Abstract
Objective
Preclinical abuse liability assessment is an essential component of tobacco regulatory science. The goal of this project was to evaluate the relative abuse liability of smokeless tobacco products in rats using aqueous extracts of those products. These extracts provide exposure to an extensive range of nicotine and non-nicotine tobacco constituents as occurs in humans.
Methods
Rats were trained to self-administer either nicotine alone or extracts of Camel Snus or Kodiak smokeless tobacco at an equivalent nicotine unit dose. In Experiment 1, the relative reinforcing efficacy of these formulations was assessed in adults and adolescents using a progressive ratio schedule under limited-access conditions. In Experiment 2, relative reinforcing efficacy was assessed in adolescents under unlimited-access conditions using behavioral economic demand curve analysis.
Results
The reinforcing efficacy of nicotine formulations was higher in adolescents than adults, but no difference was observed between formulations in either age group. Similarly, there was no difference in elasticity of demand between formulations in adolescents.
Conclusions
The present findings suggest that the abuse liability of these smokeless tobacco products is similar to nicotine alone, and that nicotine dose is the primary determinant of the reinforcing efficacy of systemic exposure to these products.
Keywords: abuse liability, self-administration, nicotine, smokeless tobacco extracts, progressive-ratio schedule, behavioral economics, rat
INTRODUCTION
The Food and Drug Administration (FDA) Center for Tobacco Products (CTP) will likely require evaluation of the abuse liability of new tobacco products prior to marketing to substantiate claims that the new product has reduced abuse potential, or that its abuse potential is at most “substantially equivalent” to currently marketed products.1 The Institute of Medicine has specifically recommended the use of animal models to evaluate tobacco products, yet few preclinical studies have specifically sought to characterize the relative abuse liability of tobacco products per se. Development of appropriate methodology for this purpose is therefore urgently needed to inform FDA policy regarding these products.1,2
Animal studies can make vital contributions to tobacco regulatory science because they allow experimental study of initiation of tobacco use in adolescents, isolation of the role of nicotine and other tobacco constituents from other factors (e.g., taste, smell, packaging), and screening of novel tobacco formulations and isolated constituents to avoid potential harmful exposure to humans. Animal models of tobacco addiction typically only examine nicotine alone and/or a small number of other isolated constituents.3,4 This approach is not sufficient to examine the abuse liability of tobacco products, which is determined by the net interaction of numerous compounds (including unidentified ones) that may contribute positively or negatively to the reinforcing efficacy of that product. Therefore, preclinical models involving exposure to a clinically-relevant mixture of constituents derived directly from tobacco products may be necessary for an accurate assessment of the abuse liability of those products.
Recent research by our lab and others have addressed this issue by using extracts of tobacco or tobacco smoke that contain an extensive mixture of tobacco constituents.5-9 Among the variety of methods that have been used, the self-administration assay is considered the gold standard for assessing the relative abuse liability of drugs.10,11 Using this assay, Costello et al.6 found that rats self-administered lower doses of nicotine in a smoke extract compared to the same nicotine doses alone under fixed-ratio (FR) schedules, but reinforcing efficacy of these formulations did not differ under progressive ratio (PR) schedules. In addition, Brennan et al.12 reported higher infusion rates for smoke extract prepared from role-your-own tobacco compared to nicotine alone under both FR and PR schedules, although these effects were not observed with a conventional cigarette smoke extract. These differences in the reinforcing effects of smoke extracts and nicotine alone may be due to the presence of certain non-nicotine constituents in extracts that have been shown to mimic or enhance nicotine's addiction-related effects when studied in isolation (e.g. minor alkaloids, MAO inhibitors3,13-15). These studies represent an important step in developing preclinical models to assess the relative abuse liability of combusted tobacco products. Similar models are needed to examine smokeless tobacco products.
Our laboratory has evaluated the abuse-related effects of smokeless tobacco (ST) extracts that contain several of the same behaviorally active non-nicotine constituents found in tobacco smoke.7,16 An important advantage of this approach is that ST extracts provide a very close representation of tobacco constituent exposure in ST users, because saliva provides a similar aqueous extraction.17 In contrast, aqueous extracts of tobacco smoke do not provide as close of an approximation of human exposure because smokers are exposed to both water-soluble and insoluble components in inhaled smoke. ST extracts are also of interest because the tobacco industry has introduced several potential “modified risk tobacco products” (MRTPs) that are claimed to be safer than conventional tobacco products due to their lower levels of toxicants. However, the relative abuse liability of MRTPs and conventional ST products has not been well characterized.
We recently reported small or no differences in the effects of ST extracts and nicotine alone on intracranial self-stimulation, nicotine discrimination, and locomotor sensitization,7,17 indicating that nicotine dose is the primary determinant of the effects of extracts in these models. The purpose of the present study was to examine the generality of these findings by examining the abuse liability of the same ST extracts using self-administration methods. Extracts were prepared from Kodiak ST, a popular conventional product, or Camel Snus, which is widely marketed as an alternative to smoking and is being evaluated as a potential MRTP (but not FDA-approved as such).18 Because there can be age differences in the behavioral effects of nicotine and non-nicotine constituents,3,19-21 we studied both adults and adolescents in a limited access (2 hr/day) model in Experiment 1. Relative reinforcing efficacy of formulations was compared using a PR schedule of reinforcement. To examine the generality of findings in Experiment 1, Experiment 2 evaluated the reinforcing effects of the same extracts and nicotine alone in an unlimited access (23 hr/day) model, using a behavioral economic approach to compare relative reinforcing efficacy. Given the lack of interaction between formulation and age in Experiment 1, only adolescents were studied in Experiment 2.
METHODS
Animals
Male adolescent and adult Holtzman rats (Harlan, Indianapolis, IN) aged 22-24 and 60-64 days old, respectively, at arrival were used. Adolescents were weaned on postnatal day 21 prior to shipment via truck. Upon arrival, all rats were individually housed in a temperature- and humidity-controlled colony room with unlimited access to food and water under a reversed 12-h light/dark cycle (lights off at 10:00 hr) for 5 days prior to surgery and during a 3- to 6-day postoperative recovery period. Food restriction started following the post-operative recovery period. Adults were given 18-20 grams/day for the entire protocol. Adolescents were initially given 10 grams/day, and the allotment increased by three grams each week to 18 grams/day for the remainder of the protocol. Pilot studies indicated this feeding regimen provided a level of restriction comparable to adults, while accommodating increased caloric needs during adolescent development. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2011 National Research Council's Guide for the Care and Use of Laboratory Animals.
Apparatus
Operant conditioning chambers for rats (model ENV-007, Med Associates, Inc (St. Albans, VT) for Experiment 1 or model H10-11R-TC, Coulbourn Instruments (Holliston, MA) for Experiment 2) were used. Two response levers were located on the front wall 6 cm above the chamber floor on either side of an aperture for food delivery (not used in this study) located 2 cm above the floor. LED stimulus lights were located 2 cm above each response lever. For rats in Experiment 2, water was continuously available via a spout mounted on the back wall of the chamber. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Infusion pumps (model PHM-100, Med Associates, for Experiment 1 or model RHSY, Fluid Metering (Syosset, NY), for Experiment 2) placed outside each cubicle delivered infusions through Tygon tubing connected to a fluid swivel mounted above the chamber, and from the swivel through a spring leash connected to a vascular access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) mounted on the back of the rat. MED-PC IV software (Med Associates) was used for operating the apparatus and recording data.
Drugs
Nicotine-alone solutions consisted of (−)-Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) dissolved in sterile saline. Aqueous tobacco extract was prepared from Kodiak Wintergreen or Camel Snus Winterchill smokeless tobacco products using general procedures described elsewhere.17 Briefly, tobacco product was mixed with saline vehicle at a concentration of 400 mg/ml (Kodiak extract) or 200 mg/ml (Camel Snus extract) for 18 h using a tube tipper. The different concentrations of the extracts reflect the higher volume of saline required for preparation of extract from Camel Snus, which is considerably more absorbent than Kodiak. We have previously found that Kodiak extracts prepared using either saline or artificial saliva contain similar levels of nicotine and the minor alkaloids nornicotine, anatabine, and anabasine17, supporting the clinical relevance of a saline extract. Use of saline extraction also simplified extract preparation and avoided possible toxic effects of artificial saliva. Following mixture of the tobacco product with saline, the resulting solution was filtered through gauze, centrifuged, and the supernate was filtered. The nicotine concentration was determined, and extract was diluted to the nicotine concentrations required for the current studies. Extract stock solutions and dilutions were prepared every 2-4 months and were kept refrigerated. We have confirmed that levels of nicotine, nornicotine, anatabine, and anabasine in ST extracts stay stable for up to one year under these storage conditions (unpublished data). The pH of the solutions was adjusted to 7.4 with dilute NaOH, and heparin (30 units/ml) was added to help maintain catheter patency. Nicotine doses are expressed as the base.
Routine Nicotine Assay
Nicotine concentrations in nicotine alone and extract solutions were measured by gas chromatography with nitrogen phosphorus detection, according to standard protocol in our laboratory.22,23 The typical measured nicotine concentration in extracts was approximately 2.0 - 3.5 mg/ml.
Surgical Procedure
Each rat was implanted with a chronic indwelling catheter into the right jugular vein under i.m. ketamine (75 - 90 mg/kg)/dexmedetomidine (0.25 mg/kg) anesthesia, described in detail elsewhere.22,24 The catheter was externalized between the scapulae and attached to the vascular-access harness (see above) that allowed connection to a fluid swivel via a tether for nicotine administration. Animals were allowed to recover for at least three days after surgery, during which time they received daily i.v. infusions of heparinized saline, ceftriaxone antibiotic (5.25 mg), and s.c. injections of buprenorphine (0.05 mg/kg; first two days only) for analgesia. Infusions of methohexital (0.1 ml, 10 mg/ml, i.v.) were administered to check patency post-session on Fridays during Experiment 1 or at the end of the study in Experiment 2. A rat was excluded from analysis if it failed to exhibit anesthesia within 3-5 sec during these patency checks.
Experiment 1
NSA acquisition
Groups of adolescent and adult rats (N = 11 - 19 per group) were allowed to self-administer nicotine alone or extracts of Camel Snus or Kodiak at a nicotine unit dose of 0.06 mg/kg/inf, beginning typically on a Monday at age PD 34 - 36 or PD 72 - 76 in adolescents or adults, respectively. This unit dose was chosen because it increased the likelihood of maintaining performance during the subsequent PR schedule phase and characterizing the inelastic portion of the demand curve in Experiment 2 (see below). A negative control group for each age was given access to saline. Infusions were available under a fixed-ratio (FR) 1 schedule. Sessions began with onset of a stimulus light above the active lever, and each lever press on that lever produced offset of the stimulus light and an infusion delivered in a volume of 0.1 ml/kg in approximately 1 sec. Each infusion was immediately followed by a 15-sec timeout during which the stimulus light remained off and lever presses were recorded but had no programmed consequence. Following the timeout, the stimulus light was illuminated to indicate drug availability. Presses on the inactive lever were recorded but had no programmed consequence. These stimulus-response contingencies are similar to those previously used for limited-access NSA.25 The active lever was baited with food powder on the first session only to ensure contact with the reinforcement contingency. Sessions were 2 hr in duration and were conducted during the dark phase of the light/dark cycle. Sessions ran five days per week for 3 weeks, resulting in a total of 13-15 sessions (equal between groups), depending on national holidays. By the end of this phase, adolescent rats were in late adolescence (PD 52 - 54). Rats were considered to have acquired self-administration if they earned at least five infusions per session and showed a mean active:inactive response ratio of at least 2:1 across the last three sessions.
Progressive-ratio phase
After the acquisition phase, rats that met acquisition criteria and still had a patent catheter (N = 10 - 14 per group) were exposed to a progressive-ratio schedule of drug delivery for two weeks, resulting in a total of 9 - 10 sessions (equal between groups), depending on national holidays. Under this schedule, the contingencies were the same as during acqusition, but the FR value increased after each drug delivery according to the sequence 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, and increased by approximately 25% thereafter. Sessions ended when the rat failed to earn an infusion within 30 min or two hours elapsed, whichever occurred first. By the end of this phase, the adolescent rats had entered early adulthood (PD 64 - 66).
Experiment 2
Acquisition phase
Four groups of adolescent rats (N = 13 - 19 per group) were used in this experiment. Adults were not used due to the lack of interaction between age and formulation in Experiment 1 and because adolescents are more amenable to study in rats than humans. Rats were trained to self-administer nicotine in daily 23-hr sessions starting at age PD 31 - 33 according to similar protocols used in our laboratory.26 This access schedule results in patterns of nicotine intake and serum nicotine concentrations that are more similar to those of smokers than shorter access sessions,24 and may provide greater sensitivity to the effects of non-nicotine constituents.27 Sessions began at the beginning of the dark phase of the light/dark cycle. Drug availability was signaled by illumination of the stimulus light above the active (right) response lever. Following completion of the response requirement, the stimulus light was extinguished and nicotine alone or extract at a nicotine unit dose of 0.06 mg/kg/inf was infused in a volume of 100 μl/kg at a rate of 50 μl/sec. A negative control group was given access to saline. Following a 7-sec time-out, the stimulus light turned on and the next nicotine infusion was available. Responses on the other (inactive) lever were recorded but had no programmed consequences. These stimulus-response contingencies are similar to those previously used for unlimited-access NSA.28 The response requirement was FR 1 for eleven sessions, with the active lever baited with food powder on the first session only. The criteria for acquisition were a minimum of 10 infusions per day and a mean ratio of active to inactive lever presses of at least 2:1 across the last three consecutive sessions. Sessions were conducted seven days per week. Rats were at age PD 41 - 43 at the end of this phase.
Demand curve analysis phase
Thirty of the rats that acquired self-administration at FR 1 (10 for each formulation) were used to assess elasticity of demand. During this phase, the FR value was increased daily until consumption decreased by at least 95% (no more than 3 infusions earned), which ensured that the lowest level of non-zero consumption amenable to demand curve analysis was similar across rats (1-3 infusions). The FR value increased according to the sequence: 2, 3, 6, 9, 15, 30, 60, and doubled thereafter. This yielded a progression of unit prices similar to that used in previous studies using a unit dose-reduction protocol in an unlimited access model.26 Unit price was altered via manipulation of FR rather than unit dose in order to be consistent with most human laboratory studies on tobacco product evaluation.29-31 In theory, both approaches should produce functionally equivalent effects on consumption.32 Rats typically completed this phase in 6 - 11 sessions, by age PD 47 - 54, thus completing the entire protocol during adolescence. Three rats were included in the nicotine group for demand curve analysis even though their active:inactive ratio did not quite meet criterion for acquisition (e.g. mean ratio of 1.7:1 instead of 2:1). These rats showed robust increases in active lever responding during FR escalation with no change or a decrease in inactive lever pressing, indicating that nicotine was serving as a reinforcer in these rats.
Data Analysis
Mean lever presses on the active and inactive lever, number of infusions, and nicotine intake across the last three sessions of each phase or at each FR value were the primary dependent measures. Breaking point (number of infusions) during the PR phase served as the primary measure of relative abuse liability in Experiment 1. These measures were analyzed by one- or two-factor ANOVA followed by Holm-Sidak post-hoc tests. For Experiment 2, mean infusions and lever presses during the acquisition phase were similarly analyzed. In addition, elasticity of demand served as the primary measure of relative abuse liability, which was determined by exponential demand curve analysis using the following equation:33
The dependent variable, Q, is the quantity consumed. The independent variable, C, is the cost of nicotine based on the unit price (FR/unit dose). The free parameters, Q0 and α are estimated from the best-fit function and refer to the maximum level of consumption at zero price (i.e., level or “intensity” of demand) and the rate of change in consumption with increases in unit price, respectively. The range of the exponential function, k, is a constant specifying the range of consumption in log units. The k value is held constant across all data sets being compared (set to 1.8 in the present study), because changes in k impact the value of α. The α parameter is considered a measure of reinforcing efficacy, such that drugs that produce rapidly declining (elastic) demand curves have higher α values and lower reinforcing efficacy than demand curves with slower declining (inelastic) demand curves. Therefore, α served as the index of elasticity of demand for, or reinforcing efficacy of, nicotine and extracts. Other demand measures of interest included: Q0, the level or intensity of demand as described above; Omax, the maximal response output; and Pmax, the unit price (responses per unit dose) at which maximal response output occurred. Demand functions were generated using a template for GraphPad Prism software (GraphPad Software, Inc; La Jolla, CA) provided by the Institutes for Behavior Resources, Inc. (Baltimore, MD) on their website (http://www.ibrinc.org/index.php?id=181).
RESULTS
Experiment 1
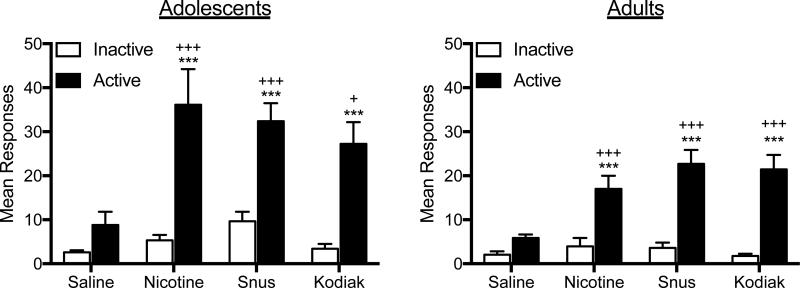
Figure 1 shows the mean number of responses on the active and inactive levers during the last three sessions of the FR 1 acquisition phase in each group (daily responses are shown in Appendix A for reference). There was a significant main effect of lever for both adolescents (F = 62.74, p < .01) and adults (F=125.1, p<0.0001), as well as a main effect of formulation (F=4.48, p<0.01 and F = 6.38, p < .001 for adolescents and adults, respectively) and lever × formulation interaction (F = 3.35, p < .05 and F = 9.32, p < .001 for adolescents and adults, respectively). For both age groups, each nicotine formulation maintained significantly higher rates of responding on the active lever compared to the inactive lever, whereas saline did not. Moreover, each nicotine formulation maintained higher rates of active lever responding than saline. There were no significant differences between nicotine alone and extracts in either age group.
Figure 1. Mean Responding on the Active and Inactive Levers During the Acquisition Phase of Experiment 1.
Note. Mean (± SEM) responses on the active and inactive lever in each group of adolescent and adult rats across the last three session under the FR 1 schedule in Experiment 1. Different from saline, +p < .05, +++p < .001. Different from inactive lever, ***p < .001.
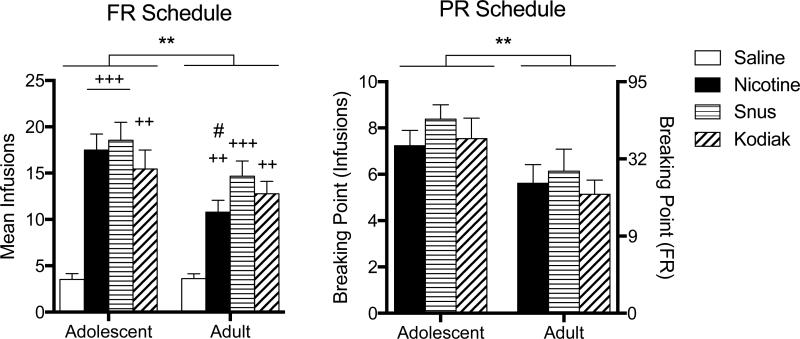
Figure 2 shows the mean infusion rate at the end of the acquisition and PR phases for each group. There was a significant main effect of age (F = 7.41, p < .01) and formulation (F = 17.16, p < .001) on mean infusions during acquisition (left panel), but no significant age × formulation interaction. Adolescents exhibited higher infusion rates overall, but a significant difference between ages in intake of each formulation was only observed for nicotine alone. Within each age, mean infusions for nicotine alone and each extract were significantly higher than saline, but no significant differences were observed between nicotine alone and extracts. Under the PR schedule, there was a significant main effect of age (F = 11.65, p < .01) on breaking point (right panel), but no significant effect of formulation or age × formulation interaction. Adolescents exhibited higher breaking points overall, but no significant difference between ages was observed for any formulation. Within each age, no significant differences were observed between nicotine alone and extracts.
Figure 2. Mean Infusions During the Acquisition and Progressive-Ratio Phases of Experiment 1.
Note. Mean (± SEM) infusions earned per session in each group of adolescent and adult rats across the last three sessions under the FR 1 schedule in Experiment 1 are shown in the left panel. Mean (± SEM) breaking points (expressed as infusions on the left y-axis and associated last completed FR on the right y-axis) across the last three sessions under the PR schedule in Experiment 1 are shown in the right panel. No saline data are shown for the PR schedule because data were only analyzed for rats that met acquisition criteria. Main effect of age, **p < .01. Different from saline, ++p < .01, +++p < .001. Different from nicotine, #p < .05.
Experiment 2
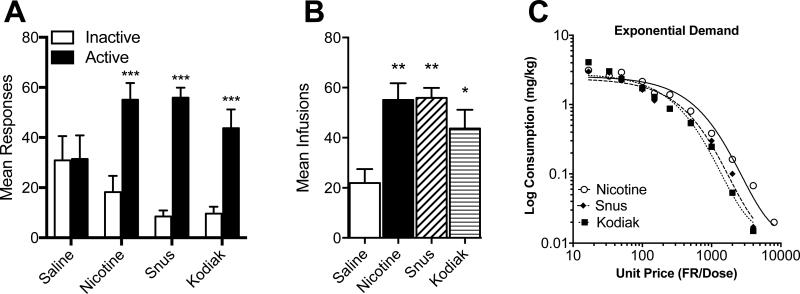
Figure 3A and 3B show mean responses on each lever and infusions per session, respectively, during the last three sessions of the acquisition phase (daily responses are shown in Appendix B for reference). There was a significant main effect of lever (F = 81.84, p < .001) and lever × formulation interaction (F = 8.79, p < .001), but no main effect of formulation. Response rates on the active lever in the nicotine alone and extract groups was not significantly higher than that in the saline group. However, active lever responding was significantly higher compared to the inactive lever in these groups, but not in the saline group (panel A). In addition, nicotine alone and both extracts maintained higher infusion rates than saline (panel B). There were no significant differences in response rates on either lever or infusion rates between nicotine alone and extracts. Figure 3C shows mean nicotine consumption in each group as a function of unit price and associated exponential curve fits during demand curve assessment. Table 1 shows the exponential demand curve parameter estimates for individual subjects in each group. As expected, nicotine consumption declined with increases in unit price in each group. The exponential demand equation described the data well, with typical r2 values greater than 0.9. Despite a somewhat faster decline in consumption (higher α values) in the extract groups, there were no significant differences between groups in any of the demand parameter estimates.
Figure 3. Mean Responses, Infusions, and Exponential Demand Curves During Experiment 2.
Note. Mean (± SEM) responses on the active and inactive lever (panel A) and mean (± SEM) infusions earned per session (panel B) in each group of adolescent rats during the last three sessions under the FR 1 schedule in Experiment 2. Panel C shows nicotine consumption in each group as a function of unit price with associated exponential demand curve fits during demand curve assessment in Experiment 2. Individual and group demand parameter estimates are shown in Table 1. Different from saline (panel A), ***p < .001. Different from saline (panel B), *p < 0.05, **p < 0.01.
Table 1.
Exponential demand curve parameters for individual subjects
| Subject | α | Q0 | Pmax | Omax | r2 |
|---|---|---|---|---|---|
| Nicotine | |||||
| 1 | 0.00024 | 3.6 | 391.7 | 429.0 | 0.95 |
| 2 | 0.00010 | 5.4 | 646.2 | 1061.4 | 0.98 |
| 3 | 0.00036 | 3.0 | 313.4 | 286.0 | 0.97 |
| 4 | 0.00068 | 3.6 | 138.3 | 151.4 | 0.97 |
| 5 | 0.00027 | 3.7 | 338.8 | 381.3 | 1.00 |
| 6 | 0.00014 | 2.3 | 1051.1 | 735.4 | 0.87 |
| 7 | 0.00015 | 2.5 | 902.6 | 686.4 | 0.97 |
| 8 | 0.00037 | 2.3 | 397.7 | 278.3 | 0.96 |
| 9 | 0.00052 | 2.0 | 325.4 | 198.0 | 0.90 |
| 10 | 0.00130 | 1.1 | 236.7 | 79.2 | 0.94 |
| Mean | 0.00041 | 2.9 | 474.2 | 428.6 | 0.95 |
| SEM | 0.00011 | 0.4 | 93.9 | 97.7 | 0.01 |
| Snus | |||||
| 1 | 0.00020 | 3.2 | 528.8 | 514.8 | 0.91 |
| 2 | 0.00110 | 0.76 | 404.9 | 93.6 | 0.71 |
| 3 | 0.00019 | 3.5 | 509.0 | 541.9 | 0.97 |
| 4 | 0.00047 | 1.8 | 400.1 | 219.1 | 0.91 |
| 5 | 0.00033 | 3.7 | 277.2 | 312.0 | 0.98 |
| 6 | 0.00310 | 4.1 | 26.6 | 33.2 | 0.93 |
| 7 | 0.00020 | 2.8 | 604.4 | 514.8 | 0.96 |
| 8 | 0.00038 | 2.7 | 329.9 | 270.9 | 0.84 |
| 9 | 0.00052 | 3.9 | 166.9 | 198.0 | 0.97 |
| 10 | 0.00047 | 4.9 | 147.0 | 219.1 | 0.96 |
| Mean | 0.00070 | 3.1 | 339.5 | 291.7 | 0.91 |
| SEM | 0.00028 | 0.4 | 58.8 | 56.6 | 0.03 |
| Kodiak | |||||
| 1 | 0.00041 | 2.3 | 358.9 | 251.1 | 0.91 |
| 2 | 0.00018 | 4.3 | 437.3 | 572.0 | 0.95 |
| 3 | 0.00018 | 3.6 | 522.3 | 572.0 | 0.97 |
| 4 | 0.00028 | 4.2 | 287.8 | 367.7 | 0.98 |
| 5 | 0.00096 | 8.0 | 44.10 | 107.2 | 0.97 |
| 6 | 0.00059 | 6.1 | 94.04 | 174.5 | 0.97 |
| 7 | 0.00035 | 2.3 | 420.4 | 294.2 | 0.95 |
| 8 | 0.00130 | 3.7 | 70.4 | 79.2 | 0.94 |
| 9 | 0.00067 | 4.0 | 126.3 | 153.7 | 0.83 |
| 10 | 0.00180 | 3.0 | 62.7 | 57.2 | 0.88 |
| Mean | 0.00067 | 4.2 | 242.4 | 262.9 | 0.94 |
| SEM | 0.00017 | 0.6 | 57.8 | 59.9 | 0.02 |
Note. The parameter k set to 1.8 log units globally.
DISCUSSION
The key finding of the present study was that the relative abuse liability of extracts of the ST products Camel Snus and Kodiak Wintergreen were similar to nicotine alone. This was observed in both adolescent and adult rats under limited-access (2 hr/day) conditions, and in adolescents under unlimited access (23 hr/day) conditions. The overall abuse liability of these nicotine formulations was greater in adolescents compared to adults, as indicated by higher infusion rates under an FR schedule and higher breaking points under a PR schedule during late adolescence/early adulthood. The present study provides a key extension of preclinical research on the abuse liability of tobacco products and has important implications for understanding the mechanisms mediating ST use and developing tobacco regulatory policy.
The present findings are consistent with our prior studies showing that these ST extracts do not differ from nicotine alone in ICSS and locomotor sensitization models.7,17 A key advantage of the systemic administration of extracts is that it avoids variation in peripheral sensory effects (eg, taste, smell) and pharmacokinetics (eg, nicotine absorption) that occur with oral exposure to ST products. Controlling these factors allows study of whether the direct CNS-mediated effects of nicotine and non-nicotine constituents might account for any differences in the reinforcing effects of ST products. As such, our present and prior findings suggest that nicotine content or yield is the primary determinant of the abuse liability of Camel Snus and Kodiak in terms of their addiction-related CNS effects. Current levels of non-nicotine constituents do not appear to contribute to the abuse-related CNS effects of these products. However, some of these constituents may contribute to abuse liability via peripheral sensory mechanisms (eg, flavorants, odorants), pharmacokinetic mechanisms, or both. Some may also begin to influence the CNS effects of these products if their levels increase significantly (eg, minor alkaloids34).
Our findings contrast with the increased abuse liability of tobacco smoke extracts compared to nicotine alone observed under some conditions in self-administration models.5 The reason for this discrepancy is unclear. The very limited chemical characterization of extracts used in the present and previous studies precludes identifying important differences in the constituent profiles of cigarette smoke versus ST extracts. However, the lack of any differences between ST extracts and nicotine alone in our studies suggests that the constituents responsible for the greater reinforcing effects of smoke extracts might be specific to smoke, or ones that are present at much higher levels in smoke than in ST. Future studies of tobacco or smoke extracts should include more thorough chemical characterization of the extracts to facilitate isolation of constituents that play a key role in moderating differences in abuse liability observed between products.
The present findings also contrast with findings from Clemens et al.4 reporting greater reinforcing efficacy of a mixture of nicotine and several minor alkaloids compared to nicotine alone under a PR schedule. Several factors may account for this discrepancy. We previously reported that Camel Snus and Kodiak extracts prepared under identical conditions contained combined levels of nornicotine, anabasine, and anatabine that were ≈ 2.5% of nicotine dose.7 Therefore, although nicotine intake was comparable between studies, the relative level of minor alkaloids in the ST extracts in the present study were likely lower than that in the alkaloid mixture used by Clemens et al. (≈ 6.3% of nicotine dose),7 which was based on alkaloid concentrations in cigarette smoke. As such, the level of minor alkaloids in the present study may have been below a threshold required to enhance reinforcing efficacy. This suggests that minor alkaloids may play a greater role in the abuse liability of combusted tobacco products than ST products. Another possible reason for the discrepancy with the findings of Clemens et al. is that other constituents in the extracts might oppose any effects of minor alkaloids that occur when administered in isolation. This may also account for recent findings indicating no differences in the reinforcing effects of nicotine alone and nicotine delivered in a cocktail containing the same minor alkaloids as that used by Clemens et al., but also containing acetaldehyde and the beta-carbolines harmane and norharmane.35 Finally, procedure differences (eg, rat strain, session duration, PR step sizes, etc.) between studies may account for the difference in findings.
Although the lack of differences in abuse liability between products might be due to an insensitivity of the procedures used in the present study, this is not likely for several reasons. First, FR or PR schedules have proven sufficiently sensitive for detecting differences in abuse liability between nicotine alone and smoke extracts and constituent mixtures,4,5 and were sensitive to age effects in the present study. Second, our studies used adolescent rats, which can be more sensitive to the behavioral effects of non-nicotine constituents than adults.3,21 Third, an unlimited access model was used in the present study, allowing time for the influence of pharmacokinetic differences among constituents to manifest, such as the longer half-life of some constituents (eg, nornicotine36) or their influence on nicotine clearance (eg, menthol37). Finally, the behavioral economic demand curve analysis that was used in the present study is considered a state-of-the-art approach for rank ordering the abuse liability of drugs.33,38,39 Moreover, the precision of this analysis was comparable to other studies in which demand curve analysis was capable of distinguishing between subpopulations of rats or smokers.26,40,41 Despite the prior utility of the procedures used in the present study, exploration of other approaches is still warranted (eg, choice assays42).
A limitation of the present study is the lack of pharmacological challenge to assess potential differences in the neural receptor mechanisms mediating the reinforcing effects of nicotine alone and ST extracts. Other studies have shown that nicotinic acetylcholine receptor (nAChR) antagonists can have weaker effects on self-administration of smoke extracts compared to nicotine alone, even when no differences in baseline self-administration are observed.5 This suggests that receptor mechanisms other than nAChRs may contribute to the reinforcing effects of smoke extracts. If there are unique receptor mechanisms mediating the reinforcing effects of ST extracts, it would suggest that their discriminative stimulus (ie, subjective) effects may differ from nicotine alone.43 In this case, the need for other self-administration assays mentioned above becomes particularly important, as differences in subjective effects might influence preference for ST extracts over nicotine alone if rats are given a choice between formulations.
An additional limitation of our study was our use of only males. Given the reports of sex differences in nicotine's reinforcing effects in both humans and animals,44-47 future studies should extend the generality of our findings to females. This would also provide insights into any effects of sex on sensitivity to non-nicotine tobacco constituents, which to our knowledge have not been studied. Examination of other nicotine unit doses also represents an important area for future work, as effects of smoke extracts and non-nicotine constituents on nicotine reinforcement can depend on the nicotine unit dose.6,12,48 Nevertheless, to the extent that manipulating unit price via increasing the FR response requirement is functionally equivalent to reducing the unit dose,49 the similar consumption of nicotine alone and extracts during the current FR escalation procedure would be expected to generalize to a dose-reduction protocol.
The present finding that the reinforcing efficacy of nicotine formulations was greater in adolescents than adults is consistent with other studies reporting greater sensitivity to nicotine reinforcement in adolescents compared to adults.19,20,50 However, this finding contrasts with other studies showing lower rates of acquisition and nicotine intake under FR schedules and lower breaking points under a PR schedule in adolescents compared to adults.51-53 This discrepancy may be due to the use of a higher training dose of nicotine and different strain of rat in the present study, as age differences in nicotine self-administration can depend on these factors.52,53 It is also important to note that adolescent rats were young adults by the end of the PR phase in Experiment 1. As such, our findings might reflect the effect of adolescent nicotine exposure on the subsequent reinforcing efficacy of nicotine in early adulthood, as opposed to greater reinforcing efficacy of nicotine during adolescence per se. Regardless of any age differences in nicotine reinforcement, there were no differences in reinforcing efficacy between formulations in either age group in the present study. To the extent that these findings generalize to other tobacco products, it would suggest that relative abuse liability between tobacco products may be similar in adolescents and adults, at least as far as CNS-mediated reinforcing effects are concerned.
IMPLICATIONS FOR TOBACCO REGULATION
This study provides important new information on the abuse liability of tobacco products in adolescents, a stated priority of the FDA CTP that had not previously been studied in a preclinical model. Specifically, our data suggest that the relative abuse liability of ST products might be similar in adolescents and adults. As such, polices that affect relative abuse liability may have a similar impact on product use in both age groups. Moreover, products deemed substantially equivalent for adults may also be so for adolescents. Our findings also provide guidance to the FDA CTP in setting standards to limit the abuse liability of ST tobacco products. For instance, the non-nicotine constituents responsible for any differences in abuse liability between ST products observed in humans may most likely be those producing peripheral sensory effects, moderating nicotine or other constituent pharmacokinetics, or both. However, because higher concentrations of other constituents (eg, minor alkaloids) could begin to influence the abuse-related CNS effects of ST products, the present findings suggest product standards for those constituents should not allow increases above their current concentrations in order to avoid this possibility.
Animal Subjects Statement
Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2011 National Research Council's Guide for the Care and Use of Laboratory Animals.
Acknowledgements
The authors thank Laura Tally and Christine Hernandez for their excellent technical assistance. The authors also thank Dr. Pete Roma from the Institutes for Behavior Resources (Baltimore, MD) and Johns Hopkins University School of Medicine for assistance with conducting the exponential demand curve analysis. Funding for this study was provided by NIH/NIDA grant U19-CA157345 (Hatsukami DH and Shields P, MPI; LeSage MG, PL) and a Career Development Award (MGL) and Translational Addiction Research Program award (ACH) from the Minneapolis Medical Research Foundation. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Appendix
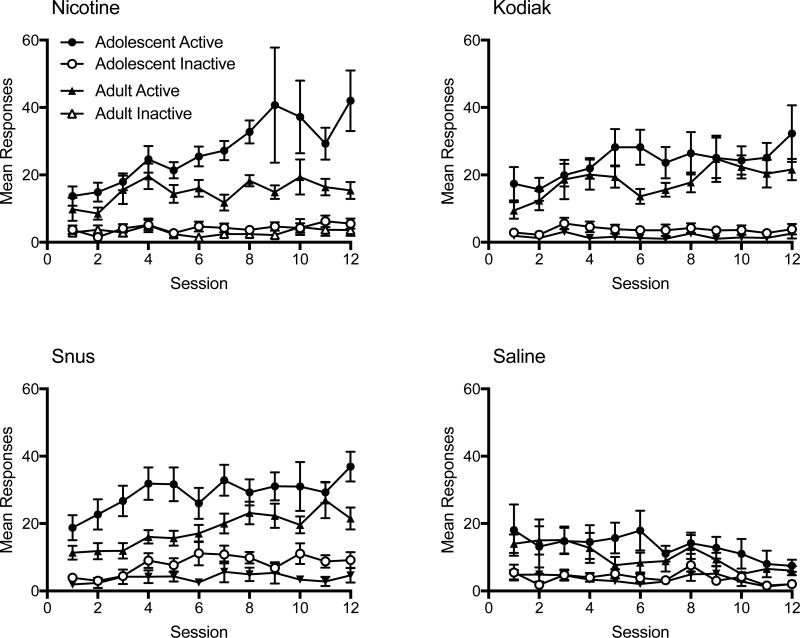
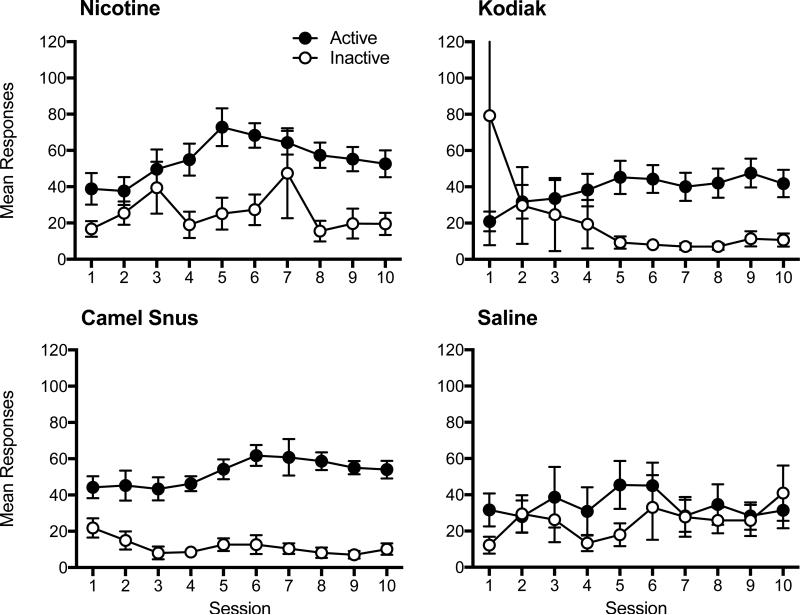
Appendix A. Daily Responding During the Acquisition Phase of Experiment 1.
Note. Mean (±SEM) responses on the active and inactive lever on consecutive sessions during the acquisition phase of Experiment 1 in each age group for each indicated formulation.
Appendix B. Daily Responding During the Acquisition Phase of Experiment 2.
Note. Mean (±SEM) responses on the active and inactive lever on consecutive sessions during the acquisition phase of Experiment 2 for each indicated formulation.
Footnotes
Conflict of Interest Disclosure Statement
Dr. LeSage has served as a paid Special Government Employee to the Food and Drug Administration Center for Tobacco Products. All other authors declare they have no conflicts of interest.
Contributor Information
Mark G. LeSage, Department of Medicine, Minneapolis Medical Research Foundation, Minneapolis, MN..
Danielle Burroughs, Department of Medicine, Minneapolis Medical Research Foundation, Minneapolis, MN..
Peter Muelken, Department of Medicine, Minneapolis Medical Research Foundation, Minneapolis, MN..
Andrew C. Harris, Department of Medicine, Minneapolis Medical Research Foundation, Minneapolis, MN..
References
- 1.Berman ML, Connolly G, Cummings MK, et al. Providing a Science Base for the Evaluation of Tobacco Products. tobacco reg sci. 2015;1(1):76–93. doi: 10.18001/TRS.1.1.8. doi:10.18001/TRS.1.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donny EC, Taylor TG, LeSage MG, et al. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14(11):1319–1338. doi: 10.1093/ntr/nts162. doi:10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30(4):705–712. doi: 10.1038/sj.npp.1300586. doi:10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- 4.Clemens K, Caillé S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12(10):1–12. doi: 10.1017/S1461145709000273. doi:10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- 5.Brennan KA, Laugesen M, Truman P. Whole tobacco smoke extracts to model tobacco dependence in animals. Neuroscience and biobehavioral reviews. 2014;47:53–69. doi: 10.1016/j.neubiorev.2014.07.014. doi:10.1016/j.neubiorev.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39(8):1843–1851. doi: 10.1038/npp.2014.31. doi:10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris AC, Tally L, Schmidt CE, et al. Animal models to assess the abuse liability of tobacco products: Effects of smokeless tobacco extracts on intracranial self-stimulation. Drug Alcohol Depend. 2015;147:60–67. doi: 10.1016/j.drugalcdep.2014.12.015. doi:10.1016/j.drugalcdep.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose V, Miller JH, Dickson SJ, et al. Tobacco particulate matter is more potent than nicotine at upregulating nicotinic receptors on SH-SY5Y cells. Nicotine Tob Res. 2007;9(8):793–799. doi: 10.1080/14622200701485117. doi:10.1080/14622200701485117. [DOI] [PubMed] [Google Scholar]
- 9.Touiki K, Rat P, Molimard R, Chait A, de Beaurepaire R. Effects of tobacco and cigarette smoke extracts on serotonergic raphe neurons in the rat. Neuroreport. 2007;18(9):925–929. doi: 10.1097/WNR.0b013e32811d6d21. doi:10.1097/WNR.0b013e32811d6d21. [DOI] [PubMed] [Google Scholar]
- 10.Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139(3):169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 11.Ator N, Griffiths R. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70(3S):55–72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 12.Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addiction Biology. 2013;20(2) doi: 10.1111/adb.12099. n/a–n/a. doi:10.1111/adb.12099. [DOI] [PubMed] [Google Scholar]
- 13.Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146(3):290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- 14.Guillem K, Vouillac C, Azar MR, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25(38):8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. doi:10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villégier A-S, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology. 2007;193(4):457–465. doi: 10.1007/s00213-007-0799-7. doi:10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- 16.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob Res. 2008;10(12):1773–1782. doi: 10.1080/14622200802443544. doi:10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris AC, Stepanov I, Pentel PR, LeSage MG. Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology. 2012;220(3):565–576. doi: 10.1007/s00213-011-2514-y. doi:10.1007/s00213-011-2514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatsukami DK, Severson H, Anderson A, et al. Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching. Tob Control. 2016;25(3):267–274. doi: 10.1136/tobaccocontrol-2014-052080. doi:10.1136/tobaccocontrol-2014-052080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. doi:10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- 20.Levin ED, Lawrence SS, Petro A, et al. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. doi:10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villégier A-S, Gallager B, Heston J, Belluzzi JD, Leslie FM. Age influences the effects of nicotine and monoamine oxidase inhibition on mood-related behaviors in rats. Psychopharmacology. 2010;208(4):601. doi: 10.1007/s00213-009-1760-8. doi:10.1007/s00213-009-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharm. 2008;16(1):86–97. doi: 10.1037/1064-1297.16.1.86. doi:10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- 23.Hieda Y, Keyler DE, VanDeVoort JT, et al. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology. 1999;143(2):150–157. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- 24.LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72(1-2):279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 25.LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79(3):507–513. doi: 10.1016/j.pbb.2004.09.002. doi:10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG. Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend. 2015;151:181–193. doi: 10.1016/j.drugalcdep.2015.03.030. doi:10.1016/j.drugalcdep.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37(9):2153–2160. doi: 10.1038/npp.2012.67. doi:10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology. 2003;170(3):278–286. doi: 10.1007/s00213-003-1539-2. doi:10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav. 2006;85(1):73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson MW, Bickel WK. The behavioral economics of cigarette smoking: The concurrent presence of a substitute and an independent reinforcer. Behav Pharmacol. 2003;14(2):137–144. doi: 10.1097/00008877-200303000-00005. doi:10.1097/01.fbp.0000063266.43827.42. [DOI] [PubMed] [Google Scholar]
- 31.DeGrandpre RJ, Bickel WK, Higgins ST, Hughes JR. A behavioral economic analysis of concurrently available money and cigarettes. J Exp Anal Behav. 1994;61(2):191–201. doi: 10.1901/jeab.1994.61-191. doi:10.1901/jeab.1994.61-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- 33.Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. doi:10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- 34.Harris AC, Tally L, Muelken P, et al. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend. 2015;153:330–334. doi: 10.1016/j.drugalcdep.2015.06.005. doi:10.1016/j.drugalcdep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith TT, Schaff MB, Rupprecht LE, et al. Effects of MAO inhibition and a combination of minor alkaloids, β-carbolines, and acetaldehyde on nicotine self-administration in adult male rats. Drug Alcohol Depend. 2015;155:1–10. doi: 10.1016/j.drugalcdep.2015.07.002. doi:10.1016/j.drugalcdep.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosheh O, Dwoskin LP, Li W-K, Crooks PA. Residence Times and Half-Lives of Nicotine Metabolites in Rat Brain after Acute Peripheral Administration of [2′-14C]Nicotine. Drug Metab Dispos. 1999;27(12):1448–1455. [PubMed] [Google Scholar]
- 37.AlSharari SD, King JR, Nordman JC, et al. Pechnick RN, editor. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PLoS ONE. 2015;10(9):e0137070–16. doi: 10.1371/journal.pone.0137070. doi:10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99(1):98–124. doi: 10.1002/jeab.7. doi:10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- 39.Hursh SR, Galuska CM, Winger G, Woods JH. The economics of drug abuse: a quantitative assessment of drug demand. Mol Interv. 2005;5(1):20–28. doi: 10.1124/mi.5.1.6. doi:10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- 40.Mackillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology. 2011;216(1):91–99. doi: 10.1007/s00213-011-2185-8. doi:10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diergaarde L, van Mourik Y, Pattij T, Schoffelmeer ANM, De Vries TJ. Poor impulse control predicts inelastic demand for nicotine but not alcohol in rats. Addiction Biology. 2011;17(3):576–587. doi: 10.1111/j.1369-1600.2011.00376.x. doi:10.1111/j.1369-1600.2011.00376.x. [DOI] [PubMed] [Google Scholar]
- 42.Shahan TA, Bickel WK, Madden GJ, Badger GJ. Comparing the reinforcing efficacy of nicotine containing and de-nicotinized cigarettes: a behavioral economic analysis. Psychopharmacology. 1999;147(2):210–216. doi: 10.1007/s002130051162. [DOI] [PubMed] [Google Scholar]
- 43.Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. Handb Exp Pharmacol. 2009;192(192):295–333. doi: 10.1007/978-3-540-69248-5_11. doi:10.1007/978-3-540-69248-5_11. [DOI] [PubMed] [Google Scholar]
- 44.Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94(1):43–50. doi: 10.1016/j.pbb.2009.07.004. doi:10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 46.Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. Nebr Symp Motiv. 2009;55:143–169. doi: 10.1007/978-0-387-78748-0_9. doi:10.1007/978-0-387-78748-0. [DOI] [PubMed] [Google Scholar]
- 47.Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: Implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114-115:70–81. doi: 10.1016/j.pbb.2013.10.020. doi:10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith TT, Rupprecht LE, Cwalina SN, et al. Effects of Monoamine Oxidase Inhibition on the Reinforcing Properties of Low-Dose Nicotine. Neuropsychopharmacology. 2016 Mar; doi: 10.1038/npp.2016.36. doi:10.1038/npp.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith TT, Sved AF, Hatsukami DK, Donny EC. Nicotine reduction as an increase in the unit price of cigarettes: A behavioral economics approach. Prev Med. 2014;68:1–6. doi: 10.1016/j.ypmed.2014.07.005. doi:10.1016/j.ypmed.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169(2):141–149. doi: 10.1007/s00213-003-1486-y. doi:10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 51.Schassburger RL, Pitzer EM, Smith TT, et al. Adolescent Rats Self-Administer Less Nicotine Than Adults at Low Doses. Nicotine Tob Res. 2016 Jan;:ntw006–ntw008. doi: 10.1093/ntr/ntw006. doi:10.1093/ntr/ntw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shram MJ, Li Z, Lê AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology. 2008;197(1):45–58. doi: 10.1007/s00213-007-1003-9. doi:10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- 53.Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33(4):739–748. doi: 10.1038/sj.npp.1301454. doi:10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]