Abstract
Background and Aims Many locally endemic species in biodiversity hotspots are restricted to edaphic conditions that are fixed in the landscape, limiting their potential to track climate change through dispersal. Instead, such species experience strong selection for germination strategies that can track suitable conditions through time. Germination strategies were compared among populations across the geographic range of a California vernal pool annual, Lasthenia fremontii. Local germination strategies were tested to determine the associations with geographic variation in precipitation patterns.
Methods This study evaluated patterns of seed germination, dormancy and mortality in response to simulated variation in the timing, amount and duration of the first autumn precipitation event using seeds from six populations that span a geographic gradient in precipitation. Next, it was tested whether the germination strategies of different populations can be predicted by historical precipitation patterns that characterize each site.
Key Results A significant positive relationship was observed between the historical variability in autumn precipitation and the extent of dormancy in a population. Marginal populations, with histories of the most extreme but constant autumn precipitation levels, expressed the lowest dormancy levels. Populations from sites with historically higher levels of autumn precipitation tended to germinate faster, but this tendency was not statistically significant.
Conclusions Germination in L. fremontii is cued by the onset of the first rains that characterize the beginning of winter in California’s Great Central Valley. However, populations differ in how fast they germinate and the fraction of seeds that remain dormant when germination cues occur. The results suggest that seed dormancy may be a key trait for populations to track increasingly drier climates predicted by climate change models. However, the low dormancy and high mortality levels observed among seeds of the southernmost, driest populations make them most vulnerable to local extinction.
Keywords: Biodiversity hotspot, climate change, climate variability, dormancy, dispersal limitation, edaphic specialist, germination niche, germination time, Lasthenia fremontii, precipitation, vernal pools
INTRODUCTION
Biodiversity hotspots are often found in regions with long-term climatic stability that allows population differentiation and speciation to occur across fine-scale environmental heterogeneity (Fjeldsa et al., 1997). In the California Floristic Province (CA-FP) of western North America, however, past climate has been relatively unstable compared with other biodiversity hotspots in Mediterranean regions (Cowling et al., 2015), and continues to be so today (Berg and Hall, 2015). In the CA-FP, individual edaphic habitat patches can be scattered across broad climatic gradients, which can promote the earliest stage of ecological speciation – population divergence (Nosil et al., 2009; Lenormand, 2012; Paun et al., 2016). In this and other biodiverse regions with strong seasonality, there is spatial variation in the extent to which climatic conditions vary within and among years (Cowling et al., 2015; Martin and Ferrer, 2015). This variation can impose strong local selection on populations for adaptation to the specific patterns of temporal variation that characterize local patches, promoting differentiation in life history characteristics.
Seed dispersal and dormancy are key life history strategies that influence the persistence of plant populations in variable environments (Venable and Lawlor, 1980; Venable and Brown, 1988). While seed dispersal allows many species to track spatiotemporal variation in the distribution of suitable habitat (Clobert et al., 2012), dispersal-limited species, such as those with fragmented habitats or naturally patchy distributions, have limited potential to shift their distributions in response to rapid environmental change. In these species, germination strategies provide mechanisms by which individuals can track suitable conditions through time by delaying seed germination until suitable conditions arise (Cohen, 1968; Venable and Lawlor, 1980; Donohue et al., 2010). Seed dormancy provides a bet-hedging strategy in variable environments by sacrificing the arithmetic mean fitness in any single year for long-term geometric mean fitness across years (Cohen, 1967; Clauss and Venable, 2000; Evans et al., 2007; Venable, 2007). Dormancy as a bet-hedging strategy has been particularly well documented in desert annuals, which are subject to high levels of variation in the timing of rainfall (e.g. Pake and Venable, 1996; Clauss and Venable, 2000; Evans et al., 2007; Gremer and Venable, 2014). Once a seed breaks from the dormant state, the time it takes to germinate in response to a cue (hereafter germination timing) determines the conditions experienced during seedling establishment, growth and reproduction (Donohue et al., 2010). Early or rapid germination is generally expected to lead to higher lifetime fecundity because a longer growth period allows plants to reach a larger size and achieve higher fecundity (Donohue, 2002; Verdu and Traveset, 2005; Donohue et al., 2010). On the other hand, early germination has been shown to reduce survival in populations that may experience early-season drought stress (Baskin and Baskin, 1972; Weekley et al., 2007). Thus, the patterns of selection on the timing of germination in non-dormant seeds will probably reflect selection by conditions that occur later in the plant’s life cycle.
Population variation in dormancy has been observed along altitudinal (e.g. Beardsell and Mullett, 1984; Weng and Hsu, 2006; Mondoni et al., 2012; Fernandez-Pascual et al., 2013) and latitudinal (e.g. Levine et al., 2008; Wagmann et al., 2012; Cochrane et al., 2015) environmental gradients. However, fewer studies have tested if the level of temporal variation in climate can predict differences in the germination strategies of populations (but see Simons, 2014), even though it is this variability that is expected to drive selection on dormancy and germination time (Clauss and Venable, 2000; Donohue et al., 2010). Because climate change is expected to bring increased climatic variability both within and between years (IPCC, 2014), understanding natural patterns of intra-specific variation in seed germination strategies will be critical for predicting how species will respond to climate change.
Here we tested for population variation in germination strategies in Lasthenia fremontii (Fremont’s goldfields; Madieae, Asteraceae), an annual herb that is endemic to seasonally flooded wetlands (hereafter vernal pools) in the Central Valley of the California Floristic Province (hereafter CA-FP) (Fig. 1A). Lasthenia fremontii is self-incompatible and disperses its seeds by wind and gravity (Ornduff, 1966; Emery, 2009). Its patchy habitat, short stature and reduced pappus (relative to its upland congeners) limit the extent to which populations can track inter-annual variation in hydrology through seed dispersal, even across fine-scale flooding gradients within pools (Emery, 2009). The time window for germination of L. fremontii seeds, which occurs between the first autumn storm and the flooding of the pools, is typically quite narrow (2 – 4 weeks), but varies among populations that are distributed across a latitudinal gradient in precipitation (Fig. 1B). Furthermore, limited gene flow among populations across the species range (Torres-Martínez and Emery, 2016) may facilitate population differentiation due to drift and local adaptation.
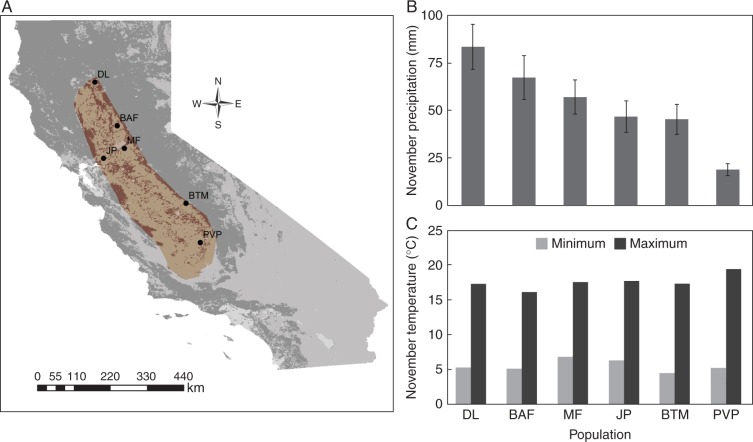
Fig. 1.
(A) The geographic locations of the six populations that were represented in the seed germination experiment, which collectively span the geographic range (shaded area) of L. fremontii in the Central Valley of the California Floristic Province. (B) The 32 year average (± 1 s.e.) for total precipitation in November (the germination period for L. fremontii) at the locations corresponding to each population included in the experiment (generated from daily precipitation data obtained from the PRISM database). (C) The 32 year averages for the minimum and maximum temperature in November for the geographic position of each population (obtained from interpolated climate data from the Worldclim database).
We tested for population differentiation in the germination strategies of L. fremontii in response to treatments that simulated different combinations of possible germination cues: (1) the timing of the first major rain event in November; (2) the amount of rainfall that the first storm brings; and (3) the length of time that water remains in the environment after the initial rain event (i.e. the duration of the water cue). We characterized germination strategies in terms of two parameters, the fraction of seeds that remain dormant in response to precipitation cues (hereafter the dormancy fraction), and the time it takes for non-dormant seeds to germinate in response to precipitation cues (hereafter germination time). We then examined whether the observed germination strategies could be predicted from historical precipitation data to test if inter-population variation in germination strategies is consistent with local adaptation to precipitation patterns. Because vernal pool seedlings emerge with traits for flood tolerance (Stone, 1990; Forrestel et al., 2015), we expect that they are highly susceptible to early-season drought. Thus, we predicted that selection has favoured delayed germination in populations that occupy particularly dry locations in the species range, as documented in other species that are susceptible to early-season drought (Baskin and Baskin, 1972; Weekley et al., 2007). We recognize that early germination can be advantageous in winter annuals that occupy dry environments because it allows seedlings to establish rapidly and reproduce before subsequent dry periods (e.g. Kimball et al., 2011). However, because soil moisture content in vernal pools can decline rapidly following the first major rain events (N. McCarten, Institute for Ecohydrology Research, CA, USA, unpubl. res.), we expect that germinating too early in dry locations would be likely to lead to drought stress in L. fremontii populations. We further predicted that L. fremontii populations from locations with a history of higher precipitation, both during the germination period and throughout the entire growing season, would exhibit faster germination time, because precipitation tends to be relatively abundant (and thus reliable) at those sites, so early germination is more likely to lead to a longer growing season and higher lifetime fecundity (Donohue, 2002; Verdu and Traveset, 2005; Donohue et al., 2010). Early germination may also provide a competitive advantage, as competition has been shown to have a major effect on fitness in L. fremontii and other endemic vernal pool plants (Emery et al., 2009; Emery and Ackerly, 2014). Finally, we tested if populations from sites with historically greater inter-annual variation in precipitation during the germination period had higher levels of dormancy, which would be consistent with the evolution of locally adaptive bet-hedging strategies in response to environmental variability.
MATERIALS AND METHODS
Study system
Vernal pools are seasonally flooded wetlands that are scattered throughout the CA-FP and support a diverse endemic flora, including L. fremontii, that are derived from primarily terrestrial ancestors (Stone, 1990). Vernal pool ‘complexes’ (hereafter referred to as populations) are patchily distributed throughout the CA-FP, and soil composition and hydrological properties vary among pools depending on their geomorphological origin, position within local watersheds, and fine-scale microtopography (Keeler-Wolf et al., 1998; Keeley and Zedler, 1998; Smith and Verrill, 1998; Zedler, 2003). Vernal pools are temporally variable environments due to the annual cycle of flooding and drought (Bliss and Zedler, 1998; Zedler, 2003) and year-to-year variation in the pattern and amount of precipitation (Bliss and Zedler, 1998; Zedler, 2003; Bauder, 2005). The hydrological regime of vernal pools has allegedly favoured the evolution of a uniquely adapted annual flora that can tolerate the stress of severe summer drought followed by severe flooding, both of which can vary greatly in extent and timing each year (Bliss and Zedler, 1998).
Like many vernal pool endemic plant species that are derived from non-aquatic ancestors (Stone, 1990), L. fremontii seeds germinate in the autumn (typically in November; Bliss and Zedler, 1998) after the first heavy rain event of the winter annual growing season but before standing water has developed in the pools. Lasthenia fremontii populations are locally dominant on the side slopes of relatively deep vernal pools and the bottoms of shallow pools (Barbour et al., 2005, 2007; Emery and Ackerly, 2014). This microhabitat is characterized by particularly variable hydrological conditions. With additional rain, seedlings at particular deep positions within pools become entirely submerged as the water table rises and will remain under water for the majority of the winter season. Seedlings at intermediate depths (or in shallow pools) may be repeatedly flooded and exposed as the water table fluctuates between storm events (Emery and Ackerly, 2014). In spring, when precipitation declines and temperatures rise, the water table rapidly recedes and L. fremonti individuals bolt, flower and set seed in the short transition period between the flooded and drought phases (Ornduff, 1966; Emery, 2009). Historical climate records (Worldclim database: Hijmans et al., 2005; PRISM databases, PRISM Climate Group, 2004) show that L. fremontii populations span a latitudinal gradient in the amount of precipitation they experience during the germination window in November (Fig. 1B), but experience relatively little variation in the minimum and maximum temperatures during the same time period (Fig. 1C). Based on these patterns, we focused on precipitation patterns as the climatic cue that may trigger different germination responses among populations from different locations within the species range.
Seed collection and maturation
In the spring of 2013, we collected seeds from six vernal pool complexes (hereafter called populations) that collectively spanned the species geographic range. We stratified our sampling effort to ensure balanced representation from the northern, central and southernmost portions of the species range (Fig. 1A). Locality information, geographic co-ordinates and ownership of each vernal pool complex that we sampled were recorded (Supplementary Data Table S1). Within each population, we randomly selected two vernal pools; in each selected pool, 30 seed heads (i.e. 30 maternal families) were randomly collected across the entire length, width and depth of the population within the pool and individually placed in coin envelopes containing silica gel (n = 60 maternal families per population). Seeds from each maternal plant were subsequently pooled within sites.
In their natural environment, L. fremontii seeds are exposed to high temperatures and dry conditions over the summer months (roughly May–October) (Supplementary Data Fig. S1), which appear to promote seed maturation and the development of seed dormancy (primary dormancy; Baskin and Baskin, 2004). To mimic these conditions, we exposed the field-collected seeds to hot, dry conditions by storing the seeds in silica gel in envelopes that were placed in a growth chamber (Percival CTH-1012, Perry, IA, USA) set to typical California summer temperature cycles (30 °C for 15 h, 15 °C for 9 h) for 3 months before beginning the germination experiments.
Measurement of precipitation-dependent germination strategies
From the pooled population of seeds from each site, we randomly selected 600 seeds that were then divided into 60 groups of ten seeds each. Unfertilized ovules and underdeveloped seeds, both of which can be identified by their shape and colour, were replaced with viable seeds to complete each experimental set. Each set was weighed to the nearest 0·005 mg using a microbalance (Mettler Toledo XP6, Greifensee, Switzerland) to provide an estimate of seed weight that could be used as a covariate to account for maternal effects. Each set of ten seeds was randomly assigned to one cell of a 6-well culture plate (Sigma-Aldrich, Cat No. Z707759-126EA, St. Louis, MO, USA) lined with a single layer of 0·34 mm thick chromatography paper (3MM Chr, Whatman, Cat No. 3030917). Each cell in the culture plate had an area of 8·96 cm2, and thus the density of ten seeds per cell is probably much lower than the typical densities of seeds in the field, where densities of germinated seedlings can reach approx. 10 seedlings cm–2 (N. Emery, pers. obs.).
After all seed sets were placed in their designated cells, the cell culture plates were placed in a single growth chamber (Percival CTH-1012) with temperature and photoperiod settings that approximate the average daily minimum and maximum values that historically characterize the Central Valley of the CA-FP in November (daylight for 10 h at 15 °C and night for 14 h at 5 °C; see Fig. 1B). We assigned each cell to one of 12 possible treatment combinations that manipulated the timing (three levels), extent (two levels) and the duration (two levels) of precipitation in a factorial design. The timing of the first rain event after summer (TAS) simulated early, mid, and late onset of the first major rain event each autumn. The levels of this treatment were imposed by adding water to the cells at 4, 6, or 8 weeks after seeds had been removed from summer conditions. The amount of water available to seeds from the first major rain event (WA) was manipulated by adding either 500 μL of deionized water (just enough to moisten the filter paper but not submerge the seeds) or 2000 μL of deionized water (generating standing water to simulate flooded conditions). Each TAS × WA combination was imposed for either 15 or 30 d to simulate brief or extended flooded or moistened conditions following the initial rain event (inundation length, or IL). The three experimental treatments (the timing, amount and duration of the first precipitation event) were applied using a complete factorial design, for a total of 12 different treatment combinations applied across 2160 seeds (grouped into 216 sets of ten seeds) representing six different populations of L. fremontii.
We evaluated the state of each seed every 2 d for the duration of the experiment, which lasted 6 months. We considered a seed to have germinated when the radicle was visible under a 10× magnifier. After recording the state of each seed in a cell as either germinated or not, all germinated seeds were removed from the culture plate and discarded. At the end of the experiment, we evaluated every seed that did not germinate to obtain estimates of dormancy and seed mortality. To obtain an estimate of the dormancy fraction for each replicate, we dissected all seeds that did not germinate to identify those that did not have an embryo, i.e. those that were inviable from the outset of the experiment. Those lacking an embryo (NE) were counted and discarded, while those that did have an embryo were further evaluated for viability by laterally dissecting the embryos and staining with 1 % tetrazolium (TZ) overnight at 30 °C (Lakon, 1949; Peters and Lanham, 2000). Ungerminated, viable seeds (i.e. those that tested positive for TZ) were used to calculate the number of seeds in the cell that were dormant (D) and those that tested negative were included in the number of seeds that had died during the experiment (M, representing the mortality fraction). Seeds that were infected with fungi during the experiment were treated as missing data (209 out of 2160 seeds). For each experimental set, we estimated the fraction of dormant seeds (FDS) out of the total number of seeds with a viable embryo, i.e. FDS = D/(G + D + M). We also estimated the mean germination time of each set as the mean number of days it took for seeds to germinate. That is, in those replicates in which germination occurred, the mean germination time (MGT) was estimated as:
where ni is the number of seeds germinated during the ith observation or time (not the cumulative number), ti is the time from the start of the experiment to the ith observation (days), and k is the time of last germination (Ranal and De Santana, 2006).
We used the number of dead seeds in each set, i.e. those with an embryo that were non-viable at the end of the experiment, to calculate the mortality rate as MR = M/(G + D + M). In addition, we calculated the ratio of inviable seeds as IR = NE/10 to provide a estimate of reproductive inefficiency.
Data analysis of precipitation-dependent germination strategies
Differences among populations in their germination responses to simulated rainfall patterns were evaluated using a general linear model (GLM) for the mean germination time and the fraction of dormant seeds. Each model included population, the timing of the first rain event (TAS), the amount of water added (WA) and the length of inundation (IL) as main effects, and all possible interactions. The total weight of each set of ten seeds was included as a covariate to control for differences among populations in maternal allocation to offspring. When treatments exhibited significant interactions with population, we conducted post-hoc tests to identify the differences among populations in their responses to different treatment levels. Tukey’s post-hoc tests were used to control for multiple comparisons when testing for significant pairwise differences between populations. The residuals from each analysis met the assumptions of analysis of variance (ANOVA) and so data were not transformed prior to analysis. All analyses were performed using SAS v. 9.4.
Dependence of germination strategies on local rainfall conditions
We characterized the patterns of precipitation typically experienced by each population during the germination period using daily rainfall data for each November between 1981 and 2013 (obtained from the PRISM database, PRISM Climate Group, 2004) (Fig. 1B). The precipitation data were extracted from the PRISM ASCII files using each population’s geographic co-ordinates and the R-package ‘raster’ (Hijmans and van Etten, 2012). We quantified the year-to-year variation in precipitation during the germination period for each population as the coefficient of variation (CV) in the total rainfall each November between 1981 and 2013. Precipitation patterns for the entire winter season were also evaluated by extracting the precipitation of the wettest quarter (BIO16) and the coldest quarter (BIO19) from the Worldclim database (Hijmans et al., 2005) using DIVAgis (Hijmans et al., 2001) (see Supplementary Data Fig. S2). Finally, total annual precipitation was extracted from the PRISM database to provide an estimate of the 32-year average total annual rainfall experienced by each population (Fig. S2). We used principal component analysis (PCA) to generate orthogonal climatic variables and selected the first two components (PC1 and PC2) to characterize the ‘germination climate niche’ of L. fremontii. We tested for relationships between each principal component and the average values for the germination time and the dormancy fraction of each population using simple regression. These analyses were conducted using SAS v. 9.4.
RESULTS
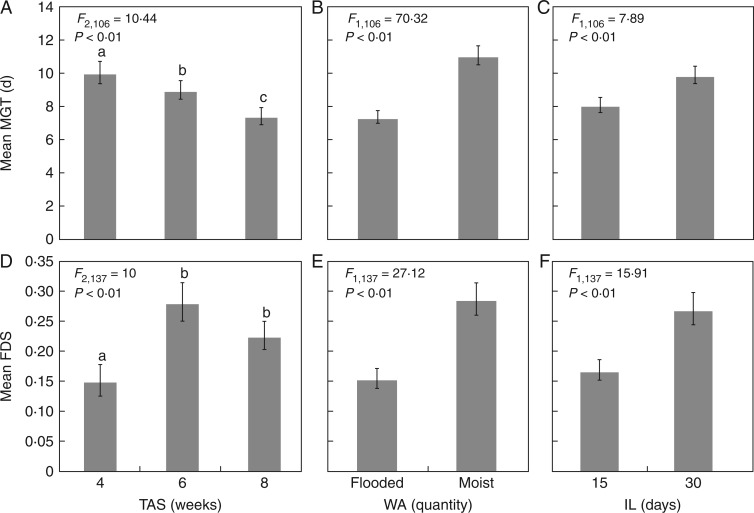
Lasthenia fremontii seeds germinated most rapidly when the time to the first precipitation event was extended (8 weeks after summer; Table 1; Fig. 2A), followed by a quick and complete imbibition of the seed coat (high volume of water, flooded conditions; Table 1; Fig. 2B), followed by a period of time for seedling establishment under wet conditions (15 d inundation length; Table 1; Fig 2C). Overall, dormancy was most common when the first precipitation event was later in the germination period (6 and 8 week treatments; Table 1; Fig. 2D), relatively light (moist conditions; Table 1; Fig. 2E) and followed by an extended inundation period (30 d inundation length; Table 1; Fig. 2F).
Table 1.
Results from ANOVA models evaluating the effects of population and all precipitation treatments on the mean germination time (MGT) and dormancy fraction (FDS) in response to different precipitation regimes
| Source | MGT |
FDS |
||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | MS | F | P | d.f. | MS | F | P | |
| Population | 5 | 97·57 | 9·76 | <0·01 | 5 | 0·27 | 8·83 | <0·01 |
| TAS | 2 | 104·34 | 10·44 | <0·01 | 2 | 0·30 | 10·00 | <0·01 |
| Population × TAS | 10 | 23·05 | 2·31 | 0·02 | 10 | 0·06 | 2·11 | 0·03 |
| WA | 1 | 702·99 | 70·32 | <0·01 | 1 | 0·82 | 27·12 | <0·01 |
| Population × WA | 5 | 9·53 | 0·95 | 0·45 | 5 | 0·02 | 0·56 | 0·73 |
| WA × TAS | 2 | 5·08 | 0·51 | 0·60 | 2 | 0·01 | 0·31 | 0·73 |
| Population × WA × TAS | 10 | 29·99 | 3·00 | 0·00 | 10 | 0·02 | 0·65 | 0·77 |
| IL | 1 | 78·91 | 7·89 | 0·01 | 1 | 0·48 | 15·91 | 0·00 |
| Population × IL | 5 | 0·47 | 0·05 | 1·00 | 5 | 0·08 | 2·61 | 0·03 |
| WA × IL | 1 | 1·61 | 0·16 | 0·69 | 1 | 0·05 | 1·68 | 0·20 |
| Population × WA × IL | 5 | 4·38 | 0·44 | 0·82 | 5 | 0·06 | 1·83 | 0·11 |
| TAS × IL | 2 | 0·85 | 0·09 | 0·92 | 2 | 0·27 | 9·04 | 0·00 |
| Population × TAS × IL | 10 | 10·03 | 1·00 | 0·45 | 10 | 0·07 | 2·27 | 0·02 |
| WA × TAS × IL | 2 | 2·39 | 0·24 | 0·79 | 2 | 0·22 | 7·39 | 0·00 |
| Population × WA × TAS × IL | 7 | 9·48 | 0·95 | 0·47 | 10 | 0·04 | 1·41 | 0·18 |
| Seed weight | 1 | 0·23 | 0·02 | 0·88 | 1 | 0·00 | 0·05 | 0·83 |
Seed weight was included as a covariate to account for maternal effects. The overall model for each variable was statistically significant (MGT: d.f. = 69, MS = 38·68, F = 3·87, P = 0·01, R2 = 0·72; FDS: d.f. = 72, MS = 0·10, F = 3·36, P < 0·01, R2 = 0·72).
TAS, time of precipitation addition after summer treatment ended (three levels); WA, amount of water added at watering event (two levels); IL, inundation length following watering event (two levels).
Fig. 2.
Lasthenia fremontii mean germination time (MGT; top row) and fraction of dormant seeds (FDS; bottom row) in response to different precipitation treatments (A, B) the timing of the first rain event (TAS) at 4, 6 or 8 weeks after the summer treatment ended; (C, D) the amount of the first rain event (WA), moist or flooded; and (E, F) the length of inundation (IL) following the initial rain event, 30 or 15 d. Error bars represent 1 s.e.
Population variation in germination responses
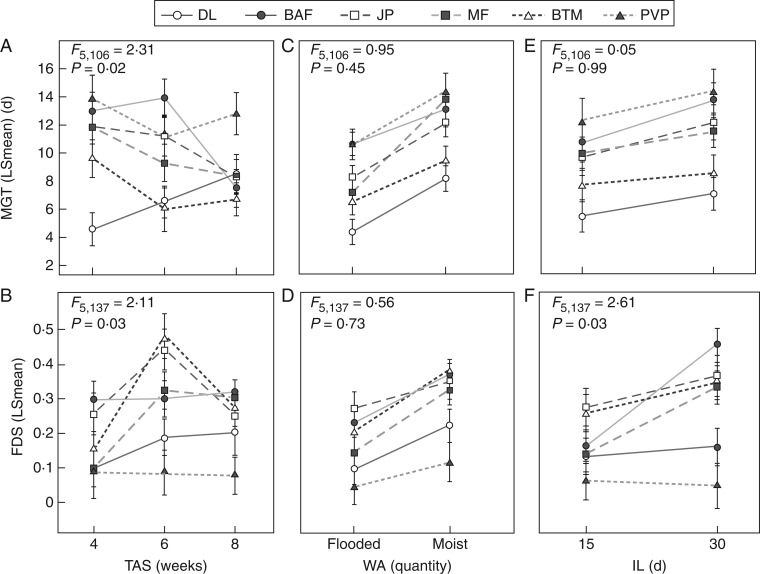
While L. fremontii populations exhibited strong overall germination responses to different levels of the experimental treatments (Table 1), we also observed that populations differed in their specific responses to treatment combinations, revealing different patterns of plasticity in germination strategies (Tables 2 and 3; Fig. 3). For example, we observed significant differences among populations in the timing of germination and fraction of dormant seeds in response to the timing of the first storm event (see Population × TAS interactions in Table 1; Fig. 3A, B). Importantly, the variation we observed among populations in the timing of germination did not vary consistently with latitude. We observed some differentiation in mean germination time between the northernmost (DL) and southernmost (PVP) population when the first rain event was early (4 week treatment) and later in the season (8 week treatment), with DL showing marginally faster germination than PVP (Fig. 3A, Tukey post-hoc tests, P = 0·067 and P = 0·02, respectively; Table 2). However, this pattern was not significant when the first rain event arrived at an intermediate length of time following the summer treatment (6 week level). Instead we observed significant differences between the second northernmost and second southernmost populations (BAF and BTM, respectively; Fig. 3A, Tukey post-hoc test, P = 0·02), and between the two northernmost populations (BAF and DL; Tukey post-hoc test, P < 0·01). In all comparisons, BAF showed the slowest germination time, while BTM and DL showed the fastest germination times.
Table 2.
Post-hoc tests for differences among populations within treatments
| Treatment | Level | d.f. | MGT | FDS | ||||
|---|---|---|---|---|---|---|---|---|
| MS | F | P | MS | F | P | |||
| TAS | 4 weeks | 5 | 62·25 | 3·9 | <0·01 | 0·08 | 2·55 | 0·03 |
| 6 weeks | 5 | 75·87 | 4·76 | <0·01 | 0·22 | 7·17 | <0·01 | |
| 8 weeks | 5 | 53·29 | 3·34 | 0·01 | 0·08 | 2·75 | 0·02 | |
| IL | 15 days | 5 | 0·09 | 2·83 | 0·02 | |||
| 30 days | 5 | 0·25 | 8·12 | <0·01 | ||||
These tests were conducted only for significant population × treatment interactions that were found in ANOVA models evaluating the effects of population identity and water treatments on the mean germination time (MGT) and fraction of dormant seeds (FDS) (see Table 1). The comparisons in this table tested for significant differences among populations within each level of each treatment.
TAS, time of precipitation addition after summer treatment ended (three levels); IL, inundation length following watering event (two levels).
Table 3.
Post-hoc tests of treatment effects within populations when the population × treatment interaction was significant in ANOVA models evaluating the effects of population identity and water treatments on the mean germination time (MGT) and fraction of dormant seeds (FDS) (see Table 1)
| Treatment | Population | d.f. | MGT |
FDS |
||||
|---|---|---|---|---|---|---|---|---|
| MS | F | P | MS | F | P | |||
| TAS | DL | 2 | 6·60 | 0·41 | 0·66 | 0·03 | 1·10 | 0·33 |
| BAF | 2 | 100·68 | 6·31 | <0·01 | 0·00 | 0·05 | 0·96 | |
| MF | 2 | 38·34 | 2·40 | 0·09 | 0·18 | 5·89 | <0·01 | |
| JP | 2 | 41·63 | 2·61 | 0·08 | 0·14 | 4·52 | 0·01 | |
| BTM | 2 | 35·86 | 2·25 | 0·11 | 0·30 | 9·96 | <0·01 | |
| PVP | 2 | 13·34 | 0·84 | 0·44 | 0·00 | 0·01 | 0·99 | |
| IL | DL | 1 | 0·00 | 0·15 | 0·70 | |||
| BAF | 1 | 0·55 | 18·14 | <0·01 | ||||
| MF | 1 | 0·25 | 8·20 | <0·01 | ||||
| JP | 1 | 0·05 | 1·79 | 0·18 | ||||
| BTM | 1 | 0·05 | 1·71 | 0·19 | ||||
| PVP | 1 | 0·00 | 0·04 | 0·85 | ||||
The comparisons in this table reflect tests for differences among treatments within each population.
TAS, time of precipitation addition after summer treatment ended (three levels); IL, inundation length following watering event (two levels).
Fig. 3.
Reaction norms showing how population mean germination time (MGT; top row) and the fraction of dormant seeds (FDS; bottom row) vary among levels of three different precipitation treatments: (A, B) the timing of the first rain event (TAS) at 4, 6 or 8 weeks after the summer treatment ended; (C, D) the amount of the first rain event (WA), moist or flooded; and (E, F) the length of inundation (IL) following the initial rain event, 30 or 15 d. Error bars represent 1 s.e.
We also observed variation among populations in the extent of dormancy expressed in response to the timing of the first rain event, but the patterns were different from those observed for mean germination time (Tables 1–3; Fig. 3). Only central populations showed significantly different levels of dormancy among the treatment levels (Table 3; Fig. 3B), while the marginal populations (DL and PVP) and BAF (the second northernmost) maintained similar levels of dormancy across all treatment levels (Table 3). Under each treatment simulating the first rain event, both DL and PVP had the lowest proportion of dormant seeds (Fig. 3B). Differences in dormancy among populations were only observed with the intermediate level of the timing of the first rain event (6 week treatment level; Fig. 3B), while all populations showed similar levels of dormancy, ranging between 10 and 30 % (Fig. 3B), when precipitation was introduced 4 or 8 weeks after the summer treatment.
In response to the amount of water received during the first rain event, populations responded in the same direction to both flooded and moist treatments. All populations showed similar, faster germination and lower dormancy when the amount of water delivered during the first precipitation event was heavy compared to light (Tables 1 and 3; Fig. 3C, D). Significant differences in mean germination time were observed between the two marginal populations (DL and PVP). The northernmost population (DL) had the fastest germination in these comparisons, while the southernmost population (PVP) had the slowest germination (Tukey post-hoc test, P = 0·02 under the moist treatment and P = 0·01 for the flooded treatment). However, the marginal populations (DL and PVP) expressed similar low levels of dormancy under both watering treatment levels compared with the more central populations (Fig. 3D), though overall these comparisons were not significant (Fig. 3D; non-significant Population × WA interaction, Table 1).
All populations expressed similar, faster germination when seeds remained in water for only 15 d compared with 30 d after the initial rain event (Table 1; Fig. 3E). In contrast, we observed significant differences among populations in their dormancy responses to the two levels of this treatment (Population × IL, F5, 137 = 2·61, P = 0·03; Table 1; Fig. 3F). In this case, populations showed a distinct centre-to-edge pattern of variation in dormancy in response to the length of inundation, with the northern- and southernmost populations (DL and PVP, respectively) having similar dormancy levels in both treatment levels while all other populations showed greater dormancy in the 30 d treatment level (Fig. 3F). Under the 30 d inundation treatment, the southernmost population (PVP) had significantly lower dormancy than BTM, MF, BAF and DL, while the northernmost population (DL) had significantly lower dormancy than the next most northern population (BAF).
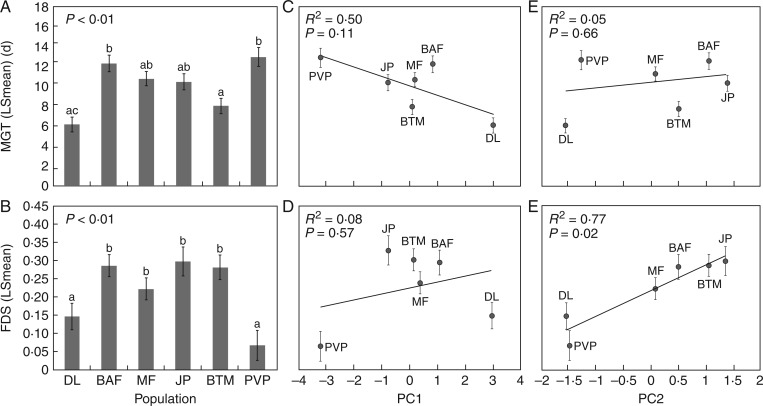
In addition to observing population variation in germination plasticity, we observed several consistent differences among populations in germination time and dormancy (Population effect, MGT, F5, 137 = 8·83, P < 0·01; FDS, F5, 106 = 9·76, P < 0·01; Table 1; Fig. 4). Interestingly, several of these responses appeared to be associated with the position of populations within the species range. Specifically, seeds from the southernmost population (PVP) took the longest time to germinate (MGT = 12 d), while the seeds from the northernmost population (DL) germinated fastest (MGT = 5 d, Fig. 4A). The other, more central populations had intermediate values for MGT, but with no clear pattern that corresponded to their latitudinal position within the species range. In contrast, the northernmost (PVP) and southernmost (DL) populations exhibited similar levels of dormancy that were significantly lower than the values measured for the remaining populations (Fig. 4B).
Fig. 4.
Population variation in germination strategies and relationships with historical precipitation data. (A) and (B) Population LSmeans (± 1 s.e.) for the mean germination time (MGT) and fraction of seeds remaining dormant (FDS), averaged across all precipitation treatments. Letters above bars identify statistically significant differences between means using Tukey’s post-hoc tests to control for multiple comparisons. Populations are arranged on the x-axis by latitude, from the most northern (left) to the most southern (right). (C) A weak negative relationship was observed between the amount of precipitation characterizing each population’s geographic location (PC1) and its grand mean germination time (± 1 s.e.), suggesting that populations from drier sites tend to take longer to germinate in response to the initial precipitation cue during the germination period, though this result was not statistically significant. (D) No significant relationship was observed between FDS and PC1, which primarily represents the total amount of precipitation experienced by each population (Table 4). (E) A very weak relationship was observed between the MGT and PC2, which primarily represents inter-annual variability in November precipitation (Table 4). (F) A significant positive relationship was observed between the mean fraction of dormant seeds in each population and PC2, indicating that populations from sites with historically more variable precipitation levels during the germination window maintain a higher level of dormancy.
Relationships between germination responses and historical local precipitation
The precipitation data used to describe historical rainfall conditions at each site over the last three decades were highly correlated (Table 4). When these variables were evaluated using PCA, PC1 explained 79 % of the variation in the data set, and was heavily weighted toward representing the amount of precipitation experienced at each site, with similarly large and positive loadings for the total precipitation in the wettest quarter, coldest quarter, calendar year and November. The second axis, PC2, explained 18 % of the variance and was most heavily loaded toward the variable representing inter-annual variation in November precipitation levels.
Table 4.
The first and second eigenvectors, and respective eigenvalues and loadings, generated from a principal components analysis (PCA) using precipitation data from the PRISM and Worldclim databases to characterize the precipitation regime at the geographic location of each L. fremontii population evaluated in the germination experiment
| Variable | PC1 (79 %) | PC2 (13 %) |
|---|---|---|
| Precipitation of the wettest quarter (BIO16) | 0·481 | 0·272 |
| Precipitation of the coldest quarter (BIO19) | 0·483 | 0·216 |
| Annual precipitation | 0·500 | 0·006 |
| November precipitation | 0·491 | −0·086 |
| Variation in November precipitation (CV) | −0·209 | 0·934 |
Simple regressions between each principal component and the mean germination trait values for each population (both germination time and dormancy fraction) revealed that the overall level of dormancy observed in a population was significantly predicted by historical levels of variability in precipitation in November (R2 = 0·77, F1,4 = 14·03, P = 0·02; Fig. 4F). Specifically, sites with higher inter-annual variation in November rainfall (i.e. larger PC2) had a larger fraction of seeds that remain dormant in our experimental trials (higher FDS). The fraction of dormant seeds was not significantly correlated with historical precipitation levels (R2 = 0·08, F1, 4 = 0·36, P = 0·57; Fig. 4D). We observed a weak negative relationship between the amount of precipitation historically experienced at each site (PC1) and the speed with which seeds germinated (Fig. 4C), but this relationship was not statistically significant (R2 = 0·50, F1, 4 = 4·12, P = 0·11), and was largely the result of the contrasting mean germination times of the northernmost and southernmost populations, DL and PVP (R2 = 0·26, P = 0·48 when outliers were removed from the analysis). Germination timing was not significantly predicted by the variance in precipitation (R2 = 0·050, F1,4 = 0·22, P = 0·66; Fig. 4E).
Population variation in seed viability and mortality
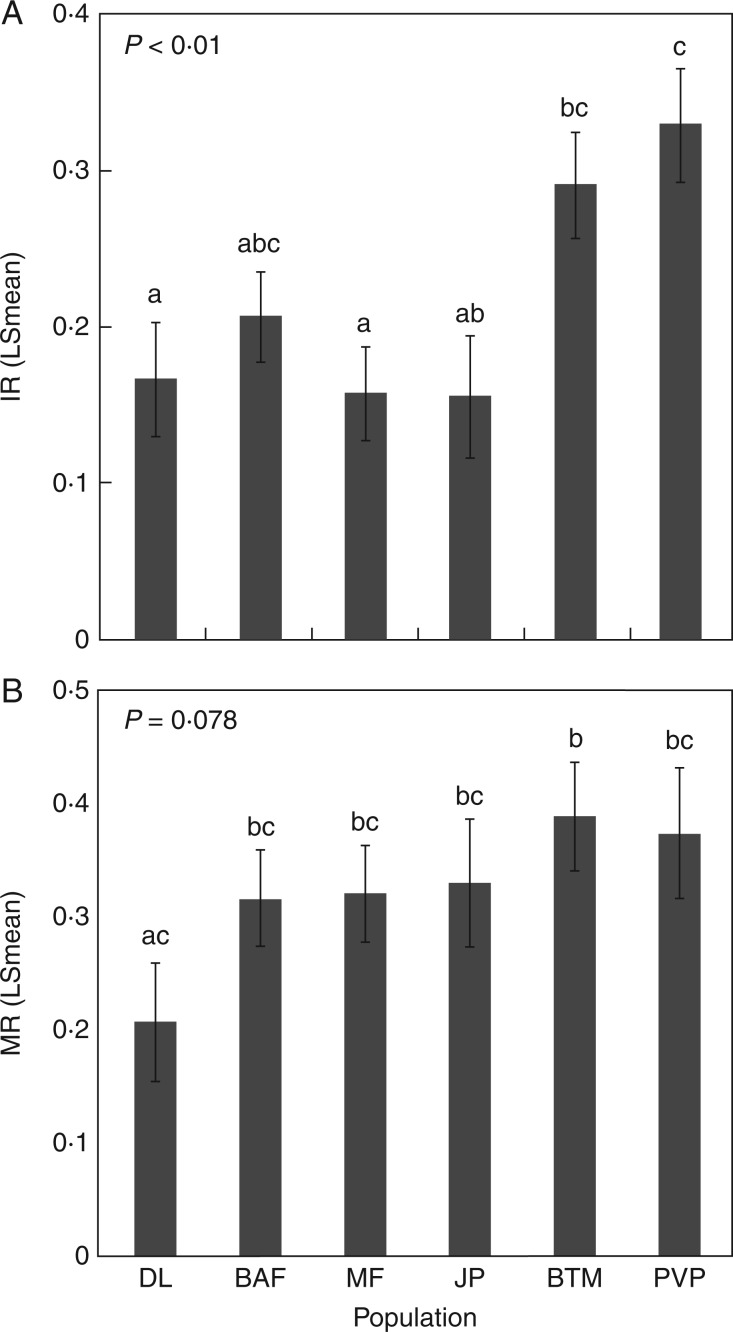
In the course of evaluating seeds before, during and after the germination experiment, we noticed substantial differences among populations in their overall seed viability and mortality rates. When seeds that did not germinate during the experiment were dissected at the end of the treatment period, we found that the proportion of seeds that lacked an embryo (inviability ratio, or IR) varied significantly among populations (Population effect: F5,143 = 6·54, P < 0·01; Fig. 5A). The populations from the southern portion of L. fremontii’s range, such as BTM and PVP, had particularly high levels of inviable seeds (29 and 32 %, respectively), compared with 15 – 20 % in all other populations (Fig. 5A). We observed similar patterns in the fraction of seeds that had an embryo, but did not germinate, and were not alive at the end of the experiment (the mortality rate, MR, as evaluated using the TZ test; see the Materials and Methods). This MR also varied among the six populations, but these differences were only marginally significant (F5, 143 = 2·03, P = 0·078). Similar to the patterns for seed inviability, the highest rates of seed mortality were in the southernmost populations, with an average of approx. 38 % and exceeding 40 % in many replicates (Fig. 5B). The northernmost population, DL, had particularly low rates of seed mortality, compared with the southern populations (Fig. 5B). Together, the viability and mortality data both indicate that overall seed quality was generally lower in L. fremontii populations from the southern edge of the species in the year in which our collections were conducted.
Fig. 5.
Variation among populations in the proportion of seeds from each population that (A) lacked an embryo (IR), or (B) had an inviable embryo (MR), after dissecting ungerminated seeds at the end of the experiment. Bars represent population LSmeans, averaged across all experimental treatments, ± 1 s.e.
DISCUSSION
The California Floristic Province (CA-FP) is a biodiversity hotspot characterized by historically high levels of topographic and climatic variability (Corander et al., 2013). This environmental instability has generated a highly heterogeneous landscape that harbours a diverse flora of edaphic endemics that are restricted to relatively isolated, patchily distributed habitat types (Millar, 2012), including vernal pool wetlands, that are considered harsh or stressful for other organisms (Stone, 1990; Kruckeberg, 2006). This complex landscape promotes population divergence due to conditions that vary among populations, such as local climate variability, which may be the earliest stages for further speciation (Lenormand, 2012). Here, we tested if this process of population divergence is under way at a key life history stage for plants – germination – in a species that is endemic to CA-FP vernal pools.
Inter-population variation in germination characteristics can be caused by genetic drift and local adaptation, both of which can be facilitated when gene flow among populations are restricted due to patchy habitat structure (Lenormand, 2012; Papaix et al., 2013). We obtained some insights into the extent to which observed differences were consistent with local adaptation by testing if germination responses could be predicted from historical precipitation data from each population’s geographic location. At least one response – the dormancy fraction – has diverged in a direction that is consistent with adaptive bet-hedging in response to the level of variation in the environment (Fig. 4D). In addition, we observed some evidence that fast and slow germination rates may be favoured in locations with particularly high and low precipitation levels, respectively, though there was no overall relationship when populations with intermediate precipitation levels were included (Fig. 4C).
The significant positive relationship between historical levels of variation in precipitation (PC2) and the fraction of dormant seeds found in L. fremontii populations (Table 4; Fig. 4F) is consistent with the hypothesis that dormancy is favoured as a bet-hedging strategy in fluctuating environments (Cohen, 1967; Clauss and Venable, 2000; Evans et al., 2007; Venable, 2007) and usually provides no advantage in environments that are temporally stable (Baskin and Baskin, 2004; but see Ellner, 1987; Metcalf, 2015). Empirical studies in desert plant populations have shown that species with higher dormancy levels exhibit lower variance in reproductive success across years, indicating that dormancy can buffer populations from demographic bottlenecks or extinction in years with unfavourable conditions (e.g. Pake and Venable, 1996; Evans et al., 2007; Gremer and Venable, 2014). Consistent with the hypothesis that it is the variability in rainfall, rather than the absolute amount of rainfall, that drives selection on dormancy, we observed a statistically significant relationship between the variability in autumn precipitation (PC2, Table 4) and the dormancy fraction (Fig. 3D), but no relationship between dormancy and the total amount of rainfall experienced at a site (Fig. 4). In L. fremontii, populations at opposite ends of the species range have similarly low levels of variation in precipitation (indicated by similar values for PC2, see Fig. 4F), even though the absolute amounts of precipitation are at opposite extremes (Fig. 1B). Thus, the relationship between precipitation variation and dormancy generates a geographic pattern in L. fremontii in which the populations at opposite extremes of the species range exhibit similar and low levels of dormancy.
Despite support for the hypothesis that patterns of precipitation variability explain population differences in dormancy, we found only weak support for the hypothesis that selection has favoured faster germination in populations that have historically experienced higher levels of precipitation. We had predicted that L. fremontii populations from relatively dry sites would have experienced selection for delayed germination due to a high risk of mortality from early-season drought (as in Baskin and Baskin, 1972; Weekley et al., 2007), while early germination would be favoured in populations from relatively wet sites (Donohue, 2002; Verdu and Traveset, 2005). We observed significant differences in the germination time measured from the sites with the historically highest and lowest levels of precipitation (DL and PVP, respectively) in a direction that is consistent with this hypothesis, with seeds from DL germinating significantly faster than those from PVP. However, there was no statistically significant relationship when we evaluated germination time with respect to historical precipitation levels (PC1) across all populations, though the trend was in the direction we had predicted (Fig. 4C).
The importance of temporal variation in driving the divergence of germination strategies among L. fremontii populations is further emphasized by the extent of plasticity we observed in response to many of the precipitation regimes we imposed in the experiment (Fig. 3). Plasticity in germination characteristics can contribute to population persistence in variable environments if seeds from more variable environments exhibit adaptive plasticity in response to germination conditions (e.g. Clauss and Venable, 2000), i.e. if seeds have mechanisms for identifying and responding to optimal conditions when they arise (Donohue et al., 2010). Most L. fremontii populations, with the exception of PVP and BAF, germinated faster when precipitation was introduced late (8 weeks after the summer), which may provide a cue for a narrow germination window and short growing season ahead. In some cases, population differences were evident in only some levels within a treatment, suggesting that populations may vary in their extent of plasticity in germination (as in Clauss and Venable, 2000). For example, significant differences among populations in germination timing were observed when the first winter storm came early (4 weeks) or an intermediate length of time (6 weeks) following the summer treatment, but not when it was most delayed (8 week treatment level; Fig. 3A). Furthermore, PVP, DL and BAF showed relatively constant levels of dormancy across all three treatment levels for the timing of the first winter storm, while several other populations exhibited a peak in dormancy at the 6 week treatment level (Fig. 3B).
While several of our results are consistent with our predictions for adaptive germination strategies to different precipitation patterns, these patterns alone are not sufficient to conclude that variation in germination strategies in L. fremontii reflect local adaptation to each population’s ‘home’ precipitation conditions. Other traits that often vary among populations due to genetic or environmental factors, such as seed size or weight, can have correlated effects on germination characteristics (e.g. Silvertown, 1981). We attempted to control for differences in maternal provisioning by including seed weight as a covariate in our analyses (though we did not detect significant relationships between this trait and the germination responses we measured; Table 1). We also know that the two germination traits that we measured – germination time and dormancy – are not correlated with each other (R2 < 0·01, P = 0·87). However, it is possible that other traits that vary among populations and covary with seed germination characteristics could be acting as confounding variables in our study. A stronger test of the adaptive value of these germination traits in these populations would require field studies that directly link dormancy and germination timing to fitness across multiple years in these different locations (e.g. Kalisz, 1986; Kelly, 1992; González-Astorga and Núñez-Farfán, 2000; Akiyama and Agren, 2014; Huang et al., 2016).
The results of this study contribute to a growing body of literature that describes the germination responses of California vernal pool endemic plant species to the timing of autumn rains. Bliss and Zedler (1998) experimentally demonstrated that several vernal pool endemics and wetland generalists had higher germination percentages (and thus lower dormancy rates) under treatments that simulated earlier autumn rain events, which is the same overall pattern that we observed among L. fremontii populations (Fig. 2). Seed dormancy has also been documented in several vernal pool endemics, including Orcuttia spp. (Griggs and Jain, 1983), Limnanthes alba (Cheng and Gordon, 2000) and Pogogyne abramsii (Zammit and Zedler, 1990), purportedly as a mechanism for persisting in the highly variable environments of vernal pools. However, to our knowledge, ours is the first study to test for intra-specific variation in germination strategies among populations of a vernal pool endemic species. Because our results indicate that germination strategies can vary substantially among populations within species, further studies of intra-specific variation in germination characteristics are warranted in other vernal pool taxa.
The presence of population variation in germination strategies of edaphic endemic species probably has important consequences for how these species will respond to future climate change. Importantly, the current germination responses in L. fremontii populations may, at least in some cases, reflect local adaptation to historical patterns of climatic variability (e.g. Fig. 4D). Like other patchily distributed, edaphic endemic plant species in the CA-FP, L. fremontii may not be able to disperse rapidly enough to track climate change, which is predicted to reach a particularly high velocity in the Central Valley of California during the 21st century (Loarie et al., 2009). Consequently, seed traits that regulate the germination process will probably play a key role defining their responses to climate change by tracking their optimal climates through time. In California, climate change is projected to increase annual variation in rainfall conditions over the next century, with extremely dry and wet seasons becoming 1·5–2 times more common (Berg and Hall, 2015; Wang and Kumar, 2015; Yoon et al., 2015). Given the variation in germination strategies observed among L. fremontii populations, we expect that seed dormancy will be the main trait to buffer populations against projected climate variation. However, the consistently wet and dry conditions are projected to continue in northern and southern peripheral populations, respectively, though perhaps becoming more extreme in magnitude compared with the last three decades (Berg and Hall, 2015; Wang and Kumar, 2015; Yoon et al., 2015). These peripheral populations currently express the lowest levels of dormancy we observed across all populations in our experimentg (Fig. 4B), suggesting that they may be particularly prone to extinction under the projected patterns of climate change. The southernmost population may be particularly susceptible to the projected extreme drought events (Wang and Kumar, 2015) that would eliminate the vernal pool environment altogether. Our observation that the southernmost populations already express a lower proportion of viable seeds (Fig. 5) further emphasizes the threat facing populations in this portion of the species range.
As highlighted in this special issue, the climatic history of biodiversity hotspots is central to explanations for the generation and maintenance of species diversity in floristically diverse regions. Furthermore, the climatic history of these regions has driven the evolution of life histories in the resident plant species that will critically define their responses to human-driven climate change. Our work shows how fine-scale heterogeneity in edaphic conditions can interact with large-scale spatiotemporal variation in climate to promote population differentiation in the life history traits of edaphic specialists. The results suggest that relatively subtle differences in climate variability experienced by populations in different habitat patches may drive the divergent evolution in germination time and dormancy. These traits, in turn, will have important consequences for population persistence in the face of increasingly variable climates projected for the near future.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Carolina Zamorano Montañez for her assistance with the experimental design of the germination experiment, Nora Castañeda for sharing her expertise with PRISM and Worldclim databases, and Suraya Williams and Maria Mercedes Levy for their technical support conducting the experiment. This work was partially supported by an Andrews Environmental Research Grant from Purdue University to L.T.M. and the National Science Foundation [DEB #1354900 to N.C.E].
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: locality information and ownership of the vernal pool complexes where L. fremontii samples were collected in 2013. Figure S1: average maximum and minimum temperatures for the years 1981–2013 from the geographic locations of the L. fremontii populations included in the germination experiment. Figure S2: precipitation variables used in the principal component analysis to describe the historical precipitation conditions at the geographic locations occupied by the L. fremontii populations in this study.
LITERATURE CITED
- Akiyama R, Ågren J. 2014. Conflicting selection on the timing of germination in a natural population of Arabidopsis thaliana. Journal of Evolutionary Biology 27: 193–199. [DOI] [PubMed] [Google Scholar]
- Barbour MG, Solomeshch AI, Buck JJ. 2007. Classification, ecological characterization, and presence of listed plant taxa of vernal pool associations in California. University of California, Davis: United States Fish and Wildlife Service Agreement/ Study No. 814205G238. [Google Scholar]
- Barbour MG, Solomeshch AI, Holland RE, et al. 2005. Vernal pool vegetation of California: communities of long-inundated deep habitats. Phytocoenologia 35: 177–200. [Google Scholar]
- Baskin JM, Baskin CC. 1972. Influence of germination date on survival and seed production in a natural population of Leavenworthia stylosa. American Midland Naturalist 88: 318–323. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Bauder ET. 2005. The effects of an unpredictable precipitation regime on vernal pool hydrology. Freshwater Biology 50: 2129–2135. [Google Scholar]
- Beardsell D, Mullett J. 1984. Seed germination of Eucalyptus pauciflora Sieb. ex. Spreng from low and high-altitude populations in Victoria. Australian Journal of Botany 32: 475–480. [Google Scholar]
- Berg N, Hall A. 2015. Increased interannual precipitation extremes over California under climate change. Journal of Climate 28: 6324–6334. [Google Scholar]
- Bliss SA, Zedler PH. 1998. The germination process in vernal pools: sensitivity to environmental conditions and effects on community structure. Oecologia 113: 67–73. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Gordon IL. 2000. The Richards function and quantitative analysis of germination and dormancy in meadowfoam (Limnanthes alba). Seed Science Research 10: 265–277. [Google Scholar]
- Clauss MJ, Venable DL. 2000. Seed germination in desert annuals: an empirical test of adaptive bet hedging. American Naturalist 155: 168–186. [DOI] [PubMed] [Google Scholar]
- Clobert J, Baguette M, Benton TG, Bullock JM. 2012. Dispersal ecology and evolution. Oxford: Oxford University Press. [Google Scholar]
- Cochrane A, Yates CJ, Hoyle GL, Nicotra AB. 2015. Will among-population variation in seed traits improve the chance of species persistence under climate change? Global Ecology and Biogeography 24: 12–24. [Google Scholar]
- Cohen D. 1967. Optimizing reproduction in a randomly varying environment when a correlation may exist between conditions at the time a choice has to be made and the subsequent outcome. Journal of Theoretical Biology 16: 1–14. [DOI] [PubMed] [Google Scholar]
- Cohen D. 1968. A general model of optimal reproduction in a randomly varying environment. Journal of Ecology 56: 219–228. [Google Scholar]
- Corander J, Majander KK, Cheng L, Merila J. 2013. High degree of cryptic population differentiation in the Baltic Sea herring Clupea harengus. Molecular Ecology 22: 2931–2940. [DOI] [PubMed] [Google Scholar]
- Cowling RM, Potts AJ, Bradshaw PL, et al. 2015. Variation in plant diversity in mediterranean-climate ecosystems: the role of climatic and topographical stability. Journal of Biogeography 42: 552–564. [Google Scholar]
- Donohue K. 2002. Germination timing influences natural selection on life-history characters in Arabidopsis thaliana. Ecology 83: 1006–1016. [Google Scholar]
- Donohue K, de Casas RR, Burghardt L, Kovach K, Willis CG. 2010. Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41: 293–319. [Google Scholar]
- Ellner S. 1987. Competition and dormancy – a reanalysis and review. American Naturalist 130: 798–803. [Google Scholar]
- Emery NC. 2009. Ecological limits and fitness consequences of cross-gradient pollen movement in Lasthenia fremontii. American Naturalist 174: 221–235. [DOI] [PubMed] [Google Scholar]
- Emery NC, Ackerly DD. 2014. Ecological release exposes genetically based niche variation. Ecology Letters 17: 1149–1157. [DOI] [PubMed] [Google Scholar]
- Emery NC, Stanton ML, Rice KJ. 2009. Factors driving distribution limits in an annual plant community. New Phytologist 181: 734–747. [DOI] [PubMed] [Google Scholar]
- Evans MEK, Ferriere R, Kane MJ, Venable DL. 2007. Bet hedging via seed banking in desert evening primroses (Oenothera, Onagraceae): demographic evidence from natural populations. American Naturalist 169: 184–194. [DOI] [PubMed] [Google Scholar]
- Fernandez-Pascual E, Jimenez-Alfaro B, Caujape-Castells J, Jaen-Molina R, Diaz TE. 2013. A local dormancy cline is related to the seed maturation environment, population genetic composition and climate. Annals of Botany 112: 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldsa J, Ehrlich D, Lambin E, Prins E. 1997. Are biodiversity ‘hotspots’ correlated with current ecoclimatic stability? A pilot study using the NOAA-AVHRR remote sensing data. Biodiversity and Conservation 6: 401–422. [Google Scholar]
- Forrestel EJ, Ackerly DD, Emery NC. 2015. The joint evolution of traits and habitat: ontogenetic shifts in leaf morphology and wetland specialization in Lasthenia. New Phytologist 208: 949–959. [DOI] [PubMed] [Google Scholar]
- González-Astorga J, Núñez Farfán J. 2000. Variable demography in relation to germination time in the annual plant Tagetes micrantha Cav. (Asteraceae). Plant Ecology 151: 253–259. [Google Scholar]
- Gremer JR, Venable DL. 2014. Bet hedging in desert winter annual plants: optimal germination strategies in a variable environment. Ecology Letters 17: 380–387. [DOI] [PubMed] [Google Scholar]
- Griggs FT, Jain SK. 1983. Conservation of vernal pool plants in California. II. Population biology of a rare and unique grass genus Orcuttia. Biological Conservation 27: 171–193. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hijmans RJ, Guarino L, Cruz M, Rojas E. 2001. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genetic Resources Newsletter 127: 15–19. [Google Scholar]
- Hijmans RJ, van Etten J. 2012. Raster: geographic analysis and modeling with raster data. R package v.2.0-12. [Google Scholar]
- Huang Z, Liu S, Bradford KJ, Huxman TE, Venable DL. 2016. The contribution of germination functional traits to population dynamics of a desert plant community. Ecology 97: 250–261. [DOI] [PubMed] [Google Scholar]
- IPCC. 2014. Climate change 2014: synthesis report Contribution of Working Groups I, II and III to the Fifth Assesment Report of the Intergovernmental Panel on Climate Change. In: Pachauri RK, Mayer LA, eds. Geneva, Switzerland: IPCC. [Google Scholar]
- Kalisz S. 1986. Variable selection on the timing of germination in Collinsia verna (Scrophulariaceae). Evolution 40: 479–491. [DOI] [PubMed] [Google Scholar]
- Keeler-Wolf T, Elam DR, Lewis K, Flint SA. 1998. California vernal pool assessment: preliminary report. Sacramento, CA: Department of Fish and Game. [Google Scholar]
- Keeley JE, Zedler PH. 1998. Characterization and global distribution of vernal pools In: Witham CW, Bauder ET, Belk D, Ferren Jr WR, Ornduff R, eds. Ecology, conservation and management of vernal pool ecosystems. Proceedings from a 1996 Conference. California Native Plant Society, Sacramento, CA, 1–14. [Google Scholar]
- Kelly CA. 1992. Spatial and temporal variation in selection on correlated life-history traits and plant size in Chamaecrista fasciculata. Evolution 46: 1658–1673. [DOI] [PubMed] [Google Scholar]
- Kimball S, Angert AL, Huxman TE, Venable DL. 2011. Differences in the timing of germination and reproduction relate to growth physiology and population dynamics of Sonoran Desert winter annuals. American Journal of Botany 98: 1773–1781. [DOI] [PubMed] [Google Scholar]
- Kruckeberg AR. 2006. Introduction to California soils and plants: serpentine, vernal pools and other geobotanical wonders. Berkeley: University of California Press. [Google Scholar]
- Lakon G. 1949. The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiology 24: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand T. 2012. From local adaptation to speciation: specialization and reinforcement. International Journal of Ecology 2012: 508458. [Google Scholar]
- Levine JM, McEachern AK, Cowan C. 2008. Rainfall effects on rare annual plants. Journal of Ecology 96: 795–806. [Google Scholar]
- Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462: 1052-U111. [DOI] [PubMed] [Google Scholar]
- Martin B, Ferrer M. 2015. Temporally variable environments maintain more beta-diversity in Mediterranean landscapes. Acta Oecologica-International Journal of Ecology 68: 1–10. [Google Scholar]
- Millar CI. 2012. Geologic, climatic, and vegetation history of California In: Baldwin BG, Goldman DH, Keil RP, Patterson R, Rosatti TJ, Wilken DH, eds. The Jepson manual: vascular plants of California. Berkeley, CA: University of California Press. [Google Scholar]
- Metcalf CJE, Burghardt LT, Koons DN. 2015. Avoiding the crowds: the evolution of plastic responses to seasonal cues in a density-dependent world. Journal of Ecology 103: 819–828. [Google Scholar]
- Mondoni A, Rossi G, Orsenigo S, Probert RJ. 2012. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany 110: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P, Harmon LJ, Seehausen O. 2009. Ecological explanations for (incomplete) speciation. Trends in Ecology and Evolution 24: 145–156. [DOI] [PubMed] [Google Scholar]
- Ornduff R.1966. A biosystematic survery of the Goldfield genus Lasthenia (Compositae: Helenieae). PhD Thesis, University of California Berkeley, CA.
- Pake CE, Venable DL. 1996. Seed banks in desert annuals: implications for persistence and coexistence in variable environments. Ecology 77: 1427–1435. [Google Scholar]
- Papaix J, David O, Lannou C, Monod H. 2013. Dynamics of adaptation in spatially heterogeneous metapopulations. PLoS One 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paun O, Turner B, Trucchi E, Munzinger J, Chase MW, Samuel R. 2016. Processes driving the adaptive radiation of a tropical tree (Diospyros, Ebenaceae) in New Caledonia, a biodiversity hotspot. Systematic Biology 65: 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Lanham B. 2000. Tetrazolium testing handbook: contribution No. 29 to the handbook on seed testing. The Association of Official Seed Analysis.
- PRISM Climate Group. 2004. PRISM Gridded Climate Data. http://prism.oregonstate.edu (last accessed 14 January 2014).
- Ranal MA, De Santana DG. 2006. How and why to measure the germination process? Revista Brasileira de Botanica 29: 1–11. [Google Scholar]
- Silvertown JW. 1981. Seed size, life-span and germination date as co-adapted features of plant life-history. American Naturalist 118: 860–864. [Google Scholar]
- Simons AM. 2014. Playing smart vs. playing safe: the joint expression of phenotypic plasticity and potential bet hedging across and within thermal environments. Journal of Evolutionary Biology 27: 1047–1056. [DOI] [PubMed] [Google Scholar]
- Smith DW, Verrill WL. 1998. Vernal pool–soil–landform relationships in the Central Valley, California In: Witham CW, Bauder ET, Belk D, Ferren Jr WR, Ornduff R, eds. Ecology, Conservation and Management of Vernal Pool Ecosystems. Proceedings from a 1996 Conference. California Native Plant Society, Sacramento, CA, 15–23. [Google Scholar]
- Stone DR. 1990. California’s endemic vernal pool plants: some factors influencing their rarity and endangerment In: Ikeda DH, Schlising RA, eds. Vernal pool plants – their habitat and biology. California State University: Studies from the Herbarium, v. 8. [Google Scholar]
- Torres-Martínez L, Emery NC. 2016. Genome-wide SNP discovery in the annual herb, Lasthenia fremontii (Asteraceae): genetic resources for the conservation and restoration of a California vernal pool endemic. Conservation Genetics Resources 8: 145–158. [Google Scholar]
- Venable DL. 2007. Bet hedging in a guild of desert annuals. Ecology 88: 1086–1090. [DOI] [PubMed] [Google Scholar]
- Venable DL, Brown JS. 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. American Naturalist 131: 360–384. [Google Scholar]
- Venable DL, Lawlor L. 1980. Delayed germination and dispersal in desert annuals: Escape in space and time. Oecologia 46: 272–282. [DOI] [PubMed] [Google Scholar]
- Verdu M, Traveset A. 2005. Early emergence enhances plant fitness: a phylogenetically controlled meta-analysis. Ecology 86: 1385–1394. [Google Scholar]
- Wagmann K, Hautekeete N-C, Piquot Y, Meunier C, Schmitt SE, Van Dijk H. 2012. Seed dormancy distribution: explanatory ecological factors. Annals of Botany 110: 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kumar A. 2015. Assessing the impact of ENSO on drought in the US Southwest with NCEP climate model simulations. Journal of Hydrology 526: 30–41. [Google Scholar]
- Weekley CW, Menges ES, Quintana-Ascencio PF. 2007. Seedling emergence and survival of Warea carteri (Brassicaceae), an endangered annual herb of the Florida scrub. Canadian Journal of Botany-Revue Canadienne De Botanique 85: 621–628. [Google Scholar]
- Weng J-H, Hsu F-H. 2006. Variation of germination response to temperature in Formosan lily (Lilium formosanum Wall.) collected from different latitudes and elevations in Taiwan. Plant Production Science 9: 281–286. [Google Scholar]
- Yoon J-H, Wang SYS, Gillies RR, Kravitz B, Hipps L, Rasch PJ. 2015. Increasing water cycle extremes in California and in relation to ENSO cycle under global warming. Nature Communications 6: 8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit C, Zedler PH. 1990. Seed yield, seed size and germination behavior in the annual Pogogyne abramsii. Oecologia 84: 24–28. [DOI] [PubMed] [Google Scholar]
- Zedler PH. 2003. Vernal pools and the concept of ‘isolated wetlands’. Wetlands 23: 597–607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.