Abstract
Radiotherapy with breast-conserving therapy plays a crucial role in the treatment of early breast cancer. However, optimal radiotherapy targets have been controversial. We therefore evaluated regional recurrence in breast cancer patients with one to three positive lymph nodes (LNs) treated with breast-conserving surgery (BCS) followed by whole-breast irradiation (WBI). From 1993 to 2010, 121 breast cancer patients with one to three positive LNs who underwent BCS followed by WBI were analyzed. All patients underwent radiotherapy with two tangential fields to the whole breast. To evaluate the radiation dose to the axillary LNs, we contoured axillary LNs area and evaluated the dose–volumetric parameters. The median follow-up time was 112.4 months (range, 15.6–248.1 months). The 5-year overall survival and disease-free survival rates were 95.6% and 86.6%, respectively. The 5-year regional recurrence–free rate (RRFR) was 97.4%. During follow-up, six patients had regional recurrence. The pathological T stage was the factor best associated with the 5-year RRFR using the log-rank test, with 100.0% in the pT1 cohort versus 94.7% in the pT2–4 cohort (P < 0.01). The radiation dose to the axillary LNs did not contribute to the RRFR. In conclusion, while the pathological T stage was the prognostic factor best associated with regional recurrence, few regional recurrences were observed in early breast cancer patients with one to three LNs treated with BCS followed by WBI. Unintentional radiation doses to the axillary LNs using standard WBI were not related to the RRFR after axillary dissection.
Keywords: radiotherapy, breast cancer, breast-conserving therapy
INTRODUCTION
Breast-conserving therapy (BCT) has been the standard treatment option for women with early breast cancer. Radiotherapy after breast-conserving surgery (BCS) plays a fundamental role in local control, and Fisher et al. [1] reported that BCS plus radiotherapy reduced the risk of local recurrence. The results of a meta-analysis performed by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed the need for radiotherapy after BCS [2]. While adjuvant radiotherapy plays a crucial role in the treatment of early breast cancer, optimal radiotherapy targets remain controversial.
The results of the EBCTCG meta-analysis showed that radiotherapy involving the chest wall and regional lymph node (LN) areas reduced recurrence and breast cancer mortality in women with positive LNs after mastectomy and axillary dissection [3]. When women with one to three, and four or more involved axillary LNs were considered separately, the beneficial effects of postmastectomy radiotherapy were evident in each group. In general, patients with positive LNs have a higher risk of axillary node recurrence after BCT than do those with negative LNs [4, 5]. To improve regional control, regional node irradiation (RNI) is required for patients with more than four positive LNs. However, the role of RNI after BCS in patients with early breast cancer with one to three positive axillary LNs remains controversial. In the MA.20 trial, Whelan et al. [6] reported that the addition of RNI, including irradiation of the internal mammary, supraclavicular and axillary LNs, to whole breast irradiation (WBI) reduced the locoregional and distant metastasis–free survival rates in breast cancer patients treated with BCT. The majority of patients in the MA.20 trial had one to three positive nodes. Poortmans et al. [7] reported in the EORTC 22922–10925 trial that internal mammary and medial supraclavicular LN irradiation together with WBI or chest wall irradiation improved the disease-free survival (DFS) and distant DFS rates. However, neither of these trials showed a significant benefit in the primary endpoint of overall survival (OS). Although the National Comprehensive Cancer Network (NCCN) guideline states that irradiation to the infraclavicular and supraclavicular areas should be strongly considered for patients with one to three positive axillary LNs [8], several other studies reported a low recurrence rate in pathological N1 breast cancers, even without RNI [5, 9, 10].
Axillary LNs are sometimes unintentionally irradiated by WBI using standard tangential field techniques; however, some researchers reported that the dose delivered to the axillary nodes was not a sufficient therapeutic dose [11, 12]. Whether an adequate radiation dose to the axillary nodes affects the prognosis for breast cancer remains unclear. No studies have reported the relationship between the axillary node dose and regional recurrence. In the following retrospective cohort study, we therefore evaluated the outcomes of BCT including postoperative WBI without RNI for breast cancer patients with one to three positive LNs and assessed prognostic factors for regional recurrence. Furthermore, we evaluated the radiation dose to the axillary LNs using WBI and investigated the possible relationship between the radiation dose and regional recurrence.
MATERIALS AND METHODS
Patients
From 1993 to 2010, 620 patients who underwent BCT were histologically diagnosed with breast cancer at our institution. Of these, 389 patients had negative LN metastasis, 133 patients had one to three positive axillary LNs, 35 patients had four or more positive LNs, and the status of LN metastasis was unknown in 63 patients. In this study, we excluded three patients whose follow-ups were interrupted within a year after BCS, and nine patients who underwent irradiation of the supraclavicular and/or parasternal LN areas, from 133 patients with one to three positive LNs. Although there were no definite criteria for supraclavicular radiotherapy (SCRT) and/or parasternal radiotherapy (PSRT) during this period in our institution, younger patients or patients with a higher percentage of positive nodes to dissected nodes were likely to receive SCRT and/or PSRT. We did not exclude patients who died or who developed recurrence within a year after BCS. Thus, we analyzed a total of 121 patients. This study was approved by the institutional review board of our institution.
Treatment
All patients underwent BCS with axillary LN dissection or sentinel LN biopsy followed by WBI. Axillary LN dissection was performed on 113 patients (93.3%), and the remaining 8 patients (6.7%) received sentinel LN biopsy without further LN dissection. Fifty-nine patients (52.2%) underwent Level 1 axillary dissection, 21 patients (18.6%) underwent Level 2 dissection, and 9 patients (8.0%) underwent Level 3 dissection. The details of the axillary treatment of the remaining 24 patients (21.2%) were unknown. Radiotherapy was performed using a 60Cobalt or 4- or 6-MV photon beam from a linear accelerator. A tangential technique with a rectangular field was used. The cranial border of the treatment field was at the sternal notch, and the caudal border was at 1 cm below the inframammary fold. WBI was performed daily using a dose of 50 Gy in 25 fractions in 117 patients, a dose of 42.56 Gy in 16 fractions in 1 patient, and a dose of 42.4 Gy in 16 fractions in 3 patients. In the case of a positive or close primary surgical margin, WBI was followed by an additional boost to the tumor bed with a dose of 10.0 Gy in 5 fractions or 10.6 Gy in 4 fractions. The axillary LN regions were not irradiated intentionally. Systemic chemotherapy was given to 61 patients (50.4%). Thirty-one of 61 patients (50.8%) received oral pyrimidine fluoride agents such as doxifluridine, tegafur/uracil, or capecitabine. Thirteen patients (21.3%) received anthracycline-based regimens, 11 patients (18.0%) received a sequence of anthracyclines and taxanes, two patients (3.3%) received taxanes alone, and another regimen was used in four patients (6.6%). Hormone therapy was given to 81 patients (66.9%).
Radiation dose to axillary LNs
To evaluate the radiation dose to the axillary LNs, we contoured three LN areas involving axillary Level 1 LNs, the interpectoral LNs, and axillary Level 2 in accordance with the European Society for Radiotherapy & Oncology (ESTRO) consensus guidelines on target volume delineations [13]. We did not contour axillary Level 3 because this level was not usually included in the field of standard tangential WBI. Computed tomography images for planning of 3D radiotherapy from 96 patients were available. All treatment plans were reproduced using the Eclipse Treatment Planning System version 8.6 (Varian Medical Systems) from the information including isocenter coordinates, field sizes, gantry and collimator angles, and monitor unit. The dose calculation algorithm used for planning was the pencil beam convolution algorithm. Treatment plans with hypofractionation were converted to standard 2 Gy equivalent dose fractions using a linear–quadratic model with alpha/beta ratio of 4 Gy. The dose–volumetric data were analyzed for the mean dose and the percentages of volumes irradiated at >45 Gy (V45Gy) and >40 Gy (V40Gy) for each LN area.
Statistical analysis
We defined regional recurrence as any tumor recurrence in the ipsilateral axilla, supraclavicular, or internal mammary areas. Ipsilateral breast tumor recurrence was defined as a failure in the ipsilateral breast. The regional recurrence–free rate (RRFR) was calculated from the date of the first BCS to the date of the event. All recurrences were diagnosed by either a clinical or radiological examination. OS was calculated from the date of the BCS to the date of death of any cause or the date of the last follow-up. DFS was calculated from the date of the first BCS to the date of any first recurrence, the development of another cancer, or death from any cause. Survival estimates were calculated using the Kaplan–Meier method. Survival rates were compared using the log-rank test between two groups. All statistical tests and P-values were two-tailed, and P-values of <0.05 were considered significant.
RESULTS
Patient characteristics
The characteristics of the patients in the study cohort are shown in Table 1. The median age at diagnosis was 52 years (range, 26–78 years). Of these patients, 61 (50.4%) and 60 (49.6%) were diagnosed with pT1 and pT2–4 tumors, respectively. Eighty-three patients (68.6%) had only one positive LN, and 38 patients (31.4%) had two or three positive LNs. The percentage of patients who were estrogen receptor–positive was 74.4% (n = 90). The median number of removed LNs was 13 (range, 2–60), and the median percentage of positive LNs was 10% (range, 2%–50%).
Table 1.
Patient characteristics
| n (%) | |
|---|---|
| Age in years | |
| ≤50 | 53 (43.8) |
| >50 | 68 (56.2) |
| Menopausal status | |
| Postmenopausal | 55 (45.5) |
| Premenopausal | 38 (31.4) |
| Perimenopausal | 3 (2.5) |
| Unknown | 25 (20.7) |
| Histology | |
| Invasive ductal carcinoma | 117 (96.7) |
| Other | 4 (3.3) |
| Pathological T stage | |
| 1 | 61 (50.4) |
| 2 | 58 (47.9) |
| 3 | 0 (0.0) |
| 4 | 2 (1.7) |
| Histological grade | |
| 1 | 22 (18.2) |
| 2 | 38 (31.4) |
| 3 | 19 (15.7) |
| Unknown | 42 (34.7) |
| Lymphovascular invasion | |
| Positive | 34 (28.1) |
| Negative | 72 (59.5) |
| Unknown | 15 (12.4) |
| Hormonal receptor status | |
| Positive | 90 (74.4) |
| Negative | 27 (22.3) |
| Unknown | 4 (3.3) |
| Number of positive LNs | |
| 1 | 83 (68.6) |
| 2 | 29 (24.0) |
| 3 | 9 (7.4) |
| Median number of dissected LNs | 13 (2–60) |
| Median percentage of positive LNs | 10% (2–50) |
LNs = lymph nodes.
OS, DFS and pattern of recurrence
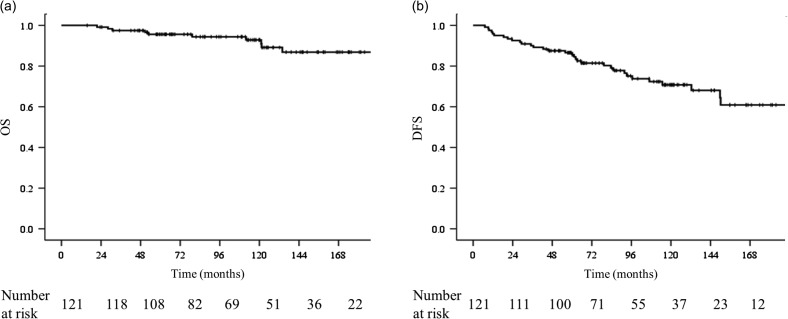
The median follow-up time was 112.4 months (range, 15.6–248.1 months) for all patients. The OS and DFS rates were 95.6% and 86.6% at 5 years and 92.9% and 70.8% at 10 years, respectively. The Kaplan–Meier curves for OS and DFS are shown in Fig. 1a and b. One patient developed contralateral breast cancer, and one patient developed another type of cancer. During follow-up, 35 recurrences in 30 patients developed in 7 ipsilateral breasts, 5 supraclavicular LNs, 1 axillary LN, and 22 distant sites, respectively. Table 2 lists the sites of the first recurrence. Two patients had regional LN recurrences only.
Fig. 1.
. Kaplan–Meier curves for (a) overall survival and (b) disease-free survival of all patients. OS = overall survival, DFS = disease-free survival.
Table 2.
Site of first recurrence
| n (%) | |
|---|---|
| Distant organ | 20 (66.7) |
| Ipsilateral breast | 5 (16.7) |
| SC + distant organ | 2 (6.7) |
| SC + ipsilateral breast | 1 (3.3) |
| SC | 1 (3.3) |
| Ax | 1 (3.3) |
| Overall site | 30 (100) |
SC = supraclavicular node, Ax = axillary node.
Regional recurrence
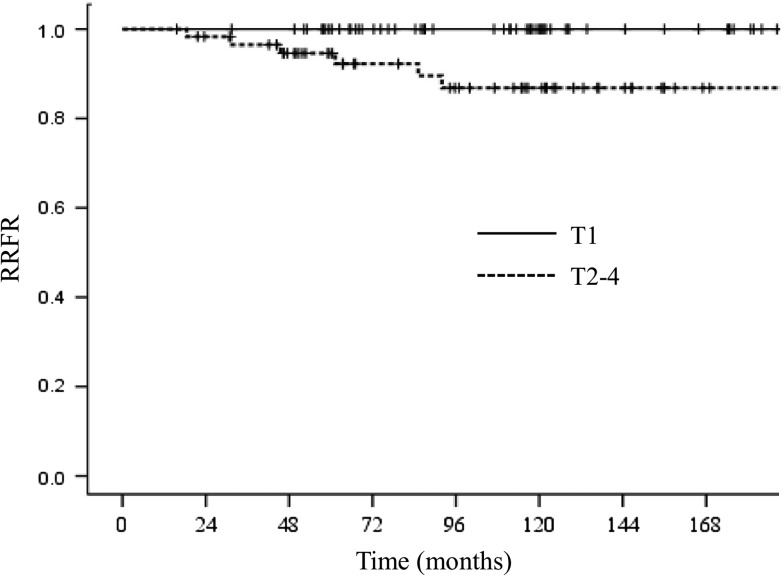
The 5- and 10-year RRFRs were 97.4% and 93.7%, respectively. During follow-up, six patients (5.0%) developed regional recurrence. One patient had two recurrences in the interpectoral LNs and in axillary Level 3, while five patients had a recurrence in the supraclavicular area. Using univariate analysis, the most significant factor associated with the RRFR was the pathological T stage, with a 5-year RRFR of 100.0% in pT1 tumors versus 94.7% in pT2–4 tumors (P < 0.01). All of the recurrences occurred in pT2 patients. The Kaplan–Meier curve analysis for the RRFR according to pathological T stage is shown in Fig. 2. No significant differences were observed for age (P = 0.30), histological grade (P = 0.54), lymphovascular invasion (P = 0.10), hormonal receptor status (P = 0.76), number of positive LNs (P = 0.07), percentage of positive LNs (P = 0.94) or using systemic chemotherapy (P = 0.10). There were very few recurrences; thus, the aforementioned factors using multivariate analysis could not be analyzed.
Fig. 2.
Kaplan–Meier curves for regional recurrence–free rate according to pathological T stage of all patients. RRFR = regional recurrence–free rate.
Radiation dose to axillary LNs
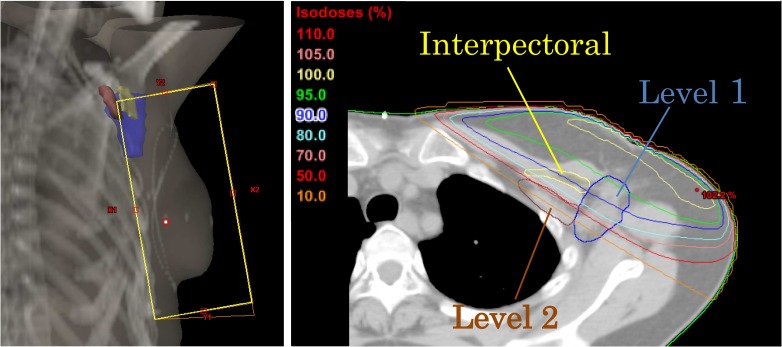
Figure 3 shows the radiation field of the tangential technique and axillary radiation dose coverage for a representative case. The dose–volume parameters for axillary Level 1, the interpectoral LNs, and Level 2 of 96 patients are shown in Table 3. The averaged mean dose to Level 1, the interpectoral LNs, and Level 2 were 35.4 ± 6.6, 30.8 ± 10.1 and 10.1 ± 7.8 Gy, respectively. From these data, the dose irradiated to the Level 2 area was negligible; thus, we conducted further studies using Level 1 and the interpectoral LNs.
Fig. 3.
Digitally reconstructed radiograph of tangential fields (left) and axillary radiation dose coverage in a representative case (right).
Table 3.
Dose–volume parameters for axillary nodes
| Level 1 | Interpectoral nodes | Level 2 | |
|---|---|---|---|
| Mean dose (Gy) | 35.4 ± 6.6 | 30.8 ± 10.1 | 10.1 ± 7.8 |
| V40 Gy (%) | 51.0 ± 25.2 | 34.7 ± 35.6 | 3.0 ± 9.8 |
| V45 Gy (%) | 31.7 ± 28.2 | 17.9 ± 27.0 | 0.6 ± 2.9 |
V40 Gy = percentage of volume receiving >40 Gy, V45 Gy = percentage of volume receiving >45 Gy.
Table 4 shows the relationship between the average mean dose to the axillary LNs and the RRFR divided into two groups according to the percentage of positive LNs. Truong et al. [14] reported that ≥20% positive LNs was associated with locoregional failure. Based upon this report, we set the cut-off value at 20%. However, the radiation doses to axillary Level 1 and the interpectoral LNs were not beneficial for the RRFR in patients with <20% or ≥20% positive LNs. The numbers of patients with <20% and ≥20% positive LNs were 77 and 19, respectively. Neither the dose to Level 1 nor to the interpectoral LNs were related to the RRFR in patients with either a higher or lower percentage of positive LNs.
Table 4.
Effect of radiation dose to axillary nodes on the 5-year RRFR
| Mean dose for Level 1 (5-year RRFR) | P value (log-rank) | Mean dose for interpectoral nodes (5-year RRFR) | P value (log-rank) | |||
|---|---|---|---|---|---|---|
| ≥35 Gy | <35 Gy | ≥35 Gy | <35 Gy | |||
| % of positive LNs | ||||||
| <20% | 92.9% | 92.9% | 0.52 | 88.9% | 93.6% | 0.72 |
| ≥20% | 100.0% | 75.0% | 0.11 | 87.5% | 100.0% | 0.39 |
RRFR = regional recurrence–free rate, LNs = lymph nodes.
DISCUSSION
Two randomized trials have demonstrated that RNI added to WBI improves DFS in patients with early-stage breast cancer. However, only 43% had one to three positive LNs in the EORTC22922/10925 trial. Therefore, the efficacy of RNI for patients with one to three positive LNs remains controversial. Several retrospective analyses have reported results of SCRT for such patients. Fortin et al. [9] reported that the regional control rate was 93.0% for WBI, but significantly higher (98.0%) for WBI and SCRT. However, Grills et al. [5] reported that 6 (3.2%) of 188 patients who received WBI without SCRT developed regional node failure, and SCRT did not affect the rate of axillary or supraclavicular failure in patients with one to three positive LNs. Several other reports showed a low recurrence rate in regional LN areas for patients with one to three positive LNs when treated with BCS followed by WBI. Bedi et al. [15] reported that 4 of 202 patients (2%) developed recurrence in the ipsilateral supraclavicular LNs and that 4 (2%) developed recurrence in the ipsilateral axillary and/or internal mammary LNs. Truong et al. [14] also reported a low incidence of regional recurrence of 5.4% (68 of 1255 patients). In our study, 6 patients (5%) treated with WBI alone developed regional recurrence, with a 5-year RRFR of 97.4% (similar to other studies). Moreover, only 2 patients (1.7%) developed their first recurrence in the regional area alone. Taken together, the results from our study indicate that the rate of regional recurrence of WBI alone is substantially low and that the absolute gains of SCRT may be negligible.
Nine patients who underwent SCRT and/or PSRT were excluded in this study. Although no definite criteria existed for SCRT and/or PSRT, these patients might be considered to have had higher risk for regional recurrence, such as younger age, or a higher percentage of positive nodes. Thus, there is a possibility that the RRFR might have been lower if the patients with SCRT and/or PSRT had been included in the analysis.
RNI may increase the risk of treatment complications such as lymphedema and pneumonitis. Powell et al. [10] reported that the 10-year risk of developing these complications was 1.8% for WBI alone versus 8.9% for WBI and SCRT after BCS for the treatment of early-stage breast cancer. Kahán et al. [16] reported that irradiation of the axillary and supraclavicular LNs favored the development of pneumonitis. The MA.20 trial also showed that patients in the RNI group had higher rates of Grade ≥2 lymphedema (8.4% and 4.5%, respectively; P < 0.01) and pneumonitis (1.2% and 0.2%, respectively; P = 0.01) [6]. Based on these observations, the adaptation of SCRT should be considered carefully.
Viani et al. [17] reported that the factors associated with supraclavicular recurrence free survival (SCRFS) were the number of positive axillary LNs, hormone receptor status, T stage, lymphovascular invasion, nuclear grade, and extracapsular extension for patients with N1 breast cancer treated by BCS with axillary LN dissection or sentinel biopsy followed by WBI. Similarly, Yu et al. [18] suggested that lymphovascular invasion and the number of involved axillary LNs had a significant effect on supraclavicular recurrence of pathological N1 tumors in breast cancer patients treated with mastectomy or BCT. They also reported that the highest level of involved axillary LNs, the percentage of positive LNs, and extracapsular extension showed significant effects on SCRFS. Both reports showed that the number of prognostic factors was significantly correlated with supraclavicular recurrence and survival. In our cohort study, we found that pathological T stage was significantly associated with regional recurrence. The 5-year RRFR for Stage ≥pT2 was 94.7%, which was lower than the pT1 stage (100.0%). Other factors, such as lymphovascular invasion, the number of positive LNs, and the percentage of positive LNs were not significantly associated with RRFR, probably because there were very few events. Because a small number of patients had regional recurrences, multivariate analyses could not be performed for these factors.
Standard tangential WBI can unintentionally irradiate some of the axillary LNs. In our study, the average mean dose to axillary Level 1 and the interpectoral LNs was 35.4 ± 6.7 Gy (70.8% ± 13.4% of the prescribed dose) and 30.8 ± 10.2 Gy (61.6% ± 20.4% of the prescribed dose), respectively. These results indicate that patients did not receive sufficient doses to axillary Level 1 or the interpectoral LNs. Furthermore, the average mean dose to Level 2 was lower; thus, standard WBI could not cover the axillary LNs. Our results are consistent with those of Reed et al. [11] and Rezink et al. [12].
The ACOSOG Z0011 trial demonstrated equivalent survival with one to two positive sentinel lymph nodes who were randomly assigned to the sentinel lymph node biopsy-alone group or the axillary dissection group [19]. They also reported that axillary recurrences were rare (0.9%) among the patients who did not receive axillary dissection, despite the 27% incidence of additional nodal disease among patients who were assigned to axillary dissection [20]. Therefore, incidental irradiation to the axillary LNs with WBI is thought to play a role in reducing the regional recurrences. However, the irradiation dose to the axillary LNs with WBI is insufficient as a treatment dose, and the practical role of WBI in reducing the regional recurrences is unclear. No studies have determined the relationship between the dose to the axillary LNs and regional recurrence in patients who are omitted axillary dissection versus those who received axillary dissection. In our study, we classified patients who received axillary dissection into two groups in accordance with the percentage of positive LNs, and we investigated the relationship between the dose to the axillary LNs and the RRFR. Neither the dose to Level 1 nor the dose to the interpectoral LNs was related to the RRFR. A possible reason is that most patients received enough axillary dissection even if the percentage of positive LNs was >20%. Truong et al. [14] reported that after mastectomy, involvement of ≥20% of the LNs was associated with locoregional failure; a few years earlier, those authors had found that >25% positive LNs was an adverse prognostic factor for locoregional recurrence when no radiotherapy was performed [21]. However, Fortin et al. [9] divided patients with one to three positive LNs into groups with <40% versus ≥40% positive LNs to investigate regional failure. In our cohort, only one patient had >40% positive LNs. Most patients may have received enough axillary dissection so that irradiation to the axillary LNs was unnecessary. When there is insufficient axillary dissection or when axillary dissection with positive sentinel LNs is omitted, an increased radiation dose to the axillary LNs may reduce regional recurrence. Further studies will be needed to resolve this issue.
Because of its retrospective design, our study may have some limitations, such as missing data for some parameters of lymphovascular invasion, nuclear grade, and hormonal receptors, as well as the absence of information for extracapsular invasion and the level of involved axillary LNs. Moreover, the parameters that determine the subtype and affect prognosis, such as the human epidermal growth factor receptor-2 status and Ki67 labeling index, were absent. Our chemotherapy regimen was very diverse and differed from recent standard regimens used in this type of cohort. One-third of the patients who received chemotherapy were treated using a single agent (such as oral fluorouracil) that is no longer a standard chemotherapeutic treatment for pathological N1 cancer. A prospective analysis will be needed to resolve these limitations.
In conclusion, while the pathological T stage was the prognostic factor best associated with regional recurrence, very few regional recurrences were observed in early breast cancer patients with one to three positive LNs treated with BCS followed by WBI. Unintentional radiation doses to axillary Level 1 and the interpectoral LNs using the tangential field of WBI were not associated with regional LN recurrence after axillary dissection.
ACKNOWLEDGEMENTS
This work was partly presented at the 23rd Annual Meeting of the Japanese Breast Cancer Society in 2–4 July 2015 at Tokyo International Forum, Tokyo.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol 2002;20:4141–9. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death:meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicini FA, Horwitz EM, Lacerna MD, et al. The role of regional nodal irradiation in the management of patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys 1997;39:1069–76. [DOI] [PubMed] [Google Scholar]
- 5.Grills IS, Kestin LL, Goldstein N, et al. Risk factors for regional nodal failure after breast-conserving therapy: regional nodal irradiation reduces rate of axillary failure in patients with four or more positive lymph nodes. Int J Radiat Oncol Biol Phys 2003;56:658–70. [DOI] [PubMed] [Google Scholar]
- 6.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015;373:307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015;373:317–27. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology https://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 9.Fortin A, Dagnault A, Blondeau L, et al. The impact of the number of excised axillary nodes and of the percentage of involved nodes on regional nodal failure in patients treated by breast-conserving surgery with or without regional irradiation. Int J Radiat Oncol Biol Phys 2006;65:33–9. [DOI] [PubMed] [Google Scholar]
- 10.Powell SN, Taghian AG, Kachnic LA, et al. Risk of lymphedema after regional nodal irradiation with breast conservation therapy. Int J Radiat Oncol Biol Phys 2003;55:1209–15. [DOI] [PubMed] [Google Scholar]
- 11.Reed DR, Lindsley SK, Mann GN, et al. Axillary lymph node dose with tangential breast irradiation. Int J Radiat Oncol Biol Phys 2005;61:358–64. [DOI] [PubMed] [Google Scholar]
- 12.Reznik J, Cicchetti MG, Degaspe B, et al. Analysis of axillary coverage during tangential radiation therapy to the breast. Int J Radiat Oncol Biol Phys 2005;61:163–8. [DOI] [PubMed] [Google Scholar]
- 13.Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol 2015;114:3–10. [DOI] [PubMed] [Google Scholar]
- 14.Truong PT, Jones SO, Kader HA, et al. Patients with T1 to T2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:357–64. [DOI] [PubMed] [Google Scholar]
- 15.Bedi C, Kron T, Willis D, et al. Comparison of radiotherapy treatment plans for left-sided breast cancer patients based on three- and four-dimensional computed tomography imaging. Clin Oncol (R Coll Radiol) 2011;23:601–7. [DOI] [PubMed] [Google Scholar]
- 16.Kahan Z, Csenki M, Varga Z, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys 2007;68:673–81. [DOI] [PubMed] [Google Scholar]
- 17.Viani GA, Godoi da Silva LB, Viana BS.. Patients with N1 breast cancer: Who could benefit from supraclavicular fossa radiotherapy. Breast 2014. [DOI] [PubMed] [Google Scholar]
- 18.Sharma R, Spierer M, Mutyala S, et al. Change in seroma volume during whole-breast radiation therapy. Int J Radiat Oncol Biol Phys 2009;75:89–93. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252:426–32; discussion 32–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truong PT, Berthelet E, Lee J, et al. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer 2005;103:2006–14. [DOI] [PubMed] [Google Scholar]