Abstract
Background and Aims Low-altitude mountains constitute important centres of diversity in landscapes with little topographic variation, such as the Southwest Australian Floristic Region (SWAFR). They also provide unique climatic and edaphic conditions that may allow them to function as refugia. We investigate whether the Porongurups (altitude 655 m) in the SWAFR will provide a refugium for the endemic Ornduffia calthifolia and O. marchantii under forecast climate change.
Methods We used species distribution modelling based on WorldClim climatic data, 30-m elevation data and a 2-m-resolution LiDAR-derived digital elevation model (DEM) to predict current and future distributions of the Ornduffia species at local and regional scales based on 605 field-based abundance estimates. Future distributions were forecast using RCP2.6 and RCP4.5 projections. To determine whether local edaphic and biotic factors impact these forecasts, we tested whether soil depth and vegetation height were significant predictors of abundance using generalized additive models (GAMs).
Key Results Species distribution modelling revealed the importance of elevation and topographic variables at the local scale for determining distributions of both species, which also preferred shadier locations and higher slopes. However, O. calthifolia occurred at higher (cooler) elevations with rugged, concave topography, while O. marchantii occurred in disturbed sites at lower locations with less rugged, convex topography. Under future climates both species are likely to severely contract under the milder RCP2.6 projection (approx. 2 °C of global warming), but are unlikely to persist if warming is more severe (RCP4.5). GAMs showed that soil depth and vegetation height are important predictors of O. calthifolia and O. marchantii distributions, respectively.
Conclusions The Porongurups constitute an important refugium for O. calthifolia and O. marchantii, but limits to this capacity may be reached if global warming exceeds 2 °C. This capacity is moderated at local scales by biotic and edaphic factors.
Keywords: Anthropogenic climate change, extinction, interspecific interactions, localized endemic, low-altitude mountain, refugia, microclimate, Southwest Australian Floristic Region (SWAFR), Ornduffia calthifolia, Ornduffia marchantii, species distribution modelling
INTRODUCTION
The world’s mountains are centres of diversity and endemism (Kessler and Kluge, 2008; Ohlemüller et al., 2008). Mountain species may also be particularly vulnerable to anthropogenic global warming and attendant climate change (Thuiller et al., 2005; La Sorte and Jetz, 2010). Upward migration of species along elevational temperature gradients is resulting in range reductions for many species, as the total area available at a given altitude generally decreases with elevation on mountains (Wilson et al., 2005; Parmesan, 2006). Where no suitable habitats are available at higher altitudes, climate change may lead to extinctions (Pauli et al., 2003; La Sorte and Jetz, 2010). Species with narrow ranges that are restricted to mountaintops may therefore be amongst the most threatened by habitat loss leading to extinction (Pounds and Crump, 1994; Dirnböck et al., 2011).
Although mountain species may be particularly vulnerable to anthropogenic climate change, steep environmental gradients and topographic complexity may provide important microrefugia for species (Byrne et al., 2008; Médail and Diadema, 2009; Tapper et al., 2014). Such refugia are often not detectable at the scale of most modelling studies, resulting in exaggerated predictions of extinction risk (Randin et al., 2009; Austin and Van Niel, 2011a; Franklin et al., 2013). However, microrefugia have facilitated the persistence of species during past climate change (Byrne et al., 2008; Provan and Bennett, 2008) and are likely to play an important role in facilitating in situ persistence under ongoing and future climate change. Identifying and protecting refugia with the highest capacity to facilitate persistence is therefore critically important for effective conservation (Keppel et al., 2012, 2015).
Low-altitude mountains often have significant conservation value but face particularly high conservation threats (Watson and Barrett, 2004; Guerin and Lowe, 2013; Barrett and Yates, 2015). Irrespective of their size, they are often the highest points in the landscape and provide unique climate and edaphic environments with attendant endemic species (Rebelo et al., 2006; Barrett and Yates, 2015). Hence, they probably constitute important microrefugia that may provide important safe havens for biodiversity under anthropogenic climate change (Ashcroft, 2010; Keppel and Wardell-Johnson, 2012).
The Southwest Australian Floristic Region (SWAFR) and Cape Floristic Region are globally significant centres of plant diversity where low-altitude mountains provide important topography, and hence potential microrefugia. Climate change is predicted to have a substantial impact on biodiversity in both regions (Midgley et al., 2002; Fitzpatrick et al., 2008; Klausmeyer and Shaw, 2009), and species in mountainous areas at higher elevations may be disproportionally vulnerable (McCullough et al., 2016). Indeed, both regions contain high concentrations of species vulnerable to climate change (Yates et al., 2010; Foden et al., 2013).
In the SWAFR, the Stirling Range (1090 m a.s.l.) and Porongurup Range (Porongurups 655 m a.s.l.) on the region’s south coast provide distinctly montane environments, with numerous endemic species restricted to higher and cooler elevations (Barrett, 1996; Barrett and Yates, 2015). Here we use species distribution modelling to forecast the likely impacts of climate change on two narrowly endemic, iconic species of the genus Ornduffia Tippery & Les in the Porongurups. We also test whether in situ edaphic (soil depth) and biotic (vegetation height) factors, which are difficult to include in species distribution modelling, significantly affect the distribution of the two species and hence the capacity of potential microrefugia. We thus determine whether the Porongurups, or other proximal locations, have the potential to act as refugia for Ornduffia species under anthropogenic climate change.
MATERIALS AND METHODS
Study site
The SWAFR constitutes a global biodiversity hotspot, with high plant species richness and endemism, and highly modified landscapes (Myers et al., 2000; Hopper and Gioia, 2004). This high diversity exists in landscapes displaying little topographic variation, with only a few areas of moderate elevation and limited scope for altitudinal migration (Hopper and Gioia, 2004; Rix et al., 2014). Granite outcrops provide important topography in this landscape, and probably acted as refugia during past periods of climate change (Schut et al., 2014; Tapper et al., 2014).
The Porongurups constitute Australia’s most massive granite outcrop, covering an area of about 12 × 3 km with several peaks exceeding 600 m in altitude (maximum: 655 m a.s.l.). It consists of a series of granite domes dating to about 1100 Mya (Abbott, 1982). The climate is Mediterranean, with cool, wet winters and hot, dry summers, and attracts considerable orographic moisture due to its size and height. The summits are mostly bare or covered with lichens, herbaceous plants and scattered shrubs. Lower down, a belt of Eucalyptus cornuta and E. megacarpa leads into extensive karri (E. diversicolor) forest on the lower slopes. This karri forest is a significant outlier for mesic species from the cooler–higher rainfall zone of the SWAFR, considerably extending the species’ ranges at their arid margins, presumably in response to favourable local climates and soil moisture created by the range’s topography (Churchill, 1968; Abbott, 1982; Schut et al., 2014). The Porongurups are a highly important, traditional ceremonial place for the Nyoongar people.
Although floristically not among the richest of the south-western Australian granite outcrops, the Porongurups include about 750 native species. These include five species of local endemics (Barrett, 1996): Brachysema subcordatum (Fabaceae), Hibbertia bracteosa (Dilleniaceae), Billardiera granulata (Pittosporaceae), Apium prostratum ssp. phillipii (Apiaceae) and Ornduffia calthifolia (Menyanthaceae). There are also several species whose distributions are centred on the Porongurups, including Ornduffia marchantii, a close relative of O. calthifolia.
Study species
Ornduffia is a genus of eight southern Australian taxa (five confined to south-western Australia) that has been recently recognized as distinct from Villarsia in the morphologically diverse and cosmopolitan family Menyanthaceae, which includes 60–70 aquatic and wetland species (Tippery and Les, 2009). Ornduffia calthifolia (Fig. 1A–C) is an erect, robust perennial, locally endemic to moist sheltered sites on the upper slopes of granite outcrops of the Porongurups (Brown et al., 1998). The fleshy-leaved species is widely recognizable, and iconic to the Porongurups (Barrett, 1996; Brown et al., 1998). The stem bearing the inflorescence (culm) rises to a metre or more. Adventitious roots arise from a fleshy underground organ, providing a reserve of water and nutrients, and enabling rapid growth under favourable conditions (Pate & Dixon, 1982). However, the species is apparently drought-susceptible, as evidenced by observations of dead plants after the extreme heat of January 1991 (Robinson and Coates, 1995). Ornduffia calthifolia is killed by fire and relies on seedling regeneration for persistence. Fire may enhance germination, but inter-fire seedling establishment also occurs (Gilfillan and Barrett, 2004).
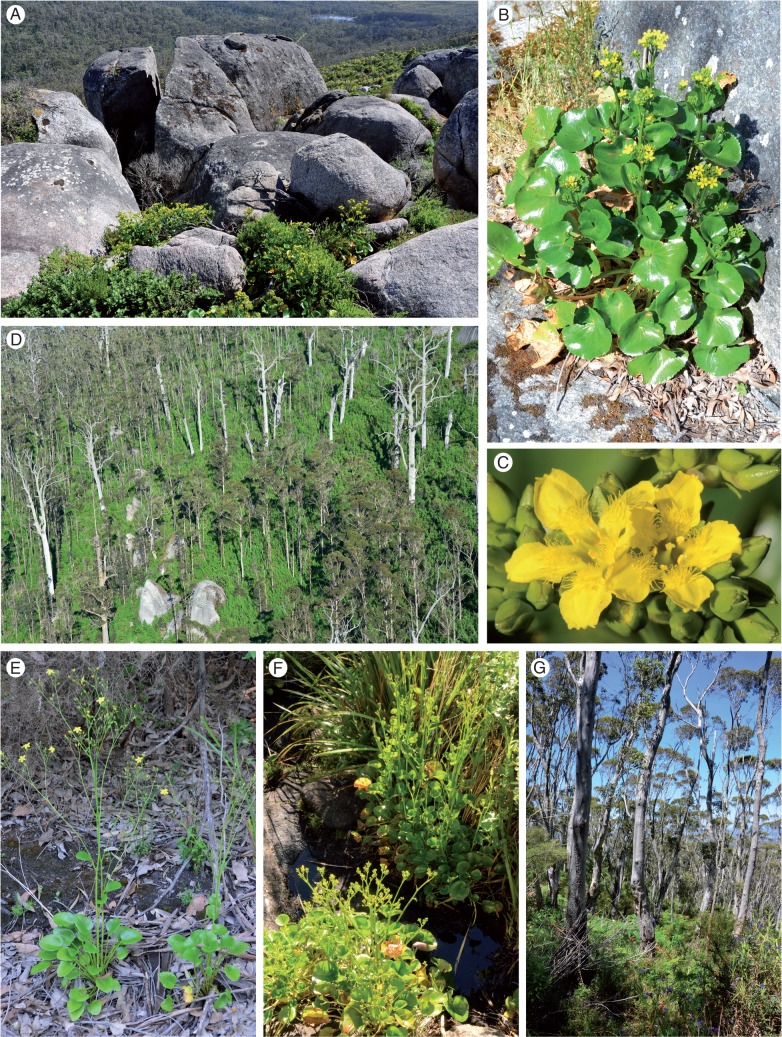
Fig. 1.
Ornduffia species and habitat in the Porongurup Range, south-western Australia. (A) Habitat of Ornduffia calthifolia (yellow-flowered plants in granite rock crevices) at 640 m elevation. (B) Single plant of O. calthifolia. (C) Flowers of O. calthifolia. (D) Karri (Eucalyptus diversicolor) forest habitat of O. marchantii at 330 m elevation. The karri trees are approx. 50 m high and the site had been burnt by high-intensity fire 5 years previously (2008). (E) Ornduffia marchantii along disturbed firebreak in karri forest at 330 m elevation. (F) Intermediate form along walk track in bullich (E. megacarpa) forest at 450 m elevation. (G) Bullich forest habitat of intermediate form of Ornduffia at 450 m elevation. The bullich trees are approx. 10 m high. Photo credits: A, Klaus Braun; B–G, Grant Wardell-Johnson.
Ornduffia calthifolia was declared as Rare Flora under the Western Australian Wildlife Conservation Act 1950 in November 1980, ranked as Endangered (EN) in 1997, and listed under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act – Gilfillan and Barrett, 2004). This species also meets World Conservation Union (IUCN, 2012) Red List Category EN (but is not currently listed), under Criteria C2a, due to a continuing decline in the number of mature individuals, with no population estimated to include more than 250 mature individuals. Nine populations and 582 mature plants were known when the species Interim Recovery Plan (Gilfillan and Barrett, 2004) was published.
Abbott (1982) originally listed O. calthifolia from karri forest at the 300-m contour in the Mira Flores estate on the southern slopes of the Porongurups, prior to recognition of a second species in the area. Ornduff (1990) described the closely related O. marchantii (Fig. 1D, E) from a small population at an elevation of 450 m in a boggy site amongst karri forest. Ornduffia marchantii is similar, but much smaller, and occurs in seasonally wet loams on lower to mid slopes at altitudes below populations of O. calthifolia (Gilfillan and Barrett, 2004). Ornduff (1990) also noted the capacity for hybridization and putative hybrids between the two species have been reported in the overlap zone (Robinson and Coates, 1995). Ornduffia marchantii is currently known from eight specimens in the WA Herbarium, with six originating from the Porongurups. The remaining two specimens are from populations in now degraded vegetation within 10 km to the north and west of the Porongurups. Extensive searches by G.W.J. did not find the species and these populations are likely to be no longer extant.
Field-based variables
In February 2012 and November 2013, we recorded the abundance of O. calthifolia and O. marchantii, and their putative hybrid, throughout the Porongurups and surrounds. We used strategic random sampling to obtain the abundance of Ornduffia species, vegetation height (m), soil depth (cm) and GPS coordinates for 606 locations (207 with O. calthifolia present, 55 with O. marchantii, 14 with the putative hybrid, and 362 absences with neither species present; note that more than one taxon occurred in some locations). Abundance of each Ornduffia taxon was recorded using a modified Braun–Blanquet scale (5 = ≥75 %, 4 = 50–74 %, 3 = 25–50 %, 2 = 10–25 %, 1 = <10 %), estimating cover in circular 27-m2 plots. Plots were at least 20 m apart. Vegetation height was estimated using a ruler and a 2-m pole. Soil depth was determined as the average of five soil depth measures from around the main stem of an individual plant by inserting a scaled (cm) soil probe (with a maximum range of 50 cm), following the approach of Houle and Phillips (1989). GPS co-ordinates were collected using a Garmin Etrex 10 GPS.
Elevation data sources
LiDAR data were obtained by airplane using a Leica ALS 50-II scanner, flying in April 2011. Flight height was approx. 1700–2200 m, resulting in 0·63 points m–2, which was interpolated into a 2-m grid using triangulation. Horizontal and vertical accuracy was <0·35 and <0·15 m, respectively. Further details can be found in Schut et al. (2014). The data were used for fine-scale, localized species distribution modelling of the Porongurups. Elevation data at 30-m resolution (1-arc second) from the Shuttle Radar Topography Mission (SRTM) were used for regional-scale modelling, which extended to other proximal ranges (e.g. Stirling Range) in the south-west.
Climatic data
Climatic variables were recorded for one year (1 November 2011 to 31 October 2012) using two climate stations with a CR200X (CS215 CSL; manufacturer: Campbell Scientific Australia Pty Ltd, Garbutt, QLD) series data logger, and sensors for air temperature and relative humidity (CS215 CSL; manufacturer: Campbell Scientific Australia), precipitation (CS702 tipping bucket rain gauge; manufacturer: Hydrological Services Pty. Ltd, Warwick Farm, NSW), solar radiation (SP210 pyranometer; manufacturer: Apogee Instruments Inc., Logan, UT, USA) and soil moisture (CS625 water content reflectometer; manufacturer: Campbell Scientific Australia).
Each climate station was equipped with an iButton data logger (DS1923 Hygrochron; manufacturer: Maxim Integrated, San Jose, CA, USA), which was attached to the climate station in a plastic cup insulated with duct tape. This was to facilitate comparison with other iButtons placed in various locations with different aspects and radiation intensities. The two climate stations were placed at different altitudes (385 m – foothill climate station, 590 m – hilltop climate station), but were both located on the northern side of the Porongurups. We compared the temperatures recorded by the iButton on the north-facing hilltop climate station with that of a south-facing iButton of similar altitude (580 m; 34°40′21·10″S, 117°50′28·19″E).
Bioclimatic variables (annual mean temperature and precipitation) were acquired from WorldClim, which are generated at a resolution of 1 km2 via interpolation of average monthly weather station data (Hijmans et al., 2005). Future condition bioclimatic variables were used to assess species distribution under a projected climate based on two representative concentration pathway scenarios – 2·6 (RCP2.6) and 4·5 (RCP4.5). RCP2.6 projections are based on the lowest emission scenario, assuming a global mean temperature increase limited to 2 °C and requiring substantial reductions in greenhouse gas emissions (van Vuuren et al., 2011). RCP4.5 assumes medium to low emissions producing a global mean temperature increase of around 3 °C (Thomson et al., 2011). For the regional-scale model, cell size was downscaled to 30 m using cubic convolution resampling to be commensurate with the SRTM elevation data and regional-scale topographic derivatives.
Topographic derivatives
A suite of raster surfaces were derived from both elevation data sources (LiDAR and SRTM) including slope (first derivative of elevation), aspect (degrees from north) and curvature (second derivative of elevation). A curvature of 0 suggests the terrain is flat, negative curvature is upwardly concave (convex) and positive curvature is upwardly convex (concave). Relative Topographic Position, a measure of terrain ruggedness, was calculated using methods described in Cooley (2015). Total solar radiation for 2011 was calculated in ArcGIS (ESRI, 2015) using the techniques outlined by Fu and Rich (2000). This approximates incoming solar radiation over the year (WH m–2) by summing monthly intervals. The Topographic Wetness Index (TWI), a surrogate for soil moisture, was calculated using eqn (1) (Gessler et al., 1995):
| (1) |
where α is calculated as (flow accumulation + 1) × (pixel area in m2) and β is the slope in radians. Flow accumulation measures the number of cells that drain into an individual cell (Olivera et al., 2002).
Species distribution modelling
Modelling was conducted at local and regional scales using 2- and 30-m resolution surfaces, respectively, using MaxEnt version 3.3.3k, which uses the concept of maximum entropy to predict potential distributions (Phillips et al., 2006). A logistic output format was used for all models, whereby suitability ranges from 0 to 1 for each grid cell. The local-scale model used elevation and topographic derivatives but not bioclimatic variables, as none are available at such fine-scale resolution. We used this model to explore habitat preferences of the two species by dividing predicted suitability into four equal-interval classes and extracting the mean value of all variables at 1000 random locations within each class. Differences between classes were tested using Tukey’s honestly significantly different test (Kramer, 1956).
At the regional scale, elevation was strongly and significantly correlated with mean annual temperature (r = −0·8, P < 0·01) and discarded (only from regional-scale modelling) to avoid masking the influence of temperature. In addition, we added mean annual precipitation to enable projections of both a warmer and drier climate under two climate change scenarios to 2070. Predictions of potential distributions (suitability) based on current conditions and climate change forecasts were calculated. As the convex hull of the sampling design covered the majority of the LiDAR image, no bias file (Fourcade et al., 2014) was deemed necessary for the local-scale model. However, to avoid potential overfitting, the convex hull of presence points was used to mitigate sampling bias (Young et al., 2011; Brown, 2014) for regional-scale modelling.
Species presence records were randomly subset, with 90 % used for model training (‘training subset’) and 10 % used as an independent source for model validation (‘testing subset’). Validation was conducted using both the training and the testing datasets by computing the area under the curve (AUC) of receiver operating characteristic (ROC) graphs (Fielding and Bell, 1997). Interpretation of discrimination potential used the ranges presented by Hosmer and Lemeshow (2000).
Ecological modelling
The abundances (Ab) of O. calthifolia (OC) and O. marchantii (OM) were the response variables. We did not model the distribution of the putative hybrid between the two species (OX) because of the low sample size (n = 14). A combination of field-based and remotely-sensed variables was used to determine the key ecological factors driving species distribution and abundance. We initially considered the following predictor variables for the starting model: soil depth (S); vegetation height (V); insolation (I) in winter (calculated as the average insolation during the month of June, Iw), summer (average insolation during the month of December, Is) and throughout the year (average insolation throughout the year, Ia); aspect (A); curvature (C); elevation (E); roughness (R); and topographic wetness index (T). The averages, variation and ranges of these variables are summarized for the three taxa in Table 1. All analyses were implemented in R software 2.15.1 (R Development Core Team).
Table 1.
Mean (standard deviation; range) of variables considered for inclusion in the starting model for all three taxa
| Soil depth | Vegetation height | Elevation | Topographic wetness index | Aspect | Curvature | Roughness | Solar radiation | |
|---|---|---|---|---|---|---|---|---|
| Ornduffia calthifolia (n = 207) | 21·2 (10·8; 3·0–50·0) | 2·5 (5·1; 0·1–25·0) | 596·2 (48·8; 447·2–669·6) | 3·7 (1·9; 0–11·2) | 189·6 (81·6; 8·3–358·9) | 14·4 (101·8; −1039·9–520·6) | 0·5 (0·09; 0·3–0·8) | 1113215 (257414; 110825–1556341) |
| Ornduffia marchantii (n = 55) | 36·8 (16·6; 10·0–50·0) | 20·9 (12·3; 3·0–40·0) | 422·2 (143·2; 238·8–648·6) | 5·3 (2·5 1·7–12·7) | 153·8 (104·3; 3·4–352·2) | −2·9 (17·8; −55·3–82·3) | 0·5 (0·08; 0·3–0·6) | 1271651 (179749 784041–1485304) |
| Putative hybrid (n = 14) | 29·4 (17·1; 12·0–50·0) | 14·4 (16·88; 3·0–40·0) | 579·7 (61·6; 447·2–648·6) | 4·9 (2·6 2·2–10·5) | 165·8 (70·4; 101·2–343·8) | –5·5 (31·6; −55·3–82·3) | 0·5 (0·09; 0·4–0·7) | 1179334 (201629; 78041–15102717) |
To avoid concurvity, we tested for correlation among explanatory variables using Pearson’s correlation coefficient, removing variables with a coefficient >0·5. If correlation was detected, we retained variables that were ecologically more relevant and more proximal (rather than distant) predictors (Dormann et al., 2013). Because winter (Iw) and summer insolation (Is) were positively correlated to each other (r = 0·518) and to annual insolation (Ia; r = 0·905 and 0·828, respectively), we excluded summer and winter insolation from starting models. In addition, strong correlations were observed between vegetation height (V), elevation (E), roughness (R) and topographic wetness index (T) (Supplementary Data Table S2). To address this, we removed R, which (in our opinion) was ecologically the least meaningful variable. Because O. calthifolia appeared to be restricted by elevation (no presences below 447 m, Table 1) and O. marchantii by vegetation height (no presences below 3 m, Table 1), we decided to include these variables instead of the correlated terms in the respective models. Because of the correlation between vegetation height and soil depth (r = 0·511), we excluded the latter from the model for O. marchantii.
We therefore built two generalized additive models (GAMs) including all retained variables. The ‘mgcv’ package (Wood, 2016) was used to implement the models with the following function calls: gam(as.factor(AbOC) ∼ s(S) + s(Ia) + s(A) + s(C) + s(E)), gam(as.factor (AbOM) ∼ s(Ia) + s(A) + s(C) + s(V)). We assumed a binomial error distribution and used a logistic-link function. Independent variables in the model were selected using backwards stepwise regression. Non-significant terms (P < 0·05) based on the likelihood ratio were consequently removed. The significance and fraction of variance explained were determined for each selected independent variable. In addition, we used the Akaike information criterion (AIC; Akaike, 1974) to test model performance. We used a second-order AIC: AICc = AIC + 2(K(K + 1)/(n – K – 1)), where K is the number of parameters in the model and n is the number of sample points (Burnham and Anderson, 1998).
RESULTS
Climate
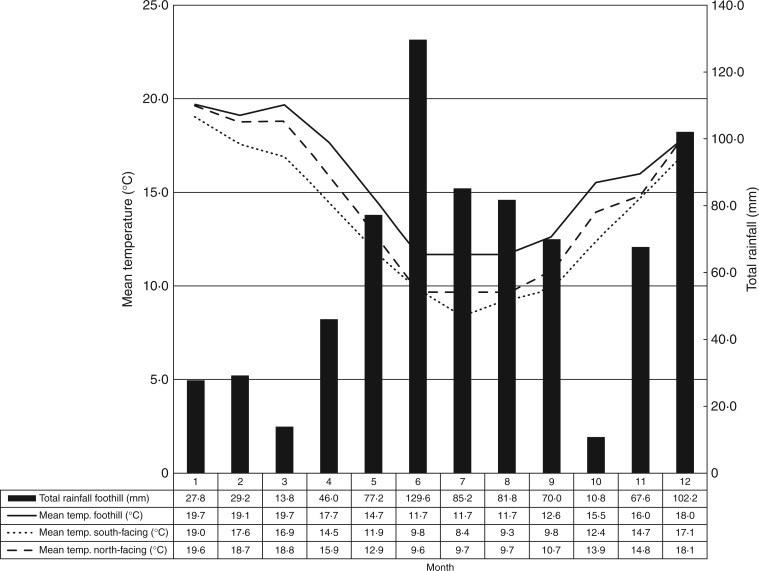
As expected for Mediterranean climates, high rainfall was recorded in winter and spring (June to September). In addition, high rainfall was recorded in November and December as a result of storm systems causing high precipitation on 3 November (27·4 mm) and from 6 to 8 December (53·8 mm) (Fig. 2). Temperatures were lower at higher elevations (the hilltop climate station at 590 m and the south-facing iButton at 580 m), where monthly average temperatures were about 2–3 °C lower than at the foothill climate station (385 m elevation). The south-facing iButton recorded consistently lower temperatures (0·95 °C on average) than the iButton facing north.
Fig. 2.
Climate diagram for the Porongurup Range from 1 November 2011 (1) to 31 October 2012 (12), showing the total rainfall for the foothill climate station (black bars) and mean monthly temperatures for iButtons at the foothill climate station (385 m elevation), at the hilltop climate station (590 m, north-facing), and at a south-facing location (580 m).
The hilltop climate station had lower average temperatures, lower precipitation, higher humidity, higher soil moisture and lower insolation than the foothill climate station (Supplementary Data Table S1). Differences were most pronounced for soil moisture (the annual average was 110·7 % higher in the hilltop climate station) and precipitation (24·5 % more total rainfall at the foothill climate station). Differences in climate variables between the two climate stations were most pronounced during winter (June to August) and least pronounced during summer (December to February).
Species distribution modelling
Elevation had the greatest explanatory power for local models (LiDAR-based) of the two species (Table 2A), although its effect was less pronounced for O. marchantii with roughness, aspect, solar radiation and TWI contributing strongly to the model (Table 2A). At the regional scale, temperature (correlated with elevation) and precipitation contributed >97 % for both species (Table 2B). Considering the qualitative interpretation of AUC values proposed by Hosmer and Lemeshow (2000), discrimination potential ranged from excellent (O. marchantti) to outstanding (O. calthifolia; Table 2).
Table 2.
Percentage contribution of each variable to the species distribution model: (A) local model (2-m resolution), (B) regional model (30-m resolution); see text for variable definitions and variable selection
| Species distribution model | Percentage contribution |
Accuracy |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annual temp. (°C × 10) | Annual precipitation (mm) | Elevation (m a.s.l.) | Roughness | Aspect (°) | Solar radiation (WH m–2) | TWI | Slope (°) | Curvature | Σ | AUCTrain* ± s.e. | AUCTest† ± s.e. | ||
| A | O. calthifolia | – | – | 85·2 | 0·7 | 0·6 | 3·1 | 3·8 | 2·1 | 4·4 | 100 | 0·991 ± 0·003 | 1·000 ± 0·000 |
| O. marchantii | – | – | 58·0 | 12·8 | 12·7 | 6·0 | 5·4 | 4·3 | 0·8 | 100 | 0·905 ± 0·027 | 0·914 ± 0·092 | |
| B | O. calthifolia | 86·3 | 13·2 | – | 0·1 | 0·2 | 0·1 | 0·1 | 0·0 | 0·0 | 100 | 0·946 ± 0·013 | 0·944 ± 0·042 |
| O. marchantii | 74·5 | 22·5 | – | 0·0 | 0·3 | 1·7 | 0·0 | 0·9 | 0·0 | 100 | 0·812 ± 0·049 | 0·857 ± 0·132 | |
*Computed using the training subset.
†Computed using the testing subset.
Under current conditions, both species preferred cooler environments. The most suitable habitats occurred at higher average elevations, being approx. 630 m for O. calthifolia and approx. 550 m for O. marchantii (Table 3). In addition, both species preferred southerly (shadier) aspects that receive less solar radiation over the year, although aspect preferences were more south-west for O. calthifolia and south-east for O. marchantii. Both species preferred steeper slopes, although this relationship was far more prominent for O. calthifolia. The two species differed in terms of curvature preference and ruggedness with O. calthifolia preferring concave, rugged terrain and O. marchantii convex, less rugged locations although curvature was not a significant variable between suitability classes within species.
Table 3.
Average statistics of variables for each model for different habitat suitability class breaks: (A) O. calthifolia, (B) O. marchantii; variables annotated with the same letter are not significantly different at α = 0·05 for those class intervals
| Class | n | Elevation (m) | Roughness | Aspect (°) | Solar radiation (WH m–2) | TWI | Slope (°) | Curvature | |
|---|---|---|---|---|---|---|---|---|---|
| A | 0·75–1·00 | 1000 | 631·7a | 0·53a | 215·5a | 1 075 442a | 2·8a | 38·0a | 6·5a |
| 0·50–0·75 | 1000 | 610·9b | 0·51b | 196·3b | 1 133 720b | 3·4b | 31·3b | 2·5a | |
| 0·25–0·50 | 1000 | 574·2c | 0·50c | 192·2b | 1 164 544c | 3·8c | 28·2c | 1·8a | |
| 0·00–0·25 | 1000 | 342·2 | 0·50c | 169·2c | 1 335 118d | 5·3d | 12·0d | 0·4a | |
| B | 0·75–1·00 | 1000 | 548·5a | 0·45a | 156·5a | 1 239 289a | 4·7a | 17·4a | –2·1a |
| 0·50–0·75 | 1000 | 427·2b | 0·46a | 146·0a,b | 1 302 324b | 5·3b | 13·2b | –1·5a | |
| 0·25–0·50 | 1000 | 361·9c | 0·49b | 139·0b | 1 300 151b | 5·2b | 12·3b | –0·4a | |
| 0·00–0·25 | 1000 | 335·3d | 0·50c | 178·2c | 1 344 218c | 5·2b | 12·5b | 0·3a |
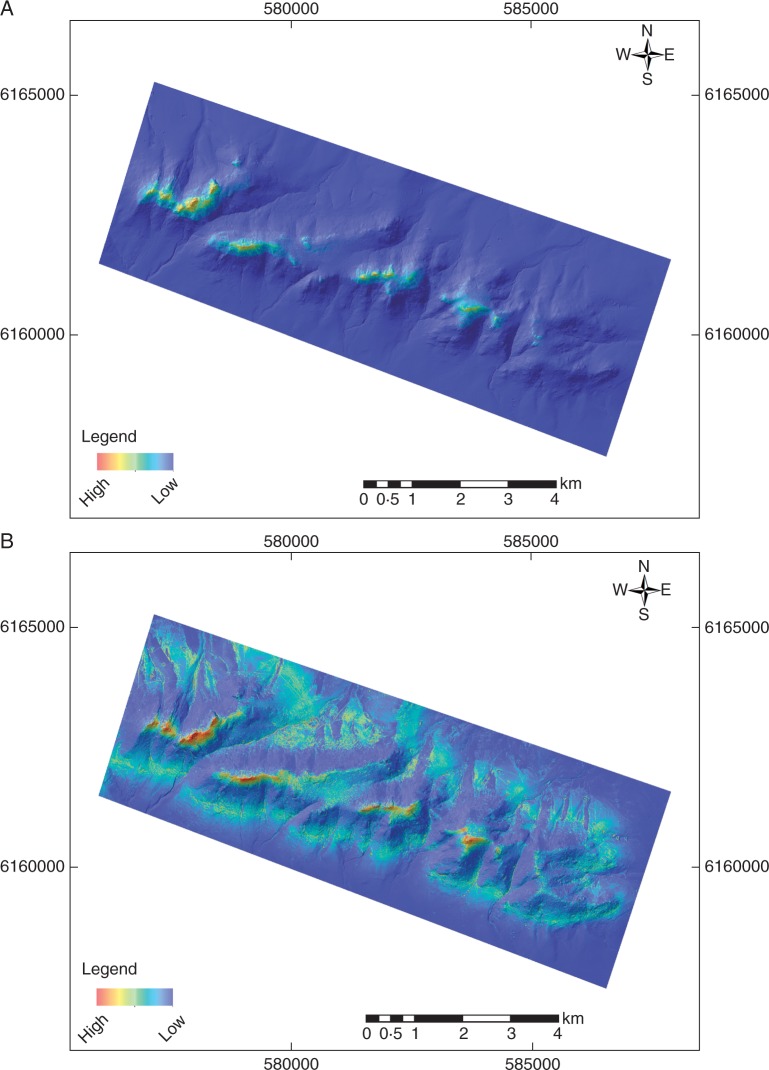
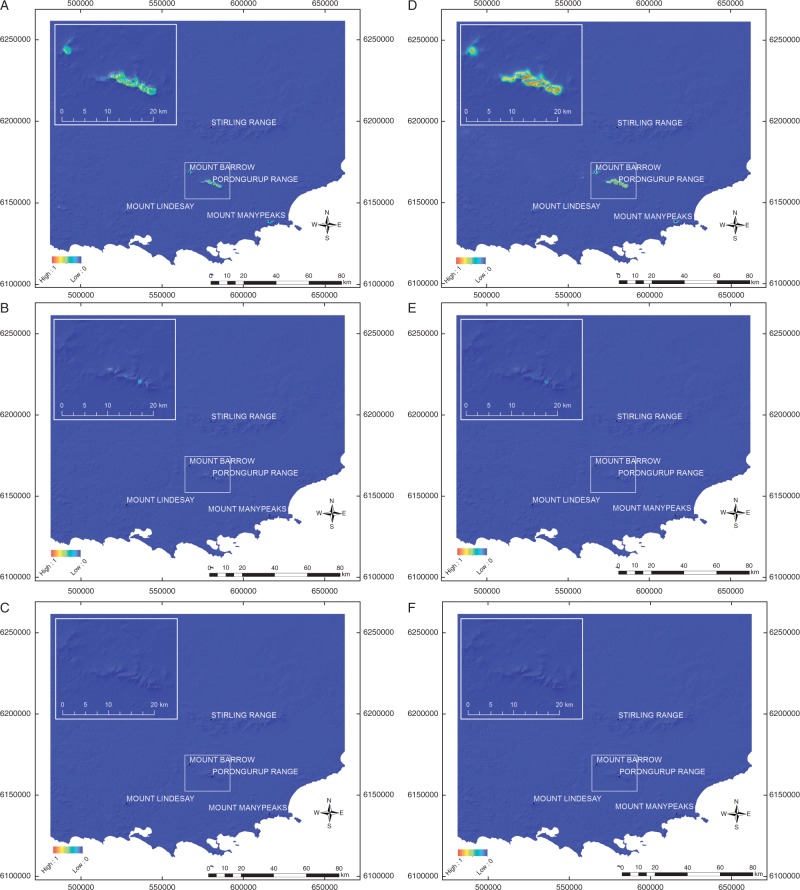
Suitable locations for O. marchantii modelled at the local scale with elevation and associated topographic derivatives were far more widespread than for O. calthifolia – the latter restricted to higher altitude areas (Fig. 3). Regional modelling also identified the Porongurups as suitable habitat with few other suitable sites (e.g. Mount Barrow and Mount Manypeaks) in the region likely to host either species (Fig. 4A). However, even under the mildest temperature prediction for 2070 (RCP2.6), the range of both species contracted significantly. Remaining sites were only moderately suitable and restricted to the Porongurups (Fig. 4B, E), suggesting no opportunities for translocation. Under more severe warming and drying (RCP4.5), modelling suggests no suitable habitat for either species by 2070 (Fig. 4C, F).
Fig. 3.
Local-scale species distribution models of Ornduffia species in the Porongurup Range based on topopgraphic derivatives of LiDAR data: (A) O. calthifolia, (B) O. marchantii.
Fig. 4.
Regional-scale species distribution models of Ornduffia species in southern Western Australia using topographic derivatives of 30-m STRM data and bioclimatic variables (resampled to 30 m): (A) O. calthifolia – current climatic conditions; (B) O. calthifolia – climatic conditions 2070 based on RCP2.6; (C) O. calthifolia – climatic conditions 2070 based on RCP4.5; (D) O. marchantii – current climatic conditions; (E) O. marchantii – climatic conditions 2070 based on RCP2.6; (F) O. marchantii – climatic conditions 2070 based on RCP4.5.
Ecological modelling
The best model for Ornduffia calthifolia included elevation, insolation, soil depth and curvature and explained almost 48 % of the observed deviance (Table 4). Although curvature was marginally not significant (P = 0·055) in the overall model, removing it significantly reduced the performance of the model (χ2 = 16·64, P = 8·4 × 10–4, likelihood ratio test). Of these variables, elevation explained the greatest amount of variance (33 %; Table 5). For O. marchantii, the best model included vegetation height and aspect, and explained 46 % of the total deviance. Vegetation height alone explained almost 38 % of the observed deviance.
Table 4.
Performance indicators of the best generalized additive models (highest deviance explained, no correlated variables) describing the abundance of Ornduffia calthifolia and O. marchantii
| d.f. | P-value | Deviance explained (%) | Adjusted r2 | |
|---|---|---|---|---|
| Ornduffia calthifolia | ||||
| Best model: AbOC ∼ s(S) + s(Ia) + s(A) + s(C) + s(E) | ||||
| Model overall | 47·8 | 0·492 | ||
| Elevation (E) | 5·71 | 7·8 × 10–7 | ||
| Insolation (Ia) | 1·00 | 5·7 × 10–10 | ||
| Curvature (C) | 4·49 | 0·06 | ||
| Soil depth (S) | 3·21 | 3·5 × 10–7 | ||
| Ornduffia marchantii | ||||
| Best model: AbOM ∼ s(A) + s(V) | ||||
| Model overall | 46·0 | 0·383 | ||
| Vegetation height (H) | 3·75 | 9·7 × 10–4 | ||
| Aspect (A) | 8·66 | 2·1 × 10–9 | ||
Table 5.
Deviance explained, P-values and degrees of freedom (d.f.) for significant variables explaining the abundance of Ornduffia calthifolia and O. marchantii
| d.f. | P-value | Deviance explained (%) | |
|---|---|---|---|
| Ornduffia calthifolia | |||
| Elevation (E) | 5·30 | 1 ·0 × 10–5 | 33 ·1 |
| Insolation (Ia) | 5·01 | <2 ·0 × 10–16 | 18 ·7 |
| Curvature (C) | 6·97 | 6 ·1 × 10–12 | 13 ·2 |
| Soil depth (S) | 3·52 | 1 ·0 × 10–7 | 7 ·6 |
| Ornduffia marchantii | |||
| Vegetation height (H) | 8·58 | 4 ·8 × 10–9 | 37 ·8 |
| Aspect (A) | 3·09 | 0 ·016 | 4 ·84 |
The putative hybrid between the two species was generally found in conditions that are intermediate between the two species (soil depth, vegetation height, TWI, solar insolation; Table 1). However, the average and range of elevation for the putative hybrid were similar to those of O. calthifolia, suggesting that the hybrid can only form in close proximity to this species.
DISCUSSION
Climate
Climatic conditions differed considerably with elevation and aspect over small distances. Such differences in soil and air temperature are well established (Rorison et al., 1986; Fridley, 2009). High variability in climatic conditions on fine scales is usually related to topographic complexity and indicates that microrefugia may be present (Dobrowski, 2011; Ashcroft et al., 2012; Keppel et al., 2015).
Species distribution modelling
Species distribution modelling of two species endemic to the Porongurups (O. calthifolia and O. marchantii) identified suitable habitat that closely matched descriptions in the literature (e.g. Robinson and Coates, 1995; Gilfillan and Barrett, 2004) as well as our validation data. Elevation, used in the local modelling as a surrogate for temperature, contributed strongly (58–85 %) to both models. At this scale, other variables, particularly aspect, roughness and solar radiation, were also of importance. At 30-m resolution (regional modelling), temperature was the key variable (74–86 %) which was also strongly (negatively) correlated to elevation. The combination of temperature and precipitation was clearly the most important driver of the regional-scale model (96–99 %).
Spatial clustering of sampling points, difficult to avoid for rare endemic species with very specific habitat preferences, probably contributes to the high model accuracy, despite random selection and the use of an independent subset. Nonetheless, projected distributions under climate change indicate that the distribution of both species will reduce markedly. Although considerable range reductions are predicted for both species, persistence is likely under the milder RCP2.6 scenario. However, both species are at high risk under the more severe RCP4.5 projection, with probable extinction and no regional opportunities for translocation.
The Porongurups constitute an important refugium for both species under anthropogenic climate change, despite being a low-altitude mountain range. Few areas outside of this mountain range contain potentially suitable climate. However, our modelling highlights important limits to refugial capacity, indicating that the range could cease to act as a refugium for both species if global warming exceeds 2 °C of pre-industrial levels. Our study therefore highlights both the potential (through provision of unique microhabitats) and the limitations (due to restricted elevational range) of low-altitude mountains to act as refugia under anthropogenic climate change. Furthermore, because of their lower elevation and the associated smaller geographical extent compared with major mountain ranges, the protected areas in which they lie tend to be smaller and surrounded by highly modified and populated landscapes (Rebelo et al., 2006; Barrett and Yates, 2015). Low-altitude mountain communities and their endemic species are therefore particularly susceptible to a whole suite of threatening processes in addition to climate change (Watson and Barrett, 2004; Barrett and Yates, 2015).
Ecological modelling
It is important to note that predictions from species distribution modelling ignore biological and edaphic factors. Based on ecological modelling, soil depth affects the distribution of O. calthifolia and vegetation height of O. marchantii. Deeper soils increase the habitat suitability for O. calthifolia, and vegetation height (probably through shade provided by the karri forest) enhances habitat suitability for O. marchantii. Our findings therefore highlight the importance of considering the effects of forest on local climate (Scheffers et al., 2014; De Frenne and Verheyen, 2016). Biological and edaphic factors therefore may significantly modify the capacity of the Porongurups to act as refugia for both species.
Hybridization has been observed between O. calthifolia and O. marchantii and presents a potential threat to both species but particularly to the rare O. calthifolia. With climate change causing upward migration of species, hybridization has recently been identified as a potential threat for narrow-range congeners (Gómez et al., 2015). Introgression of genetic material from a common to a rare species can pose considerable conservation threats through genetic assimilation of the rare species (Keppel et al., 2011; Beatty et al., 2015). Given that the hybrids were most commonly found associated with O. calthifolia, asymmetric hybridization with O. calthifolia as the maternal parent could thus be a significant threat to this rare species.
Limitations of species distribution models
Our study highlights some of the limitations of species distribution models (Pearson and Dawson, 2003; Sinclair et al., 2010). The scale at which species distribution modelling is undertaken has crucial impacts on the outcome. Unfortunately, gridded climatic data are currently only available at a resolution of 1 km (or greater), which is considerably poorer than the 2-m LiDAR used for local modelling and 30-m SRTM used for regional modelling. Although interpolation algorithms were used to change cell size, it does not replicate the resolution of the other datasets driving the model and masks local heterogeneities (e.g. microclimate) that may facilitate persistence of the species. This is particularly relevant in topographically complex areas, resulting in exaggerated predictions of extinction risk (Randin et al., 2009; Austin and Van Niel, 2011b; Franklin et al., 2013).
Species distribution models do not consider interspecific interactions. This is especially pertinent for O. marchantii, the distribution of which seems to be closely linked to the presence of karri (Eucalyptus diversicolor), which is the dominant species in the only community with vegetation heights of 20 m or more. The absence of O. marchantii in areas of currently suitable climate appears to be a result of the requirement for shade provided by high vegetation of the karri forest. In addition, most individuals of O. marchantii were found at the margins of tracks (i.e. edges of disturbance) in these karri forest sites, suggesting some level of disturbance is important to the growth of this species in the otherwise dense understorey of the karri forest. Whilst it is possible to include vegetation height as a variable in local models, its permanency, relative to topographic variables, is questionable. For example, fire could remove large proportions, and the range of the karri forest at this location is predicted to contract strongly under forecast climate change (Wardell-Johnson et al., 2015). Vegetation height therefore is unreliable for forecasting O. marchantii distributions.
Species distribution models require continuous and complete coverage (surfaces) of all variables, which is not always feasible. For example, soil depth is an important factor affecting the assembly and structure of granite outcrop plant communities (Poot et al., 2012; Schut et al., 2014; do Carmo and Jacobi, 2016). Soil depth is seemingly important for determining the distribution of O. calthifolia but cannot currently be captured using remote sensing – nor are relevant maps or GIS layers available. Consequently, models can be mis-specified if an important parameter cannot be incorporated into the model because it does not exist, or cannot easily be derived as a surface.
CONCLUSIONS
We have demonstrated that the Porongurups have the capacity to continue acting as a refugium for the target species, O. calthifolia and O. marchantii, and that edaphic (soil depth) and biotic (vegetation height) factors may have strong impacts on this capacity. This highlights the importance of considering such factors when interpreting the results of species distribution modelling. Climate change exceeding 2 °C of pre-industrial levels would have significant consequences for these two species of Ornduffia. While climate change possibly poses the most severe threat to the persistence of the two species, there are numerous other threats such as potential hybridization among the two species. Conservation planning therefore needs to consider a complex array of factors under anthropogenic climate change to facilitate persistence of low mountain endemics.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Sarah Barrett, Lisa and Klaus Braun and members of the Friends of Porongurup Range for support and enthusiasm. This work was supported by an Australian Research Council (ARC) grant (LP 0990914).
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: temperature, rainfall, relative humidity, soil moisture and insolation recorded for two climate stations from 1 November 2011 to 31 October 2012. Table S2: Pearson’s correlation coefficient (based on all 626 data points) for all variables considered for inclusion in the starting model.
LITERATURE CITED
- Abbott I. 1982. The vascular flora of the Porongurup Range south-western Australia. Western Australian Herbarium Research Notes 7: 1–16. [Google Scholar]
- Akaike H. 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control 10: 716–723. [Google Scholar]
- Ashcroft MB. 2010. Identifying refugia from climate change. Journal of Biogeography 37: 1407–1413. [Google Scholar]
- Ashcroft MB, Gollan JR, Warton DI, Ramp D. 2012. A novel approach to quantify and locate potential microrefugia using topoclimate, climatic stability, and isolation from the matrix. Global Change Biology 18: 1866–1879. [Google Scholar]
- Austin MP, Van Niel KP. 2011a. Impact of landscape predictors on climate change modelling of species distributions: a case study with Eucalyptus fastigata in southern New South Wales, Australia. Journal of Biogeography 38: 9–19. [Google Scholar]
- Austin MP, Van Niel KP. 2011b. Improving species distribution models for climate change studies: variable selection and scale. Journal of Biogeography 38: 1–8. [Google Scholar]
- Barrett S. 1996. A biological survey of the mountains in south-western Australia. Como, Western Australia: Department of Conservation and Land Management. [Google Scholar]
- Barrett S, Yates CJ. 2015. Risks to a mountain summit ecosystem with endemic biota in southwestern Australia. Austral Ecology 40: 423–432. [Google Scholar]
- Beatty GE, Barker L, Chen P-P, Kelleher CT, Provan J. 2015. Cryptic introgression into the kidney saxifrage (Saxifraga hirsuta) from its more abundant sympatric congener Saxifraga spathularis, and the potential risk of genetic assimilation. Annals of Botany 115: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Thompson-Dans C, Marchant N. (eds). 1998. Western Australia’s threatened flora. Perth, Western Australia: Department of Conservation and Land Management. [Google Scholar]
- Brown J. 2014. SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods in Ecology and Evolution 5: 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 1998. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer. [Google Scholar]
- Byrne M, Yeates DK, Joseph L, et al. 2008. Birth of a biome: insights into the assembly and maintenance of the Australian arid zone biota. Molecular Ecology 17: 4398–4417. [DOI] [PubMed] [Google Scholar]
- Churchill DM. 1968. The distribution and prehistory of Eucalyptus diversicolor F. Muell., E. marginata Donn ex Sm., and E. calophylla R. Br., in relation to rainfall. Australian Journal of Botany 16: 125–152. [Google Scholar]
- Cooley SW. 2015. GIS4Geomorphology http://www.gis4geomorphology.com (last accessed 1 December 2015).
- De Frenne P, Verheyen K. 2016. Weather stations lack forest data. Science 351: 234. [DOI] [PubMed] [Google Scholar]
- Dirnböck T, Essl F, Rabitsch W. 2011. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Global Change Biology 17: 990–996. [Google Scholar]
- do Carmo FF, Jacobi CM. 2016. Diversity and plant trait-soil relationships among rock outcrops in the Brazilian Atlantic rainforest. Plant and Soil 403: 7–20. [Google Scholar]
- Dobrowski SZ. 2011. A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology 17: 1022–1035. [Google Scholar]
- Dormann CF, Elith J, Bacher S, et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46. [Google Scholar]
- ESRI . 2015. ArcGIS Desktop: Release 10.3. Redlands, CA: Environmental Systems Research Institute. [Google Scholar]
- Fielding AH, Bell JF. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation 24: 38–49. [Google Scholar]
- Fitzpatrick MC, Gove AD, Sanders NJ, Dunn RR. 2008. Climate change, plant migration, and range collapse in a global biodiversity hotspot: the Banksia (Proteaceae) of Western Australia. Global Change Biology 14: 1337–1352. [Google Scholar]
- Foden WB, Butchart SHM, Stuart SN, et al. 2013. Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS One 8: e65427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade Y, Engler JO, Rödder D, Secondi J. 2014. Mapping species distributions with MAXENT using a geographically biased sample of presence data: a performance assessment of methods for correcting sampling bias. PLoS One 9: e97122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin J, Davis FW, Ikegami M, et al. 2013. Modeling plant species distributions under future climates: How fine scale do climate projections need to be? Global Change Biology 19: 473–483. [DOI] [PubMed] [Google Scholar]
- Fridley JD. 2009. Downscaling climate over complex terrain: high finescale (< 1000 m) spatial variation of near-ground temperatures in a montane forested landscape (Great Smoky Mountains). Journal of Applied Meteorology and Climatology 48: 1033–1049. [Google Scholar]
- Fu P, Rich PM. 2000. The solar analyst 1.0 user manual. USA: Helios Environmental Modeling Institute. [Google Scholar]
- Gessler PE, Moore ID, McKenzie NJ, Ryan PJ. 1995. Soil-landscape modelling and spatial prediction of soil attributes. International Journal of GIS 9: 421–432. [Google Scholar]
- Gilfillan S, Barrett S. 2004. Mountain Villarsia (Villarsia calthifolia) interim recovery plan. Interim Recovery Plan No. 169. Albany, Western Australia: Department of Conservation and Land Management. [Google Scholar]
- Gómez J, González-Megías A, Lorite J, Abdelaziz M, Perfectti F. 2015. The silent extinction: climate change and the potential hybridization-mediated extinction of endemic high-mountain plants. Biodiversity and Conservation 24: 1843–1857. [Google Scholar]
- Guerin GR, Lowe AJ. 2013. Multi-species distribution modelling highlights the Adelaide Geosyncline, South Australia, as an important continental-scale arid-zone refugium. Austral Ecology 38: 427–435. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Hopper SD, Gioia P. 2004. The southwest Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35: 623–650. [Google Scholar]
- Hosmer DW, Lemeshow S. 2000. Applied Logistic Regression, 2nd edn Chichester: John Wiley & Sons. [Google Scholar]
- Houle G, Phillips DL. 1989. Seed availability and biotic interactions in granite outcrop plant communities. Ecology 70: 1307–1316. [Google Scholar]
- IUCN. 2012. Guidelines for application of IUCN Red List criteria at regional and national levels: version 4.0. Gland, Switzerland and Cambridge, UK: IUCN. [Google Scholar]
- Keppel G, Wardell-Johnson GW. 2012. Refugia: keys to climate change management. Global Change Biology 18: 2389–2391. [Google Scholar]
- Keppel G, Prentis PJ, Biffin E, et al. 2011. Diversification history and hybridisation of Dacrydium (Podocarpaceae) in remote Oceania. Australian Journal of Botany 59: 262–273. [Google Scholar]
- Keppel G, Van Niel K, Wardell-Johnson GW. et al. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21: 393–404. [Google Scholar]
- Keppel G, Mokany K, Wardell-Johnson GW, Phillips BL, Welbergen JA, Reside AE. 2015. The capacity of refugia for conservation planning under climate change. Frontiers in Ecology and the Environment 13: 106–112. [Google Scholar]
- Kessler M, Kluge J. 2008. Diversity and endemism in tropical montane forests – from patterns to processes. Biodiversity and Ecology Series 2: 35–50. [Google Scholar]
- Klausmeyer KR, Shaw MR. 2009. Climate change, habitat loss, protected areas and the climate adaptation potential of species in mediterranean ecosystems worldwide. PLoS One 4: e6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer CY. 1956. Extension of multiple range tests to group means with unequal number of replications. Biometrics 12: 307–310. [Google Scholar]
- La Sorte FA, Jetz W. 2010. Projected range contractions of montane biodiversity under global warming. Proceedings of the Royal Society of London B: Biological Sciences 277: 3401–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough I, Davis F, Dingman J, et al. 2016. High and dry: high elevations disproportionately exposed to regional climate change in Mediterranean-climate landscapes. Landscape Ecology 31: 1063–1075. [Google Scholar]
- Médail F, Diadema K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. Journal of Biogeography 36: 1333–1345. [Google Scholar]
- Midgley GF, Hannah L, Millar D, Rutherford MC, Powrie LW. 2002. Assessing the vulnerability of species richness to anthropogenic climate change in a biodiversity hotspot. Global Ecology and Biogeography 11: 445–451. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GaB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Ohlemüller R, Anderson BJ, Araújo MB, et al. 2008. The coincidence of climatic and species rarity: high risk to small-range species from climate change. Biology Letters 4: 568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera F, Furnans J, Maidment D, Djokic D, Ye Z. 2002. Drainage systems In: Maidment D, ed. ArcHydro: GIS for water resources. Redlands, CA: ESRI Press, 55–86. [Google Scholar]
- Ornduff R. 1990. A new species of Villarsia (Menyanthaceae) from the Porongurup Range, Western Australia. Systematic Botany 15: 216–220. [Google Scholar]
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37: 637–669. [Google Scholar]
- Pate JS, Dixon KW. 1982. Tuberous, cormous and bulbous plants. Perth, Western Australia: University of Western Australia Press. [Google Scholar]
- Pauli H, Gottfried M, Dirnböck T, Dullinger S, Grabherr G. 2003. Assessing the long-term dynamics of endemic plants at summit habitats In: Nagy L, Grabherr G, Körner C, Thompson DA, eds. Alpine biodiversity in Europe. Berlin: Springer, 195–207. [Google Scholar]
- Pearson RG, Dawson TP. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography 12: 361–371. [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190: 231–259. [Google Scholar]
- Poot P, Hopper SD, van Diggelen JHM. 2012. Exploring rock fissures: does a specialised root morphology explain endemism on granite outcrops? Annals of Botany 110: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pounds JA, Crump ML. 1994. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conservation Biology 8: 72–85. [Google Scholar]
- Provan J, Bennett KD. 2008. Phylogeographic insights into cryptic glacial refugia. Trends in Ecology and Evolution 23: 564–571. [DOI] [PubMed] [Google Scholar]
- Randin CF, Engler R, Normand S, et al. 2009. Climate change and plant distribution: local models predict high-elevation persistence. Global Change Biology 15: 1557–1569. [Google Scholar]
- Rebelo AG, Boucher C, Helme N, et al. 2006. Fynbos Biome In: Mucina L, Rutherford MC, eds. The vegetation of South Africa, Lesotho and Swaziland. Pretoria, South Africa: SANBI, 52–219. [Google Scholar]
- Rix MG, Edwards DL, Byrne M, Harvey MS, Joseph L, Roberts JD. 2014. Biogeography and speciation of terrestrial fauna in the south‐western Australian biodiversity hotspot. Biological Reviews 90: 762–793. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Coates DJ. 1995. Declared rare and poorly known plants in the Albany District. Wildlife Management Program No. 20. Perth, Western Australia: Department of Conservation and Land Management. [Google Scholar]
- Rorison IH, Sutton F, Hunt R. 1986. Local climate, topography and plant growth in Lathkill Dale NNR. I. A twelve-year summary of solar radiation and temperature. Plant, Cell & Environment 9: 49–56. [Google Scholar]
- Scheffers BR, Edwards DP, Diesmos A, Williams SE, Evans TA. 2014. Microhabitats reduce animal’s exposure to climate extremes. Global Change Biology 20: 495–503. [DOI] [PubMed] [Google Scholar]
- Schut AGT, Wardell-Johnson GW, Yates CJ, et al. 2014. Rapid characterisation of vegetation structure to predict refugia and climate change impacts across a global biodiversity hotspot. PLoS One 9: e82778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SJ, White MD, Newell GR. 2010. How useful are species distribution models for managing biodiversity under future climates? Ecology and Society 15: art8. [Google Scholar]
- Tapper S-L, Byrne M, Yates CJ, et al. 2014. Prolonged isolation and persistence of a common endemic on granite outcrops in both mesic and semi-arid environments. Journal of Biogeography 41: 2032–2044. [Google Scholar]
- Tippery NP, Les DH. 2009. A new genus and new combinations in Australian Villarsia (Menyanthaceae). Novon 193: 404–411. [Google Scholar]
- Thomson AM, Calvin KV, Smith SJ, et al. 2011. RCP4.5: a pathway for stabilization of radiative forcing by 2100. Climatic Change 109: 77–94. [Google Scholar]
- Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC. 2005. Climate change threats to plant diversity in Europe. Proceedings of the National Academy of Sciences of the United States of America 102: 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren DP, Stehfest E, den Elzen MGJ, et al. 2011. RCP2.6: exploring the possibility to keep global mean temperature increase below 2 °C. Climatic Change 109: 95–116. [Google Scholar]
- Wardell-Johnson GW, Calver M, Burrow N, Di Virgilio G. 2015. Integrating rehabilitation, restoration and conservation for a sustainable jarrah forest future during climate disruption. Pacific Conservation Biology 21: 175–185. [Google Scholar]
- Watson J, Barrett S. 2004. Small is beautiful: conserving the nature of low-altitude mountain protected areas in South Western Australia In: Harmon D, Worboys GL. eds. Managing mountain areas: challenges and responses for the 21st century. Colledara, Italy: Andromeda Editrice, 340–345. [Google Scholar]
- Wilson RJ, Gutiérrez D, Gutiérrez J, Martínez D, Agudo R, Monserrat VJ. 2005. Changes to the elevational limits and extent of species ranges associated with climate change. Ecology Letters 8: 1138–1146. [DOI] [PubMed] [Google Scholar]
- Wood S. 2016. Package ‘mgcv’. https://cran.r-project.org/web/packages/mgcv/mgcv.pdf.
- Yates CJ, Elith J, Latimer AM, et al. 2010. Projecting climate change impacts on species distributions in megadiverse South African Cape and Southwest Australian Floristic Regions: opportunities and challenges. Austral Ecology 35: 374–391. [Google Scholar]
- Young N, Carter L, Evangelista P. 2011. A MaxEnt Model v 3.3.3.e Tutorial (ArcGIS v 10), Colarado State University.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.