Abstract
Sterilization of soft biomaterials such as hydrogels is challenging because existing methods such as gamma irradiation, steam sterilization, or ethylene oxide sterilization, while effective at achieving high sterility assurance levels (SAL), may compromise their physicochemical properties and biocompatibility. New methods that effectively sterilize soft biomaterials without compromising their properties are therefore required. In this report, a dense-carbon dioxide (CO2)-based technique was used to sterilize soft polyethylene glycol (PEG)-based hydrogels while retaining their structure and physicochemical properties. Conventional sterilization methods such as gamma irradiation and steam sterilization severely compromised the structure of the hydrogels. PEG hydrogels with high water content and low elastic shear modulus (a measure of stiffness) were deliberately inoculated with bacteria and spores and then subjected to dense CO2. The dense CO2-based methods effectively sterilized the hydrogels achieving a SAL of 10−7 without compromising the viscoelastic properties, pH, water-content, and structure of the gels. Furthermore, dense CO2-treated gels were biocompatible and non-toxic when implanted subcutaneously in ferrets. The application of novel dense CO2-based methods to sterilize soft biomaterials has implications in developing safe sterilization methods for soft biomedical implants such as dermal fillers and viscosupplements.
Keywords: dense CO2, soft hydrogels, sterilization, bacteria, spores, PEG
Introduction
The need for soft, injectable biomaterials is growing as the market for dermal fillers, viscosupplements, and tissue-engineered products continues to grow rapidly. Demonstration of sterility of these implants before human use is a regulatory requirement. Typically, sterilization of biomedical materials and implants is carried out post-manufacture using methods approved by the US Food and Drug Administration (FDA). Conventional methods of sterilizing biomedical materials include steam sterilization, ultra-high temperature treatment, ethylene oxide exposure, hydrogen peroxide exposure, and e-beam or gamma-irradiation (Russell et al., 1999). These methods are, however, plagued with a variety of drawbacks such as toxic residues, chemical changes to the material, and changes to the physical and mechanical properties of the materials being sterilized (Clayton, 1996; Dempsey and Thirucote, 1989; Dillow et al., 1999). These drawbacks severely limit the utility of these sterilization techniques for hydrogels that are high in water content and are sensitive to temperature and radiation. Post-manufacture sterilization of hydrogels therefore presents the challenge of maintaining the physicochemical, biological, and mechanical properties of the hydrogel, while at the same time achieving sterilization levels required by the regulatory authorities.
Identifying a sterilization process that can achieve the required sterility without compromising the hydrogel properties is challenging. A strategy that is commonly used to avoid the problems caused by end-point sterilization of hydrogels involves sterilizing the hydrogel precursor chemicals in a dry form or filtering the precursor solutions of the raw-materials through sterile, low pore size (typically 0.2 μm) filters before use (Russell et al., 1999). The hydrogel product is then manufactured aseptically in strictly controlled sterile cGMP conditions. Alternatively, the dry, sterile hydrogel precursor chemicals are sold along with sterile suspension buffer directly to the end user in sterile packages and can be used to form hydrogels in-situ for the prescribed application (Huebsch et al., 2005). However, sterilization of precursor chemicals is not always possible because of drawbacks of conventional methods mentioned earlier. Also, filtration of hydrogel precursor solutions may be challenging for viscous polymer precursor solutions due to issues such as adsorption of polymers to the filters and fouling of membrane filters (Belfort et al., 1994; Lo et al., 1996; Zaidi and Kumar, 2004). In addition, sterilization or filtration of precursor chemicals may not be a viable option if subsequent manufacturing steps are complicated as that might result in high risk of compromising sterility and/or increased costs of aseptic cGMP-manufacture. The US-FDA has recognized these limitations and allows the use of validated, non-conventional methods of sterilization for biomedical implants (FDA, 2008). New, reproducible, and safe methods of sterilization that can be used to sterilize soft hydrogels post-manufacture without compromising their properties, therefore, need to be developed.
Dense CO2 Sterilization
The use of dense CO2 as a sterilization medium is being explored because it is non-flammable, non-toxic, and has a low critical point (Duarte et al., 2009). The antimicrobial properties of DGs (usually CO2) have previously been demonstrated on suspensions of bacteria (Ballestra and Cuq, 1998; Calvo and Diaz, 2005; Hemmer et al., 2007; Spilimbergo and Bertucco, 2003; Spilimbergo et al., 2002, 2003a, 2003b). The inert nature of dense CO2, its low viscosity and low surface tension make it a suitable sterilization medium for polymers in general, and polymers with complex morphologies. Although, various gases (argon, N2, and N2O) have been used to sterilize Escherichia coli at 37°C and pressures between 17 and 62 bar, it was found that CO2 was most effective (Fraser, 1951).
The exact deactivation mechanism which influences the ability of CO2 to terminally sterilize bacteria is still under investigation (Dehghani et al., 2009). However, the following factors are known to affect CO2 sterilization efficiency: pressure, temperature, depressurization rate, and pressure cycling (Yoganathan et al., 2010). Dense CO2 sterilization has shown to be an effective sterilant against food products in liquid media (Dehghani et al., 2009) and solid polymeric materials (Zhang et al., 2005, 2006b). Particularly in the food industry, it has been used to pasteurize raw fluid milk at non-thermal conditions (Werner and Hotchkiss, 2006). Its effectiveness in different fields and different mediums (solid or liquid) is dictated by its temperature, pressure, density, and mass transfer properties. Irrespective of which medium dense CO2 sterilization is being used, the principles of its bacterial inactivation are likely to be one or more of the following; cytoplasmic pH decrease, explosive cell rupture due to internal pressure, modification of cell membrane and extraction of cell wall lipids, inactivation of key enzymes for cell metabolism, extraction of intracellular substances, bicarbonate conversion to carbonate, and subsequent intracellular precipitation of salts (Andras et al., 2010). The operating conditions for sterilization are species specific. The two main mechanisms which affect the sterilization of vegetative cells are mechanical cell rupture and physiological deactivation (Zhang et al., 2005, 2006b). Furthermore, dense CO2 is seen as a viable alternative to current sterilization technologies because it does not diminish the mechanical properties of medical grade polymers after the sterilization process (Jiménez et al., 2007). Much literature has been published on the effects of dense CO2 on bacteria and polymers separately, whilst studies on the sterilization effect of CO2 on polymers inoculated with bacteria are limited. Medical-grade polymers have maintained their mechanical properties after CO2 exposure (Jiménez et al., 2007). Few papers have studied the effects of CO2 sterilization on biomedical polymers, and especially its effects on hydrogels (Jiménez et al., 2008). Dillow et al. (1999) reported the sterilizing effect of dense CO2 on polymer microspheres inoculated with bacteria. Poly(lactic-co-glycolic) acid (PLGA) and polylactic acid (PLA) microspheres were sterilized using dense CO2 with and without water as a co-solvent at 140 bar and 34°C, and it was found that water reduced the sterilization time. Treatment with dense CO2 did not change the FTIR spectra of the microspheres. Jimenez et al. sterilized a poly(acrylic acid-co-acrylamide) hydrogel using dense CO2 at 276 bar and 40°C for 4 h after inoculation with Staphylococcus aureus and E. coli (Jiménez et al., 2008). Terminal sterilization of S. aureus was achieved after 30 min, and the dense CO2 did not alter the physical structure of the hydrogel. Hydrogen peroxide was also introduced into the process, and a similar bacterial log reduction was achieved with and without hydrogen peroxide.
Studies by Zhang et al. (2006b) on sterilization of gram-positive bacteria, gram-negative bacteria, and spores using supercritical CO2 indicate that spores are more resistant to dense CO2 sterilization than vegetative cells. The sterilization of spores was ineffective even with a peroxide additive. Unlike most vegetative cells, spores can withstand heat, ultraviolet (UV) light, free radicals, and chemicals. Spores may be more resistant to sterilization because they are less hydrated than vegetative cells, 10–25% water content compared to 80–90% water content in vegetative cells. The sterilization of spores such as Geobacillus stearothermophilus, Bacillus atrophaeus, and B. pumilus is, therefore, used as a standardized measure of equipment sterility (Zhang et al., 2006b). The sterilization of B. subtilis spores is also used as a bio-indicator for sterility assurance (ANSI, 1995; FDA, 1993; ISO, 2000). In the study reported here conditions that can sterilize hydrogels inoculated with B. subtilis spores were, therefore, investigated.
The aim of this study was to investigate the applicability of dense CO2-based methods for the effective sterilization of soft polymeric hydrogels without compromising their properties and without additives such as hydrogen peroxide. As a proof-of-concept, PEG-based soft hydrogels were used in this study. The structure of hydrogels treated using either conventional gamma radiation sterilization, steam sterilization, or dense CO2-based sterilization was compared. PEG hydrogels inoculated with three clinically relevant opportunistic pathogens, namely, S. epidermis (gram-positive bacteria), Pseudomonas aeruginosa (gram-negative bacteria), and B. subtilis spores were subjected to dense CO2 and the efficacy of dense CO2 as a sterilant for the selected bacteria was tested. Temperature, pressure, and process time required to achieve effective sterilization while maintaining hydrogel structure and properties were investigated. The physicochemical and viscoelastic properties of the hydrogels before and after sterilization were characterized using optical microscopy, pH measurement, swelling ratio measurements, and oscillatory shear rheometry. Preliminary evaluation of in vivo biocompatibility was done by subcutaneous implantation of the dense CO2- treated hydrogel in ferrets.
Materials and Methods
Pseudomonas aeruginosa (1 ATCC 25668), S. epidermidis (1 ATCC 14990), and B. subtilis (1 ATCC 6051) were purchased from the Microbiology Culture Collection Centre (School of Biotechnology and Biomolecular Sciences at the University of New South Wales, Sydney, NSW, Australia). Tryptone soy broth (TSB; OXOID CM0179), plate counting agar (PCA; OXOID CM009), and peptone water (OXOID CM0325) purchased from OXOID (Sydney, Australia) were used for bacteria preparation and cell counting. Cylinders of compressed CO2 grade 2.5 were supplied by Linde (Sydney, Australia). Polyethylene glycol diacrylate (10,000 Da; PEG-DA) was from SunBio (Orinda, CA). Polyethylene glycol (10,000 Da; PEG) was from Sigma–Aldrich (St. Louis, MO). Irgacure 2959 was from Ciba (Newport, DE). The UV light source, EXFO Lite, was purchased from EXFO Lifesciences (Mississauga, Ontario, Canada).
Preparation of PEG Hydrogels
Semi-interpenetrating networks (semi-IPN) of PEG-DA and PEG were prepared by polymerizing PEG-DA in the presence of PEG. Aqueous solutions of PEG-DA (100 mg/ mL) and PEG (100 mg/mL) were mixed in the volumetric ratio 3:7 and the precursor solution was exposed to UV light of intensity 10 mW/cm2 (as measured using a Radiometer centered at 365 nm) for 200 s to prepare the hydrogel. Irgacure 2959 (0.05% w/v) was used as the photoinitiator. Gels were then incubated in PBS at 37°C for 24 h. The swollen gels were softened by successive shearing through 16, 18, 20, and 22 gauge needles and stored in syringes at 4°C until further use.
Preparation of Bacteria
P. aeruginosa, S. epidermidis, and B. subtilis were grown in separate test tubes of TSB at 35°C for 24 h. The TSB suspensions were then streaked on PCA plates to isolate individual colonies. The streaked bacterial PCA plates were then incubated for 24 h at temperatures of 25, 35, and 37°C for P. aeruginosa, S. epidermidis, and B. subtilis, respectively. P. aeruginosa and S. epidermidis were then suspended in a solution of 0.1 mg/ml peptone in deionized water. Solutions were prepared of 108 colony forming units (CFU)/mL and 109 CFU/mL of P. aeruginosa and S. epidermidis, respectively. Standard plating technique with cell counting dilutions in 0.1 mg/mL peptone in deionized water was used to assess the concentration of bacteria.
Spore Formation
B. subtilis spores were prepared by transferring the B. subtilis from plates to a solution of 0.1 mg/mL peptone in deionized water. The B. subtilis suspension was then heat-shocked at 80°C for 10 min to sporulate the vegetative cells. The heat-shocked product was observed under microscope to observe cell morphology and assure complete sporulation. The B. subtilis spore suspension was centrifuged at 3,000 rpm for 15 min. The supernatant was removed and the pellet was re-suspended in 10% w/v peptone in deionized water.
Hydrogel Inoculation
The hydrogels were inoculated with 107 CFU/g before CO2 processing. The hydrogels were inoculated with suspensions of P. aeruginosa, S. epidermidis, and B. subtilis spores separately. The spores inoculated in the hydrogel looked identical to those in the peptone solution when observed using an optical microscope (see Supporting Information Fig. S1). Each inoculated sample was mixed thoroughly using a magnetic stir-bar and stirrer. Half of the inoculated hydrogels was mixed with diluent (10% w/v peptone in deionized water) then plated to enumerate the bacterial cells. The remainder of the inoculated hydrogels was stored in a sterile vial prior to CO2 processing.
Dense CO2 Treatment
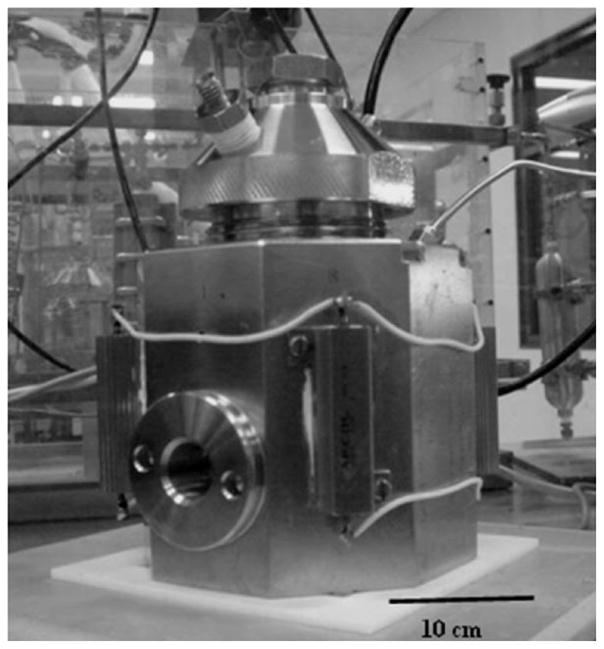
The hydrogels (~4 mL) were placed in a temperature-controlled 100 mL stainless steel reactor (Fig. 1) and exposed to dense CO2 with stirring using a magnetic stir-bar. After the sterilization process, the processed biomaterial was mixed with diluent, and the bacterial load in the diluent was enumerated using the standard plating technique. The same standard plating technique was used to enumerate the spores before the dense CO2 treatment. The B. subtilis spores were viewed under a microscope to ensure that they were still dormant, before inoculation. The CO2 processing times were kept below the required time needed for the spores to become vegetative.
Figure 1.
The dense CO2 sterilization apparatus.
Gamma Irradiation and Steam Sterilization
Gammacell 220 Excell (MDS Nordion, Ottawa, Canada) with a Co-60 source was used and the intensity of the gamma radiation was modulated using cast lead attenuators containing 6% antimony. A syringe containing the gel was enclosed in an autoclave pouch and loaded in the machine. The pouch was surrounded by ice to prevent the temperature of the gels from rising because of the gamma radiation. The rate of gamma irradiation was varied by using attenuators of different thicknesses and the total dosage was varied by controlling the amount of time the gels were exposed to the radiation. The hydrogels were steam-sterilized using a Ritter Midmark M9 Ultraclave Autosterilizer (Midmark, Versailles, OH). The gels were filled in plastic syringes and sterilized using a dry cycle that involved sterilization for 5 min at 132°C and 186 kPa followed by drying for 30 min.
Hydrogel Analysis
Optical microscopy was used to image the hydrogels before and after gamma-irradiation, steam sterilization, and dense CO2 processing. A small amount of the gel (~0.02 mL) was added to an aqueous dispersion of India Ink to visualize the gel shape and particle size. The dispersion was shaken slightly to separate the gel particles and then visualized under a microscope.
The viscoelastic shear properties of the hydrogels were measured using an AR-2000 rheometer (TA Instruments, Inc., New Castle, DE). A cone-and-plate geometry was used to apply oscillatory shear to hydrogel samples using an acrylic cone (60 mm diameter, 2° angle) and a flat metallic peltier plate heated to 37°C. Sheared gels were placed between the heated plate and the cone so that a manufacturer-specified gap of 61 μm was maintained between the cone and the plate. The gel was subjected to an oscillatory shear at 1 Hz for 2 min to equilibrate the entire gel to a uniform temperature of 37°C.
Strain sweep tests were done to ensure that the shear property measurements were in the linear region of the stress–strain curve. The viscoelastic shear properties are independent of the percentage strain in the linear region. A target shear strain value was therefore identified by measuring the viscoelastic shear properties as a function of percentage strain applied. A strain of 0.6% was typically used to measure the shear properties as a function of frequency from 1 to 10 Hz. The elastic shear modulus (G′) at 10 Hz was used to compare the mechanical properties of the hydrogels treated with different sterilization methods.
In Vivo Study
The in vivo study was performed in accordance with the PHS Policy on Human Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.). The animal use protocol was approved by the Committee on Animal Care (CAC) and the Institutional Animal Care and Use committee (IACUC) of the Massachusetts General Hospital.
Two to four subcutaneous injections (~0.2–0.3 mL) of the dense CO2-treated hydrogels were made on the dorsal surface of adult male ferrets (n = 2; Marshall Farms, North Rose, NY). Hydrogels prepared in a biological safety hood using sterile technique and disinfected with 70% ethanol were used as controls. A survival time-period of 1 month was used. Post-euthanasia, the implants were recovered and fixed in 10% formalin. Five micron thick sections were cut from paraffin-embedded tissue and the tissue response to the implant was evaluated histologically.
Results and Discussion
PEG Hydrogel Formulation
As new biomaterials for implantation continue to be developed, the need to identify methods to safely sterilize the materials without compromising their functional properties has grown. The goal of this study was to explore the utility of dense CO2 to effectively sterilize a soft, injectable hydrogel biomaterial without compromising its structure, mechanical properties and biocompatibility. To that end, we prepared a soft PEG hydrogel with high water content (≥95%). PEG was chosen for this work because it is a biocompatible polymer that has been used extensively to make hydrogels for clinical and research use (Cruise et al., 1999; Herten et al., 2009; Ifkovits and Burdick, 2007). A semi-IPN of PEG was prepared by photocrosslinking PEG-DA in the presence of PEG. The final gel was injectable using a 25-gauge needle, had a water content ≥95% (w/w) and a low elastic shear modulus (a measure of stiffness) that ranged from 20 to 40 Pa when measured at 10 Hz and 37°C.
Gamma Irradiation and Steam Sterilization of Gels
The effects of conventional sterilization methods and dense CO2-based sterilization on the gel were evaluated by comparing the morphology and mechanical properties of the gel pre- and post-sterilization. The presence of water in the hydrogel makes it unsuitable for sterilization using either ethylene oxide or hydrogen peroxide as they may leave toxic residues (Dillow et al., 1999). Therefore, sterilization using gamma irradiation and steam sterilization were evaluated.
Since it is known that gamma radiation can affect the physicochemical properties and morphology of polymers and gels (Kanjickal et al., 2008), the effect of gamma irradiation on the morphology of the gels were first tested. While the radiation dose used for sterilization depends on the type of material or polymer being sterilized, a typical dose for achieving effective sterilization is 2.5 Megarad (Mrad; Clough, 2001). Hence radiation dosages of 2.5 and 0.5 Mrad and irradiation rates of 1,527 rad/min and 4,582 rad/min were used. Thus a total of four different irradiation conditions were tested.
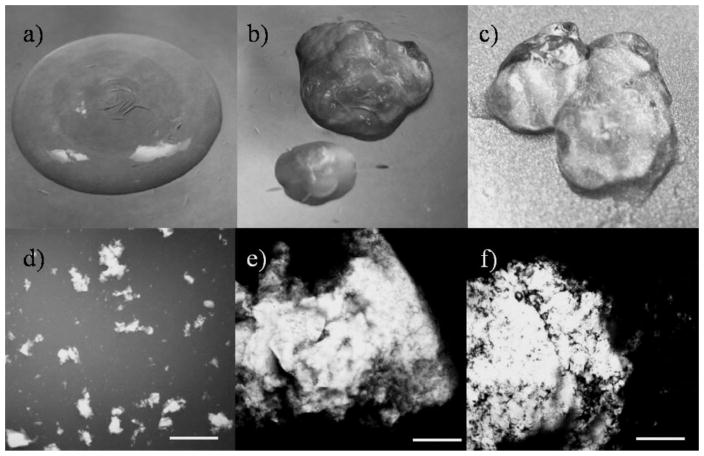
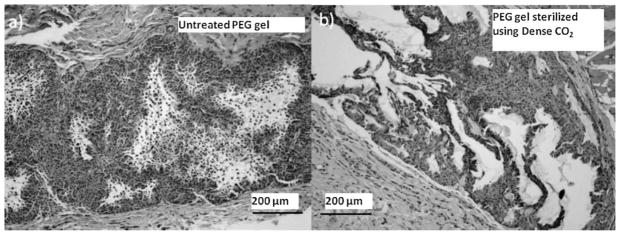
Significant changes in the gel morphology were observed upon gamma irradiation. The untreated gels were homogenous (Fig. 2a), while the gamma-irradiated gels separated into two distinct phases (Fig. 2b) – a primary phase consisting of large aggregates of gel-like particles suspended in a secondary watery phase. Figure 2b depicts the phase separation in a gel after being exposed to 0.5 Mrad of gamma radiation at an irradiation rate of 1,527 rad/min (the lowest dosage at the slowest irradiation rate tested). The gels were then added to an aqueous dispersion of India Ink which dispersed the gel particles allowing easier observation of size and shape. The untreated gels consisted of small and distinct particles ranging in size from sub-micron to a few hundred microns (Fig. 2d), while the gamma-irradiated gels had large aggregates measuring 5–10 mm. Similar results were observed for gels treated with gamma irradiation using the other three conditions tested (data not shown). Gamma irradiation has been used to prepare gels by crosslinking PEG polymers (Stringer and Peppas, 1996); it is possible that the gel aggregates seen upon irradiation of the gels may be a result of such crosslinking.
Figure 2.
Visual characterization of the gels before and after treatment with conventional sterilization methods. The gross appearance and the microstructure of the control untreated gel is shown in (a) and (d), respectively. Corresponding images for a gel treated with gamma radiation (dosage = 0.5 Mrad at 1,527 rad/min) are shown in (b) and (e), while images for gels treated with steam sterilization (132°C, 186 kPa, 5 min) are shown in (c) and (f), respectively. Scale bar = 1 mm.
Steam sterilization also adversely affected the morphology of the gels. Gels exposed to steam sterilization phase separated into sticky, aggregated gel-like particles suspended in minimal water (Fig. 2c and f). Both gamma irradiation and steam sterilization severely compromised the structure of the gels and hence were deemed unsuitable methods for sterilizing the gels. Therefore, additional characterization of sterility levels or rheological properties of the gels using these methods were not conducted.
Dense CO2 Sterilization of Gels
The operating conditions for dense CO2 sterilization are highly material and pathogen specific. Hence the operating conditions required to sterilize different pathogens incorporated in the PEG hydrogel were first identified and then the effect of dense CO2 on the properties of the gels under those conditions were tested. The gels were inoculated separately with three different clinically relevant bacterial species – S. epidermidis, P. aeruginosa, and B. subtilis. S. epidermidis are known to cause infection through skin penetration (Hedin, 1993), and P. aeruginosa can compromise human tissue once the host defenses are down (Pollack, 2000). B. subtilis was chosen because it too is a human pathogen, and its spore form is a valid bio-indicator for sterility assurance (ANSI, 1995; FDA, 1993; ISO, 2000).
B. subtilis spores are the most difficult species to sterilize (Zhang et al., 2006b). Conditions that would inactivate the spores in the PEG gels were therefore first identified and then tested on gels inoculated with vegetative bacteria. Process parameters including temperature, pressure, and processing time were varied systematically and three conditions that resulted in a log reduction of seven in spore content (i.e., sterility assurance levels [SAL] of 10−7) were identified: 70°C, 150 bar, 4 h; 70°C, 75 bar, 6 h; 80°C, 50 bar, 1 h (Table I). A log reduction of 0 (Table I) was seen when gels inoculated with spores were treated at 70°C and atmospheric pressure. The inactivation of the spores at 70°C was thus due to dense CO2 pressure and not due to high temperature alone. A SAL of 10−7 was successfully achieved for each of the bacterial species at 70°C and 75 bar in 6 h (Table I). SAL of 10−7 was achieved for the gels containing P. aeruginosa and S. epidermidis also using a lower temperature of 40°C and 250 bar in 1 h. The B. subtilis spores were resistant to inactivation under these conditions. Log-reductions of seven or higher for poly(acrylic acid-co-acrylamide) hydrogels inoculated with vegetative bacterial cells such as E. coli and S. aureus have been reported by Jiménez et al. (2008) using similar operating conditions. In contrast to the study by Jimenez et al., conditions that can successfully kill spores were also investigated in this study. Our results are consistent with results obtained for porous PLGA and PLA microspheres that have been sterilized using dense CO2 at similar pressures and temperatures without compromising their physicochemical properties (Dillow et al., 1999).
Table I.
The effect of different dense CO2 conditions on the inactivation of spores and bacteria inoculated in the PEG gel.
| Bacteria | Temperature (°C) | Pressure (bar) | Time (h) | Log reduction |
|---|---|---|---|---|
| Bacillus subtilis spores | 40 | 250 | 1 | 0 |
| 60 | 120 | 4 | 1 | |
| 60 | 150 | 4 | 1 | |
| 60 | 200 | 4 | 1 | |
| 70 | 1.01 | 4 | 0 | |
| 70 | 50 | 4 | 0 | |
| 70 | 75 | 4 | 5 | |
| 70 | 75 | 6 | 7 | |
| 70 | 100 | 2 | 2 | |
| 70 | 150 | 1 | 2 | |
| 70 | 150 | 4 | 7 | |
| 80 | 50 | 1 | 7 | |
| Pseudomonas aeruginosa | 40 | 250 | 1 | 7 |
| 70 | 75 | 6 | 7 | |
| Staphylococcus epidermidis | 40 | 250 | 1 | 7 |
| 70 | 75 | 6 | 7 |
It is interesting that we were able to achieve log reductions of seven in viable spore content without the use of an active additive such as hydrogen peroxide that has usually been required to achieve high levels of sterilization of spores using dense CO2 (White et al., 2006; Zhang et al., 2006a). However, while pure supercritical CO2 has been shown to be ineffective in killing dry spores (Hemmer et al., 2007), some studies have shown that distilled water acts as a passive additive that enhances the efficacy of killing spores (Dillow et al., 1999; Jiménez et al., 2008; White et al., 2006). The hydrogel utilized in this study has very high water content (>90% by weight) and it is possible that the water present in the hydrogel acts as a passive additive to assists dense CO2 in killing of spores. In unrelated studies, we found that residual photoinitiator (Irgacure 2959) may be present in small amounts (0.1–0.5 mg/mL) in the hydrogel along with some unreacted reactants and/or breakdown products (Karajanagi SS, unpublished data). It is unclear if these remnants in the gel impact the efficacy of dense CO2-based sterilization observed in this study.
Characterization of Dense CO2-Treated Gels
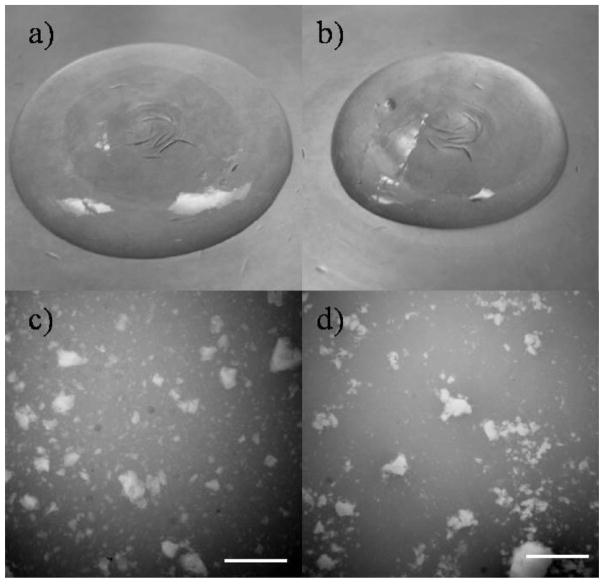
The material properties of gels treated with dense CO2 using the four conditions listed in Table II were characterized in detail. No changes were seen in the gross appearance or microstructure of the gels after treatment with dense CO2 at 70°C and 75 bar for 6 h. All treated gels were optically clear and homogenous, similar to the control (non-inoculated and untreated) gels (Fig. 3a). No phase-separation was seen and the particulate nature of the gels was preserved after the dense CO2 treatment (Fig. 3b). Furthermore, the pH of the treated gels was the same as that of the control gels (pH = 7.4). Similar results were obtained for gels treated with dense CO2 using the three other conditions (data not shown). The swelling ratio (a measure of the amount of water contained in the hydrogel per unit dry weight) of the dense CO2-treated gels at the four conditions was 33.1 ± 1.1 while that for the control gels was 33.6 ± 1.6. Thus dense CO2 treatment had a minimal effect on the water content of the gel suggesting that the water-binding capacity of the PEG polymers in the crosslinked network was unaltered after dense CO2 treatment.
Table II.
The effect of treatment with dense CO2 at different conditions on the mechanical properties of the PEG gels as measured using elastic shear modulus G′. Data are an average of at least three separate experiments (average ± standard deviation).
| Temperature (8C) | Pressure (bar) | Time (h) | Elastic shear modulus G′ (Pa)
|
|
|---|---|---|---|---|
| Before treatment | After treatment | |||
| 40 | 250 | 1 | 34.5 ±7.0 | 34.0 ±8.8 |
| 70 | 75 | 6 | 35.8 ±3.3 | 32.8 ±6.7 |
| 70 | 150 | 4 | 35.8 ±3.3 | 34.0 ±7.0 |
| 80 | 50 | 1 | 30.8 ±7.1 | 23.5 ±5.9 |
Figure 3.
Visual characterization of the gels after treatment with dense CO2. The gross appearance and the microstructure of a gel treated with dense CO2 at 40°C and 250 bar for 1 h is shown in (a) and (c), respectively. Corresponding images for a gel treated with dense CO2 at 70°C and 75 bar for 6 h are shown in (b) and (d). The similarity in appearance and microstructure to the control untreated gel (Fig. 2a and d) can be noted. Similar results were obtained for gels treated with dense CO2 using the other conditions listed in Table II. Scale bar = 1 mm.
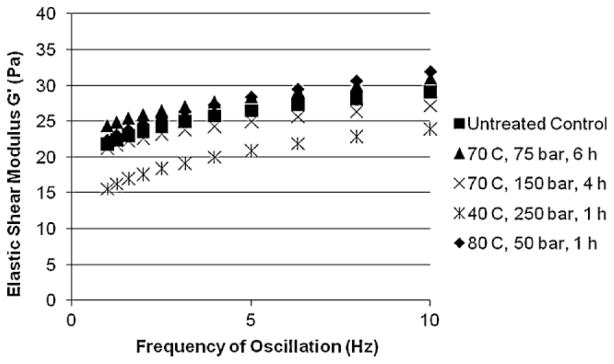
The viscoelastic properties of hydrogels have a significant impact on their injectability, in vivo function and also on the biological response they elicit (Irwin et al., 2008; Saha et al., 2008). Therefore, in addition to maintaining the homogeneity, water content, and pH of the hydrogels, an acceptable sterilization method should not adversely affect their viscoelastic properties. Viscoelastic properties of the PEG gels before and after treatment with dense CO2 were quantified using oscillatory shear rheometry. The elastic shear modulus, G′, measured as a function of frequency of oscillation (1–10 Hz) was used to compare the stiffness of the gels. Example of the data obtained for a typical run for untreated control gels and dense CO2-treated gels are shown in Figure 4. Gels treated with dense CO2 at either 40 or 70°C showed no significant changes in the G′ measured at an oscillatory frequency of 10 Hz and at 37°C (Table II). The hydrogel samples treated with dense CO2 at 80°C, 50 bar and 1 h had only a small decrease in G′. Thus, all three processing conditions that were successful in effectively eliminating the spores (70°C, 150 bar, 4 h; 70°C, 75 bar, 6 h; and 80°C, 50 bar, 1 h) were also successful in retaining the viscoelastic properties of the gels with only a slight softening seen at one condition (80°C, 50 bar, 1 h). Two process conditions (40°C, 250 bar, 1 h and 70°C, 75 bar, 6 h) that effectively eliminated S. epidermidis and P. aeruginosa and one condition (70°C at 75 bar for 6 h) that effectively eliminated all three pathogens, were also successful in simultaneously maintaining the physicochemical and viscoelastic properties of the gels.
Figure 4.
Elastic shear modulus G′ as a function of oscillatory frequency for PEG hydrogels treated with dense CO2 compared to untreated control (n ≥ 3).
Biological Response to Dense CO2-Treated Gels
To evaluate if treatment with dense CO2 affected the in vivo response to the PEG hydrogel, gels subjected to dense CO2 at 40°C and 250 bar for 1 h were implanted subcutaneously in ferrets. PEG gels that were prepared in a biological safety hood using sterile technique and not exposed to dense CO2 were used as control. The tissue response 1 month post-implantation for the dense CO2- treated gel was similar to that for the control gel (Fig. 5). Histological examination revealed the presence of the PEG hydrogel accompanied with macrophage infiltration at the implantation site including and surrounding the gel. The macrophage response was not accompanied by the presence of neutrophils. The macrophages adjacent to the implant had abundant foamy cytoplasm suggesting active phagocytosis of the material. Mild fibrosis was detected but no capsule formation around the implant was observed. Lymphocytes and plasma cells were absent and occasional giant cells were observed. No necrosis or other damage to the surrounding tissue was seen. The reaction was thus a typical foreign body reaction that has also been observed for other PEG implants (Herten et al., 2009; Strehin et al., 2009). The similarity between the biological reaction and the absence of any observable tissue toxicity in both the dense CO2-treated and the control sterile gels suggests that treatment with dense CO2 did not adversely affect the biological response elicited by the PEG hydrogels.
Figure 5.
In vivo tissue response to the PEG gels in ferrets. a: H&E stained image of the untreated PEG gel implanted subcutaneously; (b) H&E stained image of the PEG gel sterilized using supercritical CO2 (40°C, 250 bar, 1 h) implanted subcutaneously.
Conclusions
In conclusion, we have demonstrated the use of dense CO2 to effectively sterilize soft PEG hydrogels without compromising their morphology, pH, water content, and viscoelastic properties. In contrast, conventional methods of sterilization such as gamma-irradiation and steam sterilization severely compromised the morphology of the hydrogels. Dense CO2 techniques were used to sterilize PEG gels inoculated separately with three clinically relevant bacteria including spores. Overall, four processing conditions were identified at which dense CO2 was used to effectively sterilize the inoculated gels, while simultaneously preserving the appearance, morphology, water content, and pH of the gels. In three out of the four conditions the viscoelastic properties were also maintained and in one condition only a slight softening of the gel was observed. Dense CO2 treatment at 70°C and 75 bar for 6 h was successful in effectively eliminating all three pathogens and simultaneously preserving the physicochemical and viscoelastic properties of the gels. Preliminary in vivo studies showed that treatment with dense CO2 did not lead to any observable toxicity in subcutaneous tissue. These encouraging results warrant further detailed in vivo studies using dense CO2-treated hydrogels. Overall, dense CO2-based sterilization is a promising technology to effectively sterilize soft hydrogels with SAL of at least 10−6 while simultaneously preserving their material properties and biocompatibility. Dense CO2- based sterilization, therefore, may be useful as a novel method for safe and effective sterilization of hydrogels for biomedical use.
Supplementary Material
Acknowledgments
The authors would like to thank Hans Richter for assistance in carrying out the experiments involving gamma irradiation. We acknowledge use of the GammaCell (NIH S10-RR017905) in the MIT Center for Environmental Health Science (NIH P30-ES002109).
Contract grant sponsor: Eugene B. Casey Foundation
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Andras CD, Csajagi C, Orban CK, Alberta C, Abraham B, Miklossy I. A possible explanation of the germicide effect of carbon dioxide in supercritical state based on molecular-biological evidence. Med Hypotheses. 2010;74(2):325–329. doi: 10.1016/j.mehy.2009.08.043. [DOI] [PubMed] [Google Scholar]
- ANSI. Sterilization of medical devices-microbiological methods. Part 1. Estimation of population of microorganisms on products 1995 [Google Scholar]
- Ballestra P, Cuq J-L. Influence of pressurized carbon dioxide on the thermal inactivation of bacterial and fungal spores. Lebensmittel-Wissenschaft Technol. 1998;31(1):84–88. [Google Scholar]
- Belfort G, Davis RH, Zydney AL. The behavior of suspensions and macromoleucular solutions in cross-flow microfiltration. J Membrane Sci. 1994;96(1–2):1–58. [Google Scholar]
- Calvo L, Diaz C. Sterilization using supercritical carbon dioxide. Esterilización Mediante CO2 Supercrít. 2005;37(425):222–238. [Google Scholar]
- Clayton JL. Decontamination, sterilization and disinfection. Minimal Invasive Surg Nurs. 1996;10(1):13–20. [PubMed] [Google Scholar]
- Clough RL. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl Instrum Meth Phys Res Sec B Beam Interact Mater Atom. 2001;185:8–33. [Google Scholar]
- Cruise GM, Hegre OD, Lamberti FV, Hager SR, Hill R, Scharp DS, Hubbell JA. In vitro and in vivo performance of porcine islets encapsulated in interfacially photopolymerized poly(ethylene glycol) diacrylate membranes. Cell Transplant. 1999;8(3):293–306. doi: 10.1177/096368979900800310. [DOI] [PubMed] [Google Scholar]
- Dehghani F, Annabi N, Titus M, Valtchev P, Tumilar A. Sterilization of Ginseng using a high pressure CO2 at moderate temperatures. Biotechnol Bioeng. 2009;102(2):569–576. doi: 10.1002/bit.22059. [DOI] [PubMed] [Google Scholar]
- Dempsey DJ, Thirucote RR. Sterilization of medical devices: A review. J Biomater Appl. 1989;3(3):454–523. doi: 10.1177/088532828800300303. [DOI] [PubMed] [Google Scholar]
- Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R. Bacterial inactivation by using near- and supercritical carbon dioxide. Proc Natl Acad Sci USA. 1999;96(18):10344–10348. doi: 10.1073/pnas.96.18.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte ARC, Mano JF, Reis RL. Supercritical fluids in biomedical and tissue engineering applications: A review. Int Mater Rev. 2009;54(4):214–222. [Google Scholar]
- FDA; FDA US, editor Guidance on pre-market notification [510(k)] submissions for sterilizers intended for use in health care facilities. 1993. [Google Scholar]
- FDA; FDA US, editor Submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled as sterile. 2008. [Google Scholar]
- Fraser D. Bursting bacteria by release of gas pressure [8] Nature. 1951;167(4236):33–34. doi: 10.1038/167033b0. [DOI] [PubMed] [Google Scholar]
- Hedin G. Staphylococcus epidermidis – hospital epidemiology and the detection of methicillin resistance. Scand J Infect Dis Suppl (Oslo Norway: Scandinavian University Press) 1993;90:1–59. [PubMed] [Google Scholar]
- Hemmer JD, Drews MJ, LaBerge M, Matthews MA. Sterilization of bacterial spores by using supercritical carbon dioxide and hydrogen peroxide. J Biomed Mater Res B Appl Biomater. 2007;80(2):511–518. doi: 10.1002/jbm.b.30625. [DOI] [PubMed] [Google Scholar]
- Herten M, Jung RE, Ferrari D, Rothamel D, Golubovic V, Molenberg A, Hammerle CH, Becker J, Schwarz F. Biodegradation of different synthetic hydrogels made of polyethylene glycol hydrogel/RGD-peptide modifications: An immunohistochemical study in rats. Clin Oral Implants Res. 2009;20(2):116–125. doi: 10.1111/j.1600-0501.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- Huebsch N, Gilbert M, Healy KE. Analysis of sterilization protocols for peptide-modified hydrogels. J Biomed Mater Res B Appl Biomater. 2005;74B(1):440–447. doi: 10.1002/jbm.b.30155. [DOI] [PubMed] [Google Scholar]
- Ifkovits JL, Burdick JA. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13(10):2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- Irwin EF, Saha K, Rosenbluth M, Gamble LJ, Castner DG, Healy KE. Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Edn. 2008;19(10):1363–1382. doi: 10.1163/156856208786052407. [DOI] [PubMed] [Google Scholar]
- ISO. 2000. IS O14937: Sterilization of health care products. General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices.
- Jiménez A, Thompson GL, Matthews MA, Davis TA, Crocker K, Lyons JS, Trapotsis A. Compatibility of medical-grade polymers with dense CO2. J Supercrit Fluid. 2007;42(3 SPEC ISS):366–372. doi: 10.1016/j.supflu.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Zhang J, Matthews MA. Evaluation of CO2-based cold sterilization of a model hydrogel. Biotechnol Bioeng. 2008;101(6):1344–1352. doi: 10.1002/bit.21983. [DOI] [PubMed] [Google Scholar]
- Kanjickal D, Lopina S, Evancho-Chapman MM, Schmidt S, Donovan D. Effects of sterilization on poly(ethylene glycol) hydrogels. J Biomed Mater Res A. 2008;87A(3):608–617. doi: 10.1002/jbm.a.31811. [DOI] [PubMed] [Google Scholar]
- Lo YM, Yang ST, Min DB. Kinetic and feasibility studies of ultra-filtration of viscous xanthan gum fermentation broth. J Membrane Sci. 1996;117(1–2):237–249. [Google Scholar]
- Pollack M. Pseudomonas aeruginosa. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. New York: Churchill Livingstone; 2000. pp. 2310–2327. [Google Scholar]
- Russell AD, Hugo WB, Ayliffe GA, editors. Principles and practice of disinfection, preservation and sterilization. 3. Hoboken, NJ: Blackwell Science; 1999. [Google Scholar]
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilimbergo S, Bertucco A. Non-thermal bacteria inactivation with dense CO2. Biotechnol Bioeng. 2003;84(6):627–638. doi: 10.1002/bit.10783. [DOI] [PubMed] [Google Scholar]
- Spilimbergo S, Elvassore N, Bertucco A. Microbial inactivation by high-pressure. J Supercrit Fluid. 2002;22(1):55–63. [Google Scholar]
- Spilimbergo S, Bertucco A, Lauro FM, Bertoloni G. Inactivation of Bacillus subtilis spores by supercritical CO2 treatment. Innov Food Sci Emerg Technol. 2003a;4(2):161–165. [Google Scholar]
- Spilimbergo S, Dehghani F, Bertucco A, Foster NR. Inactivation of bacteria and spores by pulse electric field and high pressure CO2 at low temperature. Biotechnol Bioeng. 2003b;82(1):118–125. doi: 10.1002/bit.10554. [DOI] [PubMed] [Google Scholar]
- Strehin I, Ambrose WM, Schein O, Salahuddin A, Elisseeff J. Synthesis and characterization of a chondroitin sulfate-polyethylene glycol corneal adhesive. J Cataract Refract Surg. 2009;35(3):567–576. doi: 10.1016/j.jcrs.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Peppas NA. Diffusion of small molecular weight drugs in radiation-crosslinked poly(ethylene oxide) hydrogels. J Control Release. 1996;42(2):195–202. [Google Scholar]
- Werner BG, Hotchkiss JH. Continuous flow nonthermal CO2 processing: the lethal effects of subcritical and supercritical CO2 on total microbial populations and bacterial spores in raw milk. J Dairy Sci. 2006;89(3):872–881. doi: 10.3168/jds.S0022-0302(06)72151-8. [DOI] [PubMed] [Google Scholar]
- White A, Burns D, Christensen TW. Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol. 2006;123(4):504–515. doi: 10.1016/j.jbiotec.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Yoganathan R, Mammucari R, Foster NR. Dense gas processing of polymers. Polym Rev. 2010;50(2):144–177. [Google Scholar]
- Zaidi SK, Kumar A. Experimental studies in the dead-end ultrafiltration of dextran: Analysis of concentration polarization. Sep Purif Technol. 2004;36(2):115–130. [Google Scholar]
- Zhang J, Matthews MA, Dalal N, Fox A, Fox K, Hemmer J, Laberge M, Drews M, Stump M. Mechanisms of supercritical carbon dioxide sterilization of bacterial spores. 05AIChE: 2005 AIChE Annual Meeting and Fall Showcase; Cincinnati, OH. 2005. p. 9052. [Google Scholar]
- Zhang J, Burrows S, Gleason C, Matthews MA, Drews MJ, LaBerge M, An YHH. Sterilizing Bacillus pumilus spores using supercritical carbon dioxide. J Microbiol Meth. 2006a;66(3):479–485. doi: 10.1016/j.mimet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M, An YH. Sterilization using high-pressure carbon dioxide. J Supercrit Fluid. 2006b;38(3):354–372. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.