Abstract
Purpose
We assessed the associations between the 21-gene recurrence score assay (RS) receipt, subsequent chemotherapy use, and medical expenditures among patients with early-stage breast cancer.
Patients and Methods
Data from the Pennsylvania Cancer Registry were used to assemble a retrospective cohort of women with early-stage breast cancer from 2007 to 2010 who underwent initial surgical treatment. These data were merged with administrative claims from the 12-month periods before and after diagnosis to identify comorbidities, treatments, and expenditures (n = 7,287). Propensity score–weighted regression models were estimated to identify the effects of RS receipt on chemotherapy use and medical spending in the year after diagnosis.
Results
The associations between RS receipt and outcomes varied markedly by patient age. RS use was associated with lower chemotherapy use among women younger than 55 (19.2% lower; 95% CI, 10.6 to 27.9). RS use was associated with higher chemotherapy use among women 75 to 84 years old (5.7% higher; 95% CI, 0.4 to 11.0). RS receipt was associated with lower adjusted 1-year medical spending among women younger than 55 ($15,333 lower; 95% CI, $2,841 to $27,824) and with higher spending among women who were 75 to 84 years old ($3,489 higher; 95% CI, $857 to $6,122).
Conclusion
RS receipt was associated with reduced use of adjuvant chemotherapy and lower health care spending among women with breast cancer who were younger than 55. Conversely, among women 75 and older, RS testing was associated with a modest increase in chemotherapy use and slightly higher spending. From a population perspective, the impact of RS testing on breast cancer treatment and health care costs is much greater in younger women.
INTRODUCTION
Adjuvant chemotherapy is used in selected patients with early-stage breast cancer after initial surgical therapy to reduce the risk of cancer recurrence and to improve long-term survival. Historically, this decision has been based on relatively crude, population-based clinical and histopathologic predictors of cancer recurrence risk. However, adjuvant chemotherapy carries the risk of drug-associated toxicity, and, because most women with localized breast cancer will never have recurrent disease,1-4 they would receive no benefit from adjuvant chemotherapy despite being exposed to the toxicity risk. Therefore, a more precise “personalized” cancer recurrence prediction better informs the chemotherapy decision, both by reducing the use of chemotherapy in patients at low risk of recurrence and by identifying patients at high risk of recurrence who would clearly benefit from chemotherapy.
The 21-gene recurrence score assay (RS; Oncotype DX; Genomic Health, Redwood City, CA) was developed in 2004 to predict the risk of cancer recurrence in women with early-stage breast cancer.5-9 Continuous recurrence scores generated by the test are categorized into low, intermediate, and high risk of recurrence. A validation study determined that the marginal benefit of chemotherapy was minimal among women with low RS, indeterminate among women with intermediate RS, and high among women with a high RS.10 In 2007, the American Society of Clinical Oncology recommended RS testing in women with estrogen receptor/progesterone receptor–positive, node-negative breast cancers to identify appropriate chemotherapy candidates.11 RS use was also recommended in the National Comprehensive Cancer Network and St. Gallen practice guidelines.12
The impact of RS on health care spending is uncertain. Several economic analyses have modeled the cost consequences of testing.13-16 Most predicted RS would be either cost saving or modestly cost increasing and would improve population-based outcomes by reducing the number of patients experiencing adverse effects of chemotherapy. However, it is unknown whether these anticipated costs and benefits have been realized in practice. Therefore, the goal of this research was to assess the associations between receipt of RS testing and subsequent chemotherapy use and medical expenditures in a population-based cohort of patients with early-stage breast cancer during years when RS testing became widespread (2007 to 2010). Because adjuvant chemotherapy use is strongly correlated with patients’ age,17-19 we also investigated whether the association between RS testing and chemotherapy use varied with age.
PATIENTS AND METHODS
Patients
We used the Pennsylvania Cancer Registry (PCR), a medical provider–mandated database of cancer cases in Pennsylvania, to identify 51,385 Pennsylvania residents with breast cancer diagnosed between January 1, 2007, and December 31, 2010. We selected patients who had T-stage I to IV, node-negative, nonmetastatic, estrogen receptor/progesterone receptor–positive breast cancer. We excluded patients with more than one contemporaneously reported tumor (n = 5,620) or who had noninvasive cancer (n = 9,126). We also excluded patients who lacked clinical pathology data confirming the cancer diagnosis (n = 210), died before definitive diagnosis (n = 372), lacked cancer histology information (n = 776), had metastatic, node-positive, or node-status-unknown disease (n = 13,026), or were male (n = 184). There were 22,071 patients meeting the initial inclusion criteria.
Using Social Security numbers, we linked PCR records to administrative claims from fee-for-service Medicare, which comprised approximately two-thirds of Pennsylvania’s Medicare beneficiaries during 2007 to 2010,20 as well as to claims from Independence Blue Cross (Independence), one of Pennsylvania’s largest commercial health insurers.21 Both payers covered RS testing fully during the study period. To ensure a complete set of cost and outcomes data, we included only the 7,687 patients who had had continuous enrollment in either Medicare or Independence for at least 365 days before and after diagnosis, and who had made a claim for breast surgery within the postdiagnosis 1-year period. We excluded patients whose total medical spending was zero (n = 22), because this suggested the primary insurance coverage was provided by another insurer. Patients who did not have at least 365 days of continuous enrollment in either Medicare or Independence prior to diagnosis were also excluded (n=378). The final study cohort consisted of 7,287 patients with breast cancer (ie, 33% of the 22,071 Pennsylvanians meeting the initial selection criteria).
Predictor Variables
Patients’ demographic information and detailed clinical information (eg, cancer stage, tumor size, and so forth) were obtained from the PCR. Clinical comorbidities were abstracted from administrative claims in the year before breast cancer diagnosis using the Elixhauser classification system.22 To control for each patient’s baseline level of health care spending, prediagnosis health care costs were obtained from payment amounts as recorded on claims in the year before cancer diagnosis. Receipt of RS testing was identified in claims via Current Procedural Terminology codes and/or the National Provider Identifier of the RS manufacturer, which billed Medicare directly for the testing.
The University of Pennsylvania’s Institutional Review Board approved the study.
Chemotherapy Use and Total Spending
Receipt of chemotherapy within 1 year of diagnosis was determined from each patient’s claims via Healthcare Common Procedure Coding System codes indicating chemotherapy, using a previously validated search algorithm.23 Total 1-year medical spending was measured from the all-payer perspective, including insurer payments as well as copayments and deductibles assigned to patients from the date of cancer diagnosis through 1 year after diagnosis. Expenditures for chemotherapy drugs and their administration were calculated based on the drugs identified in the administrative claims after a classification algorithm devised by Hassett et al.23 Because we did not have access to prescription drug data, supportive care costs (eg, antiemetics or colony-stimulating factors dispensed by outpatient pharmacies, and so forth) were excluded from our cost estimates. Costs were inflated to 2010 dollars using the Gross Domestic Product price index.24
Statistical Analysis
Patients’ baseline characteristics were compared by RS receipt using t tests for continuous variables and χ2 tests for categorical variables. To control for observed confounders, we used a two-stage propensity score framework. First, we estimated the predicted probability that each patient received RS from a logistic regression model that controlled for age, insurer, tumor stage/grade/size, year of diagnosis, patient minority status, total spending in the year before diagnosis, comorbidity burden in the year before diagnosis, and the patient’s geographic region25 (ie, nine clusters of contiguous counties defined by the Pennsylvania Department of Health).
Subsequently, we modeled the associations between RS receipt and both chemotherapy receipt and medical spending with models weighted by the inverse probability of treatment derived from the propensity score.26 Because we anticipated that age would modify the effect of RS on costs and chemotherapy use because of the strong a priori association between age and chemotherapy use,17-19 our model specification included a binary indicator variable for RS receipt, indicators for the patient’s age category, and interactions between age and RS receipt. We modeled the association between RS receipt and chemotherapy receipt using logistic regression. To facilitate interpretation, we converted the results to predictive margins on the zero-to-one probability scale; this well-validated method produces more clinically interpretable results as compared with odds ratios.27 Average marginal effects of RS receipt were computed as arithmetic differences between predictive margins within each age category. SEs were calculated using the delta method and Wald tests were used to assess statistical significance.
Total 1-year medical spending and non-chemotherapy-related spending were modeled using generalized linear models with a log link. For all spending models, we ascertained the best-fitting error distribution as the γ distribution by means of the modified Park test,28 and we reported results on the dollar scale. Because a high proportion of patients did not receive chemotherapy, we modeled chemotherapy spending using a two-part model.29 The first part was a logistic regression predicting any chemotherapy spending, and the second part was a log-γ generalized linear model of non-zero chemotherapy spending. To enhance interpretation of the interaction between age and RS receipt, we then re-estimated the propensity score–weighted models of chemotherapy receipt and total medical spending, with age entering the model as a quadratic term rather than as a categorical variable. This model’s results generated a graphic display of average marginal effects of RS receipt at each decade of age.
All statistical analyses were performed using STATA 13.1 (STATA, College Station, TX).
RESULTS
Cohort Characteristics
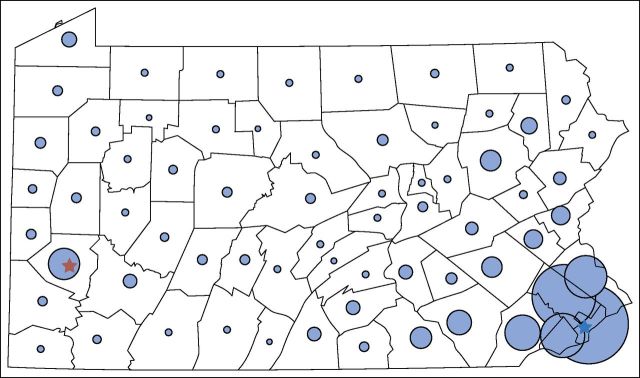
Of the 7,287 patients in the final analytic sample, 74% were insured by fee-for-service Medicare and 26% by Independence (Table 1). The mean age of the cohort was 71.6 years; 81% of the cohort was white non-Hispanic; and 45% of patients were residents of Philadelphia or its surrounding counties (Fig 1). The proportion of patients undergoing RS testing increased from 16% among patients diagnosed in 2007 to 28% of patients in 2010 (P < .001 for the difference). Relative to patients not receiving RS testing, patients receiving RS testing were younger, were more likely to have Independence coverage, had fewer comorbidities, and were more likely to have stage I cancer.
Table 1.
Patient Characteristics
| Variable Name | Overall (N = 7,287) | RS Testing | P | |
|---|---|---|---|---|
| No (n = 5,678) | Yes (n = 1,609) | |||
| Age at diagnosis, mean (SD), years | 71.6 (11.6) | 73.4 (11.2) | 65.3 (10.8) | < .001 |
| Age category, years | ||||
| < 55 | 733 (10) | 447 (7.9) | 286 (18) | < .001 |
| 55-64 | 762 (10) | 445 (7.8) | 317 (20) | |
| 65-74 | 2,526 (35) | 1,831 (32) | 695 (43) | |
| 75-84 | 2,479 (34) | 2,179 (38) | 300 (19) | |
| ≥ 85 | 787 (11) | 776 (14) | 11 (0.7) | |
| Race/ethnicity | ||||
| White, non-Hispanic | 5,896 (81) | 4,572 (81) | 1,324 (82) | .22 |
| Nonwhite or Hispanic | 612 (8.4) | 481 (8.5) | 131 (8.1) | |
| Race/ethnicity missing | 779 (11) | 625 (11) | 154 (9.6) | |
| Payer | ||||
| Medicare | 5,407 (74) | 4,453 (78) | 954 (59) | < .001 |
| Independence | 1,880 (26) | 1,225 (22) | 655 (41) | |
| Cancer stage | ||||
| I | 5,542 (76) | 4,280 (75) | 1,262 (78) | < .001 |
| II | 1,550 (21) | 1,224 (22) | 326 (20) | |
| III | 87 (1.2) | 83 (1.5) | 4 (0.2) | |
| Missing | 108 (1.5) | 91 (1.6) | 17 (1.1) | |
| Tumor grade | ||||
| 1 | 2,018 (28) | 1,622 (29) | 396 (25) | < .001 |
| 2 | 3,190 (44) | 2,331 (41) | 859 (53) | |
| 3 | 1,628 (22) | 1,350 (24) | 278 (17) | |
| 4 | 41 (0.6) | 39 (0.7) | 2 (0.1) | |
| Missing | 410 (5.6) | 336 (5.9) | 74 (4.6) | |
| Tumor size, cm | ||||
| < 1 | 2,261 (31) | 1,904 (34) | 357 (22) | < .001 |
| 1-2 | 3,919 (54) | 2,813 (50) | 1,106 (69) | |
| > 2 | 797 (11) | 680 (12) | 117 (7.3) | |
| Missing | 310 (4.3) | 281 (4.9) | 29 (1.8) | |
| Diagnosis year | ||||
| 2007 | 1,876 (26) | 1,573 (28) | 303 (19) | < .001 |
| 2008 | 1,831 (25) | 1,421 (25) | 410 (25) | |
| 2009 | 1,819 (25) | 1,408 (25) | 411 (26) | |
| 2010 | 1,761 (24) | 1,276 (22) | 485 (30) | |
| Prior year health care spending, $ | ||||
| Mean (SD) | 6,028 (12,400) | 6,261 (12,993) | 5,205 (9,993) | .003 |
| Median (IQR) | 2,244 (4632) | 2,288 (4,784) | 2,144 (4,192) | .25 |
| Elixhauser22 comorbidity count | ||||
| 0 | 1,388 (19) | 989 (17) | 399 (25) | < .001 |
| 1-3 | 4,087 (56) | 3,157 (56) | 930 (58) | |
| ≥ 4 | 1,812 (25) | 1,532 (27) | 280 (17) | |
NOTE. Data are presented as No. (% of the column’s total patients) unless otherwise specified.
Abbreviations: RS, 21-gene recurrence score assay; IQR, interquartile range.
Fig 1.
Number of patients in study cohort, by Pennsylvania county of residence. Pennsylvania county borders are indicated in black. The area of the blue circles indicates the relative numbers of patients in the study cohort from each county. The stars indicate the locations of Pennsylvania’s two largest cities: Pittsburgh (red) and Philadelphia (blue).
RS Testing and Age
The proportion of patients with breast cancer in our cohort who were undergoing RS testing declined with advancing age. Thirty-nine percent of women younger than 55 underwent RS testing, as did 42% of women 55 to 64. However, the percentage of women 65 to 74 undergoing RS testing was lower (28%), whereas only 12% of women 75 to 84 underwent RS testing, and just 1.4% of women 85 and older underwent RS testing (P < .001 for the difference across ages).
RS Testing, Age, and Chemotherapy Use
The association between RS testing and chemotherapy use varied markedly by age (Table 2). After adjustment, RS testing was associated with lower chemotherapy use among women younger than 55 (41.5% v 60.8%; difference, 19.2% ; 95% CI, 10.6 to 27.9). However, RS testing was associated with higher chemotherapy use among women 75 to 84 (14.4% v 8.7%; difference, 5.7% ; 95% CI, 0.4 to 11.0).
Table 2.
Adjusted Percentage of Patients Receiving Chemotherapy, by Age and RS Testing Receipt Status
| Age Category, years | Received RS Testing | Percentage of Subgroup Receiving Chemotherapy | Percentage Point Difference in Chemotherapy Receipt Between RS Recipients and Nonrecipients (95% CI) | P |
|---|---|---|---|---|
| < 55 | Yes (n = 286) | 41.5 | −19.2 (−27.9 to −10.6) | < .001 |
| No (n = 447) | 60.8 | |||
| 55-64 | Yes (n = 317) | 35.0 | −5.9 (−13.6 to 1.8) | .13 |
| No (n = 445) | 41.0 | |||
| 65-74 | Yes (n = 695) | 21.3 | 1.1 (−3.0 to 5.3) | .59 |
| No (n = 1,831) | 20.2 | |||
| 75-84 | Yes (n = 300) | 14.4 | 5.7 (0.4 to 11.0) | .036 |
| No (n = 2,179) | 8.7 | |||
| ≥ 85 | Yes (n = 11) | 0.0 | −2.3 (−3.4 to −1.3) | < .001 |
| No (n = 776) | 2.3 |
Abbreviation: RS, 21-gene recurrence score assay.
The adjusted probability of RS-tested women who received adjuvant chemotherapy also decreased with age. The risk-adjusted probability of an RS-tested woman younger than age 55 years proceeding to adjuvant chemotherapy was 41.5%. This proportion declined to just 14.4% among RS-tested women 75 to 84, and further declined to 0% among the 11 women 85 and older who underwent RS testing.
Medical Spending
The association between RS testing and spending also varied by age (Table 3). Testing was associated with lower total adjusted 1-year medical spending among women younger than 55 years old ($81,334 v $96,667; difference, −$15,333; 95% CI, −$27,824 to −$2,841) and with higher total spending among women who were 75 to 84 years old ($33,209 v $29,720; difference, $3,489; 95% CI, $857 to $6,122). Similar to the observed patterns of chemotherapy use by patient age, adjusted 1-year chemotherapy spending was also lower among women who underwent the RS test, with marked variations by age. Chemotherapy costs were lower among RS-tested women younger than 55 ($16,101 v $37,497; difference, −$21,396; 95% CI, −$30,701 to −$12,091), RS-tested women 55 to 64 ($10,535 v $20,210; difference, −$9,675; 95% CI, −$15,450 to −$3,601), and RS-tested women 65 to 74 ($3,905 v $6,076; difference, −$2,171; 95% CI, −$3,542 to −$800). However, there was no statistically significant difference in chemotherapy costs between RS-tested and non-RS–tested women older than 75.
Table 3.
Adjusted Mean 1-Year Health Care Spending after Breast Cancer Diagnosis, by Age and RS Testing Receipt Status
| Age, years | Received RS Testing | Total Spending | Chemotherapy Spending | Nonchemotherapy Spending | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean 1-Year Spending ($) | Difference Between RS Recipients and Nonrecipients (95% CI) | P | Mean 1-Year Spending ($) | Difference Between RS Recipients and Nonrecipients (95% CI) | P | Mean 1-Year Spending ($) | Difference Between RS Recipients and Nonrecipients (95% CI) | P | ||
| <55 | Yes (n = 286) | 81,334 | −15,333 | .002 | 16,101 | −21,396 (−30,701 to −12,091) | < .001 | 65,233 | 6,063 | .04 |
| No (n = 447) | 96,667 | (−27,824 to −2,841) | 37,497 | 59,170 | (305 to 11,821) | |||||
| 55-64 | Yes (n = 317) | 72,785 | −1,733 | .70 | 10,535 | −9,675 | .001 | 62,251 | 7,943 | .006 |
| No (n = 445) | 74,518 | (−10,561 to 7,095) | 20,210 | (−15,450 to −3,901) | 54,308 | (2,225 to 13,660) | ||||
| 65-74 | Yes (n = 695) | 39,090 | 2,442 | .07 | 3,905 | −2,171 | .002 | 35,184 | 4,613 | < .001 |
| No (n = 1,831) | 36,647 | (−233 to 5,118) | 6,076 | (−3,542 to −800) | 30,571 | (2,514 to 6,713) | ||||
| 75-84 | Yes (n = 300) | 33,209 | 3,489 | .009 | 2,093 | 206 | .78 | 31,116 | 3,283 | .001 |
| No (n = 2,179) | 29,720 | (857, 6,122) | 1,887 | (−1,238 to 1,650) | 27,832 | (1,275 to 5,292) | ||||
| ≥85 | Yes (n = 11) | 32,402 | 9,788 | .06 | 54 | −132 | .07 | 32,402 | 9,974 | .054 |
| No (n = 776) | 22,614 | (−375 to 19,950) | 186 | (−273 to 9) | 22,428 | (−185 to 20,132) | ||||
NOTE. Data reported are 2010 dollars.
Abbreviation: RS, 21-gene recurrence score assay.
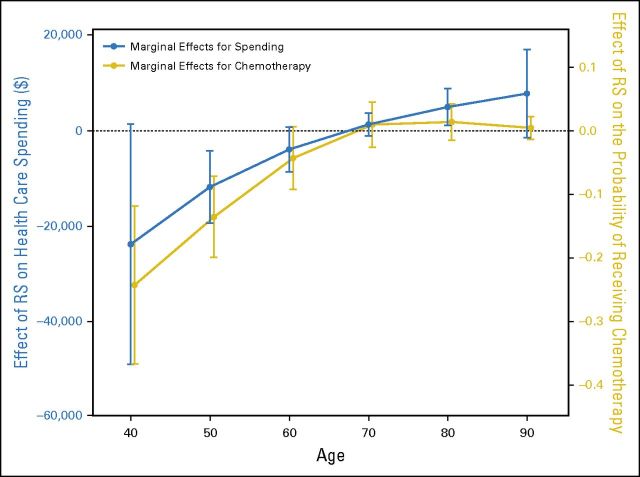
Replacing the age categories in our primary analytic model with age modeled as a quadratic term enabled calculations of the marginal probability of chemotherapy use and the marginal health care cost related to RS testing along the full age continuum. As demonstrated graphically (Fig 2), reduced chemotherapy use and concomitant health care cost savings related to RS testing were restricted to patients younger than 65.
Fig 2.
Incremental effect of RS receipt on probability of chemotherapy receipt and incremental effect of RS receipt on total spending. The x-axis indicates patient age. The gold dots and connecting lines represent the difference in probability (0 to 1 scale) of chemotherapy use among RS recipients, with probability change plotted on the right-side y-axis. The blue dots and connecting lines represent the change in total health care costs (2010 $US) among RS recipients, with cost change plotted on the left-side y-axis. The vertical lines indicate 95% CIs for the point estimates. Note that the blue and gold plots are “jittered” slightly along the x-axis to avoid overlap. RS, 21-gene recurrence score assay.
DISCUSSION
In this cohort of Pennsylvania patients with breast cancer treated between 2007 and 2010, we found that, among patients younger than 65, RS testing was associated with lower use of adjuvant chemotherapy as well as lower overall medical expenditures and lower chemotherapy spending in the year after diagnosis. These lower medical costs included the cost of RS testing itself (Medicare’s 2010 reimbursement for RS was $3,416); thus, there was strong evidence that the RS test was, on average, cost saving in younger patients. However, among patients 75 to 84, RS testing was associated with increased use of adjuvant chemotherapy and increased overall costs.
There are several possible explanations for the strong modifying effect of age on the relationships among RS testing, chemotherapy use, and costs. A fundamental goal in the development and clinical implementation of “personalized” genomic testing is to reduce the use of unnecessary treatment in patients who are unlikely to benefit. This concept is consistent with our finding that, among younger patients, RS testing was associated with a reduction of adjuvant chemotherapy use, lower total medical spending, and lower chemotherapy spending. We conclude that there is strong evidence that RS testing is meeting this important therapeutic goal among younger women.
However, our findings in older patients likely reflect a more complicated phenomenon with important ramifications for the economic impact of high-cost genomic tests. Compared with younger patients, older patients are less likely to have tumors with high RS scores10 and are more likely to have chronic medical conditions, frailty, and so on, that would complicate the receipt of chemotherapy. Hence, RS testing may be commonly used among younger patients to “rule out” patients for chemotherapy, but conversely, it may be used among older patients to “rule in” the use of chemotherapy in patients who would otherwise be considered borderline treatment candidates but who had high-risk RS results. In the latter scenario, neither a reduction in chemotherapy use nor a decrease in health care costs would be expected (on average) from the use of RS testing. Hence, at the population level, the impact of RS testing on breast cancer treatment and costs seems to be influenced greatly by patient age.
Limitations
Our results should be interpreted in the context of several limitations. First, because our data did not include results of RS testing, we could not discern whether RS recipients were treated with chemotherapy in a manner consistent with the test results. Second, we excluded patients with node-positive breast cancer because the clinical trials reporting the usefulness of RS testing in these patients were not published until midway through our study period.30 Third, our cohort undoubtedly included patients who were human epidermal growth factor receptor 2 (HER2 neu) positive, because the PCR did not begin collecting data on HER2 neu status until 2010. Adjuvant chemotherapy is recommended for women with HER2 neu positive breast cancer regardless of RS results,31 and younger women have a higher incidence of HER2 neu positive breast cancer.32 Hence, some of the cost differences between RS-tested and non–RS-tested younger women may be a result of the higher costs in HER2 neu–positive patients who did not undergo RS testing and were treated with high-cost (ie, trastuzumab-based) adjuvant chemotherapy. However, the age variation in HER2 neu prevalence is not large enough to explain our findings to entirely.33 Fourth, our data did not include outpatient prescription drug claims, and therefore, we could not measure supportive care costs derived from high-cost pharmaceuticals dispensed by outpatient pharmacies. Therefore, the chemotherapy-related cost differences we reported may underestimate the true cost differences among groups with differing rates of chemotherapy receipt. Finally, contemporary clinical use of RS testing may differ from use in the period of 2007 to 2010, although marked changes in practice patterns are unlikely.
The follow-up period (median, 3.9 years from diagnosis) was relatively short. Although it is possible that increases in the fraction of high-risk patients treated with adjuvant chemotherapy may have produced a survival benefit, it was not observable in this short time period. Longer follow-up times would be necessary to determine whether RS testing resulted in improved survival.
We do not know which unmeasured patient and physician characteristics may have motivated the decision to pursue RS testing or the use of adjuvant chemotherapy, particularly in elderly patients. We observed that only 11 of the 787 patients older than 84 received RS testing, and none of these 11 patients received chemotherapy. Because chemotherapy was used rarely (ie, < 3%) in the “oldest old,” it is unclear if RS testing in these patients influenced clinical decision-making meaningfully.
Because the majority of elderly patients in our cohort were covered by Medicare, whereas most of the younger patients were insured by Independence, it is possible that the age effect that we observed was confounded by insurance type or other (unmeasured) factors. However, our sample included 344 Medicare beneficiaries under 65, as well as 690 Independence patients older than 65, so our statistical models retained substantial ability to control for confounding by insurance type.
Strengths and Contributions
Our cohort was a large, diverse, population-based sample of patients undergoing breast cancer treatment in multiple nonexperimental clinical settings, including patients covered by Medicare as well as privately insured patients. We combined clinically detailed cancer registry data with administrative claims data to examine treatment trends across a wide age range of patients, in contrast to studies involving SEER/Medicare data that are generally restricted to patients of at least 65 years of age.
Published cost models for RS testing have hinged on whether the cost of genomic testing outweighed the savings from reduced chemotherapy use. Unsurprisingly, models derived from health systems with low chemotherapy costs found RS testing to be cost increasing,34-36 whereas studies that assumed higher chemotherapy costs found the RS test to be cost saving.37,38 Among our study’s subcohort of younger women treated in Pennsylvania, RS receipt was related to substantially lower medical spending, thus supporting the predictions of models in which chemotherapy was high cost.
Policy Implications
Our findings have important policy implications for genomic testing. It is encouraging that RS testing was associated with substantial reductions in the use of chemotherapy and medical spending among a population of younger women in real-world practice settings, because this implies that RS testing reduced unnecessary treatment—a critical goal of personalized medicine. However, among the elderly, RS testing may be less likely to rule out potential candidates for chemotherapy, but more likely to provide impetus to use chemotherapy among patients with comorbidities. This scenario is consistent with our finding that—at the population level—RS had minimal influence on overall chemotherapy rates among elderly women. Because the clinical benefit of RS testing is less clear in any patient for whom chemotherapy would be inappropriate, our results highlight the importance of careful selection of older candidates for high-cost testing.
Summary
Among women under 65 with nonmetastatic breast cancer, RS testing was associated with a lower likelihood of chemotherapy and lower medical spending in the year after cancer diagnosis. However, among women 65 or older, RS testing was associated with minimal differences in chemotherapy rates and higher medical spending. From a population perspective, RS testing has a much greater effect on clinical practice patterns and health care costs among younger women.
Footnotes
Supported by a research grant from the Commonwealth of Pennsylvania–Department of Health (Grant No. 4100059202).
The Pennsylvania Department of Health had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org
AUTHOR CONTRIBUTIONS
Conception and design: Andrew J. Epstein, Yu-Ning Wong, Nandita Mitra, Anil Vachani, Katrina Armstrong, Peter W. Groeneveld
Collection and assembly of data: Andrew J. Epstein, Sakhena Hin, Lin Yang, Peter W. Groeneveld
Data analysis and interpretation: Andrew J. Epstein, Yu-Ning Wong, Nandita Mitra, Anil Vachani, Lin Yang, Aaron Smith-McLallen, Peter W. Groeneveld
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Chemotherapy Use and Health Care Costs After Introduction of Genomic Testing in Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Andrew J. Epstein
Consulting or Advisory Role: Medicus Economics
Yu-Ning Wong
Research Funding: Pfizer, Millennium Pharmaceuticals
Travel, Accommodations, Expenses: Tokai
Nandita Mitra
No relationship to disclose
Anil Vachani
Consulting or Advisory Role: Allegro Diagnostics
Research Funding: Allegro Diagnostics, Integrated Diagnostics, Janssen Pharmaceuticals (a Johnson & Johnson co.)
Sakhena Hin
No relationship to disclose
Lin Yang
No relationship to disclose
Aaron Smith-McLallen
Employment: Independence Blue Cross
Katrina Armstrong
Leadership: GlaxoSmithKline
Peter W. Groeneveld
No relationship to disclose
REFERENCES
- 1.Peto R, Davies C, Godwin J, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Jeong JH, Bryant J, et al. National Surgical Adjuvant Breast and Bowel Project randomised clinical trials Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: Long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 4.Azim HA, Jr, de Azambuja E, Colozza M, et al. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011;22:1939–1947. doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen MT, Stessin A, Nagar H, et al. Impact of oncotype DX recurrence score in the management of breast cancer cases. Clin Breast Cancer. 2014;14:182–190. doi: 10.1016/j.clbc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Lillie SE, Brewer NT, O’Neill SC, et al. Retention and use of breast cancer recurrence risk information from genomic tests: the role of health literacy. Cancer Epidemiol Biomarkers Prev. 2007;16:249–255. doi: 10.1158/1055-9965.EPI-06-0525. [DOI] [PubMed] [Google Scholar]
- 7.Baker J. Genomic Health, Inc. Pharmacogenomics. 2007;8:397–399. doi: 10.2217/14622416.8.4.397. [DOI] [PubMed] [Google Scholar]
- 8.Zanotti L, Bottini A, Rossi C, et al. Diagnostic tests based on gene expression profile in breast cancer: from background to clinical use. Tumour Biol. 2014;35:8461–8470. doi: 10.1007/s13277-014-2366-2. [DOI] [PubMed] [Google Scholar]
- 9.Rouzier R, Pronzato P, Chéreau E, et al. Multigene assays and molecular markers in breast cancer: systematic review of health economic analyses. Breast Cancer Res Treat. 2013;139:621–637. doi: 10.1007/s10549-013-2559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 11.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 12.Untch M, Gerber B, Harbeck N, et al. 13th St. Gallen International Breast Cancer Conference 2013: Primary therapy of early breast cancer evidence, controversies, consensus - opinion of a German team of experts (Zurich 2013) Breast Care (Basel) 2013;8:221–229. doi: 10.1159/000351692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall PS, McCabe C, Stein RC, et al. Economic evaluation of genomic test-directed chemotherapy for early-stage lymph node-positive breast cancer. J Natl Cancer Inst. 2012;104:56–66. doi: 10.1093/jnci/djr484. [DOI] [PubMed] [Google Scholar]
- 14.Lamond NW, Skedgel C, Rayson D, et al. Cost-utility of the 21-gene recurrence score assay in node-negative and node-positive breast cancer. Breast Cancer Res Treat. 2012;133:1115–1123. doi: 10.1007/s10549-012-1989-5. [DOI] [PubMed] [Google Scholar]
- 15.Yang M, Rajan S, Issa AM. Cost effectiveness of gene expression profiling for early stage breast cancer: A decision-analytic model. Cancer. 2012;118:5163–5170. doi: 10.1002/cncr.27443. [DOI] [PubMed] [Google Scholar]
- 16.Lamond NW, Skedgel C, Younis T. Is the 21-gene recurrence score a cost-effective assay in endocrine-sensitive node-negative breast cancer? Expert Rev Pharmacoecon Outcomes Res. 2013;13:243–250. doi: 10.1586/erp.13.4. [DOI] [PubMed] [Google Scholar]
- 17.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) Lancet Oncol. 2012;13:e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Faul LA, Luta G, et al. Patient and physician decision styles and breast cancer chemotherapy use in older women: Cancer and Leukemia Group B protocol 369901. J Clin Oncol. 2012;30:2609–2614. doi: 10.1200/JCO.2011.40.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelblatt JS, Sheppard VB, Hurria A, et al. Cancer Leukemia Group B Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28:3146–3153. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry J. Kaiser Family Foundation Medicare advantage enrollees as a percent of total Medicare population, 2014. http://kff.org/other/state-indicator/market-share-and-enrollment-of-largest-three-insurers-large-group-market/
- 21.Henry J. Kaiser Family Foundation Market Share and enrollment of largest three insurers- large group market, 2012. http://kff.org/other/state-indicator/market-share-and-enrollment-of-largest-three-insurers-large-group-market/
- 22.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hassett MJ, O’Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst. 2006;98:1108–1117. doi: 10.1093/jnci/djj305. [DOI] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Research and Quality Medical Expenditure Panel survey. http://meps.ahrq.gov/about_meps/Price_Index.shtml.
- 25.Pennsylvania Health Care Cost Containment Council Counties by region. http://www.phc4.org/dept/dc/state.htm.
- 26.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 27.Kleinman LC, Norton EC. What’s the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44:288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 29.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23:525–542. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Albain KS, Barlow WE, Shak S, et al. Breast Cancer Intergroup of North America Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slamon D, Eiermann W, Robert N, et al. Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 33.Pritchard KI, Shepherd LE, O’Malley FP, et al. National Cancer Institute of Canada Clinical Trials Group HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 34.Holt S, Bertelli G, Humphreys I, et al. A decision impact, decision conflict and economic assessment of routine Oncotype DX testing of 146 women with node-negative or pNImi, ER-positive breast cancer in the U.K. Br J Cancer. 2013;108:2250–2258. doi: 10.1038/bjc.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klang SH, Hammerman A, Liebermann N, et al. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health. 2010;13:381–387. doi: 10.1111/j.1524-4733.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 36.Kondo M, Hoshi SL, Yamanaka T, et al. Economic evaluation of the 21-gene signature (Oncotype DX) in lymph node-negative/positive, hormone receptor-positive early-stage breast cancer based on Japanese validation study (JBCRG-TR03) Breast Cancer Res Treat. 2011;127:739–749. doi: 10.1007/s10549-010-1243-y. [DOI] [PubMed] [Google Scholar]
- 37.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 38.Tsoi DT, Inoue M, Kelly CM, et al. Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist. 2010;15:457–465. doi: 10.1634/theoncologist.2009-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]