Abstract
Background
Infants admitted to the neonatal intensive care unit (NICU), and especially those born with very low birth weight (VLBW; <1500 grams), are at risk for respiratory decompensation requiring endotracheal intubation and mechanical ventilation. Intubation and mechanical ventilation are associated with increased morbidity, particularly in urgent unplanned cases.
Methods
We tested the hypothesis that the systemic response associated with respiratory decompensation can be detected from physiological monitoring, and that statistical models of bedside monitoring data can identify infants at increased risk of urgent, unplanned intubation. We studied 287 VLBW infants consecutively admitted to our NICU and found 96 events in 51 patients, excluding intubations occurring within 12 hours of a previous extubation.
Results
In order of importance in a multivariable statistical model, we found the characteristics of reduced O2 saturation, especially as heart rate was falling, increased heart rate correlation with respiratory rate, and the amount of apnea all were significant independent predictors. The predictive model, validated internally by bootstrap, had receiver-operating characteristic area of 0.84 ± 0.04.
Conclusions
We propose that predictive monitoring in the NICU for urgent unplanned intubation may improve outcomes by allowing clinicians to intervene non-invasively before intubation is required.
INTRODUCTION
Infants born prematurely have extended stays in the neonatal intensive care unit (NICU). This is particularly true of infants born at very low birth weight (VLBW, <1500 grams), at least 65% of whom will require endotracheal intubation for administration of mechanical ventilation (Data for 2009–2010, from Vermont Oxford Network of over 900 centers, http://www.vtoxford.org/). These long ICU stays can be punctuated by clinical deterioration, including frequent apneas 1, 2 or other forms of respiratory decompensation leading to urgent unplanned intubation, in which the infant is provided mechanical ventilation through an endotracheal tube.
In addition to worsening neonatal apnea, urgent unplanned intubation can result from sepsis, respiratory distress syndrome, pneumonia, exacerbation of chronic lung disease, or critical illness from conditions such as necrotizing enterocolitis. While intubation for the purpose of mechanical ventilation is an effective intervention for respiratory decompensation, it is also associated with substantial morbidity and mortality, including pneumonia 3, barotrauma and volutrauma leading to pneumothorax or bronchopulmonary dysplasia, and oxygen toxicity leading to pulmonary and retinal injury. Early detection of respiratory decompensation may allow for early and less obtrusive treatment, such as initiation or increase of non-invasive respiratory support such as continuous positive airway pressure (CPAP), administration of bronchodilators, or evaluation and treatment of infection.
We hypothesize that some episodes of apparently sudden clinical deterioration in the NICU have precursors of altered control of heart rate and other physiological processes that require finely adaptive coupling among organs 4. This is the case with late-onset neonatal sepsis, where reduced heart rate variability and transient decelerations can precede clinical signs of illness by 24 hours 5–11. In a recent very large randomized clinical trial, we found that display of a multivariable statistical model that relates these abnormal heart rate characteristics (HRC) to the fold-increase in risk of sepsis in the next 24 hours led to a more than 20% reduction in VLBW NICU mortality 12.
We have tested the hypothesis that respiratory decompensation leading to urgent unplanned intubations can also be preceded by changes apparent from bedside physiological monitoring. Similar to the development of the HRC index, or HeRO score, we have developed logistic regression models based on physiological waveforms conventionally recorded in the NICU, including cardiac, respiratory, and pulse oximetry vital signs. Unlike the HeRO score, though, the new predictive model includes information from the respiratory as well as the cardiac system, and the interactions between the two.
RESULTS
Patient population
Times where no vital signs were recorded because of technical problems were excluded, leaving a population of 309 VLBW infants who had monitoring data available. Of the 309 VLBW infants with available data, 22 patients only had data while mechanically ventilated and were therefore excluded from the study. The total number of patients included in the study is N=287. Table 1 shows demographic information for patients in the study and that of the subset of patients who had an urgent unplanned intubation. In the population of 287 VLBW infants where data were recorded for at least 12 hours prior to intubation we found 96 unplanned intubation events in 51 patients.
Table 1.
Demographic characteristics of the study population.
| All infants in study (N = 287) | |
|---|---|
| EGA (weeks) | 27 (25, 29) |
| Males | 147 |
| Birth weight (grams) | 1010 (783, 1268) |
| Length of stay (days) | 61 (35, 95) |
| Ventilator days | 15 (2, 37) |
| PMA at discharge (weeks) | 37 (36, 39) |
| Infants with events (N = 51) | |
| Events | 96 |
| Events due to sepsis | 11 |
| Males | 32 |
| EGA (weeks) | 26 (25, 28) |
| Birth weight (grams) | 810 (708, 1060) |
| Length of stay (days) | 97 (67, 107) |
| Ventilator days | 27 (8, 43) |
| PMA at discharge (weeks) | 39 (37, 41) |
| PMA at urgent, unplanned intubation | 29 (26, 31) |
Data are presented as median (25th, 75th percentile)
An example of the analysis
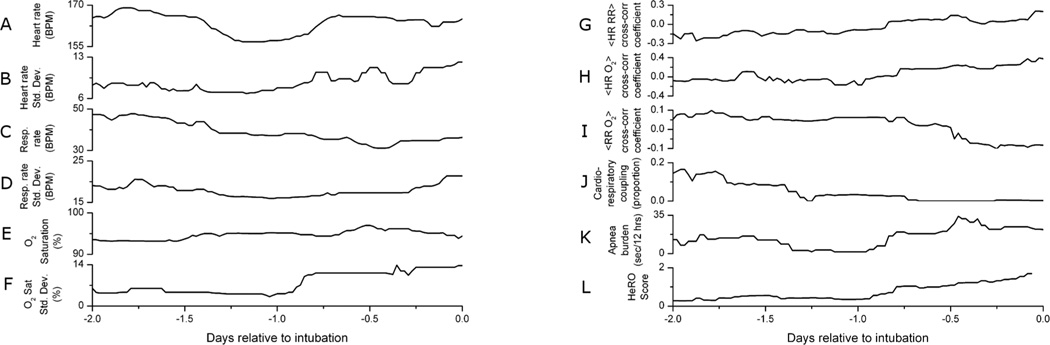
Figure 1 shows time series of cardiorespiratory parameters for a patient born weighing 1460 grams at 29 weeks estimated gestational age. This infant had an urgent unplanned intubation for respiratory acidosis nine days after birth, and parameters are shown relative to the time of this event. The clinical goal is to identify patterns that are predictive of urgent unplanned intubations in VLBW infants.
Figure 1.
Time series of physiological measures for one patient prior to unplanned intubation. Unplanned intubation occurs at zero, on the right edge of the plots. During this time period (A) mean heart rate dips, (B) heart rate standard deviation increases, (C) mean respiratory rate decreases, (D) respiratory rate standard deviation remains unchanged, (E) oxygen saturation remains unchanged, and (F) oxygen saturation standard deviation increases. Also during this time (G) correlation between heart rate and respiratory rate increases, (H) correlation between heart rate and oxygen saturation increases, (I) correlation between respiratory rate and oxygen saturation decreases, (J) cardiorespiratory coupling decreases, (K) apnea burden increases, and (L) the HeRO score increases.
The left column in Figure 1 shows mean and standard deviations of conventionally monitored vital signs, including heart rate, respiratory rate, and pulse oximetry level. During the time leading to intubation this infant’s physiological measurements present conflicting information. For example, the heart rate variability and arterial oxygen saturation are rising, consistent with improving status. Concurrently the respiratory rate is falling and pulse oximetry variability is rising, consistent with deteriorating status.
The right column of Figure 1 shows correlations between the vital signs, as well as three measures of physiological stability: the level of cardiorespiratory coupling 13, duration of time spent in apnea with associated bradycardia and desaturation (or apnea burden) 14, and the output of a model for predicting neonatal sepsis based on heart rate characteristics, the HeRO score 12. Correlations between heart and respiratory rates, and between heart rate and pulse oximetry, rise in the two days prior to urgent unplanned intubation. At the same time, the correlation between respiratory rate and pulse oximetry falls. The level of cardiorespiratory coupling one day prior to intubation is 25% of the value two days prior and falls nearly to zero by the time of intubation. The patient’s apnea burden is high throughout, and the HeRO score increases by four fold over the day leading up to intubation.
Thus the clinician has multiple streams of physiological data, all time varying, interrelated to various degrees, and often with inconsistent trajectories. This justifies an approach using multivariate time series methods.
Univariate analyses
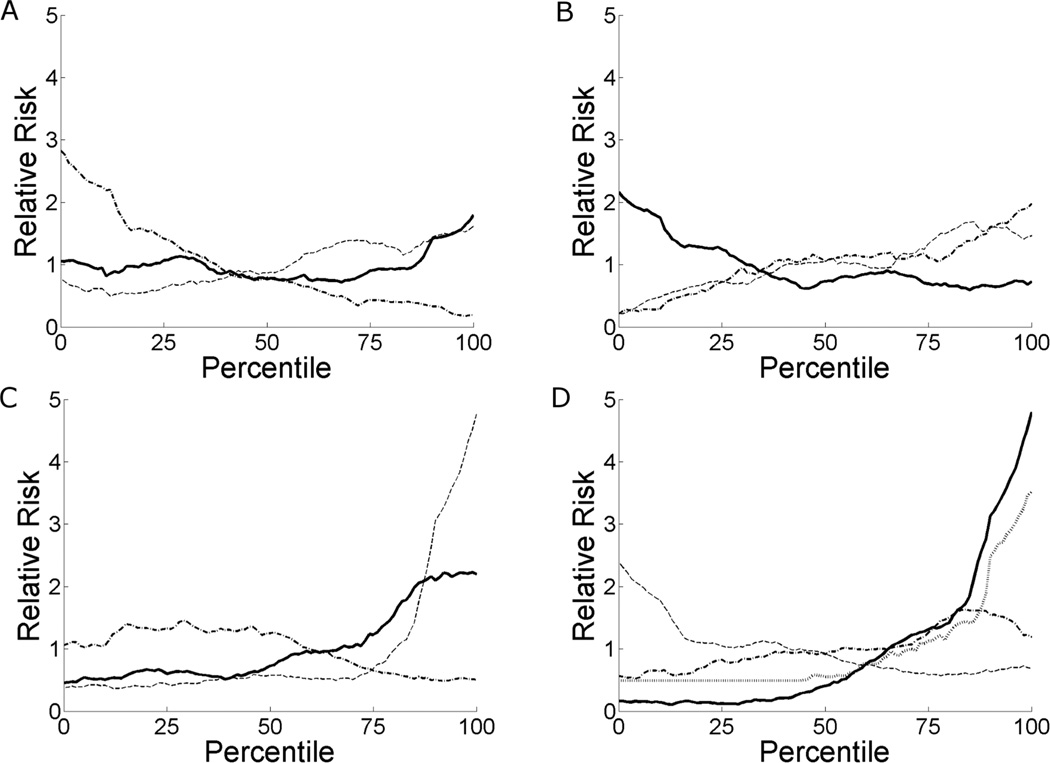
Examination of patient records indicated that patient physiology undergoes changes prior to clinically relevant incidents 15 and urgent unplanned intubation in particular. We exploit this fact by developing logistic regression models for intubation based on physiological parameters. Figure 2 shows the relative risk of unplanned intubation in the next 24 hours on the ordinate and the percentile of each physiological parameter on the abscissa. For example, the lowest and highest heart rates observed in our sample of infants are represented by the 0 and 100%-tiles, respectively. The nomenclatures µi and σi indicate the mean and standard deviation of vital sign i, respectively, and <i j> indicates the cross-correlation coefficient between vital signs i and j at zero lag. High respiratory and heart rate, and high respiratory and oxygenation variability, are associated with increased risk of intubation, as is low oxygen saturation. Risk of intubation has a non-linear relation with heart rate variability.
Figure 2.
Relative risk of unplanned intubation in the next 24 hours as a function of variable percentile. Percentiles are based on all values observed for a given variable, and variables are calculated over half-hour windows. (A) Relative risk versus percentile of mean heart rate (solid line), respiratory rate (dashed line), and pulse oximetry (dashed-dotted line). (B) Relative risk versus percentile of standard deviation of heart rate (solid line), respiratory rate (dashed line), and pulse oximetry (dashed-dotted line). (C) Relative risk versus percentile of correlation between heart rate and respiratory rate (solid line), heart rate and pulse oximetry (dashed line), and respiratory rate and pulse oximetry (dashed-dotted line). (D) Relative risk versus percentile of the HeRO score (solid line), coupling (dashed line), fraction of beats during inhale (dashed-dotted line), and apnea burden (gray dotted line).
The curves in Figure 2 indicate the importance of each physiological parameter in predicting intubation. Parameters that provide a large dynamic range between risks at 0 and 100%-tiles are good candidates for a model. Mean and variability of the pulse oximetry level, correlation between heart rate and pulse oximetry, cardiorespiratory coupling, and the HeRO score all have high dynamic ranges. The association of HeRO is in part due to the 11 of 96 urgent unplanned intubation events in response to sepsis.
We deployed candidate predictor variables in a univariate logistic regression model to predict urgent unplanned intubation in the next 24 hours. Table 2 shows the performance of these models, including the receiver-operating characteristic (ROC), significance (P value) and sign of the coefficient, and the goodness-of-fit (Chi-square). In order to account for the nonlinear relation for heart rate variability, we recast it as the absolute difference between the variability and the median variability for all patients at all times. Univariate models were based only on times where the parameter was available.
Table 2.
Performance of univariate logistic regression models for unplanned intubation.
| Variable | ROC | P value | Sign | Chi-square |
|---|---|---|---|---|
| Vital signs | ||||
| µHR | 0.53 | 0.18 | + | 0.2 |
| σHRa | 0.61 | 0.007 | + | 7.2 |
| µRR | 0.60 | 0.05 | + | 7.8 |
| σRR | 0.61 | * | + | 13.2 |
| µSpO2 | 0.70 | * | − | 37.1 |
| σSpO2 | 0.62 | * | + | 13.0 |
| Correlations | ||||
| <HR RR> | 0.65 | * | + | 15.6 |
| <HR SpO2> | 0.74 | * | + | 47.5 |
| <RR SpO2> | 0.57 | 0.06 | − | 3.7 |
| Physiological stability | ||||
| Coupling | 0.62 | 0.03 | − | 4.8 |
| CVC | 0.50 | 0.95 | − | <0.1 |
| Beat fraction | 0.58 | 0.02 | + | 5.0 |
| Apnea burden | 0.70 | * | + | 31.5 |
| HeRO score | 0.81 | * | + | 49.5 |
denotes p<0.001
A nonlinear transform was applied to heart rate variability prior to use in a logistic regression model. Specifically, the absolute difference between the measured heart rate variability and the median of all heart rate variability values was used.
Heart rate itself had little predictive information. Low and, counter-intuitively, high heart rate variability were both associated with upcoming unplanned intubation16. The HeRO score had the best association with upcoming intubation, with ROC area 0.81 and chi-square 49, followed by the cross-correlation of heart rate and O2 saturation (0.74 and 48). The former reflects the reduced heart rate variability and transient decelerations that can accompany early phases of neonatal sepsis, and the latter reflects the coordinated bradycardia and O2 desaturation that accompany neonatal apneas.
Multivariable analysis
We used the parameters whose univariate coefficients were significant (Table 2, p≤0.05) as inputs to a multivariate logistic regression model, 11 in total for 96 events. We determined this to be acceptable, as meaningful multivariate models are known to require 6–10 events per predictor17. During periods where a parameter was not available, the median value of that parameter (for all patients at all times) was used. Parameters whose multivariate regression coefficients did not reach significance were eliminated from the model, and a new model created. We note that the HeRO score, the best performing individual predictor, did not make the final model. The HeRO score does not add information to models that include the correlation between heart rate and pulse oximetry: the two measures have a moderate correlation (r = 0.45). Table 3 shows the coefficients and standard errors for each parameter used in the final model. The area under the receiver-operating curve for the final model is 0.84 ± 0.04 as determined by bootstrapping 5, 18.
Table 3.
Performance of multivariate model for unplanned intubation.
| Variable | Coefficient (Normalized)a |
Coefficient | Std. Error | P value | Chi-square |
|---|---|---|---|---|---|
| µSpO2 | −0.03 | −0.13 | 0.02 | * | 32.1 |
| <HR RR> | 24.98 | 3.51 | 0.70 | 0.017 | 24.9 |
| <HR SpO2> | 23.24 | 3.48 | 0.64 | * | 30.1 |
| Apnea burden | 0.009 | 0.04 | 0.02 | 0.02 | 5.1 |
denotes p<0.001
Coefficients normalized by standard deviation of the variable.
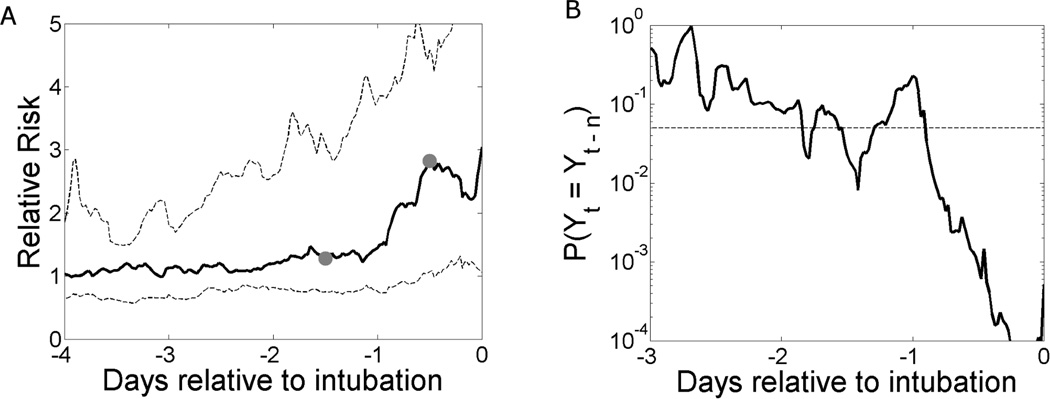
The output of the multivariate logistic regression model is the probability of urgent unplanned intubation in the next 24 hours. Figure 3(left) shows the model output for 96 events in 51 patients, normalized to the relative risk by dividing by the average rate of intubation in the next 24 hours (0.5%). The median increases by 2/3 during the day prior to intubation, and the median output 12 hours before intubation is significantly higher than that 36 hours before intubation (gray dots, p=0.001).
Figure 3.
(A) Median (solid) and 25%, 75% (dashed) model output for 96 urgent unplanned intubation events in 51 patients. The median model output 12 hours prior to intubation is significantly higher than the output 36 hours prior (grey dots, p=0.001 based on a signed rank test). (B) The probability that the null hypothesis of the Wilcoxon signed rank test is true. Paired data are separated by n = 36 hours. The dashed line shows the cutoff for rejecting the null hypothesis (p=0.05). Model outputs in the day prior to intubation are significantly higher than the values 36 hours prior.
Figure 3 (right) shows the probability (solid) that the null hypothesis of the Wilcoxon signed rank test is true, i.e., that the median model output t days before intubation is equal to the corresponding median model output (t-1.5) days before intubation. The cutoff for rejecting the null hypothesis (p=0.05) is shown as the dashed horizontal line. Model outputs throughout the 24 hour period prior to urgent unplanned intubation are significantly higher than model outputs 36–60 hours prior to intubation.
Internal validation
Bootstrapping showed the 95% confidence interval to be ±0.04 18.
Implementation of the predictive model for an individual patient
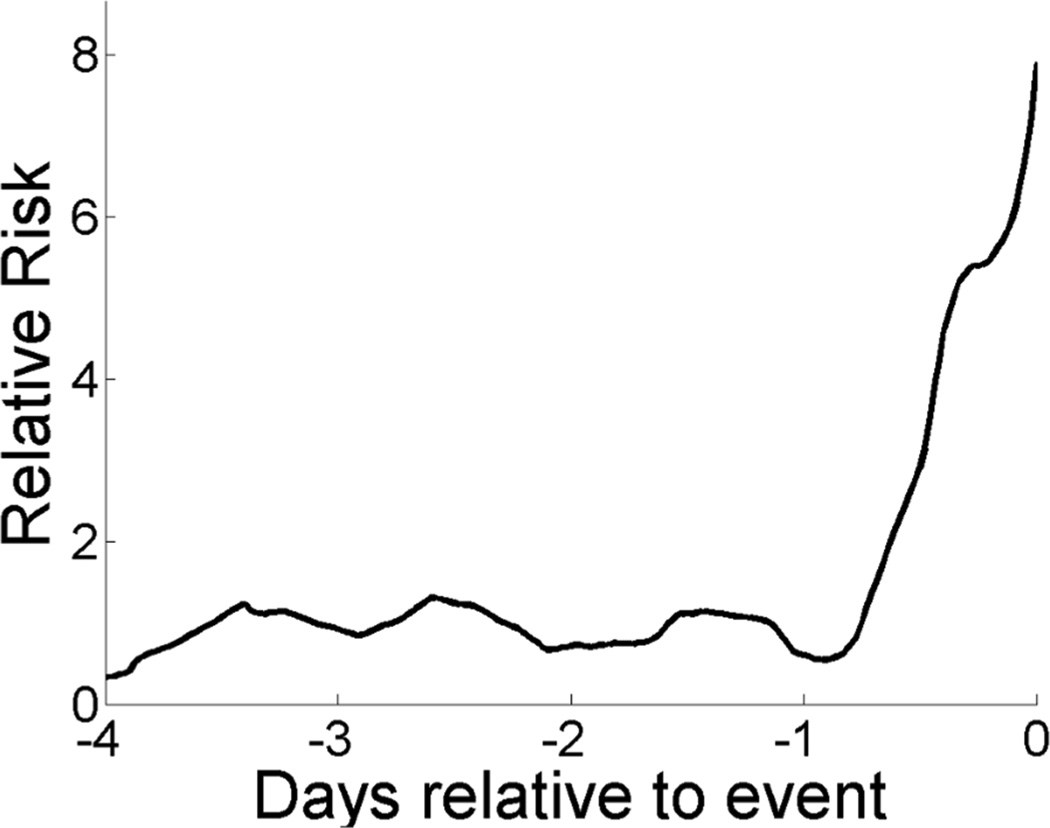
Figure 4 shows the relative risk for the patient whose records are shown in Figure 1 based on our multivariate logistic regression model, Table 3. The relative risk increases five fold from 24 and 12 hours before the event.
Figure 4.
Relative risk of urgent unplanned intubation for the patient shown in Figure 1 based on the multivariate logistic regression model defined in Table 3. From one day prior to unplanned intubation to the time of intubation, the estimated risk increases 8-fold.
DISCUSSION
We studied changes in bedside physiological monitoring parameters in premature infants at risk for respiratory decompensation. We used conventional and cross-correlation measures based on vital signs, novel variables based on cardiorespiratory waveforms, and multivariate logistic regression to predict episodes of urgent, unplanned intubation. Our predictive statistical model has good performance, with ROC area 0.84, and allowed identification of characteristics that added independent information to one another after taking them all into account. The clinical characteristics of the decompensating infant includes, in order of decreasing predictive importance based on goodness-of-fit, low O2 saturation, coincident fluctuations in heart rate and O2 saturation, correlated heart rate and respiratory rate, and increasing apneas. The heart rate-based HeRO score, which had the highest univariate predictive performance with ROC area of 0.81, was displaced in the final model by these other parameters, all of which incorporate information about respiration.
The value of these findings is in the possibility of bedside predictive monitoring for neonatal respiratory decompensation. The strengths of this analysis are that we used data that are conventionally available in the NICU and require no new sensors or contact with the infant. A limitation is that the model is not yet externally validated. In addition, further studies should investigate the impact that changing respiratory support and medication administration may have on the model output.
Inputs to the multivariate model listed in Table 3 were selected to optimize model performance and fit to the data. The significance of physiological parameters, including those not in the multivariate model, provide insight into mechanisms underlying clinical decompensation leading to the need for urgent unplanned intubation. The importance of mean and variability of pulse oximetry, and correlation between it and heart rate as well as heart rate and respiratory rate, indicates a role for hypoxemia. The association of increased HRC index with intubation indicates a decline in cardiac control through extracellular signaling 19 and the autonomic nervous system 20, 21. Decreased cardiorespiratory coupling is an indicator of critical deterioration, in agreement with the concept of systemic inflammatory response syndrome in adult patients 15.
The modern age of high-speed data analysis allows great opportunities for synthesizing the large number of data streams available to the intensive-care clinician. While the insights about the clinical picture of the decompensating infant from this study are not surprising, there is potentially great value in bedside predictive monitoring that is constantly available, requires no new contact with the infant, and optimally leverages data that are already present. Such monitoring could never replace the clinical judgment of experienced doctors and nurses; however, when considering an apparently stable infant in a busy NICU, a rising risk score might place the clinician at the right bedside at the right time.
METHODS
Patient Population
We collected cardiorespiratory waveforms and vital signs from 1438 consecutive admissions to the University of Virginia (UVA) NICU from January 2009 through June 2011. For the 320 VLBW infants, we also collected demographic data including admission and discharge dates, types and times of respiratory support, nursing documentation of apnea and bradycardia, and disposition and status at discharge. Times where no vital signs were recorded because of technical problems were excluded. The UVA Institutional Review Board gave permission for this study with waiver of consent status.
Definition of urgent unplanned intubation
Urgent unplanned intubations were defined as non-elective initiation of mechanical ventilation. Accepted causes included worsening respiratory status from primary lung disease, increasing apnea, respiratory acidosis, and increasing requirement for inspired oxygen. There is no protocol in the UVA NICU that defines when to intubate for these causes. Decisions are made on a case by case basis when less invasive treatment (e.g. CPAP) proves ineffective. Often, intubations occur overnight based on need as perceived by the NICU staff, and by their nature are considered urgent and unplanned.
We excluded planned intubations prior to surgery or other elective procedures, as well as protocol-driven surfactant administration requiring less than 12 hours of intubation. We also excluded 19 instances of re-intubation within 12 hours of a prior extubation. These clinically important events are excluded because they do not provide 12 hours of non-ventilated data on which to develop a model. Two clinical experts independently investigated patient records for each intubation, and only events deemed by both reviewers to meet our criteria for urgent unplanned intubation were included.
Data collection
Vital signs and waveforms were collected from all bedside monitors in our 45-bed NICU by a centralized server (BedmasterEx, Excel Medical, Jupiter, FL) behind the clinical firewall. Vital signs (heart rate, respiratory rate, and pulse oximetry) were calculated by the monitor by averaging over the previous 10 seconds, and collected every 2 seconds. Waveforms included signals from three electrocardiogram leads (EKG) digitized at 240 Hz, chest impedance pneumograph digitized at 60 Hz, and oximetry plethysmography digitized at 120 Hz. Data were transferred to our parallel computing and storage cluster. All babies had continuous HeRO monitoring (Medical Predictive Science Corp., Charlottesville, VA) and heart rate characteristic (HRC) indices12 were collected hourly.
Data analysis
Calculations were made on 30-minute blocks of data collected during periods of spontaneous ventilation, i.e. while the baby was not receiving mechanical ventilation. As candidate predictors, we calculated the mean and standard deviation of each vital sign – heart rate, respiratory rate, and O2 saturation – along with the cross-correlation of each vital sign with the others. From the continuous waveforms - EKG, chest impedance and oximetry plethysmography - we calculated more complex physiological and statistical measures: cardiorespiratory coupling, cardioventilatory coupling, fraction of heartbeats during inhale, apnea burden, and the HeRO score.
Cardiorespiratory coupling (hereafter referred to as “coupling”) and fraction of heartbeats during inhalation were calculated every 30 seconds over the previous 4 minutes where data were of sufficient quality for analysis13. Coupling is preferential alignment of heartbeats within the respiratory cycle, was defined as epochs where the distribution of heartbeats within the respiratory cycle had less than 0.1% chance of occurring from random numbers13. Each half hour, the fraction of measures that exhibited coupling was calculated and averaged over the previous 12 hours. The fraction of beats during inhale was defined as the mean over the previous 12 hours. Cardioventilatory coupling (CVC) is the preferential alignment of inhalation to the heartbeat, and was calculated every 30 seconds over the previous 10 minutes. We defined CVC as epochs where the relationship between inhale and the previous R-wave had less than a 5% chance of occurring from noise given the number of intervals22, 23. Each half hour, the fraction of measures that exhibited cardioventilatory coupling was calculated and averaged over the previous 12 hours.
Cardiorespiratory waveforms were automatically analyzed to detect central apnea using the methods of Lee et al 14. Briefly, breathing cessations were detected as low variance epochs in the chest impedance pneumograph after notch filtering in heart-clock time to eliminate cardiac artifact, and high-pass filtering to remove movement artifact. Heartbeats were detected using a threshold based algorithm 24 as implemented by Clifford and co-workers 25, 26. Apneas were defined as breathing cessation of at least 10 seconds with associated bradycardia (HR <100 BPM) and desaturation (SaO2 <80%) 14, 27. Apnea burden was defined as the number of seconds that the infant was apneic during the previous 12 hours.
HRC indices were collected from monitors in the NICU each hour, and values were carried over to the subsequent half hour. The HRC index is an output of a logistic regression model based on RR interval standard deviation, sample asymmetry, and sample entropy that detects reduced variability and transient decelerations and reports the fold-increase in risk of neonatal sepsis in the next 24 hours. It has been externally validated, shown to add information to the laboratory tests and clinical signs and to reduce mortality when displayed 4–12.
Development and internal validation of logistic regression models
We used these conventional and novel physiological variables as inputs to logistic regression models. Measurements within the 24 hours prior to an urgent unplanned intubation event were labeled as outcome of 1 and used as events to be predicted. All other measurements (excluding data prior to failed extubation) were labeled as outcome 0. Standard maximum likelihood estimation was used to determine the coefficients for the logistic regression model28. This approach corrects both for unequal variances and correlated responses from individual patients. More specifically, estimates of regression coefficients and other parameters of the model are obtained in standard fashion, but the p-values are corrected using the “sandwich” estimator of standard errors29. For internal validation, we used a cluster bootstrap technique whereby 1000 new samples of the same size were obtained by resampling the infants with replacement 30. The 2.5 and 97.5 percentiles of the sample of risks are used as lower and upper limits for a 95% confidence interval.
The multivariate predictive model for urgent unplanned intubation was developed by first creating univariate models for each conventional and novel physiological variable based on all available data for that variable. Variables that yielded statistically significant models (as defined by coefficients with p<0.05) were incorporated into a multivariate model. Variables whose coefficients were not significant in the multivariate model were removed. Not all parameters could be measured at all times, and we replaced missing data with the median value for all measurements of that variable from all patients when creating the multivariate model. Values were imputed in this way to allow the model to be calculated as often as possible, with the tradeoff of decreasing the accuracy of the final model.
Acknowledgments
Statement of financial support: This work was funded by National Institutes of Health grant 1RC2HD064488.
Footnotes
Disclosure: Drs. Lake and Moorman wish to disclose financial interest in Medical Predictive Science Corporation (Charlottesville, VA).
References
- 1.Darnall RA, Ariagno RL, Kinney HC. The late preterm infant and the control of breathing, sleep, and brainstem development: A review. Clin Perinatol. 2006;33:883–914. doi: 10.1016/j.clp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Barrington K, Finer N. The natural history of the appearance of apnea of prematurity. Pediatr Res. 1991;29:372–375. doi: 10.1038/pr.1991.72500. [DOI] [PubMed] [Google Scholar]
- 3.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics. 2003;112:1283–1289. doi: 10.1542/peds.112.6.1283. [DOI] [PubMed] [Google Scholar]
- 4.Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin Perinatol. 2010;37:581–598. doi: 10.1016/j.clp.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin MP, O’Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res. 2003;53:920–926. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 6.Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116:1070–1074. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 7.Griffin MP, O’Shea TM, Bissonette EA, Harrell FE, Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics are associated with neonatal mortality. Pediatr Res. 2004;55:782–788. doi: 10.1203/01.PDR.0000119366.21770.9E. [DOI] [PubMed] [Google Scholar]
- 8.Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics. 2005;115:937–941. doi: 10.1542/peds.2004-1393. [DOI] [PubMed] [Google Scholar]
- 9.Griffin MP, Lake DE, O’Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res. 2007;61:222–227. doi: 10.1203/01.pdr.0000252438.65759.af. [DOI] [PubMed] [Google Scholar]
- 10.Kovatchev BP, Farhy LS, Cao H, Griffin MP, Lake DE, Moorman JR. Sample asymmetry analysis of heart rate characteristics with application to neonatal sepsis and systemic inflammatory response syndrome. Pediatr Res. 2003;54:892–898. doi: 10.1203/01.PDR.0000088074.97781.4F. [DOI] [PubMed] [Google Scholar]
- 11.Moorman JR, Flower AA, Cao H, Kovatchev BP, Richman JS, Lake DE. Cardiovascular oscillations at the bedside: early diagnosis of neonatal sepsis using heart rate characteristics monitoring. Physiol Meas. 2011;32:1821–1832. doi: 10.1088/0967-3334/32/11/S08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristics monitoring in very low birthweight neonates: a randomized trial. J Pediatr. 2011;159:900–906. doi: 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark MT, Rusin CG, Hudson JL, et al. Breath-by-breath analysis of cardiorespiratory interaction for quantifying developmental maturity in premature infants. J Appl Physiol. 2012;112:859–867. doi: 10.1152/japplphysiol.01152.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Rusin CG, Lake DE, et al. A new algorithm for detecting central apnea in neonates. Physiol Meas. 2012;33:1–17. doi: 10.1088/0967-3334/33/1/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–1116. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MP, Scollan DF, Moorman JR. The dynamic range of neonatal heart rate variability. J Cardiovasc Electrophysiol. 1994;5:112–124. doi: 10.1111/j.1540-8167.1994.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 17.Griffin MP, Lake DE, Bissonette EA, Harrell FE, Jr, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116:1070–1074. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, McLerran D, Grizzle J. A comparison of statistical methods for clustered data analysis with Gaussian error. Stat Med. 1996;15:1793–1806. doi: 10.1002/(SICI)1097-0258(19960830)15:16<1793::AID-SIM332>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuster H, Weiss M, Willeitner AE, et al. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352:1271–1277. doi: 10.1016/S0140-6736(98)08148-3. [DOI] [PubMed] [Google Scholar]
- 20.Ellenby MS, McNames J, Lai S, et al. Uncoupling and recoupling of autonomic regulation of the heart beat in pediatric septic shock. Shock. 2001;16:274–277. doi: 10.1097/00024382-200116040-00007. [DOI] [PubMed] [Google Scholar]
- 21.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002;8:311–315. doi: 10.1097/00075198-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Larsen PD, Galletly DC. Cardioventilatory coupling in heart rate variability: the value of standard analytical techniques. Br J Anaesth. 2001;87:819–826. doi: 10.1093/bja/87.6.819. [DOI] [PubMed] [Google Scholar]
- 23.Tzeng YC, Larsen PD, Galletly DC. Mechanism of cardioventilatory coupling: insights from cardiac pacing, vagotomy, and sinoaortic denervation in the anesthetized rat. Am J Physiol Heart Circ Physiol. 2007;292:H1967–H1977. doi: 10.1152/ajpheart.01049.2006. [DOI] [PubMed] [Google Scholar]
- 24.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Mark RG, Clifford GD. Robust heart rate estimation from multiple asynchronous noisy sources using signal quality indices and a Kalman filter. Physiol Meas. 2008;29:15–32. doi: 10.1088/0967-3334/29/1/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarassenko L, Clifford G, Townsend N. Detection of ectopic beats in the electrocardiogram using an auto-associative neural network. Neural Proc Lett. 2001;14:15–25. [Google Scholar]
- 27.Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 2006;117:S47–S51. doi: 10.1542/peds.2005-0620H. [DOI] [PubMed] [Google Scholar]
- 28.White H. Maximum-Likelihood Estimation of Mis-Specified Models. Econometrica. 1982;50:1–25. [Google Scholar]
- 29.Wei LJ, Lin DY, Weissfeld L. Regression-Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 30.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]