Abstract
There have been tremendous advances in the treatment of chronic lymphocytic leukemia (CLL) over the past decade, with the goal of therapy no longer being just to palliate symptoms but now to achieve complete remission, eradicate minimal residual disease, and improve survival. During this period, there have also been major advances in identification of molecular factors associated with increased risk of progression. The clinical utility of these factors is being explored to determine whether we can identify groups of patients who should be treated earlier in their disease course and whether we can tailor therapy for groups of patients with specific molecular markers of disease. First-line chemoimmunotherapy approaches now offer prolonged survival, and there is a need to identify patients who are suitable candidates for allogeneic stem-cell transplantation that uses reduced-intensity conditioning regimens. The vast majority of CLL patients are either too old or do not have sufficiently high-risk disease to warrant these approaches, and effective therapies that can be tolerated by the more frail elderly patients with this disease are urgently needed. Numerous novel agents are being developed, and their role in the first-line treatment of frail patients or those who relapse after previous treatment is being explored in clinical trials.
INTRODUCTION
The diagnosis of chronic lymphocytic leukemia (CLL) is straightforward and outlined in detail in the International Workshop on CLL (iwCLL) guidelines.1 The diagnosis of CLL now requires a circulating B-cell count of at least 5,000 B cells/μL, although 5% of patients will present with clinical features of small lymphocytic lymphoma without the leukemic component. The presence of cells with the immunophenotype of CLL cells but with fewer than 5,000 B cells/μL and the absence of lymphadenopathy is now defined as monoclonal B lymphocytosis.2 It is currently unclear what proportion of patients with monoclonal B lymphocytosis will progress to CLL. Once diagnosed, the clinical course of CLL is extremely heterogeneous. Some patients will live for decades and never require treatment, while others require immediate treatment.3 A major focus of research has been to try to identify those clinical and biologic factors that influence the clinical course of CLL to help determine whether patients will have indolent disease or rapid progression and which patients will respond best to which treatment.
PROGNOSTIC FACTORS IN CLL AND THEIR CLINICAL RELEVANCE
Clinical staging remains an important prognostic marker; this and other markers of disease such as β2-microglobulin can be incorporated into nomograms to assess risk of progression.4 The molecular profile of CLL provides insight into disease pathogenesis and provides useful information on time to progression, need for therapy, and overall survival (OS). A molecular profile can be built from assessment of the large number of biomarkers that have been identified, the most important being cytogenetic analysis by fluorescent in situ hybridization (FISH), mutational status of the immunoglobulin heavy-chain variable gene (IgVH), use of IgVH, and expression of 70-kDa zeta-associated protein (ZAP70) and CD38.
One or more chromosome abnormalities can be found in more than 80% of CLL patients by using FISH, including del13q, del11q, trisomy 12, del17p, and del6q.5,6 The most common abnormality del13q.14 occurs in > 50% of patients, and isolated del13q is associated with a good prognosis. Two microRNA clusters, mir-15a and mir-16-1, are located within the deleted region at 13q14.7 Del11q and trisomy 12 are each found in approximately 20% of patients. Although < 10% of patients have del17p at diagnosis, this abnormality is associated with more rapid progression of disease, poor response to therapy, and short survival time. Cytogenetic changes that occur in CLL can evolve over time, and it is important to reassess these markers at subsequent time points.
Fifty percent of CLL patients have undergone somatic hypermutation in IgVH, and these patients have a more indolent clinical course and longer survival than those without somatic hypermutation.8,9 Analysis of variable region sequences demonstrates that CLL cells use a biased repertoire of V genes with over-representation of certain Ig gene segments, in particular IGHV1-69, IGHV4-34, IGHV3-7, and IGHV3-21.10,11 Patients with CLL cells that use IGHV3-21 have relatively aggressive disease, even when the expressed IGHV3-21 is mutated.12 Since it is technically difficult and expensive to determine IgVH mutational status, surrogate markers such as expression of ZAP70 and CD38 have been assessed, and both have prognostic significance. There is not an absolute relationship between ZAP70 expression and IgVH mutational status, with discrepancies occurring in up to 25% of patients.13,14 Discordant cases may have other biologic features with poor prognostic implications such as del17p, del11q or use of IGHV3-21.15 Some studies have suggested that ZAP70 status is more useful as a predictor of time to progression than mutation status,13,16 but this remains controversial. MicroRNA array analysis has revealed a 13-gene signature correlated with ZAP70 status, unmutated IgVH expression,17 and disease progression18; altered microRNA expression appears to regulate expression of genes controlling apoptosis and cell cycle progression.19 Although there is a relationship between CD38 and IgVH mutation status,9 this is not absolute, and CD38 expression may vary over time.20,21
High-risk features predictive of disease progression include del17p and del11q, IgVH unmutated status, use of the IGHV3-21 gene segment, and expression of either ZAP70 or CD38. It remains challenging to understand how these biomarkers can be used in clinical practice and whether we should alter treatment on the basis of the detection of high-risk features. Assessment of the impact of these biomarkers remains a vital component of research studies. With improvement in therapy, since some groups respond better to newer treatment combinations, the prognostic significance of some of these parameters will change.
TREATMENT OF CLL
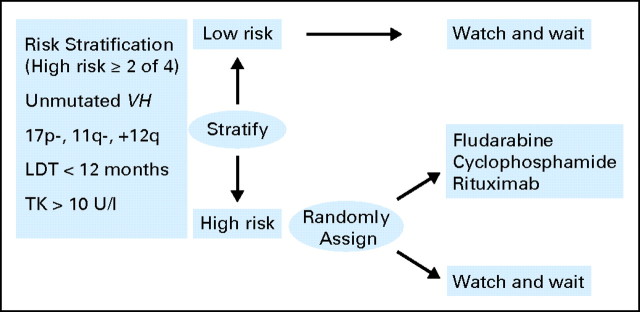
Unlike with other leukemias, once a diagnosis of CLL is made, treatment may not necessarily be initiated; previous trials22 have examined whether there is any benefit to early or immediate therapy, and a meta-analysis of these studies showed that there was no statistically significant difference in survival between those patients who were treated early versus those in whom therapy was deferred until there was a clinical indication for treatment. However, these trials enrolled patients from all risk groups and were performed by using alkylating agents. Several ongoing studies are re-evaluating this question by using a risk-adjusted strategy, so that only those with high-risk disease are randomly assigned to receive treatment with more modern regimens of chemoimmunotherapy (Fig 1). The use of prognostic factors allows this high-risk group to be readily identified. Although it is tempting to believe that these high-risk patients should be treated earlier in their disease course, there is as yet no evidence that these patients will definitely benefit from earlier treatment. Until data from the randomized trials are available, asymptomatic patients should not be offered treatment on the basis of any molecular marker before the standard criteria for treatment are reached (ie, patients with active disease, including symptomatic disease, bulky lymphadenopathy and/or splenomegaly, local compressive disease, marrow compromise, or rapid disease progression).1
Fig 1.
Clinical trial evaluating the use of prognostic factors to assess the impact of early therapy in chronic lymphocytic leukemia. Risk factors are used to stratify asymptomatic Binet stage A patients into low and high risk. High-risk patients are randomly assigned to continue with the watch-and-wait approach or to receive therapy with fludarabine, cyclophosphamide, and rituximab. VH, heavy-chain variable gene; LDT, lymphocyte doubling time; TK, thymidine kinase.
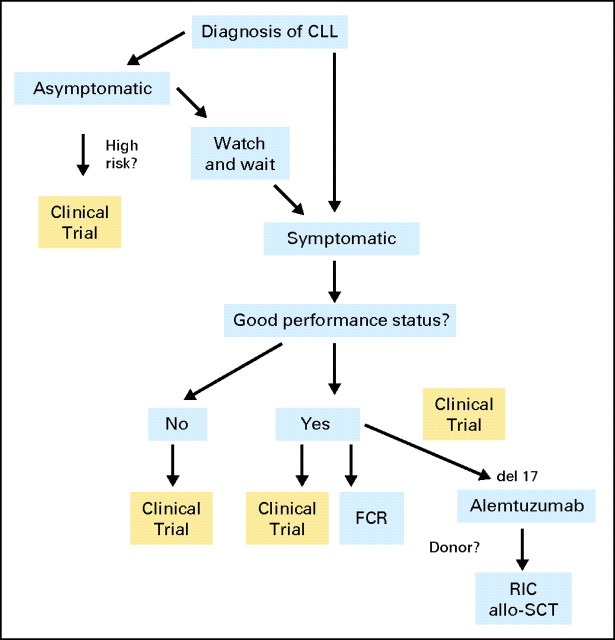
Our approach to the treatment of CLL patients is shown in Figure 2. The results of clinical trials in previously untreated CLL have demonstrated major advances over the last decade as shown in Table 1. In a comparison of single agents, fludarabine was shown to be significantly more effective than chlorambucil as initial treatment for patients with symptomatic CLL.23 Fludarabine produced higher complete response (CR) and overall response rates (ORRs) as well as longer progression-free survival (PFS). Although the initial publication did not indicate a difference in OS, the 10-year update of this trial presented at the Annual Meeting of the American Society of Hematology (ASH) in 200933 showed a significant improvement in OS in those patients who were initially treated with fludarabine compared with those who were treated with chlorambucil.
Fig 2.
How we treat chronic lymphocytic leukemia (CLL). Asymptomatic patients should be treated with a watch-and-wait approach until they are symptomatic, unless they are enrolled onto clinical trials assessing the impact of prognostic features on early treatment. Treatment of choice is fludarabine, cyclophosphamide, and rituximab (FCR), unless there is a suitable clinical trial. Patients with del17p or p53 mutations should be considered for clinical trial and offered reduced-intensity conditioning (RIC) allogeneic stem-cell transplantation (allo-SCT). Patients who are not fit for consideration for FCR should be considered for clinical trials investigating novel approaches for these patients.
Table 1.
Randomized Trials of Initial Therapy for Previously Untreated CLL
| CLL Study | Treatment | No. of Patients | CR (%) | OR (%) | PFS (months) | Reference |
|---|---|---|---|---|---|---|
| CALGB 9011 | Chlorambucil | 181 | 4 | 37 | 14 | Rai et al23 |
| Fludarabine | 170 | 20 | 63 | 20 | ||
| GCLLSG CLL5 | Chlorambucil | 100 | 0 | 51 | 18 | Eichhorst et al24 |
| Elderly patients | Fludarabine | 93 | 7 | 72 | 19 | |
| CAM 307 | Chlorambucil | 148 | 2 | 55 | 11.7 | Hillmen et al25 |
| Alemtuzumab | 149 | 24 | 83 | 14.6 | ||
| Multicenter Phase III | Chlorambucil | 157 | < 1 | 39 | 8.3 | Knauf et al29 |
| Bendamustine | 162 | 29 | 68 | 21.6 | ||
| CALGB 9712 | Fludarabine | 44 | 28 | 77 | Byrd et al31 | |
| Fludarabine/rituximab | 51 | 47 | 90 | N/D at 2 years | ||
| GCLLSG CLL4 | Fludarabine | 180 | 7 | 83 | 20 | Eichhorst et al26 |
| Fludarabine/cyclophosphamide | 182 | 24 | 94 | 48 | ||
| ECOG E2997 | Fludarabine | 137 | 5 | 59 | 19 | Flinn et al27 |
| Fludarabine/cyclophosphamide | 141 | 23 | 74 | 32 | ||
| LRF CLL4 | Chlorambucil | 366 | 7 | 72 | 20 | Catovsky et al28 |
| Fludarabine | 181 | 15 | 80 | 23 | ||
| Fludarabine/cyclophosphamide | 182 | 38 | 92 | 43 | ||
| MDACC (Phase II) | Rituximab/fludarabine/ cyclophosphamide | 300 | 72 | 95 | 80 | Tam et al30 |
| GCLLSG CLL8 | Fludarabine/cyclophosphamide | 408 | 27 | 88 | 33 | Hallek et al32 |
| Rituximab/fludarabine/cyclophosphamide | 409 | 52 | 95 | 52 |
Abbreviations: CLL, chronic lymphocytic leukemia; CR, complete response; OR, overall response; PFS, progression-free survival; CALGB, Cancer and Leukemia Group B; GCLLSG, German CLL Study Group; CAM, alemtuzumab (Campath; Genzyme, Cambridge, MA); N/D, not done; ECOG, Eastern Cooperative Oncology Group; LRF, Leukaemia Research Fund; MDACC, MD Anderson Cancer Center.
In an attempt to increase the activity of the best single agent, fludarabine has been combined with several different agents, and the most promising combination was that of fludarabine and cyclophosphamide (FC).34 Three randomized trials26–28 have compared fludarabine to FC as first-line therapy; all have shown significantly greater CR and ORR and longer PFS with FC. The best outcomes have been reported with chemoimmunotherapy, the most active combination described thus far being the three-drug regimen of fludarabine, cyclophosphamide, and rituximab (FCR). Phase II clinical studies at the MD Anderson Cancer Center evaluated FCR in previously untreated patients30,35 as well as in treated patients.36 In a series of 300 previously untreated patients, the ORR was 95%, with 72% achieving CR, 7% CR without recovery, 10% nodular partial response, and 6% partial response.30 At a median follow-up of 6 years, OS was 77% and PFS was 51%. The German CLL Study Group (GCLLSG) CLL8 randomized clinical trial32 demonstrated a significant improvement in response rates, duration of response, and OS with FCR compared with FC. The use of FCR was also associated with a higher percentage of patients having eradication of minimal residual disease (MRD); eradication of MRD was associated with longer duration of response. The combination of fludarabine and rituximab (FR) produced a 43% CR rate and a median PFS of 41 months.31 In comparison with historical controls, FR was associated with improved outcome compared with fludarabine alone.37 Regimens such as FR or pentostatin, cyclophosphamide, and rituximab (PCR) may have less hematologic toxicity than FCR, but no studies have reported outcome with FCR compared with either PCR or FR. The current US Intergroup trial will randomly assign patients requiring treatment to one of three arms: FR, FCR, or FR followed by lenalidomide. FISH is performed at entry, and patients found to have an 11q deletion are reassigned to therapy with FCR followed by lenalidomide (on the basis of accumulating evidence that the alkylating agent is particularly important for improving outcome in that subset).
Bendamustine is a potent alkylating agent developed in East Germany more than 30 years ago and recently approved for use in CLL in the United States in 2008. This agent has some structural similarity to purine analogs, but preclinical data do not clearly show purine analog–based activity.38 The drug was approved by the US Food and Drug Administration on the basis of a randomized trial of bendamustine compared with chlorambucil in previously untreated patients with CLL. The CR rate with bendamustine was 29% and the ORR was 68%, both of which were higher than those seen with chlorambucil (CR rate, 4% and ORR, 39%). The median PFS with bendamustine was 21 months, significantly longer than the 9 months seen with chlorambucil.29 Recent data from the GCLLSG suggest that bendamustine in combination with rituximab (BR) produces higher response rates than those seen with bendamustine alone.39 This combination produced a CR rate of 33% and an ORR of 89%. Although CR rates appear somewhat inferior in this phase II trial compared with those seen with FCR, ORRs appear comparable, and an ongoing first-line randomized trial is comparing the combination of BR to FCR.
The anti-CD52 monoclonal antibody, alemtuzumab, is now approved for use in previously untreated CLL, having been approved initially for fludarabine-refractory patients. A phase III randomized study was performed in 297 patients with previously untreated progressive CLL; alemtuzumab was compared with chlorambucil.25 That study demonstrated significantly superior response rates as well as longer PFS for alemtuzumab compared with chlorambucil (ORR, 83% v 56%; P < .001 and CR rate 24% v 2%; P < .001). Alemtuzumab alone or in combination is a reasonable option for patients with del17p or p53 mutations, since this agent has been shown to have efficacy in this patient population.40
There is no established role for maintenance therapy in CLL. Alemtuzumab has been assessed in this setting with some intriguing results,41 but enthusiasm has been tempered by the high toxicity observed. Ongoing clinical trials are examining maintenance therapy with alternative schedules of alemtuzumab, rituximab, or lenalidomide.
TREATMENT OF RELAPSED DISEASE
Notwithstanding the improved results with first-line therapy, the disease remains incurable, and patients with CLL are destined to relapse after primary treatment. The management of relapsed CLL patients is then dependent on several factors, including age, performance status, previous therapy, response and duration of response to therapy, and time from last therapy. The prior therapy administered and the response and duration of response to that therapy are among the most important factors in determining the next therapy. The goal of therapy, whether palliative or aggressive, must also be factored into the decision when deciding on the next line of treatment. With many potential treatments available, the sequence of treatments and the timing of procedures such as stem-cell transplantation (SCT) remain questions that are being addressed in clinical trials.
For those patients who relapse following previous treatment with alkylating agents, re-treatment with alkylating agents is unlikely to induce CR, and remissions are usually of short duration. In the Intergroup study23 comparing primary treatment with chlorambucil and fludarabine, at crossover, the ORR to fludarabine was 46% for those patients for whom chlorambucil treatment failed, whereas only 7% of patients for whom fludarabine treatment failed responded to chlorambucil, suggesting little role for alkylating-agent therapy in patients for whom treatment with purine analogs failed. Improved results in salvage were seen with the combined use of FC with the largest series reported from the MD Anderson Cancer Center.34
Modest activity is seen in previously treated CLL when rituximab is administered at standard doses, but at higher doses or at increased frequency, the ORR increased to 40% to 45%, although few patients achieved CR, and those patients had not received rituximab as part of first-line therapy.42,43 The highest response in the relapsed setting occurred with the FCR combination. In 177 previously treated patients with CLL, the ORR to FCR was 73%, with 25% achieving CR, irrespective of whether the patients had previously received any of these agents either alone or in combination. Patients who were fludarabine refractory still had ORRs of 58%, but the CR rate decreased to 6%.36 On multivariate analysis, the pretreatment factors significantly associated with achieving CR were higher platelet count, fewer prior treatments, and lower β2-macroglobulin, whereas treatment failure was associated with fludarabine refractoriness and renal impairment. These results have now been confirmed in a multicenter randomized phase III study. The REACH trial44 randomly assigned 552 patients who had relapsed after one prior treatment to receive six cycles of FCR or six cycles of FC; at a median follow-up time of 25 months, the use of FCR compared with FC significantly improved CR rate, PFS (median, 30.6 months for FCR v 20.6 months for FC), and time to next CLL treatment.
Alemtuzumab was initially approved for patients with CLL who were fludarabine refractory. In the pivotal study of alemtuzumab in 93 patients with fludarabine refractory CLL, the ORR was 33%; only 2% of patients achieved CR.45 The median time to progression for responders was 9.5 months, but there was an improvement in survival in responding patients at 32 months, compared with 16 months for all patients. Of note, alemtuzumab appears to have activity in patients who are unresponsive to chemotherapy because of the presence of p53 mutations.40 This finding was confirmed in a study of 36 patients with fludarabine-refractory CLL treated with alemtuzumab, 15 (42%) of whom had p53 mutations or deletions.46 Alemtuzumab has less activity against bulky lymphadenopathy but demonstrates impressive efficacy in clearing the peripheral blood and bone marrow compartments of disease. Alemtuzumab has shown benefit when used in combination with fludarabine.47
New treatments are urgently needed for patients who are fludarabine- and alemtuzumab-refractory or patients with fludarabine-refractory CLL with bulky lymphadenopathy who are not considered suitable for alemtuzumab treatment. Ofatumumab is a humanized monoclonal antibody targeting CD20 but binding to a different epitope than rituximab. This agent was recently approved by the US Food and Drug Administration for use in fludarabine- and alemtuzumab-refractory CLL. In that trial of heavily pretreated patients, the ORR was 58% with a median PFS of 6 months.48 The significant activity of this agent in the refractory population has raised the question of whether combining it with chemotherapy will provide more potent efficacy than current rituximab- and chemotherapy-based regimens. In a small randomized trial of 61 patients,49 all patients received FC and ofatumumab, with the latter being given at either 500 mg or 1,000 mg; ORRs were comparable, but the CR rate was 50% with 1,000 mg versus 32% with 500 mg. Neutropenia also appeared to be increased with the higher dose, occurring in 60% of patients versus 35% of patients treated with 500 mg. However, none of these comparisons were statistically significant because of the small number of patients. The CR rate with FC and ofatumumab at 1,000 mg is comparable with that achieved with FCR in the German randomized trial; however, follow-up is short and no information is yet available on PFS or survival.
There are several ongoing clinical trials exploring new agents for the treatment of CLL. These include monoclonal antibodies (GA101, lumiliximab, lucatumumab), BH3 mimetics (obatoclax, ABT-263), cyclin-dependent kinase inhibitors (flavopiridol, SNS-032), Lyn-kinase inhibitors (dasatinib, bafetinib), hypomethylating agents (azacytidine, decitabine), histone deacetylase inhibitors (parobinastat), purine analogs (8-chloroadenosine, forodesine), and small modular immunopharmaceuticals (TRU-016). Molecules inhibiting downstream signaling after B-cell receptor ligation are novel oral agents interacting at different targets including phosphatidylinositol 3-kinase inhibitors (CAL-101), Bruton's tyrosine kinase inhibitors (PCI-32765), and Syk inhibitors (fostamitinib). In addition, agents that have no direct cytotoxicity to CLL cells have also been proven to be effective in this disease, presumably by significant modulation of the microenvironment of the bone marrow (lenalidomide).
IMPACT OF PROGNOSTIC FACTORS ON TREATMENT
Several published studies have examined the impact of prognostic factors in response to therapy.28,50,51 Consistently decreased rates of response to all treatments are seen in patients with del17p. The presence of 11q deletions or unmutated immunoglobulin heavy chain gene does not predict for a lower response rate to FCR but is correlated with shorter PFS in patients achieving CR.52 Patients with del17p should be treated on clinical trials examining agents that have activity in cells without functional p53 since these patients have a response to chemotherapy combinations that is inferior to that of patients without del17p. This is the one group of patients that can reasonably be considered for allogeneic SCT even in first remission, since remission durations are short and response after relapse is poor.53
ROLE OF SCT
One major challenge remains: deciding which other patients should be considered for allogeneic SCT and when in their disease course SCT should be offered. Because of the older age of most patients with CLL, the choice is usually reduced intensity conditioning allogeneic SCT54; there is no evidence of cure with autologous SCT.55 The European Bone Marrow Transplant (EBMT) guidelines outline indications for SCT in CLL.53 Allogeneic SCT is recommended for those with p53 abnormalities and is also recommended for younger patients with CLL who fail to respond to, or relapse within 2 years of first-line chemoimmunotherapy.
IMPACT OF PERFORMANCE STATUS IN SELECTION OF TREATMENT
Among the more than 15,000 people diagnosed with CLL in the United States in 2009,56 more than two thirds were older than age 65 years at the time of diagnosis. The median age at diagnosis of CLL is 72 years, and the median age at death from CLL is 79 years. Although most patients with CLL are elderly, age alone is not a contraindication for the use of FCR.57 The limiting factors for its use are impaired renal function, poor performance status, and comorbidities. In the GCLLSG CLL8 trial (FCR v FC), there was no upper age limit, but patients had to meet eligibility criteria including creatinine clearance ≥ 70 mL/min and good physical fitness rating of ≤ 6 as assessed by the Cumulative Illness Rating Scale (CIRS). There was no difference in adverse effects when comparing patients age < 70 years or > 70 years in that trial. Thus FCR can certainly be used in older, fit patients with CLL. In the GCLLSG CLL5 trial24 in older patients, fludarabine resulted in a significantly higher ORR and CR rate than chlorambucil; there was no difference in PFS or OS. The best treatment for elderly unfit patients or those with complex comorbidities remains to be determined. Ongoing clinical trials are assessing the addition of monoclonal antibodies, including rituximab, ofatumumab or GA101 to chlorambucil compared with chlorambucil alone. Other agents being assessed in clinical trials in this patient population include bendamustine alone and in combination with rituximab, lenalidomide, ABT263, PCI-32765, and CAL-101 with rituximab.
TREATMENT OF CLL COMPLICATIONS
Autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia are common in CLL.58 There have been no controlled trials of treatment for AIHA in CLL, but corticosteroids remain the treatment of choice. In nonresponders or in those who relapse after withdrawal of steroids, cyclosporine, mycophenolate mofetil, or rituximab59,60 may be beneficial. Splenectomy is also indicated in refractory patients. AIHA occurred in 10% of patients receiving chemotherapy and occurred more often in patients treated with chlorambucil than with fludarabine, and least frequently in patients receiving the combination of fludarabine and cyclophosphamide.61 There is no contraindication to the use of FCR in patients with AIHA.
Infections are a major cause of morbidity and mortality in patients with CLL, and hypogammaglobulinemia increases with the duration of disease and may be associated with an increased incidence of bacterial infections. In a randomized cross-over study among patients with severe hypogammaglobulinemia, the incidence and severity of infections was less when patients received intravenous immunoglobulin replacement therapy.62 The frequency of infections may also increase following treatment.63 Bacterial infections remain most common, but treated patients are at risk for a wide spectrum of opportunistic infections including Listeria monocytogenes, Pneumocystis carinii, cytomegalovirus, herpes simplex virus, and mycobacteria.64,65 Long-term follow-up suggests that although purine analogs have an impact on opportunistic infections, infections are more common in patients with an incomplete response to therapy or with progressive disease, suggesting that the disease itself has more impact than the therapy.66 Opportunistic infections are also seen following treatment with alemtuzumab,45 particularly in fludarabine refractory patients, but these patients have a high incidence of infections with any therapy,67 and fewer infections were seen when alemtuzumab was used in previously untreated patients.25
Richter's transformation to high-grade non-Hodgkin's lymphoma occurs in approximately 5% to 10% of patients with CLL.68 The large cells of Richter's syndrome either arise through a transformation of the original CLL clone by the acquisition of new genetic abnormalities or, less frequently, represent a new secondary neoplasm. The clinical outcome of the disease is generally poor with median survival of months from transformation, but prognosis is better when transformation occurs in previously untreated patients. Treatment is with regimens that are effective in high-grade non-Hodgkin's lymphoma, and although numerous regimens have been proposed, there is no consensus on the best therapeutic approach for patients with Richter's syndrome.
In conclusion, chemoimmunotherapy has had a dramatic impact on the outcome of patients with CLL, has improved CR rates, eradicates MRD, and has resulted in improved survival. Remissions with first-line therapy last for years, and data are now emerging that more effective first-line regimens are associated with improved OS in CLL. Studies are also assessing whether specific treatments are indicated for groups of patients with specific cytogenetic abnormalities. However, not all patients can tolerate aggressive chemoimmunotherapy regimens, and patients continue to relapse, so new drugs are needed to effect cure in these patient populations. The encouraging array of ongoing clinical trials and investigational drugs, many of which have novel mechanisms of action, engender great optimism that cure of CLL may be accomplished within the next 20 years.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Susan O'Brien, Allos Therapeutics (C), Biogen Idec (C), Calistoga Pharmaceuticals (C), Celgene (C), Eli Lilly (C), Facet Biotech (C), Gemin X (C), GenMab (C), Genta (C), Genentech BioOncology (C), sanofi-aventis (C), Sunesis Pharmaceuticals (C) Stock Ownership: None Honoraria: John G. Gribben, Roche, Celgene, Mundipharma International, Biogen Idec, GlaxoSmithKline Research Funding: Susan O'Brien, Bayer Pharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Eli Lilly, Gemin X, Genta, Genentech BioOncology, Hana Biosciences, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawstron AC, Bennett FL, O'Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 3.Gribben JG. How I treat CLL up front. Blood. 2010;115:187–197. doi: 10.1182/blood-2009-08-207126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierda WG, O'Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 5.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 6.Kröber A, Seiler T, Benner A, et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100:1410–1416. [PubMed] [Google Scholar]
- 7.Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 9.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 10.Fais F, Ghiotto F, Hashimoto S, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauerer K, Zahrieh D, Gorgun G, et al. Immunoglobulin gene segment usage, location and immunogenicity in mutated and unmutated chronic lymphocytic leukaemia. Br J Haematol. 2005;129:499–510. doi: 10.1111/j.1365-2141.2005.05480.x. [DOI] [PubMed] [Google Scholar]
- 12.Tobin G, Thunberg U, Johnson A, et al. Somatically mutated Ig V(H)3-21 genes characterize a new subset of chronic lymphocytic leukemia. Blood. 2002;99:2262–2264. doi: 10.1182/blood.v99.6.2262. [DOI] [PubMed] [Google Scholar]
- 13.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 14.Bosch F, Muntañola A, Giné E, et al. Clinical implications of ZAP-70 expression in chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2006;70:214–217. doi: 10.1002/cyto.b.20131. [DOI] [PubMed] [Google Scholar]
- 15.Kröber A, Bloehdorn J, Hafner S, et al. Additional genetic high-risk features such as 11q deletion, 17p deletion, and V3-21 usage characterize discordance of ZAP-70 and VH mutation status in chronic lymphocytic leukemia. J Clin Oncol. 2006;24:969–975. doi: 10.1200/JCO.2005.03.7184. [DOI] [PubMed] [Google Scholar]
- 16.Del Principe MI, Del Poeta G, Buccisano F, et al. Clinical significance of ZAP-70 protein expression in B-cell chronic lymphocytic leukemia. Blood. 2006;108:853–861. doi: 10.1182/blood-2005-12-4986. [DOI] [PubMed] [Google Scholar]
- 17.Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 19.Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 2002;99:1023–1029. doi: 10.1182/blood.v99.3.1023. [DOI] [PubMed] [Google Scholar]
- 21.Montillo M, Hamblin T, Hallek M, et al. Chronic lymphocytic leukemia: Novel prognostic factors and their relevance for risk-adapted therapeutic strategies. Haematologica. 2005;90:391–399. [PubMed] [Google Scholar]
- 22.Chemotherapeutic options in chronic lymphocytic leukemia: A meta-analysis of the randomized trials: CLL Trialists' Collaborative Group. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 23.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 24.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 25.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 26.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 27.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 28.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 29.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–4384. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 30.Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 32.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 33.Rai KR, Peterson BL, Appelbaum FR, et al. Long-term survival analysis of the North American Intergroup Study C9011 comparing fludarabine (F) and chlorambucil (C) in previously untreated patients with chronic lymphocytic leukemia (CLL) Blood. 2009;114:224. (abstr 536) [Google Scholar]
- 34.O'Brien SM, Kantarjian HM, Cortes J, et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:1414–1420. doi: 10.1200/JCO.2001.19.5.1414. [DOI] [PubMed] [Google Scholar]
- 35.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 36.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 37.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: An updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 38.Gandhi V, Burger JA. Bendamustine in B-cell malignancies: The new 46-year-old kid on the block. Clin Cancer Res. 2009;15:7456–7461. doi: 10.1158/1078-0432.CCR-08-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer K, Cramer P, Stilgenbauer S, et al. Bendamustine combined with rituximab (BR) in first-line therapy of advanced CLL: A multicenter phase II trial of the German CLL Study Group (GCLLSG) Blood. 2009;114:89. (abstr 205) [Google Scholar]
- 40.Stilgenbauer S, Döhner H. Campath-1H-induced complete remission of chronic lymphocytic leukemia despite p53 gene mutation and resistance to chemotherapy. N Engl J Med. 2002;347:452–453. doi: 10.1056/NEJM200208083470619. [DOI] [PubMed] [Google Scholar]
- 41.Schweighofer CD, Ritgen M, Eichhorst BF, et al. Consolidation with alemtuzumab improves progression-free survival in patients with chronic lymphocytic leukaemia (CLL) in first remission: Long-term follow-up of a randomized phase III trial of the German CLL Study Group (GCLLSG). Br J Haematol. 2009;144:95–98. doi: 10.1111/j.1365-2141.2008.07394.x. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 43.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 44.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 45.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 46.Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood. 2004;103:3278–3281. doi: 10.1182/blood-2003-10-3729. [DOI] [PubMed] [Google Scholar]
- 47.Elter T, Borchmann P, Schulz H, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: Results of a phase II trial. J Clin Oncol. 2005;23:7024–7031. doi: 10.1200/JCO.2005.01.9950. [DOI] [PubMed] [Google Scholar]
- 48.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wierda WG, Kipps TJ, Dürig J, et al. Ofatumumab combined with fludarabine and cyclophosphamide (O-FC) shows high activity in patients with previously untreated chronic lymphocytic leukemia (CLL): Results from a randomized, multicenter, international, two-dose, parallel group, phase II trial. Blood. 2009;114:90. (abstr 207) [Google Scholar]
- 50.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: Justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–443. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 51.Grever MR, Lucas DM, Dewald GW, et al. Comprehensive assessment of genetic and molecular features predicting outcome in patients with chronic lymphocytic leukemia: Results from the US Intergroup Phase III Trial E2997. J Clin Oncol. 2007;25:799–804. doi: 10.1200/JCO.2006.08.3089. [DOI] [PubMed] [Google Scholar]
- 52.Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood. 2009;113:3168–3171. doi: 10.1182/blood-2008-10-184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dreger P, Corradini P, Kimby E, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: The EBMT transplant consensus. Leukemia. 2007;21:12–17. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 54.Gribben JG. Stem cell transplantation in chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2009;15:53–58. doi: 10.1016/j.bbmt.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gribben JG, Zahrieh D, Stephans K, et al. Autologous and allogeneic stem cell transplantation for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Cancer Institute. Surveillance Epidemiology and End Results, SEER Stat Fact Sheets. Chronic Lymphocytic Leukemia. 2010. http://seer.cancer.gov/statfacts/html/clyl.html.
- 57.Gribben JG. One step back but 2 steps forward. Blood. 2009;114:3359–3360. doi: 10.1182/blood-2009-08-234146. [DOI] [PubMed] [Google Scholar]
- 58.Dearden C. Disease-specific complications of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2008:450–456. doi: 10.1182/asheducation-2008.1.450. [DOI] [PubMed] [Google Scholar]
- 59.Gupta N, Kavuru S, Patel D, et al. Rituximab-based chemotherapy for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia. 2002;16:2092–2095. doi: 10.1038/sj.leu.2402676. [DOI] [PubMed] [Google Scholar]
- 60.D'Arena G, Laurenti L, Capalbo S, et al. Rituximab therapy for chronic lymphocytic leukemia-associated autoimmune hemolytic anemia. Am J Hematol. 2006;81:598–602. doi: 10.1002/ajh.20665. [DOI] [PubMed] [Google Scholar]
- 61.Dearden C, Wade R, Else M, et al. The prognostic significance of a positive direct antiglobulin test in chronic lymphocytic leukemia: A beneficial effect of the combination of fludarabine and cyclophosphamide on the incidence of hemolytic anemia. Blood. 2008;111:1820–1826. doi: 10.1182/blood-2007-07-101303. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths H, Brennan V, Lea J, et al. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood. 1989;73:366–368. [PubMed] [Google Scholar]
- 63.Morrison VA. Update on prophylaxis and therapy of infection in patients with chronic lymphocytic leukemia. Expert Rev Anticancer Ther. 2001;1:84–90. doi: 10.1586/14737140.1.1.84. [DOI] [PubMed] [Google Scholar]
- 64.Morrison VA, Rai KR, Peterson BL, et al. Impact of therapy with chlorambucil, fludarabine, or fludarabine plus chlorambucil on infections in patients with chronic lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B 9011. J Clin Oncol. 2001;19:3611–3621. doi: 10.1200/JCO.2001.19.16.3611. [DOI] [PubMed] [Google Scholar]
- 65.O'Brien S, Kantarjian H, Beran M, et al. Results of fludarabine and prednisone therapy in 264 patients with chronic lymphocytic leukemia with multivariate analysis-derived prognostic model for response to treatment. Blood. 1993;82:1695–1700. [PubMed] [Google Scholar]
- 66.Keating MJ, O'Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- 67.Perkins JG, Flynn JM, Howard RS, et al. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: Implications for clinical trials in this patient population. Cancer. 2002;94:2033–2039. [PubMed] [Google Scholar]
- 68.Tsimberidou AM, O'Brien S, Khouri I, et al. Clinical outcomes and prognostic factors in patients with Richter's syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24:2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]