Abstract
Controlled drug delivery systems have been successful in introducing improved formulations for better use of existing drugs and novel delivery of biologicals. The initial success of producing many oral products and some injectable depot formulations, however, reached a plateau, and the progress over the last three decades has been slow. This is likely due to the difficulties of formulating hydrophilic, high molecular weight drugs, such as proteins and nucleic acids, for targeting specific cells, month-long sustained delivery, and pulsatile release. Since the approaches that have served well for delivery of small molecules are not applicable to large molecules, it is time to develop new methods for biologicals. The process of developing future drug delivery systems, termed as the invention cycle, is proposed, and it starts with clearly defining the problems for developing certain formulations. Once the problems are well defined, creative imagination examines all potential options and selects the best answer and alternatives. Then, innovation takes over to generate unique solutions for developing new formulations that resolve the previously identified problems. Ultimately, the new delivery systems will have to go through a translational process to produce the final formulations for clinical use. The invention cycle also emphasizes examining the reasons for success of certain formulations, not just for the reasons for failure of many systems. Implementation of the new invention cycle requires new mechanisms of funding the younger generation of scientists and a new way of identifying their achievements, thereby releasing them from the burden of short-termism.
Keywords: Drug delivery, invention cycle, imagination, innovation, translation
Graphical abstract
1. INTRODUCTION
The field of drug delivery has advanced for more than six decades to evolve into its own scientific discipline. Advances in the drug delivery field were fast in its early days (i.e., 1950~1980) and the visible impacts were made through a very large number of new formulations introduced for clinical use, especially for oral and transdermal delivery systems. One of the biggest impacts that the new drug delivery systems have made is increasing patients’ convenience and compliance. In 1950s, most oral drugs had to be administered 3~4 times a day, requiring patients to take a drug at odd times, which is not easy to comply. Then, the introduction of the first controlled release formulation, known as the Spansule® technology1, changed all these. The new formulation required only two administrations, e.g., 8 am and 8 pm, which does not disrupt a normal daily schedule. Introduction of the Spansule technology prompted a host of new drug delivery systems, and the new field of drug delivery technologies was born. The four distinct drug delivery technologies, such as dissolution-, diffusion-, osmosis-, and ion exchange-controlled release, were developed during the first generation period, 1950~1980. In retrospect, those technologies developed during the first generation were low-hanging fruits of the drug delivery technology tree. The four basic mechanisms, however, are still used currently for producing many new once-a-day oral formulations.
Once the basic drug delivery mechanisms were identified and applied to making a large number of clinical products, formulation scientists started dealing with more difficult technologies for delivery of non-conventional drugs, such as insulin and other biopharmaceuticals, and nucleic acid drugs. In addition, the duration of drug release was extended from a day or a week to months for injectable, long-term depot formulations. The drug delivery technologies have also dealt with targeted delivery, i.e., delivery of a drug to specific target cells, implying delivery to non-target cells are minimized. These types of drug delivery technologies reside at the top of the drug delivery technology tree, and it has been difficult to find suitable delivery technologies for many drugs of clinical importance. The history of drug delivery technologies and the areas of drug formulations that need the attention of formulation scientists have been discussed previously2–5.
One of the pressing needs in the current drug delivery area is to develop technologies and formulations that can achieve the difficult tasks we have faced for the last few decades. These are found, as examples and not as an exhaustive list, in Table 1. The majority of new drug candidates are small molecular weight drugs which are also hydrophobic, belonging to Classes 2 and 4 of the Biopharmaceutics Classification System6. Many new drug candidates are practically insoluble in aqueous solution, making it difficult to develop into clinically useful formulations. A good example is the development of a clinical formulation for paclitaxel, a practically insoluble drug, requiring the use of ethanol and Cremophor EL (Taxol®)7. Delivery of hydrophilic, high molecular weight drugs, poses a different kind of challenge. Protein drugs are prone to be denatured, and controlling the initial burst release and sustained release of peptide drugs for months is still difficult. One of the holy grails in drug delivery is self-regulated insulin delivery, and even after more than three decades of research, it is still far from clinical application. Targeted delivery of an anticancer agent to tumors using nanoparticle systems has been studied intensively by scientists all around the world for the last few decades, but the initial promise has not been realized. This article focuses on how we, as formulation scientists, can improve the outcomes, i.e., translation of prototype systems in the laboratory to the formulations that can be clinically used by patients.
Table 1.
Barriers to overcome by the current DDSs. (From reference3).
| 1. | Delivery of poorly soluble drugs |
| Non-toxic excipients | |
| 2. | Peptide/protein/nucleic acid delivery |
| Control of the initial burst release and subsequent release rate | |
| Non-invasive delivery | |
| In vitro-in vivo correlation | |
| 3. | Self-regulated drug delivery |
| Functional in the body for months | |
| 4. | Targeted drug delivery |
| Targeting tumor cells with minimal delivery to normal cells | |
| Overcoming the blood-brain barrier |
2. THE INVENTION CYCLE
Formulation scientists develop various drug delivery systems. Many times, however, we focus too much on small details, thereby missing the big picture. Overcoming important technological difficulties facing us today requires a new approach starting from understanding the big picture where particular technological problems are understood in the context of the overall goals. When a drug delivery system is made, it is thoroughly characterized for its in vitro properties, including the drug release property. In vitro characterization is usually followed by small animal experiments for pharmacokinetic study before clinical trials. Without the clinical trials showing the efficacy and safety, no formulation can be approved by the Food and Drug Administration (FDA). One of the difficulties currently facing the drug delivery field is the lack of translation from seemingly promising drug delivery systems identified from in vitro and small animal studies to FDA-approved products. The drug delivery systems which are shown to be highly promising in mouse studies turned out to be inefficient in clinical trials. The current approach of developing drug delivery systems is somehow not working.
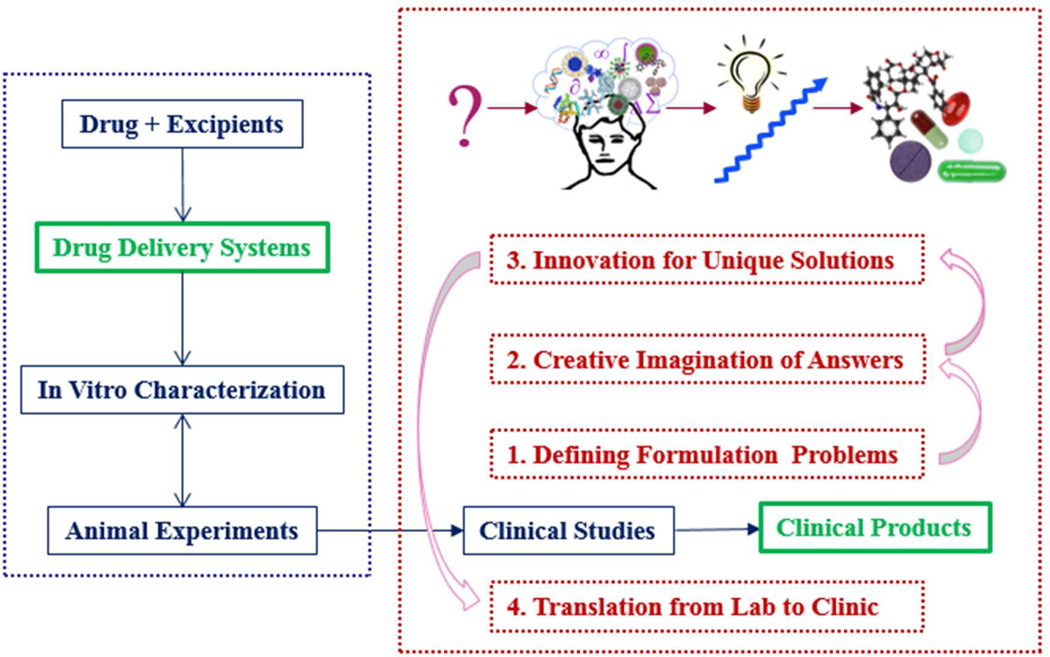
In solving any of the problems listed in Table 1 and many others, one can follow an effective pathway through the process from inspiration of developing new formulations to implementation to clinical products with a requisite set of attitudes and actions8. This process, known as the invention cycle as described by Tina Seelig8, was slightly modified to make it more relevant to developing clinically useful drug delivery systems. The overall idea of the invention cycle is described in Fig. 1. The left of Fig. 1 shows development of a drug delivery system, which is basically a mixture of a drug and excipients. The invention cycle consists of four steps: (1) defining formulation problems of bringing laboratory prototypes to the clinic; (2) creative imagination of all potential answers; (3) innovation of existing technologies for finding unique solutions; and (4) translation from the laboratory bench to the clinic for human use. Each step of the invention cycle is described in more detail in the following sections. The spine of the invention cycle in Fig. 1 is transforming drug delivery systems to clinical products, both of which are highlighted in green.
Fig. 1.
The invention cycle of developing clinically useful formulations.
2.1. Defining formulation problems of bringing laboratory prototypes to the clinic
Everything we do, whatever it is, starts from asking why? The question comes from our inherent curiosity. Humans are naturally afraid of uncertainty and ambiguity9. This may be in large part due to the survival of the species, but the curiosity has been the source of human progress. Formulation scientists have an intense curiosity to find out why seemingly promising drug delivery systems fail in clinical trials, and thus, solutions to the problems. As listed in Table 1 as examples, there is a great need to develop drug delivery systems for various important diseases. Unless we clearly define the problems, answers cannot be found. Albert Einstein said, “If I had an hour to solve a problem, I'd spend 55 minutes thinking about the problem and 5 minutes thinking about solutions”. In the drug delivery field, it seems that we have not spent enough time defining the problems facing the current challenges for developing clinically effective drug delivery systems. Our inclination is to deny the future uncertainty9, as most of our predictions of the future will turn out to be inaccurate anyway10. Thus, many of us are prone to stick to what we are familiar with or follow state-of-the-art research, even if they are not necessarily the best approach for solving the current problems. All the uncertainties of the future will be minimized, if we can identify the problems and thus, find the right answers.
A good example of the absence of clearly defined problems is the current stalemate of so-called nanotechnology-based targeted drug delivery systems. It has been very difficult in translating seemingly very promising prototype formulations for tumor targeted delivery into clinical products11. Such difficulty stems from the lack of problem identification. Almost all prototype drug delivery systems for targeted delivery failed when tested in humans12–15, and thus, the problems exist in the lack of intended functions in the human body. Yet, most research is still focused on incremental improvement in in vitro properties and small animal results. The first important step in the invention cycle is identifying the source of the problem and defining the problem clearly. For tumor targeted drug delivery, the problem exists in the lack of clear understanding of the human body reacting against the administered drug delivery systems. Yet, this problem has received only minimal attention. This may be, in part, due to the fact that most researchers in the targeted drug delivery are trained in physical chemistry or engineering, rather than physiology. Defining the problem at hand requires much more than passive collaboration among researchers in different disciplines, as described below in Section 4. Afterthoughts.
2.2. Creative imagination of the potential answers
Once we define the problems at hand, it is time to allow our creative imagination to consider all potential solutions, whether current technologies can support them or not. This is the time to maximize the benefit of harnessing the power of uncertainty by coming up with diverse, seemingly far-fetched answers. Those with a kaleidoscopic mind can break free from preconceived ideas and become creative9. None of the imagined answers have been tested or tried, and it is likely that most of them will fail. Failure, however, is a part of progress, as it will provide new information, making the next trial better. After all realistic solutions are reviewed and examined, only seemingly unrealistic solutions will remain. The seemingly implausible solutions are likely to become plausible with new technologies that are to be invented and/or with innovations of existing technologies. In the field of targeted drug delivery to tumors using nanoparticulate formulations, for example, the enhanced permeability and retention (EPR) effect has been accepted as a fact, instead of a temporary hypothesis. To date, no evidence has been found that the EPR effect actually exists in human cancer patients16. Thus, creativity here is to imagine answers that do not involve the EPR effect. Unfortunately, many researchers are still trapped in the EPR concept which was erroneously conceived. We need to move toward a more adaptive way of thinking that allows us to change our mind in the face of new evidence17.
Imagination is a mental exercise to visualize unreal, seemingly impossible, solutions to particular problems. Imagination elicits new creativity and also unleashes the potential of exploiting existing means to come up with something new. Once we imagine new answers, the steps from the current technology to the new technology become clear. The new answers here can be tested through forming mental images of the prototypes of new drug delivery systems. Imagination does not cost anything, and imagining prototypes will involve even less mental effort. The mental prototyping allows quick testing of a host of new designs, some of which can be made into real prototypes. Mental prototyping requires creativity that helps us escape from the current technology gridlock. Imagination, however, is not as easy as it sounds. Imagination requires active engagement of the mind and the ability to envision various alternative solutions that can be eventually tested experimentally to address challenges8. Starting with a solution found through creative imagination makes it easier to find real solutions. This process is known as “future back”18. It is like finding the way out through a maze by working from the end of the maze to the start, which is often far easier than the other way around. An example of this is to imagine drug delivery systems that are distributed throughout the body, instead of falsely believing that they go only to tumor cells. This allows mental prototyping of new delivery systems that can maximize the drug efficacy against tumor cells but minimize the drug effect toward normal cells. This, in turn, can lead to the design of new delivery systems that can control drug release dependent on the unique environment around tumors. Such delivery systems can also minimize the toxicity associated with the drug. The toxicity of nanoparticle drug delivery systems has not been a topic studied in depth. This is mainly due to the fact that none of the nanoparticle formulations have been effective in clinical trials, and thus, the potential toxicity has not been paid proper attention. The creative imagination step, however, can include the toxicity issue as a part of finding a solution.
2.3. Innovation of existing technologies for unique solutions
An idea is just a statement of potential solutions to a problem, while invention provides an original and useful solution to the problem19. Invention is often protected by patents. Not all patents, however, are useful in making actual products or processes. Invention presents a big solution which in itself may not be practical, but provides a fundamental understanding to problems it intends to solve, leading to various different solutions. Different solutions to slightly different problems require continuous innovation of the technology. For example, recombinant DNA technology is a new invention that made genetic engineering possible. Another example of an invention is the microprocessor. The microprocessor alone may not be useful, but it resulted in numerous useful products, processes, and products through innovation20.
Innovation is based on the existing technologies and requires focusing and reframing to generate unique solutions8. Depending on the intended use, different technologies can be combined to come up with a unique solution. Innovation comes in small steps, and incremental innovations can be made continuously. A good example is Apple’s iPhone. Combining different elements, including technologies, inventions, designs, and convenience, provides an innovative product that can change the world. In drug delivery, 3D printing of a drug delivery system21, 22 is a good example of innovation, as the technologies to make 3D printed formulations already existed, but the way the formulation is made is quite different, and hopefully it provides an alternative to the existing methods. The current nanoparticle-based formulations have not been clinically useful, but with continuous innovations, they can achieve the original goal of making tumor targeted drug delivery more effective.
For continuous innovation, it is important to keep learning. Even when the things are going well, we have to keep asking questions why things work. When causes of outcomes are not clearly understood, it is essential to keep innovating9. We tend to try to find answers only after we face failures. If we do not find the causes of our successes, the success may not be maintained, as things will be bound to change. When a new drug or a new drug delivery system works, it is critical to find out why it works, so that further innovation can be made. For example, finding out the reasons for successful development of Doxil® 16, 23 and Abraxane® 24 will help us design better drug delivery systems. Both Doxil and Abraxane formulations are relatively simple formulations, as compared with nanoparticle formulations recently developed with multiple functions and complicated structures. Thus, clinically useful formulations do not necessarily need complicated structures. Yet, it has been our inclination to make more complicated drug delivery systems with only marginal improvement, if any, over the existing formulations14. Innovation by drug delivery scientists has to be accompanied by the intended ultimate effects, i.e., treating or preventing a disease. Ensuring successful innovation requires aligning with the ultimate objectives of drug delivery. Too often, we innovate for innovation’s sake. This leads to so-called technology overshooting5, which simply makes technology more complicated without any tangible new advantages or benefits. The problems, such as cancers, diabetes, and other diseases, can be corrected only after innovation is translated into the actual products. This requires implementation, i.e., bringing innovative ideas to fruition.
2.4. Translation from laboratory bench to the clinic
Invention, innovation, and translation can be compared to a pebble tossed into the pond, the rippling effect that the pebble causes, and riding a wave formed from the ripple, respectively20. There is no textbook or standard operating procedure on how to translate ideas to fruition. Implementation is a difficult process that requires passion with persistence and an attitude that sees problems as opportunities8. Implementation of bringing new inventions and innovations to fruition is like playing golf on a difficult golf course instead of practicing golf swings on a driving range. Each shot on a golf course is different and challenging, while a driving range provides the same condition for all shots. Since the outcome of an implementation process is uncertain, one has to be prepared for an uncertain future by diversifying the efforts. Failing a drug delivery system after costly clinical trials is a major setback for everybody involved. All major disasters happened because those in charge failed to seek out more information9. Failures in clinical trials may be due to different reasons for different drug formulations, but quite often, “new” formulations fail because of unwarranted optimism bestowed to the formulations simply because they are “new”. We need to actively seek out more information and continuously ask questions, instead of falling into complacency with convenient assumptions. Once a successful formulation is developed through clinical studies, it inspires development of more formulations. In addition, it can inspire others to develop similar or better formulations that can pass the rigorous clinical studies.
3. CURRENT STATUS OF DRUG DELIVERY RESEARCH
The drug delivery area began more than six decades ago with an idea that developing new drug delivery systems for existing drugs would be more cost effective and faster than developing new drugs. It was true, at least in the beginning, that many new drug delivery systems were developed. The drug delivery research became more difficult for delivery of more complicated drugs, such as protein drugs and nucleotides, which are large in size and hydrophilic, especially for month-long delivery. New types of drugs require new delivery systems, but the progress since the 1980s has been slow. In hindsight analysis, it seems that too much attention has been focused on innovations of drug delivery systems that work only in vitro conditions or in small animal models, with a convenient assumption that it may work in humans as well. It is time to shift our focus to our ultimate goal, i.e., drug delivery systems effective in humans, rather than finding satisfaction from successes in small animal experiments.
The coin of the realm in the drug delivery field is in developing clinical products. While numerous formulation scientists have contributed to advances in the field, introduction of clinical products for non-small molecular drugs has been sporadic. Formulation scientists need to find out the causes of such slow progress. One likely cause is that the problems facing the current drug delivery field have not been clearly defined and understood. In the absence of clearly defined problems, no clear answers can be found. For the last decade or two, formulation scientists have made numerous innovations in drug delivery systems, in particular in the nanotechnology field, but they are mostly for in vitro settings and their translation to clinical products has been rare at best. The main issue is the lack of converting the laboratory design into a clinically effective formulation. This indicates that the problems facing the current drug delivery field are not known. Formulation scientists alone may not be able to clearly define the problems which arise mainly from the lack of understanding of the human body. Thus, collaboration among different disciplines, including physiology, biology, and pharmacology, is essential.
4. AFTERTHOUGHTS
Collaboration among scientists in different disciplines is easy in concept, but it remains ineffective without true dialogs among them. An expert in one field may not fully understand another field, making it difficult to define the problem and find likely answers. Thus, it is necessary to train the next generation of drug delivery scientists to understand different disciplines, as a conductor of an orchestra has experience in playing most instruments, without being great at playing all of them, to know the magic and limitations of each instrument. Changing current educational programs takes time, but it needs to be done for the future.
The current funding situation is not favorable for young scientists, especially in academia, to start their careers. The current funding rates in many countries around the globe are depressingly low. Those in charge of research funding do not seem to understand the power of the wisdom of crowds. Large groups of people, not to mention scientists, are smarter than a small group of elite, better at solving problems, fostering innovation, making intelligent decisions, and even predicting the future25. The current government funding agencies tend to promote big programs pouring in millions of dollars. When such programs do not produce intended results, they seem to pour in more research dollars. As the number of researchers increases with relatively steady level of funding, the competition becomes more intense, leading to fewer opportunities for young scientists to explore their ideas. Scientific advances occur as a result of trying many different ideas. The funding agencies should promote research projects initiated by individual investigators. It would also help if the government funding agencies do not set the scientific agenda of the future. Their track record of predicting the future of science and technology has been disappointing.
One could argue that evaluation of the outcomes of research projects is difficult and subjective. This may be true, and this is why more, smaller grants should be provided to many more investigators. Funding more investigators leads to exploring more ideas, which in turn, leads to higher probability of finding something novel. One of the difficulties the young scientists in academia are facing in building their career is the pressure of the tenure system. The tenure system is something that is designed to guarantee professors’ freedom of teaching and research. Before obtaining tenure, however, they are under serious stress of meeting certain criteria in funding and publication. This actually distracts them from doing their work, some of which may take decades to reach fruition. Again, evaluating achievements of an assistant professor without considering the number of publications and the amount of research funding may be difficult, but a new system needs to be installed to make a better environment for researchers to do their best work without worrying about the short-term productivity.
All of the above comments add uncertainty and ambiguity to the future of formulation research, and so foresight analysis will be impossible. However, the hindsight analysis of what we have done indicates that we really need to try something different. Unless we try, we will never know. This is thinking in new boxes, not thinking outside the box. Our best bet for the future of the drug delivery field is fostering the new generation of formulation scientists.
Acknowledgments
This work was supported by the Showalter Research Trust Fund and the National Institute of Health through CA129287 and GM095879.
REFERENCES
- 1.Lee PI, Li J-X. Evolution of oral controlled release dosage forms. In: Wen H, Park K, editors. Oral Controlled Release Formulation Design and Drug Delivery. Hoboken, NJ: John Wiley & Sons, Inc.; 2010. pp. 21–31. [Google Scholar]
- 2.Park K. Controlled drug delivery systems: Past forward and future back. J. Control. Release. 2014;190(0):3–8. doi: 10.1016/j.jconrel.2014.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yun YH, Byung Kook Lee, Park K. Controlled drug delivery: Historical perspective for the next generation. J. Control. Release. 2015;219:2–7. doi: 10.1016/j.jconrel.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee BK, Yun YH, Park K. Smart nanoparticles for drug delivery: Boundaries and opportunities. Chemical Engineering Science. 2015;125(0):158–164. doi: 10.1016/j.ces.2014.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park K. Drug delivery of the future: Chasing the invisible gorilla. J. Control. Release. 2016 doi: 10.1016/j.jconrel.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah VP, Amidon GL, Amidon GL, Lennernas H, Shah VP, Crison JR . A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm Res 12, 413–420, 1995—Backstory of BCS. AAPS J. 2014;16(5):894–898. doi: 10.1208/s12248-014-9620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straubinger RM. Biopharmaceutics of paclitaxel (Taxol): Formulation, activity, and pharmacokinetics. In: Suffness M, editor. TAXOL® Science and Applications. Boa Raton, FL: CRC Press; 1995. pp. 237–258. [Google Scholar]
- 8.Seelig T. Insight Out. Get Ideas Out of Your Head and Into the World. New York, NY: HarperCollins Publishers; 2015. [Google Scholar]
- 9.Holmes J. Nonsense: The Power of Not Knowing. New York, NY: Penguin Random House; 2015. p. 322. [Google Scholar]
- 10.Gardner D. Future Babble: Why Expert Predictions Fail - and Why We Believe Them Anyway. New York, NY: Penguin Group; 2011. p. 305. [Google Scholar]
- 11.Stirland DL, Nichols JW, Miura S, Bae YH. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control. Release. 2013;172(3):1045–1064. doi: 10.1016/j.jconrel.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florence AT. “Targeting” nanoparticles: The constraints of physical laws and physical barriers. Journal of Controlled Release. 2012;164(2):115–124. doi: 10.1016/j.jconrel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Florence AT. Reductionism and complexity in nanoparticle-vectored drug targeting. Journal of Controlled Release. 2012;161(2):399–402. doi: 10.1016/j.jconrel.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park K. Translation from mouse to human: Time to think in new boxes. Journal of Controlled Release. 2014;189(0):187. doi: 10.1016/j.jconrel.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Nichols JW, Bae YH. EPR: Evidence and fallacy. Journal of Controlled Release. 2014;190(0):451–464. doi: 10.1016/j.jconrel.2014.03.057. [DOI] [PubMed] [Google Scholar]
- 17.Pittampalli A. Persuadable. How Great Leaders Change Their Minds to Change the World. New York, NY: HarperCollins Publishers; 2016. p. 242. [Google Scholar]
- 18.Fisk P. Creative Genius: An Innovation Guide for Business Leaders, Border Crossers and Game Changers. United Kingdom: Capstone Publishing Ltd. (a Wiley Company): Chichester, West Sussex; 2011. p. 386. [Google Scholar]
- 19.Zimmer E. Ideas vs. inventions. http://tenonline.org/art/9010.html.
- 20.Grasty T. The difference between ‘invention’ and ‘innovation’. 2012 [Google Scholar]
- 21.Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. Journal of Controlled Release. 2015;217:308–314. doi: 10.1016/j.jconrel.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Park K. 3D printing of 5-drug polypill. Journal of Controlled Release. 2015;217:352. doi: 10.1016/j.jconrel.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Barenholz Y. DoxilR - The first FDA-approved nano-drug: Lessons learned. J. Control. Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Yardley DA. nab-Paclitaxel mechanisms of action and delivery. Journal of Controlled Release. 2013;170(3):365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Surowiecki J. The Wisdom of Crowds. New York, NY: Anchor Books; 2005. p. 306. [Google Scholar]