Abstract
Background
This study was proposed to compare the efficacy and safety of GTM-1, Rapamycin (Rap), and Carbamazepine (CBZ) in managing Alzheimer disease (AD). The impact of the above mentioned therapeutic drugs on autophagy was also investigated in our study.
Material/Methods
Firstly, 3×Tg AD mice were randomly allocated into 4 groups (each group with 10 mice), in which AD mice were separately treated with dimethylsulfoxide (DMSO, vehicle group), GTM-1 (6 mg/kg), Rap (1 mg/kg), and CBZ (100 mg/kg). Then spatial memory and learning ability of mice was tested using the Morris water maze. Routine blood tests were performed to evaluate the toxicity of these drugs. Amyloid-β42 (Aβ42) concentration was detected by ELISA and immunohistochemistry. Proteins related to autophagy were detected by Western blot.
Results
GTM-1, Rap, and CBZ significantly improved the spatial memory of 3×Tg AD mice compared to that in the vehicle group (all P<0.05). Moreover, this study revealed that CBZ dosage was related to toxicity in mice. All of the above drugs significantly increased the expression of LC3-II and reduced Aβ42 levels in hippocampi of 3×Tg AD mice (all P<0.05). On the other hand, neither GTM-1 nor CBZ had significant influence on the expression of proteins on the mTOR pathway.
Conclusions
GTM-1 can alleviate the AD syndrome by activating autophagy in a manner that is dependent on the mTOR pathway and it therefore can be considered as an alternative to Rap.
MeSH Keywords: Alzheimer Disease, Autophagy, Carbamazepine, TOR Serine-Threonine Kinases
Background
Alzheimer disease (AD), a common age-related neurodegenerative disorder, has affected a large number of elderly people around the world [1]. Since managing AD is costly and time-consuming, this disease has been considered a major challenge in both developing and developed countries [2]. AD has a number of different manifestations: cognitive impairment; disabilities; mood disturbance; and behavioral and psychological abnormalities like aggression, agitation, combativeness, delusions, hallucinations, and depression [3,4]. The above manifestations dramatically undermine quality of life, impose huge burdens on the health care system, and even contribute to earlier admissions to nursing homes [3]. The number of patients with AD is estimated to rise to approximately 9.2 million by 2050 [5]. AD is characterized by a gradual decline in cognitive functions, and brains of AD patients are scattered with fibrillogenic amyloid-β peptide (Aβ) oligomers [6]. Amyloid plaque is composed of Aβ and it is a critical pathological hallmark of AD [7]. However, standard pharmacotherapies that are able to persistently manage and control the progression of AD are still not available.
Autophagy, which consists of microautophagy, chaperon-mediated autophagy, and macroautophagy, is a key pathway related to the degradation of long-lived proteins [8]. Autophagy is essential to neuronal homeostasis and it works on pathways that are either dependent or independent on mammalian target of rapamycin (mTOR) [9,10]. Defects in autophagy functions are likely to trigger excessive accumulation of proteins in the brain [11]. Emerging evidence has demonstrated the role of autophagy in many different neurodegenerative disorders, including AD [12] and the association between autophagy and AD pathology has been shown by researchers [13]. For instance, pathologically autophagic vacuole accumulation and macroautophagic induction were discovered in AD patients and animal models [14]. Autophagy-lysosome defects, which have been suggested as a significant contributor to the progression AD, usually appear at the early stage of this disease [12]. Therefore, autophagy may be targeted for developing new AD therapies [9].
Furthermore, some compounds that induce autophagy may be promising targets for managing AD through decreasing the level of Aβ. Recently, researchers have concentrated on autophagy modulation using pharmaceuticals such as GTM-1 together with rapamycin (Rap), autophagy inducer, and autophagy enhancer [15,16]. Carbamazepine (CBZ) is another effective compound for managing AD since it is able to induce autophagy and protect against neurodegeneration in vivo [9]. CBZ is a typical anti-epileptic drug that has vigorous enhancement effects on autophagy [17]. As suggested by recent studies, CBZ is likely to eliminate autophagic substrates through increasing autophagic flux, which is significantly altered in AD patients [17]. A liver disease model constructed in mice revealed that CBZ could reduce hepatic fibrosis and hepatic load of mutant α1-antitrypsin Z by enhancing autophagic flux [11]. Moreover, CBZ can significantly decrease abnormal protein aggregation and toxicity [18], and long-term treatment of CBZ may protect mice against AD since autophagic flux was enhanced [17]. On the other hand, GTM-1 has dual functions: antagonism against the toxicity of Aβ-oligomer and autophagy induction. Additionally, GTM-1 contributes to rapid and efficient autophagy in neurons and is therefore believed to be an effective therapeutic strategy for AD [15]. As suggested by transgenic animal models, rapamycin (Rap) is an autophagy activator that may mitigate Aβ neuropathology and cognitive impairment caused by AD [17,19].
Recently, there is a trend toward managing AD assisted by autophagy regulation, and relevant data has confirmed the effectiveness of Rap, CBZ, and GTM-1 in alleviating manifestations of AD. However, the effectiveness of GTM-1 has not been confirmed because the mechanism by which it impedes the progression of AD is not clear. Thus, our study compared the efficacy and safety of Rap, CBZ, and GTM-1 in AD mice and investigated the effects of these drugs on autophagy. As a result, our study not only provides a key reference for identifying the optimal therapeutic drug for managing AD, but also shows the role of autophagy in AD.
Material and Methods
Mice and drug administration
We purchased 3×Tg AD (AD) mice from the Animal Experiment Center of Changhai Hospital, Second Military Medical University. Mice were housed in cages and each cage contained 4–5 mice. All mice were raised based on a cycle of 12 h light/12 h dark and they had ad libitum access to food and water during the treatment period. Administration of therapeutic drugs was performed when mice were 6 months of age. Each experiment was carried out based on equal numbers of male and female mice. All experimental procedures in this study complied with the guidelines provided by Changhai Hospital, Second Military Medical University and they were approved by the Institute of Animal Care. CBZ (Sigma, USA) was given to 3×Tg AD mice through daily oral administration at 100 mg/kg for 2 months. GTM-1 (6 mg/kg) was given to 3×Tg AD mice daily for 2 months, and oral Rap (1 mg/kg) (Biochempartner, Shanghai) was administrated daily for 2 months. Mice were divided into 4 groups with equal size (n=10): Vehicle group (treated with DMSO), GTM-1 group (treated with GTM-1), Rap group (treated with Rap), and CBZ group (treated with CBZ).
Morris water maze test
The Morris water maze test was carried out after treatment had been administered for 1 month. This test was implemented using an apparatus including a circular plastic tank with diameter of 1.2 m and height of 0.5 m (RWD, China). A camera was used for recording movements of mice and relevant results were automatically transferred to a computer in which the SMART 2.0 software system was used for analysis. Water temperature was maintained at 21±1°C during the experiment. A platform with diameter of 6 cm was placed 1.5 cm below the water surface and nontoxic white paint was added to make the water opaque. The Morris water maze was divided into 4 quadrants, which were marked as N (North), S (South), E (East), and W (West). S represented the location of the mice and the opposite of S was represented by N. E represented the right of the mice, whereas the left region of S was represented by W. A platform was placed in the middle of the SW quadrant. The entry point was set to equalize the distance between the initial position and the platform. Mice were trained to swim 4 times per day for 5 days. Mice were randomly placed at different entry positions in the water. Mice were placed on the platform for 15 s when they identified the platform within 120 s. Mice were wiped dry and placed in a warm cage after the training procedure was completed. Time spent identifying the platform was considered as the escape latency and recorded over the 5-day period. Swimming speed and track were recorded by the system. After the 24-h hidden platform training, the platform was removed and mice were placed in positions opposite the platform quadrant for spatial memory assessment.
Protein extraction
Mice were firstly anesthetized with 10% chloral hydrate and were killed by transcardial perfusion using cold PBS. Brains were separated from mice for future studies and half of the brain was conserved in 4% paraformaldehyde. Hippocampi were homogenized in T-PER buffer using a homogenizer (Thermo Scientific). According to the protocol, samples were centrifuged at 25 000 g and at 4°C for 30 min. Supernatant was collected as the soluble fraction for Western blot and ELISA detection, while the remaining part was homogenized in 70% formic acid and centrifuged again, according to the above instructions. After that, the acquired supernatant was considered as an insoluble fraction for ELISA.
Routine blood analysis and chemical detection
Both routine blood examination and chemical detection was performed to evaluate drug efficacy and safety reflected by the circulation, kidney, and liver system. Supernatants of blood samples obtained by centrifuging were collected for chemical testing for chemical indexes, including the concentration of white blood cells, red blood cells, hemoglobin, platelets, glutamic-pyruvic transaminase, glutamic oxalacetic transaminase, blood urea nitrogen, Cr, and blood glucose.
Western blot and ELISA
Protein collected from soluble fraction was denatured in boiling water and transferred onto polyvinylidene fluoride (PVDF) membranes once the procedure of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was completed. Membranes were washed in Tris-Buffered Saline Tween (TBST) added with 5% skim milk for 1 h and then treated with primary antibodies against mTOR (1:1000), p-mTOR (1:1000), total P70S6K (1:1000), and p-P70S6K (1:1000) at 4°C overnight. Membranes were incubated with secondary antibodies once they were completely washed. Samples together with reduced Actin, which was set as the endogenous control, were ultimately processed with enhanced chemiluminescence and quantified by Lab Works4.5 software (Mitov Software). The ELISA experiment was conducted based on the manufacturer’s protocol for detecting the expression levels of soluble and insoluble Aβ42.
Immunohistochemistry
Mice were killed when the Morris water maze test was completed. Paraffin-embedded tissue sections of 50-μm were obtained using the Leica Vibratome slicing system and stained based on the protocol. Each tissue slice was stored at 4°C and 3% H2O2 was incubated with the sections at 25°C for 30 min so that endogenous peroxidase activities can be quenched. Then sections were separately washed by TBS-A and TBS-B for 15 min and 30 min to block unspecific bindings. Next, sections were incubated with the corresponding primary anti-Aβ antibody (Covance, USA) at 4°C overnight. Then, redundant antibodies were washed and an appropriate second antibody was used for 1-h incubation at 25°C. After that, slices were incubated with second antibodies labeled by horseradish peroxidase (HRP). Finally, the avidin-biotin horseradish peroxidase system was used to develop sections with diaminobenzidine (DAB) substrate and images were analyzed by the ImageJ software.
Statistical analysis
All statistical analyses were performed with SPSS 18.0 software (Chicago, IL). Data are shown as mean ± standard deviation (SD). Categorical data were analyzed using the chi-square test. The 2-tailed t test or 1-way analysis of variance (ANOVA) was used to compare differences between or among groups and P<0.05 provided evidence for statistical significance.
Results
Toxic effects of drugs reflected by animal models
Effects of GTM-1, Rap, and CBZ on physiological indexes of 3×Tg AD mice are shown in Table 1. Indexes in the GTM-1 or Rap group obtained from the routine blood test and biochemical test were not significantly different from those in the vehicle group (all P>0.05), suggesting that the dosages of GTM-1 or Rap were not significantly associated with toxic effects. However, the amount of WBC (white blood cells), RBC (red blood cells), and PLT (platelets) in the CBZ group were significantly decreased in comparison to those in the vehicle group (all P<0.05). Compared to the vehicle group, BUN (blood urea nitrogen) levels in mice treated with CBZ were substantially increased (P<0.05). Although ALT (alanine transaminase) and AST (aspartate transaminase) levels in the CBZ group were slightly higher compared to those in other groups, these differences appeared to be insignificant. Therefore, we concluded that the dosage of CBZ had toxic effects on 3×Tg AD mice.
Table 1.
The physiological characteristics of each group.
| Index | Vehicle | DTM-1 | Rapamycin | Carbamazepine |
|---|---|---|---|---|
| Blood routine test | ||||
| WBC (×109/L) | 0.54±0.16 | 0.61±0.21 | 0.65±0.12 | 0.31±0.14* |
| RBC (×1012/L) | 10.5±1.2 | 10.92±0.68 | 10.75±1.54 | 7.8±0.54* |
| HGB (g/L) | 155.7±9.56 | 158.7±12.8 | 162.2±10.18 | 150.7±13.3 |
| PLT (×109/L) | 955.4±175.8 | 942.6±151.2 | 986.2±139.8 | 712.8±193.6* |
| Biochemical test | ||||
| ALT (IU/L) | 37.5±8.6 | 35.2±9.2 | 41.3±5.0 | 47.4±9.7 |
| AST (IU/L) | 132.5±25.7 | 138.7±15.4 | 133.1±22.5 | 153.2±20.5 |
| BUN (mmol/L) | 9.4±1.5 | 9.8±1.5 | 9.3±1.3 | 13.2±2.2* |
| Cr (μmol/L) | 36.2±7.1 | 38.4±5.9 | 37.6±6.2 | 42.8±7.5 |
| GLU (mmol/L) | 10.3±2.5 | 9.5±2.8 | 10.6±3.1 | 8.7±3.6 |
Represents P<0.05.
Effects of drugs on spatial memory of 3×Tg AD mice
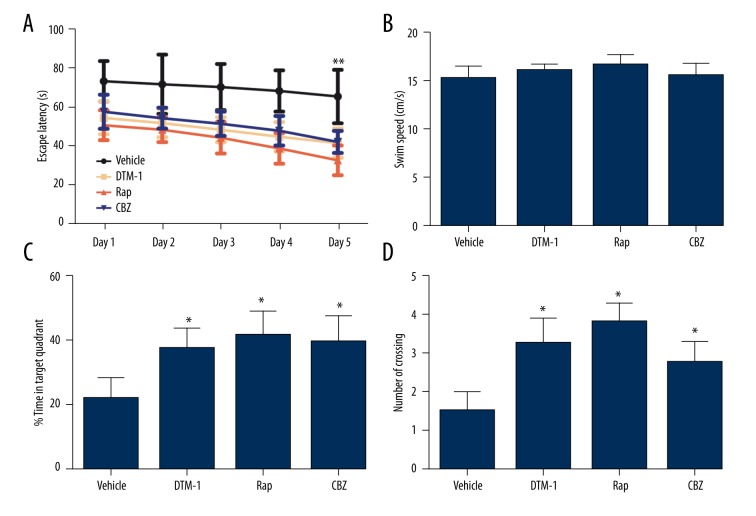
As shown in Figure 1A, GTM-1, Rap, and CBZ significantly deceased the escape latency of 3×Tg AD mice on day 5 compared to that in the vehicle group (all P<0.001), while such decline appeared to be more significant in the Rap group than those in the GRM-1 and CBZ group (all P>0.05, Figure 1B). In contrast, swimming speed did not differ significantly among these groups (all P>0.05, Figure 1B). The percentage of time in target quadrants and the number of platform crossings by mice in the GTM-1, Rap, and CBZ group were significantly higher than those in the vehicle group (all P<0.05, Figure 1C, 1D). Therefore, GTM-1, Rap, and CBZ significantly improved the spatial learning ability of 3×Tg AD mice.
Figure 1.
Spatial learning ability and behavior pattern of 3×Tg mice. GTM-1, Rap, and CBZ can significantly restore the spatial learning and memory deficits assessed by the Morris water maze test. (A) Latency in seconds for finding the hidden platform over the 5-day period test. (B) Swim speed of mice in each group did not change significantly. (C–D) The percentage of time in target quadrant and the number of crossing were significantly improved by GTM-1, Rap, and CBZ compared with the vehicle group. * Represents P<0.05, ** represents P<0.001.
Drug suppressed Aβ levels in brain
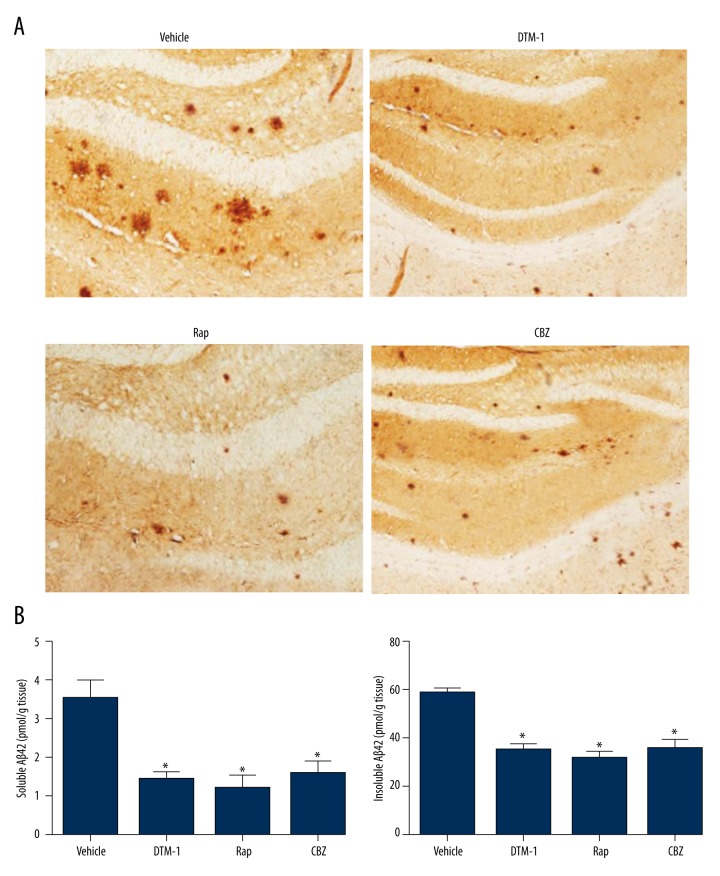
Immunohistochemistry and ELISA were carried out to determine whether GTM-1, Rap, and CBZ are able to affect Aβ levels in the brain so that the effectiveness of these drugs can be assessed. As suggested by immunohistochemistry, GTM-1, Rap, and CBZ significantly reduced the amount of amyloid plaques in hippocampus of mice compared to the vehicle group (Figure 2A). Furthermore, both soluble and insoluble Aβ42 levels were significantly lower in hippocampi of mice treated with GTM-1/Rap/CBZ when compared with the vehicle group (all P<0.05, Figure 2B).
Figure 2.
Treatments of GTM-1, Rap, and CBZ reduced the level of Aβ42 in 3×Tg mice. (A) Representative images of Aβ-stained brain sections. (B) Soluble and insoluble Aβ42 levels in hippocampi were detected by ELISA. * Represents P<0.05.
Comparing effects of therapeutic drugs on regulating the autophagy process
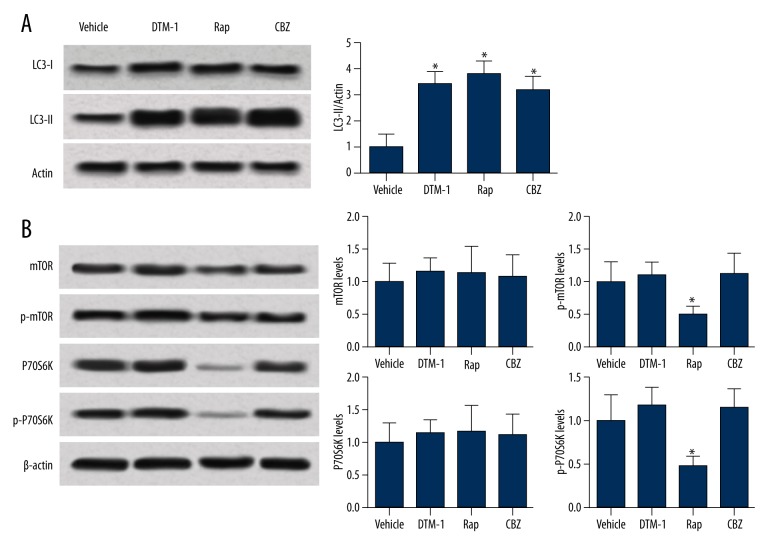
It has been reported that GTM-1, Rap, and CBZ can improve Aβ pathology through regulating the autophagy process, while such effects may differ among different therapeutic drugs. As suggested by Figure 3A, GTM-1, Rap, and CBZ up-regulated the expression level of LC3-II and therefore were able to stimulate autophagy in hippocampi of 3×Tg AD mice. Interestingly, Rap regulated neuron autophagy through suppressing the mTOR pathway as well as significantly down-regulating the expression levels of p-mTOR and p-P70S6K (all P<0.05, Figure 3B). On the other hand, both GTM-1 and CBZ affect autophagy in a manner that is independent on mTOR. No significant changes in protein expressions on the mTOR pathway were identified in the 2 groups compared with the vehicle group (all P>0.05, Figure 3B).
Figure 3.
Protein expression levels involved in autophagy were detected by Western blot. (A) Representative expressions of LC3-II in the 4 groups. GTM-1, Rap, and CBZ up-regulated the expression of LC3-II compared to the vehicle group. (B) Protein levels of autophagy, which are dependent on the mTOR pathway. Treatment of GTM-1 or CBZ did not significantly affect protein expressions on the mTOR pathway. * Represents P<0.05.
Discussion
This study used an AD mice model to explore the impacts of GTM-1, Rap, and CBZ on autophagy and then evaluated the toxicity of these therapeutic drugs. Our study proved that Rap, CBZ, and GTM-1 significantly improve the spatial learning and memory capacity of AD mice. The above results were consistent with those obtained from previous studies [15,17,20]. Histopathologically, these interventions all activate autophagy and reduce amyloid β (Aβ) levels in the hippocampus region. Although no significant difference was identified between the treatment and vehicle groups, both Rap and GTM-1 appeared to be slightly more effective than CBZ in eliminating Aβ and impeding the progression of AD.
AD is the most common form of dementia among the elderly, who usually experience progressive memory decline and other cognitive impairment [21]. A growing number of studies have demonstrated that autophagy is able to degrade the aggregation and deposition of misfolded peptides or proteins, including the Aβ peptide and the hyperphosphorylated tau protein [22]. It was also proposed that stimulating the autophagic flux has a positive role in Aβ neuropathology and memory amelioration [6,23–25]. Furthermore, mammalian target of rapamycin (mTOR) can suppress the autophagic effect on neurons, and Rap is able to inhibit the mTOR pathway [26]. Another study suggested that anti-epileptic drugs such as CBZ have a potent effect on autophagy flux enhancement, which is able to alleviate AD symptoms, as confirmed by an in vivo experiment in which APPswe/PS1deltaE9 Tg AD mouse models were built over a 3-month CBZ treatment period [17]. As recommended by a recent study, GTM-1 is a new autophagy inducer, which is able to antagonize Aβ deposition, and its positive effects on cognitive deficits have been validated [15].
The mTOR pathway is essential to protein homeostasis and most conventional AD therapies are aimed at inhibiting the mTOR pathway so that autophagy function is stimulated and aggregation of Aβ deposition can be impeded [27]. We also discovered that both CBZ and GTM-1 exerted their autophagic effects in a manner that is independent of the mTOR pathway, while Rap significantly down-regulated the level of p-mTOR and p-P70S6K. However, positive effects of Rap on Aβ oligomer degradation have not been confirmed in elderly AD mice4[6]. Since many other pathways are likely to regulate autophagy, we suspected that CBZ may exert its role in autophagy enhancement in a calpain-mediated way [28]. It was also suspected that CBZ acts as a novel molecular corrector to restore ATP-sensitive potassium (KATP) channels among congenital hyperinsulinism patients [29]. Moreover, CBZ was effective for patients with α1-antitrypsin (AT) deficiency since it decreased both ATZ hepatic load and hepatic fibrosis in mice with congenital liver diseases [11].
Another Mycobacterium tuberculosis (MTB) study in vivo revealed that CBZ was able to stimulate autophagy and its autophagic effects were independent of the mTOR pathway under the charge of cellular depletion of myo-inositol [30]. The efficiency of autophagy was unexpectedly enhanced by GTM-1 through both asparagine and thapsigargin, which eventually contributes to neuroprotection. As suggested by models constructed in 15-month-old AD mice, GTM-1 reduced Aβ deposition and improved the learning ability, as well as memory capacity, of these mice [15].
This study compared autophagic effects of several therapeutic drugs, including CBZ, Rap, and GTM-1. All of these therapeutic drugs exhibited positive effects on Aβ reduction and on alleviating AD symptoms. Nevertheless, a recent meta-analysis indicated that mood stabilizers such as CBZ and Rap were not effective but rather are harmful to AD patients [31]. More importantly, several issues in this study should be addressed by future researchers. For instance, exploratory experiments on topics including optimal dosage, potential molecular pathways of autophagy, and the effectiveness of combined use of therapeutic drugs should be encouraged in the future.
Conclusions
Our study provided solid evidence that: (1) GTM-1, Rap, and CBZ can alleviate AD symptoms and the amount of amyloid plaques; (2) CBZ and GTM-1 exhibited autophagic effects, which were independent on the mTOR pathway; (3) CBZ was associated with a certain degree of toxicity, which was proportional to its dosage. As a result, GTM-1 could be considered as an AD treatment that is free from toxicity and its autophagic effects were triggered through a non-conventional pathway. Hence, GTM-1 may replace Rap therapy due to its potent effectiveness and insignificant toxicity, particularly for AD patients who are sensitive to Rap therapies.
Footnotes
Source of support: National Natural Science Foundation (81402819) and the Scientific Foundation of Shanghai China (13ZR1408500)
References
- 1.Verdile G, Keane KN, Cruzat VF, et al. Inflammation and oxidative stress: The molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm. 2015;2015:105828. doi: 10.1155/2015/105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sykora P, Misiak M, Wang Y, et al. DNA polymerase beta deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes. Nucleic Acids Res. 2015;43:943–59. doi: 10.1093/nar/gku1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koda R, Aoyagi R, Okazaki E, et al. Acute tubulointerstitial nephritis with multiple organ involvement including fatal adrenalitis: A case report with autopsy findings. Intern Med. 2012;51:2917–22. doi: 10.2169/internalmedicine.51.8344. [DOI] [PubMed] [Google Scholar]
- 4.Vaitkevicius A, Kaubrys G, Audronyte E. Distinctive effect of donepezil treatment on P300 and N200 subcomponents of auditory event-related evoked potentials in Alzheimer disease patients. Med Sci Monit. 2015;21:1920–27. doi: 10.12659/MSM.894940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 6.Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6:e25416. doi: 10.1371/journal.pone.0025416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez MJ, Quintanilla RA. Therapeutic actions of the thiazolidinediones in Alzheimer’s disease. PPAR Res. 2015;2015:957248. doi: 10.1155/2015/957248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glick D, Barth S, Macleod KF. Autophagy: Cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu XC, Yu JT, Jiang T, Tan L. Autophagy modulation for Alzheimer’s disease therapy. Mol Neurobiol. 2013;48:702–14. doi: 10.1007/s12035-013-8457-z. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Guo X, Shen X, Kream RM, et al. Vascular dysfunction associated with type 2 diabetes and Alzheimer’s disease: A potential etiological linkage. Med Sci Monit Basic Res. 2014;20:118–29. doi: 10.12659/MSMBR.891278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hidvegi T, Ewing M, Hale P, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–32. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 12.Zare-Shahabadi A, Masliah E, Johnson GV, Rezaei N. Autophagy in Alzheimer’s disease. Rev Neurosci. 2015;26:385–95. doi: 10.1515/revneuro-2014-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Yu WH, Kumar A, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–58. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu WH, Cuervo AM, Kumar A, et al. Macroautophagy – a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu C, Zhang X, Ma W, et al. Induction of autophagy by a novel small molecule improves abeta pathology and ameliorates cognitive deficits. PLoS One. 2013;8:e65367. doi: 10.1371/journal.pone.0065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong JK, Moon MH, Lee YJ, et al. Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging. 2013;34:146–56. doi: 10.1016/j.neurobiolaging.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Zhang S, Zhang X, et al. Autophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-beta pathology in a mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2013;10:433–41. doi: 10.2174/1567205011310040008. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, Floto RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–11. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue Z, Guo Y, Zhang S, et al. Beta-asarone attenuates amyloid beta-induced autophagy via Akt/mTOR pathway in PC12 cells. Eur J Pharmacol. 2014;741:195–204. doi: 10.1016/j.ejphar.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Xue Z, Zhang S, Huang L, et al. Increased expression of Beclin-1-dependent autophagy protects against beta-amyloid-induced cell injury in PC12 cells [corrected] J Mol Neurosci. 2013;51:180–86. doi: 10.1007/s12031-013-9974-y. [DOI] [PubMed] [Google Scholar]
- 21.Peric A, Annaert W. Early etiology of Alzheimer’s disease: Tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol. 2015;129:363–81. doi: 10.1007/s00401-014-1379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan CC, Yu JT, Tan MS, et al. Autophagy in aging and neurodegenerative diseases: Implications for pathogenesis and therapy. Neurobiol Aging. 2014;35:941–57. doi: 10.1016/j.neurobiolaging.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Caccamo A, Majumder S, Richardson A, et al. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: Effects on cognitive impairments. J Biol Chem. 2010;285:13107–20. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang DS, Stavrides P, Mohan PS, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258–77. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 27.Cai Z, Zhao B, Li K, et al. Mammalian target of rapamycin: A valid therapeutic target through the autophagy pathway for Alzheimer’s disease? J Neurosci Res. 2012;90:1105–18. doi: 10.1002/jnr.23011. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Wang JH, Biel TG, et al. Carbamazepine suppresses calpain-mediated autophagy impairment after ischemia/reperfusion in mouse livers. Toxicol Appl Pharmacol. 2013;273:600–10. doi: 10.1016/j.taap.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen PC, Olson EM, Zhou Q, et al. Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J Biol Chem. 2013;288:20942–54. doi: 10.1074/jbc.M113.470948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiebler M, Brown K, Hegyi K, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med. 2015;7:127–39. doi: 10.15252/emmm.201404137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao H, Su Y, Cao X, et al. A meta-analysis of mood stabilizers for Alzheimer’s disease. J Huazhong Univ Sci Technolog Med Sci. 2010;30:652–58. doi: 10.1007/s11596-010-0559-5. [DOI] [PubMed] [Google Scholar]