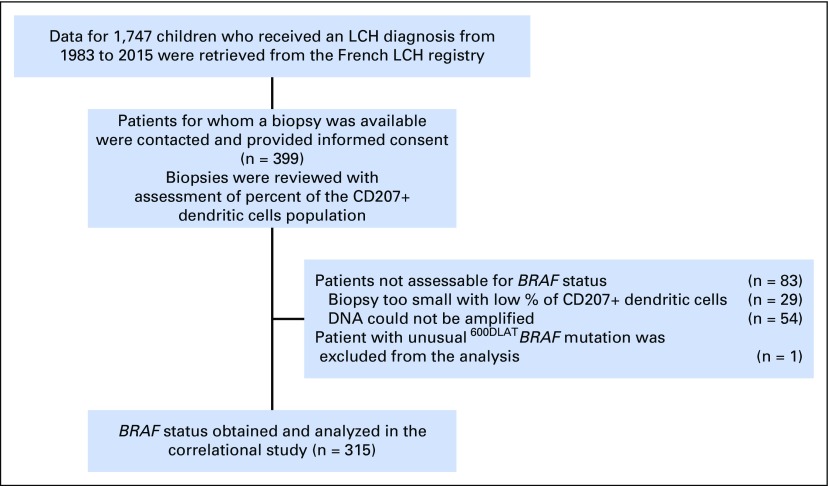
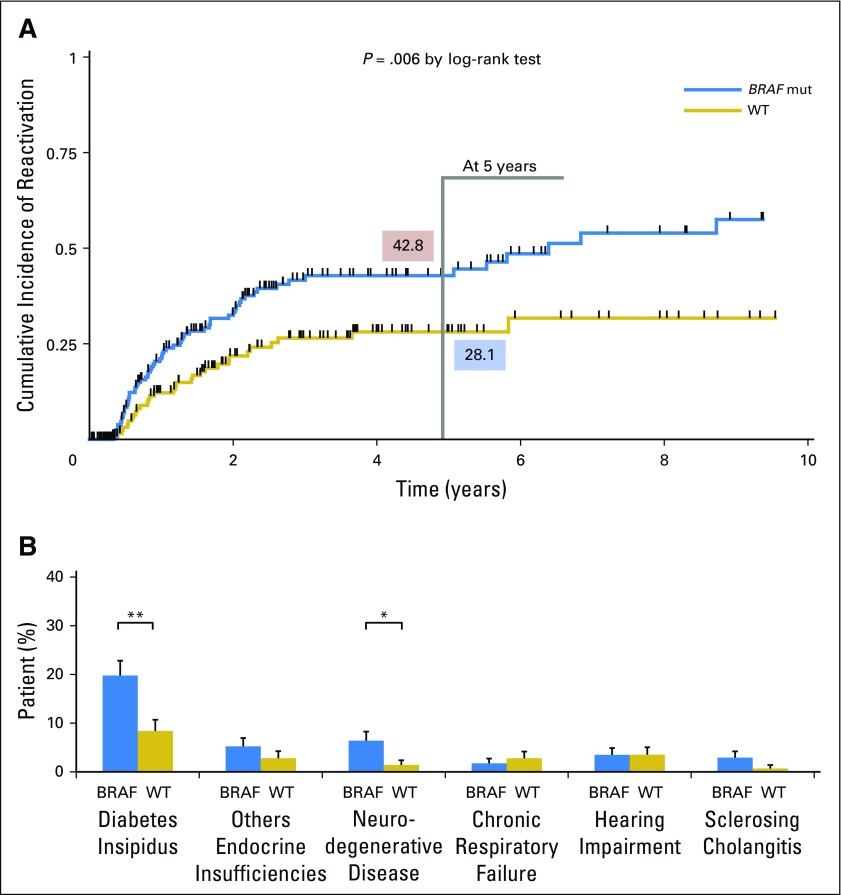
Sébastien Héritier
Sébastien Héritier
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,✉,
Jean-François Emile
Jean-François Emile
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Mohamed-Aziz Barkaoui
Mohamed-Aziz Barkaoui
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Caroline Thomas
Caroline Thomas
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Sylvie Fraitag
Sylvie Fraitag
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Sabah Boudjemaa
Sabah Boudjemaa
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Florence Renaud
Florence Renaud
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Anne Moreau
Anne Moreau
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Michel Peuchmaur
Michel Peuchmaur
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Catherine Chassagne-Clément
Catherine Chassagne-Clément
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Frédérique Dijoud
Frédérique Dijoud
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Valérie Rigau
Valérie Rigau
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Despina Moshous
Despina Moshous
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Anne Lambilliotte
Anne Lambilliotte
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Françoise Mazingue
Françoise Mazingue
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Kamila Kebaili
Kamila Kebaili
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Jean Miron
Jean Miron
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Eric Jeziorski
Eric Jeziorski
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Geneviève Plat
Geneviève Plat
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Nathalie Aladjidi
Nathalie Aladjidi
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Alina Ferster
Alina Ferster
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Hélène Pacquement
Hélène Pacquement
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Claire Galambrun
Claire Galambrun
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Laurence Brugières
Laurence Brugières
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Guy Leverger
Guy Leverger
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Ludovic Mansuy
Ludovic Mansuy
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Catherine Paillard
Catherine Paillard
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Anne Deville
Anne Deville
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Corinne Armari-Alla
Corinne Armari-Alla
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Anne Lutun
Anne Lutun
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Marion Gillibert-Yvert
Marion Gillibert-Yvert
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Jean-Louis Stephan
Jean-Louis Stephan
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Fleur Cohen-Aubart
Fleur Cohen-Aubart
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Julien Haroche
Julien Haroche
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Isabelle Pellier
Isabelle Pellier
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Frédéric Millot
Frédéric Millot
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Brigitte Lescoeur
Brigitte Lescoeur
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Virginie Gandemer
Virginie Gandemer
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Christine Bodemer
Christine Bodemer
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Roger Lacave
Roger Lacave
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Zofia Hélias-Rodzewicz
Zofia Hélias-Rodzewicz
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Valérie Taly
Valérie Taly
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Frédéric Geissmann
Frédéric Geissmann
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1,
Jean Donadieu
Jean Donadieu
1Sébastien Héritier, Mohamed-Aziz Barkaoui, Jean Miron, and Jean Donadieu, French Reference Center for Langerhans Cell Histiocytosis, Trousseau Hospital; Sébastien Héritier, Sabah Boudjemaa, Guy Leverger, and Jean Donadieu, Trousseau Hospital, Assistance Publique–Hôpitaux de Paris; Sylvie Fraitag, Despina Moshous, and Christine Bodemer, Necker Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur and Brigitte Lescoeur, Robert Debré Hospital, Assistance Publique–Hôpitaux de Paris; Michel Peuchmaur, Université Paris Diderot, Sorbonne Paris Cité; Hélène Pacquement, Institut Curie Medical Center; Guy Leverger, Université Pierre et Marie Curie; Fleur Cohen-Aubart and Julien Haroche, Pitié-Salpêtrière Hospital, Assistance Publique–Hôpitaux de Paris; Roger Lacave, Tenon Hospital, Assistance Publique-Hôpitaux de Paris; Valérie Taly, Institut National de la Santé et de la Recherche Médicale, Unités Mixte de Recherche S1147, Centre National de la Recherche Scientifique SNC 5014, Université Paris Sorbonne Cité, Paris; Sébastien Héritier, Jean-François Emile, Zofia Hélias-Rodzewicz, and Jean Donadieu, Université de Versailles Saint-Quentin-en-Yvelines, Université Paris-Saclay; Jean-François Emile, Ambroise Paré Hospital, Assistance Publique–Hôpitaux de Paris, Boulogne-Billancourt; Caroline Thomas and Anne Moreau, Centre Hospitalo-Universitaire de Nantes, Nantes; Florence Renaud, Centre Hospitalier Régional Universitaire, Université de Lille; Anne Lambilliotte and Françoise Mazingue, Centre Hospitalo-Universitaire de Lille, Lille; Catherine Chassagne-Clément, Centre Léon Bérard; Frédérique Dijoud, Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon; Kamila Kebaili, Institut d'Hémato-Oncologie Pediatrique, Lyon; Valérie Rigau, Gui de Chauliac Hospital; Eric Jeziorski, Hôpital Arnaud de Villeneuve, Montpellier; Geneviève Plat, Centre Hospitalo-Universitaire de Toulouse, Toulouse; Nathalie Aladjidi, Centre Hospitalo-Universitaire de Bordeaux, Bordeaux; Claire Galambrun, Hôpital de la Timone, Marseille; Laurence Brugières, Institut Gustave Roussy, Villejuif; Ludovic Mansuy, Brabois-Enfants Hospital, Centre Hospitalo-Universitaire de Nancy, Vandœuvre-lès-Nancy; Catherine Paillard, Centre Hospitalo-Universitaire de Strasbourg, Strasbourg; Anne Deville, Centre Hospitalo-Universitaire de Nice, Nice; Corinne Armari-Alla, Centre Hospitalo-Universitaire de Grenoble, Grenoble; Anne Lutun, Centre Hospitalo-Universitaire d’Amiens, Amiens; Marion Gillibert-Yvert, Centre Hospitalo-Universitaire de Tours, Tours; Jean-Louis Stephan, Centre Hospitalo-Universitaire de Saint Etienne, Saint Etienne; Isabelle Pellier, Centre Hospitalo-Universitaire de Angers, Angers; Frédéric Millot, Centre Hospitalo-Universitaire de Poitiers, Poitiers; Virginie Gandemer, Centre Hospitalo-Universitaire de Rennes, Rennes, France; Alina Ferster, Hôpital Universitaire des Enfants Reine Fabiola, Université Libre de Bruxelles, Brussels, Belgium; and Frédéric Geissmann, Memorial Sloan Kettering Cancer Center, New York, NY.
1