Abstract
Objective
To compare the frequency and severity of apneic events in very low birth weight (VLBW) infants before and after blood transfusions using continuous electronic waveform analysis.
Study design
We continuously collected waveform, heart rate, and oxygen saturation data from patients in all 45 neonatal intensive care unit beds at the University of Virginia for 120 weeks. Central apneas were detected using continuous computer processing of chest impedance, electrocardiographic, and oximetry signals. Apnea was defined as respiratory pauses of >10, >20, and >30 seconds when accompanied by bradycardia (<100 beats per minute) and hypoxemia (<80% oxyhemoglobin saturation as detected by pulse oximetry). Times of packed red blood cell transfusions were determined from bedside charts. Two cohorts were analyzed. In the transfusion cohort, waveforms were analyzed for 3 days before and after the transfusion for all VLBW infants who received a blood transfusion while also breathing spontaneously. Mean apnea rates for the previous 12 hours were quantified and differences for 12 hours before and after transfusion were compared. In the hematocrit cohort, 1453 hematocrit values from all VLBW infants admitted and breathing spontaneously during the time period were retrieved, and the association of hematocrit and apnea in the next 12 hours was tested using logistic regression.
Results
Sixty-seven infants had 110 blood transfusions during times when complete monitoring data were available. Transfusion was associated with fewer computer-detected apneic events (P < .01). Probability of future apnea occurring within 12 hours increased with decreasing hematocrit values (P < .001).
Conclusions
Blood transfusions are associated with decreased apnea in VLBW infants, and apneas are less frequent at higher hematocrits.
The etiology of apnea of prematurity is multifactorial; however, decreased oxygen carrying capacity may play a role.1–4 The respiratory neuronal network in neonates is immature, particularly in those born preterm, as demonstrated by their paradoxical response to hypoxemia. Although adults increase the minute ventilation in response to hypoxemia, newborns have a brief increase in ventilation followed by periodic breathing, respiratory depression, and occasionally cessation of respiratory effort.5 This phenomenon may be exacerbated by anemia in preterm newborns, where a decreased oxygen carrying capacity may result in decreased oxygen delivery to the central nervous system, a decreased efferent output of the respiratory neuronal network, and an increase in apnea.6
Several studies conducted over the past 30 years have analyzed the association between anemia and bradycardic apnea in the newborn. Some have shown no decrease in the frequency of apnea episodes following transfusions,7–9 and others have suggested that volume expansion plays a more important role than oxygen carrying capacity.1 Infants who receive more packed red blood cell (pRBC) transfusions have significantly less frequent apnea, fewer episodes requiring tactile stimulation, and fewer episodes requiring positive pressure ventilation.10 Infants in the restrictive transfusion group had a decrease in the frequency of apnea after pRBC transfusion,10 which supports the findings that transfusions significantly decrease the rate of apnea.2–4
Although it is relatively common practice for pRBCs to be given to a neonate with anemia and associated apnea and bradycardia, the literature is both limited and divided on whether blood transfusions impact the number of events. We have examined this question in a large data set of bedside monitor waveforms and vital signs.
Methods
Analysis of patient waveforms and abstraction of data from patient charts were approved by the institutional review board of the University of Virginia and classified as exempt from requiring consent. All subjects were de-identified after demographics and transfusion data were extracted from charts.
All infants with birth weights ≤1500 g admitted to the neonatal intensive care unit or intermediate care unit at the University of Virginia after January 23, 2009, and discharged by June 24, 2011, and not meeting exclusion criteria were candidates for the study. Two cohorts were examined. In the first cohort, infants who did not receive a transfusion during the admission were excluded. Data analysis was confined to 6-day time periods, 3 days before and after blood transfusion. For the second cohort, hematocrit values from all very low birth weight (VLBW) infants admitted during the time period were retrieved from the hospital clinical data repository, and data analysis was restricted to the 12 hours following the time of blood draw. For both groups, analysis of waveform and laboratory data was restricted to time periods during which infants were spontaneously breathing, whether on room air, supplemental oxygen, nasal cannula, or continuous positive airway pressure, but without mechanical ventilation.
Electrocardiographic waveforms from 3 leads and both the chest impedance pneumogram and pulse oximeter (oxyhemoglobin saturation as detected by pulse oximetry [SPO2]) signal along with GE monitor (General Electric, Wauwatosa, Wisconsin)–derived bedside alarms and vital signs were collected continuously using the BedMaster (Excel Medical, Jupiter, Florida) system and stored on a custom grid computing cluster with 100 terabyte storage, 40 gigabyte memory, and 80 processing cores. We used a custom Matlab-based interface (PMP, cgrusin@bmc.edu) for development, testing, and implementation of mathematical algorithms for analysis of apnea events.
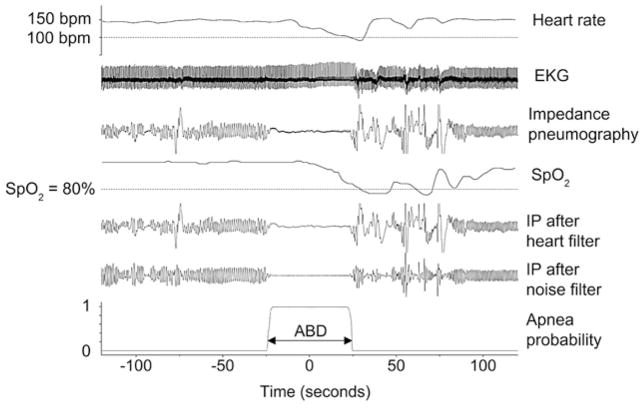
To identify central apnea events, we developed a system for electronically filtering the impedance pneumogram waveforms to remove heart activity and low frequency noise. The apnea detection methods have been described in detail elsewhere.11 In brief, we detected apneas as low-variance epochs of a digitally filtered heart-clock transform of the chest impedance signal.11 This technique removes the cardiac-related component of the chest impedance and estimates the likelihood of apnea based on adjudicated clinical case records. The algorithms have been validated clinically and have >90% agreement with the judgment of experienced clinicians. Apneas beginning within ~50 seconds (or ending within ~25 seconds) of bradycardia (heart rate <100 beats per minute) and oxygen desaturation (SPO2 <80%) were further defined as clinically significant and were labeled as “apnea-bradycardia desaturation-nn,” meaning central apnea with bradycardia and desaturation, in which the central apnea lasts at least nn seconds. As outlined by Lee et al,11 the validation process was restricted to apneas of >10 seconds and the small number of apnea-bradycardia desaturation episodes lasting >60 seconds were rare, and often accompanied by resuscitative measures. Therefore, for the purposes of this study we also restricted analysis to these extremes. Any apneas occurring within 5 minutes of another apnea were counted as a single event to reduce the possibility of overcounting multiple events that might occur during periods of nursing interventions. Figure 1 shows an example of a detected apnea-bradycardia desaturation-30 event with the original impedance pneumogram signal, the filtered impedance pneumogram, and the likelihood of apnea.
Figure 1.
An Apnea associated with bradycardia and oxygen desaturation event (“apnea-bradycardia desaturation marker at bottom) detected by our computer algorithm. Zero time is located at the mid-point of the apnea. Heart rate begins at about 150 beats per minute, and falls below 100 near t = 30. SPO2 begins near 100% and then falls below 80% near t = 35. This infant stops breathing between t ~ −25 and t ~ +25. The fluctuations in impedance pneumogram during that time interval are cardiac artifact: blood flowing in and out of the chest with each heartbeat. Filtered impedance pneumogram tracings are shown in the 5th and 6th tracings, affirming that the cardiac artifact during the apnea is properly removed by the filter. The lowest black line, which increases quickly from near 0 to near 1.0 for the ~45-second duration of the apnea, represents the probability that the infant has stopped breathing. ABD, apnea-bradycardia desaturation.
Sex, birth weight, gestational age, mother’s race or ethnic group, multiple births, and timing of various forms of respiratory support were extracted from the patient medical record. Times of administration of each red cell transfusion were extracted from transfusion slips within the bedside charts. Nursing reports of apneic events were extracted from a separate “apnea sheet” that was kept at each patient’s bedside.
Waveforms were analyzed for a total of 6 days: 3 days before and 3 days after the pRBC transfusion for all VLBW infants during periods of spontaneous ventilation. We included only those transfusions for which there were a minimum of 95% of readable data during the 24 hours before and after the start of the transfusion. Mean rates of apneas per day of computer-identified apneas for the 12 hours previous to and following a transfusion were calculated every 15 minutes and categorized into apneas of >10, >20, and >30 seconds’ duration. Transfusions that had <95% of readable waveform data for the 24 hours on either side of the transfusion were excluded from the analysis.
In a separate analysis, all hematocrit values for all VLBW infants admitted to the neonatal intensive care unit during the defined time period were retrieved electronically from the University of Virginia Hospital data repository. Values were selected only if they occurred during times when there was at least a 12-hour period immediately following drawing of the blood sample during which the patient had not received mechanical ventilation and there was at least 95% of satisfactory waveform data available for analysis.
Statistical Analysis
Differences in the rates of apneas per day before transfusion, defined as 8 hours before the start of the pRBC transfusion to 4 hours after the start of the pRBC transfusion, and rates of apnea per day after the transfusion, defined as the 4 hours after the start of transfusion of the pRBC transfusion to 16 hours after the start of the transfusion, were compared using a 2-sided signed rank test. These time epochs were chosen to allow for the time required to complete the transfusion (mean duration of transfusion = 3.6 hours).
To test the significance of the association of postmenstrual age (PMA), hematocrit, and probability of apnea following the time of hematocrit determination, we used bivariable logistic regression adjusted for repeated measures.12 We obtained 1463 hematocrit lab values from the Clinical Data Repository for unventilated VLBW infants during time periods of complete ABD event monitoring. For each hematocrit value, estimates of the probabilities were calculated using all cases where values were within 5 hematocrit units, and large-sample 95% CIs for these proportion estimates were calculated.
Results
Three hundred twenty-three VLBW infants were discharged during the study period; 67 infants received 110 blood transfusions while not on a ventilator and otherwise met inclusion criteria. Demographic data are shown in Table I computer-identified apnea events of >10, >20, and >30 seconds’ durations are listed in Table II (available at www.jpeds.com).
Table I.
Demographic data
| Grouping by GA and weight at birth | n | Mean GA (wk) | Mean body weight (g) | Mean length of stay (d) | No. of transfusions, median (range) | Mean PMA at transfusion |
|---|---|---|---|---|---|---|
| Transfusion cohort | ||||||
| ≤27 wk | 55 | 25.4 | 818 | 86 | 1 (1–6) | 29.9 |
| >27 wk | 12 | 29.8 | 1195 | 64 | 1 (1–4) | 33.4 |
| ≤1000 g | 48 | 25.3 | 763 | 90 | 1(1–6) | 32.3 |
| >1000 g | 19 | 28.1 | 1172 | 64 | 1(1–3) | 30.2 |
| Total | 67 | 26.1 | 885 | 82 | 1 (1–6) | 31.8 |
|
| ||||||
| Grouping by GA and weight at birth | n | Mean GA (wk) | Mean body weight (g) | Mean length of stay (d) | No. of hematocrits | Mean PMA at hematocrit (wk) |
|
| ||||||
| Hematocrit cohort | ||||||
| ≤27 wk | 112 | 25.2 | 793 | 93 | 973 | 32.6 |
| >27 wk | 113 | 30.1 | 1222 | 44 | 480 | 32.8 |
| ≤1000 g | 112 | 26 | 803 | 86 | 1098 | 32.8 |
| >1000 g | 113 | 30 | 1323 | 41 | 355 | 32.6 |
| Total | 225 | 27.7 | 1009 | 68 | 1453 | 32.7 |
GA, gestational age.
Table II.
Apneas before and after transfusion
| Event | Before (n) | After (n) | Rate before (mean no./d) | Rate after (mean no./d) | Both zero* | Nonzero* | P† |
|---|---|---|---|---|---|---|---|
| ABD >10 | 264 | 141 | 4.80 | 2.56 | 27 | 83 | .0003 |
| ABD >20 | 202 | 111 | 3.67 | 2.02 | 38 | 72 | .0003 |
| ABD >30 | 128 | 75 | 2.33 | 1.36 | 53 | 57 | .0037 |
| Nurse A and B | 56 | 31 | 1.02 | 0.56 | 76 | 34 | .0183 |
ABD, apnea-bradycardia desaturation.
Number of transfusions for which there were or were not apneas during the 12 hours before and/or after the transfusion. Total number of transfusions = 110.
P value of the difference in apnea rates 12 hours before and after transfusion.
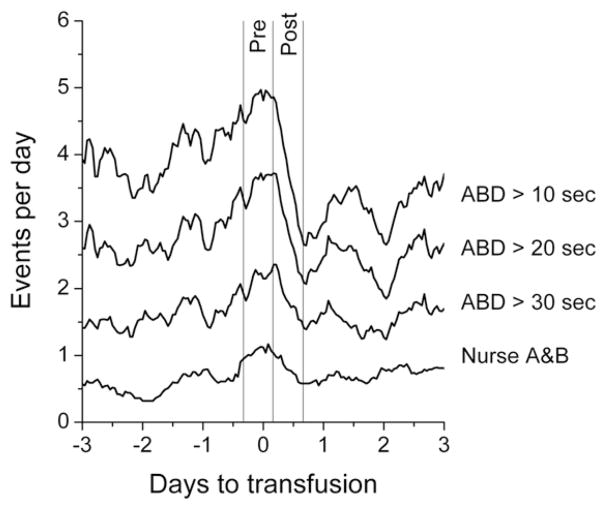
Figure 2 shows that blood transfusions were followed by a statistically significant decreased number of computer-detected apnea-bradycardia desaturation events for 10-, 20-, and 30-second apneas. Nursing records reported less than half of even the longest computer-documented apnea events before and after the pRBC transfusions (Table II).
Figure 2.
Transfusion cohort. Apnea rate, as events per day, as a function of time until and following transfusion for VLBW infants not receiving mechanical ventilation. The top 3 lines are the average numbers of events lasting >10, >20, and >30 seconds, respectively. The bottom line is the rate of events recorded on the bedside apnea and bradycardia sheets. The values at the vertical lines are the average of the event rates measured during two 12-hour periods: 8 hours before to 4 hours after the transfusion, and 4 hours after to 16 hours after the transfusion, respectively.
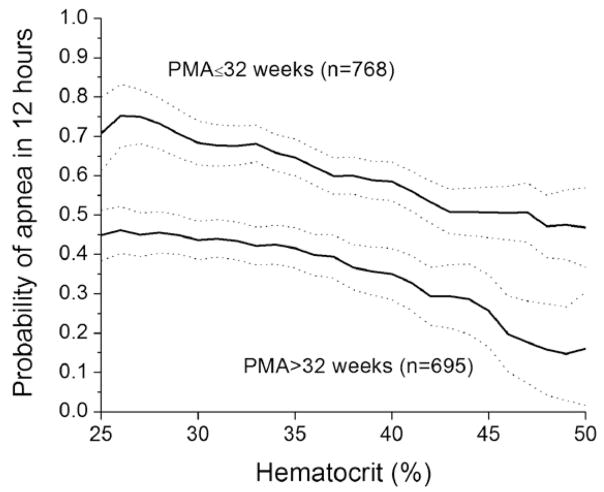
Figure 3 shows that the probability of apnea in the next 12 hours, lasting >10 seconds and associated with bradycardia and SPO2 desaturation, fell as a function of hematocrit, even when adjusted for PMA by stratifying to groups of >32 or <32 weeks. This analysis showed that both PMA and hematocrit added statistically significant information in predicting apnea in the next 12 hours (P < .001 for each; receiver operating characteristic area = 0.647).
Figure 3.
Hematocrit cohort. Probability of a >10-second apnea event (apnea-bradycardia desaturation-10) occurring in the next 12 hours as a function of hematocrit, grouped by 2 PMAs at which the hematocrits were measured. The data points are the probability of apnea in the next 12 hours in a sliding window of 10 hematocrit points centered at the value on the x-axis. The step size was 1 hematocrit point. Dashed lines are the 95% CIs.
Discussion
Using a computer algorithm to detect apnea-bradycardia desaturation events, we showed that blood transfusions decreased the frequency of apnea events in spontaneously breathing VLBW infants and that the probability of apnea is inversely related to hematocrit even after taking into account PMA at the time the hematocrit was obtained. The major strengths of this work are the size of the data set and the automated detection of apnea. The major limitation of this work is that all transfusions were considered together, without distinguishing acute from chronic blood loss, considering the possibility of coexisting diseases, or evaluating the reason for transfusing.
Over the past 30 years, there have been very few and often conflicting studies on the association of red blood cell transfusions and apnea. Many of these studies defined apnea events from nursing charts, which have been demonstrated by us13 and others14,15 to underreport and inaccurately identify apnea events. The current study, as well as a report by Belal et al,16 quantifies apnea events from a computer analysis of waveforms continuously collected from bedside monitors. Our computer analysis of a large database provides a more accurate assessment of the frequency, duration, and timing of apnea events occurring before and after blood transfusions. The conflicting outcomes observed in the few small previous studies that examined the relationship between anemia and apnea may be due to missed apnea detection by standard monitors or human error in documentation.
We identified a statistical relationship of blood transfusions and a decrease in the frequency of central apnea events for VLBW infants. The effect is statistically significant in apneas of all durations and appears to be most pronounced in the most common apneas, those of short duration. Note that even the >10-second apneas are generally considered to be pathologic, when accompanied by bradycardia and/or desaturation17; our definition required both of these criteria to be present, thereby further strengthening the validity of the observed relationship. Our waveform analysis did not permit us to determine what nursing interventions may have been required to abort the event, such as tactile stimulation or positive pressure ventilation. Moreover, we could not evaluate the influence of transfusions on obstructive apneas, as our apnea detector was limited by the availability of existing signals from bedside monitors, which did not have an assessment of airflow—a variable required to distinguish central apnea from bradycardia desaturation events resulting from airway obstruction. In future studies, we plan to evaluate other qualities of the central events, such as length of time below the thresholds, lowest heart rate and oxygen saturation, and clustering of the events, to provide additional insight into the impact of blood transfusions on the severity of apnea.
To test the hypothesis that blood transfusions are an effective clinical therapy for apnea, a prospective study of VLBW infants who were transfused to specifically treat apnea would need to be performed. It has been hypothesized that the decreased oxygen carrying capacity observed in anemia may result in decreased oxygen delivery to the central nervous system, resulting in apnea as a reflection of the paradoxical hypoxic response curve. Conversely, a study by Bifano et al1 suggested that the decreased frequency of apnea observed following a blood transfusion may be secondary to volume expansion, rather than increased oxygen carrying capacity.
We also demonstrated that the probability of future apnea is inversely related to hematocrit in the VLBW infant, which provides further evidence that decreased oxygen carrying capacity, rather than volume alone, may play a significant role in apnea of prematurity. This is especially important because the benefit of transfusion must be balanced with the associated risks, including infection, hyperkalemia, volume overload, and suppression of endogenous erythropoietin. Additionally, a recent article by Paul et al18 suggests an association between pRBC transfusions and an increased risk of necrotizing enterocolitis.
We recognize that the etiology of apnea of prematurity is multifactorial and that we did not eliminate the possibility that other confounding variables such as sepsis, medications, hyperthermia, and alteration of sleep state may have contributed to the findings.5 We also did not exclude conditions known to be associated with fixed chronic hypoxemia (ie, cyanotic congenital heart disease) or neurologic abnormalities known to manifest independently with central apnea (eg, major chromosomal abnormality or grade IV intraventricular hemorrhage). We were unable to determine the indication for the blood transfusion in this retrospective review of the clinical documentation. At our institution, the decision to order a blood transfusion for an infant is not determined by a prescribed protocol. Finally, we did not account for the few instances when there may have been a change in the type of nonventilator respiratory support immediately before or after a transfusion.
We conclude that there is a correlation between transfusions and a decreased frequency of apnea, and that the probability of future apnea is inversely related to the hematocrit.
Our system, which permits continuous collection and analysis of cardiorespiratory waveforms and oximetry data from bedside monitors, provides a more reliable assessment of apnea compared with the nursing record and has enabled us to assess more accurately the relationship of blood transfusions to frequency of apnea in VLBW infants. Furthermore, our observation that the probability of future apnea is inversely related to hematocrit suggests that decreased oxygen carrying capacity likely plays a role in the etiology of apnea of prematurity.
Acknowledgments
Funded by National Institute for Child Health and Human Development (grant 5R CZ HD064488).
Glossary
- PMA
Postmenstrual age
- pRBC
Packed red blood cell
- SPO2
Oxyhemoglobin saturation as detected by pulse oximetry
- VLBW
Very low birth weight
Footnotes
The authors declare no conflicts of interest.
References
- 1.Bifano EM, Smith F, Borer J. Relationship between determinants of oxygen delivery and respiratory abnormalities in preterm infants with anemia. J Pediatr. 1992;120:292–6. doi: 10.1016/s0022-3476(05)80447-0. [DOI] [PubMed] [Google Scholar]
- 2.Joshi A, Gerhardt T, Shandloff P, Bancalari E. Blood transfusion effect on the respiratory pattern of preterm infants. Pediatrics. 1987;80:79–84. [PubMed] [Google Scholar]
- 3.Sadisidharan P, Hemler R. Transfusion induced changes in the breathing patterns of healthy preterm anaemic infant. Pediatr Pulmonol. 1992;12:170–3. doi: 10.1002/ppul.1950120308. [DOI] [PubMed] [Google Scholar]
- 4.Stute H, Greiner B, Linderkamp O. Effect of blood transfusion on cardorespiratory abnormalities in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:194–6. doi: 10.1136/fn.72.3.f194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnall RA, Ariagno RL, Kinney HC. The late preterm infant and the control of breathing, sleep and brainstem development: a review. Clin Perinatol. 2006;33:883–914. doi: 10.1016/j.clp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Kattwinkel J. Neonatal apnea: pathogenesis and therapy. J Pediatr. 1977;90:342–7. doi: 10.1016/s0022-3476(77)80691-4. [DOI] [PubMed] [Google Scholar]
- 7.Blank JP, Sheagren TG, Vljaria J, Mangurten HH, Benawra RS, Puppala BL. The role of RBC transfusion in the premature infant. Am J Dis Child. 1984;138:831–3. doi: 10.1001/archpedi.1984.02140470031010. [DOI] [PubMed] [Google Scholar]
- 8.Poets CF, Pauls U, Bohnhorst B. Effect of blood transfusion on apneoea, bradycardia, and hypoxaema in preterm infants. Eur J Pediatr. 1997;156:311–6. doi: 10.1007/s004310050607. [DOI] [PubMed] [Google Scholar]
- 9.Westkamp E, Soditt V, Adrian S, Bohnhorst B, Groneck P, Poets CF. Blood transfusion in anemic infants with apnea of prematurity. Biol Neonate. 2002;82:228–32. doi: 10.1159/000065891. [DOI] [PubMed] [Google Scholar]
- 10.Bell EF, Stauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H, Rusin CG, Lake D, Clark MT, Guin L, Smoot TJ, et al. A new algorithm for detection central apnea in neonates. Physiol Meas. 2012;33:1–17. doi: 10.1088/0967-3334/33/1/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrell FE., Jr . Regression modeling strategies: with applications to linear models, logistic regression and survival analysis. Berlin: Springer; 2001. [Google Scholar]
- 13.Vergales B, Paget-Brown A, Callahan S, Lake D, Moorman R, Kattwinkel J. Apnea of prematurity: a comparison of nursing records with continuous electronic waveform analysis. E-PAS. 2010;1468:168. [Google Scholar]
- 14.Muttitt SC, Finer NN, Tierney AJ, Rossmann J. Neonatal apnea: diagnosis by nurse versus computer. Pediatrics. 1988;82:713–20. [PubMed] [Google Scholar]
- 15.Peabody JL, Gregory GA, Willis MM, Philip AG, Luce JF. Failure of conventional monitoring to detect apnea resulting in hypoxemia. Birth Defects Orig Article Ser. 1979;15:274–84. [PubMed] [Google Scholar]
- 16.Belal SY, Emmerson AJ, Beatty PC. Automatic detection of apnoea of prematurity. Physiol Meas. 2011;32:523–42. doi: 10.1088/0967-3334/32/5/003. [DOI] [PubMed] [Google Scholar]
- 17.Finer NN, Higgins R, Kattwinkel J, Martin RJ. The Newborn Drug Development Initiative Workshop I: summary proceedings from the Apnea of Prematurity Group. Pediatrics. 2006;117:47–51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- 18.Paul DA, Mackley A, Novitsky A, Zhao Y, Brooks A, Locke RG. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–41. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]