Abstract
BACKGROUND/OBJECTIVES
Obesity is characterized by chronic inflammation and immune dysregulation, as well as insulin resistance, but the link between obesity and adaptive immunity remains to be fully studied.
METHODS
To elucidate the role of adaptive immunity on body composition, glucose homeostasis and inflammation, recombination-activating gene 1 knockout (Rag1 − / −) mice, without mature T-lymphocytes or B-lymphocytes, were maintained on a low- or high-fat diet (LFD and HFD, respectively) for 11 weeks.
RESULTS
Rag1 − / − mice fed HFD gained significantly more weight and had increased body fat compared with wild type. Downregulation of energy expenditure as well as brown fat uncoupling protein UCP-1 and UCP-3 gene expression were noticed in HFD-fed Rag1 − / − mice compared with LFD. HFD mice had significantly decreased energy intake compared with LFD mice, consistent with decreased agouti-related protein and increased pro-opiomelanocortin gene expression levels in the hypothalamus. Moreover, compared with wild type, Rag1 − / − mice had lower interleukin (IL)-4 levels, a cytokine recently found to induce browning in white adipocytes, and higher IL-12 levels in HFD-fed Rag1 − / − mice. Despite that HFD Rag1 − / − mice were more obese, they had similar glucose, insulin and adiponectin levels, while leptin was marginally increased.
CONCLUSIONS
Mice with deficiency in adaptive immunity are obese, partly owing to decreased energy expenditure, but are metabolically normal, suggesting that mature lymphocytes have necessary roles in the development of obesity-related metabolic dysregulation.
INTRODUCTION
Obesity is associated with insulin resistance and other components of the metabolic syndrome, leading to higher mortality.1 Adipose tissue inflammation in enlarged adipose tissue has been proposed to be a pathogenic link between obesity and metabolic diseases.2–6 Local as well as systemic increase in inflammatory cytokines is a major contributor to development of insulin resistance and cardiovascular disease.7,8 Macrophages, mast cells, neutrophil, monocyte, T- and B-lymphocyte counts are increased in obese state,9–11 whereas eosinophils and some subsets of T lymphocytes, such as T helper type 2 (Th2), regulatory T cells and invariant natural killer T cells, are decreased.12 Infiltration of innate immune cells such as M1-type proinflammatory macrophages into the expanding adipose tissue or the phenotype switch from M2-type anti-inflammatory macrophages to M1-type macrophages have been shown to be key immune responses in obesity.13,14 However, recent studies have shown that adaptive immune cells may also have a role in this process and even precede innate immune response to regulate the accumulation and phenotypic physiology, but its connection with the innate immune system still remains unclear.
To elucidate the role of the adaptive immune system in adipocyte metabolism and whole-body energy homeostasis, insulin resistance, glucose homeostasis and adipose tissue inflammation, we used diet-induced obese C57BL/6J (C57) mice, which are the closest mouse model to human obesity, and recombination-activating gene 1 knockout (Rag1 − / −) mice with a Rag1tm1Mom mutation, which cannot mature T-lymphocytes or B-lymphocytes, and hence is a model of severe combined immune deficiency.15 After feeding low- and high-fat diet (LFD and HFD, respectively) to wild-type C57 mice and Rag1 − / − mice, we compared their body composition, metabolic profile and local as well as systemic inflammation, to fully assess the interaction between diet, immune genotype and obesity and its metabolic complications.
MATERIALS AND METHODS
Animals
Male, 3-week-old, C57 and Rag1 − / − mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and housed in individual cages in a room maintained at 22 °C on a 12:12-h light–dark cycle. The Rag1 − / − mice have the same genetic background with controls as they were generated from C57 mice, and all mice were purchased and shipped to us at the same time. All mice were acclimated to the facility for 3 days while being fed a regular chow diet (Formulab diet 5008, Lab Diet, Richmond, IN, USA) and water ad libitum. All animals were handled in accordance with the principles and guidelines established by the National Institutes of Health. The animal facilities and protocols reported were approved by the Beth Israel Deaconess Medical Center (BIDMC) Institutional Animal Care and Use Committee.
Experimental design
Mice were randomly assigned to four groups (n = 6 per group) as follows: C57+LFD, C57+HFD, Rag1 − / − +LFD, and Rag1 − / − +HFD. Mice in all the groups were maintained on their assigned diet for 11 weeks. The LFD used was regular animal chow diet, which provides 16.7% calories from fat (Formulab diet 5008, Lab Diet, Richmond, IN, USA). The HFD was a western diet (TD88137, Harlan Teklad, Madison, WI, USA), which contains 42.2% of calorie from milk fat, 42.8% from carbohydrates and 15.0% from protein. The macronutrient distribution, particularly the proportion of fat and distribution of saturated vs unsaturated fat in HFD was representative of diets linked to a high-risk cardiovascular disease in humans (Teklad Custom Research Diet data sheet).
Experimental procedures
Physiological characterization
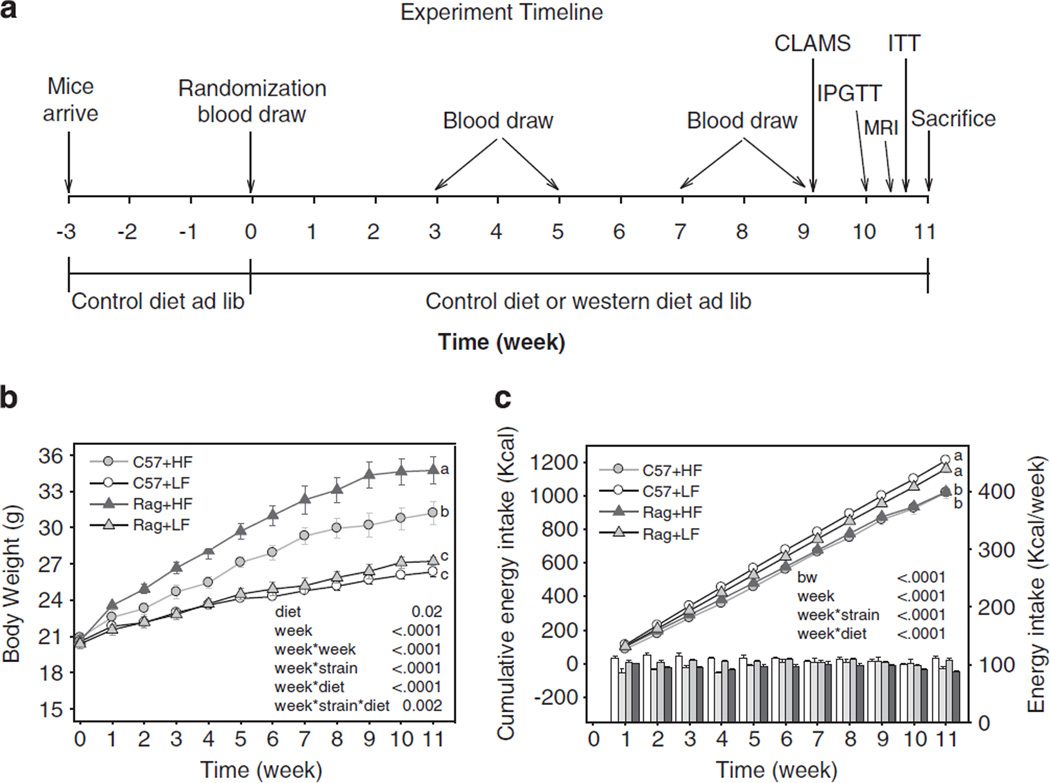
Body weights and total amount of food intake were measured weekly on the same day between 0800 and 1000 hours. with an analytical balance. Total body fat mass, lean tissue mass and water content were accessed by magnetic resonance imaging techniques (ECHO Medical Systems, Houston, TX, USA). For whole-body composition analysis, non-anesthetized mice were placed in a restraint tube and inserted into the nuclear magnetic resonance machine for determination of body adiposity 1 day before killing (Figure 1).16
Figure 1.
Experimental timeline (a), body weight (b) and energy intake (c) in C57 and Rag1 − / − mice maintained on LFD or HFD. Data were analyzed using repeated-measures analysis of variance and post-hoc multiple comparison procedures. Values are means±s.e.; n = 6 mice/group. Means with different letters are significantly different (P < 0.05).
Indirect calorimetry and activity
A Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments International, Columbus, OH, USA) was used to measure energy expenditure, oxygen consumption, carbon dioxide production and locomotor activity (XYAmb). An air sample was withdrawn every 40min. Heat (kcal h−1) produced was calculated according to the equation: (3.815+1.232 RER) × VO2, where respiratory exchange ratio (RER) was volume of CO2 (VCO2) produced per volume of O2 (VO2) consumed. VO2 or VCO2 was the volume of O2 or CO2 consumed per hour per kilogram mass of the animal. Locomotor activity was quantified as consecutive photo beam breaks along the long (XAmb) and short (YAmb) axes of the same CLAMS cages. In order for animals to acclimate to this system, they were placed in the metabolic cages 1 day before the actual recording. Mice then housed in the metabolic cages for 72 h, and an average of the data obtained during the 3 days was used for the analysis.16
Glucose and insulin tolerance test
Intraperitoneal glucose tolerance tests and insulin tolerance tests were performed. The intraperitoneal glucose tolerance tests were conducted in the morning after mice had been fasted for 16–18 h. Mice were then injected with a standard glucose bolus, as previously outlined.17 Intraperitoneal insulin tolerance tests were performed by injecting 0.75 units of insulin per kg body weight intraperitoneally.
Tissue collection and biochemical analyses
Mice were fasted for at least 4 h prior to the blood draw. Blood was collected in a chilled BD Microtainer (No Additive Tubes or Tubes with EDTA, Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Serum or plasma was isolated by centrifugation at 1800 g for 15 min at 4 °C and was stored at − 80 °C until further analysis. Plasma adiponectin, leptin and insulin were measured by enzyme-linked immunosorbent assay (Millipore, Billerica, MA, USA; ALPCO Diagnostics, Salem, NH, USA). Blood glucose was measured using the LifeScan One Touch Ultra glucometer (Johnson&Johnson, New Brunswick, NJ, USA). With the measured plasma insulin and glucose data, homeostasis model assessment for insulin resistance was calculated. Markers of inflammation, including interferon-γ (IFN-γ), interleukin (IL)-1β, IL-2, IL-4, IL-5, keratinocyte-derived chemokine/growth-related oncogene, IL-10, IL-12 total and tumor necrosis factor-α (TNF-α), were determined using a mouse TH1/TH2 9-Plex Assay Ultra-Sensitive Kit (Meso Scale Discovery (MSD) multi-spot Assay System, Gaithersburg, MD, USA) according to the manufacturer’s instructions.
After 11 weeks, mice were anesthetized with isoflurane and killed by exsanguinations. Tissues including that of the heart, liver, kidney, pancreas, spleen, intestine, hypothalamus and muscle and epididymal, perirenal, mesenteric, subcutaneous and brown adipose tissues were collected, immediately frozen in liquid nitrogen, and stored at − 80 °C.
Total RNA was extracted from hypothalamus, liver, muscle and five areas of adipose tissue using TRIZOL reagent (Invitrogen, Grand Island, NY, USA) and quantified spectrophotometrically at 260 nm. Integrity was confirmed by visualization of 18 S and 28 S rRNA on the Flash-Gel system (Lonza, Rockland, ME, USA). cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantification of mRNA expression was done in a two-step Reverse Transcriptase-Real Time PCR (RT-qPCR) using mouse-specific TaqMan Gene Expression Assays (Applied Biosystems) in 7500 Fast Real-Time PCR system using Standard real-time 7500 protocol. Data were analyzed using the 7500 system software (Applied Biosystems), and relative quantification was done using ΔΔCt method with 18S as the internal control and the C57+LFD group as the reference control.
Immunohistochemical analyses
Hematoxylin and eosin staining was performed by the Histology Core at BIDMC. To assess the number of adipocytes, at least 10 representative images were taken at × 20 magnification. Macrophages were identified in the adipose tissue using an antibody to F4/80 (AbD Serotec, Raleigh, NC, USA) and visualized using a horseradish peroxidase–conjugated secondary antibody with color development using a diaminobenzidine substrate. F4/80-positive cells were counted in at least 10 visual fields at × 20 magnification to evaluate macrophage infiltration. Images were evaluated using the SigmaScan Pro 5 software (Systat Software, Inc., Point Richmond, CA, USA).
Statistical analyses
Treatment groups were analyzed using general linear models; differences among the treatment groups were assessed by one-way analysis of variance followed by the protected least significant-differences technique (SAS version 8.2; SAS Institute, Cary, NC, USA). Changes in body weight, glucose, hormones and inflammation markers were assessed by repeated-measures analysis of variance and post-hoc multiple comparison procedures. Statistics were performed on logtransformed data for results showing unequal variances among groups. All data are presented as means ± s.e. P < 0.05 was considered statistically significant.
RESULTS
Effect of lymphocyte deficiency on body weight and body composition
There were no differences in body weight among the four groups at baseline (P = 0.79; Figure 1b). As early as 1 week after HFD, mice began to weigh more than LFD mice. Although there was no difference between LFD-fed wild-type and Rag1 − / − mice throughout the study period, Rag1 − / − mice fed HFD started to gain more weight than C57 mice fed HFD at week 1 that became significantly different from week 3. The difference persisted until the end of the experiment at week 11, with Rag1 − / − HFD mice being 10% heavier than C57 HFD mice. In association with the greater body weight gain, Rag1 − / − HFD mice had significantly more body fat mass (g and %) and less lean mass (%) compared with C57 HFD mice (Table 1a). Also, both LFD- and HFD-fed Rag1 − / − mice had lower spleen mass compared with the C57. Importantly, HFD significantly increased the mass of the entire fat depot observed (epididymal, perirenal, mesenteric, subcutaneous and brown fat) but only epididymal, mesenteric and subcutaneous fat mass was further increased by lymphocyte deficiency, not perirenal or brown fat mass.
Table 1.
| a. Body and tissue weight after 11 weeks of diet intervention | |||||||
|---|---|---|---|---|---|---|---|
| Variable | C57 | Rag | P-value | ||||
| LF (n = 6) Mean ± s.e. |
HF (n = 6) Mean ± s.e. |
LF (n = 6) Mean ± s.e. |
HF (n = 6) Mean ± s.e. |
Diet | Strain | Diet × Strain | |
| Body weight (g) | 26.3 ±0.4 c | 31.2 ±0.9 b | 27.2 ±0.3 c | 34.7 ±1.1 a | < 0.0001 | 0.01 | 0.10 |
| Fat mass (g) | 1.71 ±0.15 c | 6.68 ±0.59 b | 1.58 ±0.28 c | 9.01 ±0.87 a | 0.0003 | 0.30 | 0.17 |
| (%) | 6.60 ±0.59 c | 21.54 ±1.22 b | 5.76 ±0.88 c | 25.77 ±1.63 a | < 0.0001 | 0.20 | 0.22 |
| Lean mass (g) | 17.64 ±0.39 c | 18.40 ±0.24 b | 18.76 ±0.20 b | 19.58 ±0.24 a | 0.13 | 0.03 | 0.22 |
| (%) | 67.69 ±1.16 a | 59.99 ±1.01 b | 69.24 ±0.75 a | 56.71 ±1.19 c | 0.02 | 0.17 | 0.18 |
| Heart (g) | 0.12 ±0.00 a | 0.12 ±0.00 ab | 0.12 ±0.00 a | 0.13 ±0.00 a | 0.78 | 0.36 | 0.53 |
| (%) | 0.45 ±0.02 a | 0.38 ±0.02 b | 0.45 ±0.01 a | 0.38 ±0.02 b | 0.47 | 0.47 | 0.52 |
| Spleen (g) | 0.06 ±0.00 b | 0.08 ±0.00 a | 0.04 ±0.01 c | 0.03 ±0.00 d | 0.80 | < 0.0001 | 0.01 |
| (%) | 0.23 ±0.01 a | 0.24 ±0.01 a | 0.10 ±0.04 c | 0.08 ±0.67 b | 0.74 | < 0.0001 | 0.04 |
| Pancreas (g) | 0.27 ±0.01 ab | 0.17 ±0.03 c | 0.32 ±0.01 a | 0.24 ±0.01 b | 0.06 | 0.01 | 0.42 |
| (%) | 1.04 ±0.02 a | 0.56 ±0.10 b | 1.16 ±0.02 a | 0.69 ±0.02 b | 0.01 | 0.01 | 0.50 |
| Liver (g) | 1.19 ±0.06 bc | 1.34 ±0.05 ab | 1.07 ±0.07 c | 1.45 ±0.10 a | 0.001 | 0.93 | 0.14 |
| (%) | 4.50 ±0.22 a | 4.32 ±0.19 a | 3.91 ±0.21 a | 4.16 ±0.25 a | 0.88 | 0.10 | 0.34 |
| Kidney (%) | 1.34 ±0.03 a | 1.19 ±0.02 bc | 1.25 ±0.04 ab | 1.13 ±0.05 c | 0.33 | 0.21 | 0.48 |
| (g) | 0.35 ±0.01 b | 0.37 ±0.01 ab | 0.34 ±0.01 b | 0.39 ±0.01 a | 0.53 | 0.30 | 0.64 |
| Intestine | |||||||
| Duodenum (g) | 0.13 ±0.01 a | 0.13 ±0.01 a | 0.18 ±0.03 a | 0.15 ±0.02 a | 0.37 | 0.48 | 0.73 |
| (%) | 0.49 ±0.05 a | 0.41 ±0.02 a | 0.64 ±0.11 a | 0.43 ±0.04 a | 0.40 | 0.46 | 0.74 |
| Jejunum (g) | 0.69 ±0.02 ab | 0.53 ±0.06 c | 0.75 ±0.04 a | 0.63 ±0.05 b | < 0.0001 | 0.73 | 0.35 |
| (%) | 2.61 ±0.08 a | 1.67 ±0.16 b | 2.74 ±0.14 a | 1.81 ±0.11 b | < 0.0001 | 0.83 | 0.46 |
| Ileum (g) | 0.12 ±0.02 a | 0.09 ±0.01 a | 0.11 ±0.01 a | 0.11 ±0.02 a | 0.05 | 0.63 | 0.54 |
| (%) | 0.44 ±0.06 b | 0.28 ±0.04 b | 0.39 ±0.04 ab | 0.33 ±0.04 ab | 0.05 | 0.58 | 0.54 |
| Colon (g) | 0.14 ±0.01 b | 0.10 ±0.01 c | 0.18 ±0.01 a | 0.12 ±0.01 bc | 0.02 | 0.10 | 0.35 |
| (%) | 0.52 ±0.05 b | 0.31 ±0.03 c | 0.65 ±0.04 a | 0.35 ±0.04 c | 0.01 | 0.09 | 0.31 |
| Adipose tissue | |||||||
| Epididymal (g) | 0.50 ±0.04 c | 1.32 ±0.13 b | 0.48 ±0.03 c | 1.77 ±0.16 a | 0.01 | 0.32 | 0.18 |
| (%) | 1.92 ±0.15 c | 4.18 ±0.28 b | 1.76 ±0.09 c | 5.04 ±0.32 a | 0.0002 | 0.37 | 0.21 |
| Perirenal (g) | 0.15 ±0.01 b | 0.39 ±0.03 ab | 0.21 ±0.10 b | 0.47 ±0.04 a | 0.68 | 0.94 | 0.55 |
| (%) | 0.56 ±0.05 c | 1.23 ±0.06 ab | 0.75 ±0.35 bc | 1.33 ±0.08 a | 0.56 | 0.99 | 0.53 |
| Mesenteric (g) | 0.20 ±0.02 c | 0.37 ±0.03 b | 0.17 ±0.01 c | 0.51 ±0.05 a | 0.10 | 0.52 | 0.05 |
| (%) | 0.76 ±0.07 c | 1.19 ±0.07 b | 0.62 ±0.03 c | 1.45 ±0.09 a | 0.01 | 0.51 | 0.04 |
| Subcutaneous (g) | 0.31 ±0.01 c | 0.67 ±0.06 b | 0.29 ±0.02 c | 0.89 ±0.09 a | 0.19 | 0.35 | 0.28 |
| (%) | 1.19 ±0.05 c | 2.12 ±0.14 b | 1.05 ±0.07 c | 2.54 ±0.20 a | 0.03 | 0.34 | 0.27 |
| Brown (g) | 0.08 ±0.00 b | 0.16 ±0.02 a | 0.06 ±0.01 b | 0.17 ±0.02 a | 0.58 | 0.05 | 0.93 |
| (%) | 0.31 ±0.01 b | 0.51 ±0.05 a | 0.23 ±0.02 bc | 0.49 ±0.07 a | 0.42 | 0.04 | 0.94 |
| b. Circulating cytokine levels after 11 weeks of diet intervention | |||||||
|---|---|---|---|---|---|---|---|
| Variable | C57+LF (n = 6) Mean ± s.e. |
C57+HF (n = 6) Mean ± s.e. |
Rag+LF (n = 6) Mean ±s.e. |
Rag+HF (n = 6) Mean ±s.e. |
P-value* | ||
| Diet | Genotype | Diet × Genotype | |||||
| TNF-α | 0.83 ±0.13 b | 1.06 ±0.12 a | 1.13 ±0.09 a | 1.16 ±0.09 a | 0.09 | 0.07 | 0.04 |
| IFN-γ | 1.13 ±0.24 b | 2.01 ±0.62 a | 0.49 ±0.88 c | 0.80 ±0.17 bc | 0.12 | 0.01 | 0.85 |
| IL-1b | 2.57 ±0.32 a | 2.05 ±0.38 ab | 1.76 ±0.16 ab | 1.68 ±0.23 b | 0.32 | 0.06 | 0.48 |
| IL-2 | 4.19 ±0.48 a | 4.26 ±0.85 a | 4.55 ±0.74 a | 2.37 ±0.39 b | 0.14 | 0.27 | 0.03 |
| IL-4 | 1.19 ±0.34 a | 1.10 ±0.45 ab | 0.32 ±0.12 b | 0.34 ±0.09 b | 0.93 | 0.05 | 0.85 |
| IL-5 | 2.35 ±0.35 b | 3.33 ±0.82 b | 3.55 ±0.31 b | 5.71 ±0.67 a | 0.006 | 0.0003 | 0.34 |
| IL-6 | 5.92 ±2.08 a | 6.66 ±2.29 a | 7.88 ±2.79 a | 5.80 ±3.97 a | 0.28 | 0.28 | 0.72 |
| IL-10 | 35.8 ±8.9 ab | 38.2 ±7.1 a | 23.4 ±2.0 b | 24.6 ±3.0 ab | 0.70 | 0.30 | 0.73 |
| IL-12 total | 1178 ±54 ab | 1068 ±89 b | 1285 ±49 ab | 1373 ±96 a | 0.68 | 0.04 | 0.40 |
| IL-17 | 1.23 ±0.71 a | 2.90 ±1.32 a | 1.36 ±0.21 a | 1.03 ±0.30 a | 0.41 | 0.75 | 0.16 |
| KC/GAO | 197.2 ±31.1 a | 254.9 ±20.4 a | 192.1 ±36.0 a | 226.8 ±44.6 a | 0.05 | 0.56 | 0.48 |
| MCP1 | 32.9 ±5.7 b | 25.7 ±4.3 ba | 48.5 ±2.9 a | 40.7 ±6.8 b | 0.17 | 0.01 | 0.95 |
| MIP1α | 32.8 ±5.2 b | 38.4 ±4.9 ab | 45.8 ±1.2 a | 38.6 ±3.4 ab | 0.84 | 0.11 | 0.12 |
| VEGF | 1.25 ±0.26 ab | 1.05 ±0.16 b | 1.38 ±0.11 ab | 1.82 ±0.22 b | 0.55 | 0.03 | 0.11 |
| c. Tissue macrophage and cytokine mRNA expression levels | |||||||
|---|---|---|---|---|---|---|---|
| Variable | C57+LF (n = 6) Mean ± s.e |
C57+HF (n = 6) Mean ± s.e |
Rag+LF (n = 6) Mean ± s.e |
Rag+HF (n = 6) Mean ± s.e |
P-value* | ||
| Diet | Genotype | Diet × Genotype | |||||
| Macrophage | |||||||
| Epididymal | 1.14 ±0.30 a | 1.02 ±0.18 a | 1.00 ±0.13 a | 0.69 ±0.08 a | 0.87 | 0.64 | 0.64 |
| Mesenteric | 0.77 ±0.09 a | 0.81 ±0.35 a | 1.00 ±0.81 a | 0.81 ±0.15 a | 0.77 | 0.93 | 0.59 |
| Perirenal | 1.10 ±0.10 ab | 1.28 ±0.07 ab | 1.00 ±0.04 b | 1.33 ±0.14 a | 0.09 | 0.51 | 0.42 |
| Subcutaneous | 1.33 ±0.32 a | 2.05 ±0.74 a | 1.00 ±0.15 a | 2.34 ±0.67 a | 0.03 | 0.27 | 0.35 |
| Brown | 1.36 ±0.07 a | 1.41 ±0.03 a | 1.00 ±0.10 a | 1.35 ±0.08 a | 0.49 | 0.16 | 0.92 |
| TNF-α | |||||||
| Liver | 1.30 ±0.09 ab | 1.59 ±0.13 a | 1.00 ±0.10 b | 1.43 ±0.11 a | 0.04 | 0.23 | 0.44 |
| Epididymal | 0.58 ±0.09 a | 0.91 ±0.22 a | 1.00 ±0.29 a | 0.46 ±0.12 a | 0.19 | 0.28 | 0.02 |
| Mesenteric | 2.15 ±0.66 a | 2.25 ±0.84 a | 1.00 ±0.37 ab | 0.59 ±0.20 b | 0.48 | 0.01 | 0.28 |
| Perirenal | 0.89 ±0.09 b | 2.23 ±0.37 a | 1.00 ±0.23 b | 1.75 ±0.32 a | 0.0007 | 0.70 | 0.37 |
| Subcutaneous | 5.44 ±1.34 ab | 3.60 ±1.83 a | 1.00 ±0.40 b | 2.73 ±1.04 ab | 0.41 | 0.36 | 0.06 |
| Brown | 1.35 ±0.28 a | 1.34 ±0.24 a | 1.00 ±0.23 a | 1.46 ±0.31 a | 0.97 | 0.18 | 0.63 |
| IFN-γ | |||||||
| Epididymal | 1.22 ±0.26 a | 0.80 ±0.11 a | 1.00 ±0.28 a | 0.83 ±0.25 a | 0.53 | 0.28 | 0.54 |
| Mesenteric | 1.23 ±0.23 a | 1.23 ±0.22 a | 1.00 ±0.45 a | 1.00 ±0.20 a | 0.996 | 0.77 | 0.92 |
| Perirenal | 1.11 ±0.11 ab | 1.38 ±0.10 a | 1.00 ±0.12 b | 1.27 ±0.13 ab | 0.02 | 0.33 | 0.92 |
| Subcutaneous | 2.07 ±0.87 a | 1.80 ±0.99 a | 1.00 ±0.41 a | 1.26 ±0.43 a | 0.24 | 0.87 | 0.21 |
| Brown | 1.91 ±0.57 a | 1.51 ±0.52 a | 1.00 ±0.17 a | 1.18 ±0.22 a | 0.50 | 0.97 | 0.30 |
| IL-1α | |||||||
| Epididymal | 0.51 ±0.20 a | 1.04 ±0.27 a | 1.00 ±0.33 a | 0.41 ±0.19 a | 0.38 | 0.40 | 0.03 |
| Mesenteric | 0.90 ±0.24 a | 1.08 ±0.30 a | 1.00 ±0.50 a | 0.61 ±0.27 a | 0.29 | 0.07 | 0.15 |
| Perirenal | 4.94 ±2.83 ab | 11.37 ±4.65 a | 1.00 ±0.29 b | 4.49 ±1.77 ab | 0.04 | 0.24 | 0.90 |
| Subcutaneous | 3.48 ±0.82 a | 1.19 ±0.71 b | 1.00 ±0.51 ab | 1.48 ±1.12 ab | 0.94 | 0.84 | 0.08 |
| Brown | 0.80 ±0.20 a | 0.44 ±0.08 a | 1.00 ±0.34 a | 0.57 ±0.15 a | 0.05 | 0.62 | 0.62 |
| IL-1β | |||||||
| Epididymal | 0.80 ±0.22 a | 0.88 ±0.10 a | 1.00 ±0.22 a | 0.81 ±0.34 a | 0.17 | 0.38 | 0.09 |
| Mesenteric | 0.45 ±0.09 a | 0.76 ±0.22 a | 1.00 ±0.45 a | 0.58 ±0.16 a | 0.52 | 0.59 | 0.32 |
| Perirenal | 1.54 ±0.23 ab | 2.55 ±0.30 a | 1.00 ±0.30 b | 1.12 ±0.18 ab | 0.05 | 0.004 | 0.13 |
| Subcutaneous | 1.24 ±0.20 a | 1.32 ±0.54 a | 1.00 ±0.40 a | 2.41 ±1.05 a | 0.25 | 0.67 | 0.15 |
| Brown | 1.54 ±0.11 ab | 1.66 ±0.14 a | 1.00 ±0.18 b | 1.48 ±0.25 ab | 0.65 | 0.04 | 0.57 |
| IL-4 | |||||||
| Liver | 1.95 ±0.20 a | 2.58 ±0.41 a | 1.00 ±0.14 b | 1.76 ±0.36 a | 0.003 | 0.06 | 0.44 |
| Muscle | 0.89 ±0.08 b | 1.70 ±0.10 a | 1.00 ±0.13 b | 1.75 ±0.21 a | 0.01 | 0.47 | 0.48 |
| Epididymal | 2.02 ±0.69 a | 2.80 ±1.17 a | 1.00 ±0.15 b | 1.33 ±0.52 ab | 0.42 | 0.04 | 0.30 |
| Mesenteric | 10.01 ±3.47 a | 10.89 ±3.55 a | 1.00 ±0.44 b | 0.74 ±0.15 b | 0.70 | < 0.0001 | 0.55 |
| Perirenal | 3.70 ±0.71 ab | 10.45 ±4.74 a | 1.00 ±0.47 b | 1.51 ±1.40 b | 0.20 | 0.05 | 0.27 |
| Subcutaneous | 4.57 ±1.67 a | 3.59 ±1.99 a | 1.00 ±0.55 ab | 0.58 ±0.29 b | 0.64 | 0.05 | 0.49 |
| Brown | 2.25 ±1.03 a | 2.37 ±0.89 a | 1.00 ±0.63 ab | 0.00 ±0.00 b | 0.56 | 0.03 | 0.47 |
| IL-17a | |||||||
| Liver | Undetectable in all the groups | ||||||
| Muscle | Undetectable in all the groups | ||||||
| Epididymal | 0.17 ±0.08 a | 0.15 ±0.08 a | 1.00 ±0.91 a | 0.00 ±0.00 a | 0.68 | 0.15 | 0.90 |
| Mesenteric | 6.06 ±0.63 a | 9.59 ±4.68 a | 1.00 ±0.26 b | 0.42 ±0.20 b | 0.32 | < 0.0001 | 0.68 |
| Perirenal | Undetectable in all the groups | ||||||
| Subcutaneous | Undetectable in all the groups | ||||||
| Brown | Undetectable in all the groups | ||||||
Abbreviations: HF, high fat; LF, low fat. Means with different letters are significantly different (P < 0.05). Values in bold are significant P-values.
Abbreviations: HF, high fat; IFN-γ, interferon gamma; IL, interleukin; KC/GAO, keratinocyte chemoattractant or growth-regulated oncogene-a; LF, low fat; MCP1, monocyte chemotactic protein-1; MIP1α, macrophage inflammatory protein-1 alpha; TNF-α, tumor necosis factor alpha; VEGF, vascular endothelial growth factor.
n=3–6 for each group. Means with different letters are significantly different (P < 0.05). Values in bold are significant P-values.
Abbreviations: HF, high fat; IFN-γ, interferon gamma; IL, interleukin; LF, low fat; TNF-α, tumor necrosis factor alpha.
Adjusted for body weight. Means with different letters are significantly different (P < 0.05). Values in bold are significant P-values.
Effect of lymphocyte deficiency on energy intake, appetite and energy expenditure
In contrast to the significantly increased body weight, both C57 and Rag1 − / − mice fed HFD had significantly decreased overall energy intake compared with mice fed LFD (P = 0.0001, Figure 1c). As hypothalamus is the key organ involved in appetite regulation, we further explored hypothalamic neuropeptide gene expression. We found that mice fed on HFD had significantly decreased orexigenic agouti-related protein (AgRP; Supplementary Figure S1A) and increased anorexigenic pro-opiomelanocortin (POMC; Supplementary Figure S1B) gene expression levels, but there was no difference between C57 and Rag1 − / − mice fed HFD. In the LFD-fed mice, Rag1 − / − mice had significantly higher expression of AgRP, Neuropeptide Y (NPY; Supplementary Figure S1C) and melanocortin receptor 4 (Supplementary Figure S1D) compared with C57 mice.
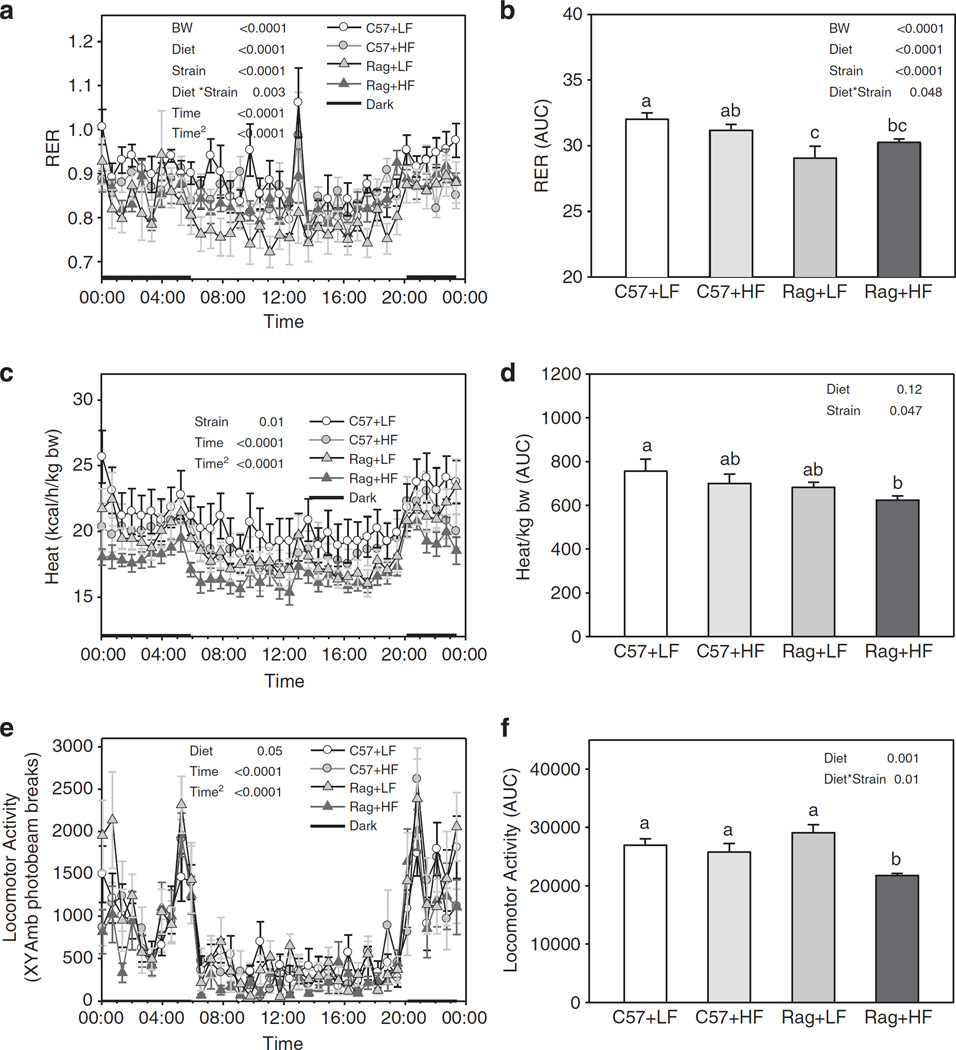
Regarding energy expenditure, CLAMS monitoring data showed that Rag1 − / − mice fed either LFD or HFD had significantly lower RER after adjusting for total body weight (P < 0.0001, Figures 2a and b) and produced less heat per kg body weight compared with C57 mice (P = 0.013, Figures 2c and d). Similar results were found after adjusting the RER data for lean body mass obtained 1 week after CLAMS data were collected (P = 0.002). In addition, Rag1 − / − mice fed HFD had significantly lower activity levels compared with all the other groups (Diet × Strain P = 0.0048, Figures 2e and f).
Figure 2.
Twenty-four hour RER (a), heat production (c), locomotor activity (e) and the respective area under the curve (b, d and f) in C57 and Rag1 − / − maintained on LFD or HFD. The mice were monitored by CLAMS. Values are means ± s.e.; n = 6 mice/group. Means with different letters are significantly different (P < 0.05).
To elucidate mechanisms underlying the decrease in heat generation and energy expenditure in Rag1 − / − mice, we measured uncoupling protein (UCP) mRNA expression levels in muscle and brown adipose tissue. We have confirmed that UCP1 mRNA expression is dominant in brown fat while UCP3 mRNA expression is dominant in muscle (Supplementary Figure S2A). Mean UCP2 mRNA expression levels were > 10 times lower compared with UCP1 and UCP3 gene expression levels in both brown adipose tissue and muscle. There were no differences in muscle UCP3 expression among groups (Supplementary Figure S2B), but Rag1 − / − mice fed HFD had significantly decreased UCP1 and UCP3 mRNA expression in brown fat compared with Rag1 − / − mice fed LFD, partly explaining the decreased energy expenditure and increased body fat in Rag1 − / − HFD mice (Supplementary Figures S2C and D).
Effect of lymphocyte deficiency on metabolic profile
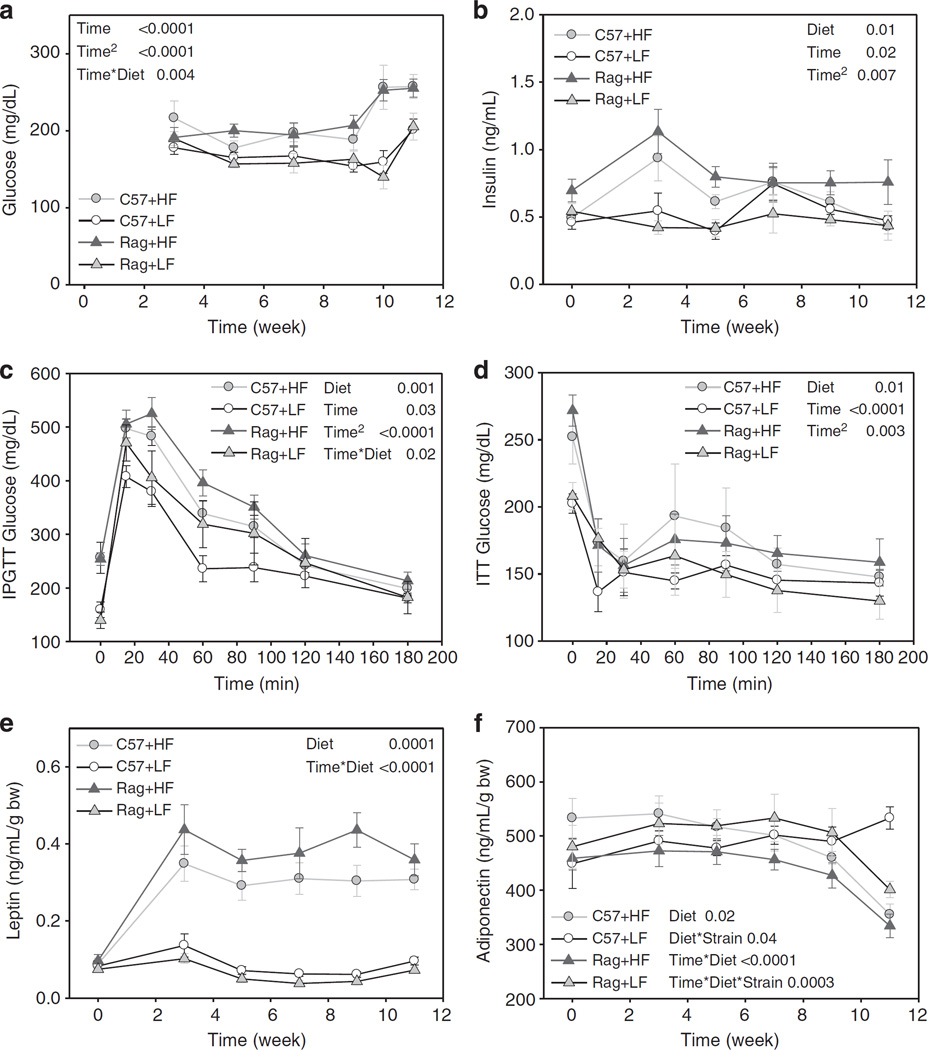
In HFD-fed mice, blood glucose and insulin levels were significantly increased compared with LFD-fed mice, but there was no difference between C57 and Rag1 − / − mice (Figures 3a and b). Ten weeks after baseline, C57 and Rag1 − / − mice fed HFD showed impaired glucose tolerance (Figure 3c) and insulin resistance (Figure 3d) compared with LFD-fed counterparts. No further differences of glucose and insulin sensitivity were found between HFD-fed C57 and Rag1 − / − mice.
Figure 3.
Plasma concentration of glucose (a), insulin (b), intraperitoneal glucose tolerance tests (IPGTTs) (c), insulin tolerance tests (ITT) (d), plasma concentration of leptin (e) and adiponectin (f) in C57 and Rag1 − / − mice maintained on LFD or HFD. Data were analyzed using repeated-measures analysis of variance and post-hoc multiple comparison procedures. Values are means± s.e.; n = 6 mice/group.
Adipokines are markers of adipocyte physiology, and therefore, leptin and adiponectin levels were measured during the study period. Mice fed HFD had significantly higher plasma leptin levels as early as week 3, consistent with their weight gain (Figure 3e). Also, HFD-fed Rag1 − / − mice had marginally higher leptin levels compared with HFD-fed C57 mice. Mice fed LFD maintained unchanged leptin levels for the whole experiment period. Meanwhile, plasma adiponectin levels were unaltered in C57 mice fed LFD, while the other groups had significantly lower adiponectin levels at the end of the experiment (Figure 3f).
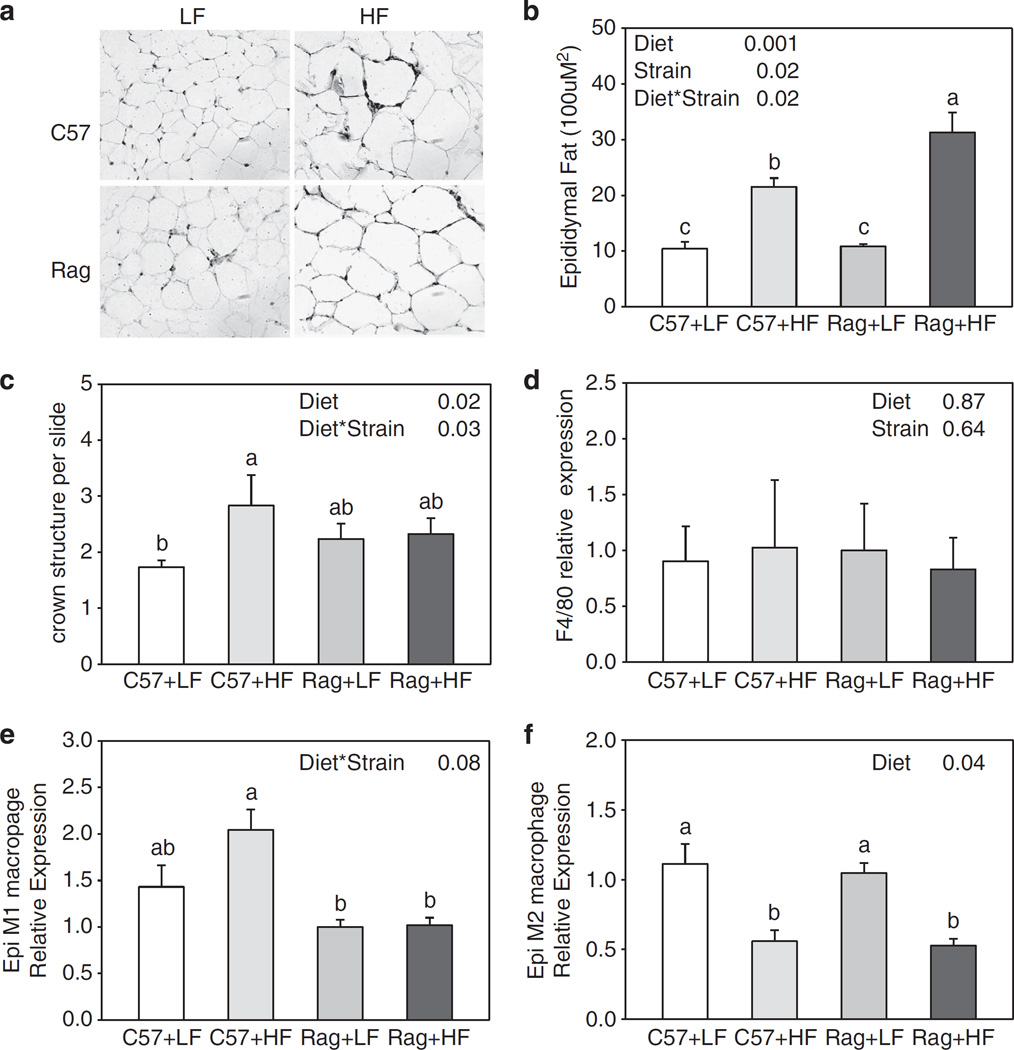
Effect of lymphocyte deficiency on local inflammation in epididymal and subcutaneous adipose tissues
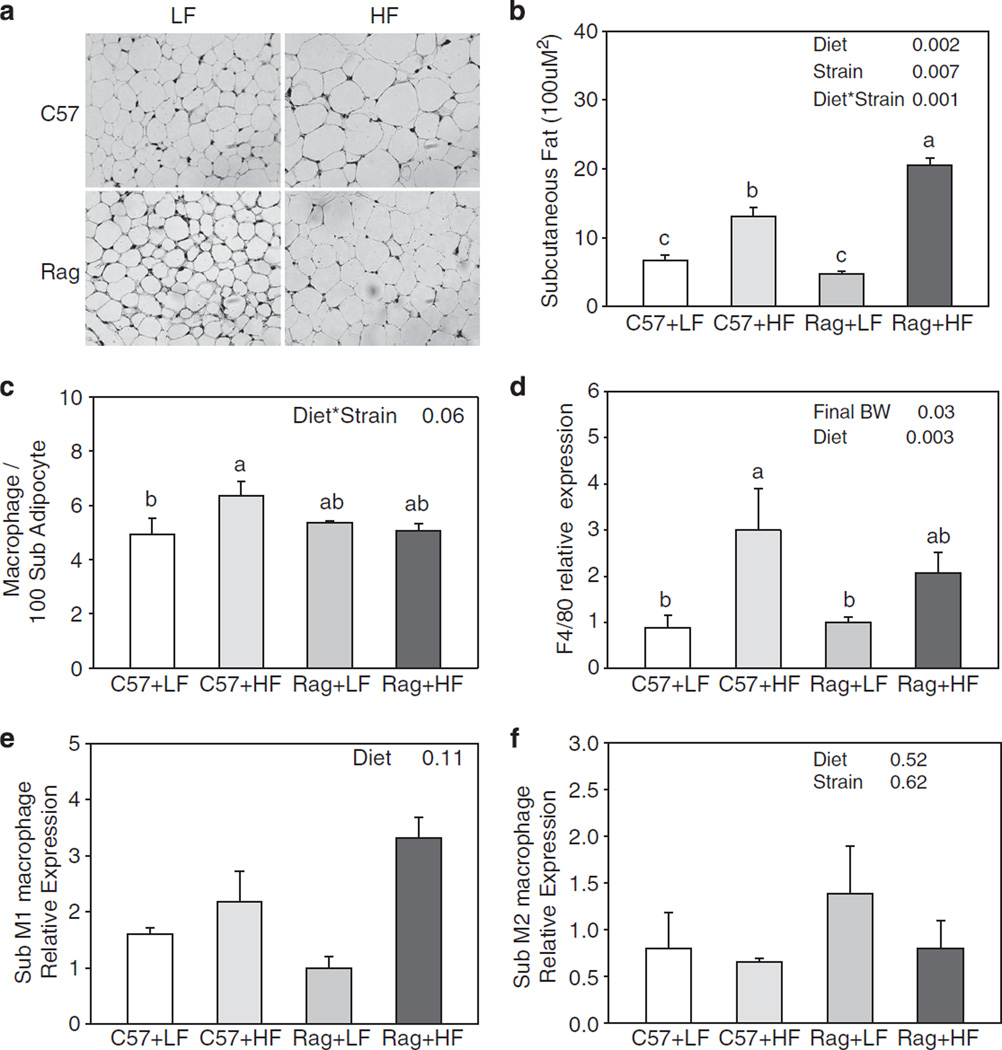
Increased fat mass in Rag1 − / − mice had led us to examine the local inflammation in adipose tissue. Morphological analysis showed that, in both epididymal and subcutaneous adipose tissue, increase in fat mass was due to adipocyte hypertrophy (Figures 4a and 5a). Adipose tissue macrophage infiltration visualized by F4/80 staining (a mouse macrophage marker) and assessment of crown-like structure in epididymal and subcutaneous adipose tissue showed that, although there was a significant elevation in macrophage infiltration in C57 mice fed HFD compared with C57 mice fed LFD, there was no difference between C57 and Rag1 − / − mice (Figures 4c and d, 5c and d). However, when the subtype of macrophages that had infiltrated each adipose tissue store was measured, both C57 and Rag1 − / − mice fed HFD had significantly decreased M2 macrophage expression compared with LFD mice in epididymal fat (Figure 4f). There was a trend in decreased M2 macrophage expression in Rag1 − / − fed HFD compared with Rag1 − / − LFD in subcutaneous fat (Figure 5f).
Figure 4.
Representative staining of F4/80-positive macrophage (a), cell area (b), the number of crown-like structure per slides (c), F4/80 (d), M1 macrophage marker (e) and M2 macrophage marker (f) gene expression of epididymal adipose tissue in C57 and Rag1 − / − mice maintained on LFD or HFD. Gene expressions were measured by real-time PCR. Relative gene expression data were analyzed by ΔΔCt method with 18S as the internal control and the C57+LF group as the reference control. Values are means ±s.e.; n = 3–6 mice/group. Means with different letters are significantly different (P < 0.05).
Figure 5.
Representative staining of F4/80-positive macrophage (a), cell area (b), the number of crown-like structure per slides (c), F4/80 (d), M1 macrophage marker (e) and M2 macrophage marker (f) gene expression of subcutaneous adipose tissue in C57 and Rag1 − / − mice maintained on LFD or HFD. Gene expressions were measured by real-time PCR. Relative gene expression data were analyzed by ΔΔCt method with 18S as the internal control and the C57+LF group as the reference control. Values are means ± s.e.; n = 3–6 mice/group. Means with different letters are significantly different (P < 0.05).
Effect of lymphocyte deficiency on systemic inflammation
To assess whether the local inflammation observed in HFD-fed mice is also reflected in terms of systemic inflammation, we measured plasma cytokine levels (Table 1b). In C57 HFD-fed mice, TNF-α and IFN-γ levels were increased, whereas MCP1 level was decreased compared with LFD-fed mice. There was no significance in any other cytokines observed in C57 mice. In contrast, deficiency in lymphocytes resulted in marked reduction in antiinflammatory cytokine IL-4 level in both LFD and HFD mice. IL-10 also had a trend towards decrease in Rag1 − / − mice although it was not statistically significant. Moreover, IL-5 and IL-12 (total) levels were significantly elevated in HFD-fed Rag1 − / − mice compared with HFD-fed C57 mice. MCP1 and MIP1α levels were also increased in Rag1 − / − mice compared with C57 mice although it was only significant in the LFD-fed group. IFN-γ, which was elevated by HFD in C57 mice, was significantly decreased by lymphocyte deficiency. These results suggest that lymphocyte deficiency results in upregulation of inflammatory cytokines even at baseline, which may have a role in development of obesity only when fed HFD.
We also measured mRNA expression of several cytokines (IL-1α, IL-1β, IL-4, IL-17a, TNF-α and IFN-γ) in five areas of adipose tissue, including epididymal, mesenteric, perirenal, subcutaneous and brown adipose tissue (Table 1c). When compared with C57 mice, Rag1 − / − mice showed significantly decreased IL-4 mRNA expression levels in all the five areas of adipose tissue (all P < 0.05), decreased IL-1β in perirenal and brown adipose tissue (P = 0.004 and P = 0.04, respectively) and decreased TNF-α (P = 0.01) and IL-17a (P < 0.0001) in mesenteric adipose tissue. Mice fed HFD showed significant increase of TNF-α (P = 0.0007), IFN-γ (P = 0.02), IL-1α (P = 0.04) and IL-1β (P = 0.05) in perirenal adipose tissue. Diet and Genotype interaction were only found for TNF-α (P = 0.02) and IL-1α (P = 0.03) in epididymal adipose tissue. TNF-α and IL-1α mRNA expression level was increased in HF-fed C57 mice while decreased in HF-fed Rag1 − / − mice compared with LF-fed counterparts. TNF-α, IFN-γ, IL-1α and IL-1β mRNA levels were significantly correlated to macrophage F4/80 mRNA levels in all the five areas of adipose tissue (all P < 0.05). We also found that IL-17a mRNA expression cannot be detected in perirenal, subcutaneous and brown adipose tissue.
DISCUSSION
We demonstrate herein that lack of adaptive immune system, as seen in Rag1 − / − mice, have adverse effect on body weight, body composition and several inflammatory cytokines but do not necessarily lead to development of metabolic diseases, such as hyperglycemia and diabetes in the short term.
The most significant change induced by lack of Rag1 in HFD-fed mice is the increase in body weight. Body composition analysis showed that the increased body weight was due to significant increase in fat mass, especially epididymal, mesenteric and subcutaneous fat. Morphological analysis of epididymal and subcutaneous fat confirmed that the enlarged fat mass was due to adipocyte hypertrophy in Rag1 − / − mice fed HFD. Animal monitoring data showed that the excess weight gain was at least in part derived from decreased energy expenditure, including reduced heat production and locomotor activity. Downregulation of brown fat UCP-1 and UCP-3 gene expression levels in Rag1 − / − HF-fed mice compared with LF-fed mice partly explains the decreased whole body thermogenesis in these animals. It is uncertain at this point how lack of adaptive immune system can lead to alteration in thermogenic genes. It is possible that there might be a direct effect, yet to be identified, or an indirect effect through lack of restrained fat accumulation in adipose tissue. Hypertrophic fat leads to hyperleptinemia and indeed Rag1 − / − mice fed HFD exert higher leptin levels compared with C57 mice, suggesting that Rag1 − / − mice are partly resistant to leptin in terms of sympathetic system,18 and thus may be gaining more weight.
Interestingly, total energy intake was lower in C57 and Rag1 −/ − mice fed HFD compared with LFD-fed mice. To explore whether the decreased food intake was related to altered appetite control in the hypothalamus, we measured the gene expression of orexigenic neuropeptides NPY and AgRP, as well as the expression of anorexigenic neuropeptide POMC.19 Consistent with decreased energy intake, HF-fed mice had significantly decreased AgRP and increased POMC gene expression levels in the hypothalamus. We hypothesize that, in context to the sympathetic nervous system, higher leptin levels in HFD mice are successfully downregulating NPY and AgRP in the hypothalamus. These findings show that there might be a dichotomy in leptin sensitivity between peripheral tissues and the central nervous system in this state of deficiency of the adaptive immune system.
Previous studies have shown similar results with ours in that Rag1 − / − and Rag2 − / − mice become more obese than wild-type mice.20,21 These immune-deficient mice had increased levels of macrophage and natural killer cells. We extended the previous findings by studying their metabolism in detail and examining a wide range of inflammatory markers in tissues as well as in circulation. In our study, pro-inflammatory M1 macrophages mRNA levels were increased in the epididymal fat of HF-fed C57 mice but not in Rag − / − mice. Anti-inflammatory M2 macrophages mRNA levels were significantly decreased in both C57 and Rag mice in epididymal fat when fed HFD. Tissue mRNA expression of cytokines showed that HFD diet induced subcutaneous mRNA expression of macrophages and perirenal mRNA expression of TNF-α and IL-1α, but there was no difference between C57 and Rag1 − / − mice. The circulating levels of IL-5 and IL-12 total were increased significantly in Rag1 − / − fed HFD. The established sources of IL-12 are stimulated macrophages, neutrophils, B-cells and dendritic cells.22 Serum levels of IL-12 showed a strong relationship with markers of low-grade inflammation and obesity in the Mexican adult population.23 IFN-γ levels were decreased, and MCP1 levels were increased in Rag1 − / − mice compared with C57. However, there were no major changes in other cytokines observed. These suggest that, although there is a change in inflammatory phenotype by HFD, the recruitment of macrophages to adipose tissue does not necessarily depend on the adaptive immune system and that the cells of the innate immune system can produce the majority of inflammatory cytokines even in the absence of the adaptive immune system. Of note, a recent study by Kim et al.24 has shown that Rag1 − / − mice are hypersensitive to TLR3 ligand and adoption of T cells to Rag1 − / − mice lowers TNF-α levels, suggesting that TNF-α is one of the essential negative regulators in adaptive immune response. In contrast, we could not observe any differences in either TNF-α mRNA expression in adipose tissue or circulating levels of TNF-α, which may explain why we did not detect any major differences in metabolism between C57 and Rag1 − / − .
Although only minor changes were observed for tissue mRNA expression of most cytokines, IL-4 mRNA levels were significantly decreased in all fat tissue deposits studied in Rag1 − / − mice as compared with C57 mice, regardless of the diet. Consistently, circulating IL-4 levels were also decreased in Rag1 − / − mice. IL-4 is a cytokine that induces differentiation of naive helper T cells to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop,25,26 leading to T-cell proliferation and stimulation and differentiation of B-cells. In addition, IL-4 and its main intracellular messenger signal transducer and activator of transcription 6 (STAT6) have been shown to have an important role in controlling peripheral nutrient metabolism and insulin sensitivity by inhibiting the peroxisome proliferator-activated receptor-α-regulated program of nutrient catabolism and attenuating adipose tissue lipid accumulation and inflammation.27,28 We have confirmed in vitro that IL-4 can activate STAT6 signaling in AML12 liver and 3T3L1 adipocyte cell line. In addition, we found that IL-4 can activate mitogen-activated protein kinase signaling in C2C12 muscle cell line and can significantly downregulate phosphorylation of glycogen synthase kinase, which mediates glycogen synthesis, in AML12 liver and 3T3L1 adipocyte (data not shown), which in conjunction with our animal study suggest that IL-4 could be a molecular link between the immune system and fat accumulation, with increased fat mass in immune-deficient Rag1 − / − mice fed a HFD. The reason for the lower IL-4 in the Rag1 − / − mice is likely be due to the lack of lymphocytes, in particular Th2 cells that produce IL-4. Previous study has confirmed that transplantation of Th2 cells reverse the metabolic dysfunction in Rag1 − / − mice and the beneficial effect of Th2 cells are maintained by STAT6 signal.20 Interestingly, a recent study has found that eosinophil-derived IL-4 is required for browning of white adipocytes and increased energy expenditure,29,30 which could provide further support to our findings on decreased energy expenditure in Rag1 − / − mice.
In terms of metabolic regulation, mice fed HFD had a higher degree of insulin resistance in both C57 and Rag1 − / − mice as expected. Prior studies have found that Rag1 − / − and Rag2 − / − mice are more insulin resistant than wild-type mice.20,21 Our study did not show significant adverse metabolic effect of Rag1 deficiency, however, despite a significant increased weight gain and decreased IL-4 levels. This finding may be related to the study of mice at an earlier time point in our study. We examined insulin and glucose tolerance after 11 weeks on a HFD, whereas the prior study examined the mice after 14 weeks. It is possible that a more prolonged period of observation would be needed for the development of insulin resistance through development of obesity and other changes seen in our Rag1 − / − mice.
A similar phenomenon has been observed in humans where 25% of obese subjects are metabolically healthy individuals and are protected from metabolic syndrome in the short term,31,32 but debate exists on what the long term implications would be.33,34
In summary, we demonstrated the role of adaptive immune system in central and peripheral systems regulating body weight, energy expenditure, systemic and tissue inflammation and metabolism. Although there was a connection between lack of adaptive immune system and a significant increase in adiposity in Rag1− / − mice fed HFD, there was no deleterious effect in terms of whole body metabolism. A decrease in energy expenditure and lack of IL-4 leading to excess fat storage may serve as underlying mechanisms driving weight gain in diet-induced obese Rag1− / − mice. Whether a longer observation period is needed for the development of metabolic complications, as seen in metabolically healthy obese subjects, remains to be studied in detail in the future.
Supplementary Material
Acknowledgments
This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award no. UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health-care centers), a discretionary grant from Beth Israel Deaconess Medical Center and award number 1I01CX000422-01A1 from the Clinical Science Research and Development Service of the VA Office of Research and Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health-care centers, the National Center for Research Resources or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
REFERENCES
- 1.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud A, Boulet MM, Veilleux A, Noel S, Paris G, Tchernof A. Abdominal subcutaneous and omental adipocyte morphology and its relation to gene expression, lipolysis and adipocytokine levels in women. Metabolism. 2014;63:372–381. doi: 10.1016/j.metabol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 4.van Beek L, Lips MA, Visser A, Pijl H, Ioan-Facsinay A, Toes R, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism. 2014;63:492–501. doi: 10.1016/j.metabol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 8.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–1552. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, et al. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 10.Krinninger P, Ensenauer R, Ehlers K, Rauh K, Stoll J, Krauss-Etschmann S, et al. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J Clin Endocrinol Metab. 2014;99:2500–2509. doi: 10.1210/jc.2013-2611. [DOI] [PubMed] [Google Scholar]
- 11.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 12.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Patel PS, Buras ED, Balasubramanyam A. The role of the immune system in obesity and insulin resistance. J Obes. 2013;2013:616193. doi: 10.1155/2013/616193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 16.Bluher S, Moschos S, Bullen J, Jr, Kokkotou E, Maratos-Flier E, Wiegand SJ, et al. Ciliary neurotrophic factorAx15 alters energy homeostasis, decreases body weight, and improves metabolic control in diet-induced obese and UCP1-DTA mice. Diabetes. 2004;53:2787–2796. doi: 10.2337/diabetes.53.11.2787. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Huang H, Choi K, Mantzoros C, Kim YB. Selective PPARgamma modulator INT131 normalizes insulin signaling defects and improves bone mass in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2012;302:E552–E560. doi: 10.1152/ajpendo.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51:2434–2440. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen AD, Herzog H, Sainsbury A. Neuropeptide Y and peptide YY: important regulators of energy metabolism. Curr Opin Endocrinol Diabetes Obes. 2011;18:56–60. doi: 10.1097/MED.0b013e3283422f0a. [DOI] [PubMed] [Google Scholar]
- 20.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, et al. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 23.Suarez-Alvarez K, Solis-Lozano L, Leon-Cabrera S, Gonzalez-Chavez A, Gomez-Hernandez G, Quinones-Alvarez MS, et al. Serum IL-12 is increased in Mexican obese subjects and associated with low-grade inflammation and obesity-related parameters. Mediators Inflamm. 2013;2013:967067. doi: 10.1155/2013/967067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris SC, Lees A, Finkelman FD. In vivo activation of naive T cells by antigen-presenting B cells. J Immunol. 1994;152:3777–3785. [PubMed] [Google Scholar]
- 26.Brown MA. IL-4 production by T cells: you need a little to get a lot. J Immunol. 2008;181:2941–2942. doi: 10.4049/jimmunol.181.5.2941. [DOI] [PubMed] [Google Scholar]
- 27.Chang YH, Ho KT, Lu SH, Huang CN, Shiau MY. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int J Obes (Lond) 2012;36:993–998. doi: 10.1038/ijo.2011.168. [DOI] [PubMed] [Google Scholar]
- 28.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Primeau V, Coderre L, Karelis AD, Brochu M, Lavoie ME, Messier V, et al. Characterizing the profile of obese patients who are metabolically healthy. Int J Obes (Lond) 2011;35:971–981. doi: 10.1038/ijo.2010.216. [DOI] [PubMed] [Google Scholar]
- 32.Calori G, Lattuada G, Piemonti L, Garancini MP, Ragogna F, Villa M, et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: the Cremona Study. Diabetes Care. 2011;34:210–215. doi: 10.2337/dc10-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care. 2013;36:2294–2300. doi: 10.2337/dc12-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta-analysis. Ann Intern Med. 2013;159:758–769. doi: 10.7326/0003-4819-159-11-201312030-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.