Abstract
Background and Aims Models of costs and benefits of dormancy (D) predict that the evolutionarily stable strategy in long-term stable environments is for non-dormancy (ND), but this prediction remains to be tested empirically. We reviewed seed traits of species in the climatically buffered, geologically stable and nutrient-impoverished campo rupestre grasslands in Brazil to test the hypothesis that ND is favoured over D. We examined the relative importance of life-history traits and phylogeny in driving the evolution of D and assessed seed viability at the community level.
Methods Germination and viability data were retrieved from 67 publications and ND/D was determined for 168 species in 25 angiosperm families. We also obtained the percentage of embryoless, viable and dormant seeds for 74 species. Frequencies of species with dormant and non-dormant seeds were compared with global databases of dormancy distribution.
Key Results The majority of campo rupestre taxa (62·5 %) had non-dormant seeds, and the ND/D ratio was the highest for any vegetation type on Earth. Dormancy was unrelated to other species life-history traits, suggesting that contemporary factors are poor predictors of D. We found a significant phylogenetic structure in the dormancy categorical trait. Dormancy diversity was highly skewed towards the root of the phylogenetic tree and there was a strong phylogenetic signal in the data, suggesting a major role of phylogeny in determining the evolution of D versus ND and seed viability. Quantitative analysis of the data revealed that at least half of the seeds produced by 46 % of the surveyed populations were embryoless and/or otherwise non-viable.
Conclusions Our results support the view that long-term climatic and geological stability favour ND. Seed viability data show that campo rupestre species have a markedly low investment in regeneration from seeds, highlighting the need for specific in situ and ex situ conservation strategies to avoid loss of biodiversity.
Keywords: Campo rupestre, Cerrado, community, ecophylogenetics, embryoless seeds, evolutionarily stable strategy, OCBIL, P-deficient soils, refugia, regeneration ecology, rupestrian grassland, seed viability
INTRODUCTION
Long-term climatic fluctuations can affect large-scale patterns of biodiversity, and centres of diversity and endemism are commonly associated with climatic and geological stability (Jansson, 2003; Carnaval and Moritz, 2008; Médail and Diadema, 2009). Therefore, identifying and protecting refugia have become a top conservation priority under projected climate change scenarios because of their ability to facilitate the survival of biota under adverse conditions (Keppel et al., 2012). However, the question of how long-term climatic and geological stability influence plant life-history traits that determine species niches has rarely been explored.
Remarkable ecological and evolutionary singularities have been shown for old, climatically buffered, infertile landscapes (OCBILs) in contrast to young, often disturbed and fertile landscapes (YODFELs; Hopper, 2009). These OCBILs occur to a greater or lesser extent in at least 12 biodiversity hotspots, and they function as biodiversity refugia containing extremely high levels of plant endemism, which can sum to 79 % (Hopper et al., 2016). In OCBILs, there is a clear predominance of slow-growing plant species that largely rely on clonal reproduction for persistence, lack specialized means for seed dispersal, have specialized nutritional mechanisms and are resilient to fire and fragmentation but not to soil removal (Hopper, 2009; Oliveira et al., 2015; Silveira et al., 2016). As a result of long-term stability, plant populations from OCBILs seem to make little investment in migration mechanisms (Hopper, 2009), but no study has yet attempted to link biome-wide regeneration strategies with both life-history traits and historical stability.
Seed germination is a key component of the regeneration niche (Grubb, 1977), and it is one of the earliest traits expressed by plants and thus is under strong natural selection (Donohue et al., 2010). Seed dormancy (D) is a state of inhibited germination (Simpson, 1990) that may confer adaptive value by helping to restrict the timing of germination to periods when environmental conditions are favourable for seedling establishment. Global datasets indicate that D tends to increase with increasing seasonality, but it is a dominant trait across all vegetation zones on Earth (Jurado and Flores, 2005; Baskin and Baskin, 2014). Since D is a condition that helps regulate the timing of seed germination, it enables seeds to avoid germination during briefly favourable periods for seedling establishment (Linkies et al., 2010; Baskin and Baskin, 2014). Delaying germination is also a bet-hedging strategy that spreads the risk of reproductive failure in unpredictably variable (stochastic) environments (Cohen, 1966; Venable, 2007; Poisot et al., 2011; Moreira and Pausas, 2012).
On the other hand, D comes at the cost of increased seed mortality before germination occurs, e.g. due to physiological ageing, predation by animals and microbial decay (Long et al., 2015). Thus, the benefits of D should outweigh its costs with increase in environmental uncertainty, whereas non-dormant seeds are expected to be more advantageous under a scenario of long-term climatic stability. In fact, models predict that the evolutionarily stable strategy in long-term constant environments is for non-dormant seeds regardless of adult longevity, the timing of reproduction and population age/stage structure (Rees, 1994). This prediction, however, remains to be tested empirically. Moreover, seed dormancy is not evenly distributed among plant functional groups such as different growth forms, dispersal modes and seed dispersal seasons (Wang et al., 2009; Baskin and Baskin, 2014; Rubio de Casas et al., 2015). This suggests that investigating life-history traits that drive D is a promising way of gaining insight into the evolution of D.
Lack of germination under favourable conditions cannot be attributed directly to seed dormancy without detailed examination of seed viability (Baskin et al., 2006; Silveira, 2013). Therefore, assessing seed viability is especially crucial in severely phosphorus (P)-impoverished landscapes, where P limitation leads to drastic constraints in plant sexual reproduction (Fujita et al., 2014). However, embryo presence and viability are often overlooked in germination studies, and disentangling the contributions of the multiple factors that result in lack of germination is crucial not only for distinguishing between dormant and non-germinable seeds but also to understanding the potential of regeneration from seeds at the community level.
Here we report the occurrence of primary dormancy (dormancy at the time of seed maturity) and seed viability for the campo rupestre, megadiverse heterogeneous grasslands (Silveira et al., 2016). The campo rupestre is an OCBIL that occurs in eastern Brazil and is traditionally included as part of the Cerrado biome, i.e. Neotropical savanna (Oliveira-Filho and Ratter, 2002), a biodiversity hotspot (Myers et al., 2000). Dated phylogenies suggested that unrelated endemic lineages of Cerrado started to diversify 9·8 million years ago (Mya) (Simon et al., 2009). Evidence also indicates that diversification of some campo rupestre lineages pre-date diversification of Cerrado lineages by several million years, suggesting that the campo rupestre was the first open habitat in eastern South America (Hughes et al., 2013). On a short-term time-scale, the climatic regime in the campo rupestre is seasonal, with markedly dry winters and wet summers and increasing aridity and temperatures towards the north of its range (Silveira et al., 2016). In the long term, however, the campo rupestre has been buffered from past climatic extremes. Although there have been shifts in temperature and aridity, campo rupestre did not undergo any significant expansion during the middle Holocene or during the Last Glacial Maximum, probably due to its strong edaphic isolation (Alves and Kolbek, 1994; Barbosa and Fernandes, 2016). The resulting vegetation stability may have favoured the existence of several areas of endemism and refugia (Collevatti et al., 2009; Bonatelli et al., 2014; Ribeiro et al., 2014; Barbosa et al., 2015). In fact, the campo rupestre is a major centre of endemism, with the highest percentage of endemic species (1951 endemic out of 4928 species, 39·6 %) among all other vegetation types in Brazil (BFG, 2015). Furthermore, campo rupestre occurs in severely P-impoverished, shallow, acidic and excessively drained quartzite-derived or ironstone soils, mostly above 900 m and up to 2033 m above sea level (Giulietti et al., 1997; Jacobi et al., 2007; Alves and Kolbek, 2010; Oliveira et al., 2015). Here, plants often experience strong winds, high irradiance, frequent fires, high daily fluctuations in temperature and water shortage during the dry season (Silveira et al., 2016).
In this study, we review the ecology and evolution of seed dormancy of campo rupestre plant species. Specifically, we tested the following hypotheses. (1) Long-term geological and climatic stability favour seeds that are non-dormant. (2) Life-history traits play a more important role than phylogeny in determining seed dormancy occurrence. (3) Plants growing in P-impoverished soils make low investments in sexual reproduction, with unusually high levels of embryoless and non-viable seeds. Further, we discuss the implications of our findings for predicting the impact of future rapid climate change in the regeneration niche of species in the campo rupestre flora.
MATERIALS AND METHODS
Literature review on campo rupestre seed germination
We conducted a comprehensive literature search using three online search services to include experimental germination studies on seeds collected at campo rupestre sites. To find relevant papers, we used a topic search in Web of Science, an all-indexes search in Scielo (a National Open-Access Scientific Library; www.scielo.br) and a broad search at Google Scholar (search terms in Supplementary Data Appendix S1), which yielded a total of 50, 9 and 1160 studies, respectively (until 5 September 2015). In addition, we included unpublished data of the present authors. We combined the results from all searches and removed duplicates.
Studies were included in the database if they met the following criteria: (1) peer-reviewed indexed literature and Masters and PhD theses written in English or Portuguese; review papers were not included; (2) germination experiments were performed with fresh seeds, given that dormancy cannot be safely determined in stored seeds (Baskin et al., 2006); (3) seeds were collected in campo rupestre sensu stricto (Silveira et al., 2016); (4) control treatments were performed whenever a dormancy-breaking treatment was conducted; and (5) species-level identification was provided by the authors.
In total, 67 studies met our criteria, providing data for 184 species in 17 families. We also report empirical data for 26 species belonging to 16 families (ten additional families) to increase representation of non-studied taxa (original data from germination and viability experiments in Supplementary Data Appendix S2). In total, we gathered information for 210 species (plus 25 subspecies, varieties or populations) belonging to 27 families. This corresponds to 20 % of the families and 4·2 % of the species in the campo rupestre. To investigate the potential for regeneration from campo rupestre seeds, a quantitative analysis was performed in which we included species only when viability tests were performed or when scarification provided reliable information on maximum seed viability. Since among-population variation in seed dormancy and germination is commonplace (Anderssen and Milberg, 1998), we considered different populations as different evolutionary units, using numbers to distinguish among them. Given that we found information on Lychnophora pinaster seeds collected in two different years, we averaged the values of viability and germinability of this species.
For each species/population examined, we recorded study site (region, location and coordinates), collection date or dispersal period, species dispersal mode (biotic or abiotic), growth form (tree, succulent, shrub or herb), microhabitat (xeric and/or mesic), experiment length (number of days), incubation temperature, germinability (germination %), germination time or rate (e.g. mean germination time, mean velocity, germination rate index), embryoless seeds (%), viable seeds (%) and pre-germination treatments when available (e.g. scarification, gibberellic acid). Species names were updated according to the Brazil Plant Species List available at Reflora (http://reflora.jbrj.gov.br) in September 2015.
Information on species geographic distribution and growth form was obtained at the specieslink network (http://www.splink.org.br) and from the Reflora database. Information on dispersal mode was inferred from fruit morphology (e.g. Barroso et al., 1999). Species microhabitat (xeric, mesic or xeric and mesic) was determined from information available in the papers or by consulting the authors and taxonomy experts. Date of seed collection or fruit dispersal phenology was used to determine the time of seed dispersal, which was further divided into four seasons according to rainfall data for the region (Madeira and Fernandes, 1999). The early rainy season is from October to December, the late rainy season from January to March, the early dry season from April to June and the late dry season from July to September. The dry season was considered unfavourable for seedling establishment due to the occurrence of frequent fires, high daily temperature fluctuations and drought (Giulietti et al., 1997; Madeira and Fernandes, 1999; Silveira et al., 2012).
Determination of primary dormancy category
There are many definitions of D. In this study, we define seed dormancy as the failure of viable seeds to germinate when environmental conditions, including water, temperature, light and gases, are favourable for germination of non-dormant seeds (Vleeshouwers et al., 1995; Bewley, 1997; Hilhorst, 2011). Due to limited information on most campo rupestre species, we did not attempt to assign dormancy classes sensu Baskin and Baskin (2004). Our primary aim was to determine whether seeds are primarily dormant or non-dormant.
Seeds were considered to be non-dormant if ≥ 70 % of viable seeds germinated in about 4 weeks. They were considered to be dormant if less than 30 % of viable seeds germinated in about 4 weeks, and pre-germination treatments improved germination. We also classified seeds as dormant for intermediate cases in which germination occurred at higher percentages only at a very limited temperature range (assumed to be conditionally dormant sensu Baskin and Baskin, 2014), and <70 % of viable seeds germinated in about 4 weeks. The D/non-dormancy (ND) classification was considered non-conclusive when: (1) seed viability was less than 10 %; (2) less than 70 % of seeds germinated in about 4 weeks and there was no reference value for seed viability and no increase in germination percentage following treatment for dormancy break. Populations classified as non-conclusive were excluded from categorical data analyses, but populations with a low percentage of viable seeds were used in quantitative analyses.
Database of seed dormancy in other vegetation zones
Baskin and Baskin (2014) collected a massive amount of information on seed dormancy and germination from all main vegetation zones worldwide. Comparison of campo rupestre data on seed dormancy with the Baskin and Baskin (2014) database allows us to situate campo rupestre within its encompassing biome and other vegetation zones. Therefore, we used the Baskin and Baskin (2014) database to extract information on occurrence of primary D in savannas (i.e. tropical dry woodlands, natural savannas and grasslands) and in three other vegetation types (tropical evergreen forest, tropical semi-deciduous forest and tropical deciduous forest) occurring in southeast Brazil that have differences and similarities of climatic patterns compared with those in campo rupestre. We then compared data for populations growing in campo rupestre with data for species growing in these four vegetation zones.
Statistical and phylogenetic analyses
The χ2 test was used to assess differences in the frequencies of D and ND between the five vegetation zones and life-history traits – i.e. dispersal mode (abiotic or biotic), dispersal period (early rainy, late rainy, early dry or late dry seasons), geographic distribution (endemic or non-endemic), growth form (herb or shrub) and microhabitat (xeric or mesic). Levels within variables with a small sample size, namely succulent and tree (within growth form category) and ‘xeric and mesic’ (within microhabitat category), were excluded from analysis to prevent loss of test reliability (Zar, 2012). Bonferroni correction (α/n) was applied in χ2 tests whenever multiple comparisons were made.
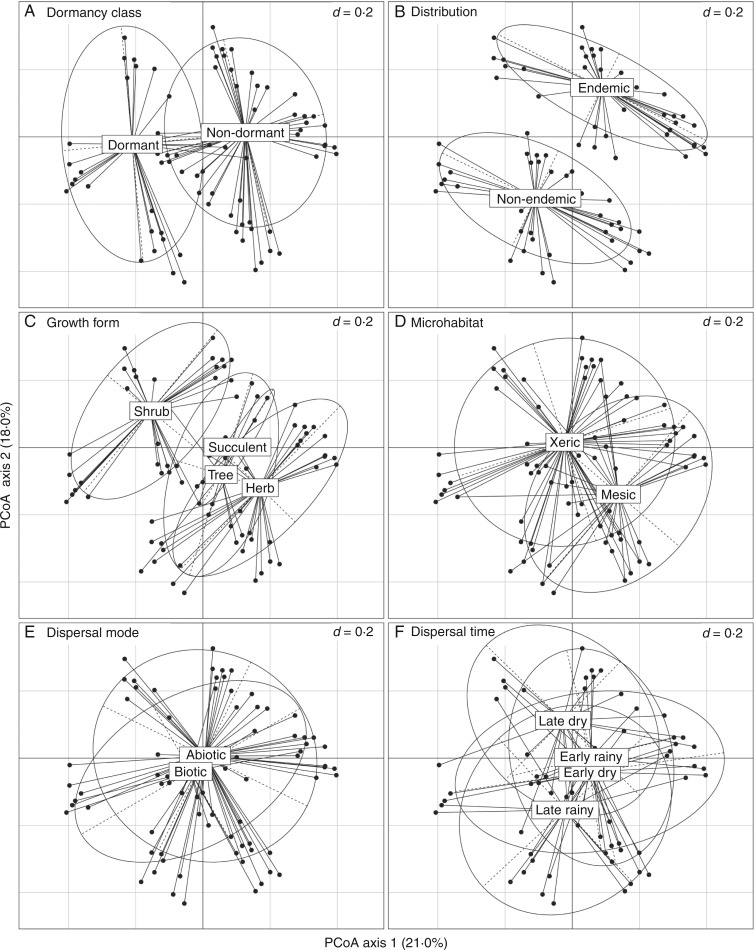
To explore the main pattern of association between all categorical variables, we computed a global distance matrix based on the above-mentioned variables and applied a principal coordinates analysis (PCoA) to compute the two main axes of variation encompassed in the global distance matrix. Finally, to visualize the groups based on the categorical variables, we plotted a factorial map, using the functions in Pavoine et al. (2009). These analyses were performed with the package ‘ade4’ (Dray and Dufour, 2007) in the R environment (R Development Core Team, 2014).
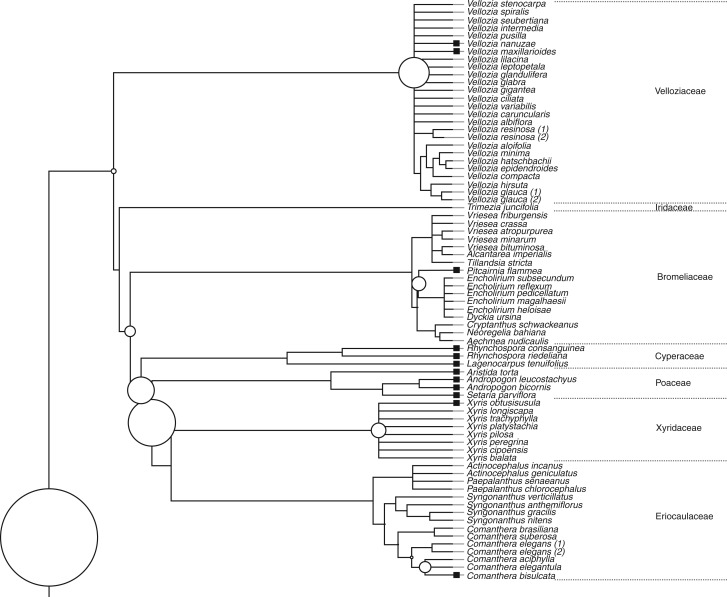
To assess the role of phylogeny in explaining seed dormancy in campo rupestre, populations were arrayed on a phylogenetic tree. Briefly, species relationships were obtained using ‘Phylomatic’ (http://phylodiversity.net/phylomatic/). We then manually corrected and improved the ultrametric tree resolution in ‘Mesquite’ (http://mesquiteproject.org/) based on a number of recent studies of molecular phylogeny. To estimate branch lengths (i.e. time since divergence), we dated 55 nodes according to several studies (Supplementary Data Appendix S3) and positioned undated nodes evenly in the tree with the ‘bladj’ algorithm of Phylocom software (Webb et al., 2008).
To assess the existence of phylogenetic signal in the dormancy categorical trait, we used two distinct approaches: (1) the Maddison and Slatkin (1991) method, which compares the minimum number of trait-state changes across a phylogenetic tree with a null model (100,000 randomizations), in which the trait states were randomized in the tips of the tree; and (2) decomposition of dormancy trait diversity among the nodes of the phylogenetic tree to test whether dormancy diversity is clustered near the root of the phylogenetic tree [tips/root skewness test based on the distance of nodes to the root of the tree, with 10 000 randomizations, according to Pavoine et al. (2010)]. To obtain the distance matrix based on the categorical dormancy trait, we used the generalization of Gower’s distance according to Pavoine et al. (2009). For quantitative traits [dormancy index D × (D + ND + 1)−1 and log(x + 1)-transformed percentages of embryoless and non-viable seeds], we examined the phylogenetic signal with Blomberg’s K test with 100 000 randomizations (Blomberg et al., 2003; Münkemüller et al., 2012). All analyses were performed in the R environment (R Development Core Team, 2014), using the ‘picante’ package for Blomberg’s K test (Kembel et al., 2010), the ‘phylo.signal.disc’ function (developed by Enrico L. Rezende, University of Roehampton, UK) for the Maddison and Slatkin (1991) method and the ‘decdiv’ function for decomposition of trait diversity among the nodes of the phylogenetic tree (Pavoine et al., 2010).
RESULTS
Ecology and evolution of seed dormancy
We were able to classify the D/ND status in 168 populations (155 species) from campo rupestre (Supplementary Data Appendices S4 and S5). The majority of populations (105; 62·5%) had non-dormant seeds, and 63 populations (37·5 %) had dormant seeds. We could not assign dormancy status in 55 species.
Frequencies of D and ND in campo rupestre were significantly different from those in all the other tropical vegetation zones. Non-dormancy was statistically more frequent in campo rupestre than in any of the other four vegetation zones. Evergreen tropical rainforest had the second highest frequency of ND, followed by tropical semi-deciduous forests and savannas and tropical deciduous forests, which had the lowest frequencies of ND (Fig. 1) (Supplementary Data Appendix S6).
Fig. 1.
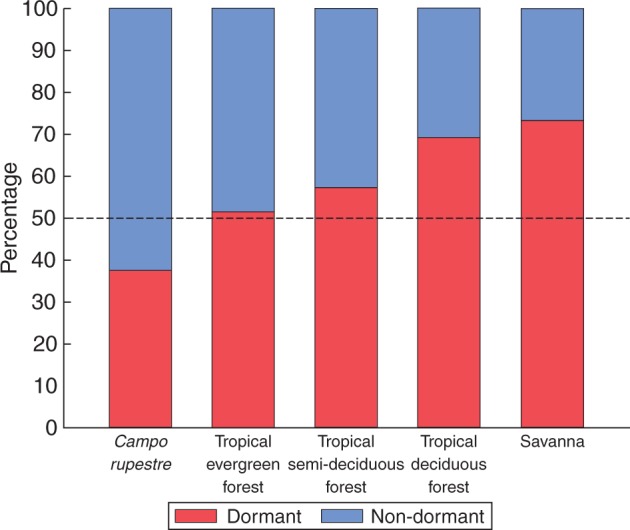
Biome-wide percentage of seed dormancy categories among campo rupestre (this study) and tropical evergreen forests, tropical semi-deciduous forests, savannas and tropical deciduous forests (Baskin and Baskin, 2014). D and ND frequencies were significantly different among all vegetation zones, except for savannas and tropical deciduous forests, according to the χ2 test (Appendix S5).
The χ2 tests did not show a correlation between seed dormancy category and any life-history traits: dispersal mode, dispersal period, geographic distribution, growth form or microhabitat (Table 1). The PCoA applied in the global distance computed with dormancy and life-history traits highlighted the lack of association between these variables. The two main axes of the PCoA explained 39 % of the total variance. The first axis separated dormant and non-dormant populations, whereas the second axis separated endemic from non-endemic populations. The other life-history traits were not associated with the first two axes, with either D or ND classification (Fig. 2).
Table 1.
Lack of association between dormancy category and life-history traits: dispersal mode (abiotic or biotic), dispersal period (early rainy, late rainy, early dry or late dry season), geographic distribution (endemic or non-endemic), growth form (herb or shrub), microhabitat (xeric or mesic). Bonferroni correction was applied in χ2 tests due to the multiple comparisons, using the significance level of P < 0·01
| Variables | χ2 test |
|
|---|---|---|
| χ2 | P | |
| Dispersal mode | 0·83 | 0·362 |
| Dispersal time | 8·077 | 0·044 |
| Geographic distribution | 2·442 | 0·118 |
| Growth form | 1·6834 | 0·194 |
| Microhabitat | 4·9452 | 0·026 |
Fig. 2.
Principal coordinates analysis (PCoA) applied to the global distance computed with the dormancy categorical trait and five life-history traits, using the generalization of Gower distance according to Pavoine et al. (2009). Each panel shows a factorial plot representing the levels of each categorical variable at the centroid of species assigned to each level. The percentage of variation explained by each axis is shown in parentheses. Vertical and horizontal grid lines are separated by 0·2 units (d) at each axis scale.
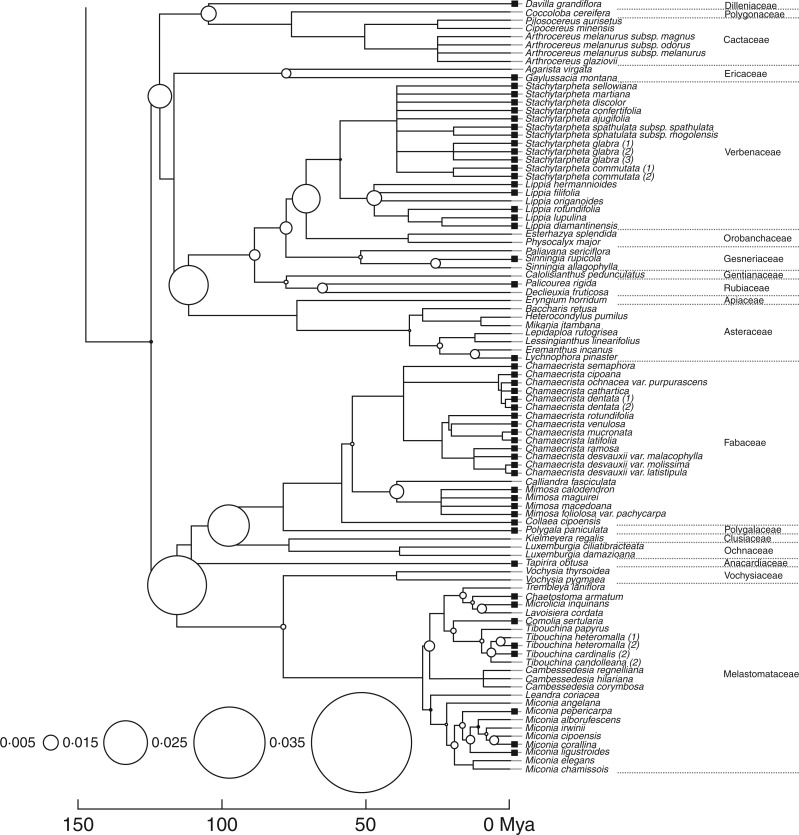
We found a strong phylogenetic signal in the dormancy categorical trait using the Maddison and Slatkin (1991) method (P < 0·00001), and the partition of dormancy diversity was highly skewed towards the root of the tree (P < 0·001; Fig. 3). The node with the highest seed dormancy trait diversity (10·2 % of total diversity) was the one that diverges between monocots and eudicots (Fig. 3). Overall, dormancy was concentrated in a few extant families, and it was especially rare in the monocots, with Poaceae and Cyperaceae having the highest dormancy percentages. Seed dormancy was more common in the eudicots, with Fabaceae and Verbenaceae showing highest percentages of species with dormant seeds. In the Melastomataceae, D appears to have evolved multiple times (Fig. 3).
Fig. 3.
Decomposition of dormancy trait diversity along the nodes of the phylogenetic tree assembled for the campo rupestre species. Black squares at the tips of the tree denote populations classified as primarily dormant. A time scale (Mya) is shown below the tree. The total quadratic entropy (TQE) for the dormancy categorical trait diversity is 0·994, and the circles at the nodes indicate the contribution of the node to total dormancy diversity. Scale is given at the bottom of the tree.
The potential for regeneration from seeds
Reliable information for embryo presence, viability and dormancy was available for 83 populations of 74 species in 21 families. Blomberg’s K test shows the phylogenetic signal in the quantitative assessment of dormancy (K = 0·646; P = 0·00001), confirming the results obtained using the Maddison and Slatkin (1991) method and diversity partitioning analysis in the dormancy categorical trait. The phylogenetic signal was also significant for the percentage of embryoless (K=1·469; P=0·00001) and non-viable (K = 0·2264; P = 0·03456) seeds. Therefore, the high prevalence of populations that produce embryoless and/or non-viable seeds seems to be phylogenetically determined (Supplementary Data Appendix S7). Overall, we found a wide variety of patterns for seeds from campo rupestre in which families had contrasting levels of dormancy, viability and embryo presence (Fig. 4).
Fig. 4.
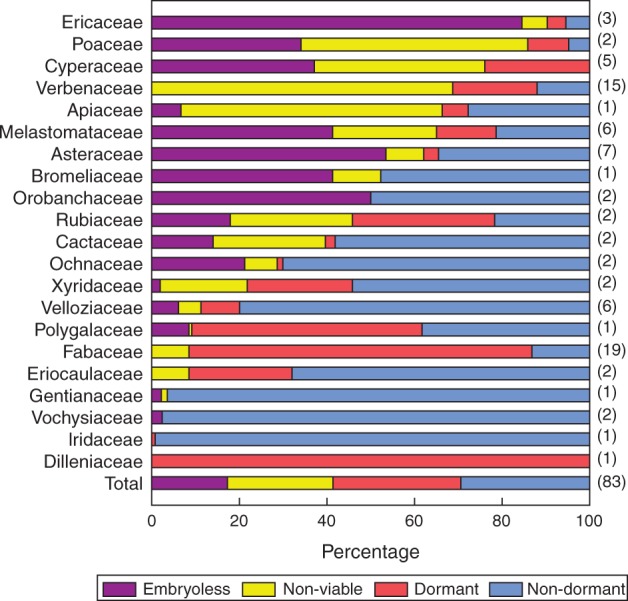
Average percentages of embryoless, non-viable, non-dormant and dormant seeds of campo rupestre species groups per family. Families are ranked by total percentage of non-germinable seeds (embryoless plus non-viable). Total percentage of viable seeds is the sum of the percentages of dormant and non-dormant seeds. Numbers in parentheses on the right side of the bars indicate numbers of sampled populations (n).
DISCUSSION
Despite the widely recognized association between centres of diversity and endemism with climatic and geological stability, very little is known about how long-term stability influences species traits that determine regeneration niches. We compiled a database with information on seed dormancy and viability in campo rupestre to test hypotheses on the life-history traits that drive the adaptive value of germination behaviour in long-term stable environments. To our knowledge, the D/ND ratio found for campo rupestre is the lowest on Earth (Baskin and Baskin, 2014). In campo rupestre, the D/ND ratio was markedly lower than that in the surrounding types of vegetation, which have gone through major and extensive area changes during periods of climatic fluctuation, especially during the Quaternary (Carnaval and Moritz, 2008; Carnaval et al., 2009; Werneck et al., 2011, 2012), thus supporting our first hypothesis that long-term geological stability and climatic buffering favours non-dormant seeds. We found no significant correlations between seed dormancy and life-history traits, thereby providing no support for our second hypothesis. Phylogeny, rather than ecology, was better correlated with seed dormancy categories, indicating that geological–climatic history played a more important role than contemporary factors in driving seed dormancy. We also found an unprecedentedly wide variation in the percentage of embryoless and non-viable seeds within a single vegetation type, which showed a strong phylogenetic signal, suggesting strong biome-wide limitations in sexual reproduction across different taxa. Notably, campo rupestre had major differences with regard to the known patterns of germination behaviour, which seems to agree with evidence of low investment in dispersal mechanisms in plant communities from OCBILs (Hopper, 2009).
Costs and benefits of seed dormancy in campo rupestre
Without accounting for the historical geological–climatic component, we would expect a seasonal environment, such as the campo rupestre, to have proportions of dormant seeds similar to that of savannas and tropical deciduous forests, and a greater proportion than that of aseasonal environments, such as the tropical evergreen rainforest and tropical semi-deciduous forests (Jurado and Flores, 2005; Baskin and Baskin, 2014). At least for Melastomataceae from campo rupestre, dormancy evolved in species dispersing seeds during the late rainy season, shortly before conditions become unfavourable for seedling establishment (Silveira et al., 2012), thereby confirming the predictions of a significant association between dispersal time and seed dormancy in seasonal environments (Rubio de Casas et al., 2015). However, our results differ from those of other studies (Bu et al., 2008; Wang et al., 2009) in that we found no correlation of D with dispersal period, suggesting that seasonality is not a strong selective force driving D in campo rupestre at the community level.
It is not yet clear how non-dormant species manage to germinate in the field at a time that is suitable for seedling establishment. Unlike other seasonal environments, life-stage transitions (e.g. germination, flowering, seed dispersal) of campo rupestre plants show tremendous diversity in phenological patterns (Madeira and Fernandes, 1999; Le Stradic, 2012; Belo et al., 2013), suggesting no obvious outcome of the complex interaction between life-history traits and environmental filters. For instance, seed dispersal and germination of two campo rupestre endemic species of Leiothrix (Eriocaulaceae) occur under unfavourable conditions for seedling establishment, resulting in enormous mortality rates of seedlings during the subsequent dry period, as predicted (Coelho et al., 2008). It seems that these Leiothrix species and many other campo rupestre species rely on long-term survival of adult plants for persistence, since long-lived perennials, resprouters and clonal plants are recurrent in this environment (Alves, 1994; Silveira et al., 2016). This strategy of persistence through long-lived individuals is markedly different from that of annual plants, which depend on seedling survival and/or formation of a seed bank for persistence and therefore delay germination, which reduces risk (Venable, 2007).
Seeds must remain viable for dormancy to be adaptive, otherwise they may die before germination occurs (Donohue et al., 2010). Our results indicate that many species produce seeds with low viability at the time of dispersal. Although some typical campo rupestre species maintain their viability for more than 1 year (Munné-Bosch et al., 2011; Cheib and Garcia, 2012; Garcia et al., 2012, 2014), some agents of mortality, such as predators, can effectively reduce seed set. Future studies should focus on quantifying seed mortality and identifying its various sources to allow more general conclusions on seed persistence in the soil (Long et al., 2015).
Phylogeny correlates with dormancy categories
All analyses consistently indicated a strong phylogenetic signal in seed dormancy, indicating that closely related species are more similar regarding D category than expected by chance. Additionally, skewness of D diversity towards the root in the phylogenetic tree shows that the rate of dormancy evolution was high in the past, during the major species divergences in angiosperms, and suggests a stable evolution of dormancy in recent lineages. This trait conservatism may be at least partly explained by campo rupestre’s history of recent speciation (Bitencourt and Rapini, 2013) and by its geographically structured speciation (Alves and Kolbek, 1994). Furthermore, in particular, transitions from ND to other D states have been considered to be uncommon throughout the evolutionary history of flowering plants, suggesting that ND is a derived state unlikely to be changed, although some such changes have occurred (Willis et al., 2014).
Since seed dormancy occurrence was not correlated to any of the life-history traits analysed here, phylogeny alone was its best predictor. In Melastomataceae, however, seed dormancy is a very labile trait, known to be influenced by life-history factors (Silveira et al., 2012). Therefore, although phylogenetic constraints probably prevent multiple evolution of seed dormancy in the vast majority of clades, some families, such as Melastomataceae, may evolve under more relaxed constraints.
Finally, we acknowledge that our database of campo rupestre seeds may be biased towards certain clades, thus limiting the extent of our conclusions on the evolution of seed dormancy. Nevertheless, biased samples are the case in all biodiversity studies (Hortal et al., 2015), including those that consider seed biology (Baskin and Baskin 2014). In addition, our database comprises several species in eight of the ten most species-rich families of campo rupestre and in eight of its nine most species-rich genera (Silveira et al., 2016). Poaceae and Cyperaceae are especially abundant in campo rupestre, and therefore they may be underrepresented in our database. However, these two families exhibited extremely low seed viability, indicating that most of their species probably do not rely on seeds as a means of reproductive assurance (Le Stradic et al., 2015). Thus, despite the limited size of the database, we are confident that it is representative of the campo rupestre vegetation.
The potential for regeneration from seeds
Seeds of taxa from campo rupestre showed diverse percentages of embryo presence and viability. At least half of the seeds produced by 46 % of the populations were embryoless and/or non-viable, thus indicating a very low potential for regeneration from seeds. Given that the soil of campo rupestre is one of the most P-deficient soils in the world (Oliveira et al., 2015), this result is in line with the hypothesis that P limitation is associated with low investment in sexual reproduction (Fujita et al., 2014). Other possible causes of the low levels of seed viability are inbreeding depression (Lamont and Wiens, 2003; Montoro and Santos, 2007; Holmes et al., 2008; Vos et al., 2012), since many campo rupestre species are locally rare (Silveira et al., 2016). In addition, the percentage of embryoless and non-viable seeds had a strong phylogenetic signal, indicating that geological–climatic historical factors also play a role in determining seed quality. Further studies are necessary to determine whether sexual versus asexual reproductive systems provide evidence for trade-offs that influence recruitment success in nutrient-impoverished environments (Vico et al., 2016).
The highly diverse patterns of seed viability across taxa also point to a great challenge in relying on seeds to preserve biodiversity of campo rupestre. The overall mean of 41 % of embryoless and non-viable seeds indicates that it will be challenging to obtain the amount of seeds necessary for the effective use of native species in landscape-scale restoration (Merritt and Dixon, 2011). To date, many restoration techniques have been inefficient in restoring plant communities of campo rupestre (Matias et al., 2009; Le Stradic et al., 2014a, b), and low seed viability is the best explanation for these failures (Le Stradic et al., 2014a). In this regard, our data show that phylogeny plays an important role in determining seed viability percentages, providing a useful tool for species selection and devising new approaches for seed-based restoration techniques.
Future scenario
There is a clear predominance of stress-tolerant strategies in campo rupestre plant communities (Negreiros et al., 2014), which may confer unusually high resistance of the species to climate change (Harrison et al., 2015). Nevertheless, we still cannot entirely appreciate the effects of climate change on this vegetation type, since there is a huge knowledge gap regarding plant regeneration. Since phylogeny seems to be the main driver of regeneration strategy, our results suggest that phylogenetic inertia determines the ability of seeds of most species to respond physiologically to specific environmental cues. The phylogenetic approach may therefore be useful to improve our ability to predict germination patterns across community and neglected clades (Ribeiro et al., 2016). This is important since the speed of climate change and the rate of habitat destruction are much higher than our ability to generate scientific data on the seed biology of native species, and effective conservation strategies are therefore necessary. However, investigations of germination timing in the field and seedling growth and survival under both present and future conditions also are necessary to better understand plant recruitment from seeds in the campo rupestre.
Altogether, our study provides support for the view that long-term climatic buffering and geological stability, along with soil infertility, are associated with a markedly low investment in regeneration from seeds (Hopper et al., 2016; Silveira et al., 2016). This view, however, raises the question of how plants adapted to long-term stability will respond to a fast-changing environment. Models of future climate scenarios have predicted large losses of environmentally suitable areas for campo rupestre until the 2080s, regardless of other existing anthropic impacts (Bitencourt et al., 2016). The high percentage of non-germinable seeds reported here, combined with the lack of specialized seed dispersal mechanisms in campo rupestre (Silveira et al., 2016), suggests that these species are not likely to migrate to suitable sites in the face of climate change. Therefore, persistence of the plant communities in current areas and ex situ conservation strategies are of utmost importance in avoiding loss of biodiversity and associated ecosystem services.
Supplementary Material
ACKNOWLEDGEMENTS
We thank A.T. Fidelis for helpful suggestions on early versions of the manuscript and FAPEMIG (APQ02317-14) for financial support. R.L.C.D. received a scholarship from CAPES and Q.S.G. and F.A.O.S. received research productivity grants from CNPq. We also thank P.H.R. Loureiro, J.V.G.L. Muniz, F.F. Trancoso and E.B. Carvalho for their major contributions to the original germination and viability experiments and P.B. Meyer for identifying most of the species. L. Echternacht, N.F.O. Mota, J.G. Rando and P.L. Viana provided the missing information on species geographic distribution, microhabitat and species phylogenetic relationships. The comments by two reviewers significantly improved an early version of the manuscript.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Appendix S1: key words used for search in three academic search engines. Appendix S2: germination experiments and viability assessments. Table S1: list of species and information concerning the collection date, locations of the studied species, number of sampled individuals and replicates used in germination and viability trials. Appendix S3: details of the resolution of polytomies and determination of node ages of the phylogenetic tree with 168 species from campos rupestres, southeastern Brazil. Table S2: estimated age of nodes numbered in Figure S1. Figure S1: phylogenetic tree with the 168 species from campos rupestres, southeastern Brazil. Appendix S4: Table S3: mean percentage of embryoless, non-viable, non-dormant and dormant seeds for 26 species of campo rupestre. Appendix S5: Table S4: dormancy or non-dormancy in seeds in 176 populations from campo rupestre. Appendix S6: Table S5: comparison of dormancy and non-dormancy absolute frequencies between different vegetation zones. Appendix S7: Figure S2: phylogenetic tree assembled for the campo rupestre species with quantitative data.
LITERATURE CITED
- Alves RJV. 1994. Morphological age determination and longevity in some Vellozia populations in Brazil. Folia Geobotanica 29: 55–59. [Google Scholar]
- Alves RJV, Kolbek J. 1994. Plant species endemism in savanna vegetation on table mountains (campo rupestre) in Brazil. Vegetatio 113: 125–139. [Google Scholar]
- Alves RJV, Kolbek J. 2010. Can campo rupestre vegetation be floristically delimited based on vascular plant genera? Plant Ecology 207: 67–79. [Google Scholar]
- Anderssen L, Milberg P. 1998. Variation in seed dormancy among mother plants, populations and years of seed collection. Seed Science Research 8: 29–38. [Google Scholar]
- Barbosa NPU, Fernandes GW. 2016. Rupestrian grassland: past, present and future distribution In: Fernandes GW, ed. Ecology and conservation of mountaintop grasslands in Brazil. Cham: Springer International Publishing, 531–544. [Google Scholar]
- Barbosa NPU, Fernandes GW, Sanchez-Azofeita A. 2015. A relict species restricted to a quartzitic mountain in tropical America: an example of microrefugium? Acta Botanica Brasilica 29: 299–309. [Google Scholar]
- Barroso GM, Morim MP, Peixoto AL, Ichaso CLF. 1999. Frutos e sementes: morfologia aplicada à sistemática de dicotiledôneas. Viçosa: Universidade Federal de Viçosa. [Google Scholar]
- Baskin JM, Baskin CC. 2004. A classification system for seed dormancy. Seed Science Research 14: 1–16. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn. San Diego: Elsevier/Academic Press. [Google Scholar]
- Baskin CC, Thompson K, Baskin JM. 2006. Mistakes in germination ecology and how to avoid them. Seed Science Research 16: 165–168. [Google Scholar]
- Belo RM, Negreiros D, Fernandes GW, Silveira FAO, Ranieri BD, Morellato PC. 2013. Fenologia reprodutiva e vegetativa de arbustos endêmicos de campo rupestre na Serra do Cipó, Sudeste do Brasil. Rodriguésia 64: 817–828. [Google Scholar]
- Bewley JD. 1997. Seed germination and dormancy. The Plant Cell 9: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BFG [Brazil Flora Group]. 2015. Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66: 1085–1113. [Google Scholar]
- Bitencourt C, Rapini A. 2013. Centres of endemism in the Espinhaço Range: identifying cradles and museums of Asclepiadoideae (Apocynaceae). Systematics and Biodiversity 11: 525–536. [Google Scholar]
- Bitencourt C, Rapini A, dos Santos LD, de Marco Junior P. 2016. The worrying future of the endemic flora of a tropical mountain range under climate change. Flora 218: 1–10. [Google Scholar]
- Blomberg SP, Garland T, Jr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Bonatelli IAS, Perez MF, Peterson AT, et al. 2014. Interglacial microrefugia and diversification of a cactus species complex: phylogeography and palaeodistributional reconstructions for Pilosocereus aurisetus and allies. Molecular Ecology 23: 3044–3063. [DOI] [PubMed] [Google Scholar]
- Bu H, Du G, Chen X, Xu X, Liu K, Wen S. 2008. Community-wide germination strategies in an alpine meadow on the eastern Qinghai-Tibet plateau: phylogenetic and life-history correlates. Plant Ecology 195: 87–98. [Google Scholar]
- Carnaval AC, Moritz C. 2008. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. Journal of Biogeography 35: 1187–1201. [Google Scholar]
- Carnaval AC, Hickerson MJ, Haddad CF, Rodrigues MT, Moritz C. 2009. Stability predicts genetic diversity in the Brazilian Atlantic forest hotspot. Science 323: 785–789. [DOI] [PubMed] [Google Scholar]
- Cheib AL, Garcia QS. 2012. Longevity and germination ecology of seeds of endemic Cactaceae species from high-altitude sites in south-eastern Brazil. Seed Science Research 22: 45–53. [Google Scholar]
- Coelho FF, Capelo C, Ribeiro LC, Figueira JEC. 2008. Reproductive modes in Leiothrix (Eriocaulaceae) in southeastern Brazil: the role of microenvironmental heterogeneity. Annals of Botany 101: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. 1966. Optimizing reproduction in a randomly varying environment. Journal of Theoretical Biology 12: 119–129. [DOI] [PubMed] [Google Scholar]
- Collevatti RG, Rabelo SG, Vieira RF. 2009. Phylogeography and disjunct distribution in Lychnophora ericoides (Asteraceae), an endangered cerrado shrub species. Annals of Botany 104: 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K, Rubio de Casas R, Burghardt L, Kovach K, Willis C. 2010. Germination, post-germination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41: 293–319. [Google Scholar]
- Dray S, Dufour AB. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22: 1–20. [Google Scholar]
- Fujita Y, Venterink HO, van Bodegrom PM, et al. 2014. Low investment in sexual reproduction threatens plants adapted to phosphorus limitation. Nature 505: 82–86. [DOI] [PubMed] [Google Scholar]
- Garcia QS, Giorni VT, Müller M, Munné-Bosch S. 2012. Common and distinct responses in phytohormone and vitamin E changes during seed burial and dormancy in Xyris bialata and X. peregrina. Plant Biology 14: 347–353. [DOI] [PubMed] [Google Scholar]
- Garcia QS, Oliveira PG, Duarte DM. 2014. Seasonal changes in germination and dormancy of buried seeds of endemic Brazilian Eriocaulaceae. Seed Science Research 24: 113–117. [Google Scholar]
- Giulietti AM, Pirani JR, Harley RM. 1997. Espinhaço range region, eastern Brazil In: Davis SD, Heywood VH, Herrera-MacBryde O, Villa-Lobos J, Hamilton AC, eds. Centres of plant diversity: a guide and strategy for their conservation. Cambridge, UK: IUCN Publication Unit, 397–404. [Google Scholar]
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews 52: 107–145. [Google Scholar]
- Harrison S, Damschen E, Fernandez-Going B, Eskelinen A, Copeland S. 2015. Plant communities on infertile soils are less sensitive to climate change. Annals of Botany 116: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilhorst HWM. 2011. Standardizing seed dormancy research In: Kermode AR, ed. Seed dormancy: methods and protocols. New York: Springer Science and Business Media (Humana Press), 43–52. [DOI] [PubMed] [Google Scholar]
- Holmes GD, James EA, Hoffmann AA. 2008. Limitations to reproductive output and genetic rescue in populations of the rare shrub Grevillea repens (Proteaceae). Annals of Botany 102: 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper SD. 2009. OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil 322: 49–86. [Google Scholar]
- Hopper SD, Silveira FAO, Fiedler P. 2016. Biodiversity hotspots and OCBIL theory. Plant and Soil 403: 167–216. [Google Scholar]
- Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ. 2015. Seven shortfalls that beset large-scale knowledge on biodiversity. Annual Review of Ecology, Evolution, and Systematics 46: 523–549. [Google Scholar]
- Hughes CE, Pennington RT, Antonelli A. 2013. Neotropical plant evolution: assembling the big picture. Botanical Journal of the Linnean Society 171: 1–18. [Google Scholar]
- Jacobi CM, Carmo FF, Vincent RC, Stehmann JR. 2007. Plant communities on ironstone outcrops: a diverse and endangered Brazilian ecosystem. Biodiversity and Conservation 16: 2185–2200. [Google Scholar]
- Jansson R. 2003. Global patterns in endemism explained by past climatic changes. Proceedings of the Royal Society B: Biological Sciences 270: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado E, Flores J. 2005. Is seed dormancy under environmental control or bound to plant traits? Journal of Vegetation Science 16: 559–564. [Google Scholar]
- Kembel SW, Cowan PD, Helmus MR. et al. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464. [DOI] [PubMed] [Google Scholar]
- Keppel G, Van Niel KP, Wardell‐Johnson GW, et al. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21: 393–404. [Google Scholar]
- Lamont BB, Wiens D. 2003. Are seed set and speciation rates always low among species that resprout after fire, and why? Evolutionary Ecology 17: 277–292. [Google Scholar]
- Le Stradic S. 2012. Composition, phenology and restoration of campos rupestres mountain grasslands, Brazil. PhD Thesis, Universidade Federal de Minas Gerais, Brazil.
- Le Stradic S, Buisson E, Fernandes GW. 2014a. Restoration of Neotropical grasslands degraded by quarrying using hay transfer. Applied Vegetation Science 17: 482–492. [Google Scholar]
- Le Stradic S, Buisson E, Negreiros D, Campagne P, Fernandes GW. 2014b. The role of native woody species in the restoration of campos rupestres in quarries. Applied Vegetation Science 17: 109–120. [Google Scholar]
- Le Stradic S, Silveira FAO, Buisson E, Cazelles K, Carvalho V, Fernandes GW. 2015. Diversity of germination strategies and seed dormancy in herbaceous species of campo rupestre grasslands. Austral Ecology 40: 537–546. [Google Scholar]
- Linkies A, Graeber K, Knight C, Leubner-Metzger G. 2010. The evolution of seeds. New Phytologist 186: 817–831. [DOI] [PubMed] [Google Scholar]
- Long RL, Gorecki MJ, Renton M, et al. 2015. The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews 90: 31–59. [DOI] [PubMed] [Google Scholar]
- Madeira JA, Fernandes GW. 1999. Reproductive phenology of sympatric taxa of Chamaecrista (Leguminosae) in Serra do Cipó, Brazil. Journal of Tropical Ecology 15: 463–479. [Google Scholar]
- Maddison WP, Slatkin M. 1991. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45: 1184–1197. [DOI] [PubMed] [Google Scholar]
- Matias SR, Pagano MC, Muzzi F, et al. 2009. Effect of rhizobia, mycorrhizal fungi and phosphate-solubilizing microorganisms in the rhizosphere of native plants used to recover an iron ore area in Brazil. European Journal of Soil Biology 45: 259–266. [Google Scholar]
- Médail F, Diadema K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. Journal of Biogeography 36: 1333–1345. [Google Scholar]
- Merritt DJ, Dixon KW. 2011. Restoration seed banks – a matter of scale. Science 332: 424–425. [DOI] [PubMed] [Google Scholar]
- Montoro GR, Santos ML. 2007. Fenologia e biologia reprodutiva de Tibouchina papyrus (Pohl) Toledo no Parque Estadual da Serra dos Pirineus, Goiás. Revista de Biologia Neotropical 4: 21–29. [Google Scholar]
- Moreira B, Pausas JG. 2012. Tanned or burned: the role of fire in shaping physical seed dormancy. PLoS One 7: e51523. doi:10.1371/journal.pone.0051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münkemüller T, Lavergne S, Bzeznik B, et al. 2012. How to measure and test phylogenetic signal. Methods in Ecology and Evolution 3: 743–756. [Google Scholar]
- Munné-Bosch S, Oñate M, Oliveira PG, Garcia QS. 2011. Changes in phytohormones and oxidative stress markers in buried seeds of Vellozia alata. Flora 206: 704–711. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Negreiros D, Le Stradic S, Fernandes GW, Rennó HC. 2014. CSR analysis of plant functional types in highly diverse tropical grasslands of harsh environments. Plant Ecology 215: 379–388. [Google Scholar]
- Oliveira RS, Galvão HC, Campos MCR, Eller CB, Pearse SJ, Lambers H. 2015. Mineral nutrition of campos rupestres plant species on contrasting nutrient-impoverished soil types. New Phytologist 205: 1183–1194. [DOI] [PubMed] [Google Scholar]
- Oliveira-Filho AT, Ratter JA. 2002. Vegetation physiognomies and woody flora of the cerrado biome In: Oliveira PS, Marquis RJ, eds. The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. New York: Columbia University Press, 91–120. [Google Scholar]
- Pavoine S, Vallet J, Dufour A, Gachet S, Daniel H. 2009. On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118: 391–402. [Google Scholar]
- Pavoine S, Baguette M, Bonsall MB. 2010. Decomposition of trait diversity among the nodes of a phylogenetic tree. Ecological Monographs 80: 485–507. [Google Scholar]
- Poisot T, Bever JD, Nemri A, Thrall PH, Hochberg ME. 2011. A conceptual framework for the evolution of ecological specialisation. Ecology Letters 14: 841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Version 3.0.3. R Foundation for Statistical Computing, Vienna. http://www.rproject.org (last accessed 25 July 2014).
- Rees M. 1994. Delayed germination of seeds: a look at the effects of adult longevity, the timing of reproduction, and population age/stage structure. American Naturalist 144: 43–64. [Google Scholar]
- Ribeiro GVT, Teixido AL, Barbosa NPU, Silveira FAO. 2016. Assessing bias and knowledge gaps on seed ecology research: implications for conservation agenda and policy. Ecological Applications, in press. doi:10.1890/15-1852.1. [DOI] [PubMed] [Google Scholar]
- Ribeiro PL, Rapini A, Damascena LS, van den Berg C. 2014. Plant diversification in the Espinhaço Range: insights from the biogeography of Minaria (Apocynaceae). Taxon 63: 1253–1264. [Google Scholar]
- Rubio de Casas RR, Donohue K, Venable DL, Cheptou PO. 2015. Gene-flow through space and time: dispersal, dormancy and adaptation to changing environments. Evolutionary Ecology 29: 813–831. [Google Scholar]
- Silveira FAO. 2013. Sowing seeds for the future: the need for establishing protocols for the study of seed dormancy. Acta Botanica Brasilica 27: 264–269. [Google Scholar]
- Silveira FAO, Ribeiro RC, Oliveira DM T, Fernandes GW, Lemos-Filho JP. 2012. Evolution of physiological dormancy multiple times in Melastomataceae from Neotropical montane vegetation. Seed Science Research 22: 37–44. [Google Scholar]
- Silveira FAO, Negreiros D, Barbosa NPU, et al. 2016. Ecology and evolution of plant diversity in the endangered campo rupestre: a neglected conservation priority. Plant and Soil 403: 129–152. [Google Scholar]
- Simon MF, Grether R, Queiroz LP, Skema C, Pennington RT, Hughes CE. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences of the USA 106: 20359–20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GM. 1990. Seed dormancy in grasses. Cambridge: Cambridge University Press. [Google Scholar]
- Venable DL. 2007. Bet hedging in a guild of desert annuals. Ecology 88: 1086–1090. [DOI] [PubMed] [Google Scholar]
- Vico G, Manzoni S, Nkurunziza L, Murphy K, Weih M. 2016. Trade-offs between seed output and life span – a quantitative comparison of traits between annual and perennial congeneric species. New Phytologist 209: 104–114. [DOI] [PubMed] [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031–1037. [Google Scholar]
- Vos JM, Keller B, Isham ST, Kelso S, Conti E. 2012. Reproductive implications of herkogamy in homostylous primroses: variation during anthesis and reproductive assurance in alpine environments. Functional Ecology 26: 854–865. [Google Scholar]
- Wang JH, Baskin CC, Cui XL, Du GZ. 2009. Effect of phylogeny, life history and habitat correlates on seed germination of 69 arid and semi-arid zone species from northwest China. Evolutionary Ecology 23: 827–846. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. 2008. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24: 2098–2100. [DOI] [PubMed] [Google Scholar]
- Werneck FP, Costa GC, Colli GR, Prado DE, Sites JW., Jr. 2011. Revisiting the historical distribution of seasonally dry tropical forests: new insights based on palaeodistribution modelling and palynological evidence. Global Ecology and Biogeography 20: 272–288. [Google Scholar]
- Werneck FP, Nogueira C, Colli GR, Sites JW, Costa GC. 2012. Climatic stability in the Brazilian Cerrado: implications for biogeographical connections of South American savannas, species richness and conservation in a biodiversity hotspot. Journal of Biogeography 39: 1695–1706. [Google Scholar]
- Willis CG, Baskin CC, Baskin JM, et al. 2014. The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytologist 203: 300–309. [DOI] [PubMed] [Google Scholar]
- Zar JH. 2012. Biostatistical analysis. New Jersey: Prentice Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.