Abstract
Using a recently discovered precatalyst, the first Pd-catalyzed Suzuki–Miyaura reactions using aryl sulfamates that occur at room temperature are reported. In complementary work, it is demonstrated that a related precatalyst can facilitate the coupling of aryl silanolates, which are less toxic and reactive nucleophiles than boronic acids with aryl chlorides. By combining our results using modern electrophiles and nucleophiles, the first Hiyama–Denmark reactions using aryl sulfamates are reported.
Graphical abstract
![]()
The discovery of Pd-catalyzed cross-coupling reactions has resulted in major advances in the synthesis of both pharmaceuticals and fine chemicals.1 Typically, aryl halides are used as the electrophile in these reactions.1 However, phenolic derivatives offer their own unique advantages.2 They not only are robust and trivial to prepare from ubiquitous phenols3 but can provide routes to prefunctionalize the electrophile through C–H activation and directed ortho-metalation.4 Although simple phenol derivatives such as aryl sulfonates have been used for many years in cross-coupling reactions,2b,5 these moieties are not useful directing groups for C–H activation. Therefore, there is interest in the use of aryl sulfamates as electrophiles in cross-coupling reactions.2b–d The first reports of Suzuki–Miyaura reactions involving aryl sulfamates utilized Ni catalysts but required elevated temperatures (>100 °C) and catalyst loadings (5–10 mol %).6 Despite further studies on Ni-catalyzed Suzuki–Miyaura reactions with aryl sulfamates,7 there is still only one system that can facilitate the coupling of a small number of naphthyl sulfamates with boronic acids at room temperature.8 In related chemistry, Percec and co-workers reported Ni systems for the room temperature coupling of aryl sulfamates with neopentylglycol boronates using catalyst loadings of 5–10 mol %.9 While these results are impressive, neopentylglycol boronates are not commercially available and are less atom efficient than boronic acids. In the last 3 years, there have been reports of Pd catalysts for Suzuki–Miyaura reactions with aryl sulfamates, but these systems also require elevated temperatures (80–100 °C) and catalyst loadings (4–10 mol %).10 These conditions can limit the utility and functional group tolerance of Suzuki–Miyaura reactions between boronic acids and aryl sulfamates.
Organoboranes, utilized in Suzuki–Miyaura reactions, are the most common nucleophiles in cross-coupling.11 This is in part due to the perceived low toxicity of boronic acids. However, recent reports suggest that some, but not all, boronic acids are mutagenic.12 In contrast, silicon-based nucleophiles have not shown similar toxicity problems, yet their application in cross-coupling is limited.13 Early Hiyama couplings required fluoride-containing additives to promote transmetalation, which limited the utility of the reactions.14 Subsequently, Denmark demonstrated that when aryl silanols and their derivatives are used as nucleophiles, only a Brønsted base is required for activation, eliminating the need for fluoride additives.15 Currently, this chemistry remains most effective with aryl bromides and iodides,16 and the scope of these reactions needs to be broadened to make the Hiyama–Denmark reaction a true alternative to Suzuki–Miyaura couplings.
Recently, we described new precatalysts for cross-coupling, (η3-1-tBu-indenyl)Pd(L)(Cl), which are compatible with both phosphine and NHC ligands and are commercially available.17 The precatalysts can be generated in situ through the reaction of the unligated dimeric precursor (η3-1-tBu-indenyl)2Pd2(μ-Cl)2 (1) with 2 equiv of free ligand, which is convenient for ligand screening. We demonstrated that our precatalysts are highly active for a range of standard cross-coupling reactions using aryl halides as electrophiles, including Buchwald–Hartwig and α-arylation reactions and Suzuki–Miyaura couplings using both aryl and alkyl organoboranes.17 Here, we demonstrate the ability of our precatalysts to broaden the scope of cross-coupling (Figure 1). Through application of ligand screening, we describe the first examples of Pd-catalyzed Suzuki–Miyaura reactions using aryl sulfamates at room temperature. Additionally, our precatalysts are compatible with less reactive nucleophiles and are capable of coupling aryl silanolates with a variety of aryl chlorides. The culmination of this work is the coupling of aryl silanolates with aryl sulfamates, which represent the first Hiyama–Denmark reactions using aryl sulfamates.
Figure 1.
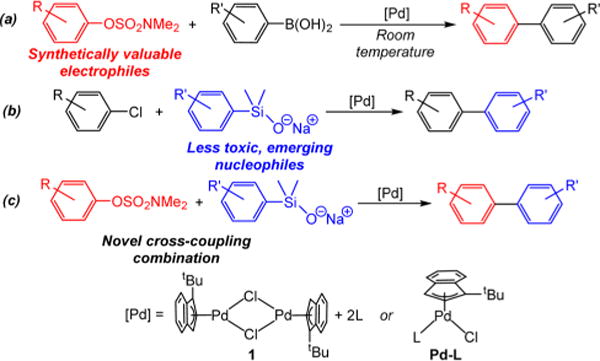
Summary of this work: (a) first examples of room-temperature Pd-catalyzed Suzuki–Miyaura couplings using aryl sulfamates; (b) Hiyama–Denmark couplings using aryl chlorides; and (c) Hiyama– Denmark couplings with aryl sulfamates.
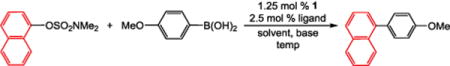
Based on pioneering work by Garg and co-workers, Ni precatalysts have been preferred for Suzuki–Miyaura reactions using aryl sulfamates.6 This is because it is proposed that oxidative addition of the aryl sulfamate is more facile for Ni compared to Pd.6b However, there have only been limited computational studies of oxidative addition of aryl sulfamates to Pd,6b and the effects of state-of-the-art ligands for cross-coupling, which can promote oxidative addition, are unclear.18 We performed a ligand screen for the coupling of 1-naphthyl sulfamate with (4-methoxyphenyl)boronic acid using our unligated dimeric precursor 1 and 2 equiv of a range of free ligands (Table 1 and SI). Using K3PO4 as the base and toluene as the solvent, no product was observed using simple phosphines such as PCy3, PtBu3, or PPh3 or the N-heterocycle carbene ligand IPr (see SI), consistent with computational predictions from Garg and co-workers.6b When Buchwald-type phosphines that are designed to increase the rate of oxidative addition18 were used as the ancillary ligand, moderate conversions were achieved at room temperature (entries 2–4). Consistent with our previous results,17 our precatalyst gives superior results compared to other related systems, such as Nolan’s (η3-allyl)Pd(L)(Cl) precatalysts (see SI).1a Using XPhos, complete conversion was achieved at 50 °C (entry 5). When methanol was used as a co-solvent, excellent conversion was obtained using XPhos at room temperature (entry 6). We suggest that there are two main reasons why the addition of methanol improves the efficiency of this reaction: (i) both the boronic acid and the base are more soluble, and (ii) methanol can potentially play a key role in the activation of the precatalyst from Pd(II) to Pd(0).19 Finally, switching the base from K3PO4 to K2CO3 provided full conversion to product at room temperature (entry 7).
Table 1.
Yields for Ligand Screening for Pd-Catalyzed Suzuki–Miyaura Reactions of Aryl Sulfamatesa
| ||||
|---|---|---|---|---|
| entry | ligand | solvent | base | yieldb (%) |
| 1 | PtBu3 | toluene | K3PO4 | <5 |
| 2 | SPhos | toluene | K3PO4 | 42 |
| 3 | RuPhos | toluene | K3PO4 | 48 |
| 4 | XPhos | toluene | K3PO4 | 61 |
| 5c | XPhos | toluene | K3PO4 | 97 |
| 6d | XPhos | toluene/MeOH | K3PO4 | 84 |
| 7d | XPhos | toluene/MeOH | K2CO3 | 98 |
Conditions: 1-naphthyl sulfamate (0.1 mmol), (4-methoxyphenyl)-boronic acid (0.15 mmol), base (0.2 mmol), 1 (0.00125 mmol), ligand (0.0025 mmol), toluene (1 mL), 25 °C, 4 h.
Yields are the average of two runs and were determined by GC.
Performed at 50 °C.
Solvent: toluene (0.66 mL), methanol (0.33 mL).
Using our optimized ligand and conditions, we expanded the substrate scope to other boronic acids and aryl sulfamates (Figure 2). In these reactions, we used the ligated XPhos precatalyst (Pd-XPhos) instead of the in situ generated system for operational simplicity and to ensure the ideal 1:1 Pd/ligand ratio. Couplings using naphthyl (Figure 2) and substituted naphthyl sulfamates (see SI) were successful with a variety of boronic acids at room temperature. Even 2-heteroaryl boronic acids, which readily undergo protodeborylation,20 yielded product in greater than 95% yield (2d–f). In general, oxidative addition of naphthyl sulfamates is easier than phenyl sulfamates,2g,21 but our optimized system facilitated the coupling of 4-(trifluoromethyl)phenyl sulfamate with both an unactivated boronic acid as well as a 2-heteroaryl boronic acid at room temperature (Figure 2). Unsubstituted phenyl sulfamate proved to be more difficult and excellent yields were only obtained when the reactions were performed at 80 °C.
Figure 2.
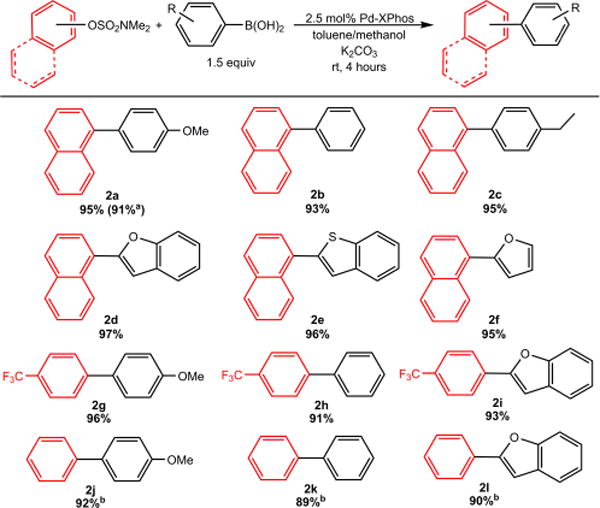
Isolated yields of products in Suzuki–Miyaura reactions using naphthyl and phenyl sulfamates. Conditions: sulfamate (0.1 mmol), boronic acid (0.15 mmol), K2CO3 (0.2 mmol), Pd-XPhos precatalyst (0.0025 mmol), toluene (0.66 mL), methanol (0.33 mL). aPerformed on 1 mmol scale in relation to aryl sulfamate. bPerformed at 80 °C for 24 hours. Yields are the average of two runs.
These results represent the first examples of Pd-catalyzed Suzuki–Miyaura reactions involving aryl sulfamates at room temperature. Furthermore, the conditions employed require a significantly lower number of equivalents of boronic acid (1.5 equiv vs 2.5 equiv) as well as external base (2.5 equiv vs 4.5 equiv) compared to state-of-art Ni catalysts.6 Our results suggest that it may be possible to develop systems for the coupling of even less activated phenol derived electrophiles such as aryl carbamates and esters, which have been proposed to be incompatible with Pd catalysts.2g
The reactions with aryl sulfamates show that our precatalysts are compatible with valuable electrophiles. We were also interested in exploring their activity with modern nucleophiles such as organosilanes. Denmark and co-workers have advanced the field of fluoride-free Hiyama coupling through their discovery of silanolates as viable nucleophiles.15 For example, using Pd(PtBu3)2, they were able to effectively couple heteroaryl iodides and bromides with various aryl and alkenyl silanolates.16a However, examples of the coupling of less reactive aryl chlorides with aryl silanolates are rare and generally require catalyst loadings of 5 mol % or more and temperatures ranging from 70 to 100 °C.16 Given the ubiquitous nature of aryl chlorides, developing systems that can perform Hiyama–Denmark reactions using these electrophiles at milder conditions would be beneficial.22
Using conditions similar to those reported by Denmark for coupling aryl iodides and bromides,16a a ligand screen was performed using 1, free ligand, 4-(trifluoromethyl)phenyl chloride, and 4-(methoxy)phenyl silanolate, which was prepared using a literature method.16a In an analogous fashion to Denmark’s results,16a the best activity was observed using PtBu3 as the ancillary ligand. Many other standard cross-coupling ligands (PPh3, PCy3, IPr) were unsuccessful at coupling the silanolate with the aryl chloride (see SI). Notably, several Buchwald-type phosphines were moderately effective in this transformation (SPhos and XPhos).
With optimal conditions and ligand found, our focus shifted to expanding the scope to other aryl chlorides (Figure 3). Using the electron-rich 4-(methoxy)phenyl silanolate,16a seven aryl chlorides were coupled using the Pd–PtBu3 precatalyst. In each case, isolated yields were greater than 85%, including deactivated substrates (3b and 3f) as well as heterocyclic aryl chlorides (3c–e,g). The scope of these reactions with 1-naphthyl (3h–o) and 4-(trifluoromethyl)phenyl (3p–v) silanolates16a is shown in Figure 3. Again, yields greater than 85% were achieved using the same conditions. No significant differences exist between the silanolate substrates, despite the differences in their electronic properties. In total, we have performed over 20 different Hiyama–Denmark reactions using aryl chlorides. Our results continue the expansion of this exciting cross-coupling reaction and highlight the improved activity observed with our precatalyst.
Figure 3.
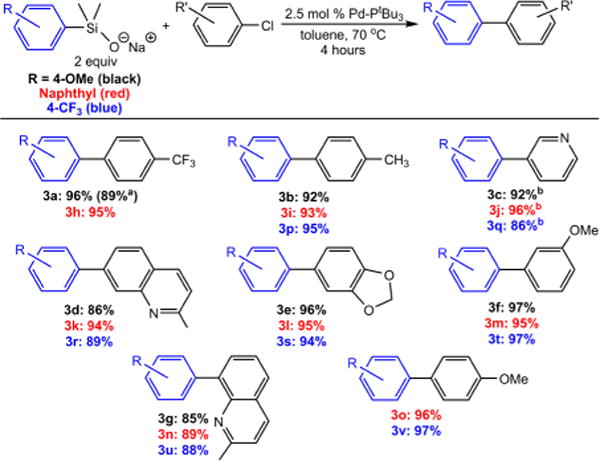
Isolated yields of products for silanolate couplings with aryl chlorides. Conditions: silanolate (0.2 mmol), aryl chloride (0.1 mmol), Pd-PtBu3 precatalyst (0.0025 mmol), toluene (1 mL), 70 °C, 4 hours. aPerformed on 1 mmol scale in relation to aryl chloride. bPerformed at 90 °C. Yields are the average of two runs.
To further show the versatility of our precatalyst system, we aimed to couple silanolates with sulfamates, a combination of substrates that has never been used together. Initial efforts to combine 1-naphthyl sulfamate with aryl silanolates proved to be unsuccessful using the conditions described in Figure 3. This is presumably related to the fact that our precatalyst ligated with PtBu3 was inactive for Suzuki–Miyaura reactions with aryl sulfamates. However, using XPhos as the ligand, some activity was observed for the coupling of 2-naphthyl sulfamate with 4-methoxyphenyl silanolate (Table 2, entry 1). Changing the ligand to RuPhos resulted in increased yields, consistent with the trend seen for Hiyama–Denmark coupling with aryl chlorides (see SI). To further improve the yield, we screened potentially useful additives. Addition of external bases did not lead to coupling, as they caused degradation of the aryl sulfamate (entries 4 and 5). When 5.0 mol % of free RuPhos was added, the yield of product increased (entry 6), as has been observed in some Buchwald–Hartwig reactions.17 It is unclear why the addition of excess RuPhos assists in the reaction, but it could reduce catalyst decomposition through the formation of unligated Pd(0) species. For our best conditions, the temperature could be lowered to 80 °C while still achieving high yields, albeit with longer reaction times (entry 7).
Table 2.
Yields for Ligand Screening for Pd-Catalyzed Coupling of Aryl Silanolates with Aryl Sulfamatesa
|
| |||||
|---|---|---|---|---|---|
| entry | ligand | Pd loading (mol %) | additive | temp (°C) |
yieldb (%) |
| 1 | XPhos | 2.5 | none | 110 | 18 |
| 2 | RuPhos | 2.5 | none | 110 | 37 |
| 3 | RuPhos | 5.0 | none | 110 | 62 |
| 4 | RuPhos | 5.0 | NaOMe | 110 | <5 |
| 5 | RuPhos | 5.0 | NaOtBu | 110 | <5 |
| 6 | RuPhos | 5.0 | RuPhosc | 110 | 95 |
| 7d | RuPhos | 5.0 | RuPhosc | 80 | 98 |
Conditions: 4– (methoxy)phenyl silanolate (0.2 mmol), 2-naphthyl sulfamate (0.1 mmol), toluene (1 mL), 8 h.
Yields are the average of two runs and were determined by GC.
5.0 mol % of RuPhos.
24 h.
This method is compatible with a variety of 2-naphthyl sulfamate derivatives (Figure 4). Using both 4-(methoxy)phenyl and naphthyl silanolates, heterocyclic sulfamates as well as sulfamates with ester and ether functionalities could be coupled with good to excellent isolated yields (4b–d). When moving to phenyl sulfamates, it was necessary to increase the equivalents of silanolate. For phenyl sulfamates with electron-withdrawing groups, good to excellent yields were obtained (4i–k), whereas for unsubstituted and 4-fluorophenyl sulfamate there was a decrease in yield (4h,l). No product was observed with 1-naphthyl sulfamate derivatives, presumably due to the increased steric bulk of these substrates. Our results represent the first use of phenol-derived electrophiles, which can be used as directing groups, in Hiyama–Denmark reactions.
Figure 4.
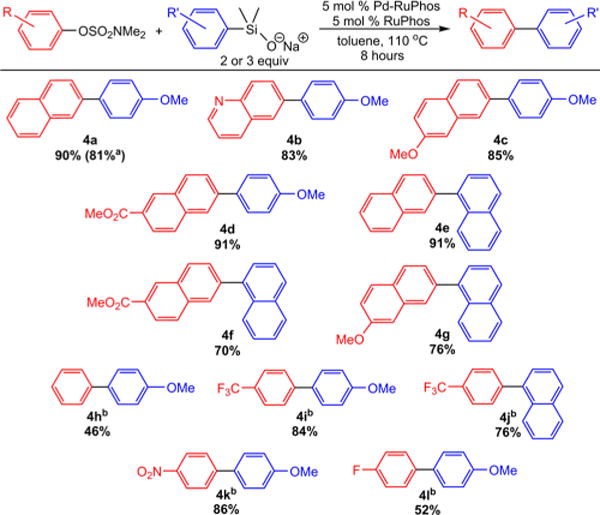
Isolated yields of products for couplings of aryl sulfamates with aryl silanolates. Conditions: silanolate (0.2 mmol), aryl sulfamate (0.1 mmol), Pd-RuPhos precatalyst (0.005 mmol), RuPhos (0.005 mmol), toluene (1 mL), 110 °C, 8 hours. aPerformed on 1 mmol scale in relation to aryl sulfamate. bSilanolate (0.3 mmol). Yields are the average of two runs.
In conclusion, we have demonstrated the utility of our (1-tBu-indenyl)Pd(L)(Cl) precatalysts by describing the room-temperature Pd-catalyzed Suzuki–Miyaura couplings of aryl sulfamates, Hiyama–Denmark reactions with aryl chlorides, and the first examples of Hiyama–Denmark couplings using aryl sulfamates.
Supplementary Material
Acknowledgments
This research was supported by the NIHGMS under Award No. R01GM120162. P.R.M. and M.M.B. thank the NSF for support as NSF Graduate Research Fellows. N.H. is a Camille and Henry Dreyfus Foundation Teacher–Scholar.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.6b02330.
Experimental procedures and characterization data (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.(a) Marion N, Nolan SP. Acc Chem Res. 2008;41:1440. doi: 10.1021/ar800020y. [DOI] [PubMed] [Google Scholar]; (b) Valente C, Calimsiz S, Hoi KH, Mallik D, Sayah M, Organ MG. Angew Chem, Int Ed. 2012;51:3314. doi: 10.1002/anie.201106131. [DOI] [PubMed] [Google Scholar]; (c) Colacot TJ, editor. New Trends in Cross-Coupling: Theory and Applications. The Royal Society of Chemistry; Cambridge: 2015. pp. 1–864. (RSC Catalysis Series No 21). [Google Scholar]
- 2.(a) Gooßen LJ, Gooßen K, Stanciu C. Angew Chem, Int Ed. 2009;48:3569. doi: 10.1002/anie.200900329. [DOI] [PubMed] [Google Scholar]; (b) Rosen BM, Quasdorf KW, Wilson DA, Zhang N, Resmerita AM, Garg NK, Percec V. Chem Rev. 2011;111:1346. doi: 10.1021/cr100259t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yu DG, Li BJ, Shi ZJ. Acc Chem Res. 2010;43:1486. doi: 10.1021/ar100082d. [DOI] [PubMed] [Google Scholar]; (d) Mesganaw T, Garg NK. Org Process Res Dev. 2013;17:29. [Google Scholar]; (e) Tobisu M, Chatani N. Top Organomet Chem. 2012;44:35. [Google Scholar]; (f) Cornella J, Zarate C, Martin R. Chem Soc Rev. 2014;43:8081. doi: 10.1039/c4cs00206g. [DOI] [PubMed] [Google Scholar]; (g) Tobisu M, Chatani N. Acc Chem Res. 2015;48:1717. doi: 10.1021/acs.accounts.5b00051. [DOI] [PubMed] [Google Scholar]
- 3.Rappoport Z. The Chemistry of Phenols. John Wiley & Sons; Chichester: 2003. [Google Scholar]
- 4.(a) Snieckus V. Chem Rev. 1990;90:879. [Google Scholar]; (b) Hartung CG, Snieckus V. Modern Arene Chemistry. Wiley-VCH: New York; 2002. [Google Scholar]; (c) Knappke CEI, Jacobi von Wangelin A. Angew Chem, Int Ed. 2010;49:3568. doi: 10.1002/anie.201001028. [DOI] [PubMed] [Google Scholar]; (d) Board J, Cosman JL, Rantanen T, Singh SP, Snieckus V. Platinum Met Rev. 2013;57:234. [Google Scholar]
- 5.(a) Norberg AM, Sanchez L, Maleczka RE., Jr Curr Opin Drug Discovery Dev. 2008;11:853. [PubMed] [Google Scholar]; (b) So CM, Kwong FY. Chem Soc Rev. 2011;40:4963. doi: 10.1039/c1cs15114b. [DOI] [PubMed] [Google Scholar]
- 6.(a) Quasdorf KW, Riener M, Petrova KV, Garg NK. J Am Chem Soc. 2009;131:17748. doi: 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Quasdorf KW, Antoft-Finch A, Liu P, Silberstein AL, Komaromi A, Blackburn T, Ramgren SD, Houk KN, Snieckus V, Garg NK. J Am Chem Soc. 2011;133:6352. doi: 10.1021/ja200398c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Chen GJ, Han FS. Eur J Org Chem. 2012;2012:3575. [Google Scholar]; (b) Ramgren SD, Hie L, Ye Y, Garg NK. Org Lett. 2013;15:3950. doi: 10.1021/ol401727y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ke H, Chen X, Zou G. J Org Chem. 2014;79:7132. doi: 10.1021/jo501291y. [DOI] [PubMed] [Google Scholar]
- 8.Beromi MM, Nova A, Balcells D, Brasacchio AM, Brudvig GW, Guard LM, Hazari N, Vinyard DJ. J Am Chem Soc. 2016 doi: 10.1021/jacs.6b11412. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Leowanawat P, Zhang N, Resmerita AM, Rosen BM, Percec V. J Org Chem. 2011;76:9946. doi: 10.1021/jo202037x. [DOI] [PubMed] [Google Scholar]; (b) Leowanawat P, Zhang N, Safi M, Hoffman DJ, Fryberger MC, George A, Percec V. J Org Chem. 2012;77:2885. doi: 10.1021/jo3001194. [DOI] [PubMed] [Google Scholar]; (c) Zhang N, Hoffman DJ, Gutsche N, Gupta J, Percec V. J Org Chem. 2012;77:5956. doi: 10.1021/jo300547v. [DOI] [PubMed] [Google Scholar]; (d) Jezorek RL, Zhang N, Leowanawat P, Bunner MH, Gutsche N, Pesti AKR, Olsen JT, Percec V. Org Lett. 2014;16:6326. doi: 10.1021/ol503061c. [DOI] [PubMed] [Google Scholar]
- 10.(a) Molander GA, Shin I. Org Lett. 2013;15:2534. doi: 10.1021/ol401021x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang ZY, Ma QN, Li RH, Shao LX. Org Biomol Chem. 2013;11:7899. doi: 10.1039/c3ob41382a. [DOI] [PubMed] [Google Scholar]
- 11.(a) Miyaura N, Suzuki A. Chem Rev. 1995;95:2457. [Google Scholar]; (b) Roughley SD, Jordan AM. J Med Chem. 2011;54:3451. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 12.(a) O’Donovan MR, Mee CD, Fenner S, Teasdale A, Phillips DH. Mutat Res, Genet Toxicol Environ Mutagen. 2011;724:1. doi: 10.1016/j.mrgentox.2011.05.006. [DOI] [PubMed] [Google Scholar]; (b) Hansen MM, Jolly RA, Linder RJ. Org Process Res Dev. 2015;19:1507. [Google Scholar]
- 13.Denmark SE, Ambrosi A. Org Process Res Dev. 2015;19:982. doi: 10.1021/acs.oprd.5b00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Nakao Y, Hiyama T. Chem Soc Rev. 2011;40:4893. doi: 10.1039/c1cs15122c. [DOI] [PubMed] [Google Scholar]; (b) Sore HF, Galloway WRJD, Spring DR. Chem Soc Rev. 2012;41:1845. doi: 10.1039/c1cs15181a. [DOI] [PubMed] [Google Scholar]
- 15.(a) Denmark SE, Chang W-TT. Cross-Coupling with Silicon Reagents: Alkenylsilanes. In: Molander GA, editor. Science of Synthesis: Cross Coupling and Heck-Type Reactions. Vol. 1. Thieme; Stuttgart: 2013. p. 431. [Google Scholar]; (b) Denmark SE, Chang W-TT. Cross-Coupling with Silicon Reagents: Heteroarylsilanes. In: Molander GA, editor. Science of Synthesis: Cross Coupling and Heck-Type Reactions. Vol. 1. Thieme; Stuttgart: 2013. p. 495. [Google Scholar]; (c) Denmark SE, Chang W-TT. Cross-Coupling with Silicon Reagents: Arylsilanes. In: Molander GA, editor. Science of Synthesis: Cross Coupling and Heck-Type Reactions. Vol. 1. Thieme; Stuttgart: 2013. p. 383. [Google Scholar]; (d) Denmark SE. Cross-Coupling Reactions of Organosilicon Compounds. In: deMeijere A, Brase S, Oestreich M, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim: 2013. Chapter 4. [Google Scholar]; (e) Tymonko SA, Smith RC, Ambrosi A, Denmark SE. J Am Chem Soc. 2015;137:6192. doi: 10.1021/jacs.5b02515. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tymonko SA, Smith RC, Ambrosi A, Ober MH, Wang H, Denmark SE. J Am Chem Soc. 2015;137:6200. doi: 10.1021/jacs.5b02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Denmark SE, Smith RC, Chang WTT, Muhuhi JM. J Am Chem Soc. 2009;131:3104. doi: 10.1021/ja8091449. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Denmark SE, Baird JD, Regens CS. J Org Chem. 2008;73:1440. doi: 10.1021/jo7023784. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Denmark SE, Baird JD. Tetrahedron. 2009;65:3120. doi: 10.1016/j.tet.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Denmark SE, Smith RC, Tymonko SA. Tetrahedron. 2007;63:5730. doi: 10.1016/j.tet.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melvin PR, Nova A, Balcells D, Dai W, Hazari N, Hruszkewycz DP, Shah HP, Tudge MT. ACS Catal. 2015;5:3680. [Google Scholar]
- 18.Martin R, Buchwald SL. Acc Chem Res. 2008;41:1461. doi: 10.1021/ar800036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melvin PR, Balcells D, Hazari N, Nova A. ACS Catal. 2015;5:5596. [Google Scholar]
- 20.Kinzel T, Zhang Y, Buchwald SL. J Am Chem Soc. 2010;132:14073. doi: 10.1021/ja1073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brauer DJ, Krueger C. Inorg Chem. 1977;16:884. [Google Scholar]
- 22.For references related to Hiyama reactions with aryl chlorides using fluoride activators, see; Yuen OY, So CM, Man HW, Kwong FY. Chem - Eur J. 2016;22:6471. doi: 10.1002/chem.201600420. and references cited therein. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


