Abstract
Introduction
Bariatric surgery patients are vulnerable to sleep-disordered breathing (SDB) early after recovery from surgery and anesthesia. We hypothesized that continuous positive airway pressure (CPAP) improves postoperative oxygenation and SDB and mitigates opioid-induced respiratory depression.
Methods
In a randomized crossover trial, patients following bariatric surgery received 30% oxygen in the Post Anesthesia Care Unit (PACU) under two conditions: atmospheric pressure (AP) and CPAP (8–10 cmH2O). During one hour of each treatment, breathing across cortical arousal states was analyzed using polysomnography and spirometry. Arousal state and respiratory events were scored in accordance with American Academy of Sleep Medicine guidelines. Data on opioid boluses in the PACU were collected. The primary and secondary outcomes were the apnea hypopnea index (AHI) and apnea following self-administration of opioids in the PACU. Linear mixed model analysis was used to compare physiologic measures of breathing.
Results
64% of the 33 patients with complete postoperative polysomnography data, demonstrated SDB (AHI>5/h) early after recovery from anesthesia. CPAP treatment decreased AHI (8±2/h vs. 25±5/h, p<0.001), decreased oxygen desaturations (5±10/h vs. 16±20/h, p<0.001), and increased the mean oxygen saturation by 3% (p=0.003). CPAP significantly decreased the respiratory depressant effects observed during sleep-wake transitions without affecting hemodynamics. The interaction effects between CPAP treatment and opioid dose for the dependent variables AHI (p<0.001), inspiratory flow (p=0.002), and minute ventilation (p=0.015) were significant.
Conclusions
This pharmaco-physiological interaction trial shows that supervised CPAP treatment early after surgery improves SDB and ameliorates the respiratory depressant effects of opioids without undue hemodynamic effects.
Precis
This prospective, randomized, cross over trial compared the apnea hypopnea index (AHI) with and without continuous positive airway pressure (CPAP) in 38 morbidly-obese patients and found that the CPAP reduced the AHI by 69% and was more effective during NREM sleep than during wakefulness. The AHI was worsened by self-administered opioid for pain and the CPAP application effectively mitigated the AHI deterioration.
INTRODUCTION
Obstructive sleep apnea (OSA), the most common type of sleep-disordered breathing (SDB), occurs in more than 75% of patients undergoing bariatric surgery1. Data suggest that OSA increases the risk of postoperative respiratory complications, including reintubation2, as a consequence of an increased vulnerability to upper airway collapse in the perioperative period. Opioids are commonly administered both intra- and postoperatively for pain control3 and induce a dose-dependent decrease in respiratory drive and upper airway dilator muscle activity4. Practice guidelines suggest attempts should be made to minimize the use of opioids for postoperative pain treatment in OSA patients. However, studies have shown OSA patients to have a lower pain threshold5 and to require high doses of analgesics and opioids to accomplish adequate analgesia, compared to patients without OSA6.
The most effective treatment for OSA is continuous positive airway pressure (CPAP). CPAP significantly reduces the number of respiratory events during sleep and improves hemoglobin oxygen saturation (SpO2)7 by increasing upper airway diameter and preventing upper airway collapse. However, it is not entirely clear if CPAP is similarly effective during the early postoperative period with lingering effects of anesthetics, neuromuscular blockade, and analgesics8. While some studies found beneficial effects of early postoperative CPAP on arterial oxygen concentration partial pressure (PaO2) and early respiratory complications after extubation9,10, other studies indicate that postoperative CPAP does not always improve breathing11,12. No information is available on the effect of CPAP on sleep- and opioid-induced respiratory depression in patients with high risk of sleep apnea in the recovery room.
We aimed to investigate if CPAP treatment early after surgery in the post anesthesia care unit (PACU) improves apnea hypopnea index (AHI) and if the use of postoperative opioids increases AHI in the PACU. In addition, we hypothesized that CPAP treatment mitigates respiratory depressant effects of opioids given for postoperative pain therapy with a specific emphasis taken on respiratory depressant effects in transitions from wakefulness to sleep13.
METHODS
Study Design and Hypothesis
This study was approved by the institutional review board of Partners Healthcare, Boston, Massachusetts under the protocol number 2011P001333. After approval, we performed this prospective, blinded, randomized, crossover study to test if CPAP applied in the PACU following bariatric surgery improves SDB and ameliorates the respiratory depressant effects of postoperative opioids. We hypothesized that CPAP, when applied in the PACU following bariatric surgery, would decrease AHI (primary outcome measure) compared to the current standard of care. The secondary hypothesis was that CPAP decreases the respiratory depressant effects of opioids given via patient-controlled analgesia in the PACU. This trial was registered with ClinicalTrials.gov identifier: NCT01697878 (principal investigator: ME; date of registration: September 21, 2012).
Patient Selection
Forty-five patients aged 18 years or older scheduled for laparoscopic Roux-en-Y gastric bypass, laparoscopic partial vertical gastrectomy, laparoscopic sleeve, or revision of gastric band to gastric bypass at Massachusetts General Hospital, Boston, Massachusetts between March 2012 and July 2014 were approached for recruitment prior to their surgery and written informed consent was obtained by study staff. Patients with known impairment of cognitive function, decision-making capacity, and/or muscle weakness were excluded from this study.
Study Treatment
Patients were fitted with an oronasal CPAP mask during their pre-anesthesia interview. After surgery, patients were transferred to a private PACU room where two members of the study team were present. Following hand-off between the anesthesia provider and the PACU nurse, patients were connected to the study equipment and polysomnography (PSG) device within a median of 24 (9 to 83, interquartile range), minutes after PACU admission. During their PACU stay, patients received treatment with 30% supplemental oxygen (fraction of inspired oxygen (FiO2) of 0.3) applied under atmospheric pressure (AP) or CPAP. AP and CPAP were applied for one hour each in a randomized crossover design using a high-flow CPAP circuit with a custom made open circuit to prevent re-breathing (Figure 1). Inspiratory gas was mixed from a high-pressure room air and oxygen source using an oxygen blender (Hans Rudolph, Kansas City, MO) to provide a constant FiO2 of 0.3 to the circuit during both CPAP and AP treatment. The outlet of the oxygen blender was connected to a reservoir balloon and the CPAP facemask without an expiration valve (Respironics, Inc, Murrysville, PA) while the open end of the circuit was either left open to the atmosphere during AP or connected to a 10 cmH2O positive end-expiratory pressure valve during CPAP. Inhaled gas was warmed and humidified by a humidifier connected downstream of the flow meter to avoid drying and bleeding of the nasal mucosa14. All study patients spent the entire PACU stay in supine position with the upper body elevated to approximately 30 degrees.
Figure 1.
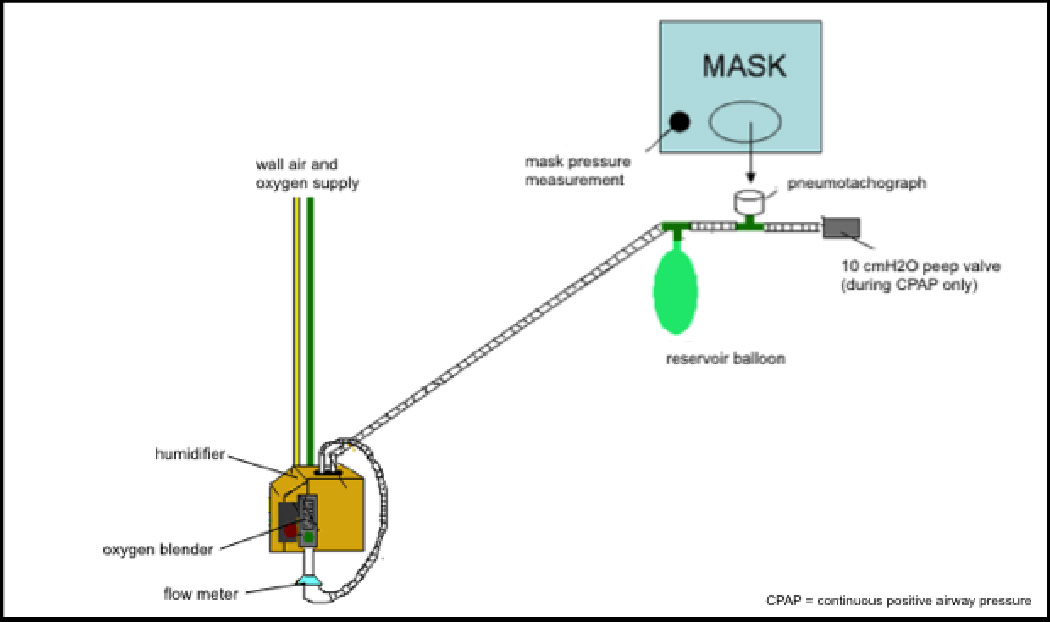
Schematic of the high flow continuous positive airway pressure (CPAP) circuit as described in Hess, D.R. et al, Eds. (2012). Respiratory Care: Principles and Practice. Sudbury, MA, Jones & Bartlett Learning. Inspiratory gas was mixed from a high-pressure room air and oxygen source using an oxygen blender (Hans Rudolph, Kansas City, MO) to provide a constant FiO2 of 0.3 to the circuit. The outlet of the oxygen blender was connected to a reservoir balloon and the CPAP facemask without an expiration valve (Respironics, Inc, Murrysville, PA). Inhaled gas was warmed and humidified by a humidifier connected downstream of the flow meter. The open end of the circuit was either left open to the atmosphere during atmospheric pressure (AP) treatment or connected to a 10 cmH2O positive end-expiratory pressure (PEEP) valve during CPAP treatment. Pneumotachograph (Hans Rudolph, Kansas City, MO) was connected serial between tubing and oro-nasal CPAP mask. Pressure probe of the polysomnography equipment was connected to the CPAP mask in parallel to the CPAP tubing.
Study Measures
PSG data was obtained using a Type 2 out-of-center PSG device (Alice PDx, Philips Respironics Inc, Murrysville, PA)15. This PSG device includes electroencephalography (EEG), electrooculography, submental and limb electromyography, as well as measures of abdominal and thoracic respiratory efforts using impedance plethysmography belts, nasal respiratory airflow, and body position. Sleep stages, arousals, and respiratory events were scored in accordance with the 2007 American Academy of Sleep Medicine Guidelines16.
Baseline severity of SDB was assessed based on recent (within six months prior to surgery) sleep lab-based PSG testing or home-based PSG testing initiated by the study team. For patients with recent PSG results, we obtained and reviewed their complete sleep study reports. If records were not available or were more than six months old, patients underwent home-based sleep testing using our out-of-center device prior to surgery.
In the PACU, PSG was applied and supervised by a study staff member trained in sleep medicine. Measurements of airway pressure and airflow (Pneumotach, Hans Rudolph Inc., Shawnee, KS) were performed continuously. AHI, mean and nadir SpO2, and oxygen desaturation index (ODI) were derived from the PSG. Peak inspiratory flow (PIF), minute ventilation (MV), tidal volume (VT), and respiratory rate (RR) were calculated from the flow tracings of the pneumotach. Timing, frequency, and dose of opioids administered by a patient-controlled analgesia (PCA) pump were documented. Blood pressure and heart rate were recorded every five minutes throughout the study and PACU stay.
Data processing
To evaluate the effect of CPAP on breathing during different arousal states in the PACU, we defined specific EEG-derived arousal states for (1) wakefulness, (2) sleep onset (i.e., transitions from wakefulness to Non-Rapid-Eye-Movement (Non-REM) sleep), and (3) stable Non-REM stage 2 sleep (NREM2) in accordance with previous reports17.
To further evaluate the effect of opioid application (i.e., 1 mg morphine IV equivalent dose per bolus) via PCA on breathing during AP vs. CPAP, the spectral EEG power for the beta, alpha, and theta frequency bands were calculated using Fast-Fourier-Transformation (FFT) as previously used by our and other groups18–21. Briefly, EEG raw signal (C3-A2) tracing derived from the PSG monitor was band limited using a 55 Hz low-pass and a 0.8 Hz high-pass filter. FFT (non-overlapping Hann 256 bit windows) was then used on 5 s epochs of the filtered EEG signal. The power content for each 20 s epoch was determined as the average power across four 5 s segments of the EEG. The spectral distribution was categorized into the following frequency bands: beta (15–21 Hz), alpha (8–13 Hz), and theta (3–7 Hz) frequency band. The power in each frequency bandwidth was expressed as a percentage of total power in each 20 s epoch.
Based on the measured EEG power spectrum, two 20 s epochs of similar beta, alpha, and theta EEG activity flanking a single opioid application were identified in the following manner: we first identified the earliest 20 s epoch with theta-dominant EEG activity (Non-REM sleep stage 1) following a single opioid application and then we identified the last theta-dominant EEG episode with similar EEG profile preceding the opioid application. In addition, the last 20 s epoch of alpha-dominant EEG activity (ie. wakefulness) based on visual examination preceding these episodes were identified. We analyzed ventilation (PIF, VT, MV, and RR) for the first three consecutive breaths during each of these 20 s epochs. Pre- and post-opioid episodes were defined as the last 10 minutes prior to opioid application and the first 10 minutes after an opioid application, respectively. Mean and nadir SpO2 and AHI were derived from the PSG measurements and averaged for all pre- and post-opioid episodes during CPAP and AP treatment. Data recorded during the 10 minutes before and after treatment switch (CPAP to AP or vice versa) were excluded from analysis to prevent any overlapping effect between treatments.
To address the primary aim of the study, data were analyzed as averages across each cortical arousal state (wakefulness, stable Non-REM sleep, and right before and after sleep onset) during AP and CPAP treatments in each individual patient. Subsequently, sequential arousal state-specific analyses were conducted. To address our secondary aim, data were averaged over 10 min pre- and post-opioid episodes. In order to analyze breathing during the transition from wakefulness to sleep, PIF, MV, VT, and RR were averaged over three consecutive breaths during 20 s epochs of similar cortical arousal states pre- and post-opioid application.
Statistical analysis
To evaluate the effect of CPAP on sleep apnea in the PACU (primary aim), we used a mixed linear model with an identity link function for normally distributed probability and defined the intercept, treatment order, and lapsed PACU time as random effects. We tested for a fixed main effect of CPAP administration on the mean individual AHI (primary outcome) as well as on number of oxygen desaturations, the mean and nadir SpO2, and the exploratory endpoints VT, PIF, MV, and RR. Comparisons of effect size were made between CPAP and AP. Random effects were excluded from the final model if they did not explain any variance of our fixed effects (p>0.05).
To evaluate a potential mitigating effect of CPAP on opioid-associated respiratory depression (secondary aim), we used the same model as for our primary aim extended by the random effects, time to first opioid administration within study, morphine equivalent dose of opioids administered within study and morphine equivalent dose of pre-study administered opioids. We tested for the fixed interaction effect of CPAP and opioid administration (“treatment group” × “before or after opioid use”) on AHI, oxygen desaturation, mean and nadir SpO2, as well as the exploratory endpoints VT, PIF, MV, and RR.
With an exploratory intention, we evaluated the fixed effect of treatment (CPAP vs. AP) on PIF, MV, and VT during the EEG-defined arousal states of wakefulness, sleep-onset and stable NREM2 sleep. We further tested for a fixed interaction effect between treatment (CPAP vs. AP) and arousal state (wakefulness vs. NREM2).
Goodness-of-fit of all models in relation to the intercept only model was determined using the likelihood ratio test (LRT).
Our power calculation was based on our previous report22. We expected a mean difference in AHI of 4 with a standard deviation of 7/h. Taking into account a two-sided significance level of 0.05 and power of 80%, we determined the required sample size to be 30 patients. Previous studies of non-invasive ventilation in postsurgical patients have reported a high rate of insufficient adherence to the CPAP treatment23. Thus, we aimed to include 45 patients in order to arrive at the desired sample size.
Statistical analyses were performed using SPSS Version 22 (SPSS Inc. Chicago, IL).
RESULTS
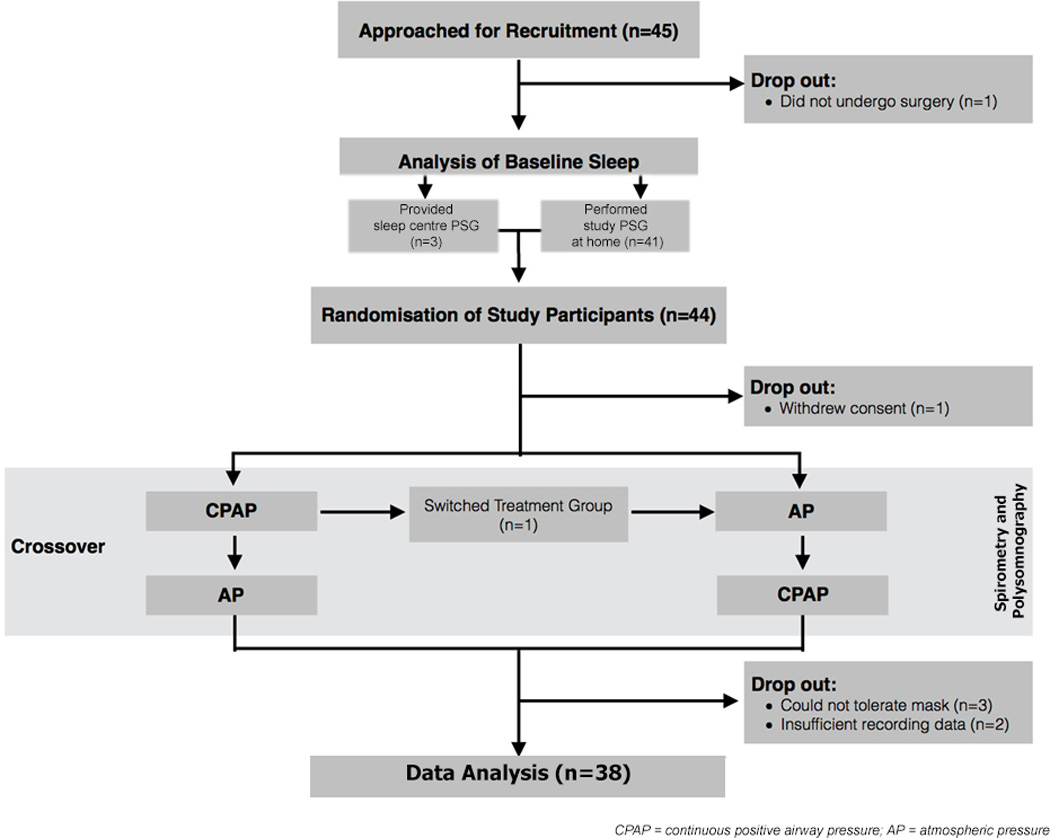
On the day of surgery, 44 patients (see Table 1 for demographics) were randomized to receive first AP or CPAP treatment. Thirty-eight of the 44 randomized study patients (18 received CPAP first and 20 received AP first) completed both treatments of the study and thus were included in the analyses of the effects of opioid and CPAP treatment on respiration and sleep. A summary of recruitment and study flow is illustrated in Figure 2.
Table 1.
Demographics and Intraoperative Management of Study Population.
| Enrolled (n=45) |
Completed Study (n=38) |
|
|---|---|---|
| Demographics | ||
| Sex | ||
| Men | 31% (14) | 32% (12) |
| Women | 69% (31) | 68% (26) |
| Age, yr | 44±13 | 43±13 |
| BMI, kg/m2 | 46±7 | 46±8 |
| Preoperative diagnosis of OSA | 36% (16) | 32% (12) |
| Previous CPAP Treatment | 24% (11) | 26% (10) |
| ASA Risk Classification | ||
| 2 | 58% (26) | 61% (23) |
| 3 | 42% (19) | 40% (15) |
| Intraoperative Management | ||
| Surgery Type | ||
| Laparoscopic Roux-en-Y Gastric Bypass | 36% (16) | 34% (13) |
| Laparoscopic Partial Vertical Gastrectomy | 48% (21) | 50% (19) |
| Laparoscopic Sleeve | 14% (6) | 16% (6) |
| Revision of Gastric Band to Gastric Bypass | 2% (1) | 0% (0) |
| Neuromuscular blocking agent administered | ||
| Cisatracurium | 66% (29) | 66% (25) |
| Rocuronium | 23% (10) | 21% (8) |
| Vecuronium | 11% (5) | 13% (5) |
| NMBA dose, mg/kg | 29±27.5 | 28±27.3 |
| Neostigmine-based reversal | 89% (39) | 89% (34) |
| Opioids applied intraoperatively | 100% (44) | 100% (38) |
| Morphine IV equivalent dose per weight, µg/kg | 125±115 | 121±121 |
ASA = American Society of Anesthesiology; BMI = Body Mass Index; CPAP = Continuous Positive Airway Pressure; IV = Intravenous; NMBA = Neuromuscular blocking agent; OSA = Obstructive Sleep Apnea
All values are presented as %(N) or Mean±SD.
Figure 2. Flow Chart of study protocol.
Baseline Sleep Apnea
26 patients completed preoperative sleep testing and 92% were found to have sleep apnea (AHI > 5/h). Among these patients, only 29% had a known diagnosis of OSA. In this group, mean preoperative AHI was 25±22/h, and 67% of the patients demonstrated predominantly obstructive events. Based on American Academy of Sleep Medicine criteria, the severity of sleep apnea was in the moderate to severe range (Figure 3).
Figure 3. Severity of obstructive sleep apnea (OSA) at baseline.
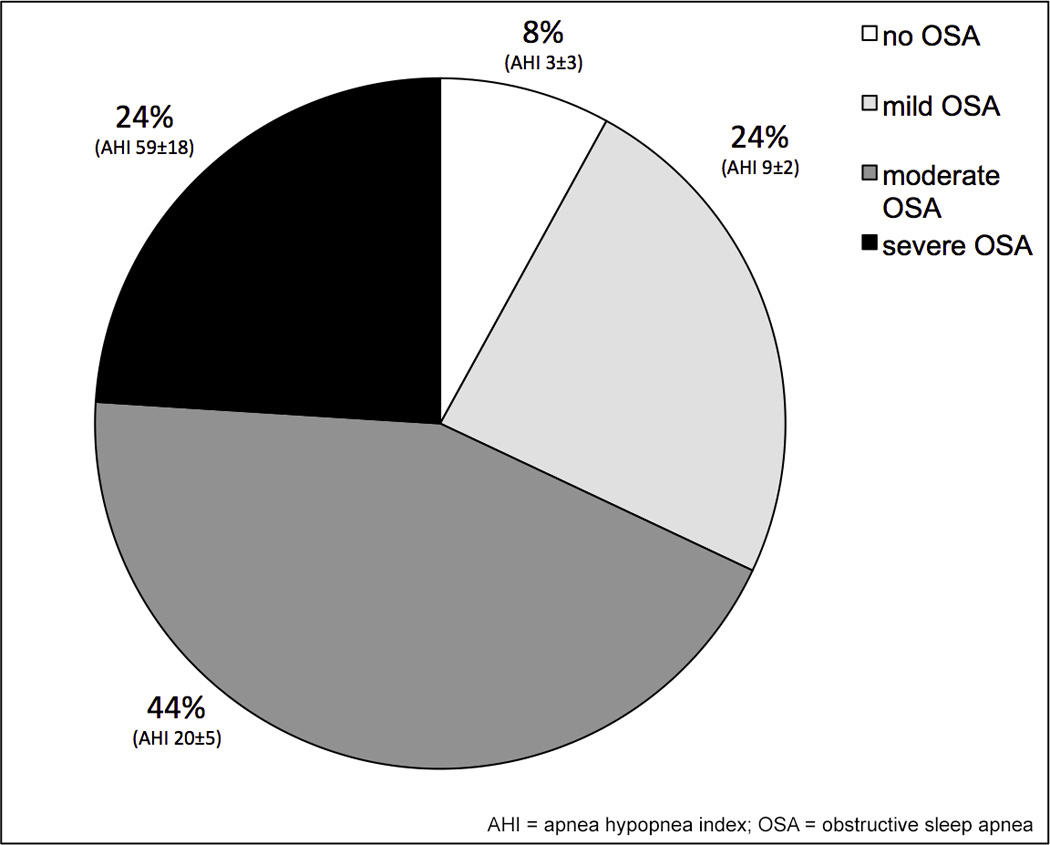
The majority of the patients scheduled for weight-loss surgery had sleep apnea (92%), which was moderate or severe in 44% and 24% of patients, respectively. Data shown as prevalence (%) and obtained from 26 patients who had completed baseline sleep apnea testing; AHI (mean ± standard deviation). AHI = apnea hypopnea index
Based on our PSG measurements in the PACU, 64% of the 33 patients with reliable PSG data for the entire treatment period demonstrated sleep apnea during daytime sleep immediately after surgery. The severity of sleep apnea measured by sleep study prior to admission and in the PACU were similar: mean AHI under AP in the PACU was 25±28/h and did not differ significantly from the AHI measured during home- or sleep lab-based testing prior to surgery (p=0.927).
Effect of CPAP during sleep and wakefulness in the PACU
Compared to AP, CPAP treatment improved sleep apnea in the PACU by reducing both AHI and ODI by 69% (regular linear model [RLM], LRT p<0.001, effect size [ES] 18.6, 95% confidence interval [CI] 7.3–29.8, p=0.002, and mixed linear model [MLM] with random intercept [RI], LRT p<0.001, ES 12.1, 95% CI 6.6–17.6, p<0.001, respectively; Figure 4), and resulted in a significantly higher nadir SpO2 (CPAP vs. AP: 93±5% vs. 89±6%; MLM with RI, LRT p<0.001, ES 3.3, 95% CI 1.3–5.2, p=0.002).
Figure 4. Effects of continuous positive airway pressure (CPAP) on postoperative sleep apnea and breathing in the PACU.
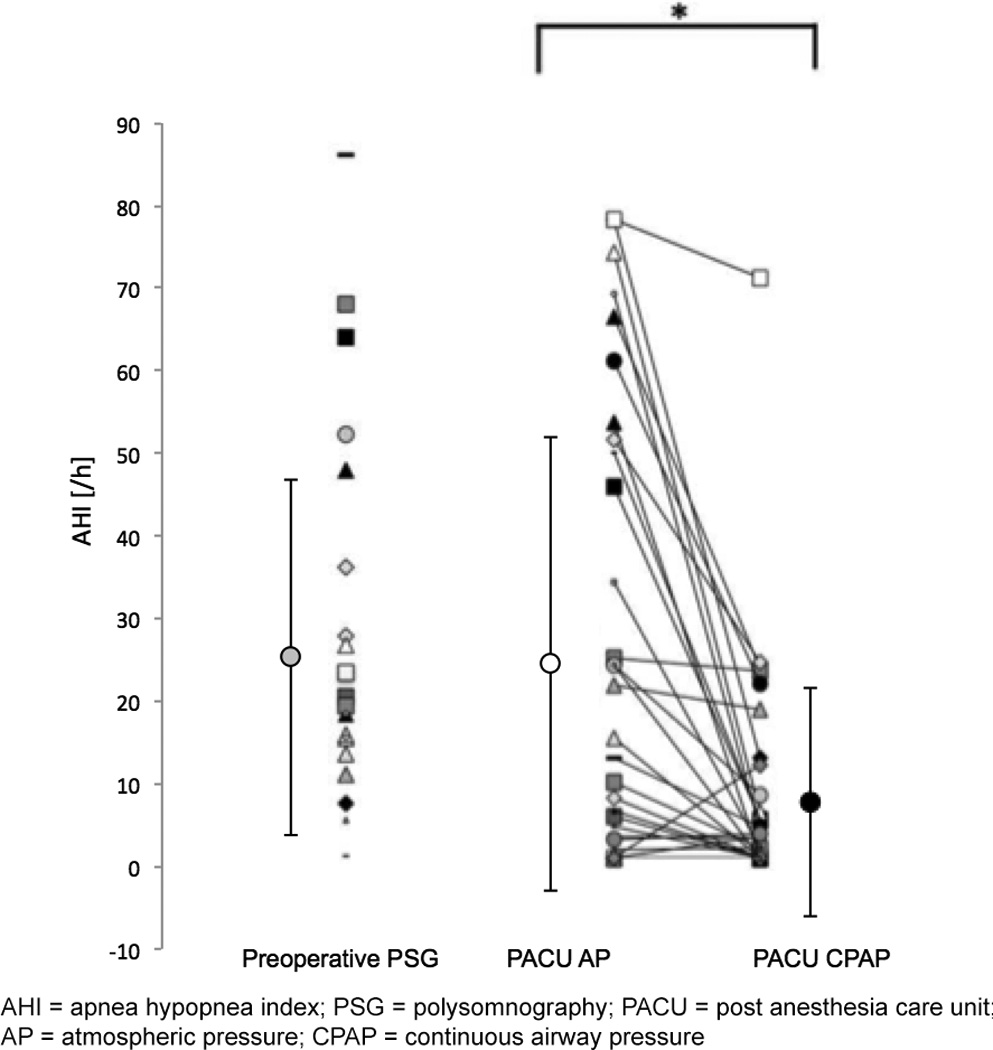
Apnea hypopnea index (AHI) during baseline preoperative evaluation visit (n=26) and in the Post Anesthesia Care Unit (PACU) following surgery (n=33). Values for AHI during PACU stay are shown as mean ± standard deviation (circle with error bars on left) and individual values (right).
Severity of sleep apnea was unchanged from baseline at the start of study in the PACU (Baseline AHI vs. PACU AP AHI, p=0.927). During the study, CPAP treatment improved AHI (p<0.05), compared to standard of care (atmospheric pressure, AP). * denotes statistically significant difference.
CPAP had stabilizing effects on breathing during wakefulness and sleep. A representative sample of combined PSG and spirometry data is shown in Figure 5. CPAP treatment was associated with significantly higher values of VT (RLM, LRT p<0.001, ES 112.3, 95% CI 64.1–160.5, p<0.001), PIF (MLM with random effects of lapsed PACU time, LRT p<0.001, ES 0.055, 95% CI 0.022–0.088, p=0.001) and MV (RLM, LRT p=0.004, ES 1.2, 95% CI 0.5–2.0, p=0.001, Figure 6) and ameliorated the respiratory depressant effects of wake-sleep transition on breathing. We observed a positive interaction effect of arousal state (wakefulness vs. NREM2) and treatment regimen (AP vs. CPAP) on VT (RLM, LRT p=0.769, ES 42.3, 95% CI 22.4–62.4, p=0.001), PIF (MLM with random effects of lapsed PACU time, LRT p<0.001, ES 0.115, 95% CI 0.029–0.200, p<0.001), MV (RLM, LRT p=0.001, ES 3.0, 95% CI 0.8–5.1, p<0.001), and RR (MLM with RI, LRT p<0.001, ES 4.0, 95% CI 2.4–5.4, p<0.001), indicating a larger effect of CPAP treatment on improving breathing during NREM sleep, compared to wakefulness. The number of arousals from sleep did not differ significantly between treatments (arousal index 9.6±1.7/h during AP compared to 9.3±1.7/h during CPAP, p=0.983) and the positive effects of CPAP were independent of randomization group (absence of order effect).
Figure 5. Polysomnography recorded in the Post Anesthesia Care Unit (PACU) during breathing at atmospheric pressure (AP) and continuous positive airway pressure (CPAP).
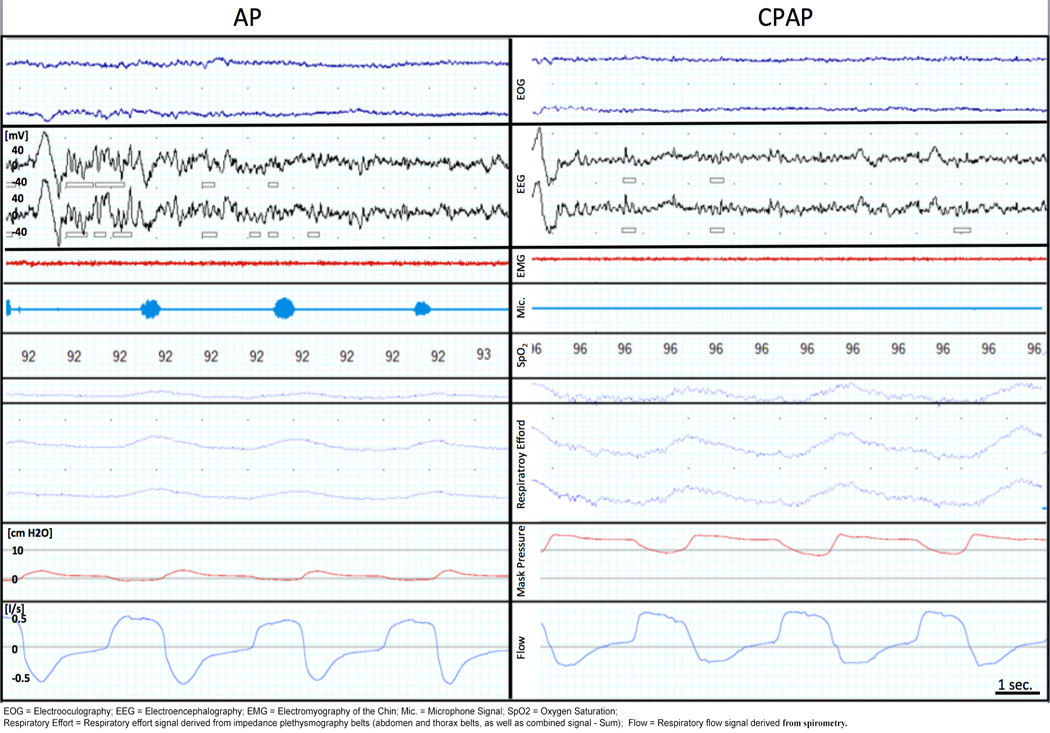
During Non-REM sleep stage 2, hypopneas with flow limitation occurred, as indicated by the flattening of the flow curve (blue curve), during AP, but not during CPAP treatment. Flow limitation was characterized by decreased tidal volume, peak inspiratory flow, and minute ventilation due to impaired upper airway patency, and was accompanied by snoring in the microphone channel [Mic.] (light blue). This resulted ultimately in upper airway collapse and apneas during sleep in some cases.
EOG = Electrooculography; EEG = Electroencephalography; EMG = Electromyography of the Chin; Mic. = Microphone Signal; SpO2 = Oxygen Saturation; Respiratory Effort = Respiratory effort signal derived from impedance plethysmography belts (abdomen and thorax belts, as well as combined signal - Sum); Flow = Respiratory flow signal derived from spirometry.
Figure 6. Effects of continuous positive airway pressure (CPAP) on breathing during wakefulness-sleep transition.
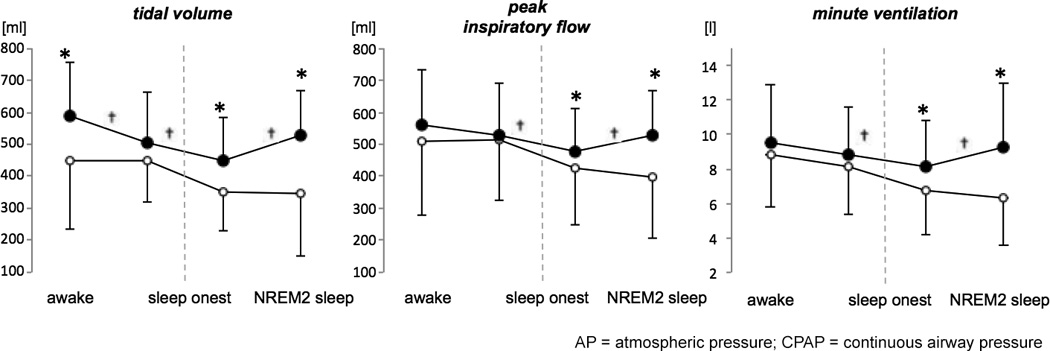
During wake and sleep transitions, tidal volume, minute ventilation and peak inspiratory flow decreased and the magnitude of respiratory depression were smaller during CPAP compared to atmospheric pressure (AP). * denotes statistically significant main effect of treatment on breathing (p<0.05). † denotes statistically significant interaction effect between treatment and sleep stage on breathing (p<0.05). Data obtained from 31 patients with complete breathing data.
CPAP did not have a significant effect on the average mean arterial pressure (AP vs. CPAP: 95±12 mmHg vs. 97±13 mmHg, p=0.16) or heart rate (AP vs. CPAP: 74±12 bpm vs. 76±13 bpm, p=0.17).
Effects of CPAP on opioid-induced respiratory depression
Of the 38 patients who completed the study, 7 did not receive opioids in the PACU. In the remaining 31 patients, opioids were administered a total of 144 times during the study period - 79 applications during AP and 65 applications during CPAP. In addition, 15 patients received morphine while study equipment was applied; on average, this amounted to a total dose of 2.1±4.5 mg morphine (or equivalent dose) between PACU admission and the initiation of the study recording. During the study treatment, the total morphine equivalent dose applied via PCA did not differ significantly during AP and CPAP treatment and amounted to 2.9±3.4 mg and 2.5±2.7 mg, respectively (p=0.608). A total of 96 applications fulfilled our strict a priori defined inclusion criteria for PSG-based analysis of the effects of opioid and CPAP on AHI, ODI, and mean and nadir SpO2. EEG activity did not differ between the pre- and post-opioid episodes (Figure 7).
Figure 7. EEG activity during measurements before and after opioid application.
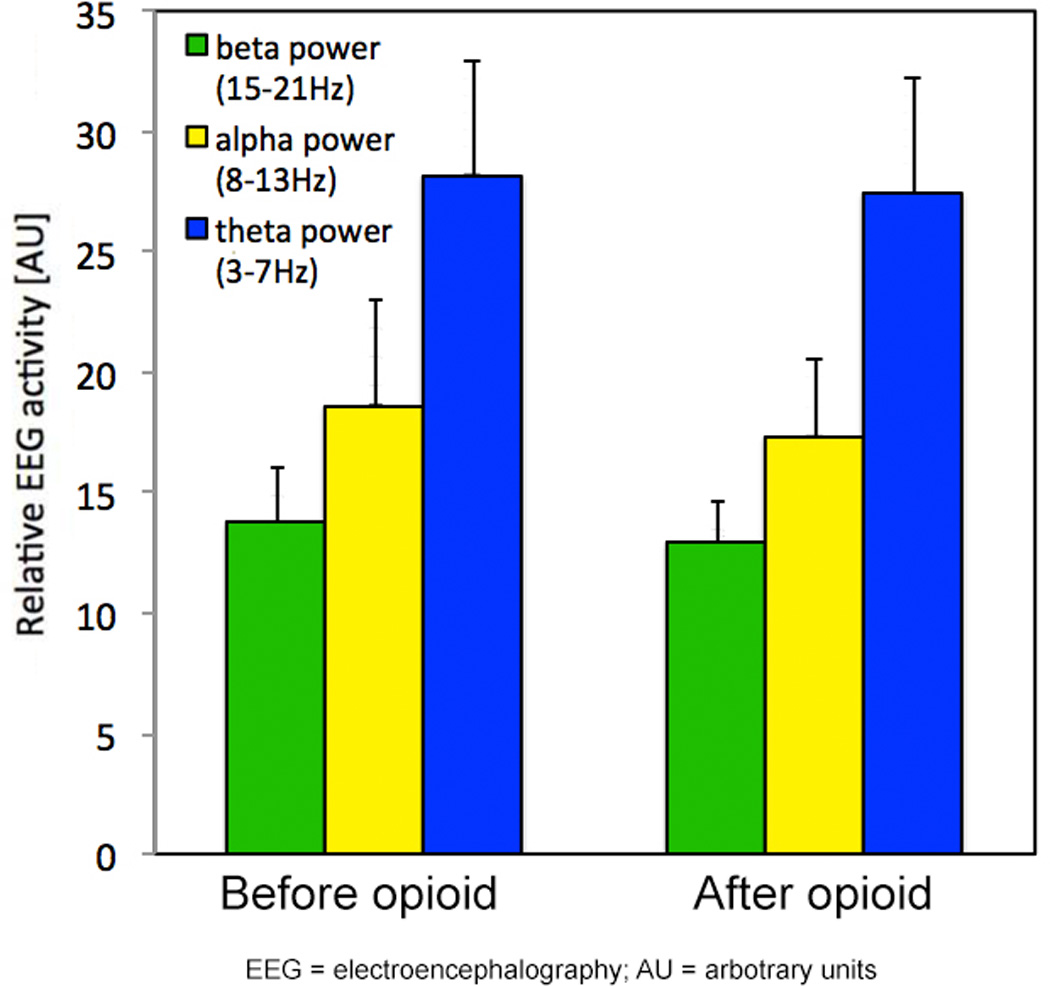
Electroencephalography (EEG) activity was quantified by EEG spectroscopy as indicators of cortical arousal. Beta-, alpha- and theta-frequency bands were calculated using Fast-Fourier-Transformation (FFT; non-overlapping Hann 256 bit windows) on 5-second epochs of the filtered EEG signal 0.8 to 55 Hz. The spectral distribution was categorized into beta (15–21 Hz), alpha (8–13 Hz), and theta (3–7 Hz) frequency band and was expressed as a percentage of total EEG power across the entire frequency band in each 20-s epoch of sleep. Alpha, beta, and theta EEG activity did not differ during measurements before and after opioid application.
AU = arbitrary units
The degree of opioid-induced respiratory depression was reduced during CPAP treatment compared with AP treatment. When self-administered during AP, opioids increased AHI by 13%, from 28±32 /h to 32±58/h (pre- vs. post-opioid). In contrast, during CPAP administration, self-administration of opioids minimally but significantly increased AHI from 6±11/h by 4%; Figure 8a). During sleep, CPAP also abolished the impairing effect of opioids on MV (10.5±5.7 L/min to 9.2±5.3 L/min during AP vs. 12.3±5.6 L/min to 12.4±6.6 L/min during CPAP; Figure 8b). In addition, CPAP improved PIF (Figure 8b), MV (Figure 8c) and VT (Figure 8d) following opioid application, which was paralleled by improved oxygenation. Of note, CPAP also reduced the impairing effects of opioids on these variables (Table 2). We observed a significant interaction effect between treatment type (CPAP vs. AP) and opioid application on AHI (RLM, p<0.001, LRT p<0.001), ODI (MLM with random effect of time to first opioid administration within study, p=0.010, LRT p<0.001), mean SpO2 (MLM with RI, p=0.029, LRT p<0.001) and nadir SpO2 (MLM with RI, p=0.006, LRT p<0.001), as well as VT (MLM with random effect of lapsed PACU time and time to first opioid administration within study, p=0.01, LRT p<0.001), PIF (MLM with RI, p=0.002, LRT p<0.001), MV (MLM with RI, p=0.015, LRT p<0.001) and RR (MLM with RI, p<0.001, LRT p<0.001).
Figure 8. Opioid-induced effects on A) apnea hypopnea index (AHI), B) tidal volume (VT), C) minute ventilation (MV), and D) peak inspiratory flow (PIF) during continuous positive airway pressure (CPAP) and atmospheric pressure (AP).
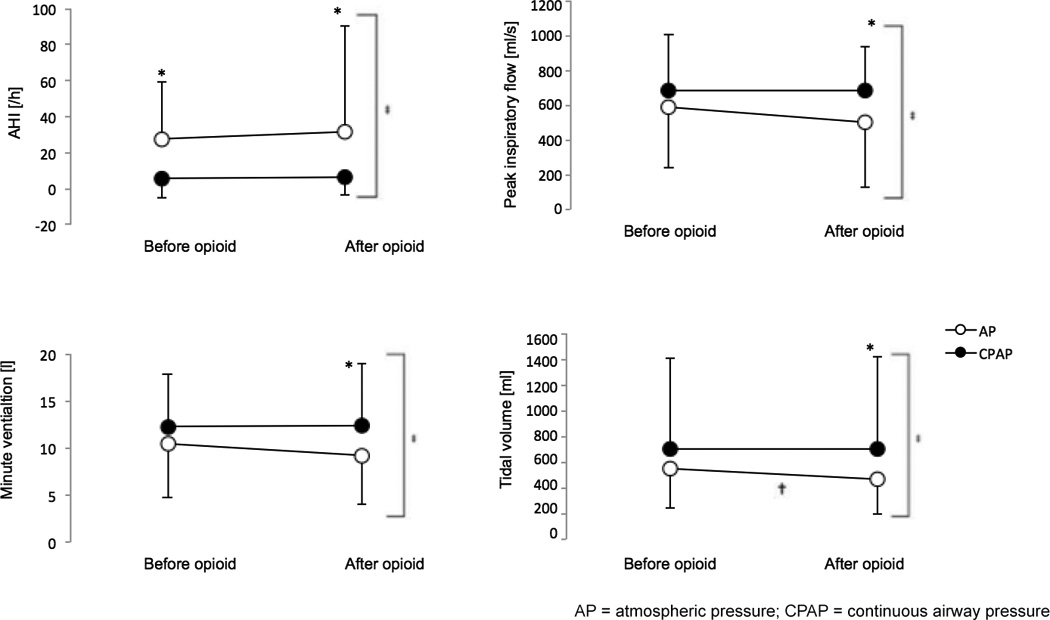
Application of opioid resulted in increases in AHI and reductions in PIF, MV, and VT during AP treatment (white circles). Administration of CPAP (black circles) decreased these opioid-induced effects. * denotes statistically significant effect of CPAP treatment. † denotes statistically significant effect of opioid administration (within treatment group). ‡ denotes statistically significant interaction effect between CPAP treatment and opioid application. Data from 31 patients who received opioids for postoperative pain therapy in the Post Anesthesia Care Unit.
Table 2.
Effect of continuous positive airway pressure (CPAP) on breathing and oxygenation during sleep before and after application of opioids.
| Before opioid application | After opioid application | |||||
|---|---|---|---|---|---|---|
| AP | CPAP | p-value | AP | CPAP | p-value | |
| ODI, /hr | 12±23 | 2±5 | 0.002 | 12±21 | 3±11 | 0.005 |
| Mean SpO2, % | 96±3 | 97±2 | 0.004 | 96±2 | 97±2 | 0.012 |
| Nadir SpO2, % | 94±5 | 95±4 | 0.022 | 93±5 | 95±4 | 0.007 |
| VT, mL | 546±306 | 700±332 | 0.004 | 466±271 | 707±332 | 0.000 |
| PIF, mL/s | 583.6±323.5 | 680.0±343.9 | 0.089 | 498.7±253.7 | 683.8±370.7 | 0.007 |
| RR, breaths/min | 20±6 | 18±4 | 0.002 | 20±6 | 17±4 | 0.000 |
AP = atmospheric pressure; CPAP = continuous positive airway pressure; ODI = oxygen desaturation index; PIF = peak inspiratory flow; RR = respiratory rate; SpO2 = oxygen saturation; VT = tidal volume. Data was obtained from 31 patients who had received opioids for postoperative pain therapy in the Post Anesthesia Care Unit.
All values are presented as Mean±SD.
Opioids given before study start did not have any effects on any of our outcomes (variance estimate of random effects p>0.05). In accordance, in contrast to the respiratory depressant effects of low-dose opioids during sleep we did not observe any effect of 1 mg morphine equivalent on PIF and VT during wakefulness. Mean PIF were 557.8±289.4 ml/s before vs. 555.6±304.7 ml/s after opioid application during AP and were 616.8±265.5 ml/s before vs. 607.8±213.4 ml/s after opioid application during CPAP (p=0.814 - before vs. after). Mean VT was 503±181 ml before vs. 504±286 ml after opioid application during AP and 604±277 ml before vs. 621±233 ml after opioid application during CPAP (p=0.900 – before vs. after). MV also did not change following low-dose opioid application (9.9±3.3 l vs. 9.7±l and 10.8±3.9 l vs. 11.6±2.9 l during CPAP; p=0.650).
DISCUSSION
This pharmaco-physiological interaction trial demonstrates beneficial effects of early postoperative CPAP treatment across the continuum of wakefulness and Non-REM sleep and on the respiratory-depressant effects of opioids used for pain management in the recovery room. CPAP mitigated opioid-induced worsening of sleep-disordered breathing early after bariatric surgery.
Effect of early postoperative CPAP on sleep disordered breathing and oxygenation
CPAP administered to patients immediately following bariatric surgery improved AHI and ventilation (PIF, VT, and MV) during sleep compared with breathing in AP, which is the standard of care for CPAP naïve patients undergoing weight-loss surgery at our institution. As expected, VT and PIF decreased during the wake-sleep transition, consistent with previous reports in patients without residual effects of anesthetics, analgesics, and neuromuscular blockade17. The magnitude of the observed reductions in VT and PIF were smaller during CPAP treatment. These effects can in part be explained by the improved airway patency associated with CPAP treatment.
The currently available data on the effect of CPAP on SDB in the early postoperative period are conflicting. Some data suggest that CPAP treatment prevents respiratory complications after abdominal9,10 and cardiac surgery24 and improves AHI and oxygen saturation during the first night after orthopedic surgery25. In another study, CPAP was applied during the first through fifth night after abdominal surgery in patients with high risk of OSA and CPAP was found to improve AHI from 30/h to below 5/h25.
Recent studies found that postoperative CPAP did not consistently improve postoperative SDB. Drummond et al did not find a beneficial effect of CPAP auto-titration on SDB after major abdominal surgery12. In their parallel-group designed study of 48 patients, the authors found no difference in ODI between AP and CPAP on the first postoperative night. However, the study investigated the effect of CPAP in patients undergoing general abdominal surgery and none of these patients had signs or symptoms of sleep apnea preoperatively. In contrast, our study focused on bariatric surgery patients, a surgical population with a high prevalence of sleep apnea. Similarly, O’Gorman et al11 studied the effects of CPAP auto-titration vs. oxygen therapy at AP in patients with OSA undergoing orthopedic surgeries. The authors surprisingly found a higher number of oxygen desaturations to an SpO2 below 90% in patients in the CPAP group compared with those in AP. Possible explanations of this negative finding include poor compliance with CPAP treatment and lower inspiratory oxygen concentration in the CPAP group. In fact, median usage of CPAP in that study was only 3 hours 4 minutes and patients may not have received any treatment during the rest of the night that was captured by PSG recordings. Furthermore, the authors were not able to control inspiratory oxygen concentration with the device during the time the patients used CPAP. In our study, we applied a standardized FiO2 0.3 across treatments by using a custom-made high-flow CPAP device in a supervised setting of a post-surgery recovery room. The CPAP pressure of 8.7±0.6 cm H2O applied in our study was similar to the pressure applied in previous studies using auto-titration CPAP devices11. A CPAP pressure of about 10 cmH2O has been reported to provide sufficient pressure transmission to the trachea26 and was found to be an effective treatment pressure for OSA when allied with supplemental oxygen27.
The available evidence on perioperative CPAP treatment in bariatric surgery patients is inconclusive. Although several studies have found beneficial effects of postoperative treatment of OSA on weight loss after bariatric surgery compared to bariatric surgery alone28–30, long-term improvement of metabolic and cardiovascular comorbidities after bariatric surgery might be linked more closely to weight loss than CPAP treatment31,32. During the early perioperative period, retrospective studies in patients undergoing bariatric surgery reported no differences in overall postoperative morbidity33, as well as no cases of death, reintubation, or cardiopulmonary complications34,35, regardless of CPAP or AP treatment. CPAP was found to improve blood oxygenation in patients after open Roux-en-Y gastric bypass in some studies36, while other authors found severe and prolonged episodes of hypoxemia during the early postoperative period despite preoperative diagnosis and treatment of OSA, including use of CPAP following bariatric surgery37. Furthermore, preoperative CPAP treatment of OSA was found to have no effect on length of stay, pulmonary complications, or mortality following bariatric surgery when CPAP was omitted during the postoperative period38.
In our study, we were able to show CPAP to provide superior respiratory stability compared with AP in the PACU immediately after bariatric surgery. We found that the mean AHI during CPAP treatment applied via full-face mask in the PACU after surgery was significantly decreased compared with the mean AHI during AP treatment (7.7/h vs. 24.9/h). This effect of CPAP might even be larger when applied via nasal mask as recently reported by Oto and coworkers39. Our findings indicate that the beneficial effects of CPAP, previously observed during the first nights after surgery,25 also extend to the period immediately following surgery despite lingering effects of anesthesia and surgery. However, whether or not these positive effects are associated with improved postoperative outcome is beyond the scope of this study.
In healthy controls, acutely administered CPAP can reduce cardiac index and cardiac stroke volume index due to PEEP. Decreased venous return on CPAP reduces cardiac preload and afterload, by reducing left ventricular transmural pressure40. Thus, hypotensive periods are a potential risk with CPAP therapy. In our study, no significant hemodynamic effects were observed when using CPAP treatment with an average pressure of 8.7±0.6 cmH2O. In fact, CPAP was well tolerated and 86% of the study patients completed the CPAP treatment phase.
Recently, CPAP therapy has been described to be of limited benefit in patients with mild OSA41. However, in our patient population, CPAP was effective, independent of OSA severity.
Opioids’ effects on breathing, airway patency, and interaction with CPAP
Opioids reduce airway patency and impair hypoxic and hypercarbic respiratory drive42. Underlying mechanisms may include direct inhibitory effects on the pre-Bötzinger complex4, as well as the retrotrapezoid nucleus and parafacial respiratory group complex, which are responsible for generating the respiratory pattern43,44 and decreasing effects on chest wall compliance via actions at various motoneurons (see45 for review).
Although traditional clinical concepts interpret opioid-induced respiratory depression in the context of “overdosing”, our data show that low doses of opioids (1 mg morphine or equivalent dose of hydromorphone) given via PCA for postoperative pain treatment after bariatric surgery decrease tidal volumes and cause a trend towards lower peak inspiratory flow and minute ventilation, as well as higher frequency of oxygen desaturations and apneas during sleep. However, we did not observe similar impairing effects of opioids on breathing during wakefulness early after surgery, when cortical arousal and excitatory inputs to the upper airway motor neurons and respiratory drive are higher8,13. Our findings indicate that the low dose of opioids applied here might be without negative effects on breathing as long as the patients stayed awake. However, we observed impairment of breathing and worsening of sleep apnea as soon as patients fell asleep and exhibited depression of cortical activity46, which was seen in all study patients during their PACU stay. The 1 mg morphine equivalent dose of opioid investigated in our study was much lower than those in previous studies and thus, effects of greater statistical significance may be observed when higher doses of opioids are given. All patients in our study slept during a significant part of PACU treatment following weight-loss surgery. Given that bariatric patients are a population at high risk of OSA, it might be reasonable to provide sleep apnea-directed management during the early postoperative period, especially while patients remain largely immobile in bed.
Of note, the respiratory depressant effects of low-dose opioid therapy given to treat postoperative pain occurred in an environment where stimulation by pain, PACU nursing interventions, and monitor alarms should offset some of the depressant effects of opioids. It is likely that the magnitude of opioid-induced respiratory depression increases as patients are discharged to a quiet room on the surgical ward.
The prevalence of OSA was high among our surgical cohort and the higher sensitivity of OSA patients to opioid-induced respiratory depression is well known47. Furthermore, the upper airway of OSA patients is more prone to collapse when under the influence of neuromuscular blockade48,49 and anesthetics50. Therefore, clinical guidelines have suggested minimizing the use of opioids in this population. However, these patients also have a lower pain threshold compared to controls5,51 and require higher doses of opioids to accomplish adequate analgesia6,52.
In this pharmaco-physiological interaction trial, we demonstrate that CPAP treatment can mitigate opioid-induced negative effects on AHI, VT and MV, potential markers of respiratory depression, and ameliorate SDB in the PACU. In contrast, among patients using daily opioid medication, CPAP does not appear to improve oxygen desaturations during sleep53. This limited effectiveness of CPAP in patients with chronic opioid use may be explained by poor compliance to CPAP treatment or tolerance to the respiratory depressant effects of opioid with chronic use. In addition, an increased number of central apneas have been reported in patients on CPAP in some studies54,53, but not in others in patients on chronic opioids55. In our study, we did not see a negative effect of low doses of opioids given via PCA on the number of central apneas in opioid naïve patients in the PACU (p=0.875).
Limitations
There are several limitations to our study and analysis. One major limitation is our use of unattended PSG performed at the patient’s home to diagnose baseline SDB in our population. Reassuringly, the positive predictive value of unattended PSG has been found to be similar to attended PSG56, and a recent multi-center trial comparing in-center to out-of-center PSG found the latter non-inferior with regards to acceptance, adherence, time to treatment, and functional improvement due to CPAP when prescribed based on an out-of-center PSG57. A second limitation arises from our study’s sample size and crossover design, which does not permit us to attribute optimal postoperative outcomes to improved AHI and oxygen saturation during CPAP treatment. A large scale, randomized controlled parallel-group design trial is needed to detect meaningful differences between both treatments. We speculate that postoperative CPAP in patients vulnerable to postoperative airway obstruction may translate to decreased intensive care unit admission rate. Recent studies indicate sleep apnea to be associated with increased risk of reintubation58,59. Of note, the incidence of OSA in our study population was high and it is unclear if CPAP improves opioid-induced respiratory depression in patient populations without sleep apnea.
A third limitation relates to the potential disruptive and arousing stimulus effect of CPAP, especially to novel users. It is possible that patients on CPAP were slightly more alert, which in turn contributed to our finding of improved AHI. The respiratory rate observed during CPAP and AP was high compared to postoperative patients on the wards, as been reported previously60,61 – presumably representing the consequence of pain, anxiety, and inflammation early after surgery in the PACU setting. However, sleep architecture as well as arousal index measured by PSG did not differ between CPAP and AP treatment conditions.
Finally, our measurement of NREM sleep is subject to potential bias as application of low doses of opioids has been shown to affect sleep behavior and decreases Non-REM sleep and rapid eye movement sleep as measured by EEG and sleep efficiency in rodents62,63 and humans64. In contrast, high doses of morphine induce sedation and are associated with slowing of the EEG65–68, in part mediated by central opioid inputs to sleep regulating brain areas (e.g. ventrolateral preoptic nucleus)63,69. In our study, we ensured the presence of at least one EEG sign other than specific theta frequency to define an episode as stable NREM2 sleep for our study (e.g. k-complex or spindle activity) such that drug effects should not bias sleep scoring under these conditions.
Conclusion
In summary, more than 90% of patients in our cohort demonstrated SDB preoperatively during nighttime at home. Two thirds of them also show sleep apnea early after bariatric surgery in the recovery room during daytime while receiving 30% supplemental oxygen (breathing in atmospheric pressure). CPAP significantly improved AHI, oxygen saturation, tidal volume, peak inspiratory flow, and minute ventilation, and mitigated the respiratory depressant effects observed during sleep and during alpha-theta transition. Supervised CPAP treatment in the PACU in patients vulnerable to sleep apnea may improve postoperative respiratory safety.
Box Summary.
What We Already Know about This Topic
Continuous positive airway pressure (CPAP) effectively reduces nocturnal obstructive respiratory events in patients with obstructive sleep apnea (OSA).
Its effectiveness for early postoperative period remains uncertain particularly in OSA patients receiving opioids for analgesia.
What This Article Tells Us That Is New
This prospective, randomized, cross over trial compared the apnea hypopnea index (AHI) with and without CPAP in 38 morbidly-obese patients and found that the CPAP reduced the AHI by 69% and was more effective during NREM sleep than during wakefulness.
The AHI was worsened by self-administered opioid for pain and the CPAP application effectively mitigated the AHI deterioration.
Acknowledgments
We are grateful to the staff of the postoperative acute care unit of the Massachusetts General Hospital, Boston, Massachusetts, USA for supporting this trial.
Funding: Supported by a research grant titled “Consequences of obstructive sleep apnea on respiratory function following weight-loss surgery: A randomized controlled trial” from the ResMed Foundation, La Jolla, California.
Financial Support:
Dean Hess discloses relationships with Philips Respironics, Bayer, McGraw-Hill, Jones and Bartlett, UpToDate, and ABIM.
Dr. Satya Krishna Ramachandran has received industry funding from Merck in 2014 for research on sleep apnea and early postoperative desaturation.
Dr. Atul Malhotra MD is funded by NIH R01 HL085188 and K24 HL 093218. He relinquished all outside personal income since May 2012.
Dr. Matthias Eikermann receives funding from Merck, Massimo, Baxter Ventures, and the Judy and Jeff Buzen fund.
Footnotes
Clinical Trial Registration NCT01697878
Conflict of Interests:
Dr. Sebastian Zaremba declares no conflict of interests.
Christina H. Shin declares no conflict of interests.
Sanjana Malviya declares no conflict of interests.
Dr. Matthew M. Hutter declares no conflict of interests.
Dean Hess declares no conflict of interests.
Stephanie D. Grabitz declares no conflict of interests.
Daniel Diaz-Gil declares no conflicts of interest.
Teresa MacDonald reports no conflict of interests.
Dr. Satya Krishna declares no conflict of interests.
Dr. Atul Malhotra MD declares no conflict of interests.
Dr. Matthias Eikermann declares no conflict of interests.
REFERENCES
- 1.Weingarten TN, Flores AS, McKenzie JA, Nguyen LT, Robinson WB, Kinney TM, Siems BT, Wenzel PJ, Sarr MG, Marienau MS, Schroeder DR, Olson EJ, Morgenthaler TI, Warner DO, Sprung J. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106:131–139. doi: 10.1093/bja/aeq290. [DOI] [PubMed] [Google Scholar]
- 2.Memtsoudis SG, Besculides MC, Mazumdar M. A rude awakening--the perioperative sleep apnea epidemic. N Engl J Med. 2013;368:2352–2353. doi: 10.1056/NEJMp1302941. [DOI] [PubMed] [Google Scholar]
- 3.Dahan A, Aarts L, Smith TW. Incidence, Reversal, and Prevention of Opioid-induced Respiratory Depression. Anesthesiology. 2010;112:226–238. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 4.Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MT, Finan PH. Sleep, respiration, and pain: a potential nexus for chronic pain risk? Anesthesiology. 2013;119:1011–1013. doi: 10.1097/ALN.0b013e3182a9521b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doufas AG, Tian L, Padrez KA, Suwanprathes P, Cardell JA, Maecker HT, Panousis P. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PLoS One. 2013;8:e54807. doi: 10.1371/journal.pone.0054807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki N, Meyer MJ, Eikermann M. Postoperative respiratory muscle dysfunction: pathophysiology and preventive strategies. Anesthesiology. 2013;118:961–978. doi: 10.1097/ALN.0b013e318288834f. [DOI] [PubMed] [Google Scholar]
- 9.Ferreyra GP, Baussano I, Squadrone V, Richiardi L, Marchiaro G, Del Sorbo L, Mascia L, Merletti F, Ranieri VM. Continuous positive airway pressure for treatment of respiratory complications after abdominal surgery: a systematic review and meta-analysis. Ann Surg. 2008;247:617–626. doi: 10.1097/SLA.0b013e3181675829. [DOI] [PubMed] [Google Scholar]
- 10.Squadrone V, Coha M, Cerutti E, Schellino MM, Biolino P, Occella P, Belloni G, Vilianis G, Fiore G, Cavallo F, Ranieri VM Piedmont Intensive Care Units N. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293:589–595. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 11.O'Gorman SM, Gay PC, Morgenthaler TI. Does autotitrating positive airway pressure therapy improve postoperative outcome in patients at risk for obstructive sleep apnea syndrome? A randomized controlled clinical trial. Chest. 2013;144:72–78. doi: 10.1378/chest.12-0989. [DOI] [PubMed] [Google Scholar]
- 12.Drummond GB, Stedul K, Kingshott R, Rees K, Nimmo AF, Wraith P, Douglas NJ. Automatic CPAP compared with conventional treatment for episodic hypoxemia and sleep disturbance after major abdominal surgery. Anesthesiology. 2002;96:817–826. doi: 10.1097/00000542-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62:799–805. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess DR, MacIntyre NR, Mishoe SC, Galvin WF, Adams AB. Respiratory Care: Principles and Practice. 2. Sudbury, MA: Jones & Bartlett Learning; 2012. [Google Scholar]
- 15.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R Portable Monitoring Task Force of the American Academy of Sleep M. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17.Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. J Appl Physiol (1985) 1992;73:2462–2469. doi: 10.1152/jappl.1992.73.6.2462. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Samet J, Caffo B, Bankman I, Punjabi NM. Power spectral analysis of EEG activity during sleep in cigarette smokers. Chest. 2008;133:427–432. doi: 10.1378/chest.07-1190. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Germain A, Hall ML, Moul DE, Nofzinger EA, Begley A, Ehlers CL, Thompson W, Kupfer DJ. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasko RC, Jr, Brunner DP, Monahan JP, Doman J, Boston JR, el-Jaroudi A, Miewald J, Buysse DJ, Reynolds CF, 3rd, Kupfer DJ. Power spectral analysis of EEG in a multiple-bedroom, multiple-polygraph sleep laboratory. Int J Med Inform. 1997;46:175–184. doi: 10.1016/s1386-5056(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 21.Eikermann M, Grosse-Sundrup M, Zaremba S, Henry ME, Bittner EA, Hoffmann U, Chamberlin NL. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology. 2012;116:35–46. doi: 10.1097/ALN.0b013e31823d010a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaremba S, Mueller N, Heisig AM, Shin CH, Jung S, Leffert LR, Bateman BT, Pugsley LJ, Nagasaka Y, Moreno Duarte I, Ecker JL, Eikermann M. Elevated upper body position improves pregnancy-related OSA without impairing sleep quality or sleep architecture early after delivery. Chest. 2015;148:936–944. doi: 10.1378/chest.14-2973. [DOI] [PubMed] [Google Scholar]
- 23.Al Jaaly E, Fiorentino F, Reeves BC, Ind PW, Angelini GD, Kemp S, Shiner RJ. Effect of adding postoperative noninvasive ventilation to usual care to prevent pulmonary complications in patients undergoing coronary artery bypass grafting: a randomized controlled trial. J Thorac Cardiovasc Surg. 2013;146:912–918. doi: 10.1016/j.jtcvs.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen-Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest. 2009;135:1252–1259. doi: 10.1378/chest.08-1602. [DOI] [PubMed] [Google Scholar]
- 25.Liao P, Luo Q, Elsaid H, Kang W, Shapiro CM, Chung F. Perioperative auto-titrated continuous positive airway pressure treatment in surgical patients with obstructive sleep apnea: a randomized controlled trial. Anesthesiology. 2013;119:837–847. doi: 10.1097/ALN.0b013e318297d89a. [DOI] [PubMed] [Google Scholar]
- 26.Kindgen-Milles D, Buhl R, Loer SA, Muller E. Nasal CPAP therapy: effects of different CPAP levels on pressure transmission into the trachea and pulmonary oxygen transfer. Acta Anaesthesiol Scand. 2002;46:860–865. doi: 10.1034/j.1399-6576.2002.460717.x. [DOI] [PubMed] [Google Scholar]
- 27.Edwards BA, Sands SA, Owens RL, White DP, Genta PR, Butler JP, Malhotra A, Wellman A. Effects of hyperoxia and hypoxia on the physiological traits responsible for obstructive sleep apnoea. J Physiol. 2014;592:4523–4535. doi: 10.1113/jphysiol.2014.277210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collen J, Lettieri CJ, Eliasson A. Postoperative CPAP use impacts long-term weight loss following bariatric surgery. J Clin Sleep Med. 2015;11:213–217. doi: 10.5664/jcsm.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Raaff CA, Coblijn UK, de Vries N, Heymans MW, van den Berg BT, van Tets WF, van Wagensveld BA. Predictive Factors for Insufficient Weight Loss After Bariatric Surgery: Does Obstructive Sleep Apnea Influence Weight Loss? Obes Surg. 2015 doi: 10.1007/s11695-015-1830-4. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Janney CA, Kilbourne AM, Germain A, Lai Z, Hoerster KD, Goodrich DE, Klingaman EA, Verchinina L, Richardson CR. The Influence of Sleep Disordered Breathing on Weight Loss in a National Weight Management Program. Sleep. 2015 doi: 10.5665/sleep.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauscher H, Formanek D, Popp W, Zwick H. Nasal CPAP and weight loss in hypertensive patients with obstructive sleep apnoea. Thorax. 1993;48:529–533. doi: 10.1136/thx.48.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakker JP, Campana LM, Montesi SB, Balachandran J, Deyoung PN, Smales E, Patel SR, Malhotra A. A pilot study investigating the effects of continuous positive airway pressure treatment and weight-loss surgery on autonomic activity in obese obstructive sleep apnea patients. J Electrocardiol. 2014;47:364–373. doi: 10.1016/j.jelectrocard.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez A, Lalor PF, Szomstein S, Rosenthal RJ. Continuous positive airway pressure in immediate postoperative period after laparoscopic Roux-en-Y gastric bypass: is it safe? Surg Obes Relat Dis. 2009;5:544–546. doi: 10.1016/j.soard.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Goucham AB, Coblijn UK, Hart-Sweet HB, de Vries N, Lagarde SM, van Wagensveld BA. Routine Postoperative Monitoring after Bariatric Surgery in Morbidly Obese Patients with Severe Obstructive Sleep Apnea: ICU Admission is not Necessary. Obes Surg. 2015 doi: 10.1007/s11695-015-1807-3. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Huerta S, DeShields S, Shpiner R, Li Z, Liu C, Sawicki M, Arteaga J, Livingston EH. Safety and efficacy of postoperative continuous positive airway pressure to prevent pulmonary complications after Roux-en-Y gastric bypass. J Gastrointest Surg. 2002;6:354–358. doi: 10.1016/s1091-255x(01)00048-8. [DOI] [PubMed] [Google Scholar]
- 36.Gaszynski T, Tokarz A, Piotrowski D, Machala W. Boussignac CPAP in the postoperative period in morbidly obese patients. Obes Surg. 2007;17:452–456. doi: 10.1007/s11695-007-9079-1. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher SF, Haines KL, Osterlund LG, Mullen M, Downs JB. Postoperative hypoxemia: common, undetected, and unsuspected after bariatric surgery. J Surg Res. 2010;159:622–626. doi: 10.1016/j.jss.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Jensen C, Tejirian T, Lewis C, Yadegar J, Dutson E, Mehran A. Postoperative CPAP and BiPAP use can be safely omitted after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:512–514. doi: 10.1016/j.soard.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Oto J, Li Q, Kimball WR, Wang J, Sabouri AS, Harrell PG, Kacmarek RM, Jiang Y. Continuous positive airway pressure and ventilation are more effective with a nasal mask than a full face mask in unconscious subjects: a randomized controlled trial. Crit Care. 2013;17:R300. doi: 10.1186/cc13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91:1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- 41.Pepin JL, Timsit JF, Tamisier R, Levy P. Is CPAP effective in reducing blood pressure in minimally symptomatic obstructive sleep apnoea? Thorax. 2014;69:1068–1070. doi: 10.1136/thoraxjnl-2014-205430. [DOI] [PubMed] [Google Scholar]
- 42.Gelberg J, Jonmarker C, Stenqvist O, Werner O. Intravenous boluses of fentanyl, 1 mug kg(−)(1), and remifentanil, 0.5 mug kg(−)(1), give similar maximum ventilatory depression in awake volunteers. Br J Anaesth. 2012;108:1028–1034. doi: 10.1093/bja/aes029. [DOI] [PubMed] [Google Scholar]
- 43.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 44.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koo CY, Eikermann M. Respiratory Effects of Opioids in Perioperative Medicine. The Open Anesthesiology Journal. 2011;5:23–34. [Google Scholar]
- 46.Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol. 1961;16:15–20. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- 47.Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125–132. doi: 10.1007/BF03009805. [DOI] [PubMed] [Google Scholar]
- 48.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol (1985) 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 49.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17:32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki N, Meyer MJ, Eikermann M. Postoperative Respiratory Muscle Dysfunction: Pathophysiology and preventive strategies. Anesthiology. 2013;118(4):961–978. doi: 10.1097/ALN.0b013e318288834f. [DOI] [PubMed] [Google Scholar]
- 51.Doufas AG, Tian L, Davies MF, Warby SC. Nocturnal intermittent hypoxia is independently associated with pain in subjects suffering from sleep-disordered breathing. Anesthesiology. 2013;119:1149–1162. doi: 10.1097/ALN.0b013e3182a951fc. [DOI] [PubMed] [Google Scholar]
- 52.Brown KA, Laferriere A, Moss IR. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology. 2004;100:806–810. doi: 10.1097/00000542-200404000-00009. discussion 5A. [DOI] [PubMed] [Google Scholar]
- 53.Farney RJ, Walker JM, Boyle KM, Cloward TV, Shilling KC. Adaptive servoventilation (ASV) in patients with sleep disordered breathing associated with chronic opioid medications for non-malignant pain. J Clin Sleep Med. 2008;4:311–319. [PMC free article] [PubMed] [Google Scholar]
- 54.Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10:637–643. doi: 10.5664/jcsm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowdhuri S, Ghabsha A, Sinha P, Kadri M, Narula S, Badr MS. Treatment of central sleep apnea in U.S. veterans. J Clin Sleep Med. 2012;8:555–563. doi: 10.5664/jcsm.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen CL, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Kapur V, Rueschman M, Zee P, Redline S. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–767. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: analysis of the nationwide inpatient sample. Obes Surg. 2013;23:1842–1851. doi: 10.1007/s11695-013-0991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mokhlesi B, Hovda MD, Vekhter B, Arora VM, Chung F, Meltzer DO. Sleep-disordered breathing and postoperative outcomes after elective surgery: analysis of the nationwide inpatient sample. Chest. 2013;144:903–914. doi: 10.1378/chest.12-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nieuwenhuijs D, Bruce J, Drummond GB, Warren PM, Wraith PK, Dahan A. Ventilatory responses after major surgery and high dependency care. Br J Anaesth. 2012;108:864–871. doi: 10.1093/bja/aes017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niesters M, Mahajan R, Olofsen E, Boom M, Garcia Del Valle S, Aarts L, Dahan A. Validation of a novel respiratory rate monitor based on exhaled humidity. Br J Anaesth. 2012;109:981–989. doi: 10.1093/bja/aes275. [DOI] [PubMed] [Google Scholar]
- 62.Bronzino JD, Kelly ML, Cordova C, Gudz M, Oley N, Stern WC, Morgane PJ. Amplitude and spectral quantification of the effects of morphine on the cortical EEG of the rat. Electroencephalogr Clin Neurophysiol. 1982;53:14–26. doi: 10.1016/0013-4694(82)90102-x. [DOI] [PubMed] [Google Scholar]
- 63.Wang Q, Yue XF, Qu WM, Tan R, Zheng P, Urade Y, Huang ZL. Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats. Neuropsychopharmacology. 2013;38:791–801. doi: 10.1038/npp.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kay DC, Pickworth WB, Neidert GL, Falcone D, Fishman PM, Othmer E. Opioid effects on computer-derived sleep and EEG parameters in nondependent human addicts. Sleep. 1979;2:175–191. doi: 10.1093/sleep/2.2.175. [DOI] [PubMed] [Google Scholar]
- 65.Zuo YF, Wang JY, Chen JH, Qiao ZM, Han JS, Cui CL, Luo F. A comparison between spontaneous electroencephalographic activities induced by morphine and morphine-related environment in rats. Brain Res. 2007;1136:88–101. doi: 10.1016/j.brainres.2006.11.099. [DOI] [PubMed] [Google Scholar]
- 66.Mayo-Michelson L, Young GA. Genetic profiles of morphine-induced EEG, EEG power spectra, and behavior in two inbred rat strains. Brain Res Bull. 1993;30:79–84. doi: 10.1016/0361-9230(93)90041-9. [DOI] [PubMed] [Google Scholar]
- 67.Kastin AJ, Pearson MA, Banks WA. EEG evidence that morphine and an enkephalin analog cross the blood-brain barrier. Pharmacol Biochem Behav. 1991;40:771–774. doi: 10.1016/0091-3057(91)90084-f. [DOI] [PubMed] [Google Scholar]
- 68.Greenwald MK, Roehrs TA. Mu-opioid self-administration vs passive administration in heroin abusers produces differential EEG activation. Neuropsychopharmacology. 2005;30:212–221. doi: 10.1038/sj.npp.1300596. [DOI] [PubMed] [Google Scholar]
- 69.Greco MA, Fuller PM, Jhou TC, Martin-Schild S, Zadina JE, Hu Z, Shiromani P, Lu J. Opioidergic projections to sleep-active neurons in the ventrolateral preoptic nucleus. Brain Res. 2008;1245:96–107. doi: 10.1016/j.brainres.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]


