Abstract
Purpose
Patients with recurrent prostate cancer after local treatment make up a heterogeneous population for whom androgen deprivation therapy (ADT) is the usual treatment. The purpose of this randomized phase II trial was to investigate the efficacy and toxicity of short-course ADT with or without bevacizumab in men with hormone-sensitive prostate cancer.
Patients and Methods
Eligible patients had an increasing prostate-specific antigen (PSA) of ≤ 50 ng/mL and PSA doubling time of less than 18 months. Patients had either no metastases or low burden, asymptomatic metastases (lymph nodes < 3 cm and five or fewer bone metastases). Patients were randomly assigned 2:1 to a luteinizing hormone-releasing hormone agonist, bicalutamide and bevacizumab or ADT alone, for 6 months. The primary end point was PSA relapse-free survival (RFS). Relapse was defined as a PSA of more than 0.2 ng/mL for prostatectomy patients or PSA of more than 2.0 ng/mL for primary radiation therapy patients.
Results
Sixty-six patients received ADT + bevacizumab and 36 received ADT alone. Patients receiving ADT + bevacizumab had a statistically significant improvement in RFS compared with patients treated with ADT alone (13.3 months for ADT + bevacizumab v 10.2 months for ADT alone; hazard ratio, 0.47; 95% CI, 0.29 to 0.77; log-rank P = .002). Hypertension was the most common adverse event in patients receiving ADT + bevacizumab (36%).
Conclusion
ADT combined with bevacizumab resulted in an improved RFS for patients with hormone-sensitive prostate cancer. Long-term follow-up is needed to determine whether some patients have a durable PSA response and are able to remain off ADT for prolonged periods. Our data provide rationale for combining vascular endothelial growth factor–targeting therapy with ADT in hormone-sensitive prostate cancer.
INTRODUCTION
The initial therapy for men with systemic prostate cancer is androgen deprivation therapy (ADT). For nonmetastatic, prostate-specific antigen (PSA) only, or low-volume metastatic disease, ADT is commonly prescribed in an intermittent fashion.1,2 Investigational strategies are needed to explore regimens that can either prolong time to castration resistance or improve quality of life by prolonging the duration of the off phase of intermittent ADT.
Angiogenesis is known to play an important role in prostate cancer pathogenesis.3 Vascular endothelial growth factor-A (VEGF-A) is present in both localized and metastatic tumors, and increased plasma concentrations of VEGF have been associated with disease progression.4-6 Preclinical studies demonstrate that VEGF inhibition with ADT results in significant suppression of tumor growth in comparison with either VEGF inhibition or ADT alone.7 These data support a role for VEGF in prostate cancer progression and also support the hypothesis that inhibition of VEGF in combination with ADT may prolong response to ADT.
Bevacizumab is a monoclonal antibody to VEGF with demonstrated efficacy in several solid tumors, including glioblastoma and colorectal, lung, ovarian, and renal cell cancer.8-13 Although the addition of bevacizumab to docetaxel improved progression-free survival and response rates in metastatic castration-resistant prostate cancer (mCRPC), it failed to improve overall survival (OS).14
The benefit of short-term ADT combined with bevacizumab in hormone-sensitive recurrent prostate cancer has not been explored. In this randomized phase II study, we compare the efficacy and toxicity of 6 months of ADT with or without bevacizumab in men with hormone-sensitive disease.
PATIENTS AND METHODS
Patient Population
Eligible patients had histologically documented prostatic adenocarcinoma treated with radical prostatectomy (RP), radiotherapy, or cryotherapy. PSA recurrence with PSA doubling time of less than 18 months and PSA ≤ 50 ng/mL was required. There was no minimum PSA requirement for RP patients. Patients treated with primary radiotherapy had to have a PSA ≥ 2.0 ng/mL. Asymptomatic metastatic disease was allowed (lymph nodes < 3 cm in greatest diameter and five or fewer bone metastases). Prior ADT was allowed if it was administered for ≤ 8 months and testosterone recovered to within 50 ng/dL of the institutional normal range. Prior chemotherapy was allowed if there were six or fewer cycles and they were given more than 6 months before enrollment. Additional eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 to 1 and predefined hematologic criteria. Exclusions that were bevacizumab specific included inadequately controlled hypertension; congestive heart failure; myocardial infarction; unstable angina; stroke; transient ischemic attack; symptomatic peripheral vascular disease; bleeding diathesis; coagulopathy; abdominal fistula, perforation, or abscess; nonhealing wound, or significant proteinuria.
Study Design and Treatment
This was a randomized, open-label phase II study conducted through the Prostate Cancer Clinical Trial Consortium at eight academic centers: Dana-Farber Cancer Institute (Boston, MA; n = 22), Memorial Sloan Kettering Cancer Institute (New York, NY; n = 20), University of Wisconsin (Madison, WI; n = 19), MD Anderson Cancer Center (Houston, TX; n = 14), Johns Hopkins University (Baltimore, MD; n = 13), Cancer Institute of New Jersey (New Brunswick, NJ; n = 11), Karmanos Cancer Institute (Detroit, MI; n = 2), and the University of Maryland (Baltimore, MD; n = 1). A total of 68 events were required to have 80% power to detect a 50% reduction in the hazard of relapse, which assumes that the median time to PSA relapse will be 6 months among patients treated with ADT alone and 12 months among patients treated with ADT + bevacizumab with one-sided α = .025. The target sample size was 100 patients. Patients were randomly assigned (2:1) to leuprolide acetate 22.5 mg intramuscularly or goserelin acetate 10.8 mg subcutaneously once every 3 months and bicalutamide 50 mg orally once per day versus leuprolide acetate 22.5 mg intramuscularly or goserelin acetate 10.8 mg subcutaneously once every 3 months, bicalutamide 10 mg orally once per day, and bevacizumab 15 mg/kg intravenously once every 3 weeks for 6 months. Randomization stratification factors included age (≤ 60 v > 60 years), prior RP (yes v no), and metastatic disease status (yes v no). The stratified permuted block method was used for randomization allocation.
Participants were evaluated at baseline, every 3 months for those on the ADT alone arm or every 3 weeks for those on the ADT + bevacizumab arm during the active treatment phase, and every 2 months during follow-up until PSA relapse. Institutional review boards of the participating institutions approved the study, which was conducted per the Declaration of Helsinki in accordance with the World Medicines Association and its amendments.
The primary end point was relapse-free survival (RFS). Relapse was defined as a PSA of more than 0.2 ng/mL for RP patients or PSA of more than 2.0 ng/mL for those who received primary radiation therapy. RFS was defined as the time from random assignment to relapse or death as a result of any cause. Among patients alive without relapse, RFS was censored at the date of the last PSA measurement. Participants remained on study during treatment unless progression defined by Prostate Cancer Clinical Trials Working Group-2 (PSA increase by ≥ 25% and at ≥ 2 ng/mL above nadir confirmed at 3 weeks or later) or progression defined by Response Evaluation Criteria in Solid Tumors version 1.1.15 Secondary end points included the percentage of patients with a PSA of less than 0.2 ng/mL at therapy completion, toxicity as determined by Common Terminology Criteria for Adverse Events version 3.0, and assessment of cytokines and angiogenic factors (CAFs). Testosterone level was recorded during follow-up until PSA progression or recovery, defined as a testosterone level of more than 200 ng/dL.
Biomarker Measurements
Plasma for biomarker assessment was collected at baseline, on day 22, at 3 months, at 6 months, and at relapse and was stored at −80°C. Concentrations of 34 CAFs were measured in duplicate at the MD Anderson Cancer Center. These factors were selected on the basis of their link to established biology, the mechanism of action of bevacizumab, and commercial availability. Samples were thawed overnight at 4°C and then centrifuged at 1500 × g to remove debris. CAFs were measured per manufacturers’ instructions with multiplex bead suspension array kits using a Bio-Plex 200 system (Bio-Rad Laboratories, Hercules, CA), including customized high-sensitivity T-cell (HSTCMAG28SPMX13) and angiogenesis (HAGP1MAG-12K-09) panels, cytokine panel II (HCYP2MAG-62K-03), a bone panel (HBNMAG-51K-03), kidney injury panel 1 (HKI5MAG-99K-02), and a soluble cytokine receptor (HSCRMAG-32K-04) panel (all from EMD Millipore, Billerica, MA). Two of 34 CAFs (6%) were rejected because of the number of out-of-range samples.
Statistical Analysis
Baseline characteristics were summarized as numbers and percentages for categorical variables and as medians with interquartile ranges for continuous variables. The primary analysis used an intention-to-treat approach to compare RFS between patients assigned to ADT + bevacizumab versus ADT alone. The stratified log-rank test initially planned for the primary analysis included the following stratification factors and resulted in eight subgroups: age (≤ 60 v > 60 years), prior RP (yes v no), and metastatic disease status (yes v no). Because of the small sample sizes of these subgroups, a stratified log-rank test was not appropriate, so a log-rank test was used. We also performed a Cox regression model adjusting for the above covariates. RFS was summarized graphically by using Kaplan-Meier methodology. Hazard ratio (HR) and 95% CIs from a Cox regression model were calculated.
CAF levels at baseline and month 6, percentage change between month 6 and baseline, and relapse and percentage change between time of relapse and baseline were summarized as medians and interquartile ranges by arm. The Wilcoxon rank sum test was used to assess whether the percentage of change in biomarker levels between month 6 and baseline differed by treatment. The Cox regression model was used to assess the association of biomarker levels at baseline, interaction between markers and treatment, and percentage of change between month 6 and baseline biomarker levels with RFS. HRs and 95% CIs were determined for subgroups from the interaction between markers and treatment test. To assess the association, we dichotomized biomarker levels at the median. Because of the limited sample size and the exploratory nature of these analyses, no correction for multiple testing was performed. Glucose, hemoglobin A1c (HgBA1c), and lipids at baseline, during treatment, at end of treatment, and after treatment (for lipids only) were compared with baseline levels by using a Wilcoxon signed rank test.
RESULTS
Baseline Patient Characteristics
In total, 102 participants were enrolled from January 2009 to December 2012. The baseline characteristics between arms were well-balanced for known prognostic factors (Table 1). The majority of the patients were older than 60 years (75%) and had Gleason 7 disease (54%). The percentage of patients treated with RP (82% ADT + bevacizumab arm v 83% ADT alone arm) and those with metastatic disease (32% ADT + bevacizumab arm v 36% ADT alone arm) was similar between arms.
Table 1.
Baseline Clinical Characteristics
| Characteristic | ADT + Bevacizumab (n = 66) | ADT Alone (n = 36) | Entire Cohort (n = 102) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median | q1, q3 | No. | % | Median | q1, q3 | No. | % | Median | q1, q3 | |
| At diagnosis | ||||||||||||
| PSA, ng/mL | 62 | 6.7 | 4.9, 11.3 | 33 | 6.5 | 4.7, 11.0 | 95 | 6.7 | 4.7, 11.2 | |||
| Gleason score | ||||||||||||
| 6 | 10 | 15 | 5 | 14 | 15 | 15 | ||||||
| 7 | 34 | 53 | 21 | 58 | 55 | 54 | ||||||
| 8-10 | 20 | 30 | 10 | 28 | 30 | 29 | ||||||
| Unknown | 2 | 3 | 0 | 0 | 2 | 2 | ||||||
| Prior RP | 54 | 82 | 30 | 83 | 84 | 82 | ||||||
| Prior RT | 52 | 79 | 33 | 92 | 85 | 83 | ||||||
| At baseline | ||||||||||||
| Age, years | 66 | 66 | 60, 69 | 36 | 63 | 60, 68 | 102 | 65 | 60, 69 | |||
| > 60 | 15 | 23 | 11 | 31 | 26 | 25 | ||||||
| ≤ 60 | 51 | 77 | 25 | 69 | 76 | 75 | ||||||
| ECOG performance status | ||||||||||||
| 0 | 57 | 86 | 34 | 94 | 91 | 89 | ||||||
| 1 | 8 | 12 | 1 | 3 | 9 | 9 | ||||||
| Unknown | 1 | 2 | 1 | 3 | 2 | 2 | ||||||
| Presence of metastatic disease | 21 | 32 | 13 | 36 | 34 | 33 | ||||||
| Bone metastases | 8 | 12 | 3 | 8 | 11 | 11 | ||||||
| Lymph node metastases | 13 | 20 | 11 | 31 | 24 | 24 | ||||||
| PSA, ng/mL | 63 | 6.0 | 3.1, 10.8 | 34 | 7.3 | 4.3, 16.0 | 97 | 6.5 | 3.6, 12.8 | |||
| PSADT, months | 65 | 4.1 | 2.6, 8.6 | 36 | 4.8 | 2.8, 0.0 | 101 | 4.6 | 2.6, 8.9 | |||
| Testosterone, ng/dL | 65 | 362 | 267, 430 | 35 | 352 | 296, 475 | 100 | 362 | 272, 463 | |||
| Alkaline phosphatase, U/L | 63 | 64 | 52, 79 | 35 | 67 | 60, 85 | 98 | 66 | 53, 81 | |||
| Hemoglobin, g/dL | 65 | 14.4 | 13.9, 15.2 | 36 | 14.6 | 14.0, 15.2 | 101 | 14.5 | 13.9, 15.2 | |||
Abbreviations: ADT, androgen deprivation therapy; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; RP, radical prostatectomy; RT, radiotherapy.
Treatment Exposure
Sixty-six patients were assigned to ADT + bevacizumab and 36 to ADT alone (Fig 1). In the ADT + bevacizumab arm, two patients never received treatment, one discontinued treatment before completion because of the development of proteinuria, and two discontinued treatment by choice. In the ADT-alone arm, one patient never received treatment and one discontinued treatment by choice. Of the seven patients who discontinued treatment, follow-up information was available for one patient.
Fig 1.
CONSORT flow diagram. ADT, androgen deprivation therapy.
Efficacy
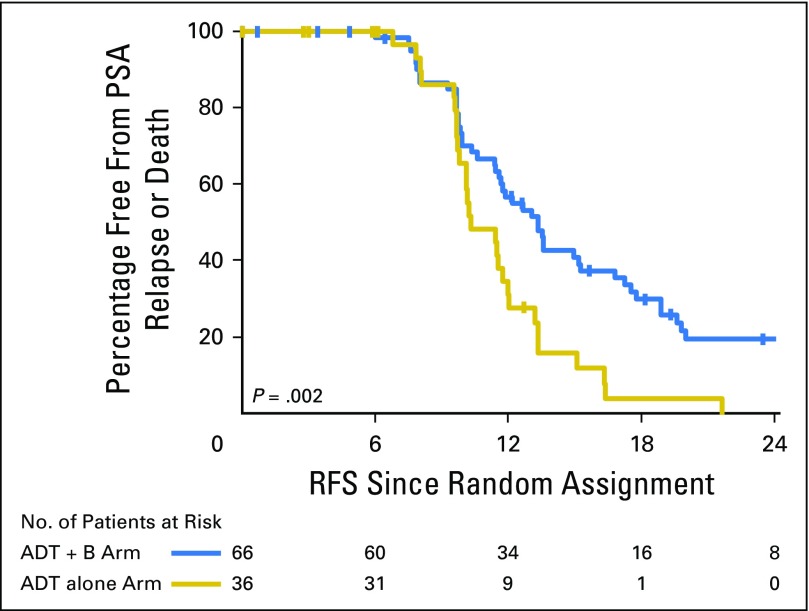
At the time of final analysis, 77 patients had experienced a PSA relapse, and patients were observed until PSA relapse. After completing treatment, 80% of patients treated with ADT + bevacizumab (n = 51 of 64; 95% CI, 67% to 89%) and 77% of patients treated with ADT alone (n = 27 of 35; 95% CI, 60% to 90%) had a PSA of less than 0.2 ng/mL. The median RFS for the ADT + bevacizumab arm was 13.3 months (range, 0 to 49 months) compared with 10.2 months (range, 0 to 21 months) for the ADT-alone arm, and this difference was statistically significant (log-rank P = .002; HR, 0.47; 95% CI, 0.29 to 0.77; Fig 2). One-year RFS was 57% (95% CI, 43% to 68%) for patients receiving ADT + bevacizumab compared with 31% (95% CI, 16% to 48%) for patients receiving ADT alone. After adjusting for age, prior RP, and metastasis status, the HR for comparing ADT + bevacizumab versus ADT alone was 0.49 (95% CI, 0.30 to 0.82; P = .01). None of the patients experienced radiographic progression before PSA progression. In patients who remained free from PSA relapse, the median follow-up was 16 months (n = 17) in the ADT + bevacizumab arm and 5 months in the ADT-alone arm (n = 8).
Fig 2.
Kaplan-Meier plot for relapse-free survival (RFS) by treatment arm. ADT, androgen deprivation therapy; B, bevacizumab; PSA, prostate-specific antigen.
Of the 65 patients with available data, 71% of those who were treated with ADT + bevizumab and 71% of those who were treated with ADT alone experienced a testosterone recovery (testosterone > 200 ng/dL). The median time to testosterone recovery was 9.9 months (range, 9.7 to 11.8 months) in the ADT + bevizumab arm and 10.1 months (range, 9.6 to 11.4 months) in the ADT-alone arm. Of the 65 patients with data on testosterone levels, 57 developed a relapse, 43 of whom relapsed before testosterone recovery. The median RFS since time of testosterone recovery was 4 months. Of patients who developed a relapse, 44% (n = 34 of 77) experienced testosterone recovery; 28% of patients (n = 7 of 25) remained in remission. Eight patients did not experience a PSA recurrence at 24 months, of whom four had testosterone recovery (range, 12.0 to 15.9 months).
Safety and Toxicity
The safety analysis includes the 99 patients who received at least one dose of the treatment drug. Table 2 summarizes all grade 2 or higher adverse events by arm. In the ADT + bevacizumab arm, 44 (69%; 95% CI, 56% to 80%) of 64 patients experienced grade 2 or 3 toxicities, of which hypertension was the most frequent (n = 23 [36%] of 64), followed by musculoskeletal pain, infection, and headache. In the ADT-alone arm, nine (26%; 95% CI, 12% to 43%) of 35 patients experienced grade 2 or 3 toxicities, of which hypertension and hot flashes were the most common. There were no grade 4 or 5 toxicities in either arm.
Table 2.
Grade 2 or Higher Adverse Events by Treatment Arm
| Adverse Event | ADT + Bevacizumab (n = 64) | ADT Alone (n = 35) | ||
|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 2 | Grade 3 | |
| Hypertension | 10 | 13 | 0 | 2 |
| Musculoskeletal pain | 5 | 3 | 0 | 1 |
| Infection | 7 | 1 | 0 | 0 |
| Headache | 6 | 1 | 1 | 0 |
| Hot flashes | 5 | 0 | 2 | 0 |
| Fatigue | 6 | 0 | 0 | 0 |
| Proteinuria | 5 | 0 | 0 | 0 |
| Weakness | 3 | 0 | 0 | 0 |
| Allergic rhinitis | 2 | 0 | 0 | 0 |
| Arthritis | 2 | 0 | 0 | 0 |
| Urinary frequency/urgency | 2 | 0 | 0 | 0 |
| Hyponatremia | 1 | 1 | 0 | 0 |
| Dyspnea | 1 | 0 | 0 | 1 |
| Hypertriglyceridemia | 1 | 0 | 1 | 0 |
| Syncope | 0 | 1 | 0 | 1 |
NOTE. Toxicities experienced by more than one patient are included.
Abbreviation: ADT, androgen deprivation therapy.
For patients in the ADT + bevacizumab arm, total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein significantly increased at 3 months and at the end of treatment compared with baseline (Appendix Table A1, online only). At 6 months after treatment, levels were not significantly different from baseline. A similar but less pronounced trend was observed in the ADT-alone arm.
Biomarkers
A total of 64 patients had CAF levels at baseline, of whom 35 (55%) had paired measurements at completion of therapy. Baseline characteristics and CAF levels were similar between arms for patients with available data (Appendix Tables A2 and A3, online only). By treatment arm, the only marker showing a change between baseline and completion of therapy that reached statistical significance was interleukin-1β (IL-1β; –5% for ADT + bevacizumab v 24% for ADT alone; P = .04; Appendix Table A4, online only).
We analyzed the association of pretreatment CAF levels with RFS (Appendix Table A5, online only). Lower leptin, IL-2, and tumor necrosis factor alpha levels were associated with significantly shorter RFS (Fig 3), and the same trends were observed in both arms separately (not shown). We next evaluated the interaction of these three CAFs with metastatic disease status and found significance only for leptin (interaction P = .006). Lower leptin levels were associated with an increased risk of relapse only in patients without metastases (15 months for greater than median v 10 months for less than or equal to median leptin; HR, 5.06; 95% CI, 2.26 to 11.32). However, leptin levels did not have an impact on outcomes in patients with metastasis (11 v 11 months; HR, 0.96; 95% CI, 0.42 to 2.23). Higher leptin was significantly associated with lower baseline testosterone (399 ng/dL for median or less v 303 ng/dL for greater than median leptin; P = .014).
Fig 3.
Kaplan-Meier plot for relapse-free survival (RFS) by baseline (A) leptin, (B) interleukin-2, and (C) tumor necrosis factor α levels.
We also analyzed whether baseline CAFs were differentially associated with RFS by treatment arm and identified a significant association for IL-6 and osteopontin (interaction P = .03 for each CAF). In patients with higher osteopontin or IL-6, type of treatment affected RFS (Fig 4): those receiving ADT + bevacizumab had a longer RFS than those who received ADT alone (osteopontin: 15 v 10 months; HR, 0.44; 95% CI, 0.23 to 0.86; IL-6: 13 v 10 months; HR, 0.45; 95% CI, 0.23 to 0.83). However, in patients with lower baseline osteopontin or IL-6, treatment did not have an impact on RFS.
Fig 4.
Kaplan-Meier plot for relapse-free survival (RFS) by baseline (A) osteopontin less than or equal to median, (B) osteopontin greater than median, (C) interleukin-6 (IL-6) less than or equal to median, and (D) interleukin-6 greater than median. Interaction P = .03 for (A,B) osteopontin and (C,D) interleukin-6. ADT, androgen deprivation therapy; B, bevacizumab.
DISCUSSION
In this study, we demonstrate that treatment with short-term ADT + bevacizumab was feasible, although it was associated with more toxicity than ADT alone in hormone-sensitive prostate cancer. When compared with ADT, the addition of bevacizumab resulted in a statistically significant improvement of RFS and improved 1-year RFS.
Although the use of antiangiogenic therapy in prostate cancer remains investigational, these results indicate that further evaluation of anti-VEGF-targeting therapy combined with ADT is warranted and may be a rational approach to prolong response to ADT even after ADT is discontinued.
A number of phase III studies have investigated antiangiogenic therapies in mCRPC, including bevacizumab, cabozantinib, sunitinib, and aflibercept (Appendix Table A6, online only).14,16-19 Although none of these agents have demonstrated improvements in OS, bevacizumab, cabozantinib, and sunitinib have demonstrated improvements in progression-free survival suggesting activity. Furthermore, docetaxel has demonstrated antiangiogenic properties, and early use in patients with hormone-sensitive disease shows improved OS.20,21 Taken together, these results suggest that with improved VEGF targeting or biomarkers of response, there may be a role for antiangiogenic therapies in prostate cancer. Further studies are warranted to identify the target population most likely to benefit.
There have been limited studies investigating the efficacy of antiangiogenic therapies in patients with hormone-sensitive prostate cancer. We previously investigated multimodality therapy with docetaxel, bevacizumab, and ADT in men for whom PSA treatment failed after local therapy and demonstrated that a subset of patients experienced a durable PSA remission.22 At a median follow-up of 27.5 months, 46% of patients were free from further therapy and 92% had testosterone ≥ 100 ng/dL.22 Furthermore, we evaluated neoadjuvant docetaxel with bevacizumab in localized high-risk prostate cancer.23 In that phase II study of 41 men undergoing RP, 29% achieved a greater than 50% reduction in tumor volume as measured by magnetic resonance imaging. The impact of chemotherapy and antiangiogenic therapy in the context of RP and radiotherapy is also an area of ongoing investigation.
In this study, the most common grade 3 toxicity was hypertension; approximately 20% of patients treated with ADT + bevacizumab experienced grade 3 events. This is higher than the rate (7%) observed in Cancer and Leukemia Group B 90401 (CALGB 90401 [Alliance] A Randomized Double-Blinded Placebo Controlled Phase III Trial Comparing Docetaxel and Prednisone With and Without Bevacizumab [IND #7921, NSC #704865] in Men With Hormone Refractory Prostate Cancer), a phase III trial of docetaxel with or without bevacizumab in mCRPC. Furthermore, we demonstrate that therapy was associated with increased lipids, which returned to baseline after discontinuation of treatment. This finding is consistent with multiple prospective studies that demonstrated lipid alterations with ADT.24,25 The effect on lipid levels was more pronounced in the ADT + bevacizumab arm. Although the study was not powered to detect differences in the two cohorts, it was reassuring that levels returned to baseline after therapy was discontinued. Bevacizumab is not being further developed for any stage of prostate cancer; however, newer anti-VEGF agents are worthy of investigation in combination with other active therapies. The balance of benefit compared with toxicities will remain important.
Given the limited efficacy of VEGF-targeted therapies in unselected patients, we measured a broad set of relevant CAFs and evaluated their association with clinical outcomes, screening for candidate markers that predicted benefit from bevacizumab. We found that lower pretreatment levels of leptin, IL-2, and tumor necrosis factor alpha were associated with shorter RFS. Of these, perhaps the most intriguing is leptin. Patients with low leptin had a higher risk for relapse, but this relationship was confined to those without metastasis. Higher leptin is linked to obesity and metabolic syndrome in humans, but studies that examined the association between leptin and the risk of prostate cancer have been contradictory.26 We hypothesized that leptin may have an impact on baseline testosterone levels and testosterone recovery after treatment. Patients with higher leptin had lower baseline testosterone and, in patients who achieved testosterone recovery, there was a trend for longer time to testosterone recovery in those with higher leptin (10.2 months for patients with less than or equal to median leptin v 9.7 months for patients with greater than median leptin).
Also of interest was our finding of a relationship between pretreatment IL-6 and osteopontin and relative benefit from bevacizumab. In patients with higher levels, those treated with bevacizumab were less likely to experience relapse. However, we found no difference in RFS by treatment in patients with lower levels. Like leptin, IL-6 is an adipokine, but its biologic significance is likely greater in castration-resistant than in castration-sensitive disease. The role of osteopontin, which is involved in bone remodeling, immune response, and inflammation, is less clear in prostate cancer.27,28 Our data are consistent with renal cell carcinoma, in which higher levels of both osteopontin and IL-6 have been linked to increased benefit from VEGF-targeted therapy.29,30 These biomarkers warrant further investigation in the context of anti-VEGF therapies.
Although this was a randomized, phase II study, there were several limitations. We did not use the stratified log-rank test intended for the primary analysis, given imbalances in the number of patients per strata. Furthermore, the biomarker analysis was exploratory in nature without adjustment for multiple comparisons.
In conclusion, we demonstrated that short-term ADT + bevacizumab was feasible and resulted in improvements in RFS compared with ADT alone. We identified biomarkers with potential prognostic and predictive potential. Given the expanding treatment armamentarium for hormone-sensitive prostate cancer, predictive biomarkers are necessary to identify patients likely to benefit from any approach incorporating anti-VEGF therapy.20 Our data suggest that ADT + bevacizumab may benefit a subset of patients with hormone-sensitive disease. We provide a rationale for combining VEGF therapy with ADT to prolong the off-ADT cycle during intermittent ADT. Long-term follow-up is needed to determine whether some patients will have durable PSA responses with testosterone recovery and be able to remain off further prostate cancer therapy.
Acknowledgment
We thank the Prostate Cancer Clinical Trials Consortium for their support of this clinical trial.
GLOSSARY TERMS
- androgen deprivation therapy (ADT):
therapy which results in a reduction of circulating testosterone levels, which may be achieved by surgical castration, by LHRH agonists or antagonists, by androgen receptor blockers, or by androgen synthesis blockers.
- angiogenesis:
the process involved in the generation of new blood vessels. Although this is a normal process that naturally occurs and is controlled by so-called on and off switches, blocking tumor angiogenesis (antiangiogenesis) disrupts the blood supply to tumors, thereby preventing tumor growth.
- bevacizumab:
also called Avastin (Genentech, South San Francisco, CA). Bevacizumab is a recombinant, humanized, monoclonal antibody that binds and neutralizes the vascular endothelial growth factor, thus acting as an antiangiogenic agent.
- vascular endothelial growth factor (VEGF):
a cytokine that mediates numerous functions of endothelial cells including proliferation, migration, invasion, survival, and permeability. VEGF is also known as vascular permeability factor. VEGF naturally occurs as a glycoprotein and is critical for angiogenesis. Many tumors overexpress VEGF, which correlates with poor prognosis. VEGF-A, -B, -C, -D, and -E are members of the larger family of VEGF-related proteins.
Appendix
Table A1.
Glucose, HgBA1c, and Lipid Trends at Baseline, During Treatment, and at the End of Treatment
| Glucose, HgBA1C, or Lipid (mg/dL)* | ADT + Bevacizumab | ADT Alone | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median | q1, q3 | P | No. | % | Median | q1, q3 | P | |
| Glucose | ||||||||||
| Baseline | 65 | 99 | 92, 110 | Reference | 36 | 98 | 90, 103 | Reference | ||
| > 110 | 15 | 23 | 6 | 17 | ||||||
| 3 months on treatment | 57 | 96 | 92, 109 | .71 | 33 | 104 | 95, 114 | .05 | ||
| > 110 | 13 | 23 | 9 | 27 | ||||||
| End of treatment | 57 | 99 | 92, 110 | .86 | 32 | 98 | 90, 112 | .90 | ||
| > 110 | 13 | 23 | 7 | 22 | ||||||
| HgBA1C | ||||||||||
| Baseline | 65 | 5.6 | 5.4, 5.9 | Reference | 35 | 5.6 | 5.4, 5.8 | Reference | ||
| > 6.5 | 6 | 9 | 2 | 6 | ||||||
| 3 months on treatment | 55 | 5.7 | 5.5, 6.0 | .24 | 32 | 5.7 | 5.5, 6.3 | .19 | ||
| > 6.5 | 4 | 7 | 5 | 16 | ||||||
| End of treatment | 53 | 5.6 | 5.3, 5.9 | .78 | 29 | 5.6 | 5.4, 5.9 | .96 | ||
| > 6.5 | 4 | 8 | 3 | 10 | ||||||
| Total cholesterol | ||||||||||
| Baseline | 65 | 177 | 156, 196 | Reference | 35 | 162 | 144, 204 | Reference | ||
| > 200 | 16 | 25 | 9 | 25 | ||||||
| 3 months on treatment | 61 | 213 | 181, 232 | < .001 | 34 | 181 | 157, 207 | .18 | ||
| > 200 | 37 | 61 | 12 | 35 | ||||||
| End of treatment | 56 | 203 | 186, 241 | < .001 | 32 | 189 | 162, 203 | .15 | ||
| > 200 | 30 | 54 | 8 | 25 | ||||||
| 6 months after treatment | 35 | 180 | 160, 210 | .21 | 10 | 167 | 134, 193 | .88 | ||
| > 150 | 10 | 29 | 2 | 25 | ||||||
| Triglycerides | ||||||||||
| Baseline | 65 | 114 | 82, 152 | Reference | 36 | 107 | 91, 153 | Reference | ||
| > 150 | 17 | 26 | 10 | 27 | ||||||
| 3 months on treatment | 61 | 136 | 102, 189 | .04 | 34 | 121 | 93, 152 | .67 | ||
| > 150 | 26 | 43 | 10 | 29 | ||||||
| End of treatment | 56 | 146 | 98, 233 | .02 | 30 | 118 | 84, 170 | .96 | ||
| > 150 | 25 | 45 | 8 | 27 | ||||||
| 6 months after treatment | 35 | 109 | 69, 177 | .66 | 10 | 109 | 83, 145 | .44 | ||
| > 150 | 12 | 34 | 2 | 25 | ||||||
| High-density lipoprotein | ||||||||||
| Baseline | 65 | 48 | 32, 54 | Reference | 36 | 46 | 42, 56 | Reference | ||
| < 40 | 13 | 20 | 4 | 11 | ||||||
| 3 months on treatment | 61 | 51 | 45, 63 | .01 | 34 | 52 | 47, 60 | .04 | ||
| < 40 | 4 | 7 | 3 | 9 | ||||||
| End of treatment | 56 | 51 | 45, 63 | .04 | 31 | 53 | 45, 57 | .13 | ||
| < 40 | 7 | 13 | 4 | 13 | ||||||
| 6 months after treatment | 35 | 109 | 69, 177 | .75 | 10 | 109 | 83, 145 | .39 | ||
| < 40 | 12 | 34 | 2 | 25 | ||||||
| Low-density lipoprotein | ||||||||||
| Baseline | 65 | 108 | 82, 124 | Reference | 35 | 92 | 75, 116 | Reference | ||
| > 129 | 14 | 22 | 4 | 11 | ||||||
| 3 months on treatment | 61 | 123 | 108, 149 | < .001 | 34 | 103 | 84, 115 | .38 | ||
| > 129 | 28 | 46 | 5 | 15 | ||||||
| End of treatment | 55 | 122 | 102, 147 | .02 | 31 | 207 | 85, 127 | .16 | ||
| > 129 | 23 | 42 | 5 | 15 | ||||||
| 6 months after treatment | 34 | 106 | 87, 133 | .29 | 10 | 98 | 74, 114 | .61 | ||
| > 129 | 10 | 29 | 2 | 25 | ||||||
NOTE. Glucose and lipid values were collected during fasting state. P values in bold are statistically significant.
Abbreviations: ADT, androgen deprivation therapy; HgBA1c, hemoglobin A1c.
The unit of measure for glucose, HgBA1c, and lipids is mg/dL.
Table A2.
Baseline Clinical Characteristics for Patients With Available Biomarker Data
| Characteristic | ADT + Bevacizumab (n = 43) | ADT Alone (n = 21) | Entire Cohort (n = 64) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median | q1, q3 | No. | % | Median | q1, q3 | No. | % | Median | q1, q3 | |
| At diagnosis | ||||||||||||
| Gleason score | ||||||||||||
| 6 | 4 | 9 | 2 | 10 | 6 | 9 | ||||||
| 7 | 23 | 53 | 13 | 62 | 36 | 56 | ||||||
| 8-10 | 15 | 35 | 6 | 28 | 21 | 33 | ||||||
| Unknown | 1 | 3 | 0 | 0 | 1 | 2 | ||||||
| Prior RP | 36 | 84 | 16 | 76 | 52 | 81 | ||||||
| Prior RT | 51 | 72 | 21 | 100 | 52 | 81 | ||||||
| At baseline | ||||||||||||
| Age, years | 43 | 66 | 60, 68 | 21 | 65 | 59, 71 | 64 | 66 | 60, 69 | |||
| > 60 | 10 | 23 | 7 | 33 | 17 | 27 | ||||||
| ≤ 60 | 33 | 77 | 14 | 67 | 47 | 73 | ||||||
| ECOG performance status | ||||||||||||
| 0 | 39 | 91 | 20 | 95 | 59 | 92 | ||||||
| 1 | 3 | 7 | 1 | 5 | 4 | 6 | ||||||
| Unknown | 1 | 2 | 0 | 0 | 1 | 2 | ||||||
| Presence of metastatic disease | 21 | 32 | 13 | 36 | 34 | 33 | ||||||
| Bone metastases | 8 | 12 | 3 | 8 | 11 | 11 | ||||||
| Lymph node metastases | 13 | 20 | 11 | 31 | 24 | 24 | ||||||
| PSA, ng/mL | 41 | 6.0 | 3.3, 11.7 | 21 | 9.2 | 6.1, 18.9 | 62 | 7.0 | 3.8, 14.6* | |||
| PSADT, months | 42 | 4.1 | 2.6, 7.6 | 21 | 4.4 | 3.0, 9.2 | 63 | 4.3 | 2.6, 8.1 | |||
| Testosterone, ng/dL | 42 | 358 | 267, 428 | 21 | 393 | 302, 491 | 62 | 358 | 277, 466 | |||
| Alkaline phosphatase, U/L | 41 | 61 | 52, 78 | 21 | 66 | 60, 70 | 62 | 65 | 53, 78 | |||
| Hemoglobin, g/dL | 42 | 14.4 | 13.8, 15.3 | 21 | 14.6 | 14.0, 15.2 | 63 | 14.4 | 13.9, 15.2 | |||
Abbreviations: ADT, androgen deprivation therapy; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time; RP, radical prostatectomy; RT, radiotherapy.
Minimum PSA was 0.3 ng/mL for the total cohort.
Table A3.
Summary of Baseline Biomarker Levels
| Biomarker | ADT + Bevacizumab (pg/mL) (n = 43) | ADT Alone (pg/mL) (n = 21) | ||
|---|---|---|---|---|
| Median | q1, q3 | Median | q1, q3 | |
| Angiopoietin-2 | 2,142 | 1,557, 2,967 | 1,813 | 1,602, 3,284 |
| BMP-9 | 119 | 82, 149 | 71 | 54, 138 |
| Collagen | 515 | 468, 593 | 511 | 451, 619 |
| EGF | 42 | 22, 68 | 52 | 19.2, 131 |
| Endoglin | 907 | 496, 1149 | 771 | 491, 944 |
| Endothelin-1 | 74 | 51, 102 | 62 | 49, 93 |
| GM-CSF | 45.3 | 17.8, 105.8 | 74.9 | 22.7, 97.7 |
| HGF | 1,564 | 1,195, 2,073 | 1,599 | 1,006, 1,787 |
| IFN-γ | 4.3 | 1.9, 7.8 | 3.68 | 1.82, 8.9 |
| IL-1β | 0.8 | 0.4, 1.4 | 0.50 | 0.3, 1.2 |
| IL-2 | 1.2 | 0.7, 2.0 | 0.68 | 0.4, 1.8 |
| IL-5 | 0.5 | 0.3, 1.0 | 0.57 | 0.4, 1.0 |
| IL-6 | 0.7 | 0.4, 1.0 | 0.52 | 0.3, 1.5 |
| IL-7 | 1.9 | 0.7, 3.1 | 2.16 | 1.1, 3.4 |
| IL-8 | 1.6 | 1.2, 2.5 | 1.66 | 0.6, 2.0 |
| IL-10 | 2.4 | 1.1, 3.3 | 1.77 | 0.7, 4.4 |
| IL-12 | 0.4 | 0.1, 1.3 | 0.68 | 0.2, 1.3 |
| IL-13 | 1.5 | 0.4, 2.8 | 1.45 | 0.4, 2.9 |
| Leptin | 13,755 | 6,570, 25,762 | 10,179 | 6,924, 15,333 |
| Osteocalcin | 10,063 | 7,975, 13,166 | 7,895 | 6,226, 18,118 |
| Osteopontin | 17,105 | 10,804, 21,462 | 19,302 | 13,709, 29,273 |
| Osteoprotegerin | 186 | 147, 254 | 169 | 124, 232 |
| PLGF | 45 | 33, 68 | 48 | 28, 66 |
| SDF-1α/β | 4,294 | 3,173, 5,463 | 4,226 | 3,745, 5,383 |
| sgp130 | 119,963 | 93,763, 139,225 | 111,333 | 92,805, 129,868 |
| sIL-6R | 17,518 | 14,537, 23,884 | 14,652 | 10,924, 19,589 |
| sVEGFR-2 | 6,166 | 4,727, 8,256 | 6,410 | 3,773, 7,562 |
| sVEGFR-3 | 2,148 | 1,528, 2,974 | 2,238 | 1,879, 2,789 |
| TIMP-1 | 193 | 164, 218 | 187 | 168, 240 |
| TNF-α | 1.5 | 1.1, 2.8 | 0.97 | 0.6, 2.9 |
| TRAIL* | 24 | 18, 36 | 22 | 18, 30 |
| VEGF-α | 381 | 256, 683 | 403 | 234, 560 |
Abbreviations: ADT, androgen deprivation therapy; BMP, bone morphogenetic protein; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; PLGF, placental growth factor; SDF, stromal cell-derived factor; sgp130, soluble gp130; sIL-6R, soluble interleukin 6 receptor; sVEGFR, soluble vascular endothelial growth factor receptor; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor; TRAIL, TNF–related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
At baseline, n = 28 for the ADT + bevacizumab arm and n = 14 for the ADT-alone arm.
Table A4.
Association of Treatment and Percentage Change of Markers From Baseline to Therapy Completion (6 months)
| Marker | ADT + Bevacizumab (pg/mL) (n = 22) | ADT Alone (pg/mL) (n = 13) | P | ||
|---|---|---|---|---|---|
| Median (%) | q1, q3 (%) | Median (%) | q1, q3 (%) | ||
| Angiopoietin-2 | −6 | –26, 1 | −1 | –15, 4 | .37 |
| BMP-9 | 19 | –2, 54 | 37 | 10, 55 | .39 |
| Collagen | 4 | –9, 23 | 0.3 | –11, 16 | .62 |
| EGF | 28 | –29, 77 | −1 | –24, 91 | .71 |
| Endoglin | −5 | –26, 45 | 1 | –25, 24 | .76 |
| Endothelin-1 | −12 | –35, 26 | 0.5 | –6, 18 | .63 |
| GM-CSF | −16 | –36, 3 | 5 | –35, 31 | .26 |
| HGF | −9 | –36, 7 | −6 | –12, 19 | .17 |
| IFN-γ | −19 | –45, 37 | 4 | –28, 73 | .24 |
| IL-1β | −5 | –41, 4 | 24 | –13, 73 | .04 |
| IL-2 | −8 | –32, 26 | 31 | –18, 105 | .08 |
| IL-5 | −30 | –46, 9 | 0 | –39, 66 | .29 |
| IL-6 | −10 | –36, 26 | 12 | –41, 38 | .55 |
| IL7 | −8 | –23, 14 | −16 | –33, 0 | .31 |
| IL8 | −11 | –29, 10 | −6 | –25, 36 | .52 |
| IL-10 | −22 | –41, 0 | 0 | –18, 107 | .11 |
| IL-12 | 0 | –12, 22 | 4 | –4, 24 | .57 |
| IL-13 | 0 | –18, 39 | 0 | –175, 175 | .71 |
| Leptin | 60 | 20, 111 | 22 | –2, 83 | .09 |
| Osteocalcin | −0.3 | –27, 26 | −25 | –40, 12 | .25 |
| Osteopontin | 45 | 2, 56 | 12 | –3, 34 | .14 |
| Osteoprotegerin | 3 | –16, 40 | 6 | –10, 21 | .78 |
| PLGF | −7 | –27, 44 | 7 | –2, 44 | .47 |
| SDF-1α/β | 6 | –9, 18 | −2 | –4, 12 | .84 |
| sgp130 | 3 | –8, 17 | 12 | –12, 36 | .21 |
| sIL-6R | 8 | –19, 17 | 4 | –5, 40 | .34 |
| sVEGFR-2 | 5 | –19, 35 | 4 | –10, 36 | .76 |
| sVEGFR-3 | 3 | –20, 30 | −10 | –21, 10 | .34 |
| TIMP-1 | 6 | –10, 22 | −0.2 | –9, 3 | .37 |
| TNF-α | −2 | –28, 27 | 42 | –15, 79 | .22 |
| TRAIL* | 3 | –11, 28 | 3 | –29, 34 | > .99 |
| VEGF-α | −22 | –44, 7 | 2 | –9, 20 | .06 |
Abbreviations: BMP, bone morphogenetic protein; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; PLGF, placental growth factor; SDF, stromal cell-derived factor; sgp130, soluble gp130; sIL-6R, soluble interleukin 6 receptor; sVEGFR, soluble vascular endothelial growth factor receptor; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
For the ADT + bevacizumab arm, n = 28 at baseline, and n = 18 at 6 months; for the ADT-alone arm, n = 14 at baseline, and n = 9 at 6 months.
Table A5.
Univariate Analysis of the Association of Markers at Baseline and RFS
| Marker | No. of Patients | No. of Events | Median RFS | P |
|---|---|---|---|---|
| Angiopoietin-2 | .68 | |||
| ≤ median | 32 | 28 | 11 | |
| > median | 32 | 28 | 12 | |
| BMP-9 | .11 | |||
| ≤ median | 32 | 27 | 12 | |
| > median | 32 | 29 | 10 | |
| Collagen | .71 | |||
| ≤ median | 32 | 28 | 12 | |
| > median | 32 | 28 | 11 | |
| EGF | .33 | |||
| ≤ median | 32 | 27 | 12 | |
| > median | 32 | 27 | 11 | |
| Endoglin | .31 | |||
| ≤ median | 33 | 29 | 12 | |
| > median | 31 | 27 | 10 | |
| Endothelin-1 | .77 | |||
| ≤ median | 32 | 28 | 11 | |
| > median | 32 | 28 | 12 | |
| GM-CSF | .80 | |||
| ≤ median | 32 | 28 | 10 | |
| > median | 32 | 28 | 12 | |
| HGF | .64 | |||
| ≤ median | 32 | 29 | 12 | |
| > median | 32 | 27 | 11 | |
| IFN-γ | .42 | |||
| ≤ median | 32 | 27 | 11 | |
| > median | 32 | 29 | 12 | |
| IL-1β | .15 | |||
| ≤ median | 32 | 28 | 11 | |
| > median | 32 | 28 | 12 | |
| IL-2 | .006 | |||
| ≤ median | 32 | 29 | 10 | |
| > median | 32 | 27 | 12 | |
| IL-5 | .33 | |||
| ≤ median | 32 | 27 | 11 | |
| > median | 32 | 29 | 12 | |
| IL-6 | .07 | |||
| ≤ median | 32 | 28 | 10 | |
| > median | 32 | 28 | 12 | |
| IL-7 | .28 | |||
| ≤ median | 32 | 27 | 11 | |
| > median | 32 | 29 | 12 | |
| IL-8 | .18 | |||
| ≤ median | 32 | 28 | 11 | |
| > median | 32 | 28 | 12 | |
| IL-10 | .37 | |||
| ≤ median | 32 | 28 | 10 | |
| > median | 32 | 28 | 12 | |
| IL-12 | .36 | |||
| ≤ median | 32 | 29 | 11 | |
| > median | 32 | 27 | 12 | |
| IL-13 | .88 | |||
| ≤ median | 32 | 28 | 12 | |
| > median | 32 | 28 | 11 | |
| Leptin | .005 | |||
| ≤ median | 32 | 29 | 10 | |
| > median | 32 | 27 | 13 | |
| Osteocalcin | .69 | |||
| ≤ median | 32 | 28 | 11 | |
| > median | 32 | 28 | 12 | |
| Osteopontin | .11 | |||
| ≤ median | 32 | 29 | 10 | |
| > median | 32 | 27 | 12 | |
| Osteoprotegerin | .31 | |||
| ≤ median | 32 | 29 | 11 | |
| > median | 32 | 27 | 12 | |
| PLGF | .73 | |||
| ≤ median | 32 | 28 | 12 | |
| > median | 32 | 28 | 11 | |
| SDF-1α/β | .59 | |||
| ≤ median | 32 | 27 | 10 | |
| > median | 32 | 29 | 12 | |
| sgp130 | .60 | |||
| ≤ median | 32 | 27 | 12 | |
| > median | 32 | 29 | 11 | |
| sIL-6R | .56 | |||
| ≤ median | 32 | 27 | 12 | |
| > median | 32 | 29 | 12 | |
| sVEGFR-2 | .44 | |||
| ≤ median | 32 | 28 | 11 | |
| > median | 32 | 28 | 12 | |
| sVEGFR-3 | .19 | |||
| ≤ median | 36 | 30 | 12 | |
| > median | 28 | 26 | 11 | |
| TIMP-1 | .30 | |||
| ≤ median | 32 | 27 | 10 | |
| > median | 32 | 29 | 12 | |
| TNF-α | .048 | |||
| ≤ median | 32 | 28 | 10 | |
| > median | 32 | 28 | 12 | |
| TRAIL | .44 | |||
| ≤ median | 21 | 19 | 10 | |
| > median | 21 | 18 | 10 | |
| VEGF-α | .52 | |||
| ≤ median | 32 | 29 | 11 | |
| > median | 32 | 27 | 12 |
NOTE. P values in bold are statistically significant.
Abbreviations: BMP, bone morphogenetic protein; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; IFN, interferon; IL, interleukin; PLGF, placental growth factor; RFS, relapse-free survival; SDF, stromal cell-derived factor; sgp130, soluble gp130; sIL-6R, soluble interleukin-6 receptor; sVEGFR, soluble vascular endothelial growth factor receptor; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor.
Table A6.
Phase III Studies of Antiangiogenic Agents in mCRPC
| Agent | Mechanism of Action | No. | Population | Treatment Arms | Primary End Point | OS (months) | P | PFS (months) | P |
|---|---|---|---|---|---|---|---|---|---|
| Bevacizumab14 | Monoclonal antibody to VEGF | 1,050 | mCRPC, chemotherapy naïve | Docetaxel + prednisone + bevacizumab | OS | 22.6 | .181 | 9.9 | < .001 |
| Docetaxel + prednisone + placebo | 21.5 | 7.5 | |||||||
| Cabozantinib18 | VEGFR-MET inhibitor | 1,028 | mCRCP, docetaxel and abiraterone and/or enzalutamide pretreated | Cabozantinib | OS | 11 | .212 | 5.5 | < .001 |
| Placebo | 9.8 | 2.8 | |||||||
| Sunitinib16 | VEGFR inhibitor | 873 | mCRPC, docetaxel pretreated | Sunitinib | OS | 13.1 | .168 | 5.6 | < .001 |
| Placebo | 11.8 | 4.1 | |||||||
| Aflibercept19 | Recombinant fusion protein, which acts as a decoy receptor for VEGF | 1,224 | mCRPC, chemotherapy naive | Docetaxel + prednisone + aflibercept | OS | 22.1 | .38 | 6.9 | .31 |
| Docetaxel + prednisone + placebo | 21.2 | 6.2 |
Abbreviations: mCRPC, metastatic castration-resistant prostate cancer; OS, overall survival; PFS, progression-free survival; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Footnotes
Supported by Genentech. Angiokine analysis was funded by The Fairweather Family Fund and Uribe Family Fund at the Dana-Farber Cancer Institute (M.-E.T.).
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Robert W. Ross, Philip W. Kantoff, Mary-Ellen Taplin
Financial support: Amado J. Zurita, Mary-Ellen Taplin
Administrative support: Philip W. Kantoff, Mary-Ellen Taplin
Provision of study materials or patients: Amado J. Zurita, Michael A. Carducci
Collection and assembly of data: Rana R. McKay, Amado J. Zurita, Lillian Werner, Justine Y. Bruce, Michael A. Carducci, Mark N. Stein, Elisabeth I. Heath, Arif Hussain, Christopher J. Sweeney, Susan F. Slovin, Mary-Ellen Taplin
Data analysis and interpretation: Rana R. McKay, Amado J. Zurita, Lillian Werner, Michael A. Carducci, Hai T. Tran, Mary-Ellen Taplin
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
A Randomized Phase II Trial of Short-Course Androgen Deprivation Therapy With or Without Bevacizumab for Patients With Recurrent Prostate Cancer After Definitive Local Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rana R. McKay
Research Funding: Pfizer (Inst), Bayer Healthcare Pharmaceuticals (Inst)
Amado J. Zurita
Consulting or Advisory Role: Incyte
Research Funding: Pfizer
Lillian Werner
No relationship to disclose
Justine Y. Bruce
No relationship to disclose
Michael A. Carducci
Consulting or Advisory Role: Medivation, Astellas Pharma, Blue Earth Diagnostics, AstraZeneca
Mark N. Stein
Research Funding: Janssen Pharmaceuticals (Inst), Medivation (Inst), Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Bavarian Nordic (Inst), AbbVie (Inst)
Elisabeth I. Heath
Honoraria: Bayer Diagnostics, Dendreon, Sanofi
Speakers’ Bureau: Sanofi Pasteur MSD, Genentech
Research Funding: Tokai Pharmaceuticals (Inst), Seattle Genetics (Inst), Agensys (Inst), Dendreon (Inst), Genentech (Inst), Millennium Pharmaceuticals (Inst), Celldex (Inst), Inovio Pharmaceuticals (Inst), Celgene (Inst)
Arif Hussain
No relationship to disclose
Hai T. Tran
Honoraria: Teva Pharmaceuticals Industries
Consulting or Advisory Role: Teva Pharmaceuticals Industries
Research Funding: Amgen
Christopher J. Sweeney
Stock or Other Ownership: Leuchemix, BIND Biosciences
Consulting or Advisory Role: Sanofi, Janssen Biotech, Astellas Pharma, Bayer Healthcare Pharmaceuticals, BIND Biosciences, Genentech, AstraZeneca
Research Funding: Janssen Biotech (Inst), Exelixis (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Leuchemix: parthenolide, dimethylaminoparthenolide; Exelixis: abiraterone plus cabozantinib combination
Robert W. Ross
Employment: Bluebird Bio
Stock or Other Ownership: Bluebird Bio
Philip W. Kantoff
Stock or Other Ownership: Bellicum Pharmaceuticals, Blend Therapeutics, Metamark Genetics
Consulting or Advisory Role: Bavarian Nordic, Janssen Pharmaceuticals, Millennium Pharmaceuticals, MorphoSys, Pfizer, Astellas Pharma, Bellicum Pharmaceuticals, BIND Biosciences, Blend Therapeutics, Endocyte, Metamark Genetics, Medivation, Merck, MTG Biotherapeutics, OncoCellMDX, OncoGenex, SOTIO, Sanofi, Tokai Pharmaceuticals, Bayer Healthcare Pharmaceuticals, Genentech, Cristal Therapeutics, Ipsen Pharmaceuticals, Omnitura, MorphoSys
Research Funding: Medivation (Inst), Sanofi (Inst), OncoGenex (Inst), Aragon Pharmaceuticals (Inst), Amgen (Inst), Astellas Pharma (Inst), Bayer (Inst), Bavarian Nordic (Inst), Dendreon (Inst), Exelixis (Inst), Janssen (Inst)
Expert Testimony: Sanofi, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Janssen Pharmaceuticals, BIND Biosciences, Bavarian Nordic, Millennium Pharmaceuticals
Susan F. Slovin
Consulting or Advisory Role: Bayer Pharmaceuticals
Mary-Ellen Taplin
Honoraria: Medivation/Astellas Pharma, Janssen Oncology, Bayer Healthcare Pharmaceuticals, Tokai Pharmaceuticals
Consulting or Advisory Role: Medivation/Astellas Pharma, Janssen Oncology, Bayer Pharmaceuticals, Tokai Pharmaceuticals
Research Funding: Genentech (Inst), Medivation/Astellas Pharma (Inst), Janssen Oncology (Inst), Bayer Healthcare Pharmaceuticals (Inst), Tokai Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Medivation/Astellas Pharma, Janssen Oncology, Bayer Healthcare Pharmaceuticals
REFERENCES
- 1.Agarwal PK, Sadetsky N, Konety BR, et al. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 2.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin J, Kelly WK. Targeting angiogenesis as a promising modality for the treatment of prostate cancer. Urol Clin North Am. 2012;39:547–560. doi: 10.1016/j.ucl.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 5.Duque JL, Loughlin KR, Adam RM, et al. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: Relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61:401–408. doi: 10.1590/s1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 6.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from Cancer and Leukemia Group B 9480. Clin Cancer Res. 2005;11:1815–1820. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson B, Gulding K, Conaway M, et al. Combination antiangiogenic and androgen deprivation therapy for prostate cancer: A promising therapeutic approach. Clin Cancer Res. 2004;10:8728–8734. doi: 10.1158/1078-0432.CCR-04-0902. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 9.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 12.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 13.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 14.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michaelson MD, Oudard S, Ou YC, et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J Clin Oncol. 2014;32:76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 17.Petrylak DP, Vogelzang NJ, Budnik N, et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2015;16:417–425. doi: 10.1016/S1470-2045(15)70025-2. [DOI] [PubMed] [Google Scholar]
- 18.Smith MR, De Bono JS, Sternberg CN, et al. Final analysis of COMET-1: Cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E). J Clin Oncol 33, 2015 (suppl 7; abstr 139) [Google Scholar]
- 19.Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): A phase 3, double-blind randomised trial. Lancet Oncol. 2013;14:760–768. doi: 10.1016/S1470-2045(13)70184-0. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney CJ, Miller KD, Sissons SE, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- 22.McKay RR, Gray KP, Hayes JH, et al. Docetaxel, bevacizumab, and androgen deprivation therapy for biochemical disease recurrence after definitive local therapy for prostate cancer. Cancer. 2015;121:2603–2611. doi: 10.1002/cncr.29398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross RW, Galsky MD, Febbo P, et al. Phase 2 study of neoadjuvant docetaxel plus bevacizumab in patients with high-risk localized prostate cancer: A Prostate Cancer Clinical Trials Consortium trial. Cancer. 2012;118:4777–4784. doi: 10.1002/cncr.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 189:S34-S42, 2013 (suppl 1); discussion S43-S44. [DOI] [PubMed]
- 25.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 26.Mistry T, Digby JE, Desai KM, et al. Obesity and prostate cancer: A role for adipokines. Eur Urol. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Anborgh PH, Mutrie JC, Tuck AB, et al. Pre- and post-translational regulation of osteopontin in cancer. J Cell Commun Signal. 2011;5:111–122. doi: 10.1007/s12079-011-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castellano G, Malaponte G, Mazzarino MC, et al. Activation of the osteopontin/matrix metalloproteinase-9 pathway correlates with prostate cancer progression. Clin Cancer Res. 2008;14:7470–7480. doi: 10.1158/1078-0432.CCR-08-0870. [DOI] [PubMed] [Google Scholar]
- 29.Nixon AB, Halabi S, Shterev I, et al. Identification of predictive biomarkers of overall survival (OS) in patients (pts) with advanced renal cell carcinoma (RCC) treated with interferon alpha (I) with or without bevacizumab (B): Results from CALGB 90206 (Alliance). J Clin Oncol 31, 2013 (suppl; abstr 4520) [Google Scholar]
- 30.Tran HT, Liu Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–837. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]