Abstract
Radiation-induced gastrointestinal syndrome, including nausea, diarrhea and dehydration, contributes to morbidity and mortality after medical or industrial radiation exposure. No safe and effective radiation countermeasure has been approved for clinical therapy. In this study, we aimed to investigate the potential protective effects of seabuckthorn pulp and seed oils against radiation-induced acute intestinal injury. C57/BL6 mice were orally administered seabuckthorn pulp oil, seed oil and control olive oil once per day for 7 days before exposure to total-body X-ray irradiation of 7.5 Gy. Terminal deoxynucleotidyl transferase dUTP nick end labeling, quantitative real-time polymerase chain reaction and western blotting were used for the measurement of apoptotic cells and proteins, inflammation factors and mitogen-activated protein (MAP) kinases. Seabuckthorn oil pretreatment increased the post-radiation survival rate and reduced the damage area of the small intestine villi. Both the pulp and seed oil treatment significantly decreased the apoptotic cell numbers and cleaved caspase 3 expression. Seabuckthorn oil downregulated the mRNA level of inflammatory factors, including tumor necrosis factor-α, interleukin (IL)-1β, IL-6 and IL-8. Both the pulp and seed oils elevated the level of phosphorylated extracellular-signal-regulated kinase and reduced the levels of phosphorylated c-Jun N-terminal kinase and p38. Palmitoleic acid (PLA) and alpha linolenic acid (ALA) are the predominant components of pulp oil and seed oil, respectively. Pretreatment with PLA and ALA increased the post-radiation survival time. In conclusion, seabuckthorn pulp and seed oils protect against mouse intestinal injury from high-dose radiation by reducing cell apoptosis and inflammation. ALA and PLA are promising natural radiation countermeasure candidates.
Keywords: seabuckthorn, radiation injury, apoptosis, inflammation
INTRODUCTION
Radiation therapy is widely used for clinical cancer treatment, especially in squamous cell carcinoma. Technological innovation has enabled the delivery of localized radiation to virtually any part of the body. However, normal tissue toxicity remains the most important dose-limiting factor in clinical radiation therapy. The biological effects of radiation on mammalian organisms are dose dependent and cumulative. Hematopoietic and gastrointestinal injuries are most commonly involved in large- or low-dose ionizing radiation [1]. The intestine is an important dose-limiting organ during the radiation therapy of abdominal and pelvic tumors. Recently, because of improvements in supportive care and stem cell rescue, the intestine is emerging as a critical organ in the determination of survival after radiation exposure from accidents, acts of war or civilian terrorism [2]. Morbidity and mortality from radiation-induced gastrointestinal syndrome occur slowly and are more amenable to radiation countermeasures.
Although efforts to identify suitable radiation countermeasures have persisted for more than 50 years, no safe and effective radiation countermeasure for acute radiation syndrome or tumor radiation therapy has yet been approved by the United States Food and Drug Administration (FDA). Thus, there is a pressing need for radiation countermeasures. The major themes of current countermeasure development have focused on free radical scavengers and the stimulation of hematopoietic progenitors. Other therapeutic avenues are also being explored, such as enhancing DNA repair or blocking cell death pathways.
Seabuckthorn (Hippophae rhamnoides L.), a natural herb, has been exploited extensively in Chinese, Tibetan and Mongolian traditional medicine for the treatment of gastric ulcer, circulatory disorders, hepatic injury, and neoplasia [3–5]. Ethanol extracts from seabuckthorn berries (named RH-3) also provide protection against radiation-induced lethality in mice and have free-radical scavenging activity [6]. Modulation of the antioxidant defense system has been suggested as the basis for their radioprotective activity [6, 7]. RH-3 has also been reported to physically interact with cellular chromatin, stabilize it, and thus possibly protect the DNA from radiation-induced damage [4, 8]. Seabuckthorn seed oil protects mice against carbon tetrachloride–induced hepatotoxicity and high-fat diet–induced atherosclerosis [9, 10]. However, the effect of seabuckthorn oil on radiation protection has not been elucidated.
In this study, we explored the potential effects of seabuckthorn oil on protecting against acute intestinal injury from high-dose radiation, possibly providing some novel evidence concerning radiation countermeasure development from this natural herb.
MATERIALS AND METHODS
Mice maintenance and treatment
Male 8-week-old C57/BL6 mice were purchased from the Model Animal Research Institute of Nanjing University and housed at five mice per cage. Mice were divided into the radiation and sham groups, and every group was divided into three subgroups to receive a 10-mg/g/day dose of seabuckthorn pulp oil, seed oil (supplied by Qinghai Tsinghua Biotry Bio-Tech Co., Ltd) or control olive oil at 8 am, once per day for 7 days before radiation; saline-treated mice were used as a non-oil control group. In addition, a 7-day pretreatment with a 5 mg/g/day dose of palmitoleic acid (PLA) or alpha linolenic acid (ALA) was used for survival experiments. All experiments were approved by the Animal Care and Use Committee of Nanjing Medical University (Ethics number: 20150112). The mice were subjected to one exposure of total-body X-ray irradiation of 7.5 Gy (RS2000 irradiator; Rad Source Technologies, Inc., Suwanee, GA, USA) at an absorbed dose rate of 1.2 Gy/min, and fresh air was continuously circulated in the irradiation chamber to avoid the generation of hypoxic conditions. Mice were kept in perforated plastic bottles for individual irradiation.
Histology and apoptosis assay
Intestinal tissues were harvested 72 h after radiation and fixed with 4% paraformaldehyde overnight. Sequentially, the tissues were embedded in paraffin for slicing. Four-micrometer-thick sections were prepared. Deparaffinization was performed by heating the sections for 25 min at 56°C. After gradual hydration through graded alcohols (95% and 75%, v/v), the sections were rinsed in distilled water. Then, the slides were blocked using 3% hydrogen peroxide and 0.01 M citrate buffer at pH = 6.0 and were heated in the microwave oven at 700 W for 10 min for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining.
To visualize DNA fragmentation, a marker of apoptosis, TUNEL staining was performed according to the manufacturer's protocol (Roche). The pretreated sections were first treated with 20 g/ml proteinase K for 10 min, 0.3% H2O2 in methanol for 10 min and 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. Then, the sections were incubated in the TUNEL reaction mixture. Next, the sections were stained with diaminobenzidine (DAB) solution for 10 min at room temperature. Finally, photographs were taken of the sections under a microscope. The number of TUNEL-positive cells and total villi cells of three adjacent sections at comparable locations of the intestine in each group was counted using Image Pro Plus software (IPP 6.0; Media Cybernetics). The apoptosis index was calculated as the percentage of TUNEL-positive cells relative to total villi cells.
Reverse transcription-polymerase chain reaction and real-time PCR
Total RNA was isolated from tissues using TRIzol (Invitrogen), and 1 μg of the RNA was then converted to cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Gene-specific primers (Table S1) were designed to flank the exon junctions to ensure that all of the expected PCR products were generated from mRNA. Real-time PCR analysis was performed using 2 × SYBR Green®PCR Master Mix and the ABI 7300 Real-time PCR system (Applied Biosystems, Foster City, CA). Each sample was analyzed in triplicate, and target genes were normalized to the reference housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or beta-actin. Fold differences were then calculated for each treatment group using normalized cycle threshold values for the control.
Western blotting
The proteins were extracted using M-PER buffer or NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo) according to the manufacturer's protocol. Then, the cell lysates were subjected to 10–15% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore). After blocking with 5% fat-free milk solution, the proteins of interest were detected using primary antibodies and corresponding HRP-linked secondary antibodies at a 1:5000 dilution. The primary antibodies used were as follows: anti-ERK antibody, anti-JNK, anti-p38, anti-pAktS308, anti-Akt and anti-cleaved caspase3 antibody (1:1000; Cell Signaling). The blots were developed using enhanced chemiluminescence (ECL) reagent (Thermo) and were exposed on a ChemiDoc MP imager (Bio-Rad). Image Lab™ software was used to quantify the band densities.
Seabuckthorn oil and high-performance liquid chromatography
Seabuckthorn oils used in this study were supplied by Qinghai Tsinghua Biotry Bio-Tech Co., Ltd. The Seabuckthorn oils were extracted by supercritical CO2 from seeds (seed oil) and the soft part (pulp oil). The extractions were carried out at a CO2 density of ~0.9 g/ml. The component analysis of seabuckthorn pulp and seed oils using high-performance liquid chromatography were performed by Intertek Testing Services Ltd., Shanghai, which has been accredited by United Kingdom Accreditation Services (UKAS) for laboratory compliance with BS/EN/ISO/IEC 17025.
Statistical analysis
All values were expressed as the mean ± standard error of mean (S.E.M.). Statistical analysis was performed using the Log-rank test and one-way analysis of variance (ANOVA) followed by Tukey's test using SPSS version 19.0 software. Differences with a P value of < 0.05 was considered statistically significant in all of the cases.
RESULTS
Seabuckthorn oil pretreatment prolonged the post-radiation survival time and attenuated acute intestinal injury
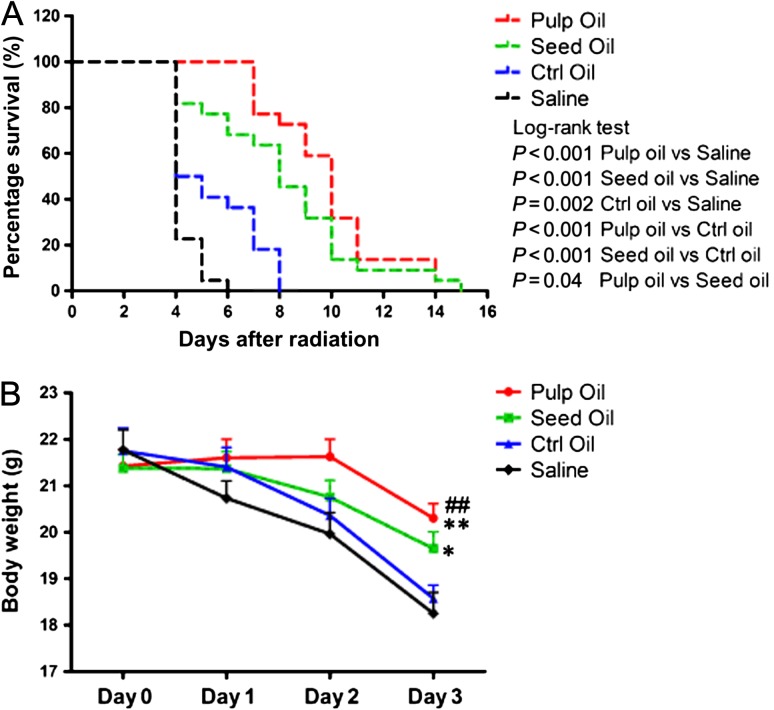
After one 7.5-Gy dose of whole-body radiation, the median survival time of mice pretreated with control olive oil was 4.5 days. Although pretreatment with seabuckthorn oil did not prevent mouse death from high-dose radiation, both seabuckthorn pulp oil and seed oil prolonged the survival time of the mice. The median survival time was 8 days and 10 days in the seed oil and pulp oil groups, respectively. Both pulp oil and seed oil pretreatment reduced weight loss after radiation (Fig. 1A–B). The main cause of early death in the saline group was severe intestinal edema and necrosis (data not shown). Seabuckthorn oil pretreatment did not relieve the reduction of erythrocytes, leukocytes and platelets (data not shown), but attenuated the radiation-induced acute intestinal injuries (Fig. 2).
Fig. 1.
Survival curves and body weight after high-dose radiation. (A) The survival curves of the mice in the control olive oil, seabuckthorn pulp oil, seabuckthorn seed oil, and saline pretreated groups after one 7.5-Gy dose of whole-body radiation. (B) The body weight curves from 1 to 3 days after radiation. Data are shown as mean ± SEM, with n = 12 per group. ##P < 0.001, pulp oil vs saline; **P < 0.01, pulp oil vs control oil; *P < 0.05, seed oil vs saline.
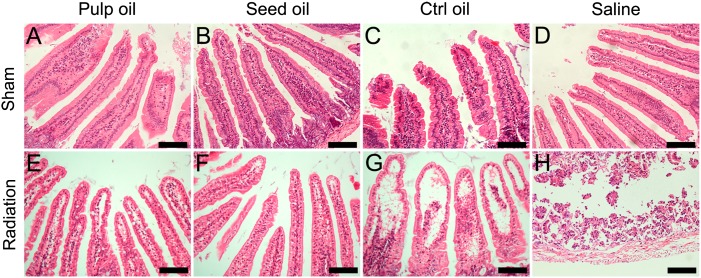
Fig. 2.
Intestinal injuries after radiation. (A–H) Structure of small intestine villi was determined by hematoxylin and eosin staining; (E–G) cell reduction and left vacuoles in villi were found in small intestine tissue treated with oils after radiation, and the degree of injury in the seabuckthorn oil group was lower than that in the control olive oil group. (H) Integral structure of intestine villi was not found in saline group. Bar = 100 μm, with n = 6 per group.
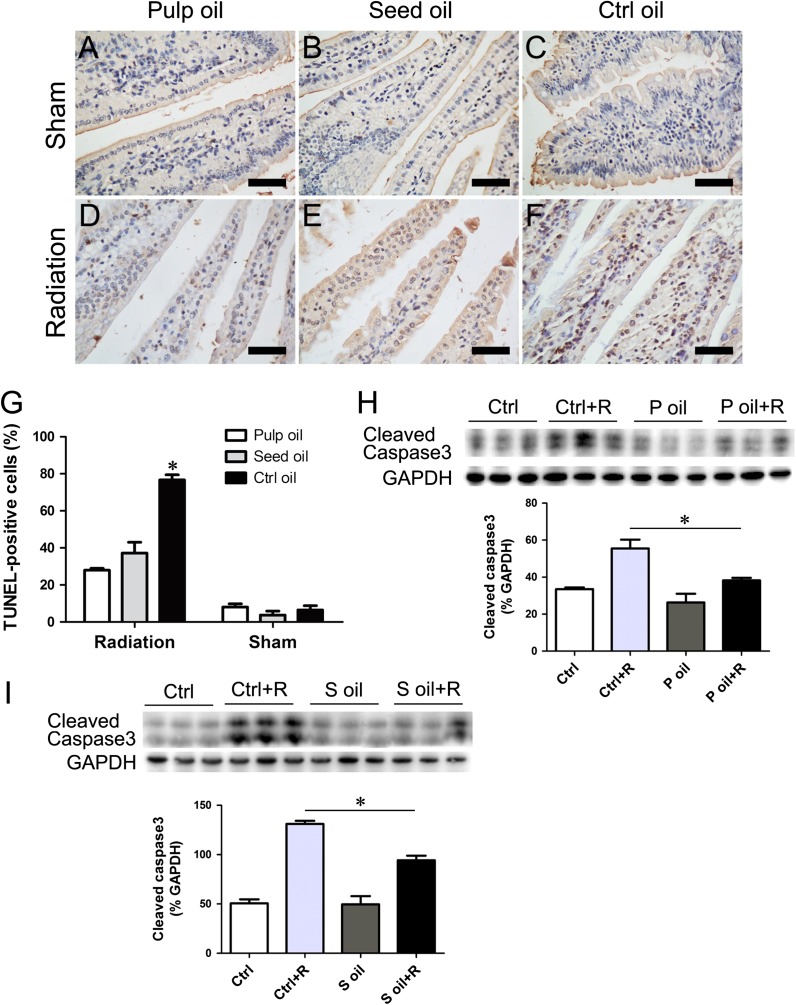
Seabuckthorn oil pretreatment decreased radiation-induced intestinal cell apoptosis and inflammatory factor expression
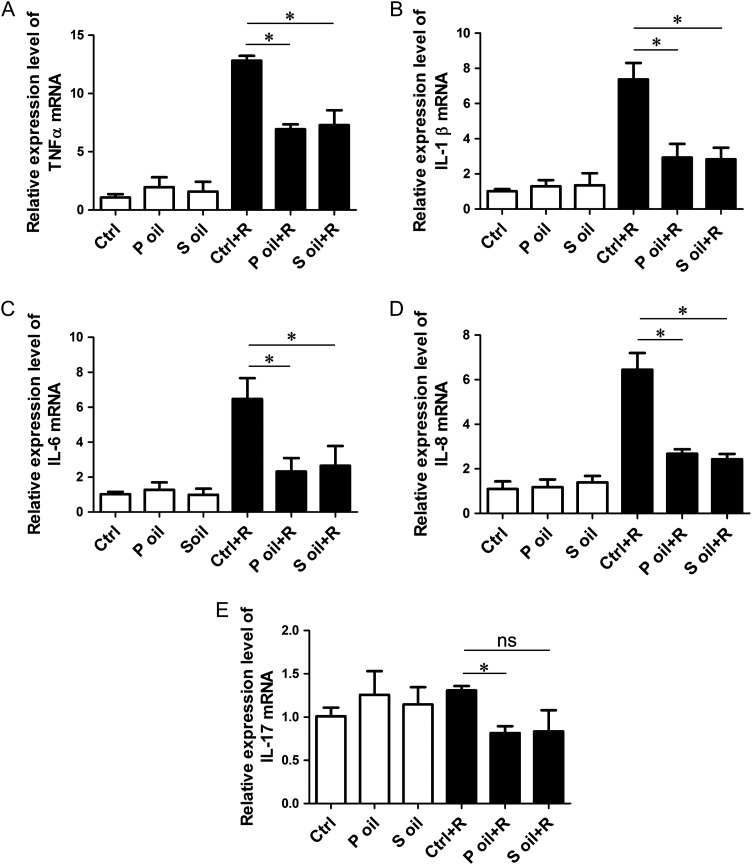
Using TUNEL staining, we found that seabuckthorn oil pretreatment significantly decreased the percentage of apoptotic cells in intestinal tissues (Fig. 3A–G). After radiation, the levels of the apoptosis marker protein cleaved caspase 3 in both the seabuckthorn pulp oil and seed oil groups were much lower than that in the control group (Fig. 3H and I). Because radiation intestinal injury involves acute inflammation, we further examined the mRNA expression levels of general inflammation factors. We found that the relative expression levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-8 in the radiation groups were higher than those in the non-radiation groups; however, the levels of these four inflammatory factors in the seabuckthorn pulp oil– and seed oil–treated groups were lower than that in the control group (Fig. 4A–D). The expression levels of another inflammatory factor, IL-17, were comparable between the radiation and non-radiation groups, except for in the pulp oil group (Fig. 4E).
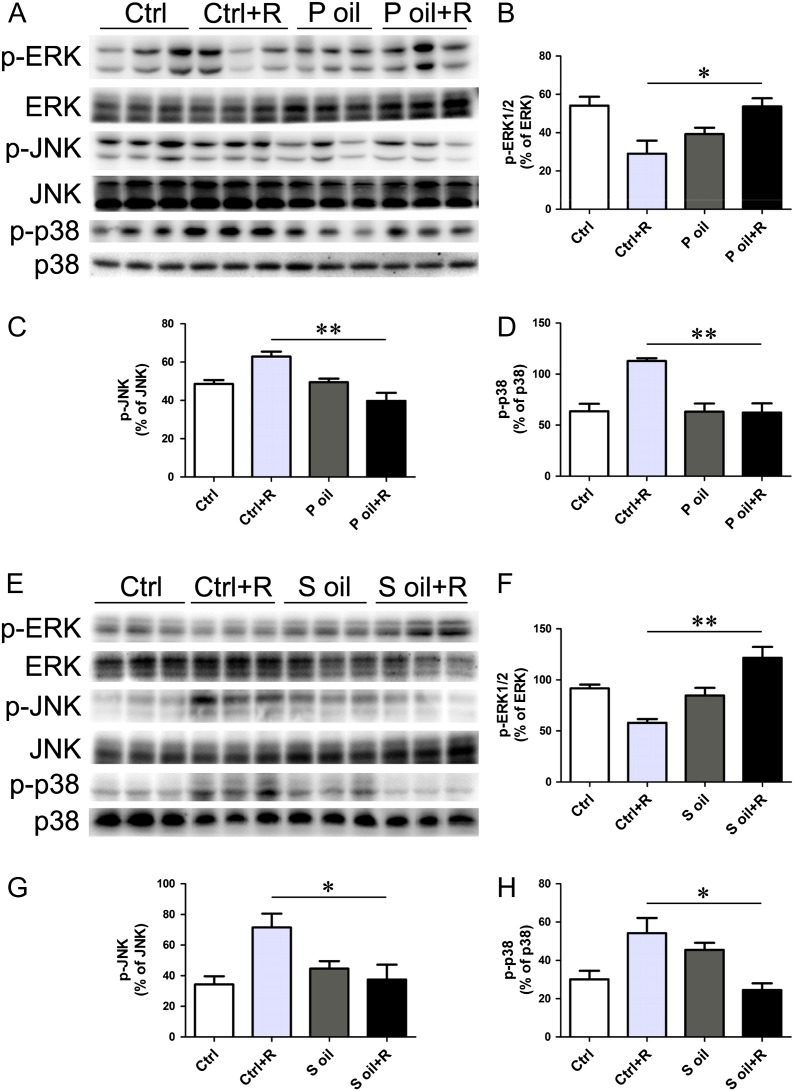
Figure 5:
Expression and activity of MAP kinases after radiation. (A–D) Pulp oil treatment did not affect the total protein level of MAP kinases, including ERK1/2, JNK and p38, but retained the activation status of ERK1/2 and inhibited the activation of JNK and p38. (E–H) Seed oil treatment did not affect the total protein level of MAP kinases, including ERK1/2, JNK and p38, but retained the activation status of ERK1/2 and inhibited the activation of JNK and p38. *P < 0.05, **P < 0.01.
Figure 3:
Reduction of apoptosis in seabuckthorn oil–treated intestines. (A–F) Apoptotic cells are detected by TUNEL staining. (G) Quantification of TUNEL-positive cells relative to total villi cells. Bar = 100 μm. Data are shown as mean ± SEM, with n = 6 per group. (H–I) Western blotting showed that radiation-induced cleaved caspase 3 was significantly inhibited by seabuckthorn pulp oil and seed oil pretreatment. *P < 0.05.
Fig. 4.
Expression levels of inflammatory cytokines after radiation. (A–E) mRNA levels of TNF-α, IL-1β, IL-6 and IL-8 were determined by quantitative real-time polymerase chain reaction. Radiation significantly increased the expression of TNF-α (A), IL-1β (B), IL-6 (C) and IL-8 (D), and both seabuckthorn pulp oil and seed oil treatment decreased these levels compared with olive oil treatment. Another inflammatory cytokine, IL-17, was not upregulated by radiation, but pulp oil treatment decreased the IL-17 level compared with olive oil treatment (D). *P < 0.05.
Seabuckthorn oil pretreatment affected the phosphorylation of mitogen-activated protein kinases in intestinal tissues
Mitogen-activated protein kinases are important proteins involved in stress-mediated biological effects. We examined the total and phosphorylated protein levels of MAP kinases, including extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 in radiation-treated intestinal tissues. Seabuckthorn oil treatment did not affect the total protein levels of MAP kinases. Both the pulp oil and seed oil treatment prevented the reduction of phosphorylated ERK levels and decreased the activation of phosphorylated JNK and p38 induced by radiation injury (Fig. 5).
Fig. 6.
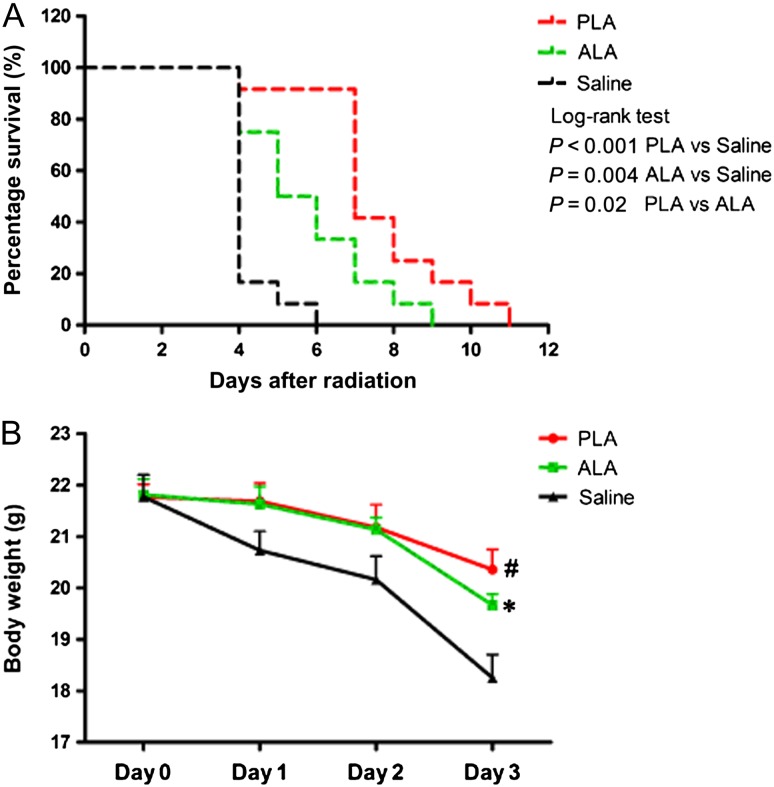
Survival curves and body weight in PLA and ALA pretreated mice after radiation. (A) The survival curves of the mice in the PLA and ALA pretreated groups after one 7.5-Gy dose of whole-body radiation. (B) The body weight curves from 1 to 3 days after radiation. Data are shown as mean ± SEM, with n = 12 per group. #P < 0.01, PLA vs saline; *P < 0.05, ALA vs saline.
Different unsaturated fatty acid composition of seabuckthorn oil compared with olive oil
To identify the potential protective components in response to radiation injuries, we analyzed the components of seabuckthorn oil and olive oil used in this study via high-performance liquid chromatography (Table 1). The main components in seabuckthorn and olive oils are unsaturated fatty acids. Palmitic acid is the main type of saturated fatty acid in the three oils. Linoleic acid (LA) is richer in seed oil at 34.64% (c.f. 4.48% in pulp oil and 18.53% in olive oil). Oleic acid (OA) was the predominant unsaturated fatty acid in olive oil, accounting for 62.65%, compared with 33.25% and 22.09% in seabuckthorn pulp oil and seed oil, respectively. A relatively richer content of 29.19% alpha linolenic acid (ALA) was found in seabuckthorn seed oil (c.f. 2.70% in pulp oil and 1.48% in olive oil). Meanwhile, a richer content of 30.37% palmitoleic acid (PLA) was only found in seabuckthorn pulp oil (c.f. 0.54% and 1.31% in seed oil and olive oil, respectively).
Table 1.
The main components of seabuckthorn pulp and seed oils and of olive oil
| Main ingredient | Pulp oil (g/100 g) | Seed oil (g/100 g) | Olive oil (g/100 g) |
|---|---|---|---|
| Saturated fatty acid | |||
| Myristic acid (C14:0) | 0.54 | 0.12 | 0.13 |
| Pentadecanoic acid (C15:0) | 0.10 | 0.14 | 0.10 |
| Palmitic acid (C16:0) | 26.36 | 8.49 | 13.73 |
| Margaric acid (C17:0) | 0.10 | 0.04 | 0.05 |
| Stearic acid (C18:0) | 1.14 | 2.18 | 1.03 |
| Arachidic acid (C20:0) | 0.30 | 0.43 | 0.23 |
| Behenic acid (C22:0) | 0.10 | 0.12 | 0.10 |
| Unsaturated fatty acid | |||
| Palmitoleic acid (ω-7, C16:1n7) | 30.37 | 0.54 | 1.31 |
| Margaric acid (C17:1n7) | 0.10 | 0.03 | 0.12 |
| Vaccenic acid (C18:1n7) | <0.01 | 1.82 | 0.43 |
| Oleic acid (ω-9, C18:1n9) | 33.55 | 22.09 | 62.65 |
| Linoleic acid (ω-6, C18:2n6) | 4.48 | 34.64 | 18.53 |
| Alpha linolenic acid (ω-3, C18:3n3) | 2.70 | 29.19 | 1.48 |
| α-Vitamin E | 0.09 | 0.06 | 0.04 |
PLA And ALA pretreatment prolonged the post-radiation survival time
To identify the anti-radiation potential of PLA and ALA, mice were pretreated with PLA and ALA for 7 days before radiation. As expected, the median survival time was prolonged to 5.5 days and 7 days in the ALA- and PLA-pretreated groups, respectively (Fig. 6A). Both PLA and ALA reduced the weight loss of mice compared with saline (Fig. 6B). The median survival time of the PLA group was statistically shorter compared with that of the pulp oil group, and the median survival time of the ALA group was statistically shorter compared with that of the pulp and seed oil groups (Fig. S1).
DISCUSSION
The clinical management of radiation-induced acute gastrointestinal injury is mostly based on symptomatic treatment [11], including antidiarrheal agents for preventing fluid loss, non-steroidal anti-inflammatory drugs and opioids for relieving pain, and glucose-containing electrolyte solution for correcting dehydration or fluid loss. Safe and effective approaches for preventing or alleviating radiation-associated enteritis are lacking. In this study, we found that seabuckthorn oil pretreatment significantly increased the post-radiation survival time and mitigated the acute intestinal injuries through reducing radiation-associated cell apoptosis and inflammation.
In this study, we found a potential anti-radiation value for seabuckthorn pulp and seed oils but not for olive oil. The fatty acid content of our pulp and seed oil was similar to that reported by Fatima et al. [12]. When comparing the detailed components of seabuckthorn oils to those of olive oil, we found distinct compositions for these three types of oils. OA, PLA and ALA were the richest fatty acids found in olive oil, seabuckthorn pulp oil and seed oil, respectively. Seabuckthorn pulp oil and seed oil, but not olive oil, showed protective effects against radiation-induced intestinal injuries. We speculate that PLA in pulp oil and ALA in seed oil might be the main potential candidates for radiation countermeasure. There is no direct report concerning the protective effects of PLA or ALA against radiation. Both pulp oil and seed pretreatment were associated with significant anti-apoptotic and anti-inflammatory effects against radiation, and the finding that PLA and ALA pretreatment increased survival time indicates that the associated mechanism involves PLA and ALA.
Cell apoptosis is one of the major biological effects induced by ionizing radiation. Caspases play essential roles in the execution of apoptosis. Among them, caspase3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins [13]. In this study, we found pretreatment with seabuckthorn oil reduced the percentage of apoptotic intestinal cells and the activation of caspase 3. Unsaturated fatty acids are the predominant components of seabuckthorn oils, and previous studies have reported the diverse effects of various unsaturated fatty acids on cell apoptosis. In most tumor cell lines, polyunsaturated fatty acids, including LA, ALA and eicosapentaenoic acid (EPA), induce tumor cell apoptosis [14, 15]. However, EPA was reported to inhibit oxidative stress–induced chondrocyte apoptosis [16]. Adequate ALA intake increases the conversion of ALA to EPA [17]. ALA was also reported to reverse endoplasmic reticulum stress-mediated apoptosis in hepatocytes [18]. These data indicate a cell type–dependent effect of ALA on cell apoptosis. Welters et al. have reported that PLA attenuates caspase activation and apoptosis following exposure to palmitate in pancreatic beta cells [19]. Our findings showed that both pulp oil and seed oil inhibited caspase 3 activation and activated the cell survival factor ERK1/2 MAP kinase, suggesting an anti-apoptotic role for ALA and PLA in radiation-induced intestinal cell apoptosis.
In addition to the anti-apoptosis effects of ALA and PLA, ALA and PLA also showed significant anti-inflammatory effects in various tissues. ALA suppresses ultraviolet-induced skin injuries via its anti-inflammatory effect through lowering prostaglandin E2 [20]. A higher dietary ALA intake attenuates the rat model of inflammatory bowel disease via reducing proinflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8 [21, 22]. PLA also shows an anti-inflammatory ability, especially in metabolic-associated inflammation. PLA reduces palmitic acid–induced macrophage activation and associated p38 MAP kinase activation, improving muscle insulin sensitivity [23]. PLA also attenuates high-fat diet–induced immunometabolic disturbances independently of PPAR-α [24]. In this study, we found a significant reduction in the radiation-induced expression of proinflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8 (and consequent p38 and JNK MAPK activation) in seabuckthorn oils rich in ALA and PLA, indicating protective anti-inflammation effects for ALA and PLA. However, further studies using ALA or PLA alone are needed to elucidate the exact role and mode of action of these two unsaturated fatty acids in radiation-associated intestinal injuries.
Our additional survival experiments showed that pretreating with PLA and ALA increased the survival time compared with the control, but the median survival time with PLA and ALA pretreatment was shorter than that with seabuckthorn oils pretreatment. We speculate that some other components of seabuckthorn oils might enhance the anti-radiation effects of PLA and ALA. Oleic acid (OA) is another rich component in pulp and seed oils. There are no reports concerning the protective effects of OA. However, OA has been reported as an anti-inflammatory fatty acid. OA inhibits pro-inflammatory responses in human arterial endothelial cells [25] and modulates the reactive oxygen species production and function of neutrophils [26, 27]. Our data showed the OA-rich olive oil improve the survival compared with saline, suggesting a protective effect of OA. We think OA may cooperate with PLA or ALA to reduce the radiation-induced intestinal injury and increase the survival time. α-Vitamin E has been suggested as a radioprotector for improving radiation-induced intestinal injury and wound healing [28, 29]. Even though the seabuckthorn oil α-Vitamin E content is relatively lower than its fatty acids content, we speculate that α-Vitamin E might produce a synergistic effects on PLA- and ALA-mediated intestinal protection.
In conclusion, seabuckthorn oils attenuate high-dose, radiation-induced acute intestinal injuries and increase the animal survival time via mitigating cell apoptosis and acute inflammation. Two unsaturated fatty acids, PLA and ALA, might be the potential radiation countermeasure candidates for clinical radiation–associated intestinal injury management and for further research into intestinal radiation protection.
ACKNOWLEDGEMENTS
Dr Wei Sun is an Assistant Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, and Dr. Xiangqing Kong is a Fellow at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
SUPPLEMENTARY DATA
Supplementary data is available at Journal of Radiation Research Online.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China (No. 81100162, No. 81270298), the Natural Science Foundation of Jiangsu province (No. BK20141024) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD2014–2016).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Koenig KL, Goans RE, Hatchett RJ, et al. . Medical treatment of radiological casualties: current concepts. Ann Emerg Med 2005;45:643–52. [DOI] [PubMed] [Google Scholar]
- 2.Lopez M, Martin M.. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother 2011;16:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guliyev VB, Gul M, Yildirim A.. Hippophae rhamnoides L.: chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J Chromatogr B Analyt Technol Biomed Life Sci 2004;812:291–307. [DOI] [PubMed] [Google Scholar]
- 4.Goel HC, Kumar IP, Samanta N, et al. . Induction of DNA-protein cross-links by Hippophae rhamnoides: implications in radioprotection and cytotoxicity. Mol Cell Biochem 2003;245:57–67. [DOI] [PubMed] [Google Scholar]
- 5.Xing J, Yang B, Dong Y, et al. . Effects of sea buckthorn (Hippophae rhamnoides L.) seed and pulp oils on experimental models of gastric ulcer in rats. Fitoterapia 2002;73:644–50. [DOI] [PubMed] [Google Scholar]
- 6.Goel HC, Prasad J, Singh S, et al. . Radioprotection by a herbal preparation of Hippophae rhamnoides, RH-3, against whole body lethal irradiation in mice. Phytomedicine 2002;9:15–25. [DOI] [PubMed] [Google Scholar]
- 7.Agrawala PK, Goel HC.. Protective effect of RH-3 with special reference to radiation induced micronuclei in mouse bone marrow. Indian J Exp Biol 2002;40:525–30. [PubMed] [Google Scholar]
- 8.Kumar IP, Namita S, Goel HC.. Modulation of chromatin organization by RH-3, a preparation of Hippophae rhamnoides, a possible role in radioprotection. Mol Cell Biochem 2002;238: 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Hsu YW, Tsai CF, Chen WK, et al. . Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem Toxicol 2009;47:2281–8. [DOI] [PubMed] [Google Scholar]
- 10.Basu M, Prasad R, Jayamurthy P, et al. . Anti-atherogenic effects of seabuckthorn (Hippophaea rhamnoides) seed oil. Phytomedicine 2007;14:770–7. [DOI] [PubMed] [Google Scholar]
- 11.Berger ME, Christensen DM, Lowry PC, et al. . Medical management of radiation injuries: current approaches. Occup Med (Lond) 2006;56:162–72. [DOI] [PubMed] [Google Scholar]
- 12.Fatima T, Snyder CL, Schroeder WR, et al. . Fatty acid composition of developing sea buckthorn (Hippophae rhamnoides L.) berry and the transcriptome of the mature seed. PLoS One 2012;7:e34099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter AG, Janicke RU.. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999;6:99–104. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Yu H, Shen Y, et al. . Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch Med Sci 2015;11:1081–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thibane VS, Ells R, Hugo A, et al. . Polyunsaturated fatty acids cause apoptosis in C. albicans and C. dubliniensis biofilms. Biochim Biophys Acta 2012;1820:1463–8. [DOI] [PubMed] [Google Scholar]
- 16.Sakata S, Hayashi S, Fujishiro T, et al. . Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J Orthop Res 2015;33:359–65. [DOI] [PubMed] [Google Scholar]
- 17.Goyens PL, Spilker ME, Zock PL, et al. . Conversion of alpha-linolenic acid in humans is influenced by the absolute amounts of alpha-linolenic acid and linoleic acid in the diet and not by their ratio. Am J Clin Nutr 2006;84:44–53. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yang X, Shi H, et al. . Effect of alpha-linolenic acid on endoplasmic reticulum stress-mediated apoptosis of palmitic acid lipotoxicity in primary rat hepatocytes. Lipids Health Dis 2011;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welters HJ, Diakogiannaki E, Mordue JM, et al. . Differential protective effects of palmitoleic acid and cAMP on caspase activation and cell viability in pancreatic beta-cells exposed to palmitate. Apoptosis 2006;11:1231–8. [DOI] [PubMed] [Google Scholar]
- 20.Takemura N, Takahashi K, Tanaka H, et al. . Dietary, but not topical, alpha-linolenic acid suppresses UVB-induced skin injury in hairless mice when compared with linoleic acids. Photochem Photobiol 2002;76:657–63. [DOI] [PubMed] [Google Scholar]
- 21.Tyagi A, Kumar U, Reddy S, et al. . Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with alpha-linolenic acid in a rat model of inflammatory bowel disease. Br J Nutr 2012;108:1612–22. [DOI] [PubMed] [Google Scholar]
- 22.Reifen R, Karlinsky A, Stark AH, et al. . alpha-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J Nutr Biochem 2015;26:1632–40. [DOI] [PubMed] [Google Scholar]
- 23.Talbot NA, Wheeler-Jones CP, Cleasby ME.. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol Cell Endocrinol 2014;393:129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souza CO, Teixeira AA, Lima EA, et al. . Palmitoleic acid (n-7) attenuates the immunometabolic disturbances caused by a high-fat diet independently of PPARalpha. Mediators Inflamm 2014;2014:582197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey KA, Walker CL, Xu Z, et al. . Oleic acid inhibits stearic acid-induced inhibition of cell growth and pro-inflammatory responses in human aortic endothelial cells. J Lipid Res 2010;51:3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatanaka E, Levada-Pires AC, Pithon-Curi TC, et al. . Systematic study on ROS production induced by oleic, linoleic, and gamma-linolenic acids in human and rat neutrophils. Free Radic Biol Med 2006;41:1124–32. [DOI] [PubMed] [Google Scholar]
- 27.Padovese R, Curi R.. Modulation of rat neutrophil function in vitro by cis- and trans-MUFA. Br J Nutr 2009;101:1351–9. [DOI] [PubMed] [Google Scholar]
- 28.Singh PK, Wise SY, Ducey EJ, et al. . alpha-Tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat Res 2012;177:133–45. [DOI] [PubMed] [Google Scholar]
- 29.Singh VK, Wise SY, Fatanmi OO, et al. . Alpha-tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation combined injury in mice. J Radiat Res 2014;55:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]