Abstract
Purpose
The primary objective was to determine safety, toxicity, and a recommended phase II dose regimen of LY2606368, an inhibitor of checkpoint kinase 1, as monotherapy.
Patients and Methods
This phase I, nonrandomized, open-label, dose-escalation trial used a 3 + 3 dose-escalation scheme and included patients with advanced solid tumors. Intravenous LY2606368 was dose escalated from 10 to 50 mg/m2 on schedule 1 (days 1 to 3 every 14 days) or from 40 to 130 mg/m2 on schedule 2 (day 1 every 14 days). Safety measures and pharmacokinetics were assessed, and pharmacodynamics were measured in blood, hair follicles, and circulating tumor cells.
Results
Forty-five patients were treated; seven experienced dose-limiting toxicities (all hematologic). The maximum-tolerated doses (MTDs) were 40 mg/m2 (schedule 1) and 105 mg/m2 (schedule 2). The most common related grade 3 or 4 treatment-emergent adverse events were neutropenia, leukopenia, anemia, thrombocytopenia, and fatigue. Grade 4 neutropenia occurred in 73.3% of patients and was transient (typically < 5 days). Febrile neutropenia incidence was low (7%). The LY2606368 exposure over the first 72 hours (area under the curve from 0 to 72 hours) at the MTD for each schedule coincided with the exposure in mouse xenografts that resulted in maximal tumor responses. Minor intra- and intercycle accumulation of LY2606368 was observed at the MTDs for both schedules. Two patients (4.4%) had a partial response; one had squamous cell carcinoma (SCC) of the anus and one had SCC of the head and neck. Fifteen patients (33.3%) had a best overall response of stable disease (range, 1.2 to 6.7 months), six of whom had SCC.
Conclusion
An LY2606368 dose of 105 mg/m2 once every 14 days is being evaluated as the recommended phase II dose in dose-expansion cohorts for patients with SCC.
INTRODUCTION
Checkpoint kinase 1 (CHK1), a multifunctional protein kinase, is a regulator of the DNA damage response.1 CHK1 is a key component of the checkpoint response after DNA damage and is essential for homologous recombination repair of double-stranded DNA breaks. It also affects the initiation of DNA replication origin firing, stabilization of replication forks, resolution of replication stress, and coordination of mitosis, even in the absence of exogenous DNA damage.2 Although CHK1 inhibitors previously have been developed as chemopotentiators, given the integral role that CHK1 plays in DNA replication and the regulation of the cell cycle, inhibitors of CHK1 may also have activity as single agents.
LY2606368 monomesylate monohydrate (hereafter referred to as LY2606368) inhibits the enzymatic activity of CHK1 with a half-maximal inhibitory concentration (IC50) of 1 nM in cell-free assays. Only CHK2 (8 nM) and RSK1 (9 nM) have an IC50 value of less than 10 nM in these assays.3 However, in nonclinical models, the biologic effects of LY2606368 seem to be driven by CHK1.3 In nonclinical studies, LY2606368 induced DNA damage as measured by replication catastrophe and increases in pH2A.X, a marker of double-stranded DNA breaks.3 LY2606368 inhibited tumor growth in cancer xenografts as monotherapy and in combination with other agents.2-4 This study consisted of the following two parts: a dose escalation of monotherapy in solid tumors and dose-expansion cohorts in patients with squamous cell carcinomas (SCCs). Here, we report the results of the dose escalation, which demonstrate, to our knowledge, the first objective responses achieved with a CHK1/CHK2 inhibitor as a single agent.
PATIENTS AND METHODS
Eligibility
Patients with advanced or metastatic nonhematologic cancer who experienced treatment failure with standard therapies and who had an Eastern Cooperative Oncology Group performance status of 0 or 1 and measurable or nonmeasurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were enrolled.5 Patients must have discontinued and recovered from the acute effects of prior therapies before enrollment. Adequate hematologic, hepatic, and renal function were required.
Exclusion criteria included symptomatic CNS malignancies, current hematologic malignancy, QTc interval greater than 470 milliseconds on screening electrocardiogram, serious cardiac conditions, systolic blood pressure less than 90 mm Hg or recurrent orthostatic hypotension, chronic use of β-adrenergic receptor blockers, serotonin-secreting carcinoid tumor or prior history of drug-induced serotonin syndrome, family history of long QT syndrome, and use of concurrent medication known to cause QTc prolongation or induce torsades de pointes.
Study Design and Treatment
This phase I, multicenter, nonrandomized, open-label trial used a 3 + 3 dose-escalation scheme to explore two dosing schedules. LY2606368 was administered as a 1-hour infusion without premedication starting at 10 mg/m2 on days 1 to 3 (schedule 1) or starting at 40 mg/m2 on day 1 (schedule 2) every 14 days.
The primary objective was to determine the safety, toxicity, and recommended phase II dose (RP2D) of LY2606368. The secondary objectives were the characterization of LY2606368 pharmacokinetics (PK), exploration of LY2606368 pharmacodynamics (PD), and documentation of antitumor activity.
This study was conducted in accordance with good clinical practices, the Declaration of Helsinki, and approval by each institution’s ethical review board. Patients provided written informed consent.
Safety Evaluations
Treatment-emergent adverse events (TEAEs) were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.6 Dose-limiting toxicity (DLT) was defined as a cycle 1 adverse event that was possibly related to study treatment with one of the following criteria: grade ≥ 3 nonhematologic toxicity, except nausea, vomiting, constipation, diarrhea, fatigue, or anorexia, that was manageable with appropriate care and resolved in ≤ 2 days, or transient (≤ 5 days) grade 3 elevations of ALT and/or AST, without evidence of other hepatic injury; grade ≥ 3 thrombocytopenia with bleeding; grade 4 hematologic toxicity of greater than 5 days in duration (except lymphopenia); any febrile neutropenia; omission or reduction of the day 2 or 3 dose as a result of an event that was possibly related to LY2606368; and any other significant toxicity deemed to be dose limiting. If a patient did not meet one of the previous criteria but experienced a drug-related TEAE that prevented the start of cycle 2 for more than 1 week from the end of cycle 1, the patient was considered to have had a DLT. Prophylactic granulocyte colony-stimulating factor use was not permitted in cycle 1 but could have been used in accordance with ASCO guidelines.7
The maximum-tolerated dose (MTD) was the highest dose level at which less than 33% of patients experienced a DLT during cycle 1. The RP2D was at or below the MTD.
PK and PD Analyses
Serial blood samples were collected over 336 hours after the start of the infusion on days 1 and 3 of cycle 1 and on day 3 of cycle 2 (schedule 1), and on day 1 of cycles 1 and 2 (schedule 2). LY2606368 plasma concentrations were quantified using a validated high-pressure liquid chromatography/mass spectrometry/mass spectrometry method. PK parameters were computed from the plasma concentration versus time data of LY2606368 by standard noncompartmental methods of analysis using WinNonlin Enterprise version 6.3 (Pharsight, Sunnyvale, CA).
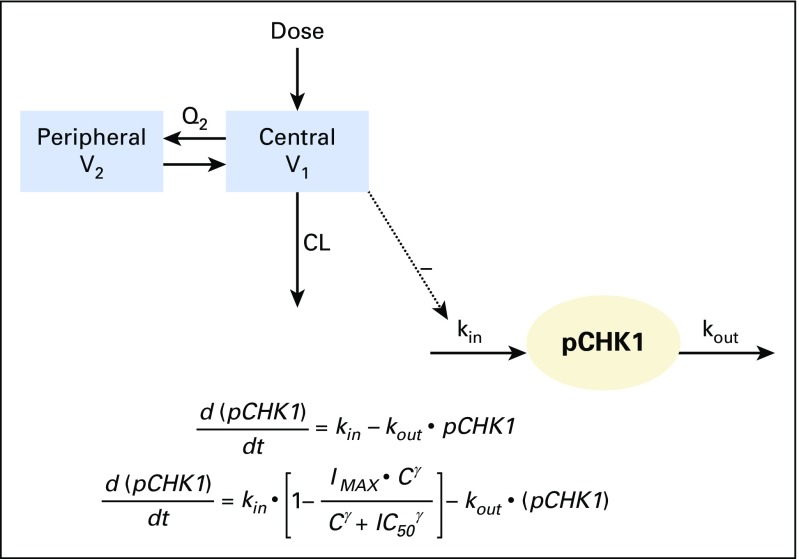
An indirect response PK/PD model (Appendix Fig A1, online only) was developed to link LY2606368 plasma concentrations with the inhibition of phosphorylated CHK1 (pCHK1) and tumor growth response in Calu-6 xenografts. A preliminary human population–based compartmental PK (popPK) analysis was conducted using NONMEM version 7.2 (ICON Development Solutions, Dublin, Ireland). This model simulated human PK profiles and was linked to the Calu-6 xenograft PD model to develop a combined PK/PD model to predict human PD profiles at the MTDs (see Appendix [online only] for PD marker methods).
Efficacy Evaluations
Responses were evaluated by RECIST version 1.1 approximately every 6 weeks.5 Objective responses were confirmed at least 4 weeks later. Statistical analysis methods can be found in the Appendix.
RESULTS
Patient Characteristics and Treatment
Forty-five patients (schedule 1, n = 27; schedule 2, n = 18) were treated. Most patients had received three or more prior systemic regimens (69%), radiotherapy (56%), and/or surgery (82%; Table 1). The most common tumor types were colon/rectal (20%) and SCC of the head and neck (SCCHN; 11%).
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | No. of Patients (%) | ||
|---|---|---|---|
| Schedule 1 (n = 27) | Schedule 2 (n = 18) | Total (N = 45) | |
| Median age, years (range) | 56 (21-77) | 62 (40-84) | 59 (21-84) |
| Sex | |||
| Female | 10 (37) | 8 (44) | 18 (40) |
| Male | 17 (63) | 10 (56) | 27 (60) |
| Race | |||
| White | 24 (89) | 15 (83) | 39 (87) |
| Black or African American | 3 (11) | 2 (11) | 5 (11) |
| Asian | 0 (0) | 1 (6) | 1 (2) |
| Cancer type | |||
| Colon/rectal | 6 (22) | 3 (17) | 9 (20) |
| SCCHN | 2 (7) | 3 (17) | 5 (11) |
| Anal | 0 (0) | 3 (17) | 3 (7) |
| Pancreas | 2 (7) | 1 (6) | 3 (7) |
| Breast | 3 (11) | 0 (0) | 3 (7) |
| All other | 14 (52) | 8 (44) | 22 (49) |
| ECOG PS | |||
| 0 | 12 (44) | 9 (50) | 21 (47) |
| 1 | 15 (56) | 9 (50) | 24 (53) |
| Prior interventions | |||
| Surgery | 21 (78) | 16 (89) | 37 (82) |
| Radiotherapy | 14 (52) | 11 (61) | 25 (56) |
| ≥ 3 prior systemic regimens | 21 (78) | 10 (56) | 31 (69) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; SCCHN, squamous cell carcinoma of the head and neck.
The median number of completed cycles was three in both schedule 1 (range, one to 12 cycles) and schedule 2 (range, one to 22 cycles). A total of 22 patients (48.9%) had dose delays (most commonly as a result of neutropenia, n = 11, and scheduling conflicts, n = 8), and 10 patients (22.2%) had dose reductions, all as a result of neutropenia. The mean dose-intensity was 91.5% and 90.3% in schedule 1 and schedule 2, respectively.
Safety
Seven patients experienced DLTs (Table 2), all of which were hematologic. The MTDs were determined to be 40 mg/m2 (schedule 1) and 105 mg/m2 (schedule 2). During schedule 2, the dose was escalated from 80 to 130 mg/m2. A dose of 130 mg/m2 exceeded the MTD, as did the subsequent dose of 120 mg/m2; therefore, the dose was decreased to 105 mg/m2, and no DLTs were observed.
Table 2.
Dose Levels and Dose-Limiting Toxicities
| Dose (mg/m2) | No. of Patients | Dose-Limiting Toxicities |
|---|---|---|
| Schedule 1: days 1-3 every 14 days | ||
| 10 | 3 | None |
| 12 | 3 | None |
| 15 | 3 | None |
| 20 | 3 | None |
| 30 | 6 | Grade 3 thrombocytopenia with bleeding (n = 1) |
| 40 | 3 | None |
| 50 | 6 | Grade 4 neutropenia > 5 days (n = 1); grade 4 neutropenia (with fever)/leukopenia > 5 days (n = 1) |
| Schedule 2: day 1 every 14 days | ||
| 40 | 3 | None |
| 60 | 3 | None |
| 80 | 3 | None |
| 130 | 2 | Grade 4 neutropenia and thrombocytopenia > 5 days (n = 1); grade 4 neutropenia and leukopenia > 5 days (n = 1) |
| 120 | 3 | Grade 4 neutropenia and leukopenia > 5 days (n = 1); grade 4 neutropenia, thrombocytopenia, leukopenia > 5 days (n = 1) |
| 105 | 4 | None |
Serious adverse events related to study treatment in schedule 1 were neutropenia (n = 4), febrile neutropenia (n = 2), leukopenia, anemia, and lung infection (n = 1 each). In schedule 2, drug-related serious adverse events were neutropenia, leukopenia, thrombocytopenia, lung infection, and epistaxis, all occurring in a single patient.
Among all cohorts and schedules, the most common all-grade TEAEs related to study treatment were neutropenia (93.3% [grade 3/4, 88.9%]), leukopenia (82.2% [grade 3/4, 71.1%]), anemia (68.9% [grade 3/4, 31.1%]), thrombocytopenia (53.3% [grade 3/4, 28.9%]), and fatigue (31.1% [grade 3/4, 2.2%]). Nausea (24.4%), oral mucositis (13.3%), and vomiting (11.1%) were also reported, but all events were grade 1 or 2. Grade 3 or 4 TEAEs related to study treatment by cohort are listed in Table 3.
Table 3.
Grade 3 or 4 TEAEs Related to Study Treatment
| TEAE | No. of Patients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schedule 1 (days 1-3 every 14 days) Dose (mg/m2) | Schedule 2 (day 1 every 14 days) Dose (mg/m2) | ||||||||||||
| 10 (n = 3) | 12 (n = 3) | 15 (n = 3) | 20 (n = 3) | 30 (n = 6) | 40 (n = 3) | 50 (n = 6) | 40 (n = 3) | 60 (n = 3) | 80 (n = 3) | 105 (n = 4) | 120 (n = 3) | 130 (n = 2) | |
| Neutropenia | 3 | 3 | 2 | 3 | 4 | 3 | 6 | 2 | 3 | 3 | 3 | 3 | 2 |
| Leukopenia | 3 | 3 | 2 | 2 | 3 | 2 | 5 | 2 | 3 | 1 | 2 | 2 | 2 |
| Anemia | 1 | — | — | 1 | 2 | 2 | 3 | — | 2 | 1 | — | 1 | 1 |
| Thrombocytopenia | — | — | — | — | 3 | 2 | 3 | — | 1 | — | — | 2 | 2 |
| Febrile neutropenia | — | — | — | — | — | 1 | 2 | — | — | — | — | — | — |
| Lung infection | — | — | — | — | — | — | 1 | — | — | — | — | — | 1 |
| Hyperuricemia | — | — | — | — | — | — | — | 1 | — | — | — | — | — |
| Epistaxis | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| Fatigue | — | — | — | — | — | — | — | — | — | — | 1 | — | — |
| Lymphopenia | — | — | 1 | — | — | — | — | — | — | — | — | — | — |
| Hyponatremia | — | — | — | — | — | — | — | — | — | 1 | — | — | — |
Abbreviation: TEAE, treatment-emergent adverse event.
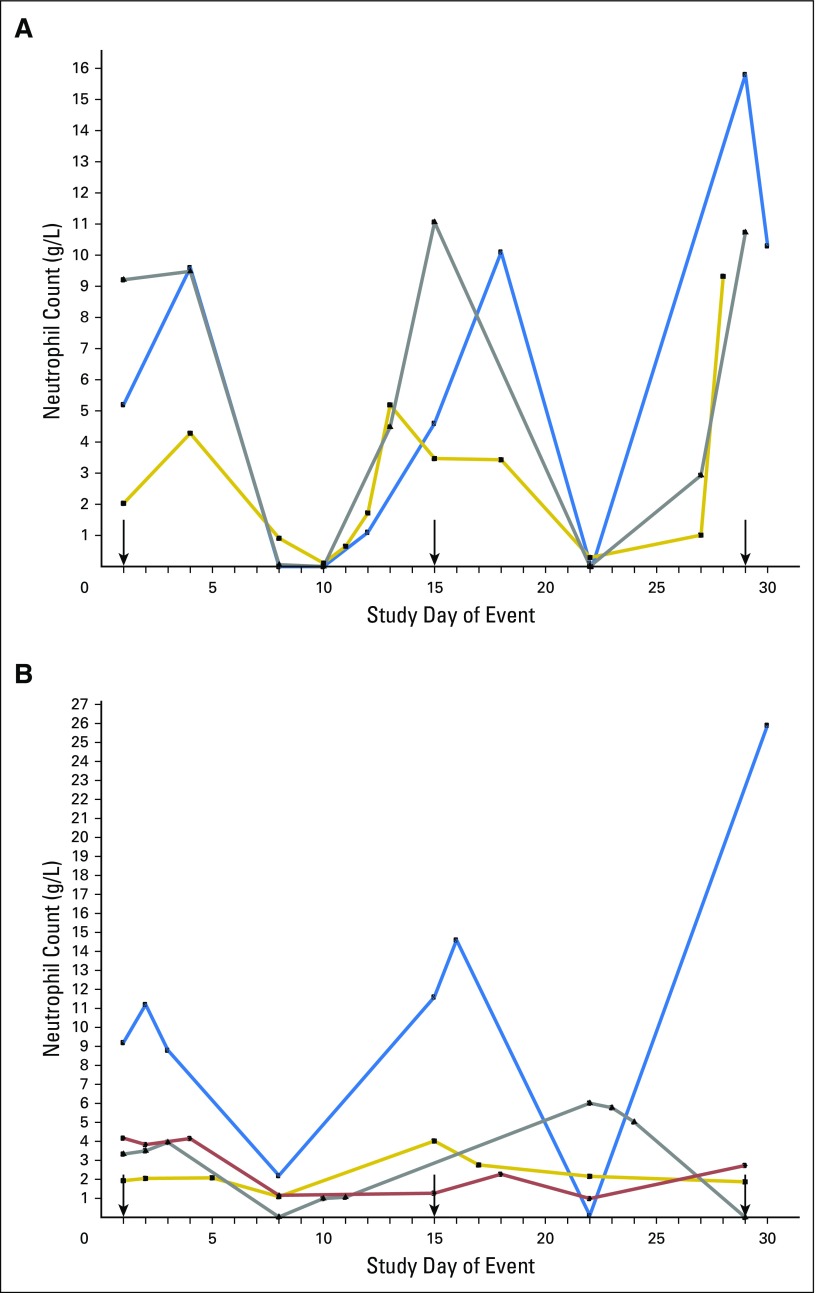
The most frequently observed toxicity was neutropenia, which was predominantly grade 4 (73.3%). The nadir consistently occurred approximately 1 week after each dose, and the duration of grade 4 neutropenia was transient (typically < 5 days; Appendix Fig A2, online only). Three patients experienced febrile neutropenia (all on schedule 1). There were no deaths or discontinuations as a result of febrile neutropenia. Granulocyte colony-stimulating factor was administered prophylactically to three patients (7%) and to treat low neutrophils in 10 patients (22%).
Most patients discontinued treatment as a result of progressive disease (n = 34, 75.6%). Five patients (11.1%) discontinued treatment as a result of investigator decision, two patients (4.4%) were lost to follow-up, two patients (4.4%) discontinued as a result of TEAEs (neutropenia in cycle 2; skin ulcer in cycle 5), one patient (2.2%) discontinued because of a protocol violation, and one patient (2.2%) died of cancer.
PK and PD
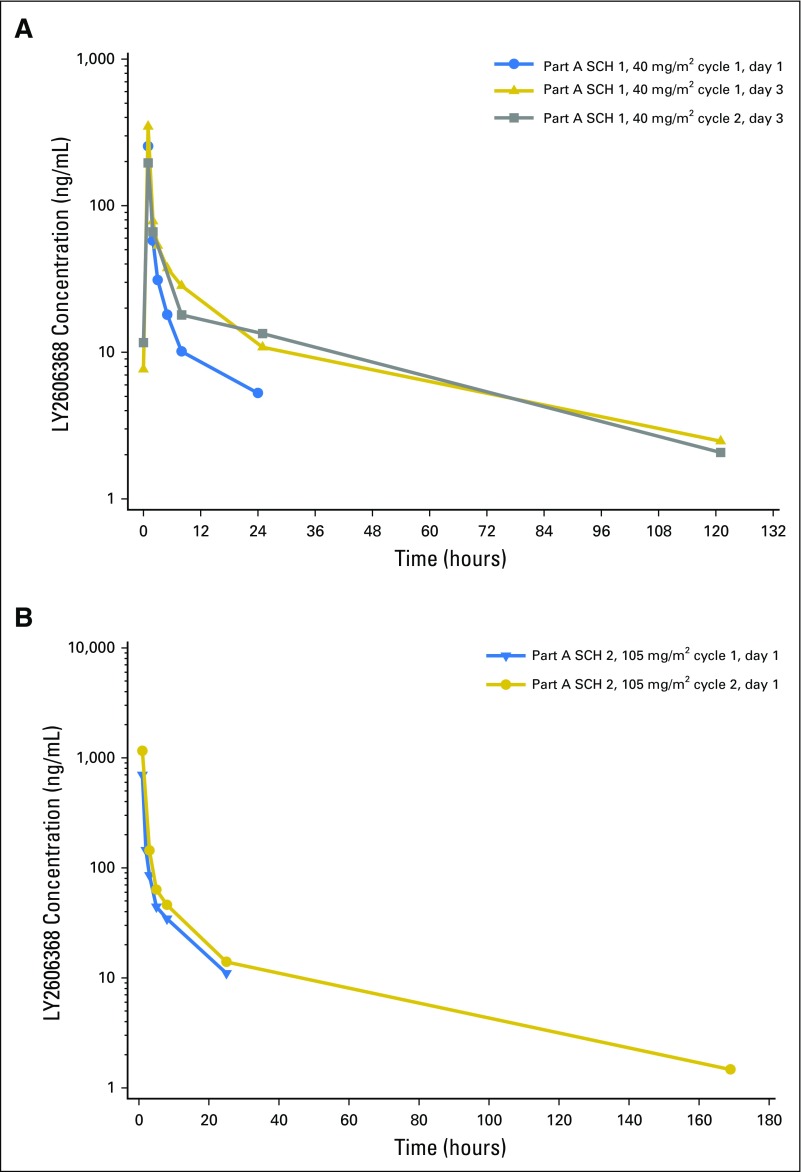
LY2606368 exposure increased in a dose-dependent manner across the dose range of 10 to 130 mg/m2 after a single dose (day 1) and multiple doses (day 3) in cycles 1 and 2 across both schedules (data not shown). Table 4 and Figure 1 summarize the PK parameters at the MTDs. The mean elimination half-life (t1/2) varied across days and cycles of treatment at the MTDs, and there was a moderate to large degree of interpatient PK variability (Table 4). The accumulation of LY2606368 was relatively minor at the MTDs (Table 4). After repeat administration on schedule 1, consistent decreases in clearance and volume of distribution at steady state were observed, indicating potential nonlinear PK behavior (Table 4). This is likely an artifact of a shorter sampling duration on day 1 (only up to 24 hours) compared with day 3 (up to 168 hours) and is supported by the larger fraction of area under the plasma concentration-time curve (AUC) from time 0 to infinity extrapolated beyond the last measurable plasma concentration [AUC(tlast-∞), %] on day 1 of cycle 1 compared with day 3 of cycles 1 and 2 (Table 4). The preliminary popPK analysis determined that a linear model best described the data supporting the time-independent PK behavior.
Table 4.
LY2606368 Pharmacokinetic Summary After Single- and Multiple-Dose Administration at the Maximum-Tolerated Dose for Each Schedule
| Parameter | Geometric Mean (CV%)* | ||||
|---|---|---|---|---|---|
| Schedule 1 (40 mg/m2) | Schedule 2 (105 mg/m2) | ||||
| Cycle 1 | Cycle 2, Day 3 | Cycle 1, Day 1 | Cycle 2, Day 1 | ||
| Day 1 | Day 3 | ||||
| No. of patients | 3 | 3 | 3† | 4 | 6‡ |
| Cmax, ng/mL | 294, 214§ | 194, 499§ | 191, 201§ | 460 (61) | 867 (58) |
| Cav,24, ng/mL | 17.3 (37) | 31.5 (44) | 31.0 (36) | 46.9 (40) | 83.4 (38) |
| AUC(0-∞), ng⋅h/mL | 516 (38) | 1,230 (80) | 1,260 (51) | 1,340 (53) | 2,410 (51) |
| AUC(0-24), ng⋅h/mL | 415 (37) | 756 (44) | 743 (36) | 1,130 (40) | 2,000 (38) |
| AUC(0-72), ng⋅h/mL | 506 (37) | 1,000 (57) | 1,100 (46) | 1,290 (46) | 2,300 (45) |
| AUC(tlast-∞), % | 18 (68) | 7 (61) | 5 (13) | 7 (115) | 4 (128) |
| Cl, L/h | 156 (47) | 65.5 (94) | 64.9 (65) | 133 (66) | 76.9 (73) |
| Vss, L | 1,890 (56) | 2,020 (46) | 1,950 (39) | 1,370 (68) | 767 (75) |
| t1/2, hours | 13.4 (27) | 25.8 (156) | 27.1 (12) | 11.9 (64) | 11.4 (124) |
| Intracycle RA for schedule 1: cycle 1, day 3 AUC(0-24)/cycle 1, day 1 AUC(0-24) | NC | 1.82 (33) | NC | NC | NC |
| Intercycle RA for schedule 1: cycle 2, day 3 AUC(0-24)/cycle 1, day 3 AUC(0-24) | NC | NC | 1.24, 0.876 | NC | NC |
| Intercycle RA for schedule 2: cycle 2, day 1 AUC(0-24)/cycle 1, day 1 AUC(0-24) | NC | NC | NC | NC | 1.96 (59)‖ |
Abbreviations: AUC(0-∞), area under the plasma concentration-time curve from time 0 to infinity; AUC(0-24), area under the plasma concentration-time curve from time 0 to 24 hours after dose; AUC(0-72), area under the plasma concentration-time curve from time 0 to 72 hours after dose; AUC(tlast-∞), fraction of AUC(0-∞) extrapolated beyond the last measurable plasma concentration; Cav,24, average plasma concentration over the 24-hour time interval after LY2606368 infusion [calculated using AUC(0-24)]; Cl, systemic clearance; Cmax, maximum plasma concentration; CV%, percent coefficient of variation; NC, not calculated; RA, accumulation ratio; t1/2, elimination half-life; Vss, volume of distribution at steady state.
Data are reported as geometric mean (CV%) or individual values (for n < 3).
One patient was dose reduced from 50 mg/m2 to 40 mg/m2 for cycle 2. These data are included in 40 mg/m2 data.
Two patients at 120 mg/m2 were dose reduced to 105 mg/m2 and are included in these data.
n = 2; one patient had a 2-hour infusion and was excluded from Cmax mean.
n = 4.
Fig 1.
Mean observed pharmacokinetic (PK) profiles of LY2606368 after single- and multiple-dose administration at the maximum-tolerated dose of schedules (SCH) 1 and 2. (A) Mean PK profile of LY2606368 40 mg/m2 (SCH 1) on days 1 and 3 of cycle 1 and day 3 of cycle 2. (B) Mean PK profile of LY2606368 105 mg/m2 (SCH 2) on day 1 of cycles 1 and 2.
The AUC from time 0 to 72 hours after dose (AUC(0-72)) values associated with the MTDs coincided with the predicted AUC(0-72) median and associated range (median, 1,896 ng⋅h/mL; range, 1,008 to 3,533 ng⋅h/mL) to achieve the maximal human tumor response to LY2606368 on the basis of the Calu-6 xenograft PK/PD model. Specifically, 105 mg/m2 on day 1 (schedule 2) in cycle 1 and cycle 2, 25% and 67% of the AUC(0-72) values, respectively, achieved the optimal predicted median AUC(0-72).
Changes in plasma concentrations of DNA and CK18 were inconclusive. At baseline, circulating tumor cells (CTCs) were detected in 29% of patients at low numbers (range, one to eight CTCs). The observed change in CTCs positive for pH2A.X was not significant (data not shown). The average postdose pH2A.X levels measured in hair follicles were not statistically different from the predose levels (Appendix Fig A3, online only).
Efficacy
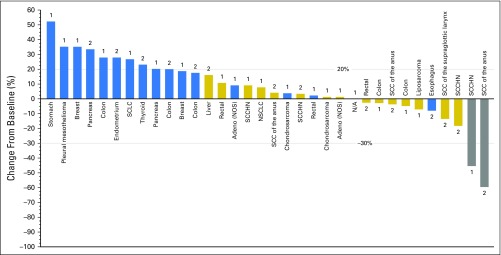
A total of 43 of 45 patients were evaluable for efficacy. Two patients had a partial response (PR; objective response rate, 4.4%), and 15 patients (33.3%) had stable disease (SD; Appendix Table A1, online only). The p53 status and human papillomavirus status of the two responding patients are unknown. Figure 2 shows the change in tumor size from baseline by best overall response. One of the responders (80 mg/m2, schedule 2, 344 days on study), who had stage IV SCC of the anus, previously received cisplatin plus fluorouracil (FU) and radiation (Figs 3A and 3B). The other responder (50 mg/m2, schedule 1, 224 days on study), who had stage IV SCCHN, previously received neoadjuvant radiotherapy, cisplatin/FU/docetaxel, FU/cetuximab/cisplatin, and docetaxel. The clinical benefit rate was 33.3% in schedule 1 and 44.4% in schedule 2. Duration of clinical benefit for the patients with PR or SD ranged from 1.2 to 7.2 months (Appendix Table A1). Three patients (6.7%) had SD for at least 4 months.
Fig 2.
Antitumor activity. Change in tumor size from baseline by best overall response. Note that 11 patients with invalid baseline or postbaseline target measurement are excluded from the plot. Blue bars indicate progressive disease; gold bars indicate stable disease; and gray bars indicate partial response. The number 1 indicates schedule 1, and 2 indicates schedule 2. Adeno, adenocarcinoma; N/A, not available; NOS, not otherwise specified; NSCLC, non–small-cell lung cancer; SCC, squamous cell carcinoma; SCLC, small-cell lung cancer; SCCHN, squamous cell carcinoma of the head and neck.
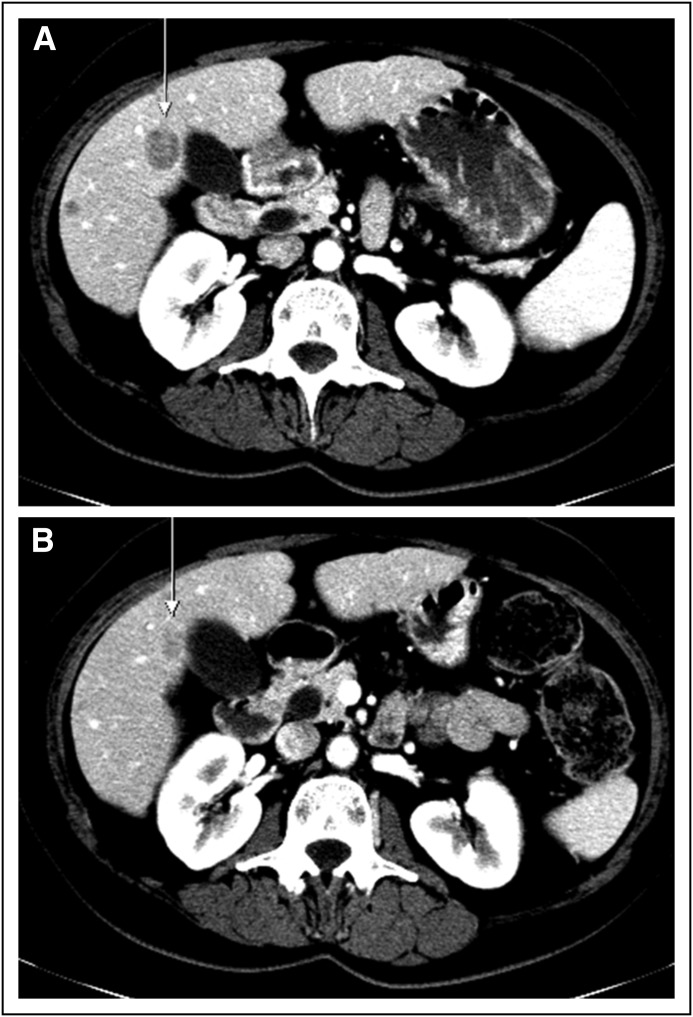
Fig 3.
(A) Pretreatment and (B) posttreatment scans from a patient with anal cancer who had a partial response. The patient was previously treated with neoadjuvant radiotherapy and cisplatin/fluorouracil. There was a 57% reduction in tumor size in cycle 3.
DISCUSSION
The MTDs of LY2606368 for schedules 1 and 2 were 40 mg/m2 and 105 mg/m2, respectively. The most common TEAE related to study treatment was neutropenia, which was predominantly grade 4 and transient. This extent of neutropenia has not been observed in monotherapy lead-in cycles before chemotherapy with either AZD7762 (CHK1/CHK2 inhibitor) or MK8776 (CHK1 inhibitor).8-10 Both have been associated with cardiotoxicity, including myocardial infarction and significant QTc changes8,10; however, in the current study, cardiac events related to study treatment were rare, with only two events (grade 2 hypotension and grade 2 sinus tachycardia).
Multiple factors were considered in selecting 105 mg/m2 administered once every 14 days as the RP2D and schedule, including safety, efficacy, predicted target inhibition, and PK/PD simulations. Each schedule had a similar safety profile and the same rate of grade 3 or 4 neutropenia (89%). One patient on each schedule achieved a PR; the patient achieving a PR on schedule 1 received 50 mg/m2, a dose later determined to exceed the MTD.
Ideally, PD would have been used to inform the selection of the RP2D; the absence of a direct PD marker to assess CHK1 modulation is a limitation of this study. In addition, changes in pH2A.X in hair may not be representative of the modulation in the tumor. An ongoing study with LY2606368 (ClinicalTrials.gov identifier: NCT02203513) includes post-treatment tumor biopsies to better assess effects in tumor. The mean changes in pH2A.X levels in hair follicles increased relative to before treatment but were not statistically significant (Appendix Fig A3). Notably, the patient achieving a PR on schedule 2 showed little change in pH2A.X in hair follicles, also suggesting that this marker may not be an appropriate surrogate. CTC analysis was hindered by the limited numbers of cells obtained from this refractory population and the high proportion of patients with tumors not typically associated with shedding CTCs.
Because the magnitude and duration of pCHK1 inhibition could not be directly determined, human PD profiles at the MTDs were generated using the preliminary human popPK model and Calu-6 xenograft PK/PD model to inform the RP2D selection. However, the predictive capability of this approach has limitations given the differences between animal models and humans (eg, protein binding, tumor size/location, target expression, immune system, and prior therapies in humans). The Calu-6 PK/PD model predicted that an average pCHK1 inhibition of 49.7% (95% prediction interval [PI], 45.0% to 54.7%) and 70.5% (95% PI, 67.5% to 73.5%) over a 72-hour period was required for the minimal and maximal tumor responses, respectively. Simulation results of human pCHK1 profiles demonstrated that the median predicted percent pCHK1 inhibition over the first 72 hours of cycle 1 at the schedule 1 and 2 MTD was similar, at 45.4% (95% PI, 26.6% to 60.6%) and 42.9% (95% PI, 22.9% to 62.6%), respectively (Appendix Fig A4, online only).
Human PK simulations also demonstrated that the LY2606368 systemic exposure correlating to CHK1 inhibition needed for maximal tumor response in preclinical xenograft models can be attained and was similar for both the schedule 1 MTD (median AUC(0-72) predicted, 1,780 ng⋅h/mL; 95% PI, 951 to 2,800 ng⋅h/mL) and schedule 2 MTD (median AUC(0-72) predicted, 1,720 ng⋅h/mL; 95% PI, 896 to 2,980 ng⋅h/mL). Moreover, the average LY2606368 plasma concentrations over the first 24 hours at the MTDs (Table 4) were greater than the IC50 (14.1 ng/mL) for pCHK1 inhibition determined in the Calu-6 PK/PD model.
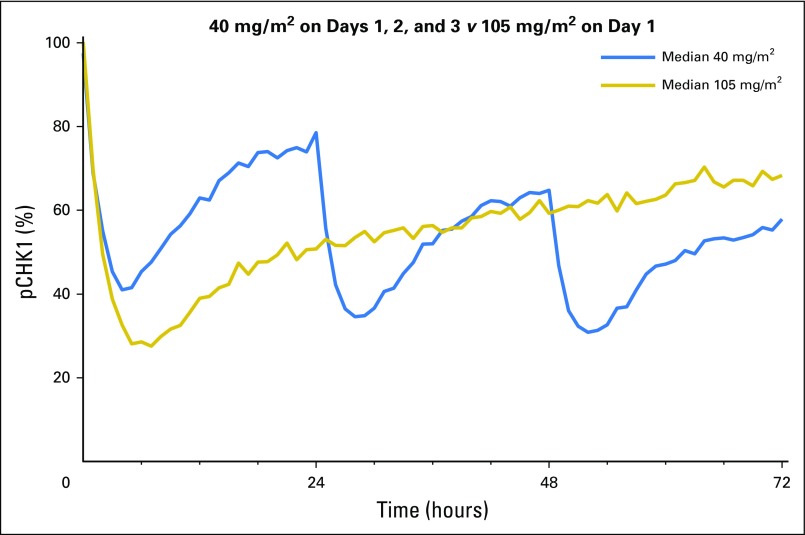
Although the magnitude of duration of pCHK1 inhibition needed for optimal clinical activity is not known, the simulated AUC and average PD profiles over 72 hours are approximately the same for both MTDs. The MTD on schedule 2 produces a greater initial magnitude of predicted percent CHK1 inhibition compared with the schedule 1 MTD. However, starting on day 2, the magnitude of predicted inhibition for schedule 1 is less than or equal to the inhibition predicted for schedule 2 (Appendix Fig A4). The profile differences may result in differences in potential efficacy for the respective schedules.
The mean LY2606368 t1/2 range (11.4 to 27.1 hours) across schedules, days, and cycles of treatment at the MTDs was similar and consistent with a t1/2 suitable for achieving acceptable systemic exposure and minimizing intra- and intercycle accumulation of LY2606368 (Table 4). Because the parameters considered for the selection of the RP2D were generally comparable between the schedules, patient convenience was also a factor in selecting schedule 2 for further study.
The two PRs observed in this study are the first reports of single-agent activity for a CHK1/CHK2 inhibitor. Other inhibitors, such as LY2603618, MK8776 (SCH 900776), AZD7762, and GDC-0425, have shown limited responses in combination with pemetrexed and cisplatin, gemcitabine, and irinotecan.8-13 Both patients with an objective response had SCC, and it is on the basis of these clinical observations that expansion cohorts in SCC were initiated in the dose-expansion phase of the study. Although the CHEK1 gene is overexpressed in some squamous tumors, it is not consistently overexpressed in tumors of squamous histology.14 It is unknown whether the observed activity of LY2606368 is dependent on histology per se or whether the better responses are a result of mechanisms contributing to greater replication stress or other drivers that have yet to be identified. Gadhikar et al15 reported that TP53 mutant/null SCCHN cell lines are resistant to cisplatin through senescence and treating them with a CHK1/CHK2 inhibitor (AZD7762) sensitizes them to cisplatin, leading to mitotic cell death. However, when LY2606368 was assessed as a single agent across a panel of nearly 400 cell lines, the potencies for growth inhibition did not correlate with squamous cell histology, CHEK1 gene expression, or TP53 mutation status (data not shown), suggesting that alternative or additional predictors of response may be operative. In particular, replication stress is emerging as a factor that may play a sensitizing role for response to monotherapy with CHK1/CHK2 inhibitors.16,17 The extent of DNA damage and S-phase defects induced by LY2606368 is reduced when drivers of DNA replication such as CDK2 or CDC25A are knocked down.3 Although replication stress is potentially important in contributing to LY2606368 sensitivity, the distinct mechanisms that may be operative specifically in SCC tumors are not known. Further studies with LY2606368 will explore these questions to gain greater insight into the mechanisms driving single-agent activity and possible biomarkers and strategies for combination chemotherapy that leverage such mechanisms.
In summary, the MTDs were 40 mg/m2 (schedule 1) and 105 mg/m2 (schedule 2) for LY2606368, and the most common toxicity was transient grade 4 neutropenia. The LY2606368 exposure over the first 72 hours at the MTD for each schedule aligned with the exposure predicted to correlate with clinical efficacy. Objective clinical responses were observed in two patients with SCC, the first objective responses observed with a CHK1/CHK2 inhibitor as monotherapy. A dose of 105 mg/m2 administered every 14 days was selected for further evaluation in the ongoing phase Ib dose-expansion part of this study in patients with SCC (anal, head and neck, and non–small-cell lung cancer). This evaluation will help further characterize the efficacy in this subset of patients.
Acknowledgment
We thank the patients who participated in this trial and the study coordinators, nurses, nurse practitioners, clinical research assistants, and doctors who assisted with the research. We also thank Goldy George and Johnique Atkins, who helped edit the article. We also acknowledge Michelle Mynderse and Andrea Humphries for their medical writing assistance, Noelle Gasco for editorial support, Elizabeth Kumm for statistical support, Eric Westin for his contributions to the CHK1 clinical program, and Rodney L. Decker for his assistance with the pharmacokinetic analysis.
Appendix
Pharmacodynamic Markers
Hair follicles were collected for the measurement of pH2A.X using immunohistochemistry (HistoRx, Branford, CT) before dose and at 1 to 2 hours, within 6 hours, and approximately 24 hours after the end of the LY2606368 infusion. Immunohistochemistry was performed using pH2A.X (Ser 139) antibody (JBW301; Millipore, Billerica, MA), pan-cytokeratin antibody (M3515; Dako, Carpinteria, CA) to identify epithelial cells and membrane/cytoplasmic regions, and DAPI (P36931; Invitrogen, Carlsbad, CA) to stain nuclei. Samples were imaged at × 20 magnification to capture complete bulb and outer sheath regions and analyzed for nuclear pH2A.X expression using automated quantitative analysis.
Circulating tumor cells (CTCs) were evaluated for pH2A.X by Genoptix (Carlsbad, CA) using the CellSearch CXC Kit (Janssen Diagnostics, Raritan, NJ) with an H2A.X antibody (05-636; Millipore) conjugated to phycoerythrin and added to the open phycoerythrin channel. Blood samples were drawn for measurement of plasma concentrations of DNA using a validated polymerase chain reaction (PCR) method and for evaluation of CK18 using an enzyme-linked immunoabsorbent assay. CTCs were isolated from whole blood using the CellTracks AutoPrep System (Janssen Diagnostics), an automated sample preparation instrument that uses a software protocol for the immunomagnetic selection and staining of CTCs using the CellSearch CXC Kit. Analysis of CTCs was performed using the CellTracks Analyzer II, a semiautomated fluorescence microscope that is used to identify and enumerate CTCs. Blood samples were drawn for measurement of plasma concentrations of circulating DNA isolated with the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) using a validated quantitative PCR method for β-actin by quantitative PCR (Transgenomic, Omaha, NE) using the following primers: 5′-CCTGGCACCCAGCACAAT-3′, 5′-GCCGATCCACACGGAGTACT-3′, and 5′-FAM and 3′-BHQ labeled probe 5′-TCATTGCTCCTCCTGAGCGC-3′ (Sigma-Aldrich, St Louis, MO). Plasma evaluation of CK18 was performed using enzyme-linked immunoabsorbent assays at Quintiles (Marietta, GA) using the M30 Apoptosence and M65 assays (Peviva, Nacka, Sweden) according to the manufacturer’s recommendations.
Statistical Analysis
Data were summarized by dose and schedule unless stated otherwise. Continuous variables were summarized using the number of patients, mean, median, standard deviation, SE, minimum, and maximum. Categorical end points were summarized using number of patients, frequency, and percentages and their SEs.
Fig A1.
LY2606368 nonclinical pharmacokinetic (PK)/pharmacodynamic (PD) model. PK model: CL, total plasma clearance; Q2, distribution clearances for compartments 1 and 2, respectively; V1 and V2, apparent volumes of distribution of the central and peripheral compartments, respectively. Indirect response PD model: C, plasma concentration of LY2606368; IC50, plasma concentration resulting in 50% pCHK1 inhibition; IMAX, maximum percentage of pCHK1 inhibition; kin, the zero-order rate constant for the activation (phosphorylation) of checkpoint kinase 1 (CHK1); kout, first-order rate constant responsible for inactivation (dephosphorylation) of CHK1; pCHK1, phosphorylated checkpoint kinase 1 enzyme; γ, Hill coefficient.
Fig A2.
Neutrophil counts in cycles 1 and 2. (A) Dose of 40 mg/m2 on days 1 to 3 every 14 days. (B) Dose of 105 mg/m2 on day 1 every 14 days. Arrows indicate the day a drug infusion was given.
Fig A3.
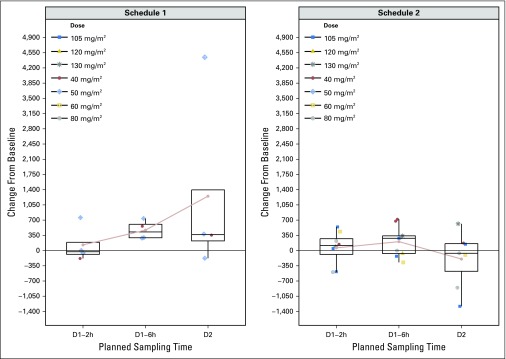
Change in pH2A.X levels in hair follicles. The average change from baseline of postdose pH2A.X levels measured in hair follicles for schedule 1 (left panel) and schedule 2 (right panel) on day (D) 1 (2 and 6 hours [h] after dose) and D2 at the indicated dosages. Each plotted data point represents a patient receiving the indicated dose. The circles connected by the light red represent the mean change from baseline at each sampling time.
Fig A4.
Median predicted human phosphorylated checkpoint kinase 1 (pCHK1; % of baseline) profiles after administration of 40 mg/m2 on days 1, 2, and 3 and 105 mg/m2 on day 1 of cycle 1.
Table A1.
Best Overall Response and Duration of Response
| Response | LY2606368 (N = 45)* |
|---|---|
| Best overall response,† No. (%) | |
| CR | 0 |
| PR | 2 (4.4) |
| SD | 15 (33.3) |
| PD | 20 (44.4) |
| NE | 8 (17.8) |
| Overall response rate (CR+PR), % | 4.4 |
| 95% CI‡ | 0.8 to 13.3 |
| Clinical benefit (CR+PR+SD), % | 37.8 |
| 95% CI‡ | 25.7 to 51.1 |
| Duration of clinical benefit, months, range | |
| Clinical benefit | 1.2-7.2 |
| Clinical benefit in SCCHN | 1.6-7.2 |
Abbreviations: CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; SCCHN, squamous cell carcinoma of the head and neck; SD, stable disease.
Total safety population.
Evaluated using Response Evaluation Criteria in Solid Tumors (RECIST v1.1). A response (CR or PR) required confirmation at least 4 weeks after initial response. Assignment of SD required 6 weeks (≥ 36 days) to elapse between first dose and disease assessment. Patients without a valid response assessment 6 or more weeks after enrollment who had not already experienced progression were assigned a best response of NE.
Clopper-Pearson exact method.
Footnotes
Supported by Eli Lilly.
Presented, in part, at the 104th Annual Meeting of the American Association for Cancer Research, Washington, DC, April 6-10, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01115790
AUTHOR CONTRIBUTIONS
Conception and design: David Hong, Jeffrey Infante, Suzanne Jones, Howard Burris, Lisa Golden, Scott Hynes, Ji Lin, Aimee Bence Lin
Administrative support: Ly M. Nguyen, Lisa Golden
Provision of study materials or patients: David Hong, Filip Janku, Howard Burris, Sarina Piha-Paul, Lisa Golden, Johanna Bendell
Collection and assembly of data: Jeffrey Infante, Filip Janku, Suzanne Jones, Ly M. Nguyen, Howard Burris, Aung Naing, Todd M. Bauer, Sarina Piha-Paul, Lisa Golden, Ji Lin, Johanna Bendell
Data analysis and interpretation: Jeffrey Infante, Suzanne Jones, Howard Burris, Todd M. Bauer, Faye M. Johnson, Razelle Kurzrock, Lisa Golden, Scott Hynes, Ji Lin, Aimee Bence Lin, Johanna Bendell
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I Study of LY2606368, a Checkpoint Kinase 1 Inhibitor, in Patients With Advanced Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
David Hong
No relationship to disclose
Jeffrey Infante
Consulting or Advisory Role: Personal Genome Diagnostics (Inst)
Research Funding: Sarah Cannon Research Institute (Inst)
Filip Janku
Consulting or Advisory Role: Deciphera, Trovagene, Sequenom, Novartis, Foundation Medicine
Research Funding: Novartis, Biocartis, Trovagene, Plexxikon, Astellas Pharma, Agios, BioMed Valley Discoveries, Deciphera
Suzanne Jones
No relationship to disclose
Ly M. Nguyen
Employment: The University of Texas MD Anderson Cancer Center
Howard Burris
No relationship to disclose
Aung Naing
Research Funding: National Cancer Institute, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor, Amplimmune, ARMO BioSciences, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Baxter (I)
Travel, Accommodations, Expenses: ARMO BioSciences
Todd M. Bauer
No relationship to disclose
Sarina Piha-Paul
No relationship to disclose
Faye M. Johnson
Research Funding: Piqur Pharmaceuticals
Razelle Kurzrock
Leadership: RScue, Rx (cofounder), Novena, Curematch,
Stock or Other Ownership: RScue, Rx (cofounder), Novena, Curematch,
Honoraria: Casdin Capital, Sequenom, NXT Strategies, Guardant Health
Consulting or Advisory Role: Sequenom, Actuate Therapeutics
Speakers' Bureau: Kiwanis
Research Funding: Genentech, Merck Serono, Foundation Medicine, Pfizer, Guardant, Sequenom
Lisa Golden
Employment: Eli Lilly
Stock or Other Ownership: Eli Lilly
Scott Hynes
Employment: Eli Lilly
Stock or Other Ownership: Eli Lilly
Travel, Accommodations, Expenses: Amgen
Ji Lin
Employment: Eli Lilly
Stock or Other Ownership: Eli Lilly
Aimee Bence Lin
Employment: Eli Lilly, Eli Lilly (I)
Stock or Other Ownership: Eli Lilly, Eli Lilly (I)
Johanna Bendell
No relationship to disclose
REFERENCES
- 1.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16:376–383. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNeely S, Beckmann R, Bence Lin AK. CHEK again: Revisiting the development of CHK1 inhibitors for cancer therapy. Pharmacol Ther. 2014;142:1–10. doi: 10.1016/j.pharmthera.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 3.King C, Diaz HB, McNeely S, et al. LY2606368 causes replication catastrophe and antitumor effects through CHK1-dependent mechanisms. Mol Cancer Ther. 2015;14:2004–2013. doi: 10.1158/1535-7163.MCT-14-1037. [DOI] [PubMed] [Google Scholar]
- 4. Wu W, Bi C, Bence Lin AK, et al: Antitumor activity of Chk1 inhibitor LY2606368 as a single agent in SW1990 human pancreas orthotopic tumor model. Proc Am Assoc Cancer Res 72, 2012 (abstr 1776)
- 5.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 6. National Cancer Institute: Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 4.02. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 7.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 8.Sausville E, Lorusso P, Carducci M, et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73:539–549. doi: 10.1007/s00280-014-2380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seto T, Esaki T, Hirai F, et al. Phase I, dose-escalation study of AZD7762 alone and in combination with gemcitabine in Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol. 2013;72:619–627. doi: 10.1007/s00280-013-2234-6. [DOI] [PubMed] [Google Scholar]
- 10.Daud AI, Ashworth MT, Strosberg J, et al. Phase I dose-escalation trial of checkpoint kinase 1 inhibitor MK-8776 as monotherapy and in combination with gemcitabine in patients with advanced solid tumors. J Clin Oncol. 2015;33:1060–1066. doi: 10.1200/JCO.2014.57.5027. [DOI] [PubMed] [Google Scholar]
- 11. Infante JR, Hollebecque A, Postel-Vinay S, et al: Phase I study of GDC-0425, a checkpoint kinase 1 inhibitor, in combination with gemcitabine in patients with refractory solid tumors. Proc Am Assoc Cancer Res 75:15s, 2015 (suppl; abstr CT139) [DOI] [PubMed] [Google Scholar]
- 12. Ho AL, Bendell JC, Cleary JM, et al: Phase I, open-label, dose-escalation study of AZD7762 in combination with irinotecan (irino) in patients (pts) with advanced solid tumors. J Clin Oncol 29, 2011 (suppl; abstr 3033) [Google Scholar]
- 13.Calvo E, Chen VJ, Marshall M, et al. Preclinical analyses and phase I evaluation of LY2603618 administered in combination with pemetrexed and cisplatin in patients with advanced cancer. Invest New Drugs. 2014;32:955–968. doi: 10.1007/s10637-014-0114-5. [DOI] [PubMed] [Google Scholar]
- 14.OmicSoft Corporation Omicsoft Oncoland TCGA dataset. http://www.omicsoft.com.
- 15.Gadhikar MA, Sciuto MR, Alves MV, et al. Chk1/2 inhibition overcomes the cisplatin resistance of head and neck cancer cells secondary to the loss of functional p53. Mol Cancer Ther. 2013;12:1860–1873. doi: 10.1158/1535-7163.MCT-13-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecona E, Fernández-Capetillo O. Replication stress and cancer: It takes two to tango. Exp Cell Res. 2014;329:26–34. doi: 10.1016/j.yexcr.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meuth M. Chk1 suppressed cell death. Cell Div. 2010;5:21. doi: 10.1186/1747-1028-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]