Abstract
Chemoradiation therapy is widely used to treat both inoperable and operable patients, and is less invasive than surgery. Although the number of long-term survivors who have received chemoradiation therapy is increasing, the long-term toxicity pattern and cumulative incidence of toxicity regarding this modality are poorly understood. Classically, chemoradiation therapy for esophageal cancer consists of an anterior–posterior field and a subsequent oblique boost field. We retrospectively analyzed patients who were treated with definitive chemoradiation therapy for esophageal cancer using this classical method from 1999 to 2008. For the assessment of toxicity, the National Cancer Institute Common Toxicity Criteria Version 3.0 was adopted. A total of 101 patients were analyzed. The median follow-up time was 16 months for all patients and 62 months for the surviving patients. Eleven patients experienced late toxicities of ≥Grade 3. Two patients died of late toxicities. The 3- and 5-year cumulative incidences for the first late cardiopulmonary toxicities of ≥Grade 3 were 17.4% and 20.8%, respectively. Cardiopulmonary effusions were observed within the first 3 years of completion of the initial treatment in seven out of eight patients. Sudden death and cardiac ischemia were observed over a 10-year period. Older age was found to be a risk factor for late toxicity after definitive chemoradiation therapy for esophageal cancer. Substantial toxicities were observed in patients who had received chemoradiation therapy for esophageal cancer using the classical method. To minimize the incidence of late toxicity, more sophisticated radiation techniques may be useful.
Keywords: esophageal cancer, chemoradiation therapy, long-term complications, cardiopulmonary toxicity, second malignancy, cumulative incidence
INTRODUCTION
Surgery is the standard treatment for operable esophageal cancer [1, 2]. However, the type of surgery required for this cancer is highly invasive, and the quality of life of patients who undergo such surgery is poor. Because patients with esophageal cancer sometimes have other severe comorbidities, less invasive therapy has been explored in patients who are not candidates for surgery. Historically, radiation therapy alone has been the standard therapy for unresectable or medically inoperable patients, but the outcomes of patients treated with this modality have not been satisfactory.
After the publication of a report concerning the intergroup randomized controlled trial (RTOG-8501), which demonstrated the superiority of concurrent chemoradiation therapy (CRT) over radiation therapy alone, combined-modality treatment became the standard nonsurgical treatment for esophageal cancer [3–5]. In terms of quality of life and acute toxicities, CRT is considered to have an advantage over surgery. In a preliminary report, the outcome of CRT was found to be similar to that of surgery [6–9]. For these reasons, CRT has become a treatment option not only for unresectable or medically inoperable patients but also for operable patients. The number of esophageal cancer patients treated with CRT has been increasing in Japan.
Unfortunately, as the number of long-term survivors increases after CRT, late complications such as heart failure, pleural effusion, pericardial effusion, and ischemic heart disease have become problems [10–12]. Some patients have died from late toxicities without cancer recurrence. Several factors have been reported as risk factors for radiation-induced late toxicities. However, especially over a 5-year period, the long-term complication pattern and cumulative incidence of toxicities have not been well described.
Radiation therapy for esophageal cancer classically consists of an anterior–posterior field and a subsequent oblique opposed field to shield the spinal cord. In the present study, we examined the incidence and pattern of long-term complications and the cumulative incidence of toxicities that occurred after definitive CRT involving the use of this classical field technique for esophageal cancer.
MATERIALS AND METHODS
Study design
This was a retrospective study regarding the late toxicities of CRT for esophageal cancer. The study was approved by the Institutional Ethical Review Board of the Kyoto University Hospital.
Patient characteristics
Between 1999 and 2008, 135 patients with thoracic esophageal cancer underwent definitive CRT at our institute. All patients had histologically confirmed esophageal cancer. Clinical stage was determined using computed tomography, X-ray fluoroscopy, and endoscopy. Endoscopic ultrasound of the esophagus, bronchoscopy, and whole-body fluorodeoxyglucose-positron emission tomography were used as appropriate. Tumor stage was classified according to the TNM classification of the Union for International Cancer Control, 6th edition.
A total of 34 patients were excluded from this analysis; 33 patients were not treated with an anterior–posterior opposed field and subsequent oblique opposed field. The characteristics of the remaining 102 patients are listed in Table 1. The median age of the patients was 65 (range, 41–82) years. One patient had adenocarcinoma, one patient had squamous cell carcinoma and adenocarcinoma simultaneously, and the remaining patients had squamous cell carcinoma.
Table 1.
Patient characteristics
| Characteristics | n = 102 |
|---|---|
| Age, median (range) | 65 (41–82) |
| Sex | |
| Male | 90 |
| Female | 12 |
| Histology | |
| Squamous cell carcinoma | 100 |
| Adenocarcinoma | 1 |
| Squamous cell carcinoma + adenocarcinoma | 1 |
| UICC T stage (6th) | |
| T1 | 18 |
| T2 | 14 |
| T3 | 37 |
| T4 | 33 |
| UICC N stage (6th) | |
| N0 | 13 |
| N1 | 89 |
| UICC M stage (6th) | |
| M0 | 73 |
| M1a | 10 |
| M1b | 19 |
| UICC clinical stage (6th) | |
| I | 4 |
| IIA | 6 |
| IIB | 17 |
| III | 46 |
| IVA | 10 |
| IVB | 19 |
| Primary site | |
| Ut | 16 |
| Ut–Mt | 20 |
| Ut–Lt | 2 |
| Mt | 30 |
| Mt–Lt | 17 |
| Mt–Ae | 2 |
| Lt | 15 |
UICC = Union for International Cancer Control; Ut = upper third of thoracic esophagus, esophagus; Mt = middle third of thoracic esophagus; Lt = lower third of thoracic esophagus; Ae = abidominal esophagus.
Radiation therapy technique
Radiation therapy was delivered using megavoltage equipment (6–15 MV). Initially, radiation therapy was performed using the anterior–posterior field technique, and total doses of 40–41.4 Gy were delivered in 1.8–2-Gy fractions. The initial clinical treatment volumes (CTVs) were basically designed as follows: the upper mediastinum, which extends from the cervical lymph nodes to ~3 cm below the tracheal bifurcation for upper-thoracic tumors; the whole mediastinum for middle-thoracic tumors; and the mediastinum and celiac and perigastric lymph nodes for lower thoracic tumors. The CTV for some early-stage patients was designed to include the primary lesion together with a 2- to 3-cm craniocaudal margin. The planning target volume (PTV) added a 0.5-cm margin to the CTV in the lateral and anterior/posterior direction and a 1-cm margin in the craniocaudal direction. The leaf margin was equivalent to the PTV plus 0.5 cm.
A radiation boost dose was delivered using an oblique opposed field to spare the spinal cord. The CTV for the boost dose consisted of the primary lesion plus 2–3 cm of the craniocaudal margin, and the metastatic lymph node plus a 0.5- to 1-cm margin. Areas of prophylactic irradiation were omitted from the CTV for the boost dose. The arrangement between the CTV and the PTV and leaf margins was the same as that used for the initial field.
Details of treatment
Details of the treatment are summarized in Table 2A and 2B. A total of 87 patients received radiation therapy at a cumulative dose of ≤60 Gy. The median radiation dose was 60 (range, 50–66.6) Gy. The median initial field length and boost field length were 24.0 (range, 13.5–30) cm and 15.4 (range, 5.5–28) cm, respectively. Four patients received an intraluminal brachytherapy median boost dose of 3.5 (range, 3–4) Gy after external beam irradiation (median 60 [range, 60–66] Gy) therapy. Total treatment time was 6–8 weeks.
Table 2A.
Details of the radiation therapy technique
| Parameters | |
|---|---|
| External beam radiation dose | |
| Median | 60 Gy |
| Range | 50–66.6 Gy |
| Initial field length | |
| Median | 24 cm |
| Range | 13.5–30 cm |
| Boost field length | |
| Median | 15.4 cm |
| Range | 5.5–28 cm |
Table 2B.
Combined chemotherapy regimens
| Chemotherapy regimen | n = 102 |
|---|---|
| CDDP + 5-FU | 63 |
| CDDP + 5-FU continuous infusion | 22 |
| 5-FU | 4 |
| CBDCA + 5-FU | 5 |
| CDGP + 5-FU | 3 |
| S-1 | 2 |
| Others | 3 |
CDDP = cisplatin; 5-FU = 5-fluorouracil; CBDCA = carboplatin; CDGP = nedaplatin; S-1 = TS-1; tegafur, gimeracil, oteracil potassium.
The chemotherapy regimens used mainly consisted of platinum agents plus 5-fluorouracil (5-FU) chemotherapy. A total of 83 patients received chemotherapy according to the KROSG-0101/JROSG-021 chemotherapy protocol [13]. Six patients received salvage surgery; among them, one patient died from postoperative complications. Two patients underwent salvage endoscopic therapy.
Follow-up
After the initial therapy, CT and endoscopic observation was performed every 1–3 months in the first year, 3–6 months in the second to fifth year, and annually after 5 years. When symptoms appeared, other surveys were mandatory for the attending physicians. Locoregional recurrence, distant metastasis, and cardiopulmonary toxicities were evaluated by means of barium swallow, upper gastrointestinal endoscopy, ultrasound scan, chest X-ray, or a thoraco-abdominal computed tomography scan. For the assessment of toxicity, the National Cancer Institute Common Toxicity Criteria Version 3.0 was adopted. On the basis of onset time, CRT toxicities were classified as acute toxicities or late toxicities at 6 months after the start of the treatment.
Statistical analysis
Analysis of survival and the incidence of late complications was performed using the Kaplan–Meier method. Univariate analysis of late toxicity was carried out using the Cox hazard model. Two-group analysis of the cumulative incidence of differences in late toxicity was conducted using the Grey test. The time to event was calculated from the start of radiation therapy. Univariate analysis was performed in relation to age, tumor location, initial field length, boost field length, chemotherapy regimen, total dose, and sex. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [14].
RESULTS
Survival
Overall survival as estimated using the Kaplan–Meier method is shown Fig. 1. The median follow-up time was 16 months. The complete response rate in the initial evaluation was 30%. The median follow-up period for surviving patients was 62 (range, 2–140) months, and the median survival time for all patients was 16 months. The 3- and 5-year overall survival rates for all patients were 32.9% and 26.8%, respectively.
Fig. 1.
Overall survival of all patients.
Acute toxicity (excluding hematological toxicity)
Non-hematological acute toxicities of ≥Grade 3 are summarized in Table 3A. They were as follows: six Grade 4 and two Grade 5 esophageal fistulas; one Grade 4 and one Grade 5 hepatic failure; one Grade 5 radiation pneumonitis; and one Grade 5 sudden death within 6 months after the start of CRT. Two patients died of aorta–esophageal fistulas at 55 and 82 days after the start of CRT. All patients who experienced esophageal fistulas had clinical T4–T3 stage disease at diagnosis. One patient died from acute hepatic failure at 3 months after the start of radiation therapy and before the assessment of therapeutic response, which was highly suggestive of 5-FU–related acute toxicity. One patient died from radiation pneumonitis at 4 months after treatment without cancer recurrence. One patient with cancer died suddenly at 6 months after treatment.
Table 3A.
Acute toxicities of >Grade 3
| Grade | 3 | 4 | 5 |
|---|---|---|---|
| Esophageal fistula | 0 | 6 | 2 |
| Hepatic failure | 0 | 1 | 1 |
| Radiation pneumonitis | Not assessed | 0 | 1 |
| Sudden death | 0 | 0 | 1 |
Late toxicity
The late complications of ≥Grade 3 that occurred after CRT are summarized in Tables 3B and 4. Seventeen late toxicities of ≥Grade 3 were observed in 11 patients. Two patients died of late toxicities. One patient died suddenly at 150 months after treatment without cancer recurrence. One patient died from acute myeloid leukemia at 77 months after treatment without esophageal cancer recurrence.
Table 3B.
Late toxicities of >Grade 3
| Grade | 3 | 4 | 5 |
|---|---|---|---|
| Pleural effusion | 8 | 0 | 0 |
| Pericardial effusion | 4 | 0 | 0 |
| Pneumonitis | 1 | 0 | 1 |
| Cardiac ischemia | 1 | 1 | 0 |
| Sudden death | 0 | 0 | 1 |
| Secondary malignancy | 0 | 0 | 1 |
Table 4.
Characteristics of patients with late toxicities of >Grade 3
| Number | Grade | Age (years) | Primary site | Late toxicities | Onset (months) | Outcome | Cause of death | Chemotherapy regimen | Radiation dose (Gy) | Initial field length (cm) | Boost field length (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 70 | Mt–Lt | Sudden death | 150 | Died without relapse | Sudden death | CDDP + 5-FU continuous infusion | 50 | 22 | 9.0 |
| 2 | 5 | 67 | Ut–Mt | Secondary malignancy | 77 | Died with secondary malignancy | AML | CDDP + 5-FU | 60 | 24 | 18.0 |
| 3 | Pleural effusion | 35 | |||||||||
| 3 | 4 | 73 | Ut–Mt | Cardiac ischemia | 57 | Died without relapse | ARDS | CDDP + 5-FU continuous infusion | 60 | 24 | 12.1 |
| 3 | Pleural effusion | 40 | |||||||||
| 3 | Pericardial effusion | 40 | |||||||||
| 4 | 3 | 74 | Mt | Cardiac ischemia | 151 | Died with lung cancer (out of field) | Lung cancer | CDDP + 5-FU continuous infusion | 55 | 26 | 19.8 |
| 5 | 3 | 58 | Lt | Pleural effusion | 22 | Died with relapse | Esophageal cancer | CDDP + 5-FU | 60 | 29 | 20.6 |
| 3 | Pericardial effusion | 22 | |||||||||
| 6 | 3 | 74 | Ut–Mt | Pleural effusion | 20 | Died without relapse | Infectious pneumonitis | CDDP + 5-FU | 64 | 21.5 | 10.0 |
| 7 | 3 | 80 | Ut–Mt | Pleural effusion | 19 | Died with relapse | Esophageal cancer | CDDP + 5-FU | 60 | 24 | 11.3 |
| 8 | 3 | 71 | Mt | Pleural effusion | 19 | Died with relapse | Esophageal cancer | CDDP + 5-FU continuous infusion | 60 | 27.1 | 14.0 |
| 3 | Pericardial effusion | 19 | |||||||||
| 9 | 3 | 68 | Mt–Lt | Pleural effusion | 14 | Died with relapse | Esophageal cancer | CDDP + 5-FU | 60 | 28.2 | 20.2 |
| 10 | 3 | 81 | Lt | Pleural effusion | 13 | Alive without relapse | S-1 | 54 | 21 | 10.0 | |
| 3 | Pericardial effusion | 13 | |||||||||
| 11 | 3 | 59 | Ut | Radiation pneumonitis | 10 | Died with relapse | Esophageal cancer | CDDP + 5-FU | 60 | 25 | 15.6 |
AML = acute myeloid leukemia; Ut = upper third of thoracic esophagus; Mt = middle third of thoracic esophagus; Lt = lower third of thoracic esophagus; CDDP = cisplatin; 5-FU = 5-fluorouracil; S-1 = TS-1; tegafur, gimeracil, oteracil potassium.
Regarding cardiopulmonary effusions, there were four Grade 3 pericardial effusions and eight Grade 3 pleural effusions. All four patients who had Grade 3 pericardial effusions also had Grade 3 pleural effusions; both pericardial and pleural effusion were observed simultaneously at 13, 16, 23 and 40 months after treatment. In our study, no patients died from chronic heart failure during the follow-up period. All cases of cardiopulmonary effusions were manageable with diuretics, chest tube drainage, or pleurodesis. No cases of cardiopulmonary effusion were observed in patients whose primary tumor was exclusively located in the upper third of the thoracic esophagus. As for other cardiopulmonary toxicities, one acute myocardial infarction, one cardiac arrest, and one radiation pneumonitis that required oxygen support were observed.
The cumulative incidence and timing of late toxicity
The estimated cumulative incidence of the first late cardiopulmonary toxicity episodes of ≥Grade 3 as calculated using the Kaplan–Meier method is shown in Fig. 2. The overall 3- and 5-year cumulative incidence rates were 17.4% and 20.8%, respectively. The first late toxicities occurred within the first 3 years of the completion of the initial treatment in eight out of 11 patients. In particular, the first pericardial and pleural effusions were observed within the first 3 years of completion of the initial treatment in seven out of eight patients.
Fig. 2.
Cumulative incidence of first cardiopulmonary late toxicities.
The factor of late toxicity
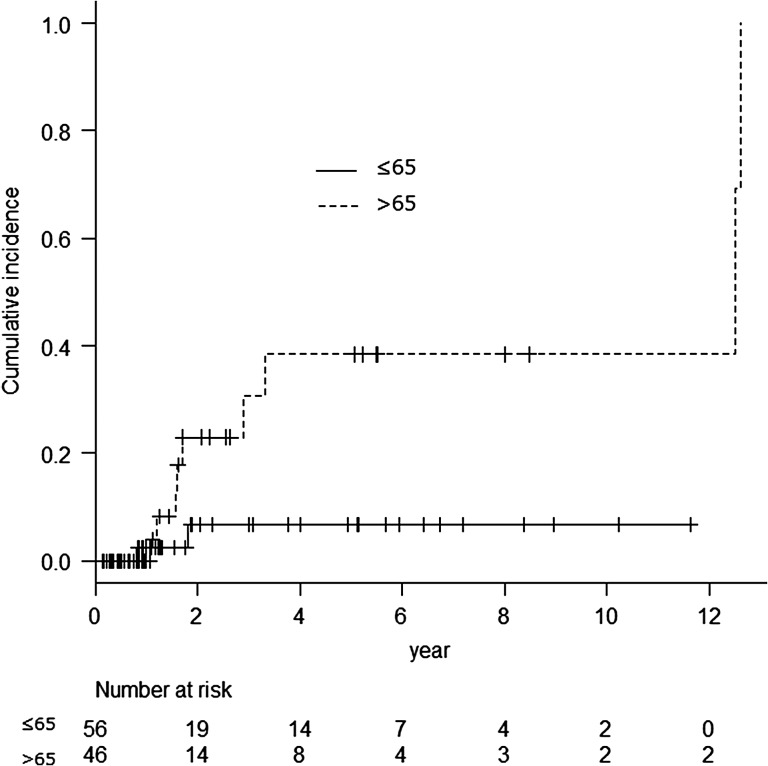
Univariate analysis was performed in relation to age, location, initial field length, boost field length, sex, chemotherapy regimen, and total dose. Results regarding the analysis of first late toxicities are shown in Table 5. The cut-off point for continuous variables was the median value. The patients whose primary tumor was located exclusively in the thoracic esophagus did not have pleural effusion or pericardial effusion; however, the primary tumor location was not a risk factor regarding late cardiopulmonary toxicity in this study. In univariate analysis, age was found to be the only risk factor for late cardiopulmonary toxicity. When the patients were divided into two groups based on age (≤65 years and >65 years), the older group was found to have experienced significantly more late toxicity (Grey test, P = 0.029; Fig. 3). The 5-year incidence of late toxicity was 6.8% in the younger group and 38.4% in the older group.
Table 5.
Univariate analysis results regarding first late toxicities
| P-value | HR | 95% CI | |
|---|---|---|---|
| Age (years) >65/≤65 | 0.046 | 4.93 | 1.03–23.64 |
| Initial field length (cm) >24.4/≤24.4 | 0.49 | 1.57 | 0.43–5.70 |
| Boost field length (cm) >15.4/≤15.4 | 0.93 | 0.94 | 0.27–3.28 |
| Sex male/female | 0.99 | ||
| Chemotherapy platinum containing/non-containing | 0.41 | 2.63 | 0.26–2.63 |
| Radiation dose (Gy) ≥60/<60 | 0.96 | 0.95 | 0.11–8.35 |
| Location, Ut/lower than Ut | 0.56 | 0.52 | 0.058–4.68 |
Ut = upper third of thoracic esophagus.
Fig. 3.
Cumulative incidence of cardiopulmonary late toxicities divided by age. Grey test, P = 0.029.
DISCUSSION
Surgery is the standard treatment for operable esophageal cancer. However, the type of surgery required for this cancer is highly invasive. Because patients with esophageal cancer sometimes have other severe comorbidities, less invasive therapy has been explored not only in patients who are not candidates for surgery but also in operable patients. After a number of studies had reported good outcomes regarding CRT for esophageal cancer [6–9], this modality has gradually become the treatment of choice for esophageal cancer, not only in inoperable patients but also in operable patients. In terms of quality of life and acute toxicities, CRT is considered to have an advantage over surgery. However, in long-term follow-up, late toxicities associated with CRT for esophageal cancer have become a problem [10–12]. The most common late CRT toxicities are pericardial effusion and pleural effusion. Although almost all of these complications can be managed with diuretics, needle drainage, or pleurodesis, they not only affect patient quality of life but can also occasionally be fatal [8, 15]. In previous studies, although treatment regimens, field set-up, patient background, and evaluation of late toxicity were individualized, 5–16% of patients experienced some type of severe cardiopulmonary late toxicity after classical radiation therapy; this comprised irradiation using an anterior–posterior field and a subsequent booster dose delivered to an oblique opposed field [10–12]. In the present study, 12.1% of patients experienced late toxicities, and their cumulative rate at 5 years was ~20%. Morota et al. and Kato et al. reported that the cumulative rate of late toxicities was ~30% [8, 12]. With 10 years’ additional follow-up, we found that the cumulative incidence of severe cardiopulmonary effusions did not increase year on year, but rather had plateaued at ~3 years. The most severe cardiopulmonary toxicities were also seen within 3 years in previous studies [10–12].
We performed univariate analysis concerning the risk factors for late cardiopulmonary toxicities; age was found to be the only significant risk factor. Previous studies have also reported that older patients are at high risk of late complications [12, 16]. Generally, elderly patients have a low performance status and multiple comorbidities. In a previous report, patients whose performance status was 2 had a greater risk regarding cardiopulmonary effusions than patients with a performance status of 0–1 [16]. However, in general, the performance status of patients who have received platinum-containing CRT is assumed to be 0–1; this was also the assumption made in our study. Mild cardiopulmonary comorbidities may not be a risk factor for cardiopulmonary complications, as previously reported [16, 17].
The incidences of ischemic heart disease and sudden death that have been reported in previous studies were 1.4–3.7% [10–12, 15], and they were 3% in our study. Although sudden death may be the result of radiation-induced heart arrhythmias, if it is associated with Grade 5 ischemic heart disease, cardiac ischemia can be seen not only at <3 years after CRT but also at >10 years after CRT, unlike pericardial effusion and pleural effusion. This finding is in accordance with the findings of previous studies involving late complications associated with radiation therapy for Hodgkin's lymphoma and breast cancer [18]. In an analysis of postoperative radiation therapy for breast cancer, a history of prior ischemic heart disease and greater age increased the risk of post–radiation therapy ischemic heart disease [19]. We should be aware that patients who have such a background tend to be treated using CRT, but they are at high risk of post-radiation ischemic heart disease. In previous studies, chronic heart failure was reported to have occurred at rates of 1.4–3.7% [10–12]. Sometimes patients died from chronic heart failure. However, in our study, no patients died from this condition during the follow-up period.
Although tumor location was not a significant risk factor for late cardiopulmonary toxicities in univariate analysis in our study, cardiac toxicities and pleural effusions only occurred after irradiation of the middle to lower third of thoracic esophageal cancer. The middle to lower thoracic area may be the organ at risk (OAR) of cardiopulmonary effusions, because the radiation field is terminated at the subcarina level in upper third thoracic esophageal cancer.
Secondary malignancies also remain a concern after CRT. In our analysis, one patient died from acute myeloid leukemia at 77 months after treatment without cancer recurrence. Chang et al. also reported two acute myeloid leukemia cases at 55 and 20 months after definitive CRT for esophageal cancer [20]. The necessity of longer follow-up times should be emphasized after CRT. In our study one patient died from lung cancer, but the lung cancer primary site was located outside of the irradiation field.
To reduce the risk of these late toxicities, we should make efforts to improve the quality of radiation therapy because most late toxicities are assumed to be caused by radiation therapy. Using a more precise therapy technique such as radiation therapy involving multiple field irradiation, intensity-modulated radiation therapy [21], or particle beam therapy [22] may also be beneficial. With these techniques, we can deliver an optimal radiation dose to the clinical target without increasing the dose delivered to the OAR. In a retrospective study, Lin et al. reported that overall survival, non-cancer-related death, and the cumulative incidence of cardiac death had been significantly improved after intensity-modulated radiation therapy relative to 3D radiation therapy [21]. This study suggested that the use of the latest precision radiation therapy techniques can increase overall survival, not only by improving local tumor control but also by reducing radiation-related toxicities by avoiding OARs. Another method that can be used to reduce the dose to OARs is the elimination of elective nodal irradiation, although this approach is still controversial [23].
Although many Japanese radiation oncologists continue to prescribe a total dose of 60 Gy for esophageal cancer [15], a randomized study has demonstrated the superiority of radiation therapy at total doses of 50.4 Gy over 64.8 Gy [24]. To take into account long-term complications, Japanese radiation oncologists should consider a reduction in the prescribed dose to reduce OAR dose exposure. A Japanese Phase II study that will evaluate CRT at a total dose of 50.4 Gy, including salvage treatment (JCOG0909), has been planned and has ended accrual. The findings of this study could result in the prescribed dose being changed for esophageal cancer in Japan.
We evaluated the long-term toxicity pattern and cumulative incidence associated with CRT for esophageal cancer using an anterior–posterior field and a subsequent oblique boost field. CRT is an effective and less invasive therapy than surgery, but has substantial long-term toxicity. We should assess the results of CRT with long-term toxicity in mind. Further investigation aimed at finding ways to minimize the incidence of late toxicity, such as using more sophisticated radiation therapy techniques and/or reducing the prescribed doses, is warranted.
The present study had a number of limitations. It was retrospective and involved only one institute; it also entailed the use of a non-uniform protocol, and lacked detailed patient background data. However, because the long-term complication patterns and the cumulative incidence of definitive CRT for esophageal cancer have not been well described, we believe that our study has value.
CONCLUSION
Approximately 20% of patients who received definitive CRT for esophageal cancer using the classical method had substantial long-term toxicity. With 10 years’ additional follow-up, we found that the cumulative incidence of severe cardiopulmonary effusions did not increase year on year, but rather had plateaued at ~3 years. Older age was a risk factor for long-term toxicities. To minimize the incidence of late toxicity, more sophisticated radiation techniques may be useful.
ACKNOWLEDGEMENTS
The authors wish to thank Dr Michihide Mitsumori for his leadership regarding radiation therapy for esophageal cancer.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Igaki H, Kato H, Tachimori Y, et al. . Clinicopathologic characteristics and survival of patients with clinical Stage I squamous cell carcinomas of the thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg 2001;20:1089–94. [DOI] [PubMed] [Google Scholar]
- 2.Morita M, Yoshida R, Ikeda K, et al. . Advances in esophageal cancer surgery in Japan: an analysis of 1000 consecutive patients treated at a single institute. Surgery 2008;143:499–508. [DOI] [PubMed] [Google Scholar]
- 3.al-Sarraf M, Martz K, Herskovic A, et al. . Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol 1997;15:277–84. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Guo MD, Herskovic A, et al. . Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- 5.Herskovic A, Martz K, al-Sarraf M, et al. . Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–8. [DOI] [PubMed] [Google Scholar]
- 6.Ariga H, Nemoto K, Miyazaki S, et al. . Prospective comparison of surgery alone and chemoradiotherapy with selective surgery in resectable squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2009;75:348–56. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Sato A, Fukuda H, et al. . A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009;39:638–43. [DOI] [PubMed] [Google Scholar]
- 8.Kato K, Muro K, Minashi K, et al. . Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II–III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys 2011;81:684–90. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsu A, Boku N, Muro K, et al. . Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol 1999;17:2915–21. [DOI] [PubMed] [Google Scholar]
- 10.Ishikura S, Nihei K, Ohtsu A, et al. . Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol 2003;21:2697–702. [DOI] [PubMed] [Google Scholar]
- 11.Kumekawa Y, Kaneko K, Ito H, et al. . Late toxicity in complete response cases after definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Gastroenterol 2006;41:425–32. [DOI] [PubMed] [Google Scholar]
- 12.Morota M, Gomi K, Kozuka T, et al. . Late toxicity after definitive concurrent chemoradiotherapy for thoracic esophageal carcinoma. Int J Radiat Oncol Biol Phys 2009;75:122–8. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura Y, Mitsumori M, Hiraoka M, et al. . A randomized phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer: short-term infusion versus protracted infusion chemotherapy (KROSG0101/JROSG021). Radiother Oncol 2009;92:260–5. [DOI] [PubMed] [Google Scholar]
- 14.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura Y, Jingu K, Itasaka S, et al. . Clinical outcomes of radiotherapy for esophageal cancer between 2004 and 2008: the second survey of the Japanese Radiation Oncology Study Group (JROSG). Int J Clin Oncol 2016;21:88–94. [DOI] [PubMed] [Google Scholar]
- 16.Shirai K, Tamaki Y, Kitamoto Y, et al. . Dose–volume histogram parameters and clinical factors associated with pleural effusion after chemoradiotherapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys 2011;80:1002–7. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Fujiwara Y, Nomura M, et al. . Predictive factors for pericardial effusion identified by heart dose–volume histogram analysis in oesophageal cancer patients treated with chemoradiotherapy. Br J Radiol 2015;88:20140168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darby SC, Cutter DJ, Boerma M, et al. . Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darby SC, Ewertz M, McGale P, et al. . Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 20.Chang H, Liaw CC, Chang HK.. Therapy-related acute myeloid leukemia after concurrent chemoradiotherapy for esophageal cancer: report of two cases. Tumori 2009;95:371–3. [DOI] [PubMed] [Google Scholar]
- 21.Lin SH, Wang L, Myles B, et al. . Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugahara S, Tokuuye K, Okumura T, et al. . Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys 2005;61:76–84. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Zhang X, Zhao F, et al. . Involved-field radiotherapy for esophageal squamous cell carcinoma: theory and practice. Radiat Oncol 2016;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minsky BD, Pajak TF, Ginsberg RJ, et al. . INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–74. [DOI] [PubMed] [Google Scholar]