Abstract
Purpose
To provide recommendations on appropriate clinical trial designs in non–muscle-invasive bladder cancer (NMIBC) based on current literature and expert consensus of the International Bladder Cancer Group.
Methods
We reviewed published trials, guidelines, meta-analyses, and reviews and provided recommendations on eligibility criteria, baseline evaluations, end points, study designs, comparators, clinically meaningful magnitude of effect, and sample size.
Results
NMIBC trials must be designed to provide the most clinically relevant data for the specific risk category of interest (low, intermediate, or high). Specific eligibility criteria and baseline evaluations depend on the risk category being studied. For the population of patients for whom bacillus Calmette-Guérin (BCG) has failed, the type of failure (BCG unresponsive, refractory, relapsing, or intolerant) should be clearly defined to make comparisons across trials feasible. Single-arm designs may be relevant for the BCG-unresponsive population. Here, a clinically meaningful initial complete response rate (for carcinoma in situ) or recurrence-free rate (for papillary tumors) of at least 50% at 6 months, 30% at 12 months, and 25% at 18 months is recommended. For other risk levels, randomized superiority trial designs are recommended; noninferiority trials are to be used sparingly given the large sample size required. Placebo control is considered unethical for all intermediate- and high-risk strata; therefore, control arms should comprise the current guideline-recommended standard of care for the respective risk level. In general, trials should use time to recurrence or recurrence-free survival as the primary end point and time to progression, toxicity, disease-specific survival, and overall survival as potential secondary end points. Realistic efficacy thresholds should be set to ensure that novel therapies receive due review by regulatory bodies.
Conclusion
The International Bladder Cancer Group has developed formal recommendations regarding definitions, end points, and clinical trial designs for NMIBC to encourage uniformity among studies in this disease.
INTRODUCTION
There is a significant unmet need for new therapies in non–muscle-invasive bladder cancer (NMIBC), as evidenced by the fact that in more than 30 years, only three drugs have been approved by the US Food and Drug Administration (FDA) or European Medicines Agency for treatment of the disease: thiotepa (1959), bacillus Calmette-Guérin (BCG; 1990), and valrubicin (1998). Studies in NMIBC are hampered by lack of consensus on trial end points and appropriate control arms among regulatory bodies and confusion resulting from perceived difficulties related to these factors.
In recent years, the American Urological Association (AUA), FDA, European Association of Urology, and others have tried to address these issues and proposed trial designs to support the development of new therapies for NMIBC. Recommendations put forth have been based primarily on expert commentary and not on review of the available literature or on formal consensus of panel members.1,2 Furthermore, some of these proposed recommendations have been challenged by other bladder cancer experts.3 Although phase II marker lesion studies are an efficient design for screening the activity of new drugs, they are difficult to carry out because of ethical issues.4,5
The International Bladder Cancer Group has been systematically addressing these issues through recent publications defining various clinical trial design elements, including definitions of low-, intermediate-, and high-risk NMIBC; standards of care for each of these risk strata6,7; and definitions of outcomes such as recurrence, treatment failure, and disease progression.6,8 The purpose of this review is to expand upon this work and provide recommendations on appropriate clinical trial designs in NMIBC based on current literature, clinical practice guidelines, and expert consensus.
METHODS
We searched the Cochrane Library, Medline, and Embase (date range, 1995 to 2015) to identify published clinical trials, reviews, clinical practice guidelines, and meta-analyses that examined elements related to the design of clinical trials in NMIBC as of March 2015. Keywords included “nonmuscle invasive bladder cancer,” “clinical trials,” “study designs,” “high-risk,” “intermediate-risk,” “low-risk,” “BCG failure,” “BCG refractory,” and “intravesical treatment.” We reviewed identified articles as well as associated reference lists for additional applicable literature; we largely selected publications from the past 10 years but did not exclude commonly referenced and highly regarded older publications. The initial list of selected articles was further enhanced by individual suggestions of abstracts from annual congresses of the AUA, European Association of Urology, Society of Urologic Oncology, and American Society of Clinical Oncology, as well as relevant book chapters.
We met on 3 separate days, with discussions focused on patient eligibility criteria, baseline evaluations, efficacy end points, study designs, ideal comparators, appropriate sample sizes, and the magnitude of effect that would be considered clinically meaningful. Recommendations provided are based on amalgamation of the literature that the group deemed relevant as well as on group consensus and expert opinion.
RESULTS
NMIBC is a complex disease, and the cost of a clinical trial in this area can vary considerably based on what is considered standard of care versus research only. With extensive experience in clinical trials, we list our broad recommendations in Table 1. Inclusion criteria should not be too restrictive as to lead to recruitment difficulties, and end points should be practical and clinically meaningful. The pathologic reporting system should be clearly specified, and variant histology should be excluded. Wherever feasible, opportunities for translational biomarker research should also be considered.
Table 1.
General Recommendations for Clinical Trials in NMIBC From the IBCG
| Recommendation |
|---|
| Inclusion criteria should not be too restrictive |
| Although specific eligibility criteria are essential for patient accrual and ensuring generalizability of the study findings, from a statistical perspective, these criteria do not reduce bias, nor do they increase the power of a clinical trial (unless there are certain subgroups that benefit from the treatment and other subgroups that do not). Hence, these criteria should not be so restrictive as to lead to difficulties in patient recruitment or generalizability of findings to the nontrial clinical practice setting. |
| Clearly specify the pathologic reporting system |
| The WHO 2004 system (high or low grade), in conjunction with the 1973 system (G1, G2, or G3), is recommended. Central pathology review is also recommended (especially in high-grade T1) but not mandated. Variant histology should be excluded to avoid inappropriate treatment.9 |
| Ensure clinical trial end points are meaningful and practical |
| It is important that trial end points are clinically meaningful and related to the disease process, are practical so that they can be assessed in all patients in the same way, and occur frequently enough for the study to have adequate statistical power. |
| Carefully consider tissue end points |
| We do not recommend mandatory biopsies and prefer that these be performed for cause (eg, suspicious lesion or positive urinary marker such as urinary cytology). The exception is CIS where response is being documented, and thus, a study biopsy at 6 or 12 months is recommended by regulatory bodies. |
| Use of urinary markers is not mandated |
| Although we do not mandate the use of urinary markers in clinical trials of NMIBC, if used, the protocol should clearly specify what should be done if the marker is positive. |
| Consider opportunities for translational biomarker research |
| We recognize that the amount of tissue available with NMIBC trials is minimal. Nonetheless, where feasible and appropriate, molecular biomarkers should be explored in the context of a clinical trial, including correlative tissue studies and blood and urine marker studies. However, patient enrollment based on biomarker status is discouraged, unless the trial is specifically designed to assess the prognostic or predictive value of a particular marker. |
Abbreviations: CIS, carcinoma in situ; IBCG, International Bladder Cancer Group; NMIBC, non–muscle-invasive bladder cancer.
Trial Design for High-Risk NMIBC: BCG Naïve
Patient eligibility.
The inclusion criteria for trials in this population are: histologically confirmed T1 and/or high-grade tumor and/or carcinoma in situ (CIS)6,10-12 that has never been treated with BCG immunotherapy (Table 2). This cohort could include patients who previously received but stopped BCG more than 3 years before study entry, because clinical experience suggests that response rates in these patients are similar to those in BCG-naïve patients.
Table 2.
Summary of Clinical Trial Design Elements for NMIBC According to Risk Category
| Element | NMIBC Population | |||
|---|---|---|---|---|
| High Risk | Intermediate Risk | Low Risk | ||
| BCG Naive | BCG Failure | BCG Naive | ||
| Patient eligibility | ||||
| Inclusion criteria |
|
▪ Depends on type of BCG failure (Table 5) |
|
▪ Histologically confirmed solitary, primary low-grade Ta tumor < 3 cm in diameter |
| Exclusion criteria |
|
|
|
|
| Baseline evaluations | Document:
|
Document:
|
|
|
| Study design |
|
|
|
▪ Randomized superiority trial (noninferiority trial feasible but likely not practical) |
| Comparator or control arm |
|
Investigator choice may include valrubicin, gemcitabine, mitomycin, thermochemotherapy, BCG plus IFN, and taxanes |
|
TURBT plus an immediate postoperative chemotherapeutic instillation |
| Primary end point |
|
|
▪ Time to recurrence or RFS | ▪ Time to recurrence or RFS |
| Secondary |
|
|
|
▪ Toxicity |
| Study duration | Minimum 2 years: 1 year of active treatment followed by a minimum of 1 year of monitoring and follow-up | Minimum 2 years: 1 year of active treatment followed by a minimum of 1 year of monitoring and follow-up | Minimum 2 years: 1 year of active treatment followed by a minimum of 1 year of monitoring and follow-up | Minimum 2 years: 1 year of active treatment followed by a minimum of 1 year of monitoring and follow-up |
| Patient follow-up and monitoring |
|
|
|
|
| Clinically meaningful magnitude of effect | ▪ Absolute difference of 10% in the percentage of patients with recurrence at 2 years | BCG refractory or unresponsive CIS:
|
▪ Absolute difference of 10% in the percentage of patients with recurrence at 2 years | ▪ Absolute difference of 6% in the percentage of patients with recurrence at 2 years |
| Sample size | 150 recurrences per 450 patients |
|
BCG control:
|
110 recurrences per 600 patients |
Abbreviations: BCG, bacillus Calmette-Guérin; CIS, carcinoma in situ; CR, complete response; IFN, interferon; NMIBC, non–muscle-invasive bladder cancer; OS, overall survival; QOL, quality of life; RFS, recurrence-free survial; TURBT, transurethral resection of the bladder tumor.
Baseline evaluations.
At time of study enrollment, we recommend documenting patient demographics; cytologic results; presence or absence of CIS; stage, grade, size, and number of tumors; and details and dates of initial presenting transurethral resection of the bladder tumor (TURBT) and any prior therapies. Complete resection of all visible tumor (except in CIS) is recommended, which may require more than one TURBT.10,11 An appropriate upper tract evaluation is mandatory at baseline and should be repeated at periodic intervals during the study period. Although photodynamic diagnosis is more sensitive for the detection of malignant tumors,13 it is not mandatory for study inclusion.
Study designs and ideal comparators.
The superiority trial design is preferred, because it aims to show that the new therapy is more effective than the current standard (BCG). A noninferiority trial designed to show that the new product is not unacceptably less effective than the standard of care by a prespecified amount (noninferiority margin) has the following problems: the noninferiority margin is subjective and difficult to set, because one could argue that any loss in efficacy is unacceptable, and small margins require a large number of patients. Despite these challenges, it may be relevant to pursue a product that is less efficacious than BCG if it is expected to cause fewer adverse effects or lead to improved quality of life.
Because high-risk NMIBC is associated with a high risk of progression and mortality if left untreated, placebo-controlled trials are unethical. The ideal comparator arm is BCG induction plus maintenance, which is the current, guideline-recommended standard of care for high-risk NMIBC.6,10-12 Both recent evidence and guidelines suggest that full-dose BCG maintenance, administered once per week for 3 weeks , at 3 months after the first BCG dose of induction course (ie, 6 weeks after completion of induction BCG), and at 6 months, and then every 6 months for 3 years, as used in the SWOG 8507 and European Organization for Research and Treatment of Cancer (EORTC) 30911 and 30962 trials, is the most appropriate maintenance schedule.10,14-16 For the purpose of designing clinical trials, a minimum of 1 year of BCG maintenance is sufficient for the active control arm. Given that BCG is so highly efficacious, novel immunologic agents (eg, checkpoint inhibitors) may ideally be studied in combination with BCG.
Chemotherapy (mitomycin, epirubicin) could potentially be considered as a control arm in patients not eligible for BCG. No more than 12 months of chemotherapeutic instillations are advised, because a systematic review of randomized trials found short, intensive chemotherapeutic instillation schedules to be as effective as longer-term schedules.17
End points.
Primary end points should include time to recurrence or recurrence-free survival (RFS) for fully resected papillary disease (because these patients have no evidence of disease at study entry) and complete response (CR) rate and duration of response for CIS (because these patients have active disease at study entry; Table 3 lists definitions). The appropriate time period for evaluation of CR is 6 months, because evidence suggests that more than 60% of patients can convert from positive to negative with the first BCG maintenance course despite an absence of response during the initial 3 months after induction therapy,14 except if there is progression of disease at 3 months. Patients who have a recurrent, high-grade T1 tumor at 3 months are to be considered high risk and should be counseled accordingly.
Table 3.
Definitions of Primary and Secondary End Points for Clinical Trials in High-Risk NMIBC
| End Point | Description |
|---|---|
| Primary | |
| Recurrence | Reappearance of high-risk disease (high grade, T1, or CIS) after the start of therapy |
| CR | Histologic disappearance of malignancy on bladder biopsy and normal cytology and cystoscopy |
| Secondary | |
| Progression8 | Presence or development of any of the following:* |
| Stage | Development of or increase in stage to: |
| Lamina propria invasion (eg, increase from Ta to T1 or CIS to T1) | |
| Muscle invasive disease (stage ≥ T2) | |
| Lymph node (N+) or distant metastasis (M1) disease (patient must have previously been diagnosed with N0 and/or M0 disease) | |
| Grade | Increase in grade from low to high† (including CIS) |
| Disease worsening8 | Cystectomy or change in therapy indicative of more advanced disease, including systemic chemotherapy or radiation therapy |
| DSS | Time from random assignment to death resulting from bladder cancer |
| OS | Time from random assignment to death resulting from any cause |
Abbreviations: CIS, carcinoma in situ; CR, complete response; DSS, disease-specific survival; NMIBC, non–muscle-invasive bladder cancer; OS, overall survival.
In clinical trials, it is mandatory that type of progression (stage or grade) and degree or level of stage progression be explicitly reported.
WHO 2004 classification.
Secondary clinical trial end points may include time to progression (Table 3),8 disease-specific survival, overall survival, toxicity, disease worsening, and quality of life. Although progression should be assessed, it is relatively uncommon during BCG therapy (Table 4) and often occurs 5 or more years after treatment. Therefore, this outcome is unlikely to occur frequently enough to have sufficient power to detect a difference in treatment efficacy even if one exists. It may suggest that a new and potentially better treatment does not reduce NMIBC progression, when in fact, the results are inconclusive because of the lack of power. Thus, disease worsening should also be documented (Table 3).
Table 4.
Estimated Recurrence and Progression Rates Based on NMIBC Risk Category and Treatment Based on EORTC Data*
| Treatment by Disease State | Recurrence Rate (%) | Progression Rate (%) | |||||
|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 12 Months | 24 Months | 6 Months | 12 Months | 24 Months | |
| Low risk | |||||||
| TURBT alone | 10 | 15 | 20 | 25 | 0 | 1 | 1 |
| TURBT plus perioperative chemotherapy | 3 | 5 | 10 | 15 | 0 | 1 | 1 |
| Intermediate risk (BCG naïve)† | |||||||
| TURBT plus chemotherapy | 10 | 20 | 30 | 40 | 2 | 3 | 5 |
| TURBT plus BCG induction plus maintenance | 10 | 20 | 25 | 30 | 2 | 3 | 4 |
| High risk (BCG naïve) | |||||||
| TURBT plus reTURBT as needed plus BCG induction plus maintenance | 10 | 20 | 25 | 30 | 3 | 5 | 10 |
| High risk, BCG failure (BCG unresponsive) | |||||||
| TURBT + reTURBT as needed | The IBCG recommends using the following benchmarks:‡ | ||||||
| BCG-unresponsive CIS: an initial CR rate of 50% at 6 months and a durable response rate of 30% at 12 months and 25% at 18 months would be clinically meaningful | |||||||
| BCG unresponsive papillary disease: a recurrence-free rate of 30% at 12 months and 25% at 18 months would be clinically meaningful | |||||||
Abbreviations: BCG, bacillus Calmette-Guérin; CR: complete response; EORTC, European Organisation for Research and Treatment of Cancer; IBCG, International Bladder Cancer Group; NMIBC, non–muscle-invasive bladder cancer; TURBT, transurethral resection of the bladder tumor.
With the exception of the high-risk BCG-failure category, estimates are based on results from EORTC studies and meta-analyses.
Defined numbers are not available from literature, because trials have used varying definitions.
Trial duration.
For time-to-event end points, such as recurrence, patients must undergo follow-up long enough for the required number of events to be observed. Because a large proportion of disease recurrences in patients with high-risk NMIBC occur within the first 2 years after the start of therapy (approximately 30%),16 we recommend that the minimum study duration for each patient be 2 years, comprising 1 year of active treatment followed by at least 1 year of monitoring and follow-up.
Patient follow-up and monitoring.
Cystoscopy every 3 months is mandatory in the follow-up of high-risk patients in a study. Cytology is recommended but not mandatory, except in CIS.18 To assess CR, bladder biopsies at 6 months are mandatory for CIS, but they are only recommended for cause in papillary disease. Although photodynamic diagnosis and narrow-band imaging may be used, it may not be reasonable to mandate their use. An appropriate upper-tract evaluation should be considered at the end of the study period to rule out an upper-tract tumor.
Clinically meaningful magnitude of effect.
Reasonable and realistic efficacy thresholds should be set. Given the high efficacy of BCG in this setting, the International Bladder Cancer Group considers an absolute reduction of 10% in the percentage of patients with recurrence at 2 years as the magnitude of effect for a clinical trial to be considered positive.
Sample size.
For time-to-event end points, the power to detect a prespecified difference (reduction in the risk of the event) in superiority studies depends on the number of events that are observed, which is uniquely determined by the size of the hypothesized difference and the type I and II errors (α and β). The number of patients required is not unique. A sufficient number should be entered and undergo follow-up long enough for the required number of events to be observed. For example, if the 2-year RFS rate in the control arm is 70% (Table 4), 150 recurrences are required to detect an increase in RFS to 80% in the experimental arm, with a reduction of 37% in the relative risk (RR) of recurrence (hazard ratio [HR], 0.63; α = 0.05; β = 0.20). A minimum of approximately 450 patients is required.
For noninferiority studies, the power to reject the null hypothesis of a difference of a given size (noninferiority margin) depends on the observed number of events. Because noninferiority margins are small, large sample sizes are required.
Trial Design for High-Risk NMIBC: BCG Failure
Patient eligibility.
BCG failure has been broadly defined as any recurrence or progression during therapy.6 However, this term is heterogeneous, encompassing a number of differing clinical scenarios. To date, comparing salvage therapies in this population has been hindered by the lack of standard definitions, inconsistent methods of reporting results, and studies that have frequently combined different classes of failure.19 There are currently a number of published concepts of how to categorize disease that reappears during or after intravesical BCG.1,6,10,20-22 Although many of these definitions take into account the timing of BCG failure, they do not consider the type of BCG schedule administered. Hence, it is possible that patients classified as those for whom BCG has failed are simply those who have received inadequate BCG therapy.
Table 5 summarizes our classification of BCG failures, dividing them into the following four types: BCG refractory, BCG relapsing, BCG intolerant, and BCG unresponsive. The rationale for waiting until the 6-month evaluation time point to identify high-risk NMIBC as truly BCG refractory is that an additional 25% to 67% who do not respond to an initial induction course will respond to a second course of BCG.14,24,25 Recent evidence suggests that BCG-relapsing disease is associated with better outcomes than BCG-refractory disease26; this should also be considered when designing trials for the population of patients for whom BCG has failed. The BCG-unresponsive category represents a group of patients for whom further BCG is not indicated, and radical cystectomy is a true option; thus, they could be considered for single-arm studies. Although patients would be considered BCG unresponsive if they were to experience relapse at cystoscopy 6 months after the last BCG exposure, there are often delays in referral to and enrollment onto trials. Thus, we recommend that study designs account for this window (eg, for trial enrollment, patients can be within 9 months of the last BCG exposure, thereby allowing a 3-month lead time for referral). All patients enrolled onto trials of novel therapeutics for BCG failures must be informed that treatments other than cystectomy in this population are considered oncologically inferior at present.19
Table 5.
Classification of BCG Failures
| Classification | Description |
|---|---|
| Refractory | Persistent high-grade disease at 6 months despite adequate BCG* treatment. This category also includes any stage or grade progression by 3 months after the first BCG cycle (ie, high-grade T1 at 3 months after initial Ta, T1, high-grade disease, or CIS). |
| Relapsing | Recurrence of high-grade disease after achieving a disease-free state at 6 months after adequate BCG.* Although this category has previously been subdivided based on time to recurrence after stopping BCG (ie, early [< 12 months], intermediate [1-2 years], or late [> 24 months]), for the purpose of being included in the BCG-unresponsive category, patients should be within 6 months of the last BCG exposure (eg, patient receiving maintenance therapy). |
| Intolerant | Disease persistence as a result of inability to receive adequate BCG* because of toxicity. With current attention to abrogation of BCG adverse effects, we expect this category to represent a small portion of the BCG-treated population. |
| Unresponsive23 | BCG refractory and BCG relapsing disease. The term BCG unresponsive, which essentially includes BCG refractory and BCG relapsing (within 6 months of last BCG exposure), is meant to denote a subgroup of patients at highest risk of recurrence and progression for whom additional BCG therapy is not a feasible option. These patients can be considered for single-arm studies.† |
Abbreviations: BCG, bacillus Calmette-Guérin; CIS, carcinoma in situ.
For clinical trials, adequate BCG therapy is when a patient has received at least five of six induction instillations and at least one maintenance (two of three instillations) in a 6-month period.
Because there are often delays in referral to and enrollment in trials, we recommend that study designs account for a window from tumor recurrence, and patients can be within 6 to 9 months of the last BCG exposure, thereby allowing a 3-month lead time for referral.
Baseline evaluations.
Baseline evaluations similar to those proposed previously are recommended (Table 2). The timing of recurrence and type of BCG schedule administered before failure should also be documented.
Study designs and ideal comparators.
Other than radical cystectomy, there is currently no accepted standard of care for this population, especially for those in the BCG-unresponsive category. Given the high risk of disease progression, a placebo-controlled arm is not ethical. In the setting of unmet medical needs, of no approved standard of care, and where placebo control is not acceptable, single-arm trials have been allowed and could provide sufficient evidence of benefit of a new therapy for BCG failures, provided that the results are robust.
A randomized trial using an investigator-choice comparator is also feasible for examining potential new therapies for this population. Yates et al19 recently summarized bladder-preserving intravesical treatments studied in patients for whom BCG therapy failed (not included in this summary are mycobacterial cell wall–DNA complex [MCNA] and valrubicin [FDA-approved treatment for patients with BCG-refractory CIS who are not candidates for cystectomy]; Table 6). Any of these salvage therapies could be considered as potential comparators (active controls) in trials of novel therapies for this population.
Table 6.
Studies of Bladder-Preserving Intravesical Therapies in Patients for Whom BCG Has Failed19
| Study | Treatment Modality | No. of Patients | No. of Patients Experiencing Failure | Follow-Up | NED (%) | RFS (%) | Recurrence (%) | Progression (%) | Radical Cystectomy (%) | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Dalbagni27 | IV gemcitabine | 30 | 26 | 19 months (range, 0-35 months) | 50 | 21 | 40 | 3.5 | 37 | Phase II trial |
| Dalbagni28 | IV gemcitabine | 18 | 16 | 12 weeks | 39 | — | — | — | — | Phase I trial |
| Bartoletti29 | IV gemcitabine | 116 | 40 | 13.6 months | — | 32.5 | — | Recurrence in 32.5% of patients with BCG failure v 21% in BCG-naïve group; 43.7% of high-risk patients experiencing failure developed recurrence v 25% of intermediate-risk patients experiencing failure; phase II study | ||
| Mohanty30 | IV gemcitabine | 35 | 35 | 18 months | 60 | — | 31.4 | 8.75 | — | — |
| Di Lorenzo31 | IV gemcitabine | 80 | 80 | 15.5 months (range, 6-22 months) | — | 19 | 52.5 | 33 | 33 | Recurrence and 2-year DFS better for GC v BCG (P = .002 and P < .008, respectively); phase III RCT |
| Addeo32 | IV gemcitabine | 54 | 46 | 36 months | — | — | 28 | 11 | — | Recurrence free: 72% GC v 61% mitomycin; DFS in favor of GC (P = .0021); phase III RCT |
| McKiernan33 | IV docetaxel | 18 | 18 | 12 weeks | 28 | — | 72 | 5.5 | — | Phase I study |
| Laudano34 | IV docetaxel | 18 | 18 | 48.3 months | 22 | 44-61 | 61 | 5.5 | 33 | Long-term follow-up of McKiernan et al33; median DFS, 13.3 months |
| Barlow35 | IV docetaxel | 33 | 33 | 29 months | 61 | 32-45 | 39 | — | — | 5-year DSS, 83%; 5-year OS, 71% |
| Bassi36 | IV paclitaxel | 16 | 16 | 1 week | 60 | — | 40 | — | — | Phase I study |
| McKiernan37 | IV paclitaxel | 18 | 18 | 6 weeks | 56 | — | 44 | 0 | 22 | Phase I study |
| Joudi38 | BCG plus IFN-α | 1,007 | 467 | 24 months | 45 | — | — | — | — | Of BCG-failure group, 45% disease free v 59% BCG naïve (P < .001); phase II trial |
| Witjes39 | Thermochemotherapy | 51 | 34 | 27 months | 51 | — | 49 | — | 10.2 | Synergo working party study |
| Nativ40 | Thermochemotherapy | 111 | 111 | 16 months (range, 2-74 months) | — | — | 56-85 | 3 | — | 2-year recurrence rate of 61% if no maintenance v 39% for maintenance (P = .01) |
| Halachmi41 | Thermochemotherapy | 56 | 19 | 20 months (range, 2-49 months) | 67 | 49.3 | 33.3 | 7.9 | 12 | Kaplan-Meier–estimated probability of recurrence of 50.7% at 2 years for BCG-failure cohort v 42.9% |
| Waidelich42 | Photodynamic therapy | 24 | 24 | 36 months (range, 12-51 months) | 29 | — | 70.8 | 16.6 | 12.5 | — |
| Berger43 | Photodynamic therapy | 31 | 10 | 23.7 months (range, 1-73 months) | — | 40 | 60 | — | — | — |
| Breyer44 | Mitomycin plus gemcitabine | 10 | 9 | 26 months | 60 | — | 40 | 10 | 0 | Only 10 patients |
NOTE. Data adapted.19
Abbreviations: BCG, bacillus Calmette-Guérin; DFS, disease-free survival; DSS, disease-specific survival; GC, gemcitabine and cisplatin; IFN-α, interferon α; IV, intravesical; NED, no evidence of disease; OS, overall survival; RCT, randomized controlled trial; RFS, recurrence-free survival.
End points.
The appropriate primary end points for clinical trials of high-risk BCG failures are freedom from high-risk recurrence at 1 year for papillary disease and CR at 6 months for CIS. While this is usually sufficient, for regulatory approval, durability of this effect at 12 months may be considered and the requirement for an end of study biopsy with the agency should be discussed. The recommended secondary end points are similar to those proposed for trials of high-risk, BCG-naïve NMIBC (Table 2).
Trial duration and patient follow-up.
Trial duration and patient follow-up should be similar to those recommended previously (Table 2). Patients who do not achieve a CR or who experience a high-risk recurrence by 3 months (6 months for CIS) should be removed from the trial, and radical cystectomy should be recommended, because the prognosis of these patients is adversely affected by delayed surgery.45-48
Clinically meaningful magnitude of effect.
For patients with BCG-unresponsive CIS, we recommend an initial CR rate of 50% at 6 months and durable response rates of 30% at 12 months and 25% at 18 months as clinically meaningful. For patients with papillary disease that is BCG unresponsive, we consider recurrence-free rates of 30% at 12 months and 25% at 18 months as clinically meaningful. These recommendations are consistent with the results of studies of other salvage therapies for BCG failures, which have noted 1- to 2-year RFS rates ranging from 18% to 43%.49-54
In a recent FDA–AUA public workshop, some panel members felt that an initial CR rate of 40% to 50% at 6 months and a durable response rate of at least 30% for 18 to 24 months, with the lower bound of the 95% CI excluding 20%, could be clinically meaningful in the BCG-refractory CIS population.1,2 We are in partial agreement with these recommendations but feel that the 30% durable response at 18 to 24 months criterion is likely too high and may not be realistically achievable.
These recommendations are meant to guide clinical trial development and should not be taken as set-in-stone directives that could potentially eliminate the development of agents that may help patients avoid cystectomy.3 Also, although progression is actually the clinically meaningful end point in this patient population, it is not practical to power these trials for progression.
Sample size.
For randomized studies with time-to-event end points, the same principles discussed previously apply here. In a nonrandomized setting, the sample size can be calculated based on the DFS rate at a fixed point in time (eg, at 1 year) using a one-stage Fleming or A’Hern design. The parameters outlined in Appendix Table A1 (online only) must be specified. On the basis of these values, one can calculate the required number of patients and the minimum number of patients who should be disease free for the drug to be worthy of further study. For example, for CIS and the CR rate at 6 months, P0 = 40%, P1 = 60%, and α = β = 0.10; at least 20 (50%) of 40 patients should have achieved a CR at 6 months. For patients with papillary disease, P0 = 20%, P1 = 40%, and α = β = 0.10; at least 10 (30%) of 33 patients should be recurrence free at 1 year.
Trial Design for Intermediate-Risk NMIBC: BCG Naïve
Patient eligibility.
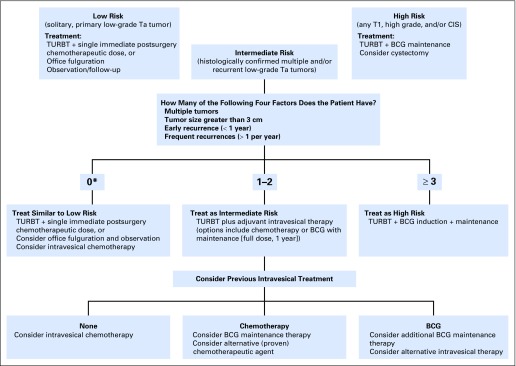
Inclusion criteria for clinical trials of novel therapies in intermediate-risk disease are listed in Table 2. We recently defined intermediate-risk disease as multiple or recurrent low-grade Ta tumors and provided guidance on further stratifying these patients into categories of lower versus higher risk of recurrence or progression based on key factors (Appendix Fig A1, online only).7
Baseline evaluations, study designs, and ideal comparators.
Baseline evaluations similar to those proposed previously (Table 2) are recommended. Randomized superiority or noninferiority trial designs are appropriate, and full-dose BCG induction plus maintenance administered once per week for 3 weeks, at 3 months after the first BCG dose of induction course (ie, 6 weeks after completion of induction BCG), and repeated at 6 months and 12 months (so a total of 1 year maintenance) is the ideal comparator arm, particularly for higher-risk patients with intermediate-risk disease (Appendix Fig A1). Evidence suggests that BCG with maintenance is superior to maintenance chemotherapy in intermediate-risk disease.15,55-57 EORTC 30962 found that full-dose BCG maintenance (SWOG schedule) for 1 year was associated with the best outcomes in patients with intermediate-risk disease, with no further improvement when maintenance was continued to 3 years.16 Intravesical chemotherapy is an appropriate comparator for lower-risk patients with intermediate-risk disease (Appendix Fig A1),7 because most guidelines recommend adjuvant chemotherapy as an option for intermediate-risk NMIBC.6,10-12
End points, trial duration, and patient monitoring.
End points, trial duration, and patient follow-up should be similar to those recommended for the high-risk, BCG-naïve population (Table 2).
Clinically meaningful magnitude of effect.
Similar to the high-risk, BCG-naïve trial design, an absolute reduction of 10% in the percentage of patients with recurrence at 2 years represents an appropriate magnitude of effect for a clinical trial to be considered positive.
Sample size.
For studies with BCG as the control arm, the sample size is the same as for high-risk, BCG-naïve studies. For studies with chemotherapy as the control arm, 250 recurrences are required to detect an increase in RFS at 2 years from 60% to 70% in the experimental arm, with a reduction of 30% in the RR of recurrence (HR, 0.70; α = 0.05; β = 0.20). A minimum of approximately 500 patients is required.
Trial Design for Low-Risk NMIBC
Patient eligibility.
The inclusion criterion for low-risk NMIBC trials is a histologically confirmed, solitary, primary low-grade Ta tumor smaller than 3 cm in diameter (Table 2).6,10-12
Baseline evaluations, study designs, and ideal comparators.
Baseline evaluations similar to those proposed previously are recommended. Given the relatively good prognosis of this population, a large sample size will be required for noninferiority trials, and therefore, randomized superiority trials are more feasible. TURBT plus an immediate postoperative chemotherapeutic instillation is the ideal comparator arm given that it is the current guideline-recommended standard of care.6,10,11 However, given the extremely low risk of progression in this population, placebo may be considered in select trials.
End points, trial duration, and patient follow-up.
The recommended primary end point is time to recurrence or RFS. From a practical perspective, the only feasible secondary end point in this population is toxicity. The trial duration should be similar to that recommended previously (Table 2). Regarding follow-up, cystoscopy at 3 months and then every 6 months is advised. An upper-tract evaluation is not mandatory given the rarity of upper-tract recurrences in this population.
Clinically meaningful magnitude of effect.
An absolute reduction of 6% in the percentage of patients with recurrence at 2 years is the magnitude of effect for a clinical trial to be considered positive (an RR reduction of 42% based on Table 4), because the current standard of care (TURBT plus a single immediate chemotherapeutic instillation) is effective. Note that this population is at low risk of progression (Table 4) and mortality.
Sample size.
Approximately 110 recurrences are required to detect an increase in RFS at 2 years from 85% to 91% in the experimental arm, with a reduction of 42% in the RR of recurrence (HR, 0.58; α = 0.05; β = 0.20). A minimum of approximately 600 patients is required.
DISCUSSION
The optimal design of clinical trials in NMIBC continues to be an area of much discussion. Through an extensive literature review and discussions and consensus gained during group meetings, we have developed realistic recommendations for the design of clinical trials in NMIBC. The goals are to provide a template that will encourage the conduct of trials for the development of highly needed new therapies for NMIBC and to ensure uniformity in reporting and analysis of such trials.
Acknowledgment
We thank Julie Tasso and Sandra Steele from Bridge Medical Communications for their administrative and editorial assistance.
Appendix
Table A1.
Parameters to Be Set for Sample-Size Calculation
| Parameter | Description |
|---|---|
| P0 | Largest DFS rate that if true implies that the drug does not warrant further study |
| P1 | Lowest DFS rate that if true implies that the drug does warrant further study |
| α | Probability of concluding the drug is active if it has a true DFS rate ≤ P0 |
| β | Probability of concluding the drug is inactive if it has a true DFS rate of at least P1 |
Abbreviation: DFS, disease-free survival.
Fig A1.
International Bladder Cancer Group algorithm for the management of intermediate-risk non–muscle-invasive bladder cancer.7 Recommendations provided have been simplified for ease of use and will need to be customized to each individual patient, taking into account patient diagnosis, histology, age, previous history, and overall condition. For example, a 75-year-old man with numerous comorbidities who experiences two small (< 1 cm) low-grade recurrences more than 1 year after initial therapy may be a candidate for office fulguration and observation rather than bacillus Calmette-Guérin (BCG) maintenance or intravesical chemotherapy as suggested in this algorithm. *A score of 0 refers to a solitary, recurrent (> 1 year) low-grade tumor. Data adapted.7 CIS, carcinoma in situ; TURBT, transurethral resection of the bladder tumor.
Footnotes
Listen to the podcast by Dr Galsky at www.jco.org/podcasts
Supported by Sanofi Pasteur.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Ashish M. Kamat, Andreas Böhle, Joan Palou, Donald L. Lamm, Maurizio Brausi, Mark Soloway, Raj Persad, Roger Buckley, J. Alfred Witjes
Collection and assembly of data: Ashish M. Kamat, Andreas Böhle
Data analysis and interpretation: Ashish M. Kamat, Richard J. Sylvester, Andreas Böhle, Mark Soloway, Roger Buckley, Marc Colombel
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Definitions, End Points, and Clinical Trial Designs for Non–Muscle-Invasive Bladder Cancer: Recommendations from the International Bladder Cancer Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Ashish M. Kamat
Honoraria: Photocure, Sanofi, Abbott Laboratories, Taris
Consulting or Advisory Role: Taris, Merck Sharp & Dohme, Telesta Therapeutics, Spectrum Pharmaceuticals
Research Funding: FKD Therapies, Photocure (Inst), Heat Biologics (Inst)
Patents, Royalties, Other Intellectual Property: CyPRIT (Cytokine Panel of Response to Intravesical Therapy; Inst)
Richard J. Sylvester
No relationship to disclose
Andreas Böhle
Consulting or Advisory Role: Sanofi Pasteur
Joan Palou
No relationship to disclose
Donald L. Lamm
Honoraria: Sanofi Pasteur
Maurizio Brausi
Honoraria: Janssen Pharmaceuticals, Sanofi Pasteur
Consulting or Advisory Role: Janssen Pharmaceuticals, Sanofi Pasteur
Travel, Accommodations, Expenses: Janssen Pharmaceuticals, Sanofi Pasteur
Mark Soloway
Honoraria: Sanofi Canada
Consulting or Advisory Role: Sanofi Canada
Raj Persad
Honoraria: Sanofi Pasteur, Spire Healthcare
Travel, Accommodations, Expenses: Sanofi Pasteur
Roger Buckley
Honoraria: Sanofi Pasteur, AbbVie, Astellas Pharma, Janssen Pharmaceuticals
Consulting or Advisory Role: Sanofi Pasteur
Marc Colombel
Honoraria: Sanofi Pasteur
Consulting or Advisory Role: Sanofi Pasteur
Travel, Accommodations, Expenses: Sanofi Pasteur
J. Alfred Witjes
Honoraria: Spectrum Pharmaceuticals, Ipsen, MEL, Sanofi Pasteur, Astellas Pharma, Merck Sharp & Dohme, Nucleix
Consulting or Advisory Role: MEL, Sanofi Pasteur, Astellas Pharma, Merck Sharp & Dohme, Ipsen, Nucleix
Travel, Accommodations, Expenses: Spectrum Pharmaceuticals, Ipsen, MEL, Sanofi Pasteur, Astellas Pharma, Merck Sharp & Dohme
REFERENCES
- 1.Jarow JP, Lerner SP, Kluetz PG, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: Report of a Food and Drug Administration and American Urological Association public workshop. Urology. 2014;83:262–264. doi: 10.1016/j.urology.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 2. FDA/AUA Bladder Cancer Workshop: Clinical trial design issues: Development of new therapies for non-muscle invasive bladder cancer. https://www.auanet.org/university/live-course-wip.cfm?id=476id=476&video=202&agenda=2117.
- 3.Amrhein J, Kamat AM, Morales A. Re: Jarrow JP et al: Clinical trial design for the development of new therapies for non-muscle-invasive bladder cancer: Report of a Food and Drug Administration and American Urological Association public workshop (Urology 2014;83:262-265) Urology. 2014;84:494–495. doi: 10.1016/j.urology.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 4.van der Meijden AP. The use of the marker tumor concept in Ta, T1 bladder cancer: is it justified? Urol Oncol. 2002;7:31–33. doi: 10.1016/s1078-1439(01)00156-9. [DOI] [PubMed] [Google Scholar]
- 5.McCullough LB. Ethical issues in the use of tumor markers in clinical investigation of the management of bladder cancer. Urol Oncol. 2002;7:35–37. doi: 10.1016/s1078-1439(01)00157-0. [DOI] [PubMed] [Google Scholar]
- 6.Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol. 2011;186:2158–2167. doi: 10.1016/j.juro.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 7.Kamat AM, Witjes JA, Brausi M, et al. Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014;192:305–315. doi: 10.1016/j.juro.2014.02.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamm D, Persad R, Brausi M, et al. Defining progression in nonmuscle invasive bladder cancer: It is time for a new, standard definition. J Urol. 2014;191:20–27. doi: 10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 9.Porten SP, Willis D, Kamat AM. Variant histology: role in management and prognosis of nonmuscle invasive bladder cancer. Curr Opin Urol. 2014;24:517–523. doi: 10.1097/MOU.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 10.Babjuk M, Burger M, Zigeuner R, et al. European Association of Urology EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 11.American Urological Association Guideline for the management of nonmuscle invasive bladder cancer (stages Ta,T1, and Tis): 2007 update. http://www.auanet.org/education/guidelines/bladder-cancer.cfm. [DOI] [PubMed]
- 12.National Comprehensive Cancer Network Clinical practice guidelines in oncology: Bladder cancer, version 1. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 13.Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013;64:846–854. doi: 10.1016/j.eururo.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 15.Sylvester RJ, Brausi MA, Kirkels WJ, et al. EORTC Genito-Urinary Tract Cancer Group Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin, and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766–773. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: One-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester RJ, Oosterlinck W, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non-muscle-invasive bladder cancer: A systematic review of the published results of randomized clinical trials. Eur Urol. 2008;53:709–719. doi: 10.1016/j.eururo.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamat AM, Vlahou A, Taylor JA, et al. Considerations on the use of urine markers in the management of patients with high-grade non-muscle-invasive bladder cancer. Urol Oncol. 2014;32:1069–1077. doi: 10.1016/j.urolonc.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Yates DR, Brausi MA, Catto JW, et al. Treatment options available for bacillus Calmette-Guérin failure in non-muscle-invasive bladder cancer. Eur Urol. 2012;62:1088–1096. doi: 10.1016/j.eururo.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 20.Nieder AM, Brausi M, Lamm D, et al. Management of stage T1 tumours of the bladder: International consensus panel. Urology. 2005;66:108–125. doi: 10.1016/j.urology.2005.08.066. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 21.Martin FM, Kamat AM. Definition and management of patients with bladder cancer who fail BCG therapy. Expert Rev Anticancer Ther. 2009;9:815–820. doi: 10.1586/era.09.35. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell MA, Boehle A. Treatment options for BCG failures. World J Urol. 2006;24:481–487. doi: 10.1007/s00345-006-0112-0. [DOI] [PubMed] [Google Scholar]
- 23.Lerner SP, Dinney C, Kamat AM, et al. Short communication: Clarification of bladder cancer disease states following treatment of patients with intravesical BCG. Bladder Cancer. 2015;1:29–30. doi: 10.3233/BLC-159002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Reijke TM, Kurth KH, Sylvester RJ, et al. European Organization for the Research and Treatment of Cancer-Genito-Urinary Group Bacillus Calmette-Guerin versus epirubicin for primary, secondary or concurrent carcinoma in situ of the bladder: Results of a European Organization for the Research and Treatment of Cancer–Genito-Urinary Group Phase III Trial (30906) J Urol. 2005;173:405–409. doi: 10.1097/01.ju.0000150425.09317.67. [DOI] [PubMed] [Google Scholar]
- 25.Sylvester RJ, van der Meijden AP, Witjes JA, et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90–107. doi: 10.1016/j.urology.2005.06.135. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 26.Herr HW, Milan TN, Dalbagni G. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: A prospective cohort outcomes study. Urol Oncol. 2015;33:108.e1–108.e4. doi: 10.1016/j.urolonc.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 27. Dalbagni G, Russo P, Sheinfeld J, et al: Phase I trial of intravesical gemcitabine in bacillus Calmette-Guérin–refractory transitional-cell carcinoma of the bladder. J Clin Oncol 20:3193-3198, 2002. [DOI] [PubMed]
- 28. Dalbagni G, Russo P, Bochner B, et al: Phase II trial of intravesicalgGemcitabine in bacille Calmette-Guérin–refractory transitional cell carcinoma of the bladder. J Clin Oncol 24:2729-2734, 2006. [DOI] [PubMed]
- 29. Bartoletti R, Cai T, Gacci M, et al: Intravesical gemcitabine therapy for superficial transitional cell carcinoma: Results of a phase II prospective multicenter study. Urology 66:726-731, 2005. [DOI] [PubMed]
- 30. Mohanty NK, Nayak RL, Vasudeva P, et al: Intravesicle gemcitabine in management of BCG refractory superficial TCC of urinary bladder-our experience. Urol Oncol 26:616-619, 2008. [DOI] [PubMed]
- 31.Di Lorenzo G, Perdonà S, Damiano R, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer: A multicenter prospective randomized trial. Cancer. 2010;116:1893–1900. doi: 10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]
- 32. Addeo R, Caraglia M, Bellini S, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: Evaluation of efficacy and tolerance. J Clin Oncol 28:543-548, 2010. [DOI] [PubMed]
- 33. McKiernan JM, Masson P, Murphy AM, et al: Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol 24:3075-3080, 2006. [DOI] [PubMed]
- 34. Laudano MA, Barlow LJ, Murphy AM, et al: Long-term clinical outcomes of a phase I trial of intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to standard intravesical therapy. Urology 75:134-137, 2010. [DOI] [PMC free article] [PubMed]
- 35. Barlow LJ, McKiernan JM, Benson MC: The novel use of intravesical docetaxel for the treatment of non-muscle invasive bladder cancer refractory to BCG therapy: A single institution experience. World J Urol 27:331-335, 2009. [DOI] [PubMed]
- 36. Bassi PF, Volpe A, D'Agostino D, et al: Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder: Results of a phase I study. J Urol 185:445-449, 2011. [DOI] [PubMed]
- 37. McKiernan JM, Barlow LJ, Laudano MA, et al: A phase I trial of intravesical nanoparticle albuminbound paclitaxel in the treatment of bacillus Calmette-Guerin refractory nonmuscle invasive bladder cancer. J Urol 186:448-451, 2011. [DOI] [PubMed]
- 38. Joudi FN, Smith BJ, O'Donnell MA: Final results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol 24:344-348, 2006. [DOI] [PubMed]
- 39. Witjes J, Hendricksen K, Gofrit O, et al: Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: Experience of the European Synergo working party. World J Urol 27:319-324, 2009. [DOI] [PMC free article] [PubMed]
- 40. Nativ O, Witjes JA, Hendricksen K, et al: Combined thermochemotherapy or recurrent bladder cancer after bacillus Calmetteuerin. J Urol 182:1313-1317, 2009. [DOI] [PubMed]
- 41. Halachmi S, Moskovitz B, Maffezzini M, et al: Intravesical mitomycin combined with hyperthermia for patients with T1G3 ransitional cell carcinoma of the bladder. Urol Oncol 29:259-264, 2011. [DOI] [PubMed]
- 42. Waidelich R, Stepp H, Baumgartner R, et al: Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. J Urol 165:1904-1907, 2001. [DOI] [PubMed]
- 43. Berger AP, Steiner H, Stenzl A, et al: Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: A single center study. Urology 61:338-331, 2003. [DOI] [PubMed]
- 44. Breyer BN, Whitson JM, Carroll PR, et al: Sequential intravesical gemcitabine and mitomycin C chemotherapy regimen in patients with non-muscle invasive bladder cancer. Urol Oncol 28:510-514, 2010. [DOI] [PMC free article] [PubMed]
- 45.Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: Do risk factors define feasibility of bladder-sparing approach? Eur Urol. 2008;53:146–152. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 46.Thalmann GN, Markwalder R, Shahin O, et al. Primary T1G3 bladder cancer: organ preserving approach or immediate cystectomy? J Urol. 2004;172:70–75. doi: 10.1097/01.ju.0000132129.87598.3b. [DOI] [PubMed] [Google Scholar]
- 47.van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur Urol. 2011;60:493–500. doi: 10.1016/j.eururo.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 48.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296–1299. [PubMed] [Google Scholar]
- 49.Dalbagni G, Russo P, Bochner B, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729–2734. doi: 10.1200/JCO.2005.05.2720. [DOI] [PubMed] [Google Scholar]
- 50.Skinner EC, Goldman B, Sakr WA, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guérin. J Urol. 2013;190:1200–1204. doi: 10.1016/j.juro.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morales A, Herr H, Steinberg G, et al. Efficacy and safety of MCNA in patients with nonmuscle invasive bladder cancer at high risk for recurrence and progression after failed treatment with bacillus Calmette-Guérin. J Urol. 2015;193:1135–1143. doi: 10.1016/j.juro.2014.09.109. [DOI] [PubMed] [Google Scholar]
- 52.Rosevear HM, Lightfoot AJ, Birusingh KK, et al. National BCG/Interferon Investigator Group Factors affecting response to bacillus Calmette-Guérin plus interferon for urothelial carcinoma in situ. J Urol. 2011;186:817–823. doi: 10.1016/j.juro.2011.04.073. [DOI] [PubMed] [Google Scholar]
- 53.Steinberg G, Bahnson R, Brosman S, et al. The Valrubicin Study Group Efficacy and safety of valrubicin for the treatment of bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. J Urol. 2000;163:761–767. [PubMed] [Google Scholar]
- 54.Gallagher BL, Joudi FN, Maymí JL, et al. Impact of previous bacille Calmette-Guérin failure pattern on subsequent response to bacille Calmette-Guérin plus interferon intravesical therapy. Urology. 2008;71:297–301. doi: 10.1016/j.urology.2007.09.050. [DOI] [PubMed] [Google Scholar]
- 55.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 56.Hinotsu S, Akaza H, Naito S, et al. Maintenance therapy with bacillus Calmette-Guérin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumor for non-muscle-invasive bladder cancer. BJU Int. 2011;108:187–195. doi: 10.1111/j.1464-410X.2010.09891.x. [DOI] [PubMed] [Google Scholar]
- 57.Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]