj.onc.1208540. [DOI] [PubMed] [Google Scholar]
- 18.Minkovsky A, et al. The Mbd1-Atf7ip-Setdb1 pathway contributes to the maintenance of X chromosome inactivation. Epigenetics Chromatin. 2014;7:12. doi: 10.1186/1756-8935-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csankovszki G, Nagy A, Jaenisch R. Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. J Cell Biol. 2001;153(4):773–784. doi: 10.1083/jcb.153.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minajigi A, et al. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science. 2015;349(6245):aab2276. doi: 10.1126/science.aab2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minkovsky A, et al. A high-throughput screen of inactive X chromosome reactivation identifies the enhancement of DNA demethylation by 5-aza-2′-dC upon inhibition of ribonucleotide reductase. Epigenetics Chromatin. 2015;8:42. doi: 10.1186/s13072-015-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatnagar S, et al. Genetic and pharmacological reactivation of the mammalian inactive X chromosome. Proc Natl Acad Sci USA. 2014;111(35):12591–12598. doi: 10.1073/pnas.1413620111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20(5):614–622. doi: 10.1101/gr.103200.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hata A, Chen YG. TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8(9):a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katagiri T, Watabe T. Bone morphogenetic proteins. Cold Spring Harb Perspect Biol. 2016;8(6):a021899. doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill CS. Inhibiting the inhibitor: The role of RNF12 in TGF-β superfamily signaling. Mol Cell. 2012;46(5):558–559. doi: 10.1016/j.molcel.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 27.Shin J, et al. Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature. 2010;467(7318):977–981. doi: 10.1038/nature09457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicklas D, Saiz L. Characterization of negative feedback network motifs in the TGF-β signaling pathway. PLoS One. 2013;8(12):e83531. doi: 10.1371/journal.pone.0083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen ZJ, et al. Antisense targeting of TGF-beta1 augments BMP-induced upregulation of osteopontin, type I collagen and Cbfa1 in human Saos-2 cells. Exp Cell Res. 2007;313(7):1415–1425. doi: 10.1016/j.yexcr.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Morris SM, et al. TGF-β signaling alters the pattern of liver tumorigenesis induced by Pten inactivation. Oncogene. 2015;34(25):3273–3282. doi: 10.1038/onc.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakefield LM, Hill CS. Beyond TGFβ: Roles of other TGFβ superfamily members in cancer. Nat Rev Cancer. 2013;13(5):328–341. doi: 10.1038/nrc3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh CA, et al. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521(7551):232–236. doi: 10.1038/nature14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gokhman D, Livyatan I, Sailaja BS, Melcer S, Meshorer E. Multilayered chromatin analysis reveals E2f, Smad and Zfx as transcriptional regulators of histones. Nat Struct Mol Biol. 2013;20(1):119–126. doi: 10.1038/nsmb.2448. [DOI] [PubMed] [Google Scholar]
- 34.Makhlouf M, et al. A prominent and conserved role for YY1 in Xist transcriptional activation. Nat Commun. 2014;5:4878. doi: 10.1038/ncomms5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KH, Evans S, Ruan TY, Lassar AB. SMAD-mediated modulation of YY1 activity regulates the BMP response and cardiac-specific expression of a GATA4/5/6-dependent chick Nkx2.5 enhancer. Development. 2004;131(19):4709–4723. doi: 10.1242/dev.01344. [DOI] [PubMed] [Google Scholar]
- 36.Tritto P, et al. Loss of Pol32 in Drosophila melanogaster causes chromosome instability and suppresses variegation. PLoS One. 2015;10(3):e0120859. doi: 10.1371/journal.pone.0120859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, et al. Drosophila CAF-1 regulates HP1-mediated epigenetic silencing and pericentric heterochromatin stability. J Cell Sci. 2010;123(Pt 16):2853–2861. doi: 10.1242/jcs.063610. [DOI] [PubMed] [Google Scholar]
- 38.Henderson DS, Banga SS, Grigliatti TA, Boyd JB. Mutagen sensitivity and suppression of position-effect variegation result from mutations in mus209, the Drosophila gene encoding PCNA. EMBO J. 1994;13(6):1450–1459. doi: 10.1002/j.1460-2075.1994.tb06399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Shibahara K, Stillman B. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature. 2000;408(6809):221–225. doi: 10.1038/35041601. [DOI] [PubMed] [Google Scholar]
- 40.Liachko I, Tye BK. Mcm10 is required for the maintenance of transcriptional silencing in Saccharomyces cerevisiae. Genetics. 2005;171(2):503–515. doi: 10.1534/genetics.105.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dziak R, Leishman D, Radovic M, Tye BK, Yankulov K. Evidence for a role of MCM (mini-chromosome maintenance)5 in transcriptional repression of sub-telomeric and Ty-proximal genes in Saccharomyces cerevisiae. J Biol Chem. 2003;278(30):27372–27381. doi: 10.1074/jbc.M301110200. [DOI] [PubMed] [Google Scholar]
- 42.Galdieri L, Vancura A. Acetyl-CoA carboxylase regulates global histone acetylation. J Biol Chem. 2012;287(28):23865–23876. doi: 10.1074/jbc.M112.380519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi H, McCaffery JM, Irizarry RA, Boeke JD. Nucleocytosolic acetyl-coenzyme A synthetase is required for histone acetylation and global transcription. Mol Cell. 2006;23(2):207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 44.Yu PB, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuny GD, et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18(15):4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lessing D, et al. A high-throughput small molecule screen identifies synergism between DNA methylation and Aurora kinase pathways for X reactivation. Proc Natl Acad Sci USA. 2016;113(50):14366–14371. doi: 10.1073/pnas.1617597113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang L, Kirby JE, Sunwoo H, Lee JT. Female mice lacking Xist RNA show partial dosage compensation and survive to term. Genes Dev. 2016;30(15):1747–1760. doi: 10.1101/gad.281162.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickins RA, et al. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37(11):1289–1295. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 49.Hubert CG, et al. Genome-wide RNAi screens in human brain tumor isolates reveal a novel viability requirement for PHF5A. Genes Dev. 2013;27(9):1032–1045. doi: 10.1101/gad.212548.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding Y, et al. Cancer-specific requirement for BUB1B/BUBR1 in human brain tumor isolates and genetically transformed cells. Cancer Discov. 2013;3(2):198–211. doi: 10.1158/2159-8290.CD-12-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26(2):149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 52.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32(2):73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.