Abstract
Objective
The abnormal brain functional connectivity (FC) has been assumed to be a pathophysiological aspect of major depressive disorder (MDD). However, it is poorly understood, regarding the underlying patterns of global FC network and their relationships with the clinical characteristics of MDD.
Methods
Resting-state functional magnetic resonance imaging data were acquired from 16 first episode, medication-naïve MDD patients and 16 healthy control subjects. The global FC network was constructed using 90 brain regions. The global topological patterns, e.g., small-worldness and modularity, and their relationships with depressive characteristics were investigated. Furthermore, the participant coefficient and module degree of MDD patients were measured to reflect the regional roles in module network, and the impairment of FC was examined by network based statistic.
Results
Small-world property was not altered in MDD. However, MDD patients exhibited 5 atypically reorganized modules compared to the controls. A positive relationship was also found among MDD patients between the intra-module I and helplessness factor evaluated via the Hamilton Depression Scale. Specifically, eight regions exhibited the abnormal participant coefficient or module degree, e.g., left superior orbital frontal cortex and right amygdala. The decreased FC was identified among the sub-network of 24 brain regions, e.g., frontal cortex, supplementary motor area, amygdala, thalamus, and hippocampus.
Limitation
The limited size of MDD samples precluded meaningful study of distinct clinical characteristics in relation to aberrant FC.
Conclusions
The results revealed altered patterns of brain module network at the global level in MDD patients, which might contribute to the feelings of helplessness.
Keywords: Major depressive disorder, Helplessness, Functional connectivity, Small-worldness, Modularity
1. Introduction
Major depressive disorder (MDD) is a complex disease that can be characterized by a variety of symptoms: negative moods, cognitive deficits, and somatic symptoms (APA, 2013). The array of symptoms can make diagnoses a difficult problem, a fact exacerbated by how little is presently known about the actual neurobiological mechanisms underlying the symptoms of MDD. Functional neuroimaging studies found abnormal neurological activities in some specific brain areas of MDD patients, including prefrontal cortex (Frodl et al., 2009; Lui et al., 2009), parahippocampal (Peng et al., 2011), and thalamus (Frodl et al., 2009). Recent reports have further observed atypical functional connectivity (FC) networks among MDD patients. For instance, Veer et al. (2010) noted that a decrease of resting-state FC may be involved in the dysfunctional affects and cognition networks among MDD patients. Likewise, in other sampled MDD patients, atypical FC was found within the default mode network (DMN) involving medial prefrontal cortex, precuneus/posterior cingulate cortex (Guo et al., 2013; Sheline et al., 2010), subgenual cingulate cortex, thalamus (Greicius et al., 2007), and bilateral caudate (Bluhm et al., 2009).
However, most previous studies do not actually examine functional interconnections of the brain at global level in patients with MDD. Graph theoretical analysis is a novel method used to explore the integration and segregation of global brain information for both small-world model analysis and modular analysis (Bullmore and Sporns, 2009; Wang et al., 2010). Using small-world properties, Zhang et al. (2011) reported that the MDD patients at first episode showed lower path length and higher global efficiency in the whole brain networks. However, replicating these findings in recent studies using unipolar depression including both first episode and recurrent samples failed (Lord et al., 2012), as did those among samples with late-life depression (Bohr et al., 2012). Using the module organization, Tao et al. (2013) reported significant rearrangement in the first episode and recurrent MDD, but the measures of modularity showed no significant alteration among MDD patients in other studies (Bohr et al., 2012; Lord et al., 2012). Conversely, abnormal topological properties of module networks have been found in depressed patients in most previous studies. For instance, Lord et al. (2012) reported that the increased participant index of nodes was significantly distributed in the frontal and parietal temporal regions, while the decreased participant index was located in the occipital, temporal and inferior-frontal regions in first episode and recurrent episode unipolar depression subjects. The increased nodal centralities were found in the caudate nucleus and DMN regions, while the reduced nodal centralities were found in the occipital, frontal (orbital part) and temporal regions of first episode MDD patients (Zhang et al., 2011). In another study of late-life depression, hub analysis of nodes indicated that posterior medial parietal regions were more highly connected with the distant neighbors, thereby increasing the average Euclidean distance of connectivity paths as compared to the healthy controls (Bohr et al., 2012). Due to somewhat conflicting reports in both small-world and modular properties (Bohretal., 2012; Lordetal., 2012; Taoetal., 2013; Zhang et al., 2011), further investigation into the topological alterations of the global brain regions in the relatively specific depression samples may offer some novel insights.
In the meantime, the relationship between clinical symptoms and patterns of FC networks is still remaining unclear, especially for the core symptoms of helplessness, hopelessness, and worthlessness that often accompany MDD's negative moods (Beck et al., 1979). The bias cognition model emphasizes that the negative evaluations of environment, future and selfness may specifically contribute to the symptoms of helplessness, hopelessness, and worthlessness in MDD patients (Beck et al., 1979). These clinical symptoms can be quantitatively measured using 24-item Hamilton Depression Scale (HAMD) (Hamilton, 1967; Zhang, 1998), the scoring values of which may be acquired across five points (from 0 through 4) by a semi-structured clinician-rated interview (Zhang et al., 2011; Zhang, 1998). Some studies have paid more attention to both feelings of hopelessness (Becker-Weidman et al., 2009; Young et al., 1996) and worthlessness among MDD patients (Rimes and Watkins, 2005; Spijker et al., 2010), but studies on helplessness were conducted only in animal models, and these findings indicated abnormal activities in the prefrontal cortex (Hoyle et al., 2011; Petty et al., 1994), hippocampus (Kohen et al., 2005; Lachman et al., 1992), and some subcortical areas (Shumake et al., 2003, 2010). Interestingly though, the aforementioned neuroimaging studies indicated that most of the brain areas related to the helplessness were within the abnormal FC networks. Actually, it is valuable to further explore the alterations of brain FC network underlying core symptoms such as helplessness in MDD patients. This will help extend our understanding of the symptoms in MDD from clinical cognitive psychology to neurobiology at a global brain network level.
Both small-worldness and modularity are the novel quantitative measures of topological properties in the global brain level, which offer some helpful indices for investigating the underlying neurological bases of MDD symptoms. The current study tests two hypotheses on these metrics: (1) the global brain network or functional modularity would be disrupted in MDD patients, and (2) the topological pattern alterations may be related with some core clinical factors of MDD, such as helplessness. To test these hypotheses, first, we conducted small-world analysis by constructing the global functional network of MDD and further explored its relationship with clinical factors. Next, we applied modular analysis to measure the local properties of the global functional network in MDD patients and investigated the relationship between local properties and clinical factors. Similarly, we also examined the patterns of impaired brain networks in MDD patients by using the network-based statistic (NBS) method.
2. Methods
2.1. Subjects
In total, 16 medication-naïve patients with MDD were recruited from outpatient clinics at the Huashan Hospital and Shanghai Mental Health Centre, China. Healthy subjects matched with MDD patients for age, sex, and education level were recruited via advertisement. Both 16 MDD patients and 16 healthy controls were then interviewed independently by two psychiatrists. All participants were verified as being right-handed with the right eye being dominant. Eligibility screening procedures included the Structured Clinical Interview for DSM-IV (SCID), 24-item HAMD (Hamilton, 1967; Zhang, 1998), and 14-item Hamilton anxiety scale (HAMA). Written informed consent was obtained from all subjects prior to their inclusion in the study. All protocols of this study were approved by the Investigational Review Board (IRB00002733-Shanghai Mental Health Center, China).
Inclusion criteria for depressed subjects were as follows: aged 25–50 years, satisfying DSM-IV diagnosis criteria of MDD, first episode, medication-naïve, 24-HAMD score > 20 (Hamilton, 1967), 14-HAMA score < 7, and outpatient treatment. Depressed patients were excluded according to the following criteria: meeting criteria of any current or past Axis I disorder of DSM-IV (e.g., schizophrenia, schizoaffective disorder, bipolar disorder, or anxiety disorder as primary diagnosis), any prescription or psychotropic medications in the past 4 weeks, clinically verified feelings of being acutely suicidal, homicidal or requiring inpatient treatment, meeting criteria for substance dependence within the past year (except for caffeine or nicotine), positive urinary toxicology screening at baseline, use of alcohol in the past week, serious medical or neurological illness, current pregnancy or breastfeeding, and metallic implants or other contraindications to magnetic resonance imaging (MRI).
Healthy subjects were included if they met the following criteria: aged 25–50 years, no history of psychiatric illness or substance abuse/dependence, no family history of major psychiatric or neurological illness in the first degree relatives, not currently taking any prescription or psychotropic medications, no use of alcohol in the past week, and no serious medical or neurological illness on record. Exclusion criteria for healthy subjects included those who were pregnant or breastfeeding, or those who had metallic implants or other contraindications that would inhibit MRI.
2.2. MRI acquisition
All participants were instructed to lie down and remain motionless, keep eyes closed, and relax. Structural images and functional images were acquired by a 3.0-T General Electric Signa scanner using a standard whole-head coil. A high-resolution T1-weighted spoiled grass gradient sequence with the following parameters was used: repeat time (TR) = 5.9 ms, echo time (TE) = 1.4 ms, flip angle = 15°, field of view = 24 × 24 cm2, image resolution = 1 × 1 × 1 mm3, and slice gap = 0. The functional magnetic resonance imaging (fMRI) was acquired by multi-slice echo planar imaging (EPI) sequence using parameters: TR = 3000 ms, TE = 30 ms, flip angle = 90°, field of view = 24 × 24 cm2, image resolution = 3.75 × 3.75 × 5.0 mm3, and slice gap = 0. Each brain volume was comprised of 22 axial slices, and each functional run contained 100 volumes. The scanning time of fMRI was 5 min.
2.3. Imaging data preprocessing
Image preprocessing was performed by the SPM8 package (http://www.fil.ion.ucl.ac.uk/spm; Wellcome Trust Centre for Neuroimaging, University College London, United Kingdom). The preprocessing steps included discarding the first 5 volumes, and correcting both the intravolume acquisition time differences between slices and the intervolume geometric displacement from head motion. None of the participants were excluded based on the criterion of displacement of more than 3 mm, or an angular rotation of greater than 3° in any direction. Following these procedures, the image data were spatially normalized to the standard space of the Montreal Neurological Institute and resampled to 3 × 3 × 3 mm3. The resulting data was further temporally bandpass filtered (0.01 < f < 0.08 Hz), to reduce physiological and high frequency noises (Biswal et al., 1995). Finally, to further reduce the effects of confounding factors, the variance in image data were accounted for using six head-motion parameters, white matter signal, and cerebrospinal fluid signal (Fox et al., 2005; Sheline et al., 2010). We did not regress out the global signal in this study, since several studies found that it may be a controversial step leading to false positive results (Achard et al., 2006; Fox et al., 2009; Lynall et al., 2010; Murphy et al., 2009; Supekar et al., 2008).
2.4. Functional network construction
A network is composed of a collection of nodes connected by edges. To define the nodes, we partitioned the brain into 90 cortical and subcortical regions of interest (ROIs), comprised of 45 ROIs for each hemisphere, based on the Automated Anatomical Labeling (AAL) brain atlas (Tzourio-Mazoyer et al., 2002) (Table S1). We then defined the network edges as interregional resting-state FC between each pair of ROIs. Pearson correlation coefficients between all pairs of 90 brain regions were calculated based on the wavelet coefficients (Wang et al., 2013). The brain correlation matrices were obtained in the native space. After registering a brain template to each individual brain, we will have brain regions defined in individual native space. From that, we calculate the correlation matrices for each subject in the native space. The result of all these steps was a 90 × 90 correlation matrix for each subject (Fig. S1). The details are enclosed in Supplementary materials.
2.5. Small-world properties
Small-world properties were measured by the clustering coefficient Cp and the path length Lp, which computed the respective extent of interconnectivity of a network at local and global levels (Watts and Strogatz, 1998). The small-worldness was used to measure the balance between integration and segregation of network (Humphries and Gurney, 2008). Besides, efficiency metrics were also used to measure the small-world properties. The global efficiency (Eglob) quantifies the extent of information transmission at the global networks, and the local efficiency (Eloc) quantifies the extent at individual node levels (Latora and Marchiori, 2001).
Individual correlation matrices were transformed to the binary format at a wide range of network sparsity levels for extensive evaluation. Network sparsity measures the percentage of the number of existing edges in all possible connections and is used as threshold. Small-world properties were compared between groups at each sparsity level. See Supplementary materials for details of the procedures.
2.6. Network modularity
A module in the complex network is defined as a subset of nodes tightly connected within the modules but sparsely connected between the modules. The modularity of a network was computed using a modified greedy optimization algorithm (Chen et al. 2008; Newman and Girvan, 2004; Shi et al., 2013), details of which are provided in Supplementary materials. The modules are non-overlapping, with each node assigned to only one module. The process of modularity optimization does not need to specify either the number or the size of the modules. To assess the inter- and intra-modular connectivity, IMCs,t was used as the intermodule connectivity between module s and module t. Likewise, MCs was used as the intra-module connectivity within module s, being the sum of edge weights within the module (Shi et al., 2013).
To measure the potential regional role played by each brain region, the participation coefficient (PC) and module degree (MD) for each region (or node) were respectively defined as the indices of their inter- and intra-module connection density (Guimera and Amaral, 2005). For example, for a node i in module s, the PC will be close to 0 if all weights are largely intramodular, while a high value of MDi indicates strong within-module connectivity for node i (Rubinov and Sporns, 2010). Details of mathematical definitions are listed in Supplementary materials.
2.7. Network based statistic (NBS)
We used the NBS approach to further localize the impaired brain sub-network based on the specific pairs of brain regions, in which the FC was found to be altered in MDD patients (Zalesky et al., 2010). In NBS, region pairs showing between-group differences in FC were determined, and those connected sub-networks with significant changes in MDD patients were localized. See the details in Supplementary materials.
2.8. Statistical analysis
The statistical significance of network property differences between groups was evaluated with a non-parametric permutation test (Zhang et al., 2011). Small-world properties were compared at each sparsity level. Since individual subjects have correspondingly different modular distributions, we refer the modular distribution, as computed from the mean correlation matrix of control subjects, as the baseline. The modular measurements (e.g., MD and PC) were computed for the individual subjects with same modular organization, which makes a group comparison feasible. Permutation tests were also used to evaluate the significance level of connectivity networks in NBS alterations (Zhang et al., 2011). Notably, age, gender and age-gender interaction were removed by linear regression prior to further statistical analysis.
Multivariate linear regressions were performed to evaluate the relationships between the aforementioned topological measures and the recorded clinical variables (e.g., HAMD score, HAMA score, age of onset, and course of illness) (Zhang et al., 2011). The scale of HAMD was broken into seven factors based on its Chinese-language version: anxiety/somatization, change of weight, cognitive dysfunction, atypical circadian rhythm, retardation, sleep disorder, and desperation (Zhang et al., 2011; Zhang, 1998). The factor desperation was further analyzed by three items, including feelings of helplessness, hopelessness, and worthlessness (Zhang et al., 2011; Zhang, 1998). Both the total score and the factor scores of 24-HAMD scale were analyzed with the appropriate metric measures. Likewise, age, gender, and age-gender interaction were further controlled as the confounding factors.
3. Results
The demographic and clinical status of all subjects are listed in Table 1. In terms of age, gender distribution, and education level (p > 0.05), patients with MDD did not differ significantly from healthy subjects, as the controls were matched as closely as possible to the MDD patients during the sample selection. Details of HAMD measure in MDD group are listed in (Supplementary material Table S2).
Table 1.
Demographic and clinical characteristics.
| Depressed patients (n = 16) |
Healthy subjects (n = 16) |
Significance (p) |
|
|---|---|---|---|
| Age (years) | 34.43 ± 6.72 | 33.75 ± 6.36 | 0.77 |
| Gender (male/ female) |
7/9 | 7/9 | – |
| Education level | 15.63 ± 1.99 | 15.81 ± 2.48 | 0.82 |
| Age on set (years) | 33.25 ± 6.98 | – | – |
| Duration of illness (weeks) |
8.01 ± 4.19 | – | – |
| 24-item HRSD Score | 30.88 ± 7.69 | 3.81 ± 1.05 | <0.0001a |
| 14-item HAMA Score | 5.62 ± 0.72 | 3.31 ± 1.25 | <0.0001a |
Significance was evaluated using two sample t-test.
Denote significance found.
3.1. Small-world properties
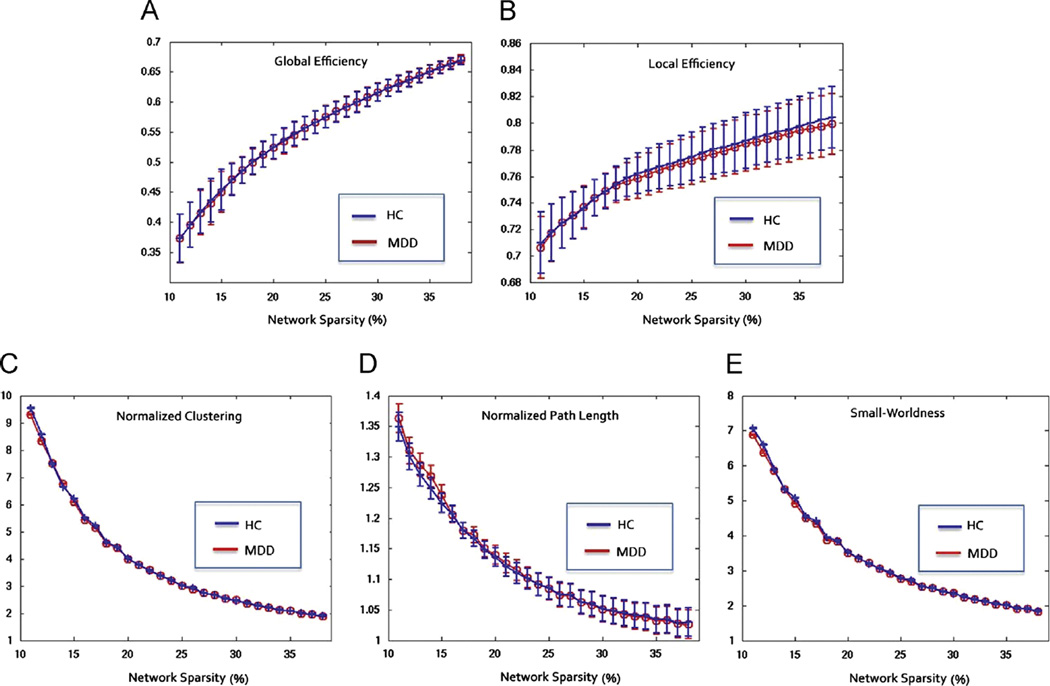
The functional networks showed economical small-world properties (high Eloc and Eglob) in the two groups. No significant group differences were presented at both Eloc and Eglob (p > 0.05, Fig. 1A and B). There were also no significant differences observed between the two groups in terms of global brain topological metrics, including normalized clustering coefficient, path length, and small-worldness (p > 0.05, Fig. 1C–E).
Fig. 1.
Network properties of major depressive disorder patients (MDD, red) and healthy controls (HC, blue). (A) Global efficiency, (B) local efficiency, (C) normalized clustering, (D) normalized path length, and (E) small-worldness as a function of network sparsity. Mean and standard deviation of network properties are also illustrated at each sparsity level. No significant difference was found between the two groups within sparsity of 11–38% (p > 0.05, permutation test).
3.2. Modular organizations
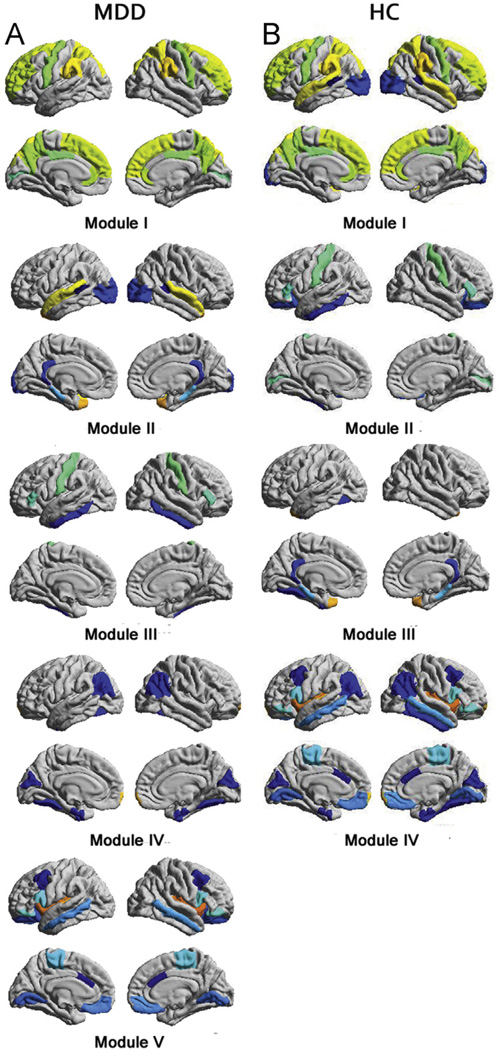
Typical brain modularity was presented in the two groups along-side significantly different patterns of modular organization found between the two groups (representations of modularity shown in Fig. 2). We detected four functional oriented modules in the healthy controls (Fig. 2B), each with a corresponding number of regions: Module I has 34 regions, Module II has 24 regions, Module III has 14 regions, and Module IV has 18 regions. In detail, Module I includes the dorsal and medial superior frontal gyrus (SFGdor and SFGmed), superior, inferior, medial, and middle orbitofrontal cortex (ORBsup, ORBinf, ORBmed, and ORBmid), middle frontal gyrus (MFG), anterior cingulum gyrus (ACG), middle cingulum gyrus (MCG), posterior cingulum gyrus (PCG), angular (ANG), precuneus (PCUN), inferior temporal gyrus (ITG), middle temporal gyrus (MTG), and inferior parietal lobule (IPL). This module is mainly responsible for default mode network (DMN) in resting-state (Buckner et al., 2008) and also associated with cognition control and strategic/executive functions, and also partly involved in primary sensor and motor functions. Module II contains the precentral gyrus (PreCG), postcentral gyrus (PoCG), paracentral lobule (PCL), supplement motor area (SMA), and superior parietal gyrus (SPG), which are primarily involved in sensory-motor function. It also includes the superior occipital gyrus, middle occipital gyrus and inferior occipital gyrus (SOG, MOG, and IOG), which are related with visual processing function. Module III includes the superior temporal pole (TPOsup), middle temporal pole (TPOmid), superior temporal gyrus (STG), heschl gyrus (HES), insula (INS), and rolandic operculum (ROL), which are the main components of auditory-verbal operation. Module IV mainly includes left inferior orbitofrontal cortex (ORBinf.L), olfactory (OLF), hippocampus (HIP), parahippocampal gyrus (PHG), amygdala (AMYG), thalamus (THA), caudate (CAU), putamen (PUT), and pallidum (PAL), which are known largely involved in mnemonic and affective processing.
Fig. 2.
Modular organizations of the global brain functional network in major depressive disorder (MDD) group (A) with 5 modules, and in healthy control group (B) with 4 modules. (A) Brain modular organization of MDD patients. Module I: default model network/cognition control; Module II: executive and strategic processing. Module III: sensory-motor/auditory and verbal functions; Module IV: visual and verbal functions; Module V: mnemonic and affective processing. (B) Brain modular organization of healthy controls. Module I: default model network/cognition control, and executive and strategic regions; Module II: sensory-motor/visual processing; Module III: auditory-verbal operation. Module IV: mnemonic and affective processing. The colors in each module denote different brain regions.
Among the MDD patients, five functional oriented modules were found (Fig. 2A). Module I included 26 regions (e.g., SFGdor, SFGmed, ORBmed, ORBsup, ACG, MCG, PCG, ANG, PCUN, ITG and MTG) responsible for cognition control and DMN functions. Module II contained 10 regions (e.g., MFG, ORBmid, opercular inferior frontal gyrus (IFGoperc), triangular inferior frontal gyrus (IFGtriang), and IPL), which collectively are related to executive and strategic functions during cognition processing. Module III consists of 18 regions, including the PreCG, PoCG, PCL, SMA, STG, HES, INS and ROL, which correspond to sensory-motor, auditory and verbal functions. Totally 18 regions were included in module IV, consisting of SOG, MOG, IOG, SPG, and lingual gyrus (LING), which are known to be involved in both visual and verbal functions. Finally, module V was comprised of 16 regions, including ORBinf.L, HIP, AMGY, THA, CAU, and PUT, which are related to mnemonic and affective processing.
3.3. Brain modularity
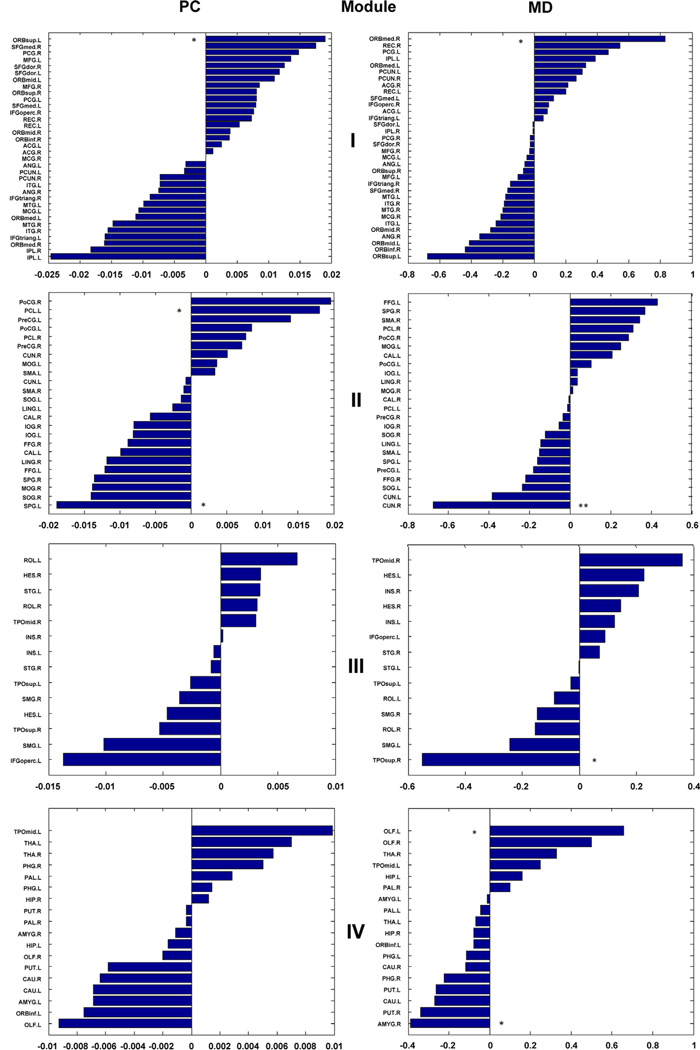
Global brain modularity was not significantly different between the patients with MDD and the healthy controls (p = 0.80, t = − 0.25). We observed no significant alteration of both inter- and intra-connectivity at the global module level in the MDD patients' networks (p > 0.05). However, we explored disease-related regional alterations for the global intra- and inter-module networks connectivity. Compared to the healthy controls, three regions showed significant inter-module alterations (Fig. 3), in which the regions with increased PC were located in the left superior orbital frontal cortex and the left paracentral lobule, and the region with decreased PC was located in the left superior parietal gyrus in the networks of patients with MDD. In MDD patient's networks, five regions were identified with significant intra-module alterations (Fig. 3): the regions with increased MD were the left olfactory and right medial orbital frontal cortex, and the regions with decreased MD were the right amygdala, right cuneus and right superior temporal pole.
Fig. 3.
Regional roles of module network. The left bars of each bar chart with negative values indicate that the connectivity of healthy controls was larger than that of major depressive disorder patients. The right bars of each bar chart with positive values indicate that the connectivity of MDD patients was larger than that of healthy controls. Stars denote brain regions with significant differences between the two groups (*p < 0.05, **p < 0.01, FDR corrected). In MDD patients, 3 brain regions with significantly altered participant coefficient (PC) were shown in the left bar charts (PC column), and 5 brain regions with significantly changed intra-module degree (MD) values were shown in the right bar charts (MD column).
3.4. Altered sub-functional connectivity in MDD
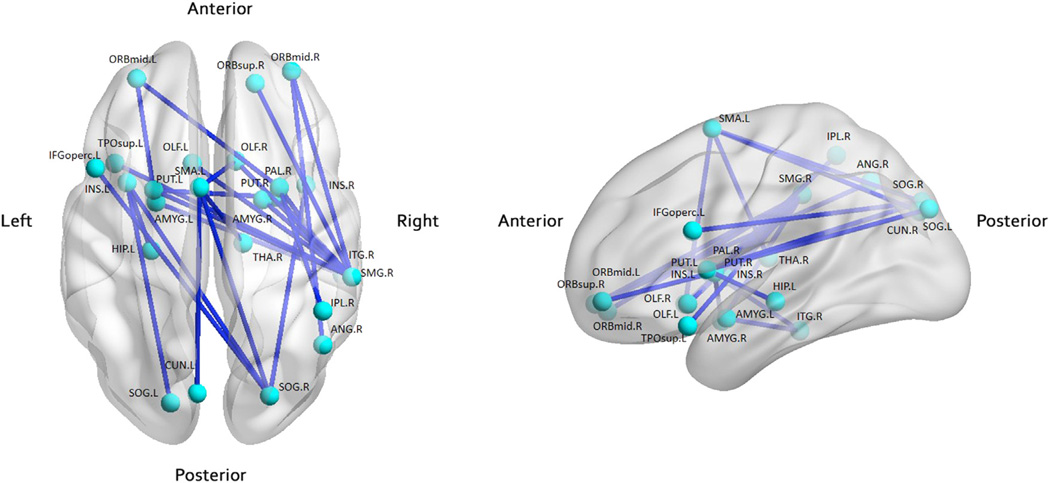
We identified a single connected network with 24 nodes and 24 connections via NBS, which showed a significantly decreased FC in MDD patients as compared to the healthy controls (p < 0.05, Fig. 4, Table S3). The respective nodes included several cognition and affective processing regions, such as the right superior orbital frontal cortex and right amygdala. Several altered regions, such as right middle orbital frontal cortex and supplementary motor area, were involved in strategic and executive function in the sub-network. Interestingly, the core regions of the putative “hate circuit”, e.g., frontal, insular, and putamen (Tao et al., 2013), were also found to be altered in the sub-functional network.
Fig. 4.
Sub-functional network showing decreased functional connectivity in MDD patients. Left shows the top view, and right shows the lateral view. Decreased functional connections are marked in blue. These connections compose a single connected network with 24 nodes and 24 connections in the MDD patients (p < 0.05, corrected). Visualization was done with BrainNet Viewer (http://www.nitrc.org/projects/bnv/; details provided in Table S3).
3.5. Relationships between network measures and clinical variables
There was significant correlation between the local efficiency of network and helplessness score from the HAMD (r = 0.55, p < 0.05). Likewise, the local efficiency was also significantly correlated with the hopelessness score from the HAMD (r = 0.69, p < 0.05). The mean value of intra-connectivity showed significant correlation with both the sleep score (r = 0.58, p < 0.05) and the helplessness score of the HAMD (r = 0.54, p < 0.05) within module I. The mean value of inter-connectivity between modules I and IV showed significant correlations with the sleep score of the HAMD (r = 0.70, p < 0.05).
4. Discussion
To the best of our knowledge, the current study is the first to investigate the role of global module networks and their relationship with core symptoms in MDD patients. Our results indicated that there were no measurably significant differences in the small-world properties between the healthy controls and the MDD patients (Fig. 1), though the local efficiency of small-world among MDD patients showed a positive correlation with feelings of helplessness evaluated by the HAMD in this study. This has some interesting implications which is worth further exploring the connection between the module patterns of MDD and its core symptoms, as local efficiency is associated with short-range connections between nearby regions that regulate the modularized information processing of a network (Latora and Marchiori, 2001).
In assessing the relationship between the global module network and the core symptoms of MDD, it is important to note that the composition of module in MDD patients was significantly different from that found among the healthy controls. Among MDD patients, module I appeared to be responsible for DMN, while cognition control and strategic/executive function was divided into modules I and II (Fig. 2A). Conversely, among the healthy controls, the integrated cognition processing was done by one entire module I (Fig. 2B). This difference implies that MDD patients may not efficiently adjust thoughts and actions due to the separation of module I. MDD patients have previously been characterized by helpless cognition, viewing themselves as personal helplessness (Maiden, 1987) and then acting with a passive mood (Peterson et al., 1993; Salomons et al., 2012). The findings of this study indicated that the mean value of intra-connectivity within module I was positively correlated with the helplessness score of HAMD (r = 0.54, p < 0.05). This correlation implies that the more tightly the Module I connects, the more severe the feeling of helplessness will be in MDD. By extension, the cognition of helplessness may then be related to the atypical functional network of module I among MDD patients.
There may also be additional insights from investigation of the regional roles of the inter- and intra-module networks among MDD patients by both the participant coefficient (PC) and module degree (MD), since they both may contribute to the alteration of module organization (Fig. 3). This is actually quite a novel venue for studying MDD. Our study found that the increased PC in MDD was located in the left superior orbital frontal cortex and left paracentral lobule. The superior orbital frontal cortex is part of the DMN, while the paracentral lobule is related with the DMN due to the included portions of the frontal and parietal lobes. In our study, the decreased PC was found in the left superior parietal gyrus. Similarly, previous studies reported that MDD patients exhibited a trend of spatial distribution for brain regions, by either measurement of the participant index for nodes (Lord et al., 2012), or nodal centrality (Zhang et al., 2011). The increased PC was located in regions of DMN among MDD patients, further confirming the separation of the DMN organization in module I. Meanwhile, our results showed that MDD patients increased MD in the left olfactory and right medial orbital frontal cortex, and decreased MD in the right amygdala, right cuneus, and right superior temporal pole. The MD was opposite to PC for the single node, which was used to measure the regional intra-module degree of each brain region. In sum, since the regional alterations indicated by both PC and MD were mainly located in module I or module V, the alterations may be responsible for abnormal cognition processing (the function of module I) and affective processing (the function of module V) that often define aspects of MDD, such as helpless cognition and affect reaction.
Another finding of this study is that the FC of sub-network showed decreasing trend in MDD patients (Table S3, Fig. 4), which may be also one of the underlying reasons behind the altered modules. Two significant findings in the sub-network are worth considering. First, cortical regions with decreased FC were mainly located in module I among MDD patients, such as right superior and middle orbital frontal cortex which reduced its connectivity with other cognition and affective processing regions. These findings are markedly different from the sub-network characterized among MDD patients in a previous study (Zhang et al., 2011). The differences in our present findings may be explainable by differences in the samples. Some data provides novel evidence of the relationship between supplementary motor area (SMA) cortical thickness and feelings of helplessness: it is even suggested that SMA mediates the helpless cognition of motor behavior over a chronic and poorly controlled stressor (Salomons et al., 2012). The decreasing FC reported in this study between SMA and other affective processing regions, such as the cuneus of DMN, thalamus, superior occipital gyrus, and olfactory region, may be useful to explain the helpless symptoms in MDD.
Another second finding is that the subcortical regions with decreased FC included the right amygdala, thalamus and hippocampus of module V in MDD patients. The FC of the amygdala with other brain regions was shown to be abnormally decreased among patients with MDD, such as prefrontal cortex (Bohr et al., 2012; Long et al., 2013; Yue et al., 2013). The decreasing FC between amygdala and prefrontal cortex was earlier reported to underlie the abnormal processing of fear and anxiety, and is related with depression (Prater et al., 2013). The thalamus has likewise been found to be the source of abnormal function in previous studies (Lui et al., 2009; Peng et al., 2011; Zobel et al., 2005). In our study, thalamus showed decreasing FC with SMA. The abnormal FC of SMA has been thought to be related with feelings of helplessness, as described above (Salomons et al., 2012). Specifically, hippocampus may potentially mediate the relationship between helplessness and depression via regulation of gene expression (Kohen et al., 2005; Lachman et al., 1992). In this study, the decreased FC between hippocampus and insula was also found among MDD patients. The insula is one of key regions in negative affective processing circuit, e.g., “hate circuit” (Tao et al., 2013). Tao et al. (2013) speculated that the depressed patients reduced their cognition control over negative feelings due to uncoupling of their hate neural circuit. It then stands to reason that the decreased FC with these subcortical regions may also be responsible for negative moods that accompany MDD, such as fear or anxiety, hate, and helplessness.
Though several of the explanations and findings we have outlined offer intriguing possibilities, previous studies on animal models have likewise indicated that there may be a neurobiological susceptibility to depression caused by changes in brain systems (Shumake and Gonzalez-Lima, 2003). Neurotransmitter studies found that a serotonergic (5-hydroxytryptamine, 5-HT) excess of medial frontal cortex was causally related to learned helplessness (Petty et al., 1994). Likewise, quantitative histochemistry studies showed opposite metabolic changes of cytochrome oxidase between the habenula and the ventral tegmental area (VTA) in helpless rat (Shumake et al., 2003, 2010). Finally, other genetic studies in animal models confirmed a correlation between helplessness and depression (Smalheiser et al., 2011; Yacoubi et al., 2012) as evidenced by differential gene expression in the hippocampus (Kohen et al., 2005; Lachman et al., 1992) and the frontal cortex (Hoyle et al., 2011). Taken together, these findings suggest that the prefrontal cortex, hippocampus, and some subcortical regions may be responsible for the organization alteration of brain functional network that underlies both helplessness and depression. In this study, both module and NBS analyses showed that, among MDD patients, most of these regions have the disrupted functional activities, including altered participant coefficient/module degree, and atypical FC pairs of sub-networks. Meanwhile, our current findings confirmed the relationship between altered intra-module I and helplessness in MDD. Although these findings need to be further investigated, it seems that the altered topological patterns of modules in MDD may actually underpin the helpless symptoms experienced by MDD patients.
There are several limitations to the current study that are worth indicating. First, the small size of samples imposes some restrictions on our potential conclusions. However, MDD patients in this study should, theoretically, be ideal samples to examine the early manifestation of MDD, without confounding factors including duration and medication effect. While this size of samples is comparable to many previous first-episode drug-naïve neuroimaging studies in depression, our results require replication in the larger-sized treatment-naive samples. Second, the shorter scanning time (5 min) may possibly result in unreliable data of the resting-state. In our procedures, we asked all the subjects to relax before scanning. The scan began only after the subjects showed signals of relaxation. During the scan, the foam pads were further used for immobilization of the head within the head coil. By the aforementioned steps, the stable data can be acquired with the balance between the resting-state and motion control. Third, the altered functional module is also involved in various brain regions related to negative mood (e.g., fear, anxiety and hate), atypical cognition control processing, and somatic symptoms (e.g. insomnia) in MDD, all of which will be valuable to investigate by the follow-up studies.
We focused on the alteration of modules since they were previously identified as a sensitive marker in brain networks (He et al., 2009). Our study showed that MDD patients had atypical reorganization of the module compared to the corresponding healthy controls. A positive relationship was found between the inter-module I and the helplessness among MDD patients. Furthermore, we found two major alterations of modular patterns in MDD patients: (1) the atypical participant coefficient or module degree was found in eight nodes of modules, e.g., the left superior orbital frontal cortex and the right amygdala; (2) a decreased functional connectivity among the sub-network of 24 brain regions (including frontal cortex, supplementary motor area, amygdala, thalamus, and hippocampus). In general, these findings confirm our hypothesis that MDD is accompanied by disrupted functional modularity, though the precise nature of the relationship is still not entirely clear. Nonetheless, our current findings may help explain how altered brain module might induce feelings of helplessness among MDD patients.
Supplementary Material
Acknowledgments
The authors thank Lena Palaniyappan for the English language assistance.
Role of funding source
This work was supported by Science Fund of Shanghai Jiao Tong University (Grant nos. 11XJ21006 and YG2012MS11), Fund of Science and Technology Commission of Shanghai Municipality (Grant no. 134119a6200), Overseas Talent Project of Shanghai health bureau (Grant no. GWHW201208), the “12th Five-year Plan” of National Key Technologies R&D Program (Grant no. 2012BAI01B04), National Natural Science Foundation of China, China (Grant nos. 81201049 and 91232719). In addition, this grant is also partially supported by NIH grants (Grant nos. EB006733, EB008374, EB009634, AG041721, MH100217, and AG042599).
Footnotes
Conflict of interest
No conflict declared.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jad.2014.05.061.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington D.C.: American Psychiatric Association; 2013. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- Becker-Weidman EG, Reinecke MA, Jacobs RH, Martinovich Z, Silva SG, March JS. Predictors of hopelessness among clinically depressed youth. Behav. Cogn. Psychother. 2009;37:267–291. doi: 10.1017/S1352465809005207. [DOI] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, Neufeld R, Osuch E. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin. Neurosci. 2009;63:754–761. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Bohr IJ, Kenny E, Blamire A, O’Brien JT, Thomas AJ, Richardson J, Kaiser M. Resting-state functional connectivity in late-life depression: higher global connectivity and more long distance connections. Front Psychiatry. 2012;3:116. doi: 10.3389/fpsyt.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb. Cortex. 2008;18:2374–2381. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Scheuerecker J, Albrecht J, Kleemann AM, Muller-Schunk S, Koutsouleris N, Moller HJ, Bruckmann H, Wiesmann M, Meisenzahl E. Neuronal correlates of emotional processing in patients with major depression. World J. Biol. Psychiatry. 2009;10:202–208. doi: 10.1080/15622970701624603. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Amaral LAN. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, Long L, Chen H, Gao Q, Xiao C. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;41:24–29. doi: 10.1016/j.pnpbp.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PloS One. 2009;4:e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle D, Juhasz G, Aso E, Chase D, del Rio J, Fabre V, Hamon M, Lanfumey L, Lesch KP, Maldonado R, Serra MA, Sharp T, Tordera R, Toro C, Deakin JF. Shared changes in gene expression in frontal cortex of four genetically modified mouse models of depression. Eur. Neuropsychopharmacol. 2011;21:3–10. doi: 10.1016/j.euroneuro.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Gurney K. Network ‘small-world-ness': a quantitative method for determining canonical network equivalence. PLoS One. 2008;3:e0002051. doi: 10.1371/journal.pone.0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R, Kirov S, Navaja GP, Happe HK, Hamblin MW, Snoddy JR, Neumaier JF, Petty F. Gene expression profiling in the hippocampus of learned helpless and nonhelpless rats. Pharmacogenomics J. 2005;5:278–291. doi: 10.1038/sj.tpj.6500322. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Weiner ED, Ramazankhana R, Hartnick C, Edwards E, Henn FA. Hippocampal neuropeptide Y mRNA is reduced in a strain of learned helpless resistant rats. Brain Res. Mol. Brain Res. 1992;14:94–100. doi: 10.1016/0169-328x(92)90015-4. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Long H, Liu B, Hou B, Wang C, Li J, Qin W, Wang D, Zhou Y, Kendrick KM, Yu C. The long rather than the short allele of 5-HTTLPR predisposes Han Chinese to anxiety and reduced connectivity between prefrontal cortex and amygdala. Neurosci. Bull. 2013;29:4–15. doi: 10.1007/s12264-013-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord A, Horn D, Breakspear M, Walter M. Changes in community structure of resting state functional connectivity in unipolar depression. PLoS One. 2012;7:e41282. doi: 10.1371/journal.pone.0041282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Parkes LM, Huang X, Zou K, Chan RC, Yang H, Zou L, Li D, Tang H, Zhang T, Li X, Wei Y, Chen L, Sun X, Kemp GJ, Gong QY. Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology. 2009;251:476–484. doi: 10.1148/radiol.2512081548. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. J. Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden RJ. Learned helplessness and depression: a test of the reformulated model. J. Gerontol. 1987;42:60–64. doi: 10.1093/geronj/42.1.60. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME, Girvan M. Finding and evaluating community structure in networks. Phys. Rev. E: Stat. Nonlin. Soft Matter Phys. 2004;69:026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Peng DH, Jiang KD, Fang YR, Xu YF, Shen T, Long XY, Liu J, Zang YF. Decreased regional homogeneity in major depression as revealed by resting-state functional magnetic resonance imaging. Chin. Med. J. (Engl) 2011;124:369–373. [PubMed] [Google Scholar]
- Peterson C, Maier SF, Seligman MEP. Learned helplessness. USA: Oxford University Press; 1993. [Google Scholar]
- Petty F, Kramer G, Wilson L, Jordan S. in vivo serotonin release and learned helplessness. Psychiatry Res. 1994;52:285–293. doi: 10.1016/0165-1781(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress. Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimes KA, Watkins E. The effects of self-focused rumination on global negative self-judgements in depression. Behav. Res. Ther. 2005;43:1673–1681. doi: 10.1016/j.brat.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Perceived helplessness is associated with individual differences in the central motor output system. Eur. J. Neurosci. 2012;35:1481–1487. doi: 10.1111/j.1460-9568.2012.08048.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Wang L, Peng Z, Wee CY, Shen D. Altered modular organization of structural cortical networks in children with autism. PLoS One. 2013;8:e63131. doi: 10.1371/journal.pone.0063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Colorado RA, Barrett DW, Gonzalez-Lima F. Metabolic mapping of the effects of the antidepressant fluoxetine on the brains of congenitally helpless rats. Brain Res. 2010;1343:218–225. doi: 10.1016/j.brainres.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav. Cogn. Neurosci. Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR, Lugli G, Rizavi HS, Zhang H, Torvik VI, Pandey GN, Davis JM, Dwivedi Y. MicroRNA expression in rat brain exposed to repeated inescapable shock: differential alterations in learned helplessness vs. non-learned helplessness. Int. J. Neuro-Psychopharmacol. 2011;14:1315. doi: 10.1017/S1461145710001628. [DOI] [PubMed] [Google Scholar]
- Spijker J, de Graaf R, Ten Have M, Nolen WA, Speckens A. Predictors of suicidality in depressive spectrum disorders in the general population: results of the Netherlands Mental Health Survey and Incidence Study. Soc. Psychiatry Psychiatr. Epidemiol. 2010;45:513–521. doi: 10.1007/s00127-009-0093-6. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput. Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Guo S, Ge T, Kendrick KM, Xue Z, Liu Z, Feng J. Depression uncouples brain hate circuit. Mol. Psychiatry. 2013;18:101–111. doi: 10.1038/mp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst. Neurosci. 2010;41 doi: 10.3389/fnsys.2010.00041. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zuo X, Dai Z, Xia M, Zhao Z, Zhao X, Jia J, Han Y, He Y. Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol. Psychiatry. 2013;73:472–481. doi: 10.1016/j.biopsych.2012.03.026. [DOI] [PubMed] [Google Scholar]
- Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front Syst. Neurosci. 2010;4:16. doi: 10.3389/fnsys.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Yacoubi ME, Popa D, Martin B, Zimmer L, Hamon M, Adrien J, Vaugeois J-M. Genetic association between helpless trait and depression-related phenotypes: evidence from crossbreeding studies with H/Rouen and NH/Rouen mice. Int. J. Neuro-Psychopharmacol. 2012;15:363. doi: 10.1017/S1461145711000605. [DOI] [PubMed] [Google Scholar]
- Young MA, Fogg LF, Scheftner W, Fawcett J, Akiskal H, Maser J. Stable trait components of hopelessness: baseline and sensitivity to depression. J. Abnorm. Psychol. 1996;105:155–165. doi: 10.1037//0021-843x.105.2.155. [DOI] [PubMed] [Google Scholar]
- Yue Y, Yuan Y, Hou Z, Jiang W, Bai F, Zhang Z. Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PloS One. 2013;8:e75058. doi: 10.1371/journal.pone.0075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang MY. Rating Scales Manual for Mental Health. Hunan: Hunan Science and Technology Press; 1998. [Google Scholar]
- Zobel A, Joe A, Freymann N, Clusmann H, Schramm J, Reinhardt M, Biersack H-J, Maier W, Broich K. Changes in regional cerebral blood flow by therapeutic vagus nerve stimulation in depression: an exploratory approach. Psychiatry Res.: Neuroimaging. 2005;139:165–179. doi: 10.1016/j.pscychresns.2005.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.