Abstract
Background Centres of endemism have received much attention from evolutionists, biogeographers, ecologists and conservationists. Climatic stability is often cited as a major reason for the occurrences of these geographic concentrations of species which are not found anywhere else. The proposed linkage between endemism and climatic stability raises unanswered questions about the persistence of biodiversity during the present era of rapidly changing climate.
Key Questions The current status of evidence linking geographic centres of endemism to climatic stability over evolutionary time was examined. The following questions were asked. Do macroecological analyses support such an endemism–stability linkage? Do comparative studies find that endemic species display traits reflecting evolution in stable climates? Will centres of endemism in microrefugia or macrorefugia remain relatively stable and capable of supporting high biological diversity into the future? What are the implications of the endemism–stability linkage for conservation?
Conclusions Recent work using the concept of climate change velocity supports the classic idea that centres of endemism occur where past climatic fluctuations have been mild and where mountainous topography or favourable ocean currents contribute to creating refugia. Our knowledge of trait differences between narrow endemics and more widely distributed species remains highly incomplete. Current knowledge suggests that centres of endemism will remain relatively climatically buffered in the future, with the important caveat that absolute levels of climatic change and species losses in these regions may still be large.
Keywords: Paleoendemism, neoendemism, plants, diversity, climate change velocity, climatic stability
INTRODUCTION: CLIMATIC STABILITY, ENDEMISM, AND CONSERVATION UNDER CLIMATE CHANGE
Centres of endemism, or geographic regions with concentrations of species not found anywhere else, have long been of central interest to biogeographers, evolutionary biologists and ecologists (Nelson, 1978). Evolutionary and ecological processes responsible for producing centres of endemism are complex and reflect interacting aspects of climatic, geological and biogeographic history. Climate, and particularly its patterns of stability and change over evolutionary time, is a key ingredient in most interpretations of the origins and maintenance of centres of high plant and animal endemism (e.g. Dynesius and Jansson, 2000; Jansson, 2003; Jetz et al., 2004; Linder, 2008; Sandel et al., 2011; Feng et al., 2016). Old and historically stable climates lacking histories of glaciation or extreme temperature fluctuation (climatic ‘refugia’) tend to enable the survival of old and narrowly distributed taxa, a phenomenon that has been called paleoendemism (Stebbins and Major, 1965). The proliferation of relatively young and narrowly distributed species, or neoendemism (Stebbins and Major, 1965), may be promoted by many factors, including insularity, topography and novel environments (e.g. Winkworth et al., 2005; Linder, 2008; Verboom et al., 2009). Climatic stability may play an important role in enabling the survival of these neoendemic lineages. Climate and associated disturbance regimes also contribute to the evolution of niche and life-history attributes, such as the annual life cycle in herbs and the resprouting or reseeding habit in shrubs, that may feed back to modulate the rates of speciation and extinction shaping patterns of endemism (e.g. Hopper and Gioia, 2004).
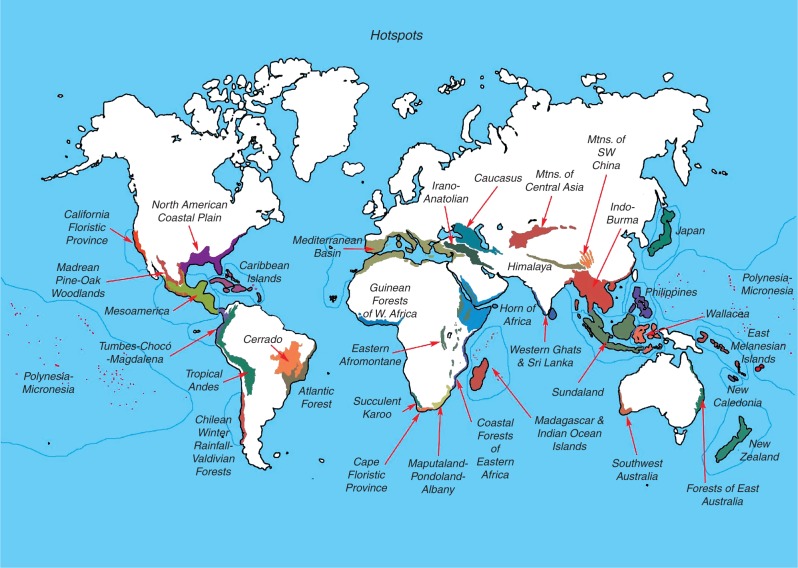
Major world centres of endemism experiencing high rates of habitat loss were christened ‘biodiversity hotspots’ by Myers et al. (2000), who outlined a bold strategy for setting global land protection priorities that has since been adopted in modified form by some of the world’s largest non-governmental conservation organizations, such as Conservation International. In their original analysis, biodiversity hotspots were identified as each having >1500 endemic plant species as well as ≥ 70 % loss of primary vegetation. The 25 hotspots thus identified at that time comprise only 1·5 % of the world’s terrestrial land surface but hold more than one-third of the world’s vertebrate animals in four major groups, as well as half to two-thirds of the plants and vertebrates on the IUCN Red List (Myers et al., 2000). The number of recognized global hotspots was later expanded to 35 (Mittermeier et al., 2011) and then 36 (Critical Ecosystem Partnership Fund, 2016; Fig. 1). The concept behind using threatened hotspots to set broad global conservation priorities is that the cost of land protection generally scales by area, while the benefits can be measured by how many of the world’s species are protected, making it cost-effective to minimize extinction rates by focusing on high concentrations of species not found anywhere else. Protecting centres of endemism before they suffer significant habitat loss would be a proactive conservation strategy to complement the threatened hotspots approach advocated by Myers et al. (2000) and others. The related concepts of complementarity and irreplaceability, which evaluate units of land based on how many species they contain that are not protected anywhere else or do not exist anywhere else, are cornerstones of systematic conservation planning (Margules and Pressey, 2000).
Fig. 1.
Currently recognized global hotspots of plant endemism, which are defined as having >1500 endemic plant species and >70 % habitat conversion. Stephen D. Nash© Conservation International.
In recent years, while a proliferation of new studies have addressed the identification of endemism hotspots and their use in conservation planning, process-based understanding of hotspots has also grown, but at a more modest pace. In a semi-quantitative survey of the top 100 Google Scholar search results for ‘endemism hotspot’ (or ‘endemism centre’), we found that only a minority of studies (<20 %) discussed the possible causes of endemism hotspots, and even fewer (<10 %) contained analyses linking geographic or phylogenetic patterns of endemism to measures of climate, climatic history or other factors related to the potential causes of high endemism. Of these latter few studies, most implicated stable or benign climates as a main or contributing factor (Fjeldsaå et al., 1997; Carnaval et al., 2009; Kier et al., 2009; Sandel et al., 2011; Särkinen et al., 2012) although past or persistent insularity also played an important role (e.g. James, 1961; Bossuyt et al., 2004. Kier et al., 2009; Särkinen et al., 2012).
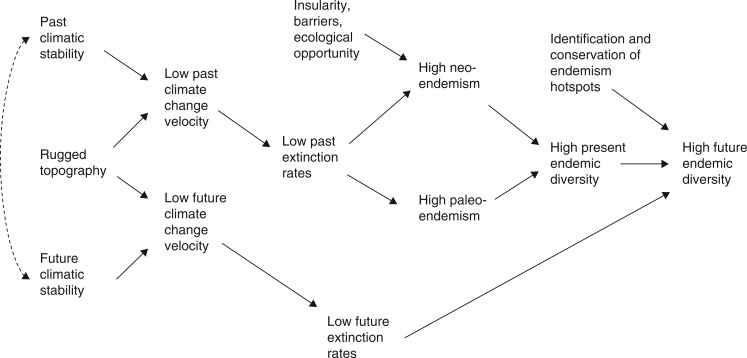
Climate change has, needless to say, brought a new perspective to the goals and strategies of conservation, in which protection of natural lands from conversion is no longer a secure end point (e.g. Coetzee et al., 2009; Klausmeyer and Shaw, 2009; Ackerly et al., 2010; Game et al., 2011). Challenged with the certainty of massive change, conservationists must now face questions that might once have been regarded as purely academic about factors promoting the long-term maintenance or loss of biological diversity. It is in the context of anticipated rapid biotic change in the near future that we ask: are centres of plant or animal endemism frequently associated with stable climatic refugia? What kinds of plant species are found in high-endemism regions; do their histories conform more closely to the paleoendemic or neoendemic models, respectively suggesting greater roles for reduced extinction or enhanced speciation? Do they tend to be extreme climatic or edaphic specialists, and are their niches evolutionarily conservative or labile? Do climatic microrefugia created by small-scale topographic relief also play an important role in protecting endemic species? From existing evidence, can we conjecture whether climatic refugia with high concentrations of endemic species will remain relatively stable and retain their biological diversity under rapid climate change in the near future, given appropriate conservation measures (Fig. 2)?
Fig. 2.
Hypothesized linkages of climatic stability to present and future biodiversity. Climatic stability refers to relative lack of change in the macroclimate, caused by such influences as warm ocean currents, low latitude and low continentality. Climate change velocity refers to the speed at which organisms must move to keep pace with climate change. Rugged topography causes climate change velocity to be lower for any given degree of climatic stability. Paleoendemism means old endemic lineages; we argue that these exist in places where they survived due to the combination of climatic stability and topography, which combine to produce low climate change velocity. Neoendemism means young endemic lineages; we argue that these exist where (1) they evolved, as a result of insularity, barriers and ecological opportunity, and (2) they survived, because of climatic stability and topography combining to produce low climate change velocity. Solid arrows, proposed positive cause and effect relationships. Dashed double-headed arrow, proposed positive correlation.
CLIMATIC STABILITY IN THE CONTEXT OF OTHER INTERACTING CAUSES OF ENDEMISM
Ever since Wallace (1878) famously linked the latitudinal diversity gradient to the absence of glaciation in the tropics, Pleistocene climatic fluctuations have been at the heart of theories relating species diversity and endemism to stable climates. Within the unglaciated parts of the world, climatic oscillations during and since the Pleistocene have varied in their magnitude owing to latitude, continentality and idiosyncratic variation in the global atmospheric and marine circulation system. Several global analyses have found that high concentrations of small-ranged species are found in regions with low estimated values of climatic oscillation during and since the Pleistocene (e.g. Jansson, 2003; Sandel et al. 2011). Molecular evidence also often points toward the importance of Pleistocene refugia for the existence of modern-day hotspots of both genetic and species diversity (e.g. Carnaval et al., 2009). However, climatic stability over longer time scales than the Quaternary may also be important for endemism. Protection from post-Eocene cooling, aridification and climatic extremes has been implicated as contributing to the high concentrations of old endemic lineages in some regions, for example south-western Australia, the North American Coastal Plain, the Cape region of South Africa and Amazonia (Phillips et al., 2001; Sorrie and Weakley, 2001; Hopper and Gioia, 2004; Linder, 2008).
Proximity to persistent warm or mild ocean currents plays an important role in generating regional climatic stability and creating terrestrial centres of endemism. This stabilizing maritime influence is considered to be an important reason for the high endemism of islands (Kier et al., 2009) and of today’s Mediterranean climate regions (Hopper and Gioia, 2004; Lancaster and Kay, 2013), for example. Likewise, the North American Coastal Plain, second only to California in the USA as a centre of plant endemism in spite of its relative absence of topography, has been relatively stable climatically for millions of years (Noss et al., 2015). During the late Pleistocene, a strengthened Loop Current made the region warmer in winter during stadial than interstadial intervals, with relatively minor changes in vegetation throughout these cycles (Grimm et al., 2006; Donders et al., 2011).
Mountainous terrain is characteristic of most major centres of endemism, an effect that may be explained in part by the heterogeneity of environments found in rugged regions (Jetz et al., 2004). More importantly, though, mountainous topography also contributes to an effectively stable climate and to high endemism by enabling species to survive periods of climatic oscillation through short-distance dispersal along elevational gradients (Jansson, 2003; Ohlemuller et al., 2008) or into more favourable topographic positions (Dobrowski, 2011) or microclimatic refugia such as depressions of karst surfaces (Bátori et al., 2017). Recently, the proposed buffering effect of mountainous topography has been quantified using a simple metric termed climate change velocity, which is lowest where stable (often coastal) climates coincide with mountain ranges (Loarie et al., 2008, 2009). Climate change velocity has been shown to be a strong predictor of the global richness of small-ranged species, i.e. of centres of endemism (Sandel et al., 2011, 2017; Z. Ma, B. Sandel, G. Feng, L. Mao, S. Normand, A. Ordonez and J.-C. Svenning, pers. comm.; see next section for further discussion).
Climatic instability and long-term change may also play roles in generating endemism, both by generating novel ecological opportunities for speciation (e.g. Särkinen et al., 2012) and by creating shifting patterns of habitat fragmentation that are conducive to allopatric speciation, followed in some cases by secondary contact and hybridization (e.g. Hopper and Gioia, 2004). Long-term climatic drying led to the emergence of fire as an important influence on plant evolution in many parts of the world in the late Miocene (Keeley and Rundel, 2005; Beerling and Osborne, 2006), and, in turn, fire-adapted and exceptionally endemic-rich floras developed in southern Africa (Goldblatt and Manning, 2002), the Brazilian cerrado (Simon et al., 2009), the North American Coastal Plain (Noss et al., 2015) and elsewhere. However, even when climatic changes were responsible for bursts of diversification, some amelioration of Quaternary climatic oscillations was probably necessary for the persistence of the newly evolved lineages, as evidenced by the overall global association between Quaternary climatic stability and high levels of endemism (Jansson, 2003; Sandel et al., 2011, 2017). For example, long-term persistence of the seasonal wet–dry cycle characteristic of savannas probably provided a stable evolutionary environment for strongly fire-adapted species in savanna regions (Keeley and Rundel, 2005; Bond, 2015).
Insularity and geographic barriers are perhaps the most often-cited causes of high levels of endemism. Large and isolated oceanic islands (e.g. Kier et al., 2009), the five widely separated regions of the world with Mediterranean-type climates (Cowling et al., 1996), localized areas of cool montane climates within warmer regions (e.g. Ohlemuller et al. 2008) and island-like terrestrial habitats such as outcrops of serpentine soil (Anacker and Harrison, 2012) exemplify the power of spatial isolation at global to regional scales to promote allopatric divergence, continued range restriction and high endemism. Nevertheless, climate may still play a significant interacting role. The stabilizing maritime influence as well as the physical separation of islands contributes to their exceptional levels of endemism (Kier et al., 2009). Evolutionary transitions to serpentine endemism in the Californian flora have tended disproportionately to occur in the most benign climates (Anacker and Harrison, 2012). Seasonally dry tropical forest in the Andes occurs as ‘islands’ of endemic-rich vegetation that have remained relatively stable for long periods of evolutionary time, surrounded and separated by younger vegetation (Särkinen et al., 2012), providing another example of the interactions of climatic stability, environmental change and geographic separation in generating high endemism.
CLIMATIC STABILITY AS MEASURED BY CLIMATE CHANGE VELOCITY
The combined influences of long-term climatic stability and topography have recently been quantified using the concept of ‘climate change velocity’, defined as how fast species must move to maintain constant climatic envelopes in the face of a changing climate (Loarie et al., 2008, 2009). Climate change velocity is faster – that is, organisms must move greater distances in any given time period to keep pace with a changing climate, and are therefore more likely to become extinct under historic or modern climate change – in unstable interior climates and in flatter regions than in more stable coastal climates or more rugged regions. An over-riding role for past climatic stability in generating high endemism is suggested by the result that low climate change velocity over the past 22 000 years is associated with high richness of relatively small-ranged animal species (Sandel et al., 2011). High mobility gives organisms greater capacity to track shifting climates, and, accordingly, the relationship of climate change velocity to endemism is stronger in less mobile than more mobile animal groups (Sandel et al., 2011). Looking into the future, geographic patterns in loss of species under climate change may be expected to be predictable in part by climate change velocity (Loarie et al., 2008, 2009).
New evidence shows that low Quaternary climate change velocity also predicts high levels of endemism in grasses (Sandel et al., 2017), in agreement with many other analyses and interpretations of plant endemism (e.g. Jansson, 2003; Linder, 2008; Noss et al., 2015; Feng et al., 2016). Endemism in grasses is also higher in climates that are atypical for their regions, such as in mountains surrounded by lowlands (Sandel et al., 2017), paralleling the findings of other studies (e.g. Ohlemuller et al., 2008). Surprisingly, however, climate change velocity is not related to the percentage of annuals in global endemic grass floras, which might be considered an index of dispersal potential; rather, this percentage depends only on the current climate (Sandel et al., 2017). Within regions of generally high climate change velocity, the percentage of exotics in the grass flora is positively related to past climate change velocity, underscoring concerns that the high dispersal capacity required to track climate change will generally favour exotics over natives (Dukes and Mooney, 1999).
Endemism can be measured in phylogenetic terms, using metrics that consider both the range sizes of the species in a region and the branch lengths separating them from other species. Such measures of phylogenetic endemism may be expected to better reflect the old events shaping a region’s diversity, notably the survival or extinction of paleoendemics with few close relatives, than endemism at the level of species (Rosauer et al., 2009). Quaternary climate change velocity is a strong predictor of phylogenetic endemism in North American trees (Ma et al., 2016) and, indeed, the stability–endemism relationship is stronger for phylogenetic diversity than species diversity (Z. Ma, B. Sandel, G. Feng, L. Mao, S. Normand, A. Ordonez and J.-C. Svenning, pers. comm.). Phylogenetic endemism in trees is also related to modern temperature (Ma et al., 2016), in accordance with expectations that climatic niches are conserved and that ancient climates were predominantly warm (Wiens and Donoghue, 2004).
The balance of evidence, we conclude, strongly supports a linkage between climatic stability and endemism, together with an important interacting role for rugged topography.
CLIMATIC STABILITY AND NEO- VS. PALEOENDEMISM
Since the pioneering work of G. L. Stebbins (Stebbins and Major, 1965; Stebbins, 1974), who memorably posed the question of whether the tropics are rich in species because they are a ‘cradle’ of high speciation or a ‘museum’ of low extinction, the question of rapid and recent species origination (neoendemism) vs. the persistence of ancient lineages (paleoendemism) has been a fundamental framework for discussions of endemism. Centres of high endemism have been interpreted as primarily containing either ancient relictual taxa, young and rapidly diversifying taxa, or mixtures of both ancient and young taxa segregating along different environmental gradients. For example, Stebbins and Major (1965) and Raven and Axelrod (1978) concluded that the neoendemic element of California’s flora was concentrated in regions with the relatively young Mediterranean climate and high topographic relief, while the paleoendemic element was concentrated in the mesic and stable northern and coastal regions. Similarly, Medail and Quezel (1999) considered ‘vicariant’ (young) endemics to be most abundant in the geologically young and heterogeneous eastern Mediterranean, and ‘relictual’ (old) endemics to predominate in the more stable western basin. More formal analyses of endemism patterns have reached similar conclusions about geographic concentrations of species classified a priori as neo- and paleoendemics, for example in China (Lopez-Pujol et al., 2011) and parts of Europe (Casazza et al., 2008). In a very different system, climatically stable deep-water refugia for coral reefs in the Indo-Australian Archipelago both preserved old fish species during Quaternary glacial cycles and, when reefs were disconnected during drops in sea level, promoted high rates of speciation (Pellissier et al., 2014).
Phylogenetic methods have been a powerful tool for refining the understanding of endemism hotspots, and in some cases have overturned traditional ideas about the ages and origins of endemic lineages. For example, in the California Floristic Province, the proliferation of ‘neoendemics’ in California-centred genera was long attributed to rapid diversification under the modern Mediterranean-type climate (Raven and Axelrod, 1978). However, molecular phylogenetic examination has recently shown that diversification in many of these genera began much earlier, in the Miocene (Baldwin, 2014), and that reduced extinction in a stable pre-Mediterranean climate may have contributed more to high diversification in these groups than rapid recent speciation (Lancaster and Kay, 2013). Likewise, the ancestors of endemic plants in peninsular Florida were thought to have arisen through allopatric speciation during Pleistocene sea level oscillations (James, 1961), but dated phylogenies of some taxa have shown their origins to be Miocene or Pliocene (Germain-Aubrey et al., 2014), albeit that these speciation events were probably still related to isolation on islands during high sea level stands. The North American Coastal Plain was long considered to be geologically young and climatically unstable, but molecular and other analyses indicate that some of its flora is of Tertiary origin (Sorrie and Weakley, 2001; Noss, 2013; Noss et al., 2015). The long-term climatic stability and high paleoendemism of the North American Coastal Plain is indicated by the monotypic status of 43 (77 %) of the 56 plant genera endemic to the region (Sorrie and Weakley, 2001; Noss et al., 2015; B. Sorrie and A. Weakley, pers. comm.). In contrast, the extremely endemic-rich cerrado vegetation of Brazil is surprisingly young, having diverged from sister groups in wet forest mostly <4 million years ago as the flammable savanna biome expanded worldwide (Simon et al., 2009).
New phylogenetically based methods have made it possible to include lineage ages and/or diversification rates in analyses of endemism hotspots and their environmental correlates. In a global analysis of inferred speciation and extinction rates in plants, Linder (2008) found that ‘mature radiations’ tend to be found in climatically and geologically stable environments, while ‘recent and rapid’ radiations are found in young environments; some of the world’s hyperdiverse floras, such as the Cape and Neotropical floras, combine both of these elements.
Within part of the Mediterranean Basin endemism hotspot, high values of relative phylogenetic endemism, a continuous metric of the prevalence of old endemic lineages (Rosauer et al., 2009; Z. Ma, B. Sandel, G. Feng, L. Mao, S. Normand, A. Ordonez and J.-C. Svenning, pers. comm.), were geographically correlated with high rainfall, whereas neoendemics were concentrated in areas of high topographical relief (Molina-Venegas et al., 2017), largely consistent with earlier interpretations (Medail and Quezel, 1999). Within California, the youngest neoendemics were associated with the geologically recent desert environment (Kraft et al., 2012), again largely supporting previous conclusions (Raven and Axelrod, 1978).
Genetic diversity within widespread species may follow many of the same historically driven patterns as endemic species diversity, with the highest levels found in climatic refugia (e.g. Carnaval et al., 2009). Phylogeographic analysis and climatic modelling of the widespread cerrado tree Dimorphandra mollis (Fabaceae) indicated that it survived Pleistocene climatic fluctuations in a large climatically stable refugium. Significantly for the questions posed here, this historic refugium is predicted to remain relatively stable in the future (Souza et al., 2017). Maintenance or loss of genetic diversity through past episodes of climate-driven expansion and contraction is an especially important aspect of the capacity of species to adapt to future environmental changes (Schierenbeck, 2017).
We conclude that climatic stability has demonstrable links to both paleo- and neoendemism, although the linkage is more straightforward for paleoendemism, and many additional factors contribute to generating neoendemism.
CLIMATIC STABILITY AND SPECIES TRAITS
Concern for rare and endemic species has led to many efforts to identify biological traits that distinguish them from more common species (e.g. Kruckeberg and Rabinowitz, 1985; Kunin and Gaston, 1997). However, there are multiple forms of rarity (Rabinowitz et al., 1986), and few studies in the rarity literature have focused on identifying traits of species restricted to large-scale geographic centres of endemism. To the extent that geographic centres of endemism are the product of climatic stability, species found in them may have attributes that equip them poorly to survive climatic instability. Such stability-associated attributes might include narrow climatic tolerances, poor dispersal or dormancy capacities, high habitat specialization, dependence on highly specific disturbance regimes, obligate mutualisms, low population sizes or low levels of genetic diversity (Dynesius and Jansson, 2000; Jansson, 2003; Keeley et al., 2011; Sandel et al., 2011). By this reasoning, unless centres of endemism remain especially climatically stable in the future (Fig. 2), they may soon become hotspots of extinction.
In the old, climatically stable, nutrient-poor and exceptionally endemic-rich campo rupestre grassland vegetation of Brazil, plants were found to have the lowest levels of seed dormancy measured in any vegetation type on earth. In addition, seed viability was found to be low, consistent with an evolutionary strategy of relatively low investment in seed reproduction. The tendency toward low seed dormancy showed a strong phylogenetic signal, meaning it is unlikely to evolve upward even if the changing climate increases the adaptive value of dormancy (Dayrell et al., 2017).
In contrast, in the endemic-rich vernal pool grasslands of California, the endemic annual plant Lasthenia fremontii (Asteraceae) showed adaptive variation in dormancy strategy, consistent with the interpretation that multiyear seed dormancy is beneficial to persistence in the face of moderate interannual climatic variability. Populations from the sites with the highest levels of historical variation in autumn rainfall showed the greatest rates of multiyear seed dormancy in experimental trials (Torres-Martinez et al., 2017).
We conclude that there is currently very little evidence on whether species in endemism hotspots tend to show particular traits reflective of climatic stability, which could influence their chances of future persistence.
CLIMATIC MICROREFUGIA
During past and modern climatic fluctuations, species may have persisted in localized areas in which changes in climate were more moderate than in the broader surrounding regions. These climatic ‘microrefugia’ may have served as nuclei for population re-expansion, stepping stones for dispersal and/or reservoirs of genetic diversity (Rull, 2009; Keppel et al., 2012; Hannah et al., 2014). Microrefugia are created, in part, by the climatic influences of relatively large-scale geographic features such as mountainous topography and proximity to coasts. For plants and butterflies in Europe, Ohlemuller et al. (2008) concluded that high concentrations of small-ranged species tend to occur in cool mountaintop microrefugia where cold-adapted species have been able to survive unusually warm periods during the last 10 000 years. The regionally rare climates supporting the current centres of endemism are predicted to shrink disproportionately under future climate change, leading to elevated vulnerability for their endemic species.
Importantly, microrefugia may also be created by small-scale topographic variation down to the level of metres (Dobrowski, 2011), as well as by localized biological influences such as overstorey shading (De Frenne et al., 2013). These small-scale influences on the climates experienced by organisms are not represented in GIS layers, and thus are not well captured in models of species distributional shifts under climate change (e.g. Franklin et al., 2013; Keppel et al., 2017). For endemic plants in the South-west Australia biodiversity hotspot, subtle topographic variability can provide important climatic refugia within generally low-relief landscapes, and soil depth and vegetation height may add significantly to this refugial capacity (Keppel et al., 2017). For cool-adapted plants in eastern Europe, depressions created by karstic weathering are important climatic microrefugia under past and expected future climate change (Bátori et al., 2017). The evolution of many species in edaphic communities such as glades may have been shaped more by the unique local microclimate, for example alternating extremes of wet and dry on thin soils, than by the regional macroclimate (Noss, 2013).
We conclude that microrefugia play crucial roles in hosting endemic species, both within regional climatic refugia and in regions that are not refugia. These microrefugia are likely to become of even greater significance under present and future climate change, making their identification and protection urgent.
CLIMATIC STABILITY IN THE PAST VS. THE FUTURE
To function as reliable foci of conservation efforts, biodiversity hotspots must persist over time. For plants and animals at a global scale, Jansson (2003) found that the climatically stable hotspots of endemism show a significant tendency to remain more stable than other regions in the future, evidenced by a negative correlation between present-day endemism and anticipated temperature increases over the next century derived from the Hadley climate model. Even in these relatively stable refugia, however, the predicted temperature increases were in the order of 3–5 °C, a magnitude of change that might not be survivable through dispersal, especially given the interactive effects of habitat loss and fragmentation (see also Keppel and Wardell-Johnson, 2015; Keppel et al., 2015). By one estimate, the world’s major endemism hotspots will lose up to 43 % of their endemic plant species in the next century (Malcolm et al., 2006).
Considering total species richness rather than endemism, Sommer et al. (2010) found that rising global temperatures will increase the ‘capacity for species richness’ in temperature-limited high-latitude regions, but will decrease this capacity in the warm, water-limited tropical and sub-tropical regions of the world where diversity is currently highest. These authors caution that increases in the climatic capacity for richness are not necessarily beneficial for diversity; specialist species adapted to harsh environmental conditions will be vulnerable to increased competition from generalists, and endemics adapted to long-term climatic stability may be especially poorly adapted to survive change, resulting in disproportionate losses of small-ranged species.
For grasses at the global scale, Sandel et al. (2017) find, similarly to Jansson (2003), that inferred past climate change velocity is strongly correlated with predicted climate change velocity over the next century, leading to a tendency for endemism hotspots to be more climatically stable into the future than other regions. Underscoring the conclusions of Jansson (2003), Sandel et al. (2017) find that the absolute values of change are massive even in these relatively stable areas: climate change velocity in grass ‘endemism hotspots’ (regions with >10 % endemism) is predicted to increase from an average of 3·1 m year−1 to 1090 m year−1. They note, however, that the long-term averages of past climate change velocity mask considerable variation. In particular, during the warming episode beginning 11 700 years ago, which followed the Younger Dryas cool period and marks the start of the Holocene, local short-term velocities were thousands of times above long-term averages. While these historic periods of rapid warming suggest somewhat greater potential for present-day species assemblages to survive rapid modern warming, Sandel et al. (2017) note that other aspects of current global change do not have precedents, such as the elevated levels of multiple greenhouse gases and the presence of interacting anthropogenic stresses.
We conclude that centres of endemism appear likely to remain relatively climatically buffered in the future, with the important caveat that absolute levels of change will still be large.
CONCLUSIONS
We find widespread new support for the classic idea that centres of endemism occur in regions where past climatic fluctuations have been mild due to ocean currents and other large-scale climatic influences, as well as where mountainous topography and microclimatic diversity contribute to buffering lineages against extinction during historical climatic oscillations. The concept of climate change velocity is an especially valuable way to quantify and test the effect of climatic stability on geographic patterns of endemism.
One of the areas where our knowledge remains least complete is the question of trait differences between narrow endemics in hotspots and widely distributed species. Future work is needed to compare species inside and outside of endemism hotspots in several respects: their intrinsic traits such as dispersal rates, dormancy capacities and genetic diversity; extrinsic traits, such as habitat associations and adaptation to disturbance regimes; and extinction rates over evolutionary and modern time. To the extent that past climatic stability has influenced trait evolution, species in endemism hotspots may be at elevated risk in the future.
In conclusion, the key conservation implications of the endemism–stability linkage are: first, that it is critical to protect large stable climatic macrorefugia, which appear to be well indicated by endemism hotspots; secondly, that it is also critical to identify and protect microrefugia, which facilitate persistence of species both inside and outside of the larger scale macrorefugia; and, thirdly, that nothing is more critical than minimizing global warming and anthropogenic habitat destruction. Rapid warming will make even relatively stable climatic refugia unsuitable for the long-term sustainability of biodiversity, while continuing loss of natural habitat will eliminate even the most climatically stable macro- and microrefugia.
ACKNOWLEDGEMENTS
We thank the editors of the Annals of Botany for the opportunity to present this Viewpoint.
LITERATURE CITED
- Ackerly DD, Loarie SR, Cornwell WK, et al. 2010. The geography of climate change: implications for conservation biogeography. Diversity and Distributions 16: 476–487. [Google Scholar]
- Anacker BL, Harrison S. 2012. Climate and the evolution of serpentine endemism in California. Evolutionary Ecology 26: 1011–1023. [Google Scholar]
- Anacker BL, Rajakaruna N, Ackerly DD, Vasey MC, Harrison S, Keeley JE. 2012. Ecological strategies in California chaparral: interacting effects of soils, climate, and fire on specific leaf area. Plant Ecology and Diversity 4: 179–188. [Google Scholar]
- Baldwin BG. 2014. Origins of plant diversity in the California Floristic Province. Annual Review of Ecology, Evolution, and Systematics 45: 347–369. [Google Scholar]
- Bátori Z, Vojtkó A, Farkas T, et al. 2017. Large- and small-scale environmental factors drive distributions of cool-adapted plants in karstic microrefugia. Annals of Botany 119: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling DJ, Osborne CP. 2006. The origin of the savanna biome. Global Change Biology 12: 2023–2031. [Google Scholar]
- Bond WJ. 2015. Fires in the Cenozoic: a late flowering of flammable ecosystems. Frontiers in Plant Science 5: 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt F, Meegaskumbura M, Beenaerts N, et al. 2004. Local endemism within the Western Ghats–Sri Lanka biodiversity hotspot. Science 306: 479–481. [DOI] [PubMed] [Google Scholar]
- Carnaval AC, Hickerson MJ, Haddad CFB, Rodrigues MT, Moritz C. 2009. Stability predicts genetic diversity in the Brazilian Atlantic Forest hotspot. Science 323: 785–789. [DOI] [PubMed] [Google Scholar]
- Casazza G, Zappa E, Mariotti MG, Médail F, Minuto L. 2008. Ecological and historical factors affecting distribution pattern and richness of endemic plant species: the case of the Maritime and Ligurian Alps hotspot. Diversity and Distributions 14: 47–58. [Google Scholar]
- Coetzee BWT, Robertson MP, Erasmus BFN, van Rensburg BJ, Thuiller W. 2009. Ensemble models predict important bird areas in southern Africa will become less effective for conserving endemic birds under climate change. Global Ecology and Biogeography 18: 701–710. [Google Scholar]
- Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianotsou M. 1996. Plant diversity in mediterranean-climate regions. Trends in Ecology and Evolution 11: 362–366. [DOI] [PubMed] [Google Scholar]
- Crisp MD, Laffan S, Linder HP, Monro A. 2001. Endemism in the Australian flora. Journal of Biogeography 28: 183–198. [Google Scholar]
- Critical Ecosystem Partnership Fund. 2016. Announcing the world’s 36th biodiversity hotspot: the North American Coastal Plain http://www.cepf.net/news/top_stories/Pages/Announcing-the-Worlds-36th-Biodiversity-Hotspot.aspx (last accessed 31 October 2016).
- Dayrell RLC, Garcia QS, Negreiros D, Baskin CC, Baskin JM, Silveira FAO. 2017. Phylogeny strongly drives seed dormancy and quality in a climatically buffered hotspot for plant endemism. Annals of Botany 119: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowski SZ. 2011. A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology 17: 1022–1035. [Google Scholar]
- Donders TH, deBoer HJ, Finsinger W, et al. 2011. Impact of the Atlantic warm pool on precipitation and temperature in Florida during North Atlantic cold spells. Climate Dynamics 36: 109–118. [Google Scholar]
- Donoghue JF. 2011. Sea level history of the northern Gulf of Mexico coast and sea level rise scenarios for the near future. Climatic Change 107: 17–33. [Google Scholar]
- Dukes JS, Mooney HA. 1999. Does global change increase the success of biological invaders? Trends in Ecology and Evolution 14: 135–139. [DOI] [PubMed] [Google Scholar]
- Dynesius M, Jansson R. 2000. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovich climate oscillations. Proceedings of the National Academy of Sciences, USA 97: 9115–9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mao L, Swenson NG, Svenning J-C. 2016. High plant endemism in China is partially linked to reduced glacial–interglacial climate change. Journal of Biogeography 43: 145–154. [Google Scholar]
- De Frenne P, Rodríguez-Sánchez F, Coomes DA, et al. 2013. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences, USA 110: 18561–18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldsaå J, Ehrlich D, Lambin E, Prins E. 1997. Are biodiversity ‘hotspots’ correlated with current ecoclimatic stability? A pilot study using the NOAA-AVHRR remote sensing data. Biodiversity and Conservation 6: 401–422. [Google Scholar]
- Franklin J, Davis FW, Ikegami M. 2013. Modeling plant species distributions under future climates: how fine scale do climate projections need to be? Global Change Biology 19: 473–483. [DOI] [PubMed] [Google Scholar]
- Game ET, Lipsett-Moore G, Saxon E, Peterson N, Sheppard S. 2011. Incorporating climate change adaptation into national conservation assessments. Global Change Biology 17: 3150–3160. [Google Scholar]
- Germain-Aubrey CC, Soltis PS, Neubig KM, Thurston T, Soltis DE, Gitzendanner MA. 2014. Using comparative biogeography to retrace the origins of an ecosystem: the case of four plants endemic to the central Florida scrub. International Journal of Plant Sciences 175: 418–431. [Google Scholar]
- Goldblatt P, Manning JC. 2002. Plant diversity of the Cape region of southern Africa. Annals of the Missouri Botanical Garden 89: 281–302. [Google Scholar]
- Grimm EC, Watts WA, Jacobson GL, Jr, Hansen BCS, Almquist HR, Dieffenbacher-Krall AC. 2006. Evidence for warm wet Heinrich events in Florida. Quaternary Science Reviews 25: 2197–2211. [Google Scholar]
- Hannah L, Flint L, Syphard AD, Moritz MA, Buckley LB, McCullough IM. 2014. Fine-grain modeling of species’ response to climate change: holdouts, stepping-stones, and microrefugia. Trends in Ecology and Evolution 29: 390–397. [DOI] [PubMed] [Google Scholar]
- Hopper SD, Gioia P. 2004. The southwestern Australian floristic region: evolution and conservation of a global hotspot of biodiversity. Annual Review of Ecology, Evolution, and Systematics 35: 623–650. [Google Scholar]
- James CW. 1961. Endemism in Florida. Brittonia 13: 225–244. [Google Scholar]
- Jansson R. 2003. Global patterns in endemism explained by past climatic change. Proceedings of the Royal Society B: Biological Sciences 270: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz W, Rahbek C, Colwell R. 2004. The coincidence of rarity and richness and the potential signature of history in centers of endemism. Ecology Letters 7: 1180–1191. [Google Scholar]
- Keeley JE, Rundel PH. 2005. Fire and the Miocene expansion of C4 grasslands. Ecology Letters 8: 683–690. [Google Scholar]
- Keeley JE, Pausas JG, Rundel PW, Bond WJ, Bradstock RA. 2011. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science 16: 406–411. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wardell-Johnson GW. 2015. Refugial capacity defines holdouts, microrefugia and stepping-stones: a response to Hannah et al. Trends in Ecology and Evolution 30: 233–234. [DOI] [PubMed] [Google Scholar]
- Keppel G, Van Niel KP, Wardell-Johnson GW, et al. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21: 393–404. [Google Scholar]
- Keppel G, Mokany K, Wardell-Johnson GW, Phillips BL, Welbergen JA, Reside AE. 2015. The capacity of refugia for conservation planning under climate change. Frontiers in Ecology and the Environment 13: 106–112. [Google Scholar]
- Keppel G, Robinson T, Wardell-Johnson GW, et al. 2017. Low-altitude mountains as refugium for two narrow endemics in the Southwest Australian Floristic Region biodiversity hotspot. Annals of Botany 119: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier G, Kreft H, Lee TM, et al. 2009. A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Sciences, USA 106: 9322–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausmeyer KR, Shaw MR. 2009. Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide. PLoS One 4: e6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft NJB, Baldwin BG, Ackerly DD. 2010. Range size, taxon age and hotspots of neoendemism in the California flora. Diversity and Distributions 16: 403–413. [Google Scholar]
- Kruckeberg AR, Rabinowitz D. 1985. Biological aspects of endemism in higher plants. Annual Review of Ecology and Systematics 16: 447–479. [Google Scholar]
- Kunin WE, Gaston KJ. 1997. The biology of rarity: causes and consequences of rare–common differences. London: Chapman and Hall. [Google Scholar]
- Lancaster LT, Kay KM. 2013. Origin and diversification of the California flora. Evolution 67: 1041–1054. [DOI] [PubMed] [Google Scholar]
- Linder HP. 2008. Plant species radiations: where, when, why? Philosophical Transactions of the Royal Society B: Biological Sciences 363: 3097–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462: 1052–1055. [DOI] [PubMed] [Google Scholar]
- Loarie SR, Carter BE, Hayhoe K, et al. 2008. Climate change and the future of California’s endemic flora. PLoS One 3: e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pujol J, Zhang F-M, Sun H-Q, Ying T-S, Ge S. 2011. Centres of plant endemism in China: places for survival or for speciation? Journal of Biogeography 38: 1267–1280. [Google Scholar]
- Ma Z, Sandel B, Svenning J-C. 2016. Phylogenetic assemblage structure of North American trees is more strongly shaped by glacial–interglacial climate variability in gymnosperms than in angiosperms. Ecology and Evolution 6: 3092–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm JR Liu CNeilson RP, Hansen L, Hannah L.. 2006. Global warming and extinctions of endemic species from biodiversity hotspots. Conservation Biology 20: 538–548. [DOI] [PubMed] [Google Scholar]
- Margules CR, Pressey RL. 2000. Systematic conservation planning. Nature 405: 243–253. [DOI] [PubMed] [Google Scholar]
- Medail F, Quezel P. 1999. Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conservation Biology 13: 1510–1513. [Google Scholar]
- Mittermeier RA, Turner WR, Larsen FW, Brooks TW, Gascon C. 2011. Global biodiversity conservation: the critical role of hotspots In: Zachos FE, Habel JC, eds. Biodiversity hotspots: distribution and protection of conservation priority areas. Heidelberg: Springer Verlag, 3–22. [Google Scholar]
- Molina-Venegas R, Aparicio A, Lavergne S, Arroyo J. 2017. Climate and topographical correlates of plant palaeo- and neoendemism in a Mediterranean biodiversity hotspot. Annals of Botany 119: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Nelson G. 1978. From Candolle to Croizat: comments on the history of biogeography. Journal of the History of Biology 11: 269–305. [DOI] [PubMed] [Google Scholar]
- Noss RF. 2013. Forgotten grasslands of the South: natural history and conservation. Washington DC: Island Press. [Google Scholar]
- Noss RF, Platt WJ, Sorrie BA, et al. 2015. How global biodiversity hotspots may go unrecognized: lessons from the North American Coastal Plain. Diversity and Distributions 21: 236–244. [Google Scholar]
- Ohlemüller R, Anderson BJ, Araújo MB, et al. 2008. The coincidence of climatic and species rarity: high risk to small-range species from climate change. Biology Letters 4: 568–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier L, Leprieur F, Parravicini V, et al. 2014. Quaternary coral reef refugia preserved fish diversity. Science 344: 1016–1019. [DOI] [PubMed] [Google Scholar]
- Phillips JA. 2001. Marine macroalgal biodiversity hotspots: why is there high species richness and endemism in southern Australian marine benthic flora? Biodiversity and Conservation 10: 1555–1577. [Google Scholar]
- Rabinowitz D, Cairns S, Dillon T. 1986. Seven forms of rarity and their frequency in the flora of the British Isles In: Soulé ME, ed. Conservation biology: the science of scarcity and diversity. Sunderland, MA: Sinauer, 182–204. [Google Scholar]
- Raven P, Axelrod D. 1978. Origin and relationships of the California flora. Berkeley, CA: University of California Publications in Botany. [Google Scholar]
- Roberts CM, McClean CJ, Veron JE, et al. 2002. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295: 1280–1284. [DOI] [PubMed] [Google Scholar]
- Rosauer D, Laffan SW, Crisp MD, Donnellan SC, Cook LG. 2009. Phylogenetic endemism: a new approach for identifying geographical concentrations of evolutionary history. Molecular Ecology 18: 4061–4072. [DOI] [PubMed] [Google Scholar]
- Rull V. 2009. Microrefugia. Journal of Biogeography 36: 481–484. [Google Scholar]
- Russell DA, Rich FJ, Schneider V, Lynch-Stieglitz J. 2009. A warm thermal enclave in the Late Pleistocene of the south-eastern United States. Biological Reviews 84: 173–202. [DOI] [PubMed] [Google Scholar]
- Sandel B, Arge L, Dalsgaard B, et al. 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science 334: 660–664. [DOI] [PubMed] [Google Scholar]
- Sandel B, Monnet A-C, Govaerts R, Vorontsova M. 2017. Late Quaternary climate stability and the origins and future of global grass endemism. Annals of Botany 119: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Särkinen T, Pennington RT, Lavin M, Simon MF, Hughes CE. 2012. Evolutionary islands in the Andes: persistence and isolation explain high endemism in Andean dry tropical forests. Journal of Biogeography 39: 884–900. [Google Scholar]
- Schierenbeck KA. 2017. Population-level genetic variation and climate change in a biodiversity hotspot. Annals of Botany 119: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MF, Grether R, de Queiroz LP, Skema C, Pennington RT, Hughes CE. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proceedings of the National Academy of Sciences, USA 3092–3106: 20359–20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer JH, Kreft H, Kier G, Jetz W, Mutke J, Barthlott W. 2010. Projected impacts of climate change on regional capacity for global plant species richness. Proceedings of the Royal Society B: Biological Sciences 277: 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrie BA, Weakley AS. 2001. Coastal Plain plant endemics: phytogeographic patterns. Castanea 66: 50–82. [Google Scholar]
- Souza HAV, Collevatti RG, Lima-Ribeiro MS, Lemos-Filho JP, Lovato MB. 2017. A large historical refugium explains spatial patterns of genetic diversity in a Neotropical savanna tree species. Annals of Botany 119: 239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. 1974. Flowering plants: evolution above the species level. Cambridge, MA: Harvard University Press. [Google Scholar]
- Stebbins GL, Major J. 1965. Endemism and speciation in the California flora. Ecological Monographs 35: 1–35. [Google Scholar]
- Torres-Martinez L, Weldy P, Levy M, Emery NC. 2017. Spatiotemporal heterogeneity in precipitation patterns explains population-level germination strategies in an edaphic specialist. Annals of Botany 119: 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboom GA, Archibald JK, Bakker FT, et al. 2009. Origin and diversification of the Greater Cape flora: ancient species repository, hot-bed of recent radiation, or both? Molecular Phylogenetics and Evolution 51: 44–53. [DOI] [PubMed] [Google Scholar]
- Wallace AR. 1878. Tropical nature and other essays. London: MacMillan. [Google Scholar]
- Whittaker RH. 1961. Vegetation history of the Pacific Coast states and the ‘central’ significance of the Klamath region. Madrono 16: 5–23. [Google Scholar]
- Wiens JJ, Donoghue MJ. 2004. Historical biogeography, ecology and species richness. Trends in Ecology and Evolution 19: 639–644. [DOI] [PubMed] [Google Scholar]
- Winkworth RC, Wagstaff SJ, Glenny D, Lockhart PJ. 2005. Evolution of the New Zealand mountain flora: origins, diversification and dispersal. Organisms, Diversity and Evolution 5: 237–247. [Google Scholar]