Abstract
Treatment strategies for nasal extranodal NK/T-cell lymphoma (ENKTL), including sequential chemotherapy followed by radiotherapy (SCRT), concurrent chemoradiotherapy (CCRT), or radiotherapy alone (RT), remain varied. The purpose of this study was to assess the treatment outcome, the toxicity, and the potential prognostic factors for patients with early-stage nasal ENKTL treated using definitive RT (minimum of 50 Gy) with or without chemotherapy. From 1998 to 2014, 37 patients were included in the study. Eight patients were treated with RT alone, 1 with CCRT, and 28 with SCRT. Local regional control (LRC), progression-free survival (PFS), and overall survival (OS) were calculated using the Kaplan–Meier method. RT resulted in an overall response rate of 91.2%, with a complete response rate of 78.4%. After a median follow-up time of 36.8 months, the 3-year LRC, PFS and OS were 87.4%, 64.0% and 76.3%, respectively. Acute severe toxicity (Grade 3) of mucositis was observed in 6 (16.2%) of the 37 patients. In univariate analyses, extensive disease (Stage I/II with local invasiveness) and the presence of B symptoms were significantly associated with a poor PFS, whereas extensive disease was significantly associated with a poor OS. Multivariate analysis identified the presence of extensive disease as an independent predictor of PFS (P < 0.001) and OS (P = 0.015). High-dose RT with or without chemotherapy reported promising locoregional control and a favorable outcome for patients with early-stage nasal ENKTL without local invasiveness. Further investigation of new treatment strategies for patients with local invasiveness is warranted.
Keywords: nasal NK/T-cell lymphoma, radiotherapy, chemotherapy, prognosis
INTRODUCTION
Extranodal NK/T-cell lymphoma (ENKTL), a rare subtype of lymphoma, is recognized as a distinct entity in the World Health Organization (WHO) classification [1]. ENKTL accounts for <1% of lymphomas in western countries, but it is relatively common in Latin America [2] and Asia, where it accounts for ~3–10% of lymphomas [3, 4]. The tumor cells of ENKTL are characterized by immunochemical staining of CD3 epsilon+, CD2+, CD56+ and CD20−. The presence of Epstein–Barr virus (EBV) infection detected through in situ hybridization for EBV-encoded small ribonucleic acid (RNA) is a distinctive feature of ENKTL [5]. Because EBV infection is implicated in disease pathogenesis of ENKTL, the presence of EBV was incorporated into the disease definition of the 2008 revised version of the WHO guidelines [5–7]. Clinically, ENKTLs, nasal type, were defined as those involving the entire upper-aerodigestive tract (UAT) and non-UAT regions, whereas nasal ENKTL was defined as primary tumors involving the nasal cavity, paranasal sinuses, or nasopharynx.
Ann Arbor stages and International Prognostic Index (IPI) scores have been used to predict the outcome of ENKTL in previous studies; however, they did not report optimal prognostic values for progression-free survival (PFS) or overall survival (OS) of ENKTL [8, 9]. In a multicenter study including 262 patients with ENKTL, Lee et al. proposed a new prognostic model for stratifying these patients to four different risk groups depending on the presence of B symptoms, stage, serum lactate dehydrogenase (LDH) level and regional lymphadenopathy, and revealed that patients with three or four of the aforementioned factors (Group 4) had a much worse outcome compared with the other three groups [10]. Further, Kim et al. observed that local invasiveness was significantly associated with the poor disease-free survival and OS in patients with ENKTL, and classified these patients into two different stages (limited disease and extensive disease) based on disease extents and local invasiveness [11].
ENKTL was relatively refractory to anthracycline-based chemotherapy in comparison with B-cell non-Hodgkin's lymphoma (NHL). Previous studies have reported that among patients with early-stage nasal ENKTL, combined chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone) and radiotherapy (RT) resulted in a 5-year OS rate of 50% [12–15]. L-asparaginase has been known to be an effective cytotoxic agent for ENKTL, and several L-asparginase–incorporated chemotherapy regimens have been reported to have promising clinical results associated with ENKTL treatment [16–18]. In addition to chemotherapy, local RT alone has been demonstrated to provide a satisfactory response and local control for Stage I/II NHL [19–21]. A RT dose of 24–40 Gy was reported to achieve an optimal treatment response and local control for B-cell NHL [19], but a higher RT dose (~45–55 Gy) is required for nasal ENKTL [20, 21]. However, the optimal dose of RT for nasal ENKTL remains unclear.
In this study, we assessed the relationship between Lee's prognostic score, Kim's new stage stratification, treatment modalities and treatment response, and the clinical outcome of patients with Stage IE/IIE nasal ENKTL who received definitive RT (at least 50 Gy) with or without chemotherapy.
MATERIALS AND METHODS
Clinicopathologic features of patients
A total of 40 patients with nasal ENKTL who received RT with or without chemotherapy in our institution between October 1998 and December 2014 were retrospectively analyzed. Among them, 37 patients with Ann Arbor Stage IE/IIE were included in this study, whereas 3 patients with Ann Arbor Stage III–IV advanced disease were excluded.
Patients were confirmed histologically to have nasal ENKTL, which is characterized by classical pathological findings of angiocentric infiltration and necrosis. The immunochemical stains of the tumor cells of a nasal ENKTL were positive for cytoplasmic CD3 epsilon, CD2 and CD56 and were negative for CD20. We performed in situ hybridization for EBV-encoded early small RNA (EBER) for tumor samples of 31 patients to determine the presence of an EBV infection, and demonstrated positive EBER in 27 patients.
Pretreatment evaluation involved history taking, comprehensive physical examination, complete blood cell count, blood chemistry analysis (including LDH), bone marrow studies, and head and neck imaging computed tomography (CT) or magnetic resonance imaging (MRI). In the present study, all patients with nasal ENKTL received ear, nose and throat examination through transnasal endoscopy and physical examination before treatment (either chemotherapy or radiotherapy). From 2006, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) scans were used for lymphoma staging in our institution, and 19 of these 37 patients had undergone PET scans for initial staging.
Patients were staged based on the Ann Arbor stages and the new stage classification through Kim's stratification [8, 11]. ‘Limited disease’ was defined as Stage I/II disease without local invasiveness, whereas ‘extensive disease’ was defined as Stage I/II disease with local invasiveness. Local invasiveness is defined by a lymphoma with bony invasion or perforation, or invasion of the overlying skin. Because our patients were all Ann Arbor Stage IE/IIE, we modified Lee's ENKTL prognostic score [10] (including three prognostic factors: presence of B symptoms, elevated serum LDH level, and regional lymphadenopathy) and divided our patients into two subgroups: Group I (with 0 or 1 prognostic factor) and Group II (with 2 or 3 prognostic factors).
The study protocol was approved by the Research Ethical Committee of the National Taiwan University Hospital (NTUH: 201410063RINB). The patients’ medical data were anonymized prior to access and analysis. The institutional review board has waived the need for written informed consent from study subjects because all potentially patient-identifying information was removed prior to data analysis.
Radiotherapy
Patients were treated with a 6 MV photon beam, but some received a 6–9 MeV electron beam for superficial lesions. Ten patients were treated with a 2D technique, 5 patients were treated with a 3D conformal technique, 14 patients received intensity-modulated radiotherapy (IMRT), 5 patients received volumetric-modulated arc therapy (VMAT), and 3 patients were treated with Tomotherapy (Table 1). The radiation prescription dose ranged from 50 Gy to 71 Gy, with a median dose of 50 Gy, in 1.8–2.0 Gy per daily fraction (Table 1).
Table 1.
Treatment characteristics of 37 patients with nasal extranodal NK/T-cell lymphoma
| Treatment strategies | Number of patients | % | |
|---|---|---|---|
| Treatment sequence | |||
| RT alone | 8 | 21.6 | |
| C/T→RT or CCRT | 24 | 64.9 | |
| CCRT | 1 | 2.7 | |
| C/T→RT→C/T (Sandwich) | 4 | 10.8 | |
| C/T regimen | |||
| CHOP (or CHOP like) | 16 | 55.2 | |
| L-VP | 8 | 27.6 | |
| Others | 5 | 17.2 | |
| RT dose (Gy) | 50 (median) | ||
| 50–60 | 33 | 89.2 | |
| ≥60 | 4 | 11.8 | |
| RT technique | |||
| 2D | 10 | 27.0 | |
| 3D | 5 | 13.5 | |
| IMRT | 14 | 37.8 | |
| VAMT | 5 | 13.5 | |
| Tomotherapy | 3 | 8.1 | |
| RT fielda | |||
| Extended-field | 28 | 75.7 | |
| Limited-field | 9 | 24.33 | |
C/T = chemotherapy, RT = radiotherapy, CCRT = concurrent chemoradiotherapy, LN = lymph nodes, IMRT = intensity-modulated radiation therapy, VMAT = volumetric-modulated arc therapy, HSCT = hematopoietic stem cell transplantation, CHOP = cyclophosphamide + adriamycin + vincristine + prednisolone, L-VP = L-asparaginase + vincristine + prednisolone.
aRT field: limited-field: primary involved regions; extended-field: primary involved regions plus cervical lymphatics.
Radiotherapy to primary involved regions, including the nasal cavity, nasopharynx, and paranasal sinuses, was defined as limited-field RT. RT to the primary involved regions and cervical lymphatics (usually Level I, II, III and IV) was defined as extended-field RT. For patients who were treated with 3DRT, IMRT, VMAT and Tomotherapy, the contouring was based on a simulation CT scan. PET-avid lesions were included in the treatment field if PET was available. An example of extended-field dose distribution is demonstrated in Supplementary Figure 1. Among the 37 patients, 9 (24.3%) received limited-field RT, and 28 (75.7%) received extended-field RT.
Chemotherapy
In the current study, 8 (21.6%) patients with nasal ENKTL were treated with RT alone (RT alone group), and the other 29 (78.4%) patients received at least one course of chemotherapy (combined modality group). Treatment modalities for our patients are summarized in Table 1. Among these 29 patients, 16 (55.2%) received an anthracycline-based regimen such as CHOP or a CHOP-like regimen, 8 (27.6%) were treated with an L-VP (L-asparaginase, vincristine, prednisolone) regimen, and 5 (17.2%) received other chemotherapy regimens including concurrent CCRT with cisplatin, cyclophosphamide only, DVP (dexamethasone, etoposide, cisplatin), or SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide). Among the 13 patients who were treated with an L-asparaginase–containing chemotherapy regimen, 8 patients received L-VP, 1 received SMILE, and 4 patients received L-CHOP.
Statistical analysis
The treatment response was assessed using Cheson's criteria [22]. The treatment response was evaluated using radiographic (mainly CT or MRI) studies after completing RT or RT and chemotherapy. Toxicity was evaluated using the Common Toxicity Criteria for Adverse Events (CTCAE) 4.0. OS was measured from the date of diagnosis to death from any cause or the time from the date of diagnosis to the last follow-up (i.e. censor date). PFS was measured from the date of diagnosis to the date of disease progression or death from any cause, or the time from the date of diagnosis to the last follow-up (i.e. censor date). Local regional control (LRC) was defined as the time to local progression or death, or the time from the date of diagnosis to the last follow-up (i.e. censor date). The OS, PFS and LRC were calculated using the Kaplan–Meier method, and the comparison between survival curves was calculated using a log-rank test. Hazard analyses for OS and PFS were calculated by forward stepwise regression using the Cox regression model. A P value of <0.05 (two-tailed) was considered statistically significant.
RESULTS
Clinicopathologic features and treatment response
The clinicopathologic features of ENKTL patients are listed in Table 2. Twenty-three patients were classified as Group I (with 0 or 1 prognostic factor) and 14 patients as Group II (with 2 or 3 prognostic factors), using the modified NK/T-cell lymphoma prognostic index proposed by Lee et al. [8]. Twenty-seven patients (73.0%) were classified as having limited disease and 10 patients as having extensive disease, using the new stage system proposed by Kim et al. [9].
Table 2.
Demographic characteristics of 37 patients with nasal extranodal NK/T-cell lymphoma
| Characteristics | No. of patients | % | |
|---|---|---|---|
| Total | 37 | 100.0 | |
| Age | 52 (median) | ||
| >60 | 10 | 27.0 | |
| ≤60 | 27 | 73.0 | |
| Gender | |||
| Male | 26 | 70.3 | |
| Female | 11 | 29.7 | |
| Ann Arbor stage | |||
| IE | 29 | 78.4 | |
| IIE | 8 | 21.6 | |
| B symptoms | |||
| No | 25 | 67.6 | |
| Yes | 12 | 32.4 | |
| ECOG performance status | |||
| 0 | 17 | 45.9 | |
| 1 | 20 | 54.1 | |
| Regional lymphadenopathies | |||
| No | 29 | 78.4 | |
| Yes | 8 | 21.6 | |
| LDHa | |||
| Normal | 12 | 32.4 | |
| Elevated | 23 | 62.2 | |
| IPI score | |||
| 0 | 8 | 21.6 | |
| 1 | 24 | 64.9 | |
| 2 | 5 | 13.5 | |
| PET staging | |||
| No | 18 | 48.6 | |
| Yes | 19 | 51.4 | |
| New stageb | |||
| Limited | 27 | 73.0 | |
| Extensive | 10 | 27.0 | |
| ENKTL prognostic indexc | |||
| Group I | 23 | 62.2 | |
| Group II | 14 | 37.8 | |
No. = number, ECOG = Eastern Cooperative Oncology Group, IPI = International Prognostic Index, LDH = lactate dehydrogenase.
aLDH normal range: 140–271 U/L.
bLimited disease as Stage I/II upper aerodigestive tract (UAT) disease without invasiveness; extensive disease as Stage I/II disease with local invasiveness or Stage III/IV disease of UAT or non-UAT disease.
cModified from prognostic index proposed by Lee et al., J Clin Oncol 2006;24:612–8 [10].
Thirty-five patients completed the course of RT. One patient did not complete the RT course because of rapid disease progression, and the other patient died from incidentally found Type III dissected aortic aneurysm rupture. RT with and without chemotherapy resulted in an overall response rate of 91.2%, with a complete response (CR) rate of 78.3% (29 of 37 patients). The CR rate was 75% and 79.3% for the RT alone group and for the combined modality group, respectively. Six (16.2%) out of the 37 patients receiving RT with or without chemotherapy experienced acute Grade 3 mucositis, and 2 (5.4%) experienced Grade 3 dermatitis. For 22 patients receiving a modern RT technique such as IMRT, VMAT or Tomotherapy (1 limited-field RT; 21 extended-field RT), we demonstrated an incidence of acute Grade 3 mucositis of 5/22 (22.7%) and dermatitis of 1/22 (4.5%). In contrast to this, among 15 patients who underwent 2D or 3D RT (8 limited-field RT; 7 extended-field RT), 1 patient (6.7%) experienced acute Grade 3 mucositis and 1 patient (6.7%) developed acute Grade 3 dermatitis. There were no medical records of ophthalmic or central nervous system toxicities. No Grade 3–4 late toxicity or treatment-related death was reported (Supplementary Table 1).
Clinical outcomes and prognostic factors
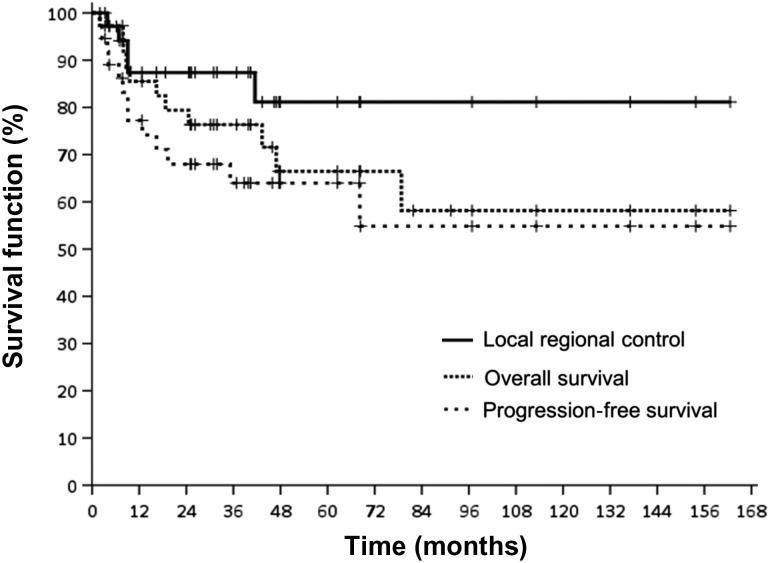
The median follow-up time for the 37 patients was 36.8 months. The 3-year LRC, PFS and OS for all patients were 87.4%, 64.0% and 76.3%, respectively (Fig. 1, Supplementary Table 2). The 3-year LRC, PFS and OS for patients who underwent RT alone were 83.3%, 72.9% and 72.9%, respectively, whereas the results for patients who underwent combined modality treatments were 88.6%, 62.9% and 77.8%, respectively. Among 27 patients with limited disease, combined modality treatments group (n = 19) had a trend of improved 3-year LRC, PFS and OS, but none of these differences were statistically significant when compared with the RT alone group (n = 8) (Supplementary Table 3).
Fig. 1.
Overall survival (OS), progression-free survival (PFS) and local regional control (LRC) for all patients.
During the follow-up period, 11 (29.7%) of 37 patients had a recurrence of the disease. Of these patients, 1 patient had local regional recurrence only, 7 patients had distant metastasis without evidence of local regional disease, and 3 patients had both local and distant failure. Among the 11 patients with recurrence or metastases, 3 (27.3%) achieved partial remission after salvage chemotherapy, and 4 received palliative radiotherapy for their metastatic lesions. Two patients underwent salvage peripheral blood stem cell transplantation (PBSCT, one autologous and one allogeneic) for recurrent disease; both were alive after PBSCT. Of all the patients, six patients died of lymphoma, two patients died of infection, one patient died of another cancer, one patient died of GVHD after transplantation, and one patient died of cardiac events (aortic aneurysm rupture). Among the three patients who died of intercurrent disease, two patients received RT alone (one with infection and another with aortic aneurysm rupture), whereas the other patient receiving induction chemotherapy with two courses of CHOP followed by RT developed infection-associated mortality more than 6 months after completing chemotherapy. These results indicate that the causes of death for these three patients were not related to the adverse effects of chemotherapy.
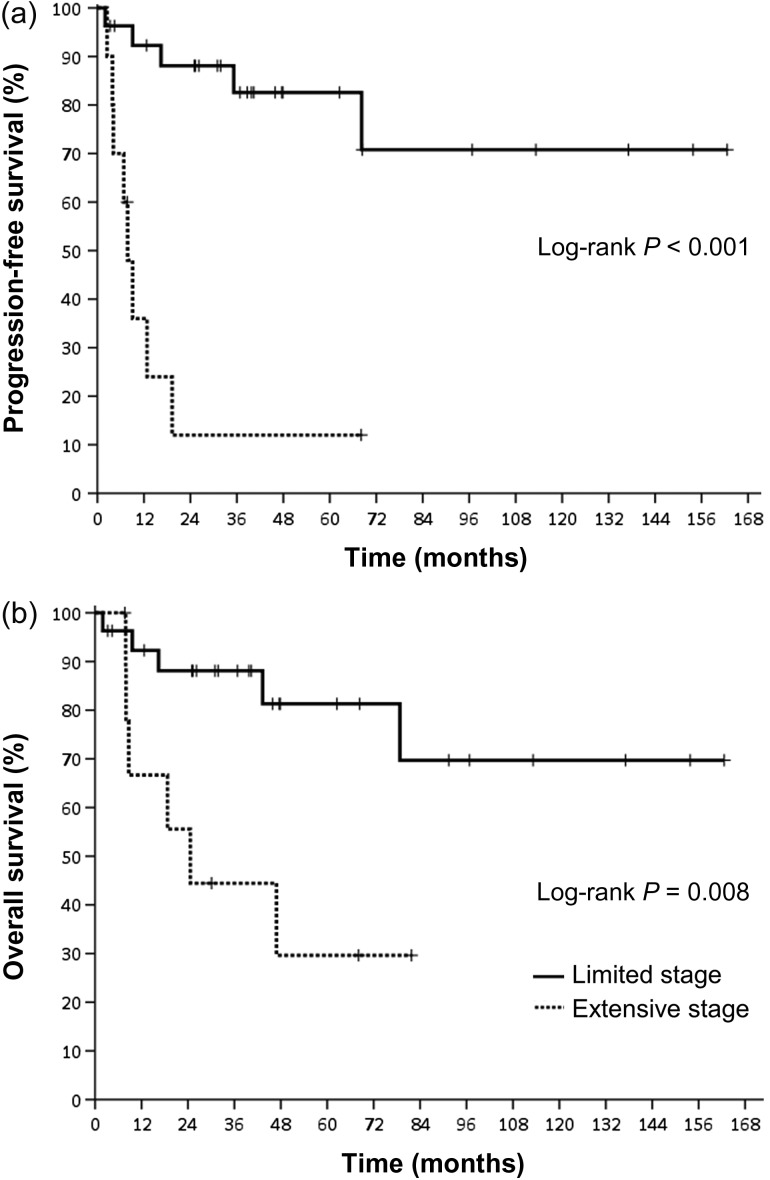
As demonstrated in Table 3, extensive disease (Stage I/II with local invasiveness) and the presence of B symptoms were significantly associated with a poor PFS, whereas extensive disease was significantly associated with a poor OS in the univariate analysis. As shown in Fig. 2 and Supplementary Table 2, patients with limited disease had significantly better PFS and OS than those with extensive disease (3-year PFS: 88.1% vs 12.0%, P < 0.001; 3-year OS: 88.1% vs 44.4%, P = 0.008). In a multivariate analysis using the Cox regression model, the presence of extensive disease was identified as an independent predictor of PFS (hazard ratio [HR] = 11.177 P < 0.001) and OS (HR = 4.446, P = 0.015, Table 3).
Table 3.
Hazard analysis for overall survival and progression-free survival (by Cox regression)
| Overall survival | Progression-free survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Univariate analysis | ||||||
| ECOG (0 vs 1) | 0.383 | 0.101–1.450 | 0.158 | 0.291 | 0.080–1.062 | 0.062 |
| Ann Arbor stage (I vs II) | 0.514 | 0.135–1.947 | 0.327 | 0.583 | 0.310–1.094 | 0.093 |
| B symptoms (No vs Yes) | 0.311 | 0.095–1.021 | 0.054 | 0.285 | 0.095–0.860 | 0.026 |
| New Stage (Extensive vs Limited) | 2.109 | 1.159–3.835 | 0.015 | 3.343 | 1.791–6.240 | <0.001 |
| ENKTL prognostic index(Group I vs Group II) | 0.631 | 0.348–1.145 | 0.13 | 0.645 | 0.373–1.114 | 0.115 |
| Multivariate analysis | ||||||
| New Stage (Extensive vs Limited) | 4.446 | 1.344–14.706 | 0.015 | 11.177 | 3.208–38.942 | <0.001 |
HR = hazard ratio, CI = confidence interval, CR = complete response, PR = partial response.
Fig. 2.
(a) Progression-free survival and (b) overall survival according to new stage.
DISCUSSION
Chemotherapy followed by radiotherapy has been established as the standard treatment for NHL [23]. The optimal treatment strategies for nasal ENKTL have not been recognized yet because of the relative resistance to anthracycline-based chemotherapy and the rarity of the disease. Many studies have investigated different treatment sequences (chemotherapy followed by RT, RT alone, RT combined with chemotherapy, and sandwich therapy) and chemotherapy regimens for ENKTL [10, 13, 24, 25]. However, most studies were limited by small sample size and heterogeneity of treatment. In this study, we demonstrated that RT with and without chemotherapy results in an overall response rate of 91.2% (CR rate, 78.3%) for patients with Stage IE/IIE nasal ENKTL, and that the 3-year LRC, PFS and OS were 87.4%, 64.0% and 76.3%, respectively. Our findings were in line with recently published results of studies investigating the effects of RT alone or RT with chemotherapy on ENKTL, including nasal ENKTL (summarized in Table 4), with a reported overall response rate of 69–96% [2, 13, 21, 25–29].
Table 4.
Comparison of clinical outcomes of early-stage nasal extranodal NK/T-cell lymphoma in recent publications
| Author | No. of pts | Ann Arbor stage (%) | Treatment types (No. of pts) | CT regimen (No. of pts) | RT dose(cGy) | Response (%) | 5-year OS (%) | 5-year PFS (%) | ≥ Grade 3 Toxicity (n)a |
|---|---|---|---|---|---|---|---|---|---|
| Avilés et al. (2013)b [2] | 427 |
|
|
CMED (318) | 5500 |
|
|
|
|
| Li et al. (2013) [13] | 73 |
|
|
CHOP like | 46 | Median PFS 3yrs | |||
| Wang et al. (2012) [21] | 42 |
|
|
CHOP like (19) | 5000 (median) |
95.2 | 78 (2-year) | 74 (2-year) | 7 |
| Jayoung Lee et al. (2013) [25] | 24 |
|
|
|
4500 (median) |
83.3 | 70.3 | 66.2 |
|
| Jieun Lee et al. (2013)b [26] | 43 |
|
|
|
|
|
|
|
|
| Jiang et al. (2012) [27] | 26 |
|
C/T→RT→C/T | L-VP (26) | 5600 | 88.5 | 88.5 (2-year) |
80.6 (2-year) |
2 |
| Oh et al. (2015) [28] | 62 |
|
CCRT→C/T |
|
4000 | 96.7 | 83.1 (3-year) |
77.1 (3-year) |
3 |
| Yang et al. (2015) [29] | 1273 |
|
|
|
5000 (median) |
93.3 |
|
|
– |
| Present study | 37 |
|
|
|
5000 (median) |
91.2 | 76.3 (3-year) |
64.0 (3-year) |
6 |
C/T = chemotherapy, RT = radiotherapy, No. of pts = Number of patients, ProMACE-CytaBOM = cyclophosphamide + doxorubicin + etoposide + cytozar + bleomycin + vincristine + methotrexate + prednisone, CHOP = cyclophosphamide + adriamycin + vincristine + prednisolone, L-VP = L-asparaginase + vincristine + prednisolone, CMED = cyclophosphamide + methotrexate + etoposide + dexamethasone, VIPD = etoposide + ifosfamide + cisplatin + dexamethasone, VIDL = etoposide + ifosfamide + dexamethasone followed by intramuscular injection of L-asparaginase, MIDLE = methotrexate + etoposide + ifosfamide + mesna + L-asparaginase, OS = overall survival, PFS = progression-free survival, SCRT = sequential chemoradiotherapy, CCRT = concurrent chemoradiotherapy.
aAcute toxicity according to CTCAE or RTOG, n as number of toxicity events.
bResponse, OS, PFS, toxicity according to different treatment types.
For nasal ENKTL, combined chemoradiotherapy (sequential or concurrent) has been reported to result in a favorable outcome [2, 9]. Recently, Oh et al. revealed that among 62 patients with Stage IE/IIE ENKTL, nasal type, that CCRT (median RT dose: 40 Gy, chemotherapy with cisplatin) followed by consolidation chemotherapy resulted in a 3-year LRS of 92.4%, PFS of 77.1% and OS of 83.1% [28]. However, Ma et al. [15] and Li et al. [30] revealed that adding anthracycline-based chemotherapeutic regimens with RT in Stage IE/IIE nasal ENKTL treatment did not result in a superior OS compared with RT alone. Lee et al. also reported that there were no differences in OS and PFS between CCRT and chemotherapy followed by RT for nasal ENKTL [26]. In a recent multicenter retrospective study, Yang et al. demonstrated that there was no difference in 5-year OS between RT alone (88.8%) and combined modality treatment (chemotherapy followed by RT (86.9%) or chemotherapy following RT (86.3%)) for early-stage nasal ENKTL with low-risk characteristics (age <60 years, Stage I, ECOG 0 or 1, serum LDH level within normal limits, and no primary tumor invasion) [29]. However, for high-risk early-stage nasal ENKTL, RT followed by chemotherapy provided a better 5-year OS (72.2%) than RT alone (59.6%) or induction chemotherapy followed by RT (58.3%) [29]. In the present study, we showed that among patients with limited stage, there were no statistically significant differences in LRC, PFS or OS between RT alone and combined modality treatment groups.
Considering L-asparaginase–containing chemotherapy regimens have been found to be effective for both early-stage and advanced-stage NK/T-cell lymphoma [31, 32], L-VP followed by RT was first used for nasal ENTKL in our institution from 2009. In this study, 8 (27.6%) of the 37 patients received an L-VP regimen as the chemotherapy regimen. We observed that L-VP and RT led to an improved 3-year LRC (100.0% vs 76.9%) and PFS (75.0% vs 58.7%) trend, but the OS remained unaffected when anthracycline-based regimens with RT were compared with patients received L-VP and RT (Supplementary Table 2). Our results further supported the results of a Phase II trial examining the efficacy of ‘sandwich’ L-VP treatment with RT for Stage I/II nasal ENKTL [27]. In their study, the overall response rate was 88.5%, and the 2-year PFS and OS were 80.6% and 88.5%, respectively [27].
Previous studies have revealed that high-dose RT was associated with superior disease control for nasal ENKTL [33, 34]. For example, Koom et al. observed that the local failure rate was 64% and 38% for patients who received an RT dose of <45 Gy and those who received a minimum of 45 Gy, respectively [33]. Furthermore, Huang et al. reported that the 5-year OS and PFS values were higher in patients receiving ≥54 Gy (5-year OS 75.5% vs 46.1%, P = 0.019; 5-year DFS 60.3% vs 33.4%, P = 0.004) [34]. These findings are supported by our current study, wherein all of the 37 patients receiving ≥50 Gy had a 3-year LRC of 87.4%. A minimum RT dose of 50 Gy must be used to treat nasal ENKTL because the use of a modern RT technique can reduce toxicity with escalating radiation dose [18, 19, 35].
Considering the uncertainty and inconsistency of RT field targeting in extranodal NHL, the International Lymphoma Radiation Oncology Group (ILROG) proposed simulation guideline, volume delineation, dose, and treatment planning of RT for extranodal NHL based on the literature reviews and consensus recommendation [36]. In this consensus of ILROG, Yahalom et al. suggest that for lymphoma of the nasal cavity and paranasal sinus (e.g. nasal ENKTL), the RT volume delineation should be based on the physical, fiberscopic and imaging findings because the primary lesions of nasal ENKTL might not be detected by CT, MRI or PET scan [36]. In the current study, all patients with nasal ENKTL received ear, nose and throat examination through transnasal endoscopy and physical examination before treatment (either chemotherapy or RT). This approach assisted the more precise definition of the target RT field, and might explain the reason our patients receiving ≥50 Gy had a 3-year LRC of 87.4%.
In this study, we demonstrated that the presence of an extensive disease in Stage IE/IIE nasal ENKTL was an independent poor prognostic factor of both PFS and OS in a multivariate analysis. These findings are in accordance with a previous study on prognostic factors for ENKTL that suggested Stage I/II with local invasiveness had a worse survival than that without local invasiveness [11]. However, Lee et al. reported that the significance of local invasiveness was lost in patients without lymph node involvement [10]. In this study, we observed that modified ENKTL prognostic index score Group II was associated with a significantly poor LRC and trend of worse PFS and OS in survival analyses (Supplementary Table 2). These findings suggested that Stage IE/IIE nasal ENKTL with local invasiveness or with 2 or 3 prognostic factors (B symptoms, elevated serum LDH level and lymphadenopathies) may require aggressive chemoradiotherapy. Recently, Kim et al. proposed a new prognostic index (PINK) for NK/T-cell lymphoma based on the clinical outcome of patients with Stage I–IV nasal and non-nasal ENKTL who received non-CHOP regimens [37]. The PINK consists of four prognostic factors, including age >60 years, Stage III–IV disease, distant lymph node involvement, and non-nasal type disease [37]. However, in the current study, we only included patients with Stage I or Stage IIE nasal ENKTL. Patients with Stage III–IV disease or disease originating from other upper-aerodigestive tract were not included in this study. Excluding 8 patients receiving RT alone and 16 patients receiving CHOP or CHOP-like regimens, 10 patients were classified as a low-risk group and 3 patients were classified as an intermediate-risk group according to the prognostic factor of age >60 years in PINK. We showed that the 3-year OS rates in the low risk and intermediate-risk groups were 70% and 100%, respectively (P = 0.272).
Our two relapsed patients exhibited disease-free survival after PBSCT (one autologous and one allogeneic). Previous studies have also reported that allogeneic hematopoietic stem cell transplantation could result in a 3-year OS of 40–55% for advanced ENTKL [38, 39]. The small number of patients and heterogeneity of treatment of this study may limit the conclusion because ENKTL is a rare disease.
Although the number of enrolled patients was small, the patient population was homogenous (Stage I and IIE, and primary lesions involved nasal cavity, nasopharynx and paranasal sinuses). This study showed that in patients with early-stage nasal ENKTL with limited disease or good modified ENKTL prognostic scores, high-dose RT (≥50 Gy) results were promising for locoregional control and favorable outcome. For patients with extensive disease or with poor modified ENKTL prognostic scores, more intensive treatment strategies, including RT with a new chemotherapy regimen such as that containing L-asparaginase, is warranted.
SUPPLEMENTARY DATA
Supplementary data is available at Journal of Radiation Research online.
ACKNOWLEDGMENTS
The authors thank the Cancer Registry, Cancer Administration and Coordination Center, National Taiwan University Hospital for providing the necessary patient information.
FUNDING
This study was supported by the Ministry of Science and Technology, Taiwan [grant numbers 104-2314-B-002-189-MY3 and MOST 104-2811-B-002-058], National Taiwan University, Taiwan [grant numbers NTUH 105-S3036, 105-S3059 and 105-N3275I] and the Ministry of Health and Welfare, Taiwan [grant number MOHW104-TD-B-111-04].
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Jaffe ES, Krenacs L, Raffeld M.. Classification of cytotoxic T-cell and natural killer cell lymphomas. Semin Hematol 2003;40:175–84. [DOI] [PubMed] [Google Scholar]
- 2.Avilés A, Neri N, Fernández R, et al. . Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma: results of a clinical trial. Med Oncol 2013;30:637. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki R, Takeuchi K, Ohshima K, et al. . Extranodal NK/T-cell lymphoma: diagnosis and treatment cues. Hematol Oncol 2008;26:66–72. [DOI] [PubMed] [Google Scholar]
- 4.Lee MY, Tan TD, Feng AC, et al. . Clinicopathological analysis of 598 malignant lymphomas in Taiwan: seven-year experience in a single institution. Am J Hematol 2006;81:568–75. [DOI] [PubMed] [Google Scholar]
- 5.Harabuchi Y, Imai S, Wakashima J, et al. . Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer 1996;77:2137–49. [DOI] [PubMed] [Google Scholar]
- 6.Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia 2005;19:2186–94. [DOI] [PubMed] [Google Scholar]
- 7.Chan JK, Quintanilla-Martinez L, Ferry JA, et al. . Extrannodal NK/T-cell lymphoma, nasal type In: Swerdlow SH, Campo E, Harris NL, et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC, 2008, 285–8. [Google Scholar]
- 8.Kim TM, Park YH, Lee SY, et al. . Local tumor invasiveness is more predictive of survival than International Prognostic Index in stage I(E)/II(E) extranodal NK/T-cell lymphoma, nasal type. Blood 2005;106:3785–90. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi M, Tobinai K, Oguchi M, et al. . Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T-cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol 2009;27:5594–600. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Suh C, Park Y-H, et al. . Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol 2006;24:612–8. [DOI] [PubMed] [Google Scholar]
- 11.Kim TM, Heo DS.. Extranodal NK/T-cell lymphoma, nasal type: new staging system and treatment strategies. Cancer Sci 2009;100:2242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim WS, Song SY, Ahn YC, et al. . CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma. Ann Oncol 2001;12:349–52. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Feng X, Li T, et al. . Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol 2013;37:14–23. [DOI] [PubMed] [Google Scholar]
- 14.Cheung MM, Chan JK, Lau WH, et al. . Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys 2002;54:182–90. [DOI] [PubMed] [Google Scholar]
- 15.Ma HH, Qian LT, Pan HF, et al. . Treatment outcome of radiotherapy alone versus radiochemotherapy in early stage nasal natural killer/T-cell lymphoma. Med Oncol 2010;27:798–806. [DOI] [PubMed] [Google Scholar]
- 16.Yong W, Zheng W, Zhu J, et al. . L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol 2009;88:647–52. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi M, Kwong YL, Kim WS, et al. . Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 2011;29:4410–6. [DOI] [PubMed] [Google Scholar]
- 18.Jaccard A, Gachard N, Marin B, et al. . Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood 2011;117:1834–9. [DOI] [PubMed] [Google Scholar]
- 19.Lowry L, Smith P, Qian W, et al. . Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: a randomised phase III trial. Radiother Oncol 2011;100:86–92. [DOI] [PubMed] [Google Scholar]
- 20.Bi XW, Li YX, Fang H, et al. . High-dose and extended-field intensity modulated radiation therapy for early-stage NK/T-cell lymphoma of Waldeyer's ring: dosimetric analysis and clinical outcome. Int J Radiat Oncol Biol Phys 2013;87:1086–93. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Li YX, Wang WH, et al. . Mild toxicity and favorable prognosis of high-dose and extended involved-field intensity-modulated radiotherapy for patients with early-stage nasal NK/T-cell lymphoma. Int J Radiat Oncol Biol Phys 2012;82:1115–21. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, et al. . Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. [DOI] [PubMed] [Google Scholar]
- 23.Miller TP, Dahlberg S, Cassady JR, et al. . Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Eng J Med 1998;339:21–6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Zhu MY, Wang L, et al. ‘Sandwich’ chemotherapy (CT) with radiotherapy (RT) improves outcomes in patients with stage IE/IIE extranodal natural killer (NK)/T-cell lymphomas. Asian Pac J Cancer Prev 2013;14:4061–6. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Cho SG, Chung SM, et al. . Retrospective analysis of treatment outcomes for extranodal NK/T-cell lymphoma (ENKL), nasal type, stage I–IIE: single institute experience of combined modality treatment for early localized nasal extranodal NK/T-cell lymphoma (ENKL). Ann Hematol 2013;92:333–43. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Kim CY, Park YJ, et al. . Sequential chemotherapy followed by radiotherapy versus concurrent chemoradiotherapy in patients with stage I/II extranodal natural killer/T-cell lymphoma, nasal type. Blood Res 2013;48:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang M, Zhang H, Jiang Y, et al. . Phase 2 trial of ‘sandwich’ L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer 2012;118:3294–301. [DOI] [PubMed] [Google Scholar]
- 28.Oh D, Ahn YC, Kim SJ, et al. . Concurrent chemoradiation therapy followed by consolidation chemotherapy for localized extranodal natural killer/T-cell lymphoma, nasal type. Int J Radiat Oncol Biol Phys 2015;93:677–83. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Zhu Y, Cao JZ, et al. . Risk-adapted therapy for early-stage extranodal nasal-type NK/T-cell lymphoma: analysis from a multicenter study. Blood 2015;126:1424–32. [DOI] [PubMed] [Google Scholar]
- 30.Li YX, Yao B, Jin J, et al. . Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 2006;24:181–9. [DOI] [PubMed] [Google Scholar]
- 31.Jaccard A, Petit B, Girault S, et al. . L-asparaginase–based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann Oncol 2009;20:110–6. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi M, Suzuki R, Kwong YL, et al. . Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci 2008;99:1016–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koom WS, Chung EJ, Yang WI, et al. . Angiocentric T-cell and NK/T-cell lymphomas: radiotherapeutic viewpoints. Int J Radiat Oncol Biol Phys 2004;59:1127–37. [DOI] [PubMed] [Google Scholar]
- 34.Huang MJ, Jiang Y, Liu WP, et al. . Early or up-front radiotherapy improved survival of localized extranodal NK/T-cell lymphoma, nasal-type in the upper aerodigestive tract. Int J Radiat Oncol Biol Phys 2008;70:166–74. [DOI] [PubMed] [Google Scholar]
- 35.Tomita N, Kodaira T, Tachibana H, et al. . A comparison of radiation treatment plans using IMRT with helical tomotherapy and 3D conformal radiotherapy for nasal natural killer/T-cell lymphoma. Br J Radiol 2009;82:756–63. [DOI] [PubMed] [Google Scholar]
- 36.Yahalom J, Illidge T, Specht L, et al. . International Lymphoma Radiation Oncology Group. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:11–31. [DOI] [PubMed] [Google Scholar]
- 37.Kim SJ, Yoon DH, Jaccard A, et al. . A prognostic index for natural killer cell lymphoma after non-anthracycline–based treatment: a multicentre, retrospective analysis. Lancet Oncol 2016;17:389–400. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki R, Suzumiya J, Nakamura S, et al. . Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant 2006;37:425–31. [DOI] [PubMed] [Google Scholar]
- 39.Kwong YL. High-dose chemotherapy and hematopoietic SCT in the management of natural killer-cell malignancies. Bone Marrow Transplant 2009;44:709–14. [DOI] [PubMed] [Google Scholar]