Abstract
Purpose
Merkel cell carcinoma (MCC) is a relatively rare, potentially aggressive cutaneous malignancy. We examined the clinical and histologic features of primary MCC that may correlate with the probability of a positive sentinel lymph node (SLN).
Methods
Ninety-five patients with MCC who underwent SLN biopsy at the University of Michigan were identified. SLN biopsy was performed on 97 primary tumors, and an SLN was identified in 93 instances. These were reviewed for clinical and histologic features and associated SLN positivity. Univariate associations between these characteristics and a positive SLN were tested for by using either the χ2 or the Fisher's exact test. A backward elimination algorithm was used to help create a best multiple variable model to explain a positive SLN.
Results
SLN positivity was significantly associated with the clinical size of the lesion, greatest horizontal histologic dimension, tumor thickness, mitotic rate, and histologic growth pattern. Two competing multivariate models were generated to predict a positive SLN. The histologic growth pattern was present in both models and combined with either tumor thickness or mitotic rate.
Conclusion
Increasing clinical size, increasing tumor thickness, increasing mitotic rate, and infiltrative tumor growth pattern were significantly associated with a greater likelihood of a positive SLN. By using the growth pattern and tumor thickness model, no subgroup of patients was predicted to have a lower than 15% to 20% likelihood of a positive SLN. This suggests that all patients presenting with MCC without clinical evidence of regional lymph node disease should be considered for SLN biopsy.
INTRODUCTION
Merkel cell carcinoma (MCC) is a relatively rare, potentially aggressive, cutaneous malignancy with a high incidence of local recurrence and regional and distant metastasis. The majority of patients with MCC (70%) present with disease clinically localized to the skin (American Joint Committee on Cancer stage I or II), 25% present with palpable regional lymphadenopathy (stage III), and 5% with distant metastasis (stage IV).1–4 The most common location of metastasis is the draining lymph node basin (27% to 60%).1–2,5 Various clinical, histologic, and immunohistochemical factors have been considered as prognostic indicators, but the presence or absence of lymph node disease is currently the most consistent predictor of survival in patients with MCC.1–2,6–10 Although the optimal treatment of MCC has yet to be determined, sentinel lymph node (SLN) biopsy is emerging as an important staging tool to assess the regional lymph node basin and guide additional therapy.
SLN biopsy is a well-described and widely used technique for identifying subclinical metastasis to regional lymph nodes in patients with melanoma. Factors have been identified that predict the risk of having a positive SLN in patients with melanoma to better select which patients should undergo SLN biopsy.11–12 The clinical and histologic characteristics of primary MCC, however, have yet to be examined with regard to their ability to predict SLN positivity. Our objectives were to examine the clinical and histologic features of primary MCC that may correlate with the probability of a positive SLN. This information could then be used to potentially identify a group of patients at low risk of a positive SLN who could be spared the procedure and conversely to identify patients who would most benefit from the procedure to guide additional treatment decisions.
METHODS
Patients
This study was approved by the institutional review board at the University of Michigan. Our prospective MCC database was queried for patients who underwent SLN biopsy at the University of Michigan after evaluation in the multidisciplinary MCC clinic. The majority of patients (n = 88) were evaluated between July 2006 (opening of the clinic) and February 2010. Seven additional patients treated between 1999 and 2005 who underwent SLN biopsy and have continued follow-up in our clinic were included. Patients were categorized by the presence or absence of metastatic MCC in at least one SLN. Our SLN biopsy technique has been previously described.11 Histologic evaluation of the SLN(s) included serial sectioning, hematoxylin and eosin staining, and cytokeratin-20 and pancytokeratin (AE1/AE3/CAM5.2) immunostains.
Clinical variables evaluated included patient sex, age, presence or absence of immunosuppression (medication- or disease-induced immunosuppression), history of other skin cancer (yes/no), history of other nonskin cancer (yes/no), site of the primary MCC (categorized as head/neck, arm, leg/buttock, trunk), and the clinical size in centimeters (categorized as < 1, 1 to 2, > 2 cm). Histologic features evaluated were greatest horizontal dimension within a transversely serially sectioned specimen (measured in millimeters), tumor thickness (measured in millimeters from the granular layer of the epidermis to the deepest extent of tumor invasion [ie, Breslow depth]), anatomic level of invasion (ie, Clark level), number of mitoses per squared millimeter, tumor growth pattern (circumscribed or infiltrative), and presence or absence of ulceration and angiolymphatic invasion. Mitotic rate was determined by counting the number of dermal mitoses in 1 mm2, starting in the field with the most mitoses. Tumors with a circumscribed growth pattern demonstrated well circumscribed tumor nodules with pushing borders. An infiltrative growth pattern was characterized by strands, cords, trabeculae, and single cells of tumor infiltrating dermal collagen and/or soft tissue. Tumors displaying both patterns were classified as infiltrative. All primary lesions and SLN biopsy slides were reviewed by a dermatopathologist in the MCC program at the University of Michigan. An 11-point profile, including the histologic features listed in this Methods section, was reported for each primary MCC.10
Statistical Analysis
The event of interest was the presence of at least one positive SLN (PSLN). Possible univariate associations between the characteristics and a PSLN were tested for by using either the χ2 or the Fisher's exact test if data were sparse. Continuous characteristics were also analyzed categorically and by using the two-sample t test. For clinical characteristics found to be significantly associated with a PSLN, the magnitude of that association was calculated by the odds ratio (OR). Point estimates and 95% CIs were reported.
To derive a probabilistic model for the occurrence of at least one PSLN and to account for potential correlation and confounding between the clinical and histologic features, multivariate logistic regression was used. A backward elimination algorithm was used to help create a best multiple variable model to predict a PSLN. The model begins by including all characteristics and iteratively removes nonsignificant characteristics until only significant characteristics remain. However, SLN occurrences that were missing information for a particular characteristic were excluded from the sample on which the model was built when that characteristic was included. All analyses were performed by using SAS software (Version 9; SAS Institute, Cary, NC).
RESULTS
Ninety-five patients with clinically node-negative MCC who underwent SLN biopsy were identified. Two patients each had two primary MCCs draining to distinct lymph node basins; therefore, SLN biopsy was performed for 97 tumors. The procedure failed to identify a sentinel node in four instances. These were excluded, and the analyzable sample size was, therefore, 93 occurrences. Table 1 lists the distribution of clinical and histologic characteristics including the percentage of occurrences with at least one PSLN for each characteristic considered. Age at diagnosis ranged from 38 to 91 years (median, 73 years). Overall, at least one PSLN was identified in 42 occurrences (45.2%). When at least one PSLN was discovered, 29 occurrences (69%) had only one PSLN, seven occurrences (17%) had two PSLNs, and six occurrences (14%) had three PSLNs.
Table 1.
Clinical and Histologic Features and Associated SLN Positivity
| Characteristic | Total Occurrences |
|
|---|---|---|
| No. | % SLN positive | |
| Sex | ||
| Male | 50 | 50.0 |
| Female | 43 | 39.5 |
| Age quartiles, years | ||
| < 64 | 27 | 48.2 |
| 64-72 | 22 | 45.5 |
| 73-78 | 25 | 32.0 |
| ≥ 79 | 19 | 57.9 |
| Immunosuppressed | ||
| Yes | 11 | 36.4 |
| No | 82 | 46.3 |
| Other skin cancer | ||
| Yes | 39 | 43.6 |
| No | 54 | 46.3 |
| Other cancer | ||
| Yes | 22 | 31.8 |
| No | 71 | 49.3 |
| Primary tumor site | ||
| Head and neck | 39 | 46.2 |
| Arm | 22 | 36.4 |
| Leg and buttock | 19 | 52.6 |
| Trunk | 13 | 46.2 |
| Clinical size, cm* | ||
| < 1 | 42 | 23.8 |
| 1-2 | 29 | 58.6 |
| > 2 | 22 | 68.2 |
| Greatest horizontal dimension quartiles, mm* | ||
| ≤ 3.75 | 19 | 26.3 |
| 3.76-7.00 | 19 | 42.1 |
| 7.01-10.50 | 18 | 50.0 |
| > 10.50 | 17 | 70.6 |
| Unknown | 20 | 40.0 |
| Tumor thickness, mm* | ||
| ≤ 2.00 | 13 | 23.1 |
| 2.01-4.00 | 24 | 33.3 |
| 4.01-5.99 | 19 | 52.6 |
| ≥ 6.00 | 28 | 64.3 |
| Unknown | 9 | 33.3 |
| Clark level | ||
| II/III | 4 | 0 |
| IV | 37 | 43.2 |
| V | 43 | 53.5 |
| Unknown | 9 | 33.3 |
| Mitotic rate, per mm2* | ||
| < 10.0 | 11 | 36.4 |
| 10.0-30.0 | 33 | 36.4 |
| 30.1-50.0 | 17 | 64.7 |
| > 50 | 7 | 71.4 |
| Unknown | 25 | 40.0 |
| Growth pattern* | ||
| Circumscribed | 43 | 30.2 |
| Infiltrative | 42 | 61.9 |
| Unknown | 8 | 37.5 |
| Ulceration | ||
| Absent | 75 | 45.3 |
| Present | 9 | 55.6 |
| Unknown | 9 | 33.3 |
| Angiolymphatic invasion | ||
| Absent | 52 | 38.5 |
| Present | 38 | 52.6 |
| Unknown | 3 | 66.7 |
| Total SLN removed | ||
| 1 | 42 | 42.9 |
| 2 | 29 | 44.8 |
| ≥ 3 | 22 | 50.0 |
Abbreviation: SLN, sentinel lymph node.
P < .05
Univariate Analysis
Results from the univariate analyses of clinical and histologic characteristics with SLN positivity are summarized in Table 2. SLN positivity was significantly associated with the clinical size of the lesion, greatest horizontal histologic dimension, tumor thickness, mitotic rate, and growth pattern. Patients with lesions clinically greater than 2 cm in size were nearly seven times more likely to have a PSLN than patients with lesions less than 1 cm. Patients with lesions in which the greatest horizontal histologic dimension was in the fourth quartile (> 10.5 mm) were 6.7 times more likely to have a PSLN than patients with lesions in the first quartile (≤ 3.75 mm). Patients with histologically thick lesions, greater than 6 mm in depth, were six times more likely to have a PSLN than those with lesions 2 mm or less in tumor thickness. When considered as a continuous covariate, a 1-mm increase in tumor thickness was associated with a 24% increase in the odds of having a PSLN. When mitotic rate was considered as a continuous covariate, an increase of 1 mitosis/mm2 was associated with a 3% increase in the odds of having a PSLN. However, as noted in Table 1, mitotic rate information was missing for 25 lesions, or 27% of the data set, because of a modification in the MCC primary tumor profile during the study period. Finally, tumors with an infiltrative growth pattern were nearly four times more likely to have a PSLN when compared with tumors with a circumscribed growth pattern.
Table 2.
Univariate Associations of Significant Clinical Features With at Least One PSLN
| Characteristic | Univariate Analysis |
||
|---|---|---|---|
| Odds Ratio | 95% CI | P | |
| Clinical size, cm | |||
| < 1 | 1.0 | ||
| 1-2 | 4.5 | 1.6 to 12.6 | .0038 |
| > 2 | 6.9 | 2.2 to 21.5 | .0010 |
| Greatest horizontal dimension quartiles, mm | |||
| ≤ 3.75 | 1.0 | ||
| 3.76-7.00 | 2.0 | 0.5 to 8.0 | .3083 |
| 7.01-10.50 | 2.8 | 0.7 to 11.1 | .1428 |
| > 10.50 | 6.7 | 1.6 to 28.9 | .0105 |
| Categoric-variable tumor thickness, mm | |||
| ≤ 2.00 | 1.0 | ||
| 2.01-4.00 | 1.7 | 0.4 to 7.8 | .5168 |
| 4.01-5.99 | 3.7 | 0.8 to 17.9 | .1029 |
| ≥ 6.00 | 6.0 | 1.3 to 27.0 | .0196 |
| Continuous-variable tumor thickness, mm | |||
| 1-mm increase | 1.24 | 1.09 to 1.43 | .0018 |
| Continuous-variable mitotic rate, per mm2 | |||
| 1-mitosis/mm2 increase | 1.03 | 1.006 to 1.06 | .0176 |
| Growth pattern | |||
| Circumscribed | 1.0 | ||
| Infiltrative | 3.8 | 1.5 to 9.2 | .0040 |
Abbreviation: PSLN, positive sentinel lymph node.
Multivariate Analysis
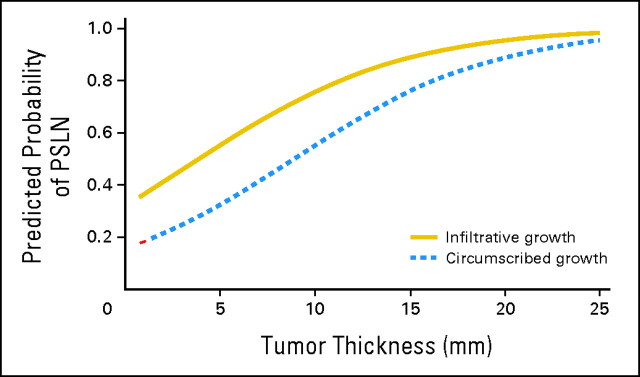
Two parsimonious multiple variable models were generated to predict a PSLN and are listed in Table 3. Two models, separating tumor thickness and mitotic rate, were necessary because of the strong colinearity between these variables. Histologic growth pattern was the only other significant characteristic, when modeled simultaneously with either tumor thickness (model 1) or mitotic rate (model 2). As the subset of data differs between the models, because of the different patterns of missing data for tumor thickness and mitotic rate, it is impossible to compare the models directly. Rather, it is clear that growth pattern along with either tumor thickness or mitotic rate is associated with a PSLN in our data. Lesions with an infiltrative growth pattern were between 2.5 (model 1, P = .0607) and 3.7 (model 2, P = .0167) times more likely to have a PSLN than lesions with circumscribed growth. From model 1, a 1-mm increase in tumor thickness increased the odds of having a PSLN by 21%. From model 2, an increase of 1 mitosis/mm2 increased the odds of having a PSLN by 4%. Figure 1 shows the predicted probability of a PSLN on the basis of tumor thickness and growth pattern (model 1). For either circumscribed or infiltrative growth pattern, increasing tumor thickness increased the probability of a PSLN. For a particular tumor thickness, a lesion with infiltrative growth was more likely to have a PSLN than a lesion with circumscribed growth.
Table 3.
Best Multiple Variable Model Candidates to Explain at Least One PSLN
| Characteristic | Analysis |
||
|---|---|---|---|
| Odds Ratio | 95% CI | P | |
| Model 1* | |||
| Growth pattern | |||
| Circumscribed | 1.0 | ||
| Infiltrative | 2.53 | 0.96 to 6.67 | .0607 |
| Tumor thickness, mm | |||
| 1-mm increase | 1.21 | 1.05 to 1.38 | .0076 |
| Model 2† | |||
| Growth pattern | |||
| Circumscribed | 1.0 | ||
| Infiltrative | 3.67 | 1.27 to 10.66 | .0167 |
| Mitotic rate, per mm2 | |||
| 1 mitosis/mm2 increase | 1.04 | 1.01 to 1.06 | .0151 |
Abbreviation: PSLN, positive sentinel lymph node.
Eighty-four of 93 occurrences used.
Sixty-eight of 93 occurrences used.
Fig 1.
Predicted probability of at least one positive sentinel lymph node (PSLN) by tumor thickness and growth pattern.
Tumor thickness and mitotic rate were significantly positively correlated in our population (Pearson correlational coefficient r = 0.36; P = .0025), accounting for the colinearity during multiple variable modeling. Although the multivariate analyses presented account for the potential correlation and confounding between the clinical and histologic features, tumor thickness was significantly higher for lesions with infiltrative growth (mean, 7.3 mm) compared with circumscribed growth (mean, 4.4 mm), on average +2.9 mm (95% CI, 1.0 to 4.9). The average mitotic rate was also higher for lesions with infiltrative growth compared to circumscribed growth, but the difference was not statistically significant.
DISCUSSION
There is wide agreement that the presence of lymph node metastases is an adverse prognostic indicator in MCC. Allen et al1 found that disease stage was the only independent predictor of survival (stage I, 81%; stage II, 67%; stage III, 52%; stage IV, 11%). For MCC, an orderly progression of metastasis has been proposed.13–14 Nodal involvement typically precedes the development of distant metastasis. Although palpable nodal involvement at presentation is reported to occur in approximately 25% of patients, an additional 30% to 50% develop nodal disease in the course of their disease.15 In many instances, lymph node recurrence in patients presenting with clinically negative lymph nodes likely occurs from micrometastatic disease in the lymph nodes at the time of treatment of the primary tumor.1,16 How to manage the regional lymph node basin/s before clinically apparent involvement is a compelling question. Early aggressive treatment for MCC, including prophylactic lymph node dissection and adjuvant radiotherapy (RT), has been advocated.17 However, both are associated with risks.10,18
SLN biopsy has been proposed to stage the lymph node basin and to guide additional treatment.19 For instance, patients with a negative SLN biopsy can be spared the morbidity of additional surgery or RT. Furthermore, SLN biopsy may allow for a more homogenous stratification of patients for clinical trials. The prognostic value of SLN biopsy in patients with MCC has been confirmed at a number of institutions.1,20–21 As a prognostic tool, the procedure is invaluable because of the lack of other reliable prognostic indicators in patients presenting with clinically localized disease. To date, there are no patient characteristics or any clinical or histologic features of the primary tumor that can be used to accurately determine which patients are at increased risk for nodal or systemic metastasis.
The purpose of this study was to determine if specific clinical and/or histologic features of MCC were predictive of SLN positivity. In addition, could a subset of patients be identified who had a significantly low risk of a PSLN and thus be spared the procedure? The feasibility of SLN biopsy in MCC has been previously shown.22 Of the 97 patients with MCC who underwent SLN biopsy at our institution, the procedure failed to identify a sentinel node in only four (4%). At least one PSLN was identified in 45.2% of successful SNL biopsies. Of numerous studies with more than five patients undergoing SLN biopsy for MCC, the SLN positivity rate varied from 11% to 57%.1,20–24 Although our SLN positivity rate is within the range of rates reported in the literature, it is higher than other large series. Allen et al1 and Mehrany et al21 reported positivity rates of 22% (ie, 12 of 54 patients) and 33% (ie, 20 of 60 patients), respectively. This difference in positivity rate could be explained, at least in part, by differences in the histologic examination of SLNs. Immunohistochemical analysis, particularly cytokeratin-20, is paramount for high sensitivity and specificity in the interpretation of SLNs for MCC. Neither Allen et al1 nor Mehrany et al21 comment on whether immunohistochemical staining of the SLN(s) was part of the protocols. Smaller studies describing examination of the SLNs with serial sectioning and immunohistochemistry with cytokeratin-20 report similar SLN positivity rates to that in this study. In particular, Shnayder et al23 and Maza et al24 report positivity rates of 40% and 47.8%, respectively. Routine use of immunohistochemistry allows identification of micrometastases in the SLN that are composed of only rare single cells that would have been missed on routine hematoxylin-eosin–stained sections, as shown in Figure 2. Given the high rate of regional and distant disease in MCC, we regard these single-cell micrometastases as biologically relevant. Differences in the clinical size of tumors may also account for variability in SLN positivity rates. We may be referred a higher number of large tumors, which would result in a higher rate of SLN positivity. Because size is not specifically available for patients who underwent SLN biopsy in the studies by Allen et al1 and Mehrany et al,21 it is not possible to assess if this may be a contributing factor in the differing PSLN rates.1,21 Furthermore, there is variability in the reporting of clinical size, categoric versus continuous variable, and source of measurement that comparisons across studies are difficult.
Fig 2.
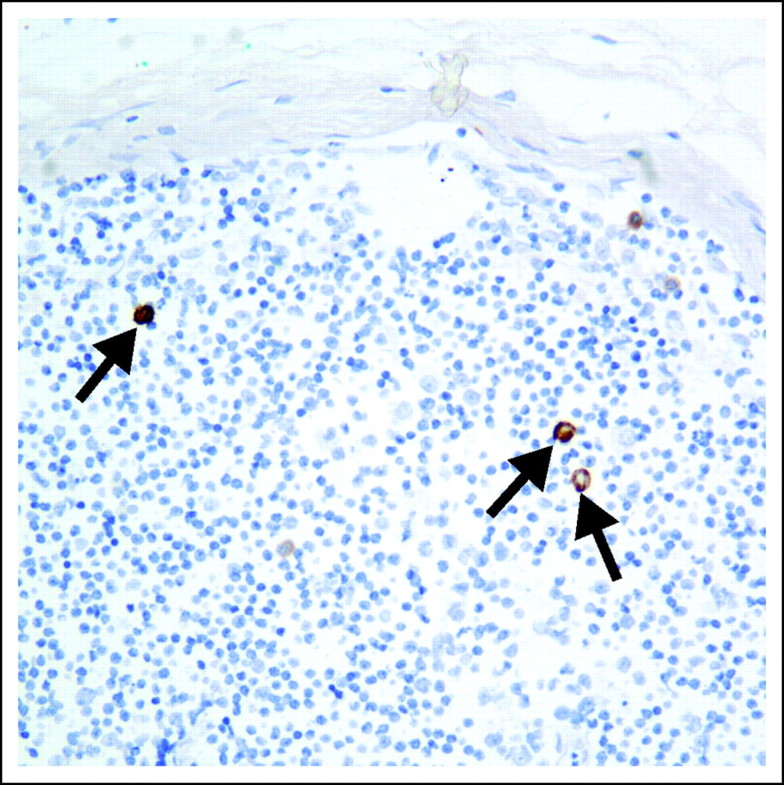
Cytokeratin-20 immunostain identifies Merkel cell carcinoma micrometastases composed of only rare single cells in a sentinel lymph node (×200).
In our univariate analysis, we observed that factors related to growth of the primary tumor including clinical size, greatest horizontal histologic dimension, tumor thickness, and mitotic rate were significantly associated with a PSLN. However, Allen et al1 found the incidence of clinically occult nodal disease not to be associated with the clinical size of the tumor. Accurate clinical size of the primary tumor may be difficult to obtain and/or verify in patients referred to tertiary care centers, because excisional biopsies have frequently been performed before referral. Tumor thickness may be a more reproducible and accurate measurement of growth, which in this study was significantly associated with a PSLN. It remains to be seen whether tumor thickness correlates with prognosis and overall survival. Recent studies report a positive association of tumor thickness with poor outcome.8
One of the compelling findings in our study was that, unlike in melanoma, there was no subgroup of small primary lesions at low enough risk of a PSLN not to warrant the procedure. Those tumors that were smallest by any measurement of size (clinical size < 1 cm, greatest horizontal histologic dimension ≤ 3.75 mm, tumor thickness ≤ 2 mm, or a lower mitotic rate [< 10 per mm2]) still had a PSLN rate between 23.1% and 36.4%. This is in contrast to the findings of Stokes et al25 that patients with MCC ≤ 1 cm in diameter are unlikely to harbor regional lymph node metastases. In their report of 52 patients with clinical tumor ≤ 1 cm and no clinical regional lymph node metastasis at presentation, none had nodal metastases diagnosed by either SLN biopsy or elective lymph node dissection. However, on the basis of our finding that 23.8% of patients with tumors ≤ 1 cm in clinical diameter have a PSLN, we conclude, as have others, that patients with small clinical lesions must be considered candidates for SLN biopsy.26
Interestingly, the histologic growth pattern emerged as an important parameter when assessing factors predicting a PSLN in MCC. An infiltrative growth pattern significantly increased the likelihood of a PSLN. In addition, there was strong correlation between growth pattern and tumor thickness. Tumor thickness was significantly higher in lesions with an infiltrative pattern compared to a circumscribed pattern. Andea et al8 have previously reported that the histologic growth pattern of MCC has prognostic significance. They found tumor architecture, infiltrative versus (nodular) circumscribed, to be an independent predictor of survival in MCC. The circumscribed pattern was associated with longer survival, whereas the infiltrative pattern had a poorer prognosis. The histologic growth pattern needs to be studied more extensively to better understand why it carries prognostic significance for MCC.
In our study, there was no single best model predicting SLN positivity in MCC. The two models generated were not derived from exactly the same subset of data and, therefore, cannot be compared directly. For model 1 (Table 3), despite correlation between histologic growth pattern and tumor thickness, a lesion with an infiltrative growth pattern at a particular tumor thickness was more likely to have a PSLN than a tumor with a circumscribed pattern. Moreover, with either growth pattern, increasing tumor thickness was associated with a greater probability of a PSLN. Extrapolating the curves in Figure 1 to a tumor thickness less than 1 mm, this subcategory of very thin primary tumors would still have a predicted probability of a PSLN between approximately 15% to 30%, dependent on the growth pattern. This lends additional support to having a low threshold for SLN biopsy for primary MCC even in patients with small primary tumors. These models may provide guidance by estimating the risk of nodal metastasis and may aid patients and physicians in the management of primary MCC.
In conclusion, increasing clinical size, increasing tumor thickness (continuous variable), increasing mitotic rate (continuous variable), and infiltrative tumor growth pattern were significantly associated with a greater likelihood of a PSLN in MCC in univariate analysis. In multivariate analysis, two competing models that used growth pattern with either tumor thickness or mitotic rate could be used to predict a PSLN. In addition, when using the growth pattern and tumor thickness model, there was no subgroup of patients predicted to have a lower than 15% to 20% likelihood of a PSLN. This suggests that all patients presenting with MCC without clinical evidence of regional lymph node disease or distant metastases should be considered candidates for SLN biopsy. In the future, novel tumor markers or characteristics may be identified that better predict which patients are at risk for occult nodal metastasis to guide selection of patients for this procedure. Furthermore, the impact of SLN biopsy on survival remains a question to be answered.
Acknowledgment
We thank Nisha Meireles for database management.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer L. Schwartz, Kent A. Griffith, Sandra L. Wong, Riley S. Rees, Timothy M. Johnson, Christopher K. Bichakjian
Provision of study materials or patients: Sandra L. Wong, Scott A. McLean, James A. Hayman, Carol R. Bradford,Christopher K. Bichakjian
Collection and assembly of data: Jennifer L. Schwartz, Lori Lowe, Douglas R. Fullen, Christopher K. Bichakjian
Data analysis and interpretation: Jennifer L. Schwartz, Kent A. Griffith, Lori Lowe, Sandra L. Wong, Scott A. McLean, Christopher D. Lao, James A. Hayman, Carol R. Bradford, Christopher K. Bichakjian
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 2.Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: Case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204–208. doi: 10.1007/s10434-001-0204-4. [DOI] [PubMed] [Google Scholar]
- 3.Ott MJ, Tanabe KK, Gadd MA, et al. Multimodality management of Merkel cell carcinoma. Arch Surg. 1999;134:388–392. doi: 10.1001/archsurg.134.4.388. discussion 392-393. [DOI] [PubMed] [Google Scholar]
- 4.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual (ed 7) New York, NY: Springer; 2010. Merkel cell carcinoma; pp. 315–323. [Google Scholar]
- 5.Voog E, Biron P, Martin JP, et al. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999;85:2589–2595. doi: 10.1002/(sici)1097-0142(19990615)85:12<2589::aid-cncr15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Llombart B, Monteagudo C, López -Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–634. doi: 10.1111/j.1365-2559.2005.02158.x. [DOI] [PubMed] [Google Scholar]
- 7.Andea A, Coit DG, Busam K. An analysis of morphologic parameters as prognostic markers in Merkel cell carcinoma. Am J Dermatopathol. 2006;28:228. [Google Scholar]
- 8.Andea AA, Coit DG, Amin B, et al. Merkel cell carcinoma: Histologic features and prognosis. Cancer. 2008;113:2549–2558. doi: 10.1002/cncr.23874. [DOI] [PubMed] [Google Scholar]
- 9.Sandel HD, 4th, Day T, Richardson MS, et al. Merkel cell carcinoma: Does tumor size or depth of invasion correlate with recurrence, metastases, or patient survival? Laryngoscope. 2006;116:791–795. doi: 10.1097/01.mlg.0000208615.93883.b2. [DOI] [PubMed] [Google Scholar]
- 10.Bichakjian CK, Lowe L, Lao CD, et al. Merkel cell carcinoma: Critical review with guidelines for multidisciplinary management. Cancer. 2007;110:1–12. doi: 10.1002/cncr.22765. [DOI] [PubMed] [Google Scholar]
- 11.Sondak VK, Taylor JMG, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: Lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11:247–258. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 12.Paek SC, Griffith KA, Johnson TM, et al. The impact of factors beyond Breslow depth on predicting sentinel lymph node positivity in melanoma. Cancer. 2007;109:100–108. doi: 10.1002/cncr.22382. [DOI] [PubMed] [Google Scholar]
- 13.Yiengpruksawan A, Coit DG, Thaler HT, et al. Merkel cell carcinoma: Prognosis and management. Arch Surg. 1991;126:1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]
- 14.Smith DE, Bielamowicz S, Kagan AR, et al. Cutaneous neuroendocrine (Merkel cell) carcinoma: A report of 35 cases. Am J Clin Oncol. 1995;18:199–203. doi: 10.1097/00000421-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock CL, Bland KI, Laney RG, 3rd, et al. Neuroendocrine (Merkel cell) carcinoma of the skin: Its natural history, diagnosis, and treatment. Ann Surg. 1988;207:201–207. doi: 10.1097/00000658-198802000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton DL, Cochran AJ. The case for lymphatic mapping and sentinel lymphadenectomy in the management of primary melanoma. Br J Dermatol. 2004;151:308–319. doi: 10.1111/j.1365-2133.2004.06133.x. [DOI] [PubMed] [Google Scholar]
- 17.Kokoska ER, Kokoska MS, Collins BT, et al. Early aggressive treatment for Merkel cell carcinoma improves outcome. Am J Surg. 1997;174:688–693. doi: 10.1016/s0002-9610(97)00193-1. [DOI] [PubMed] [Google Scholar]
- 18.Uren RF, Howman-Giles R, Thompson JF, et al. Interval nodes: The forgotten sentinel nodes in patients with melanoma. Arch Surg. 2000;135:1168–1172. doi: 10.1001/archsurg.135.10.1168. [DOI] [PubMed] [Google Scholar]
- 19.Eng TY, Boersma MG, Fuller CD, et al. A comprehensive review of the treatment of Merkel cell carcinoma. Am J Clin Oncol. 2007;30:624–636. doi: 10.1097/COC.0b013e318142c882. [DOI] [PubMed] [Google Scholar]
- 20.Messina JL, Reintgen DS, Cruse CW, et al. Selective lymphadenectomy in patients with Merkel cell (cutaneous neuroendocrine) carcinoma. Ann Surg Oncol. 1997;4:389–395. doi: 10.1007/BF02305551. [DOI] [PubMed] [Google Scholar]
- 21.Mehrany K, Otley CC, Weenig RH, et al. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28:113–117. doi: 10.1046/j.1524-4725.2002.02901.x. discussion, 117. [DOI] [PubMed] [Google Scholar]
- 22.Hill AD, Brady MS, Coit DG. Intraoperative lymphatic mapping and sentinel lymph node biopsy for Merkel cell carcinoma. Br J Surg. 1999;86:518–521. doi: 10.1046/j.1365-2168.1999.01046.x. [DOI] [PubMed] [Google Scholar]
- 23.Shnayder Y, Weed DT, Arnold DJ, et al. Management of the neck in Merkel cell carcinoma of the head and neck: University of Miami experience. Head Neck. 2008;30:1559–1565. doi: 10.1002/hed.20899. [DOI] [PubMed] [Google Scholar]
- 24.Maza S, Trefzer U, Hofmann M, et al. Impact of sentinel lymph node biopsy in patients with Merkel cell carcinoma: Results of a prospective study and review of the literature. Eur J Nucl Med Mol Imaging. 2006;33:433–440. doi: 10.1007/s00259-005-0014-1. [DOI] [PubMed] [Google Scholar]
- 25.Stokes JB, Graw KS, Dengel LT, et al. Patients with Merkel cell carcinoma tumors ≤ 1.0 cm in diameter are unlikely to harbor regional lymph node metastasis. J Clin Oncol. 2009;27:3772–3777. doi: 10.1200/JCO.2008.20.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarnaik AA, Zager JS, Cox LE, et al. Routine omission of sentinel lymph node biopsy for Merkel cell carcinoma ≤ 1 cm is not justified. J Clin Oncol. 2010;28:e7. doi: 10.1200/JCO.2009.25.9937. [DOI] [PubMed] [Google Scholar]