Abstract
Purpose
To determine the dose of cetuximab that can be safely combined with irinotecan for treatment of pediatric and adolescent patients with refractory solid tumors.
Patients and Methods
This open-label, phase I study enrolled patients ages 1 to 18 years with advanced refractory solid tumors, including tumors of the CNS. Patient cohorts by age group (children, ages 1 to 12 years; adolescents, ages 13 to 18 years) received escalating weekly doses of cetuximab (75, 150, 250 mg/m2) in a 3 + 3 design, plus irinotecan (16 or 20 mg/m2/d) for 5 days for 2 consecutive weeks every 21 days. The primary end points were establishing the maximum-tolerated dose (MTD), recommended phase II dose (RPIID), and pharmacokinetics of the combination. Preliminary safety and efficacy data were also collected.
Results
Twenty-seven children and 19 adolescents received a median of 7.1 and 6.0 weeks of cetuximab therapy, respectively. Cetuximab 250 mg/m2 weekly plus irinotecan 16 mg/m2/d (pediatric) or 20 mg/m2/d (adolescent) have been established as the MTD/RPIID. Dose-limiting toxicities included diarrhea and neutropenia. Mild to moderate (grade 1 to 2) acneiform rash occurred in a majority of patients; no grade 3 to 4 rashes were observed. Cetuximab demonstrated dose-dependent clearance in both children and adolescents, similar to that in adults. There were two confirmed partial responses, both in patients with CNS tumors. Stable disease was achieved in 18 patients overall, including 10 patients with CNS tumors (38.5%).
Conclusion
The cetuximab/irinotecan combination can be given safely to children and adolescents with cancer. Promising activity, particularly in CNS tumors, warrants phase II evaluation of this regimen.
INTRODUCTION
Cancer incidence in children ages 0 to 19 years in the United States is 16.7/100,000 cases; 30% of these cancers are solid tumors.1 Diagnostic and treatment advances have improved outcomes substantially, with the 5-year overall survival rate now approaching 80% for all childhood cancers.2,3 However, treatment options for metastatic, refractory, or high-risk disease (such as glioblastoma multiforme, diffuse pontine glioma, or metastatic alveolar rhabdomyosarcoma) are limited, and survival remains poor.
The epidermal growth factor receptor (EGFR) is overexpressed in several pediatric malignancies, including Wilms tumor,4,5 osteosarcoma,6 rhabdomyosarcoma,7 and a variety of CNS tumors, making it a relevant therapeutic target.8–11 In addition, EGFR is associated with the growth and survival of tumor stem/progenitor cells, providing a rationale for EGFR-targeted agents in the treatment of refractory malignancies.12
Cetuximab (ERBITUX; ImClone Systems, New York, NY; and Bristol-Myers Squibb, Princeton, NJ) is a chimeric monoclonal antibody with high affinity and specificity for the EGFR. It blocks ligand binding, inhibits receptor activation, and, as an immunoglobulin G1, may mediate antibody-dependent cell-mediated cytotoxicity.13,14 Cetuximab has demonstrated activity as a single agent and in combination with chemotherapy or radiotherapy in multiple solid tumors.15–21 The majority of adverse events associated with cetuximab are mild to moderate. Dermatologic manifestations such as acneiform rash are common22; other events of special interest include infusion reactions and hypomagnesemia. To date, no data are available on the efficacy or tolerability of cetuximab in pediatric patients.
Clinical studies of irinotecan in children with relapsed or refractory solid tumors have reported encouraging responses in rhabdomyosarcoma, nephroblastoma, neuroblastoma, and gliomas.23–27 These studies established an administration schedule of irinotecan 20 mg/m2/d, 5 days/wk for 2 consecutive weeks, every 3 weeks.27 Diarrhea and abdominal cramps were the predominant toxicities. Severe hematologic toxicity was infrequent.
Cetuximab has been shown to enhance the antitumor activity of irinotecan in preclinical models28 and in the clinic. In patients with irinotecan-refractory metastatic colorectal cancer, the combination is significantly more active than single-agent cetuximab (overall response rate, 22.9% v 10.8%; P = .007).15,17 The toxicity profile of cetuximab and irinotecan in combination was as expected from the individual agents, and cetuximab did not seem to exacerbate irinotecan-associated toxicities.
This phase I study evaluated the safety, tolerability, and pharmacokinetics of cetuximab in combination with irinotecan and determined the maximum-tolerated dose (MTD) and recommended phase II dosing (RPIID) of this combination in children (group A, 1 to 12 years of age) and adolescents (group B, 13 to 18 years of age) with refractory solid tumors.
PATIENTS AND METHODS
Patients
Children (group A, ages 1 to 12 years) or adolescents (group B, ages 13 to 18 years) with solid tumors, including primary CNS tumors and non-Hodgkin's lymphoma, and with progressive disease after standard therapy or without standard therapy were eligible. Although the overall goal of the trial was to study a pediatric population younger than 18 years, the separate subgroup of patients younger than 12 years was created in response to the specific information request on this age group by regulatory agencies. Other key inclusion criteria were Karnofsky performance score of ≥ 50 for patients older than 10 years or a Lansky play scale of ≥ 50 for patients 10 years of age or younger; life expectancy of ≥ 8 weeks; no immunotherapy, radiotherapy, or chemotherapy within 2 weeks of first cetuximab dose (4 weeks from any prior investigational therapy, 6 weeks from prior nitrosoureas, mitomycin, or liposomal doxorubicin) and resolution or recovery to baseline from all previous therapy-related toxicities. Adequate bone marrow function (absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 75,000/μL), hepatic function (total serum bilirubin ≤ 1.5× the upper limit of normal [ULN], alkaline phosphatase, AST, and ALT levels ≤ 2.5× the ULN), and renal function (serum creatine ≤ 1.5 times the ULN) were required.
Key exclusion criteria included autologous bone marrow or peripheral-blood stem-cell transplantation ≤ 3 months before study entry or allogeneic hematopoietic stem-cell transplantation ≤ 6 months before study entry; evidence of acute or chronic graft-versus-host disease; radiation therapy to ≥ 25% of bone marrow; brain metastases from non-CNS primary tumors (at the request of regulatory authorities); use of phenytoin, phenobarbital, primidone, carbamazepine, or valproic acid; and prior therapy with an anti-EGFR monoclonal antibody (prior EGFR-targeting small-molecule tyrosine kinase inhibitor treatment was acceptable).
The study protocol was reviewed and approved by the institutional review boards of the participating sites in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was obtained from all patients or their parents/legal guardians, with patient assent when appropriate.
Design
This multicenter, open-label, phase I study evaluated weekly escalating doses of cetuximab 75 mg/m2, 150 mg/m2, or 250 mg/m2 (administered intravenously over 60 minutes) in combination with irinotecan at 20 mg/m2/d (administered intravenously over 60 minutes), fixed dose, for 5 consecutive days in weeks 1 and 2. The cetuximab dose-escalation limits were based on adult studies showing 75 mg/m2 to be the minimum dose required to measure drug exposure and the 250 mg/m2 dose to produce acceptable exposure and safety. Cycles were repeated every 21 days. Premedication with diphenhydramine was mandated before the first cetuximab dose and was recommended thereafter. Dose reduction of irinotecan (to 12 mg/m2/d) and/or cetuximab was permitted in the event of specified toxicities. Intrapatient cetuximab dose escalation was permitted beginning with the second cycle of therapy, provided no toxicity worse than grade 1 occurred with the first dose. Patients with disease control were permitted to continue treatment as long as toxicity was acceptable.
Within each age group, cohorts of three patients were treated at each dose level with observation for at least 21 days before opening the next dose level for enrollment. Escalation to the next dose level was permitted if no first-cycle dose-limiting toxicities (DLT) were observed. If one patient developed DLT during cycle 1, up to three additional patients were enrolled at that dose level. The MTD was defined as that dose at which no more than one of six patients within a cohort experienced a DLT. The MTD and RPIID were determined independently for each of the two age groups.
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.29 The following were considered dose-limiting: febrile neutropenia; grade 4 neutropenia (absolute neutrophil count < 500/μL) or thrombocytopenia (< 25,000/μL) for more than 7 consecutive days; grade 3 to 4 nausea, vomiting, or diarrhea despite use of adequate supportive care; any other grade 3 or worse nonhematologic toxicity, excluding alopecia, rash, and fatigue/asthenia; infusion reaction/hypersensitivity reaction; more than five omitted irinotecan doses within a cycle secondary to toxicity; and more than 4-week delay in treatment because of toxicity and discontinuation of cetuximab secondary to dermatologic toxicity.
Patients experiencing treatment-related diarrhea were managed according to local institutional standards, including atropine for early-onset diarrhea and loperamide for late onset diarrhea. Testing for UGT1A1 polymorphisms and the use of prophylactic cefixime for management of diarrhea were encouraged.
Patient Evaluation
Patient histories, physical examinations with assessment of Karnofsky performance status/Lansky play scale scores, and serum chemistry profiles were conducted at baseline and before each cycle. Hematologic profiles were obtained at baseline and weekly thereafter. Adverse events were assessed continuously. Archival tumor tissue samples were obtained for baseline assessment of EGFR expression in all patients (except those with brainstem or intrinsic pontine gliomas); evidence of EGFR expression in the tumor was not required for enrollment. Samples were screened for EGFR copy number by fluorescent in situ hybridization and sequenced to identify mutations in exon 18 to 21 of EGFR and codons 12 and 13 of K-RAS. The assessments for human anticetuximab antibodies using a double-antigen radiometric assay were performed at baseline, 6 weeks, and at follow-up.
Tumor imaging and assessment was performed for all treated patients at baseline and before every other cycle of therapy. Responses in patients with non-CNS primary tumors were determined using the Response Evaluation Criteria in Solid Tumors guidelines,30 whereas responses in patients with CNS tumors were evaluated using WHO criteria.31,32
Pharmacokinetic Studies
Blood samples for pharmacokinetic assessment of cetuximab serum concentrations were obtained from the arm contralateral to the infusion site at 0, 1, 2.5, 3, 4, 6, 8, 24, 48, 96, 168, 264, and 364 hours from the start of the cetuximab infusion. Cetuximab serum concentrations were determined using a validated enzyme-linked immunosorbent assay procedure. Pharmacokinetic parameters of cetuximab were derived from serum concentration-time data by noncompartmental methods using the Kinetica software version 4.4 (Thermo Electron, Philadelphia, PA).33
No pharmacokinetic studies of irinotecan or SN-38 were deemed necessary, given the lack of interactions with cetuximab consistently observed in prior phase I studies.34,35
RESULTS
Between August 2005 and March 2008, 48 patients were enrolled across nine centers throughout the United States, with 27 children in group A and 21 adolescent patients in group B. Two patients in group B were not treated; thus the treated population for this study consisted of 46 patients (27 patients in group A and 19 patients in group B). Patient characteristics are listed in Table 1. Sex distribution was nearly even. A significant proportion of patients in both age groups had primary CNS tumors (63.0% and 47.4% in groups A and B, respectively). All patients were heavily pretreated, and all but one patient (97.8%) had been previously treated with chemotherapy; 58.7% had received two or more prior regimens, including irinotecan in one case. No patients had received previous anti-EGFR therapy. Median duration of treatment was 7.1 weeks (range, 0.9 to 133+ weeks) among children and 6.0 weeks (range, 1.0 to 26 weeks) among adolescents. All patients in both age subgroups were assessable for toxicity. Forty of 46 patients were assessable for response, including 24 patients (88.9%) in group A and 16 patients (84.2%) in group B. Of the six patients who were not assessable for response, three patients had experienced hypersensitivity to the first cetuximab infusion precluding further treatment, one patient withdrew consent, one patient lacked on-study tumor evaluations, and one patient experienced a fatal pneumonia/neutropenia/sepsis event.
Table 1.
Patient Characteristics
| Characteristic | Group A, Ages 1 to 12 Years (n = 27) |
Group B, Ages 13 to 18 Years (n = 19) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Sex | ||||
| Male | 12 | 44.4 | 10 | 52.6 |
| Female | 15 | 55.6 | 9 | 47.4 |
| Age, years* | ||||
| Median | 8 | 16 | ||
| Range | 1-12 | 13-18 | ||
| Performance status† | ||||
| ≥ 50-70 | 8 | 29.6 | 3 | 15.8 |
| > 70-100 | 19 | 70.4 | 16 | 84.2 |
| CNS primary tumor | 17 | 63.0 | 9 | 47.4 |
| Ependymoma | 2 | 7.4 | 1 | 5.3 |
| Glioma, brainstem | 8 | 29.6 | 1 | 5.3 |
| Glioma, high grade | 3 | 11.1 | 4 | 21.1 |
| Other‡ | 4 | 14.8 | 3 | 15.8 |
| Non–CNS primary tumor | 10 | 37.0 | 10 | 52.6 |
| Ewing sarcoma | 0 | — | 1 | 5.3 |
| Hepatoblastoma | 4 | 14.8 | 0 | — |
| Neuroblastoma | 2 | 7.4 | 0 | — |
| Osteosarcoma | 2 | 7.4 | 0 | — |
| Rhabdomyosarcoma | 0 | — | 1 | 5.3 |
| Wilms tumor | 1 | 3.7 | 0 | — |
| Other§ | 1 | 3.7 | 8 | 42.1 |
| Time from diagnosis, months | ||||
| Median | 14.8 | 16.3 | ||
| Range | 1.3-81.1 | 2.4-207.3 | ||
| Prior chemotherapy regimens | 26 | 96.3 | 19 | 100 |
| 0 | 1 | 3.7 | 0 | — |
| 1 | 10 | 37.0 | 8 | 42.1 |
| 2 | 8 | 29.6 | 2 | 10.5 |
| 3 | 8 | 29.6 | 9 | 47.4 |
| Prior radiation therapy | 19 | 70.4 | 16 | 84.2 |
| Prior surgery | 20 | 74.1 | 18 | 94.7 |
| Patients assessable for toxicity | 27 | 100 | 19 | 100 |
| Patients assessable for response | 24 | 88.9 | 16 | 84.2 |
| EGFR expression (IHC) | ||||
| Positive | 14 | 51.9 | 11 | 57.9 |
| Weak (1+) | 0 | — | 4 | 21.1 |
| Moderate (2+) | 5 | 18.5 | 4 | 21.1 |
| Strong (3+) | 9 | 33.3 | 3 | 15.8 |
| Negative | 4 | 14.8 | 5 | 26.3 |
| Not evaluated‖ | 9 | 33.3 | 3 | 15.8 |
Abbreviations: EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
At time of treatment.
Karnofsky performance status or Lansky play scale.
Other CNS tumor types included chordoma, high-grade neuroepithelial neoplasm, pineal neuroectodermal tumor (n = 2), atypical teratoid rhabdoid tumor, anaplastic astrocytoma, and choroid plexus carcinoma.
Other non-CNS tumors included malignant peripheral nerve sheath tumor, desmoplastic small round-cell tumor, retinoblastoma, epithelioid sarcoma, lymphoepithelioma, nasopharyngeal carcinoma, temporal bone mesenchymal chondrosarcoma, and metastatic signet ring adenocarcinoma.
Tumors not evaluated for EGFR expression are either brainstem or pontine glioma for which tissue was not available.
Toxicity
Among the 27 patients in group A, one DLT (diarrhea) occurred at the initial cetuximab dose level of 75 mg/m2 with irinotecan 20 mg/m2/d, and two events (neutropenia, diarrhea) occurred with subsequent cetuximab escalation to 150 mg/m2 (Fig 1). These events were attributed to irinotecan, and according to protocol guidelines, the dose of irinotecan was reduced to 16 mg/m2/d and enrollment continued. With the reduced dose of irinotecan, the dose of cetuximab was escalated to 250 mg/m2 weekly without further dose-limiting events. Among the 19 adolescent patients in group B, there was one dose-limiting event with both cetuximab 75 mg/m2 and 250 mg/m2 in combination with irinotecan 20 mg/m2/d; these events were diarrhea and hypokalemia, respectively (Fig 1). In both age groups, cetuximab was well-tolerated at the dose level of 250 mg/m2. The MTD and RPIID of these agents in combination were cetuximab 250 mg/m2 with irinotecan 16 mg/m2/d for children and cetuximab 250 mg/m2 with irinotecan 20 mg/m2/d for adolescents.
Fig 1.
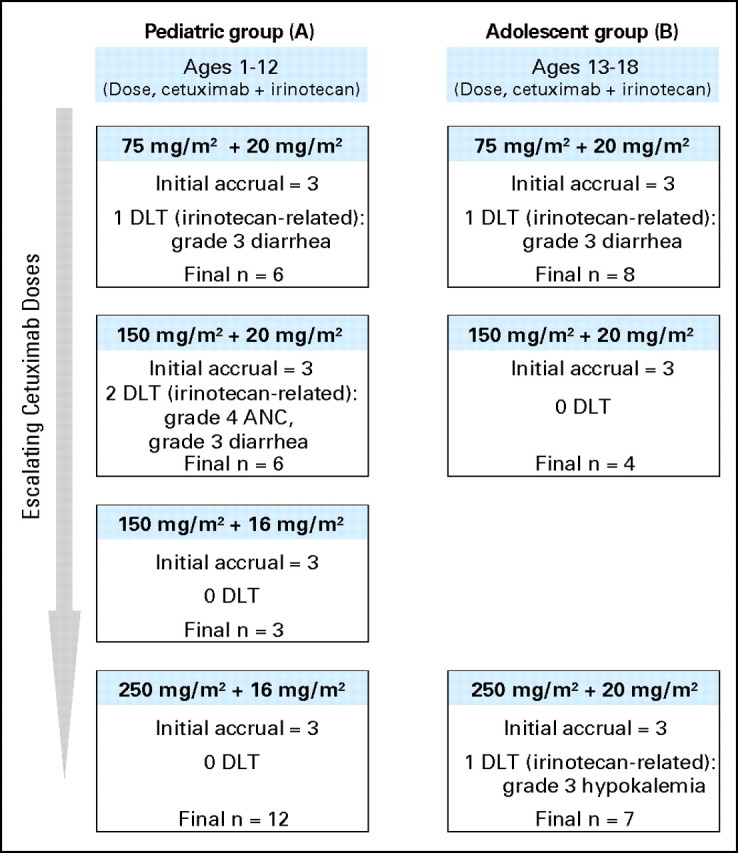
Overall accrual schema (including patients not assessable for toxicity and those withdrawn from the study) and dose-limiting toxicities (DLTs) observed.
The overall adverse event profile for the combination was similar between the two age subgroups and consistent with the adult experience (Table 2). No relevant dose-dependent cetuximab toxicities were observed (Appendix Table A1, online only). The most common grade 3 to 4 toxicities were hematologic, though these events were uncomplicated and manageable. The most common nonhematologic grade 3 to 4 toxicities included diarrhea, nausea, vomiting, abdominal pain, dehydration, and anorexia. The UGT1A1*28 allele conferring increased susceptibility to irinotecan-related toxicities was not present in any of the patients tested. Mild to moderate acneiform rash was common, but no patient experienced grade 3 to 4 rash. Severe infusion reactions with the first cetuximab infusion occurred in three of 19 adolescents. One patient in the pediatric group experienced grade 4 hypomagnesemia.
Table 2.
Safety Profile of the Cetuximab/Irinotecan Combination by Age Group
| Adverse Event | Group A, Ages 1 to 12 Years (n = 27) |
Group B, Ages 13 to 18 Years (n = 19) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Grade |
Grade 3 or 4 |
Any Grade |
Grade 3 or 4 |
|||||||||
| No. | Total Assessable | % | No. | Total Assessable | % | No. | Total Assessable | % | No. | Total Assessable | % | |
| Hematologic | ||||||||||||
| Neutropenia* | 19 | 23 | 82.6 | 9 | 23 | 39.1 | 11 | 15 | 73.3 | 8 | 15 | 53.3 |
| Leukopenia* | 23 | 27 | 85.2 | 10 | 27 | 37.0 | 14 | 18 | 77.8 | 8 | 18 | 44.4 |
| Thrombocytopenia* | 13 | 27 | 48.1 | 6 | 27 | 22.2 | 10 | 18 | 55.6 | 4 | 18 | 22.2 |
| Anemia* | 24 | 27 | 88.9 | 7 | 27 | 25.9 | 12 | 18 | 66.7 | 2 | 18 | 11.1 |
| Nonhematologic, grade 3 or 4 ≥ 5% | ||||||||||||
| Diarrhea | 24 | 27 | 88.9 | 7 | 27 | 25.9 | 15 | 19 | 78.9 | 4 | 19 | 21.1 |
| Nausea | 13 | 27 | 48.1 | 2 | 27 | 7.4 | 16 | 19 | 84.2 | 5 | 19 | 26.3 |
| Vomiting | 19 | 27 | 70.4 | 5 | 27 | 18.5 | 14 | 19 | 73.7 | 2 | 19 | 10.5 |
| Abdominal pain | 16 | 27 | 59.3 | 3 | 27 | 11.1 | 9 | 19 | 47.4 | 1 | 19 | 5.3 |
| Headache | 14 | 27 | 51.9 | 0 | 12 | 19 | 63.2 | 1 | 19 | 5.3 | ||
| Fatigue | 8 | 27 | 29.6 | 0 | 7 | 19 | 36.8 | 2 | 19 | 10.5 | ||
| Anorexia | 9 | 27 | 33.3 | 2 | 27 | 7.4 | 8 | 19 | 42.1 | 5 | 19 | 26.3 |
| Dehydration | 6 | 27 | 22.2 | 5 | 27 | 18.5 | 5 | 19 | 26.3 | 1 | 19 | 5.3 |
| Special interest | ||||||||||||
| Acneform rash† | 18 | 27 | 66.7 | 0 | 12 | 19 | 63.2 | 0 | ||||
| Infusion reaction‡ | 4 | 27 | 14.8 | 0 | 6 | 19 | 31.6 | 3 | 19 | 15.8 | ||
| Hypomagnesemia* | 6 | 27 | 22.2 | 1 | 27 | 3.7 | 7 | 15 | 46.7 | 0 | ||
Incidence of these events calculated relative to the number of patients who underwent the laboratory tests.
Composite term made up of the Medical Dictionary for Regular Activities (MedDRA) terms, including rash, rash pustular, rash erythematous, dermatitis acneform, dermatitis exfoliative, rash papular, rash pruritic, rash generalized, rash macular, rash maculopapular, acne, dry skin, acne pustular, and skin desquamation.
Composite term made up of select MedDRA preferred terms, including infusion-related reaction, hypersensitivity, anaphylactic reaction, anaphylactic shock, anaphylactoid reaction. The terms dyspnea, pyrexia and rigors were included as infusion reactions if the onset of these toxicities occurred on the first day of study treatment.
Antitumor Activity
Of the 26 patients with primary CNS tumors, confirmed partial responses were seen in two patients (7.7%) and stable disease was seen in 10 patients (38.5%), for an overall clinical benefit rate of 46.2% in this tumor subset (Table 3). At the time of data cutoff, the two partial responses observed in this patient population had been sustained for 6.4 months and 27 months (as of September 2008, the latter patient continues on treatment with a confirmed partial response). Among the 20 patients with non-CNS tumors, the best response seen was stable disease among eight patients (40.0%). Patients achieving disease stabilization (CNS and non-CNS tumors, n = 18) received a median of four treatment cycles (3 weeks per each treatment cycle).
Table 3.
Response by Tumor Type
| Best Response* | Primary CNS Tumors (n = 26) |
Non-CNS Solid Tumors (n = 20) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Complete response | 0 | — | 0 | — |
| Partial response | 2 | 7.7 | 0 | — |
| Stable disease | 10 | 38.5 | 8 | 40.0 |
| Disease progression | 10 | 38.5 | 11 | 55.0 |
| Not assessable† | 4 | 15.4 | 1 | 5.0 |
Based on all treated patients.
Includes unable to determine.
No EGFR or K-RAS mutations were detected in any tissue samples. There seemed to be no correlation between response and any biomarker status (scores for EGFR immunohistochemistry or EGFR fluorescent in situ hybridization or sequence mutations in EGFR or K-RAS). Elevated EGFR copy number was observed in a single sample from a subject with stable disease. Meaningful interpretation of these analyses is limited by the small sample size.
Pharmacokinetics
Complete pharmacokinetic data were available for 23 (85%) of 27 patients in group A and 12 (63%) of 19 patients in group B (Table 4, Fig 2A). Single-dose cetuximab pharmacokinetic was evaluated based on concentration-time profile up to 168 hours after the start of the cetuximab infusion. Within the dose range studied, cetuximab pharmacokinetics were nonlinear, evidenced by the disproportional increase in area under the curve of concentration-time profiles compared with dose (Fig 2B). In the noncompartmental pharmacokinetic analysis, clearance decreased with increasing dose in both age groups (Table 4). In group A, clearance decreased from 0.057 L/h · m2 to 0.015 L/h · m2 as cetuximab dose increased from 75 mg/m2 to 250 mg/m2. Similar results were observed in group B patients. Mean volume of distribution at steady-state across all dose and age groups was approximately 2 L/m2, suggesting minimal distribution of cetuximab into the extracellular space. Pharmacokinetic parameters between the two age groups seemed to have similar distributions within dose, ranging from 75 to 250 mg/m2; however, given the relatively small sample size of the adolescent group, formal statistical comparisons were not performed.
Table 4.
Summary of Selected Cetuximab Pharmacokinetic Parameters
| Cetuximab Dose | No. of Patients | AUC(0-inf) (μg/mL • h) |
Clearance (L/h • m2) |
Vdss (L/m2) |
|||
|---|---|---|---|---|---|---|---|
| Mean* | CV (%) | Mean† | SD | Mean† | SD | ||
| 75 mg/m2 | |||||||
| Ages 1-12 years | 7 | 1,598 | 48 | 0.057 | 0.041 | 2.081 | 0.666 |
| Ages 13-18 years | 4 | 1,925 | 23 | 0.040 | 0.010 | 2.138 | 0.453 |
| 150 mg/m2 | |||||||
| Ages 1-12 years | 7 | 8,871 | 21 | 0.017 | 0.004 | 1.860 | 0.564 |
| Ages 13-18 years | 2 | 7,027 | 3 | 0.021 | 0.001 | 1.887 | 0.262 |
| 250 mg/m2 | |||||||
| Ages 1-12 years | 9 | 17,706 | 34 | 0.015 | 0.005 | 2.157 | 0.362 |
| Ages 13-18 years | 6 | 13,410 | 38 | 0.020 | 0.009 | 2.179 | 0.250 |
Abbreviations: AUC, area under the curve; Vdss, volume of distribution at steady state; CV, coefficient of variation; SD, standard deviation; inf, infinity.
Geometric mean for AUC(0-inf).
Arithmetic mean for clearance and Vdss.
Fig 2.
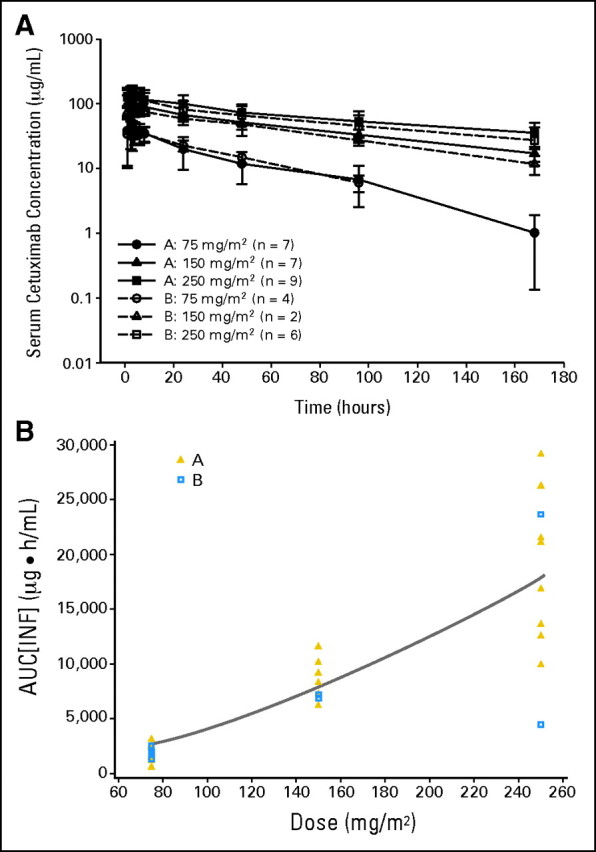
Cetuximab pharmacokinetics. (A) Mean (± standard deviation) serum concentration-time profiles for cetuximab after a single infusion for patients ages 1 to 12 years (group A, gold triangles) and 13 to 18 years (group B, blue squares). (B) Cetuximab area under the curve (AUC) versus dose for patients ages 1 to 12 years (group A) and 13 to 18 years (group B). INF, infinity.
Immunogenicity Analysis
Of 42 cetuximab-treated patients with one time point analyzed for human anticetuximab antibodies reactivity, 26 were assessable with pre- and post-treatment samples. One subject (4%) exhibited anticetuximab antibodies, a rate consistent with observations in adults.
DISCUSSION
In this first clinical evaluation in pediatric patients, cetuximab showed encouraging efficacy in a heavily pretreated population. Cetuximab, in combination with irinotecan, was both tolerable and feasible for pediatric patients with CNS or refractory solid tumors. In children, the MTD was reached at cetuximab 250 mg/m2 plus irinotecan 16 mg/m2, which was determined to be the RPIID for this group. The MTD for adolescent patients was cetuximab 250 mg/m2 plus irinotecan 20 mg/m2; difficulties in accrual curtailed the prespecified expanded enrollment at this highest-dose cohort, establishing this as the RPIID, with a total of seven patients treated. This dose of cetuximab is similar to standard adult regimens, although no loading dose was given in this study.
The safety profile was similar across age groups and consistent with the known safety profile of each individual drug in adult patients. Reported toxicities were generally mild to moderate. Hematologic toxicities, including those of grade 3 or 4 severity, were generally uncomplicated and reversible. The most common nonhematologic toxicities included diarrhea, nausea, vomiting, abdominal pain, dehydration, and anorexia. Mild to moderate acneform rash was common, but no patient experienced grade 3 or 4 nonhematologic, noninfusion-related toxicity. The frequency of infusion reactions was comparable to those observed in adults. All grade 3 reactions occurred during the first infusion and resolved within minutes to hours of onset.
Analysis of cetuximab pharmacokinetics indicated nonlinear elimination and similar profiles between age groups. Estimates of clearance, area under the curve, and volume of distribution at steady-state in both the pediatric and adolescent subgroups were similar to those previously reported for adults.36 The dose-dependent elimination of cetuximab can be explained by receptor-mediated clearance, where the receptors seemed to be saturated at higher doses.37 Similar nonlinear cetuximab pharmacokinetics have been described in adults for cetuximab doses ranging from 50 to 500 mg/m2.22,33,36,38 Consistent with previous studies, no pharmacokinetic interaction between cetuximab and irinotecan was observed.34,35
Although assessment of antitumor efficacy is limited in the context of this phase I study, clinical benefit data associated with this combination seem encouraging. Disease control was seen in 12 (46.2%) of 26 patients with primary CNS disease, and disease stabilization was seen in eight (40%) of 20 patients in the non-CNS solid tumor cohort. There was no apparent correlation between EGFR or K-RAS biomarker status and response, though the sample size was relatively small.
Two patients with refractory and progressive CNS tumors demonstrated sustained partial responses for 6.4 or 27+ months and received 15 and 45 treatment cycles (cetuximab 75 mg/m2 and irinotecan 20 mg/m2), respectively, during this timeframe. The patient with a partial response of 27+ months duration had an EGFR-negative anaplastic astrocytoma of the thalamus. Although seemingly counterintuitive, responses to cetuximab in patients with tumors classified as EGFR-negative by immunohistochemistry have been described before, and this test is now widely considered inadequate to predict likelihood of response.39,40 Objective neurologic improvement was observed, and there was near-complete resolution of a right hemispheric lesion. On the basis of the sustained response and limited therapeutic options, treatment of this patient continued beyond trial completion.
Management of children with CNS or refractory solid tumors remains a clinical challenge and warrants evaluation of novel therapies. The EGFR is a rational therapeutic target in these tumors. Studies with gefitinib (IRESSA; AstraZeneca Pharmaceutical, Wilmington, DE), an EGFR-targeted tyrosine kinase inhibitor, have also suggested efficacy in children with refractory solid tumors,41,42 supporting further pediatric studies of this therapeutic class. A phase II trial of cetuximab plus irinotecan and radiation therapy in patients with poor-prognosis and refractory CNS tumors is underway.
Although the recommended irinotecan dose for children from this phase I study is 16 mg/m2/d for 5 days/wk for 2 consecutive weeks (with cycles repeated every 21 days), irinotecan could be given at 20 mg/m2/d in adolescents. Given the extensive prior experience of the 20 mg/m2 dose of irinotecan on the protracted dosing schedule, one could consider this, with close monitoring for toxicity, in younger patients who are less heavily pretreated.
K-RAS mutational status, which has emerged as a reliable predictor of cetuximab efficacy in adult patients with metastatic colorectal cancer,43–45 has not been systematically evaluated in pediatric tumors. Its relevance to cetuximab response in this patient population is unknown. In the current study, no activating K-RAS mutations were detected; therefore, additional investigation within this population is warranted to better identify a potential response biomarker for future trials.
Appendix
Table A1.
Safety Profile of the Cetuximab/Irinotecan Combination by Dose Cohort
| Adverse Event | 75 mg/m2 C, 20 mg/m2 I (n = 14) |
150 mg/m2 C, 20 mg/m2 I (n = 10) |
150 mg/m2 C, 16 mg/m2 I (age 1-12 years only, n = 3) |
250 mg/m2 C, 16 mg/m2 I (ages 1-12 years only, n = 12) |
250 mg/m2 C, 20 mg/m2 I (ages 13-18 years only, n = 7) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any |
Grade 3/4 |
Any |
Grade 3/4 |
Any |
Grade 3/4 |
Any |
Grade 3/4 |
Any |
Grade 3/4 |
|||||||||||
| No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | No. | Total Assessable | |
| Hematologic* | ||||||||||||||||||||
| Neutropenia | 9 | 12 | 6 | 12 | 5 | 7 | 4 | 7 | 3 | 3 | 0 | 3 | 8 | 10 | 3 | 10 | 5 | 6 | 4 | 6 |
| Leukopenia | 10 | 13 | 5 | 13 | 7 | 10 | 5 | 10 | 2 | 3 | 0 | 3 | 12 | 12 | 4 | 12 | 6 | 7 | 4 | 7 |
| Thrombocytopenia | 6 | 13 | 1 | 13 | 4 | 10 | 3 | 10 | 2 | 3 | 0 | 3 | 6 | 12 | 2 | 12 | 5 | 7 | 4 | 7 |
| Anemia | 10 | 13 | 4 | 13 | 7 | 10 | 1 | 10 | 3 | 3 | 2 | 3 | 11 | 12 | 2 | 12 | 5 | 7 | 0 | 7 |
| Nonhematologic | ||||||||||||||||||||
| Diarrhea | 12 | 14 | 4 | 14 | 8 | 10 | 4 | 10 | 2 | 3 | 0 | 3 | 11 | 12 | 3 | 12 | 6 | 7 | 0 | 7 |
| Nausea | 11 | 14 | 2 | 14 | 5 | 10 | 3 | 10 | — | — | 7 | 12 | 1 | 12 | 6 | 7 | 1 | 7 | ||
| Vomiting | 11 | 14 | 3 | 14 | 5 | 10 | 1 | 10 | 1 | 3 | 0 | 3 | 10 | 12 | 3 | 12 | 6 | 7 | 0 | 7 |
| Abdominal pain | 7 | 14 | 0 | 14 | 6 | 10 | 2 | 10 | 1 | 3 | 0 | 3 | 9 | 12 | 2 | 12 | 2 | 7 | 0 | 7 |
| Headache | 9 | 14 | 1 | 14 | 4 | 10 | 0 | 10 | 2 | 3 | 0 | 3 | 7 | 12 | 0 | 12 | 4 | 7 | 0 | 7 |
| Fatigue | 5 | 14 | 1 | 14 | 4 | 10 | 0 | 10 | 1 | 3 | 0 | 3 | 4 | 12 | 0 | 12 | 1 | 7 | 1 | 7 |
| Anorexia | 6 | 14 | 3 | 14 | 3 | 10 | 2 | 10 | — | — | 6 | 12 | 1 | 12 | 2 | 7 | 1 | 7 | ||
| Dehydration | 3 | 14 | 2 | 14 | 3 | 10 | 1 | 10 | — | — | 3 | 12 | 3 | 12 | 2 | 7 | 0 | 7 | ||
| Special Interest | ||||||||||||||||||||
| Acneform rash | 8 | 14 | 0 | 14 | 4 | 10 | 0 | 10 | 3 | 3 | 0 | 3 | 8 | 12 | 0 | 12 | 7 | 7 | 0 | 7 |
| Infusion reaction | 3 | 14 | 2 | 14 | 1 | 10 | 1 | 10 | 2 | 3 | 0 | 3 | 2 | 12 | 0 | 12 | 2 | 7 | 0 | 7 |
| Hypomagnesemia* | 4 | 12 | 0 | 12 | 3 | 9 | 0 | 9 | 0 | 3 | 0 | 3 | 4 | 12 | 1 | 12 | 2/6 | 0/6 | ||
Abbreviations: C, cetuximab; I, irinotecan.
Indicated relative to the number of patients who underwent the laboratory tests.
Footnotes
Supported by Bristol-Myers Squibb. Editorial assistance for the development of this manuscript was provided by Clinical Insights Inc, with the financial support of Bristol-Myers Squibb and ImClone Systems, Inc.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL; and the 13th International Symposium in Pediatric Neuro-Oncology, June 29-July 2, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Christopher Harbison, Bristol-Meyers Squibb (C); Xiaofei Zhou, Bristol-Meyers Squibb (C); Haolan Lu, Bristol-Meyers Squibb (C); Christiane Langer, Bristol-Myers Squibb (C); Martin Weber, Bristol-Meyers Squibb (C) Consultant or Advisory Role: None Stock Ownership: Stephen P. Hunger, Bristol-Meyers Squibb, Bristol-Meyers Squibb; Haolan Lu, Bristol-Meyers Squibb; Christiane Langer, Bristol-Myers Squibb; Martin Weber, Bristol-Meyers Squibb Honoraria: None Research Funding: James A. Whitlock, Bristol-Meyers Squibb; Jessica Boklan, Bristol-Meyers Squibb Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Tanya M. Trippett, John Kuttesch, Christiane Langer, Martin Weber, Lia Gore
Administrative support: Tanya M. Trippett, Lia Gore
Provision of study materials or patients: Tanya M. Trippett, Cynthia Herzog, James A. Whitlock, John Kuttesch, Rochelle Bagatell, Stephen P. Hunger, Jessica Boklan, Amy A. Smith, Howard M. Katzenstein, Lia Gore
Collection and assembly of data: Tanya M. Trippett, James A. Whitlock, John Kuttesch, Stephen P. Hunger, Christopher Harbison, Martin Weber, Lia Gore
Data analysis and interpretation: Tanya M. Trippett, Rochelle Bagatell, Jessica Boklan, Christopher Harbison, Xiaofei Zhou, Haolan Lu, Christiane Langer, Martin Weber, Lia Gore
Manuscript writing: Tanya M. Trippett, Rochelle Bagatell, Haolan Lu, Martin Weber, Lia Gore
Final approval of manuscript: Tanya M. Trippett, Cynthia Herzog, James A. Whitlock, Johannes Wolff, Rochelle Bagatell, Stephen P. Hunger, Jessica Boklan, Amy A. Smith, Robert J. Arceci, Howard M. Katzenstein, Christopher Harbison, Haolan Lu, Christiane Langer, Martin Weber, Lia Gore
REFERENCES
- 1.United States Cancer Statistics Working Group. United States Cancer Statistics: 1999-2004 Incidence and Mortality Web-based Report. Atlanta, GA: United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2007. http://www.cdc.gov/uscs. [Google Scholar]
- 2.McGregor LM, Metzger ML, Sanders R, et al. Pediatric cancers in the new millennium: Dramatic progress, new challenges. Oncology. 2007;21:809–820. [PubMed] [Google Scholar]
- 3.National Cancer Institute. Surveillance Epidemiology and End Results (SEER) http://seer.cancer.gov/
- 4.Ghanem MA, Van Der Kwast TH, Den Hollander JC, et al. Expression and prognostic value of epidermal growth factor receptor, transforming growth factor-alpha, and c-erb B-2 in nephroblastoma. Cancer. 2001;92:3120–3129. doi: 10.1002/1097-0142(20011215)92:12<3120::aid-cncr10173>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Yokoi A, McCrudden KW, Huang J, et al. Human epidermal growth factor receptor signaling contributes to tumor growth via angiogenesis in her2/neu-expressing experimental Wilms' tumor. J Pediatr Surg. 2003;38:1569–1573. doi: 10.1016/s0022-3468(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 6.Hughes DP, Thomas DG, Giordano TJ, et al. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer Res. 2004;64:2047–2053. doi: 10.1158/0008-5472.can-03-3096. [DOI] [PubMed] [Google Scholar]
- 7.Ganti R, Skapek SX, Zhang J, et al. Expression and genomic status of EGFR and ErbB-2 in alveolar and embryonal rhabdomyosarcoma. Mod Pathol. 2006;19:1213–1220. doi: 10.1038/modpathol.3800636. [DOI] [PubMed] [Google Scholar]
- 8.Ganigi PM, Santosh V, Anandh B, et al. Expression of p53, EGFR, pRb and bcl-2 proteins in pediatric glioblastoma multiforme: A study of 54 patients. Pediatr Neurosurg. 2005;41:292–299. doi: 10.1159/000088731. [DOI] [PubMed] [Google Scholar]
- 9.Tabori U, Rienstein S, Dromi Y, et al. Epidermal growth factor receptor gene amplification and expression in disseminated pediatric low-grade gliomas. J Neurosurg. 2005;103(suppl):357–361. doi: 10.3171/ped.2005.103.4.0357. [DOI] [PubMed] [Google Scholar]
- 10.Bredel M, Pollack IF, Hamilton RL, et al. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–1792. [PubMed] [Google Scholar]
- 11.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 12.Mimeault M, Hauke R, Mehta PP, et al. Recent advances in cancer stem/progenitor cell research: Therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 14.Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham D, Humbled Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 16.Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Nowacki M, Lang I, et al. Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. J Clin Oncol. 2007;25(suppl):164s. abstr 4000. [Google Scholar]
- 18.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 19.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 20.Vermorken JB, Trigo J, Hitt R, et al. Open-Label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 21.Pirker R, Szczesna A, von Pawel J, et al. FLEX: A randomized, multicenter, phase III study of cetuximab in combination with cisplatin/vinorelbine (CV) versus CV alone in the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2008;26(suppl):6s. abstr 3. [Google Scholar]
- 22.Blick SK, Scott LJ. Cetuximab: A review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal canacer. Drugs. 2007;67:2585–2607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- 23.Vassal G, Couanet D, Stockdale E, et al. Phase II trial of irinotecan in children with relapsed or refractory rhabdomyosarcoma: A joint study of the French Society of Pediatric Oncology and the United Kingdom Children's Cancer Study Group. J Clin Oncol. 2007;25:356–361. doi: 10.1200/JCO.2006.06.1960. [DOI] [PubMed] [Google Scholar]
- 24.Shitara T, Shimada A, Hanada R, et al. Irinotecan for children with relapsed solid tumors. Pediatr Hematol Oncol. 2006;23:103–110. doi: 10.1080/08880010500457152. [DOI] [PubMed] [Google Scholar]
- 25.Cosetti M, Wexler LH, Calleja E, et al. Irinotecan for pediatric solid tumors: The Memorial Sloan-Kettering experience. J Pediatr Hematol Oncol. 2002;24:101–105. doi: 10.1097/00043426-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Friedman HS, Keir ST, Houghton PJ. The emerging role of irinotecan (CPT-11) in the treatment of malignant glioma in brain tumors. Cancer. 2003;97(suppl):2359–2362. doi: 10.1002/cncr.11305. [DOI] [PubMed] [Google Scholar]
- 27.Furman WL, Stewart CF, Poquette CA, et al. Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol. 1999;17:1815–1824. doi: 10.1200/JCO.1999.17.6.1815. [DOI] [PubMed] [Google Scholar]
- 28.Prewett MC, Hooper AT, Bassi R, et al. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 29.National Cancer Institute. Common Terminology Criteria for Adverse Events v 3.0 (CTCAE) 2006. Aug 9, http://ctep.cancer.gov/forms/CTCAEv3.pdf.
- 30.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 31.Gnekow AK. Recommendations of the Brain Tumor Subcommittee for the reporting of trials. Med Pediatr Oncol. 1995;24:104–108. doi: 10.1002/mpo.2950240209. [DOI] [PubMed] [Google Scholar]
- 32.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 33.Fracasso PM, Burris H, 3rd, Arquette MA, et al. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: Pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986–993. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]
- 34.Ettlinger DE, Mitterhauser M, Wadsak W, et al. In vivo disposition of irinotecan (CPT-11) and its metabolites in combination with the monoclonal antibody cetuximab. Anticancer Res. 2006;26:1337–1341. [PubMed] [Google Scholar]
- 35.Delbaldo C, Pierga JY, Dieras V, et al. Pharmacokinetic profile of cetuximab (Erbitux) alone and in combination with irinotecan in patients with advanced EGFR-positive adenocarcinoma. Eur J Cancer. 2005;41:1739–1745. doi: 10.1016/j.ejca.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Tan AR, Moore DF, Hidalgo M, et al. Pharmacokinetics of cetuximab after administration of escalating single dosing and weekly fixed dosing in patients with solid tumors. Clin Cancer Res. 2006;12:6517–6522. doi: 10.1158/1078-0432.CCR-06-0705. [DOI] [PubMed] [Google Scholar]
- 37.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 38.Baselga J, Pfister D, Cooper MR, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 39.Chung KY, Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 40.Lenz H-J, Van Cutsem E, Khambata-Ford S, et al. Multicenter phase II and translational study of cetuximab in metastatic colorectal carcinoma refractory to irinotecan, oxaliplatin, and fluoropyrimidines. J Clin Oncol. 2006;24:4914–4921. doi: 10.1200/JCO.2006.06.7595. [DOI] [PubMed] [Google Scholar]
- 41.Daw NC, Furman WL, Stewart CF, et al. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: A Children's Oncology Group Study. J Clin Oncol. 2005;23:6172–6180. doi: 10.1200/JCO.2005.11.429. [DOI] [PubMed] [Google Scholar]
- 42.Freeman BB, 3rd, Daw NC, Geyer JR, et al. Evaluation of gefitinib for treatment of refractory solid tumors and central nervous system malignancies in pediatric patients. Cancer Invest. 2006;24:310–317. doi: 10.1080/07357900600632058. [DOI] [PubMed] [Google Scholar]
- 43.Van Cutsem E, Kohne C-H, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 44.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2008;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 45.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]


