Abstract
Lipids encompass a wide variety of molecules such as fatty acids, sterols, phospholipids, and triglycerides. These molecules represent a highly efficient energy resource and can act as structural elements of membranes or as signaling molecules that regulate metabolic homeostasis through many mechanisms. Cells possess an integrated set of response systems to adapt to stresses such as those imposed by nutrient fluctuations during feeding-fasting cycles. While lipids are pivotal for these homeostatic processes, they can also contribute to detrimental metabolic outcomes. When metabolic stress becomes chronic and adaptive mechanisms are overwhelmed, as occurs during prolonged nutrient excess or obesity, lipid influx can exceed the adipose tissue storage capacity and result in accumulation of harmful lipid species at ectopic sites such as liver and muscle. As lipid metabolism and immune responses are highly integrated, accumulation of harmful lipids or generation of signaling intermediates can interfere with immune regulation in multiple tissues, causing a vicious cycle of immune-metabolic dysregulation. In this review, we summarize the role of lipotoxicity in metaflammation at the molecular and tissue level, describe the significance of anti-inflammatory lipids in metabolic homeostasis, and discuss the potential of therapeutic approaches targeting pathways at the intersection of lipid metabolism and immune function.
Keywords: lipids, obesity, diabetes, insulin resistance, signaling lipids, inflammation
The term “lipid” is used to identify a large set of hydrophobic and amphiphilic molecules such as free fatty acids, sterols, fatty acid esters, and phospholipids. These molecules are involved in forming fundamental structures in cells and tissues, providing energy for the metabolic needs of organisms, and regulating several homeostatic processes within and outside of cells, including organelle homeostasis, immune function, inter-organ communication, energy metabolism, and cell survival. However, when the balance in their metabolism and composition is altered by environmental or metabolic stress, lifestyle, and genetic or epigenetic factors, lipids can also become critical components of pathophysiological cascades that are detrimental to healthy cell and tissue function. Hence, although lipids play fundamental physiological roles, in excess or in improper composition, they can be highly damaging, leading to organelle dysfunction, cell death, chronic inflammation, and disturbances in energy and substrate metabolism and survival responses. In this review, we primarily focus on the roles of lipid classes that regulate immune responses and signaling mechanisms, which perpetuate a vicious cycle of metabolic and inflammatory disturbances leading to disease.
LIPID-ASSOCIATED METAFLAMMATION
Lipotoxicity, generally defined as an increased concentration of harmful lipids, impairs cellular homeostasis and disrupts tissue function. This is a vast area of study that encompasses many fundamental processes in the cell and involves multiple mechanistic models. Here, we will focus mainly on lipotoxicity as it relates to the integration of metabolic and immune responses, which is critical for health and also plays a role in metabolic diseases (1). Chronic low-grade metabolic inflammation, termed “metaflammation,” is considered one of the hallmarks of metabolic diseases such as obesity and diabetes, and it occurs in several metabolic tissues, including adipose tissue, liver, muscle, brain, and gut. Among other potential mechanisms, it is now well-established that immunometabolic pathways are highly responsive to lipids and linked to lipotoxicity (1). Just as lipotoxicity gives rise to metaflammation, alterations in lipid metabolism and signaling can also converge on common immune and stress responses (2), thus creating vicious pathological cycles that contribute to many diseases.
Perturbations in fatty acid and cholesterol fluxes lead to higher representation of harmful lipid classes in cells and in the circulation, especially saturated fatty acids and oxidized cholesterol (Fig. 1). These species have been studied extensively for their effects on cellular function, including inflammatory responses and organ performance. For example, the saturated fatty acid, palmitate, can be imported into cells via fatty acid transport protein 1 (FATP1), and overexpression of FATP1 in the heart leads to lipotoxicity-mediated cardiomyopathy (3). Forced exposure to fatty acids can also be a driver for pathologies at other sites or in other metabolic diseases, and the discussion below is predominantly framed in this context.
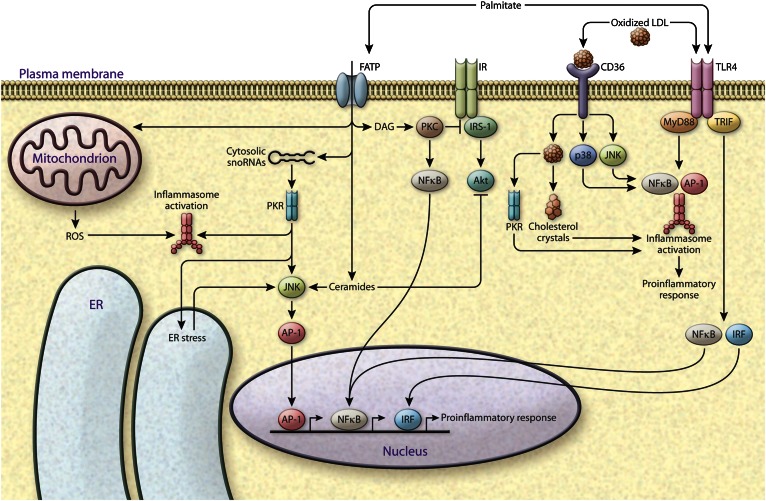
Fig. 1.
Coupling of toxic and pro-inflammatory lipids and innate immune response. Accumulation of toxic lipid classes causes deterioration of metabolic regulation, and the effects of such lipids converge on inflammatory and stress pathways. Saturated fatty acids such as palmitate have been extensively studied for their effects in increasing inflammation and inhibiting insulin action. Palmitate is taken up by the cells via FATPs, and is involved in upregulation of cytosolic snoRNAs, which are implicated in ER stress. PKR is a potential kinase that links palmitate-mediated snoRNA upregulation to ER stress induction. PKR also activates inflammasomes and JNK, and promotes AP-1-mediated inflammatory gene expression. Lipotoxicity can also lead to ROS production from mitochondria, which is linked to inflammasome activation. Palmitate directly contributes to the synthesis of DAGs and ceramides. DAGs activate stress kinases, PKCs, and the NFκB pathway; ceramides activate JNK signaling; and both DAGs and ceramides cause insulin resistance via inhibition of IRS1 and AKT, respectively, downstream of insulin receptor (IR). Palmitate can also activate TLR4 signaling, which leads to activation of inflammasomes and induction of inflammatory gene transcription factors interferon regulatory factor (IRF), NFκB, and AP-1. Cholesterol is taken up by the cells via scavenger receptors (e.g., CD36). Accumulation of oxidized cholesterol or cholesterol crystals also leads to induction of TLR4, PKR, and stress kinase (JNK and p38) signaling or inflammasome activation and pro-inflammatory gene expression, which are central players in atherosclerosis progression.
The mechanisms underlying the harmful effects of excess lipid flux are related in part to the impact of lipids on the biophysical properties of cellular organelles. For example, the endoplasmic reticulum (ER), which is one of the major hubs for lipid biosynthesis and esterification, is a critical organelle mediating both metabolic and inflammatory adaptive responses to proteotoxic, nutritional, and energy-related stresses. In the setting of chronic nutrient stress, lipid synthesis is dysregulated in the ER, leading to changes in phospholipid composition of the ER membrane. These changes cause disruption of calcium signaling, prolonged ER stress, and decreased translation of ER-associated proteins (4, 5). Similarly, saturated fatty acids and cholesterol loading increase ER stress and associated cell death (6–9). ER stress responses also intersect with inflammatory pathways via activation of numerous inflammatory kinases, such as JNK, protein kinase R (PKR), and IKK (10, 11), and activation of inflammatory mediators and the inflammasome (Fig. 1). The adaptive responses of the ER and the unfolded protein response exhibit a peculiar pattern of defects in obesity and diabetes, in the context of chronic inflammation. This is evident in both type 1 and type 2 diabetes (10, 12–17). For instance, ER stress propagation via increased induced nitric oxide synthase (iNOS) activity and subsequent inactivation of the key ER regulator, inositol-requiring protein 1 (IRE1α), via nitrosylation (18) represents one example of the indirect impact of lipotoxicity on chronic inflammatory processes.
Beyond the alteration of organelle function, lipotoxicity can also influence metaflammation and hormone action via direct effects on intracellular signaling pathways. For example, palmitate exposure is implicated in synthesis of diacylglycerols (DAGs) (19), which can activate novel protein kinase C (PKC) isoforms (20) (Fig. 1) such as PKC-θ and PKC-ε, which have been linked to T cell activation and LPS responses (21, 22) as well as insulin action and metabolic responses (23). While the exact mechanisms underlying these signaling events and the lipid species that engage PKCs remains under debate (23, 24), there is strong evidence supporting the involvement of PKCs in both metabolic and inflammatory responses that are relevant to obesity and type 2 diabetes. Palmitate accumulation also leads to ceramide biosynthesis, which can activate inflammatory pathways and inhibit insulin action (Fig. 1). Ceramides inhibit Akt-mediated insulin signaling as well as mitochondrial fatty acid oxidation by disrupting mitochondrial electron transport (25–28). Furthermore, inhibition of ceramide synthesis via myriocin treatment improves glucose and energy metabolism via recovery of insulin signaling in liver and muscle (29). Interestingly, toll-like receptor 4 (TLR4) signaling can also lead to increased expression of ceramide biosynthetic enzymes (30), suggesting the importance of this pathway in mediating metaflammation and insulin resistance and the reciprocal and highly integrated operation of lipid and immune signaling pathways (Fig. 1).
Finally, lipids can influence cell fate and function by engaging receptors on the cell surface or stress kinases within the cytoplasm. Fatty acids such as palmitate can directly activate inflammatory pathways by increasing TLR4 signaling (31) and by stimulating signaling molecules such as PKR (10) (Fig. 1). In response to harmful lipids such as palmitate and oxidized cholesterol, PKR can activate JNK, leading to engagement of downstream transcription factor activator protein 1 (AP-1) and expression of genes that mediate inflammation and apoptosis and promote inflammasome activity (32–34). It is unlikely that a single receptor or molecular event underlies these lipotoxic responses; however, it is possible to envision common signaling intermediates that mediate the vast array of downstream biological outcomes of lipotoxicity. One such potential mechanism involves upregulation of small nucleolar RNAs (snoRNAs) in the cytoplasm (35). Interestingly, PKR is the only kinase that also has direct double stranded RNA binding activity, and snoRNAs are enriched in PKR immunoprecipitates after palmitate treatment, suggesting that palmitate sensing by PKR may involve direct binding of snoRNAs to PKR for its activation in the metabolic disease context (36). How snoRNAs are involved in signaling lipotoxicity at the molecular level is yet unclear, but cells with impaired ability to produce snoRNAs are resistant to lipotoxicity-induced ER stress and death (37), indicating the potential of snoRNAs serving as mediators of broad lipotoxic responses. This is one of the most interesting emerging areas of research related to mechanisms of lipotoxicity and metabolic regulation.
LIPOTOXICITY-ASSOCIATED INFLAMMATION IN METABOLIC TISSUES
Continuous cycles of nutritional exposures and changing environmental factors require integrated metabolic, stress, and immune responses in many critical organs. Under chronic energy and substrate excess, metabolic stress is unresolved, yielding maladaptive outcomes, such as unresolved inflammation, impaired hormone action, lipid accumulation, and loss of function. Here, we will not discuss specific conditions such as insulin resistance, fatty liver disease, and cardiovascular pathologies, which are covered in detail in excellent recent reviews (2, 19, 38–42), beyond specific examples relevant to metaflammation in a few representative sites (Fig. 2).
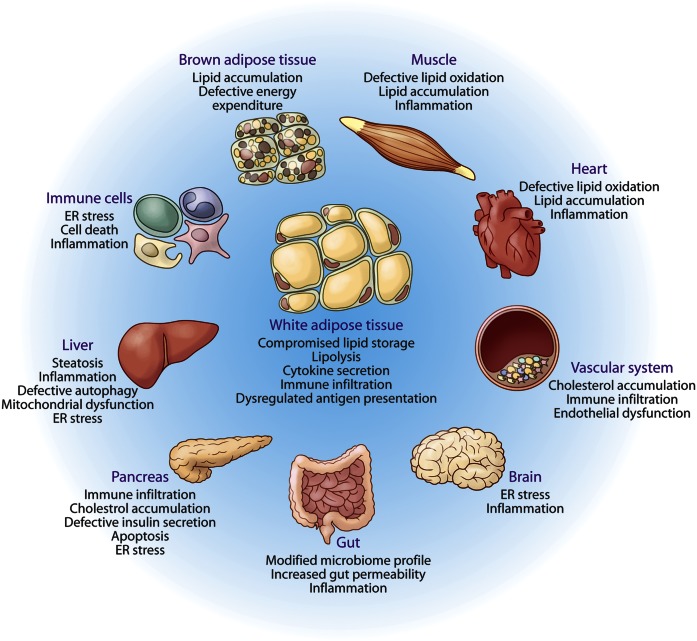
Fig. 2.
Integrated organ pathology resulting from lipotoxicity and metabolic inflammation. White adipose tissue is the designated lipid storage depot of higher organisms. In the presence of prolonged nutrient excess or metabolic disturbances, the storage capacity of white adipose tissue is exhausted, leading to ectopic deposition of lipids and lipotoxicity in several organs, such as muscle, liver, pancreas, and heart, as depicted and explained in detail in the text. Because the lipid homeostatic pathways converge with stress and immune responses, such responses in affected tissue systems are activated by harmful lipid species. The outcomes of lipotoxicity differ in the various target tissues, for example NASH in liver, cardiac failure in the heart, altered feeding behavior and appetite due to lipotoxicity in brain, degenerative changes in muscle and BAT, etc. Furthermore, lipotoxicity leads to sustained and unresolved inflammation, organelle dysfunction and stress, which can lead to a vicious cycle of metabolic deterioration.
Adipose tissue
Fatty acids are esterified and compartmentalized in dynamic organelles called lipid droplets (LDs), such that their use can be coupled to cellular metabolism and signaling (43). During times of excess nutrient availability, LDs act as a depot for excess fatty acids and cholesterol that are otherwise harmful to the cells (43). In higher organisms, this can occur in all cells, but the most dramatic example is the adipose tissue, a specialized organ for lipid deposition. In the setting of increased metabolic demand, adipocytes hydrolyze neutralized lipids in LDs through lipolysis, liberating fatty acids for use in other tissues, feeding mitochondrial fatty acid oxidation pathways, and creating metabolic intermediates that serve as substrates or signaling molecules (24). The proper functioning of this rheostat is necessary to keep all other metabolic organs in check. In the presence of excess nutrients and energy, or in obesity, the capacity of adipose tissue can be overwhelmed, causing stress, injury, and abnormalities in function. For example, insulin resistance under these conditions leads to higher levels of basal lipolysis and a decreased capacity to synthesize and esterify fatty acids for storage or neutralization via downregulation of synthetic machinery (44–47). This dysfunctional state contributes to systemic lipotoxicity, as released or absorbed excess dietary fatty acids move into circulation and are deposited into organs that are not well-equipped to store lipids (Fig. 2). The purpose of esterification is to prevent harmful effects of fatty acids by forming a neutral pool (48), and accordingly, increasing the storage capacity of LDs in adipocytes and macrophages by driving synthesis can protect against diabetes and insulin resistance (49–51). However, above a certain threshold, the accumulation of such lipids induces stress and metaflammation, mediated by several stress kinases, such as PKC, JNK, and PKR, molecular sensors or receptors, such as TLRs, and signaling proteins, such as cyclic AMP-responsive element-binding protein 3-like protein 3 (CREBH) and SOCS proteins (10, 31, 52–57). In addition, obesity leads to a local lipotoxic environment through dysregulated release of fatty acids, leading to alteration of the secretory output and an immune phenotype of adipose tissue characterized by increased release of cytokines, such as TNFα and MCP-1, decreased secretion of anti-inflammatory adipokines, such as adiponectin, and recruitment of inflammatory macrophages, T-cells, and other immune effectors (38). Reciprocally, pro-inflammatory cytokines such as TNFα regulate lipid metabolism in adipocytes via increasing lipolysis (58), which continuously exposes the organs to fatty acids. Increased lipolysis from adipose tissue is also linked to secretion of the adipokine, aP2 (FABP4) (59, 60), which is an important mediator of immunometabolic responses locally at the adipose tissue, and links the lipolytic state to glucose metabolism in the liver and elsewhere (61–63). Overall, while adipocytes are the specialized site for neutralizing fatty acids, this capacity is not infinite and these cells are not impervious to excess lipid accumulation, which drives inflammatory responses locally and at distant tissues. The mechanisms and specific mediators in this context are not fully understood, and are an area of great interest.
Studies of mouse models of obesity have demonstrated that the lipotoxic milieu of adipose tissue promotes drastic changes in the resident immune cell profile and function. Specifically, adipose tissue is infiltrated by macrophages, which shift from an anti-inflammatory profile (also referred to as M2-like polarized) to a pro-inflammatory (also known as M1-like polarized) phenotype, and several immune cell types are implicated in adipose tissue dysfunction, such as B and T lymphocytes, neutrophils, eosinophils, mast cells, and NK cells (64). As explained above, palmitate, ceramide, and DAGs are broadly-studied lipid mediators that lead to activation of immune cells (Fig. 1), further suggesting a lipotoxic inflammatory connection in adipose tissue. Apart from direct activation of sensors and stress kinases, lipids can also act as antigens that are presented to immune cells by CD1d. Recently, CD1d-positive adipocytes have been implicated in lipid-derived antigen presentation to a subclass of natural killer T (NKT) cells called invariant NKT (iNKT) cells (65). However, the role of specific iNKT cells in regulation of adipose tissue inflammation has been a subject of debate, with some studies suggesting a beneficial effect of iNKT cells (65, 66) and others implicating iNKT cells in skewing the adipose phenotype to a more inflammatory and dysfunctional state (67). The identity of lipid molecules that are subject to antigen presentation and how they mount an inflammatory cascade is an exciting and understudied area and may provide clarity on the role of this particular mechanism on adipose tissue and systemic metabolism. Overall, these findings indicate that lipids can directly or indirectly modulate both innate and acquired immune responses due to close interactions between these systems, with implications for systemic metabolic homeostasis.
Compared with white adipose tissue, there is much less information on the lipotoxic events in the brown adipose tissue (BAT), which has a lower storage but higher turnover capacity. BAT is best known for its function in nonshivering thermogenesis and energy expenditure. At cold temperatures, BAT increases fatty acid uptake and fatty acid oxidation to keep up with thermogenic needs. Recently, a surprisingly large capacity for postprandial lipid uptake via triglyceride-rich proteins was demonstrated in BAT, suggesting another layer of metabolic regulation in BAT function that can lead to intracellular stress and intermediary products that would require a strong defense mechanism (68). Indeed, it is known that in dietary and genetic models of metabolic disease such as ob/ob mice, BAT becomes a white adipose-like tissue due to increased triglyceride accumulation, leading to inflammation and dysfunction (69, 70) (Fig. 2). The mechanisms that are in place to preserve the functional integrity of BAT and defend against inflammation and stress and how these relate to pathophysiology of metabolic disease are critical but incompletely answered questions and open to further research.
Liver
The liver is one of the most highly explored organs in the context of lipotoxicity. Because nonalcoholic fatty liver disease (NAFLD) is highly prevalent, and is closely associated with a cluster of metabolic diseases such as obesity, insulin resistance, and diabetes, understanding hepatic lipotoxicity is of paramount importance. A subclassification of NAFLD is nonalcoholic steatohepatitis (NASH), which is characterized by the presence of inflammatory cells in liver histology. NASH progression is mediated by interplay between lipid-mediated toxicity and inflammatory responses leading to liver injury (71) (Fig. 2). The signaling events in this process involve DAGs and ceramide (72, 73), which are discussed above for their significance in inflammatory signaling. Numerous studies have also demonstrated the importance of inflammatory responses in hepatic lipotoxicity associated with local or systemic TNFα and IL-1β exposure (74, 75) or as a result of dysbiosis (76–78). In liver, the ER is a pivotal node at the crossroads of inflammation and lipid metabolism. ER dysfunction may contribute to metabolic dysregulation of liver through iNOS-mediated tyrosine nitrosylation of IRE1 (18) or engaging inflammatory signaling cascades (10, 79, 80) and the inflammasome (81). Similarly, JNK as well as the δ and γ isoforms of p38 are critical mediators of inflammation and metabolic deterioration in mouse models of obesity or NASH, highlighting engagement of the stress pathways in lipotoxic responses (82–84). In mouse models of fatty liver disease, the mitochondria exhibit dysfunctional β-oxidation. This might be partially due to increased ER-mitochondria connections and associated calcium overload in mitochondria in obesity (85). In these contexts, it is unequivocal that inflammatory pathways are highly critical to obesity-induced metabolic dysfunction, and blocking these can reverse the disease. A recent study in mouse models as well as humans also showed that weight loss can result in dramatic resolution of obesity-induced liver inflammation along with insulin action (86). Interestingly, this study also showed that residual adipose tissue inflammation and insulin resistance persists for extended periods of time despite weight loss at this site (86).
One emerging aspect of hepatic lipotoxicity is free cholesterol accumulation due to disturbances in cholesterol homeostasis and transport (87–89), which has been underappreciated until recently in liver metabolic dysfunction. This phenomenon is supported by association studies in humans demonstrating dysregulation of cholesterol metabolism genes and free cholesterol levels with NAFLD (90). Excess free cholesterol causes damage in liver through JNK-1- and TLR4-dependent mechanisms (91). Cholesterol loading of mitochondria in liver is also argued to contribute to TNFα-mediated steatohepatitis (92). Additionally, Kupffer cells, the liver resident macrophages, have been shown to form crown-like structures around dying hepatocytes and accumulate cholesterol in NASH (93). However, whether lipogenesis or inflammation constitute the initiating events in NASH and are causal to pathology has been the subject of much debate. While the value of such debate itself could be debated, time course analysis of inflammatory and lipogenic changes in mouse models of hepatosteatosis (94) and inhibition of steatosis by liver macrophage depletion (75) suggest that inflammatory events occur very early in the course of disease. In agreement with this, recent studies examining arginase 2-deficient (Arg2−/−) mice suggest that inflammation can lead to de novo hepatic lipogenesis (95). Arg2−/− mice develop spontaneous steatosis with inflammation and increased lipogenic gene expression. Depletion of liver macrophages leads to decreased LD accumulation in the liver, suggesting that inflammation may precede lipid accumulation (96). Other studies have suggested that this may not be the case and even concluded that inflammation may not be a major player in this context due to lack of inflammatory connections to liver insulin resistance in the respective mouse models studied (97–99). The likely scenario is that lipogenesis and inflammatory responses cannot be separated in real life, they regulate each other, both contribute to abnormal liver metabolism and disease, and both are part of a regulatory as well as maladaptive cycle (2). For example, high-fat diet feeding exacerbates inflammation and liver injury in Arg2−/− mice, suggesting a further contribution of lipotoxicity in forming a futile cycle. Also, adipose-derived acetyl-CoA has been shown to accumulate in the liver in the setting of insulin resistance, and this is linked to aberrant hepatic glucose production (100). In this case, the effect of acetyl-CoA was dependent on IL-6 action, as neutralization of this cytokine reversed the disease phenotype demonstrating yet another example of inflammatory pathways interacting with lipids. Many other examples exist in literature and are reviewed elsewhere (2, 101).
In discussing tissue lipid accumulation, it is important to note that LD formation in hepatocytes may also be an adaptive response in the liver as it is in adipose tissue, and to a certain tolerable threshold and composition, may represent a way to preserve the function of the hepatocytes. Consistent with this, hepatic LD formation should not be expected to be, and in fact is not, uniformly associated with adverse metabolic outcomes (102–104).
Skeletal muscle and heart
High levels of circulating fatty acids and triglycerides are associated with muscle insulin resistance in mice and humans (105–107). In both experimental models and in obese and diabetic individuals, there is increased TNFα expression and accumulation of inflammatory cells in the muscle tissue (Fig. 2), which can, at least in part, be rescued by exercise (108, 109). In addition, adipocytes can accumulate in the muscle tissue, providing an opportunity for paracrine communication (110). Chronic exposure to a high concentration of saturated fatty acids leads to inhibition of insulin receptor signaling through inhibition of IRS-1 via decreased tyrosine phosphorylation in muscle cells (107, 111, 112). Saturated fatty acids also engage several stress and inflammatory signaling molecules, such as PKC, JNK, ERK, STAT3, and iNOS, and increase IL-6, TNFα, and IL-1β expression, which, in turn, impact metabolism (113, 114). DAG and long-chain acyl-CoAs have been implicated as the culprits in muscle lipotoxicity because they activate stress kinases such as PKC that contribute to insulin resistance (111, 115, 116). Chronic metabolic stress leads to mitochondrial dysfunction, and lipid accumulation is suggested to occur due to defective mitochondrial β-oxidation (117). However, this notion has been challenged by studies in which lipid trafficking into mitochondria is perturbed by knocking out malonyl-CoA decarboxylase (MCD) (118). Mice lacking MCD are resistant to developing diet-induced glucose intolerance, although they have increased intramuscular lipid accumulation. These data led to the proposal that the problem of mitochondrial dysfunction in this context may in fact be increased, yet maladaptive, β-oxidation, such that a blockade in the TCA cycle and consecutive accumulation of acyl-CoAs and acylcarnitines in the mitochondria would result in insulin resistance (118). Regardless, impaired mitochondrial function can also promote chronic inflammatory responses through many mechanisms, including generation of excess reactive oxygen species (ROS) and activation of the inflammasome (119, 120) (Fig. 1). Overall, lipid-mediated changes during insulin resistance in muscle converge with immune pathways and directly or indirectly regulate inflammatory signaling. An intriguing exception where excess lipid accumulation in muscle does not correlate with metabolic deterioration occurs in athletes, suggesting active adaptive mechanisms including robust mitochondrial β-oxidation (121). In depth understanding of such adaptive mechanisms could help identify targets for alleviating metabolic pathologies.
The heart has a robust capacity to utilize fatty acids for metabolic and functional demands (39). However, a prolonged increase in circulating fatty acids and triglycerides and accumulation of pericardial adipose tissue can trigger inflammatory signaling in the heart and cause cardiac dysfunction in metabolic disease that features mitochondrial dysfunction and increased ROS and NFκB activity (Fig. 2) (122). Epicardial fat can also secrete pro-inflammatory cytokines to drive inflammatory cell infiltration and exacerbate heart disease (123–125). TLR4 has been implicated as the mediator of fatty acid-induced lipid accumulation and inflammatory responses in the heart (126). Similarly, excessive triglyceride accumulation in the cardiac muscle was demonstrated to cause exhaustion of mitochondrial capacity via deregulation of PPARα signaling involved in oxidative pathways (127, 128) and accumulation of harmful lipids, such as DAG and ceramides, which can activate PKCs and inhibit insulin signaling (129). Interestingly, driving triglyceride synthesis via DGAT1 expression in cardiac tissue renders mice resistant to developing lipotoxic cardiomyopathy and decreases accumulation of DAG and ceramide (130), suggesting an adaptive role for increasing lipogenic capacity or altering the profile of lipids in cardiomyocytes.
Lipotoxicity-mediated inflammation also drives cardiovascular disease pathogenesis in the context of atherosclerosis. Exposure to saturated fatty acids, such as palmitate, or modified lipoproteins leads to ER stress in macrophages, which drives apoptosis and further promotion of inflammation in atherosclerotic plaques (131). Dysregulation of cholesterol fluxes and accumulation of oxidized LDL particles in arterial walls leads to inflammatory cell recruitment and atherogenic plaques (132). In an attempt to limit plaque formation, macrophages take up oxidized LDL particles, which causes formation of foam cells that secrete inflammatory cytokines and leads to a futile cycle of inflammation and formation of more foam cells. Cholesterol crystals are also indicators of progressed atherosclerotic plaques, and can activate the inflammasome in macrophages in the context of atherosclerosis (133) (Fig. 1). The role of cholesterol and inflammation in cardiovascular disease is extensively reviewed elsewhere (132, 134, 135) and will not be covered further here. It is however important to note that diabetes is a central risk factor for cardiovascular disease and our current understanding of how diabetes drives myocardial perturbations is insufficient. Further study on diabetes-associated changes in heart and the significance of cardiovascular lipotoxicity and inflammation may help to determine alternative and novel clinical approaches to cardiovascular disease.
Other organs
As has been reviewed elsewhere (136–138), lipotoxicity plays an important role in islet dysfunction in obesity. Although fatty acids regulate insulin secretion from islets at different levels, such as vesicle trafficking and calcium influx via their metabolites (139), chronic elevation of such lipids leads to β-cell failure together with inflammatory etiology (140). Human data suggest that type 2 diabetic patients have increased IL-1β expression and macrophage recruitment in their islets (140–142). It has also been proposed that β-cell failure in type 2 diabetes has an inflammatory component that is promoted by lipotoxicity (Fig. 2) (143). Moreover, in a study in which mice were infused with ethyl palmitate in order to elevate circulating palmitate levels, glucose-stimulated insulin secretion was defective in a TLR4/MyD88-dependent manner (144). Palmitate elevation also led to macrophage infiltration of the islets. These observations laid the groundwork for a new perspective for lipotoxicity-induced inflammation in islets in type 2 diabetes. Recent findings also suggest a role for abnormal cholesterol metabolism in β-cell failure in type 2 diabetes patients (145, 146). A role for cholesterol accumulation in islet inflammation and β-cell dysfunction was shown in mice that were deficient in cholesterol transporters ABCA1 and ABCG1. In this model, excessive accumulation of cholesterol led to macrophage recruitment and increased IL-1β expression as well as defective glucose-stimulated insulin secretion (147). This intriguing hypothesis that dysregulated cholesterol fluxes drive metabolic inflammation may have profound translational implications for the utility of cholesterol management strategies in β-cell preservation and diabetes.
Similarly, many of the pathways defined earlier apply to the CNS, in that obesity and exposure to excess lipids can create chronic inflammation and cause ER stress in the brain (148–150) (Fig. 2). In both humans and preclinical models, obesity-induced inflammatory changes are evident in the brain (151, 152), and hypothalamic ER stress contributes to defective insulin and leptin action (148, 150, 153). Interestingly, amelioration of ER stress via chemical chaperones can reverse some of these effects (153). One lipotoxic inducer of hypothalamic ER stress was proposed to be ceramide. CNS levels of ceramide are increased in obese mouse models, and central administration of these lipids leads to weight gain via inhibition of BAT function (154). Saturated fatty acids have been shown to increase hypothalamic inflammation via TLR4 (149). In contrast, increasing fatty acid oxidation in hypothalamic neurons can decrease palmitate-induced inflammation and toxicity (155). These observations highlight the need for more research to understand the mechanism of lipotoxic inflammation in the CNS leading to metabolic dysregulation.
Finally, nutritional input, for example exposure to a high-fat diet, leads to drastic changes in the gut microbiome (Fig. 2), and these alterations contribute to the development of metabolic disease. The most compelling evidence to support this postulate is that, in mice, fecal transplantation from obese to lean experimental groups is sufficient to induce weight gain (156). In obese mice, circulating levels of microbial factors such as LPS were found to be increased, potentially due to increased gut permeability (Fig. 2) (157). Such factors can engage the TLR signaling pathways, which are established mediators of metaflammation. A recent study suggested that feeding mice a diet that is rich in saturated fatty acids increases LPS and other microbial factors in the circulation, and this leads to white adipose tissue inflammation through TLR4 signaling (158). These observations suggest the presence of an extra layer of regulation of metaflammation involving gut-adipose tissue communication in dietary lipid recognition. Gut-derived lipid signals such as N-acylphosphatidylethanolamines (NAPEs), which are produced upon fat ingestion, might also impact immunometabolic outcomes. For example, NAPEs act in the gut-brain axis to decrease food intake (159) and they can also suppress inflammation (160, 161). Hence, both through its endocrine impact on systemic metabolism and by hosting the microbiota, the gut is a critical determinant of metabolic and organismal health. Future research in this area is also exciting and promising in understanding the systemic impact of lipids and their interactions with the immune and other response systems.
BIOACTIVE LIPIDS AND REGULATION OF INFLAMMATION
In recent years, emerging studies have offered a new understanding of lipid function as soluble signals (lipokines) regulating biological processes outside cells (Fig. 3). Interestingly, several classes of these have been shown to be important for resolution of inflammation. One such lipokine with a significant role in metabolic regulation is the fatty acid C16:1n7-palmitoleate (162). When synthesis of palmitoleate is increased as a result of adipose tissue-specific upregulation of de novo lipogenesis, its levels in circulation rise, resulting in suppression of inflammation and liver lipogenesis and stimulation of muscle glucose uptake (162–164). Furthermore, palmitoleate generation in macrophages alleviates lipotoxicity-induced ER stress and cell death and, consequently, progression of atherosclerosis (131). Another endogenously produced lipid family with potentially vast metabolic actions includes the fatty acid hydroxy fatty acids (FAHFAs), which were identified in adipose tissue and the circulation of mice in which adipose tissue de novo lipogenesis was also experimentally increased (165). A subclass of these lipids, called palmitic acid hydroxy stearic acids, has been implicated in mitigation of adipose tissue inflammation and exert anti-diabetic and insulin-sensitizing activities in mice, showing FAHFAs as another bioactive lipid signal that controls immunometabolic aspects of diabetes.
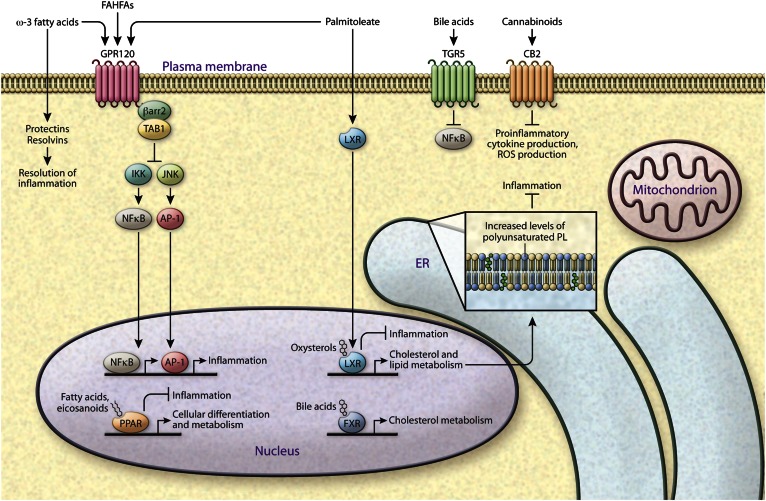
Fig. 3.
Immunometabolic signaling capacity of lipids. The discovery of beneficial roles for specific lipids has changed the perspective on metabolic disease from being excessive fat storage disease to dysregulation of fat composition, and suggested the presence of more complex regulation of lipid subclasses in maintaining homeostatic signaling. The figure depicts several lipids that have anti-inflammatory signaling properties through various distinct mechanisms. For example, lipid ligands bind a variety of nuclear receptors: PPAR ligands are fatty acyl derivatives, LXR ligands are oxysterols, and FXR ligands are bile acids, all with ability to activate an anti-inflammatory program. Lipids are also involved in cell-to-cell and inter-organ communication. Palmitoleate, FAHFAs, and ω-3 fatty acids can activate GPR120 signaling leading to inhibition of JNK- and IKK-mediated inflammation. FAHFAs and palmitoleate are endogenous lipids identified in mouse models of increased adipose tissue de novo lipogenesis, while ω-3 fatty acids are acquired from food intake. The ω-3 fatty acids can further be metabolized into resolvins and protectins, which are involved in resolution of inflammation. Bile acids and endocannabinoids bind to their respective receptors, TGR5 and CB2, to inhibit inflammation and can impact metabolic homeostasis.
The ω-3 fatty acids, which can be acquired via the diet, have also been shown to inhibit metabolic inflammation and alleviate insulin resistance (166). Specifically, ω-3 fatty acids stimulate a lipid sensor, G protein-coupled receptor 120 (GPR120), and inhibit TNFα and TLR4-mediated inflammation. Treatment of mice with ω-3 decreases adipose tissue inflammation and improves insulin sensitivity (166). Discovery of a dysfunctional variant of GPR120 in humans that is associated with obesity and insulin resistance also strengthened the hypothesis that GPR120 signaling is a viable target for metabolic disease treatment and that this pathway may involve signaling by endogenous monounsaturated fatty acids such as palmitoleate (167). Additional beneficial roles are attributed to ω-3 fatty acids through their metabolism into resolvins and protectins (168, 169). Defects in the production and action of these molecules can impair resolution of inflammation and lead to impaired cellular and organismal function (169). Endocannabinoids are a class of monoacylglycerols that are well-known for their effect in increasing appetite via activating their receptors in the CNS and directly impacting adipose tissue and liver in the periphery to regulate metabolism and inflammatory responses. While activation of cannabinoid receptor type 1 (CB1) has undesirable effects in the context of metabolic disease (170–172), CB2 signaling can promote anti-inflammatory outcomes (173, 174) (Fig. 3).
In addition to serving as signaling molecules, an alternative mechanism by which lipids regulate metabolism and alleviate inflammation is through direct engagement of transcription factors (Fig. 3). For example, fatty acyl derivatives and eicosanoids are well-known ligands for nuclear receptors such as PPARs (175). PPARs, in turn, regulate transcription associated with immunological and metabolic outcomes, such as fatty acid oxidation, lipid biosynthesis, and attenuation of inflammation (176–178). Oxysterols are endogenous ligands for liver X receptors (LXRs), which are critical in liver and macrophage function in the context of metabolic and cardiovascular diseases and regulate whole body cholesterol metabolism and have anti-inflammatory roles that are partially mediated by modulation of ER membrane composition (179, 180). In addition to acting through TGR5 to inhibit NFκB-mediated inflammation (181), bile acids bind to and activate farnesoid X receptor (FXR), which is a major regulator of transcription related to bile acid and cholesterol metabolism (182), and FXR activation inhibits inflammation and fibrosis in a mouse model of NASH (183).
It is clear that while the field is in its early stages, there is tremendous potential in exploring the vast diversity of lipids for their specific biology and how signaling and structural functions are intertwined. Overall, the discovery of lipid species with beneficial or detrimental effects and associated pathways involved in immunometabolic functions has already expanded the perspective on lipids that were conventionally associated with lipotoxicity, and uncoupled lipid availability from metabolic dysfunction. Identification of these lipids also offers unique opportunities to exploit them for preventive and therapeutic strategies, especially in the context of chronic immunometabolic diseases.
THERAPEUTIC POTENTIAL OF TARGETING INFLAMMATORY LIPIDS IN METAFLAMMATION
Lipid metabolism and immune responses are closely integrated in multiple tissue systems through conserved pathways, perhaps due to once advantageous evolutionary adaptations at times of frequent periods of famine and high occurrence of infectious disease. In the current era, when there is an excess of available nutrients and decreased prevalence of infectious disease, excess nutrients can drive immune pathways leading to chronic low-level sterile metaflammation, making these adaptations disadvantageous remnants of evolution. The accelerated increase in the prevalence of obesity and associated diseases calls for deeper understanding of the complex makeup of lipid and immune responses and their interaction in order to develop therapies targeting each.
The contribution of lipotoxicity, inflammation, and associated stress responses to metabolic disease involves interplay of several dysregulated pathways that are highly integrated and coregulated. Because of this complexity, it is challenging to distinguish which phenomena are initiating and which lie downstream, and how they can be best targeted for intervention. For example, insulin resistance leads to uncontrolled lipolysis, which is one of the earlier events in systemic lipotoxicity; thus insulin-sensitizing therapies such as TZDs can help increase lipid storage in adipose tissue to overcome lipotoxic effects (184, 185). The finding that adipose triglyceride lipase (ATGL)-deficient mice are insulin sensitive despite triglyceride accumulation (186) suggests that lipases can also be targeted for metabolic disease treatment. Fatty acids released by ATGL activity, however, are also implicated in PPARα activation and contribute to cardiac muscle homeostasis (127), and hence the prolonged general inhibition of lipolysis might have undesirable effects. Further understanding of the lipolytic pathway and the details and molecular components of its contribution to signaling and metabolism will help determine if and how lipolysis can be pharmacologically targeted in a more specific and restricted manner toward treatment of diabetes. For example, lipolysis has been linked to increased FABP4 secretion from adipose tissue via a nonclassical mechanism (59, 60, 187, 188). FABP4 secretion is regulated by ATGL and hormone-sensitive lipase activity and subsequent increase in fatty acid availability (60). Interestingly, there is strong correlation between circulating FABP4 levels and metabolic disease in preclinical models and in humans (59, 189–199). Mechanistically, secreted FABP4 has been demonstrated to increase liver glucose production and insulin secretion and decrease cardiomyocyte contractility (59, 200, 201), and hence, may explain some of the detrimental effects of uncontrolled lipolysis. These effects of circulating FABP4 support the possibility of using neutralizing antibodies to treat metabolic disease, and this approach has proven successful in preclinical models (59, 202, 203).
As metaflammation is the hallmark of chronic metabolic disease, immunoregulatory or anti-inflammatory therapies can help reduce lipid-induced inflammation, cellular dysfunction, and death. The most prominent and common example of this is the use of cholesterol-lowering drugs, such as statins, that can help to decrease the harmful effects of free cholesterol accumulation in liver and macrophages (204, 205). This topic is covered in detail elsewhere (134, 206). Anti-inflammatory medications, such as colchicine and methotrexate, have been associated with decreased cardiovascular disease (207, 208). Salicylates can inhibit the NFκB pathway and have been shown to improve glucose metabolism and diabetes (209, 210). TNFα was one of the first inflammatory molecules shown to play a role in metabolic disease (211). Targeting this pathway in preclinical models proved successful (212–228), although comprehensive human studies are lacking, and the existing limited studies have reported both failures and successes in humans (229). The limitations of the human studies with existing anti-cytokine reagents were recently reviewed in an excellent article (230). There are also exciting, but not yet fully exploited, possibilities by antagonizing lipid-sensing pathways, i.e., using TLR4 antagonists (231) or PKR inhibitors (232) to alleviate inflammation along with other beneficial effects associated with the target functions. In these areas, studies in humans are also highly limited at the moment. Because lipotoxicity impairs ER function and leads to a prolonged unfolded protein response, which can engage stress and inflammatory pathways, it may also be considered for potential intervention strategies. The use of agents that alleviate ER stress, such as tauroursodeoxycholic acid (TUDCA) and 4-phenylbutyric acid, has been tested in metabolic contexts with beneficial results on liver and CNS function (153, 233–235). TUDCA treatment in experimental models of acute pancreatitis and ischemia reperfusion in liver also demonstrated decreased JNK activity along with attenuated inflammatory responses (236, 237). In obese mice treated with TUDCA or 4-phenylbutyric acid, liver JNK activity was decreased along with improved insulin sensitivity and glucose metabolism (233). Interestingly, TUDCA treatment in humans also improved liver and muscle insulin resistance (238) warranting further clinical studies (239), some of which are currently underway (e.g., NCT01829698, https://www.clinicaltrials.gov/ct2/show/NCT01829698).
An exciting and emerging translational area relates to bioactive lipids, such as palmitoleate, FAHFAs, and ω-3 fatty acids that have all been shown to have anti-diabetic and anti-inflammatory effects in mouse models of metabolic disease (discussed above). Hence supplementation with such molecules or finding agonists that target pathways associated with these lipids, such as GPR120 signaling (240, 241), could help reduce lipid-induced metaflammation in a variety of immunometabolic diseases, including obesity and diabetes. The ω-3 fatty acids are further metabolized into resolvins, which are involved in resolution of inflammation (169), a persistent process in diabetes. Hence resolvin supplementation or targeting pathways of resolvin synthesis could help alleviate systemic and local adipose tissue inflammation (242) and represent an effective approach against diabetes. Similar opportunities may also arise from exploring the products of gut microbiota (243, 244). Considering the potential transformational impact of these approaches to manage chronic disease, further research and preclinical as well as clinical testing of these concepts represent a highly promising area of further translational possibilities.
SUMMARY
Extensive research on immunometabolic disorders has established a strong connection between inflammatory pathways and lipids. While the current challenge remains to be the effective development and/or testing of translational tools for prevention and treatment, the future looks more promising than ever with the diversity of new possibilities against chronic metabolic diseases.
Acknowledgments
The authors thank Dr. Kathryn Claiborn for critical reading and editing of this work, and Bruce Worden for assistance with scientific illustrations.
Footnotes
Abbreviations:
- AP-1
- activator protein 1
- Arg2−/−
- arginase 2-deficient
- ATGL
- adipose triglyceride lipase
- BAT
- brown adipose tissue
- DAG
- diacylglycerol
- ER
- endoplasmic reticulum
- FAHFA
- fatty acid hydroxy fatty acid
- FXR
- farnesoid X receptor
- GPR120
- G protein-coupled receptor 120
- iNKT
- invariant natural killer T
- iNOS
- induced nitric oxide synthase (iNOS)
- LD
- lipid droplet
- LXR
- liver X receptor
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- NKT
- natural killer T
- PKC
- protein kinase C
- PKR
- protein kinase R
- ROS
- reactive oxygen species
- snoRNA
- small nucleolar RNA
- TLR4
- toll-like receptor 4
- TUDCA
- tauroursodeoxycholic acid
This work was supported in part by the Office of Extramural Research, National Institutes of Health Grants RO1DK052539, RO1HL125753, and RO1AI116901, a research grant from the Juvenile Diabetes Research Foundation, and sponsored research agreements from Union Chimique Belge (UCB), and Servier. M.E.E. is supported by a Catharina Foundation Postdoctoral Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 2.Fu S., Watkins S. M., and Hotamisligil G. S.. 2012. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 15: 623–634. [DOI] [PubMed] [Google Scholar]
- 3.Chiu H. C., Kovacs A., Blanton R. M., Han X., Courtois M., Weinheimer C. J., Yamada K. A., Brunet S., Xu H., Nerbonne J. M., et al. . 2005. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 96: 225–233. [DOI] [PubMed] [Google Scholar]
- 4.Fu S., Yang L., Li P., Hofmann O., Dicker L., Hide W., Lin X., Watkins S. M., Ivanov A. R., and Hotamisligil G. S.. 2011. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 473: 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu S., Fan J., Blanco J., Gimenez-Cassina A., Danial N. N., Watkins S. M., and Hotamisligil G. S.. 2012. Polysome profiling in liver identifies dynamic regulation of endoplasmic reticulum translatome by obesity and fasting. PLoS Genet. 8: e1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borradaile N. M., Han X., Harp J. D., Gale S. E., Ory D. S., and Schaffer J. E.. 2006. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47: 2726–2737. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y., Wang D., Topczewski F., and Pagliassotti M. J.. 2006. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 291: E275–E281. [DOI] [PubMed] [Google Scholar]
- 8.Feng B., Yao P. M., Li Y., Devlin C. M., Zhang D., Harding H. P., Sweeney M., Rong J. X., Kuriakose G., Fisher E. A., et al. . 2003. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 5: 781–792. [DOI] [PubMed] [Google Scholar]
- 9.Pineau L., Colas J., Dupont S., Beney L., Fleurat-Lessard P., Berjeaud J. M., Berges T., and Ferreira T.. 2009. Lipid-induced ER stress: synergistic effects of sterols and saturated fatty acids. Traffic. 10: 673–690. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T., Furuhashi M., Li P., Cao H., Tuncman G., Sonenberg N., Gorgun C. Z., and Hotamisligil G. S.. 2010. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 140: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu P., Han Z., Couvillon A. D., Kaufman R. J., and Exton J. H.. 2006. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26: 3071–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., and Hotamisligil G. S.. 2004. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 306: 457–461. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani Y., Kaneto H., Kawamori D., Yoshiuchi K., Hatazaki M., Matsuoka T. A., Ozawa K., Ogawa S., Hori M., Yamasaki Y., et al. . 2005. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 280: 847–851. [DOI] [PubMed] [Google Scholar]
- 14.Boden G., Duan X., Homko C., Molina E. J., Song W., Perez O., Cheung P., and Merali S.. 2008. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 57: 2438–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregor M. F., Yang L., Fabbrini E., Mohammed B. S., Eagon J. C., Hotamisligil G. S., and Klein S.. 2009. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 58: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan-Murphy B., and Urano F.. 2012. ER stress as a trigger for beta-cell dysfunction and autoimmunity in type 1 diabetes. Diabetes. 61: 780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tersey S. A., Nishiki Y., Templin A. T., Cabrera S. M., Stull N. D., Colvin S. C., Evans-Molina C., Rickus J. L., Maier B., and Mirmira R. G.. 2012. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 61: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L., Calay E. S., Fan J., Arduini A., Kunz R. C., Gygi S. P., Yalcin A., Fu S., and Hotamisligil G. S.. 2015. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 349: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass C. K., and Olefsky J. M.. 2012. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 15: 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitz-Peiffer C., Browne C. L., Oakes N. D., Watkinson A., Chisholm D. J., Kraegen E. W., and Biden T. J.. 1997. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 46: 169–178. [DOI] [PubMed] [Google Scholar]
- 21.Coudronniere N., Villalba M., Englund N., and Altman A.. 2000. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc. Natl. Acad. Sci. USA. 97: 3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aksoy E., Amraoui Z., Goriely S., Goldman M., and Willems F.. 2002. Critical role of protein kinase C epsilon for lipopolysaccharide-induced IL-12 synthesis in monocyte-derived dendritic cells. Eur. J. Immunol. 32: 3040–3049. [DOI] [PubMed] [Google Scholar]
- 23.Perry R. J., Samuel V. T., Petersen K. F., and Shulman G. I.. 2014. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 510: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zechner R., Zimmermann R., Eichmann T. O., Kohlwein S. D., Haemmerle G., Lass A., and Madeo F.. 2012. FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratford S., Hoehn K. L., Liu F., and Summers S. A.. 2004. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279: 36608–36615. [DOI] [PubMed] [Google Scholar]
- 26.Turpin S. M., Nicholls H. T., Willmes D. M., Mourier A., Brodesser S., Wunderlich C. M., Mauer J., Xu E., Hammerschmidt P., Bronneke H. S., et al. . 2014. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20: 678–686. [DOI] [PubMed] [Google Scholar]
- 27.Raichur S., Wang S. T., Chan P. W., Li Y., Ching J., Chaurasia B., Dogra S., Ohman M. K., Takeda K., Sugii S., et al. . 2014. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20: 687–695. [Erratum. 2014. Cell Metab 20: 919.] [DOI] [PubMed] [Google Scholar]
- 28.Summers S. A. 2006. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 45: 42–72. [DOI] [PubMed] [Google Scholar]
- 29.Yang G., Badeanlou L., Bielawski J., Roberts A. J., Hannun Y. A., and Samad F.. 2009. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297: E211–E224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., et al. . 2011. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J. Y., Sohn K. H., Rhee S. H., and Hwang D.. 2001. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J. Biol. Chem. 276: 16683–16689. [DOI] [PubMed] [Google Scholar]
- 32.Takada Y., Ichikawa H., Pataer A., Swisher S., and Aggarwal B. B.. 2007. Genetic deletion of PKR abrogates TNF-induced activation of IkappaBalpha kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 26: 1201–1212. [DOI] [PubMed] [Google Scholar]
- 33.Kang R., and Tang D.. 2012. PKR-dependent inflammatory signals. Sci. Signal. 5: pe47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng K., Liu L., Wei D., Lv Y., Wang G., Xiong W., Wang X., Altaf A., Wang L., He D., et al. . 2015. P2X7R is involved in the progression of atherosclerosis by promoting NLRP3 inflammasome activation. Int. J. Mol. Med. 35: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel C. I., Holley C. L., Scruggs B. S., Sidhu R., Brookheart R. T., Listenberger L. L., Behlke M. A., Ory D. S., and Schaffer J. E.. 2011. Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab. 14: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youssef O. A., Safran S. A., Nakamura T., Nix D. A., Hotamisligil G. S., and Bass B. L.. 2015. Potential role for snoRNAs in PKR activation during metabolic stress. Proc. Natl. Acad. Sci. USA. 112: 5023–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scruggs B. S., Michel C. I., Ory D. S., and Schaffer J. E.. 2012. SmD3 regulates intronic noncoding RNA biogenesis. Mol. Cell. Biol. 32: 4092–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen E. D., and Spiegelman B. M.. 2014. What we talk about when we talk about fat. Cell. 156: 20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg I. J., Trent C. M., and Schulze P. C.. 2012. Lipid metabolism and toxicity in the heart. Cell Metab. 15: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuel V. T., and Shulman G. I.. 2012. Mechanisms for insulin resistance: common threads and missing links. Cell. 148: 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborn O., and Olefsky J. M.. 2012. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18: 363–374. [DOI] [PubMed] [Google Scholar]
- 42.Cao S. S., and Kaufman R. J.. 2013. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin. Ther. Targets. 17: 437–448. [DOI] [PubMed] [Google Scholar]
- 43.Farese R. V. Jr., and Walther T. C.. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 139: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morigny P., Houssier M., Mouisel E., and Langin D.. 2016. Adipocyte lipolysis and insulin resistance. Biochimie. 125: 259–266. [DOI] [PubMed] [Google Scholar]
- 45.Moraes R. C., Blondet A., Birkenkamp-Demtroeder K., Tirard J., Orntoft T. F., Gertler A., Durand P., Naville D., and Begeot M.. 2003. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology. 144: 4773–4782. [DOI] [PubMed] [Google Scholar]
- 46.Nadler S. T., Stoehr J. P., Schueler K. L., Tanimoto G., Yandell B. S., and Attie A. D.. 2000. The expression of adipogenic genes is decreased in obesity and diabetes mellitus. Proc. Natl. Acad. Sci. USA. 97: 11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranganathan G., Unal R., Pokrovskaya I., Yao-Borengasser A., Phanavanh B., Lecka-Czernik B., Rasouli N., and Kern P. A.. 2006. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J. Lipid Res. 47: 2444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V. Jr., Ory D. S., and Schaffer J. E.. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen H. C., Stone S. J., Zhou P., Buhman K. K., and Farese R. V. Jr. 2002. Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 51: 3189–3195. [DOI] [PubMed] [Google Scholar]
- 50.Franckhauser S., Munoz S., Pujol A., Casellas A., Riu E., Otaegui P., Su B., and Bosch F.. 2002. Increased fatty acid re-esterification by PEPCK overexpression in adipose tissue leads to obesity without insulin resistance. Diabetes. 51: 624–630. [DOI] [PubMed] [Google Scholar]
- 51.Koliwad S. K., Streeper R. S., Monetti M., Cornelissen I., Chan L., Terayama K., Naylor S., Rao M., Hubbard B., and Farese R. V. Jr. 2010. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J. Clin. Invest. 120: 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzer R. G., Park E. J., Li N., Tran H., Chen M., Choi C., Solinas G., and Karin M.. 2011. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 147: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirosumi J., Tuncman G., Chang L., Gorgun C. Z., Uysal K. T., Maeda K., Karin M., and Hotamisligil G. S.. 2002. A central role for JNK in obesity and insulin resistance. Nature. 420: 333–336. [DOI] [PubMed] [Google Scholar]
- 54.Han M. S., Jung D. Y., Morel C., Lakhani S. A., Kim J. K., Flavell R. A., and Davis R. J.. 2013. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 339: 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang S., Rutkowsky J. M., Snodgrass R. G., Ono-Moore K. D., Schneider D. A., Newman J. W., Adams S. H., and Hwang D. H.. 2012. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 53: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentile C. L., Wang D., Pfaffenbach K. T., Cox R., Wei Y., and Pagliassotti M. J.. 2010. Fatty acids regulate CREBh via transcriptional mechanisms that are dependent on proteasome activity and insulin. Mol. Cell. Biochem. 344: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Emanuelli B., Peraldi P., Filloux C., Chavey C., Freidinger K., Hilton D. J., Hotamisligil G. S., and Van Obberghen E.. 2001. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J. Biol. Chem. 276: 47944–47949. [DOI] [PubMed] [Google Scholar]
- 58.Kawakami M., Murase T., Ogawa H., Ishibashi S., Mori N., Takaku F., and Shibata S.. 1987. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J. Biochem. 101: 331–338. [DOI] [PubMed] [Google Scholar]
- 59.Cao H., Sekiya M., Erikci Ertunc M., Burak M. F., Mayers J. R., White A., Inouye K., Rickey L. M., Ercal B. C., Furuhashi M., et al. . 2013. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17: 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ertunc M. E., Sikkeland J., Fenaroli F., Griffiths G., Daniels M. P., Cao H., Saatcioglu F., and Hotamisligil G. S.. 2015. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J. Lipid Res. 56: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotamisligil G. S., Johnson R. S., Distel R. J., Ellis R., Papaioannou V. E., and Spiegelman B. M.. 1996. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 274: 1377–1379. [DOI] [PubMed] [Google Scholar]
- 62.Furuhashi M., Fucho R., Gorgun C. Z., Tuncman G., Cao H., and Hotamisligil G. S.. 2008. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 118: 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao H., Maeda K., Gorgun C. Z., Kim H. J., Park S. Y., Shulman G. I., Kim J. K., and Hotamisligil G. S.. 2006. Regulation of metabolic responses by adipocyte/macrophage Fatty Acid-binding proteins in leptin-deficient mice. Diabetes. 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 64.Mathis D. 2013. Immunological goings-on in visceral adipose tissue. Cell Metab. 17: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huh J. Y., Kim J. I., Park Y. J., Hwang I. J., Lee Y. S., Sohn J. H., Lee S. K., Alfadda A. A., Kim S. S., Choi S. H., et al. . 2013. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol. Cell. Biol. 33: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch L., Nowak M., Varghese B., Clark J., Hogan A. E., Toxavidis V., Balk S. P., O’Shea D., O’Farrelly C., and Exley M. A.. 2012. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 37: 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu L., Parekh V. V., Gabriel C. L., Bracy D. P., Marks-Shulman P. A., Tamboli R. A., Kim S., Mendez-Fernandez Y. V., Besra G. S., Lomenick J. P., et al. . 2012. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl. Acad. Sci. USA. 109: E1143–E1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartelt A., Bruns O. T., Reimer R., Hohenberg H., Ittrich H., Peldschus K., Kaul M. G., Tromsdorf U. I., Weller H., Waurisch C., et al. . 2011. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17: 200–205. [DOI] [PubMed] [Google Scholar]
- 69.Peng X. G., Ju S., Fang F., Wang Y., Fang K., Cui X., Liu G., Li P., Mao H., and Teng G. J.. 2013. Comparison of brown and white adipose tissue fat fractions in ob, seipin, and Fsp27 gene knockout mice by chemical shift-selective imaging and (1)H-MR spectroscopy. Am. J. Physiol. Endocrinol. Metab. 304: E160–E167. [DOI] [PubMed] [Google Scholar]
- 70.Nisoli E., Briscini L., Giordano A., Tonello C., Wiesbrock S. M., Uysal K. T., Cinti S., Carruba M. O., and Hotamisligil G. S.. 2000. Tumor necrosis factor alpha mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl. Acad. Sci. USA. 97: 8033–8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hebbard L., and George J.. 2011. Animal models of nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 8: 35–44. [DOI] [PubMed] [Google Scholar]
- 72.Kumashiro N., Erion D. M., Zhang D., Kahn M., Beddow S. A., Chu X., Still C. D., Gerhard G. S., Han X., Dziura J., et al. . 2011. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA. 108: 16381–16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magkos F., Su X., Bradley D., Fabbrini E., Conte C., Eagon J. C., Varela J. E., Brunt E. M., Patterson B. W., and Klein S.. 2012. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 142: 1444–1446.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldstein A. E., Werneburg N. W., Canbay A., Guicciardi M. E., Bronk S. F., Rydzewski R., Burgart L. J., and Gores G. J.. 2004. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 40: 185–194. [DOI] [PubMed] [Google Scholar]
- 75.Negrin K. A., Roth Flach R. J., DiStefano M. T., Matevossian A., Friedline R. H., Jung D., Kim J. K., and Czech M. P.. 2014. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS One. 9: e107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W. Z., Strowig T., Thaiss C. A., Kau A. L., Eisenbarth S. C., Jurczak M. J., et al. . 2012. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J., Liu X., Gao B., Karin M., Tsukamoto H., Brenner D., and Kisseleva T.. 2014. New approaches for studying alcoholic liver disease. Curr. Pathobiol. Rep. 2: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henao-Mejia J., Elinav E., Thaiss C. A., Licona-Limon P., and Flavell R. A.. 2013. Role of the intestinal microbiome in liver disease. J. Autoimmun. 46: 66–73. [DOI] [PubMed] [Google Scholar]
- 79.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P., and Ron D.. 2000. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 287: 664–666. [DOI] [PubMed] [Google Scholar]
- 80.Timmins J. M., Ozcan L., Seimon T. A., Li G., Malagelada C., Backs J., Backs T., Bassel-Duby R., Olson E. N., Anderson M. E., et al. . 2009. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 119: 2925–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu B., Nakamura T., Inouye K., Li J., Tang Y., Lundback P., Valdes-Ferrer S. I., Olofsson P. S., Kalb T., Roth J., et al. . 2012. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 488: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.González-Terán B., Matesanz N., Nikolic I., Verdugo M. A., Sreeramkumar V., Hernández-Cosido L., Mora A., Crainiciuc G., Sáiz M. L., Bernardo E., et al. . 2016. p38gamma and p38delta reprogram liver metabolism by modulating neutrophil infiltration. EMBO J. 35: 536–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malhi H., Bronk S. F., Werneburg N. W., and Gores G. J.. 2006. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 281: 12093–12101. [DOI] [PubMed] [Google Scholar]
- 84.Seki E., Brenner D. A., and Karin M.. 2012. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 143: 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arruda A. P., Pers B. M., Parlakgul G., Guney E., Inouye K., and Hotamisligil G. S.. 2014. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 20: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmitz J., Evers N., Awazawa M., Nicholls H. T., Bronneke H. S., Dietrich A., Mauer J., Bluher M., and Bruning J. C.. 2016. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 5: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Rooyen D. M., Larter C. Z., Haigh W. G., Yeh M. M., Ioannou G., Kuver R., Lee S. P., Teoh N. C., and Farrell G. C.. 2011. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 141: 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao L., Chen Y., Tang R., Chen Y., Li Q., Gong J., Huang A., Varghese Z., Moorhead J. F., and Ruan X. Z.. 2011. Inflammatory stress exacerbates hepatic cholesterol accumulation via increasing cholesterol uptake and de novo synthesis. J. Gastroenterol. Hepatol. 26: 875–883. [DOI] [PubMed] [Google Scholar]
- 89.Simonen P., Kotronen A., Hallikainen M., Sevastianova K., Makkonen J., Hakkarainen A., Lundbom N., Miettinen T. A., Gylling H., and Yki-Jarvinen H.. 2011. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J. Hepatol. 54: 153–159. [DOI] [PubMed] [Google Scholar]
- 90.Min H. K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J., Kellum J., Warnick R., Contos M. J., and Sanyal A. J.. 2012. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gan L. T., Van Rooyen D. M., Koina M. E., McCuskey R. S., Teoh N. C., and Farrell G. C.. 2014. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J. Hepatol. 61: 1376–1384. [DOI] [PubMed] [Google Scholar]
- 92.Marí M., Caballero F., Colell A., Morales A., Caballeria J., Fernandez A., Enrich C., Fernandez-Checa J. C., and García-Ruiz C.. 2006. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 4: 185–198. [DOI] [PubMed] [Google Scholar]
- 93.Ioannou G. N., Haigh W. G., Thorning D., and Savard C.. 2013. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 54: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiri-Sverdlov R., Wouters K., van Gorp P. J., Gijbels M. J., Noel B., Buffat L., Staels B., Maeda N., van Bilsen M., and Hofker M. H.. 2006. Early diet-induced non-alcoholic steatohepatitis in APOE2 knock-in mice and its prevention by fibrates. J. Hepatol. 44: 732–741. [DOI] [PubMed] [Google Scholar]
- 95.Navarro L. A., Wree A., Povero D., Berk M. P., Eguchi A., Ghosh S., Papouchado B. G., Erzurum S. C., and Feldstein A. E.. 2015. Arginase 2 deficiency results in spontaneous steatohepatitis: a novel link between innate immune activation and hepatic de novo lipogenesis. J. Hepatol. 62: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang W., Metlakunta A., Dedousis N., Zhang P., Sipula I., Dube J. J., Scott D. K., and O’Doherty R. M.. 2010. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 59: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galbo T., Perry R. J., Jurczak M. J., Camporez J. P., Alves T. C., Kahn M., Guigni B. A., Serr J., Zhang D., Bhanot S., et al. . 2013. Saturated and unsaturated fat induce hepatic insulin resistance independently of TLR-4 signaling and ceramide synthesis in vivo. Proc. Natl. Acad. Sci. USA. 110: 12780–12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jurczak M. J., Lee A. H., Jornayvaz F. R., Lee H. Y., Birkenfeld A. L., Guigni B. A., Kahn M., Samuel V. T., Glimcher L. H., and Shulman G. I.. 2012. Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J. Biol. Chem. 287: 2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samuel V. T., Liu Z. X., Wang A., Beddow S. A., Geisler J. G., Kahn M., Zhang X. M., Monia B. P., Bhanot S., and Shulman G. I.. 2007. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perry R. J., Camporez J. P., Kursawe R., Titchenell P. M., Zhang D., Perry C. J., Jurczak M. J., Abudukadier A., Han M. S., Zhang X. M., et al. . 2015. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 160: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samuel V. T., Petersen K. F., and Shulman G. I.. 2010. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 375: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V. Sr., et al. . 2007. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6: 69–78. [DOI] [PubMed] [Google Scholar]
- 103.Minehira K., Young S. G., Villanueva C. J., Yetukuri L., Oresic M., Hellerstein M. K., Farese R. V. Jr., Horton J. D., Preitner F., Thorens B., et al. . 2008. Blocking VLDL secretion causes hepatic steatosis but does not affect peripheral lipid stores or insulin sensitivity in mice. J. Lipid Res. 49: 2038–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benhamed F., Denechaud P. D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J., et al. . 2012. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 122: 2176–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Itani S. I., Ruderman N. B., Schmieder F., and Boden G.. 2002. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 51: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 106.Boden G., Chen X., Ruiz J., White J. V., and Rossetti L.. 1994. Mechanisms of fatty acid-induced inhibition of glucose uptake. J. Clin. Invest. 93: 2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dresner A., Laurent D., Marcucci M., Griffin M. E., Dufour S., Cline G. W., Slezak L. A., Andersen D. K., Hundal R. S., Rothman D. L., et al. . 1999. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greiwe J. S., Cheng B., Rubin D. C., Yarasheski K. E., and Semenkovich C. F.. 2001. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 15: 475–482. [DOI] [PubMed] [Google Scholar]
- 109.Lambert C. P., Wright N. R., Finck B. N., and Villareal D. T.. 2008. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J. Appl. Physiol. 105: 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nawrocki A. R., and Scherer P. E.. 2004. The delicate balance between fat and muscle: adipokines in metabolic disease and musculoskeletal inflammation. Curr. Opin. Pharmacol. 4: 281–289. [DOI] [PubMed] [Google Scholar]
- 111.Griffin M. E., Marcucci M. J., Cline G. W., Bell K., Barucci N., Lee D., Goodyear L. J., Kraegen E. W., White M. F., and Shulman G. I.. 1999. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 48: 1270–1274. [DOI] [PubMed] [Google Scholar]
- 112.Virkamäki A., Korsheninnikova E., Seppälä-Lindroos A., Vehkavaara S., Goto T., Halavaara J., Häkkinen A. M., and Yki-Järvinen H.. 2001. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 50: 2337–2343. [DOI] [PubMed] [Google Scholar]
- 113.Wei Y., Chen K., Whaley-Connell A. T., Stump C. S., Ibdah J. A., and Sowers J. R.. 2008. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294: R673–R680. [DOI] [PubMed] [Google Scholar]
- 114.Plomgaard P., Bouzakri K., Krogh-Madsen R., Mittendorfer B., Zierath J. R., and Pedersen B. K.. 2005. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 54: 2939–2945. [DOI] [PubMed] [Google Scholar]
- 115.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. . 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277: 50230–50236. [DOI] [PubMed] [Google Scholar]
- 116.Grevengoed T. J., Klett E. L., and Coleman R. A.. 2014. Acyl-CoA metabolism and partitioning. Annu. Rev. Nutr. 34: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morino K., Petersen K. F., and Shulman G. I.. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 55(Suppl 2): S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., et al. . 2008. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7: 45–56. [DOI] [PubMed] [Google Scholar]
- 119.Zhou R., Yazdi A. S., Menu P., and Tschopp J.. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature. 469: 221–225. [DOI] [PubMed] [Google Scholar]
- 120.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M. T., Brickey W. J., and Ting J. P.. 2011. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goodpaster B. H., He J., Watkins S., and Kelley D. E.. 2001. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 86: 5755–5761. [DOI] [PubMed] [Google Scholar]
- 122.Dirkx E., Schwenk R. W., Glatz J. F., Luiken J. J., and van Eys G. J.. 2011. High fat diet induced diabetic cardiomyopathy. Prostaglandins Leukot. Essent. Fatty Acids. 85: 219–225. [DOI] [PubMed] [Google Scholar]
- 123.Mazurek T., Zhang L., Zalewski A., Mannion J. D., Diehl J. T., Arafat H., Sarov-Blat L., O’Brien S., Keiper E. A., Johnson A. G., et al. . 2003. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 108: 2460–2466. [DOI] [PubMed] [Google Scholar]
- 124.Baker A. R., Silva N. F., Quinn D. W., Harte A. L., Pagano D., Bonser R. S., Kumar S., and McTernan P. G.. 2006. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kremen J., Dolinkova M., Krajickova J., Blaha J., Anderlova K., Lacinova Z., Haluzikova D., Bosanska L., Vokurka M., Svacina S., et al. . 2006. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J. Clin. Endocrinol. Metab. 91: 4620–4627. [DOI] [PubMed] [Google Scholar]
- 126.Dong B., Qi D., Yang L., Huang Y., Xiao X., Tai N., Wen L., and Wong F. S.. 2012. TLR4 regulates cardiac lipid accumulation and diabetic heart disease in the nonobese diabetic mouse model of type 1 diabetes. Am. J. Physiol. Heart Circ. Physiol. 303: H732–H742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haemmerle G., Moustafa T., Woelkart G., Buttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., et al. . 2011. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 17: 1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zechner R. 2015. FAT FLUX: enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol. Med. 7: 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guzzardi M. A., and Iozzo P.. 2011. Fatty heart, cardiac damage, and inflammation. Rev. Diabet. Stud. 8: 403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu L., Shi X., Bharadwaj K. G., Ikeda S., Yamashita H., Yagyu H., Schaffer J. E., Yu Y. H., and Goldberg I. J.. 2009. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J. Biol. Chem. 284: 36312–36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., et al. . 2009. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weber C., and Noels H.. 2011. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17: 1410–1422. [DOI] [PubMed] [Google Scholar]
- 133.Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nunez G., Schnurr M., et al. . 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goldstein J. L., and Brown M. S.. 2015. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 161: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Libby P., Lichtman A. H., and Hansson G. K.. 2013. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 38: 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee Y., Hirose H., Ohneda M., Johnson J. H., McGarry J. D., and Unger R. H.. 1994. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc. Natl. Acad. Sci. USA. 91: 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eizirik D. L., Miani M., and Cardozo A. K.. 2013. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 56: 234–241. [DOI] [PubMed] [Google Scholar]
- 138.Biden T. J., Boslem E., Chu K. Y., and Sue N.. 2014. Lipotoxic endoplasmic reticulum stress, beta cell failure, and type 2 diabetes mellitus. Trends Endocrinol. Metab. 25: 389–398. [DOI] [PubMed] [Google Scholar]
- 139.Prentki M., Matschinsky F. M., and Madiraju S. R.. 2013. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 18: 162–185. [DOI] [PubMed] [Google Scholar]
- 140.Donath M. Y., Boni-Schnetzler M., Ellingsgaard H., and Ehses J. A.. 2009. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda). 24: 325–331. [DOI] [PubMed] [Google Scholar]
- 141.Larsen C. M., Faulenbach M., Vaag A., Volund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., and Donath M. Y.. 2007. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356: 1517–1526. [DOI] [PubMed] [Google Scholar]
- 142.Richardson S. J., Willcox A., Bone A. J., Foulis A. K., and Morgan N. G.. 2009. Islet-associated macrophages in type 2 diabetes. Diabetologia. 52: 1686–1688. [DOI] [PubMed] [Google Scholar]
- 143.Imai Y., Dobrian A. D., Morris M. A., and Nadler J. L.. 2013. Islet inflammation: a unifying target for diabetes treatment? Trends Endocrinol. Metab. 24: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eguchi K., Manabe I., Oishi-Tanaka Y., Ohsugi M., Kono N., Ogata F., Yagi N., Ohto U., Kimoto M., Miyake K., et al. . 2012. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 15: 518–533. [DOI] [PubMed] [Google Scholar]
- 145.Brunham L. R., Kruit J. K., Verchere C. B., and Hayden M. R.. 2008. Cholesterol in islet dysfunction and type 2 diabetes. J. Clin. Invest. 118: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hermans M. P., Ahn S. A., and Rousseau M. F.. 2010. log(TG)/HDL-C is related to both residual cardiometabolic risk and beta-cell function loss in type 2 diabetes males. Cardiovasc. Diabetol. 9: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kruit J. K., Wijesekara N., Westwell-Roper C., Vanmierlo T., de Haan W., Bhattacharjee A., Tang R., Wellington C. L., LütJohann D., Johnson J. D., et al. . 2012. Loss of both ABCA1 and ABCG1 results in increased disturbances in islet sterol homeostasis, inflammation, and impaired beta-cell function. Diabetes. 61: 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X., Zhang G., Zhang H., Karin M., Bai H., and Cai D.. 2008. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 135: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Milanski M., Degasperi G., Coope A., Morari J., Denis R., Cintra D. E., Tsukumo D. M., Anhe G., Amaral M. E., Takahashi H. K., et al. . 2009. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Posey K. A., Clegg D. J., Printz R. L., Byun J., Morton G. J., Vivekanandan-Giri A., Pennathur S., Baskin D. G., Heinecke J. W., Woods S. C., et al. . 2009. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 296: E1003–E1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thaler J. P., Yi C. X., Schur E. A., Guyenet S. J., Hwang B. H., Dietrich M. O., Zhao X., Sarruf D. A., Izgur V., Maravilla K. R., et al. . 2012. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Freeman L. R., Haley-Zitlin V., Rosenberger D. S., and Granholm A. C.. 2014. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr. Neurosci. 17: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ozcan L., Ergin A. S., Lu A., Chung J., Sarkar S., Nie D., Myers M. G. Jr., and Ozcan U.. 2009. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9: 35–51. [DOI] [PubMed] [Google Scholar]
- 154.Contreras C., Gonzalez-Garcia I., Martinez-Sanchez N., Seoane-Collazo P., Jacas J., Morgan D. A., Serra D., Gallego R., Gonzalez F., Casals N., et al. . 2014. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Reports. 9: 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McFadden J. W., Aja S., Li Q., Bandaru V. V., Kim E. K., Haughey N. J., Kuhajda F. P., and Ronnett G. V.. 2014. Increasing fatty acid oxidation remodels the hypothalamic neurometabolome to mitigate stress and inflammation. PLoS One. 9: e115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., and Gordon J. I.. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 157.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., et al. . 2007. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- 158.Caesar R., Tremaroli V., Kovatcheva-Datchary P., Cani P. D., and Backhed F.. 2015. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 22: 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gillum M. P., Zhang D., Zhang X. M., Erion D. M., Jamison R. A., Choi C., Dong J., Shanabrough M., Duenas H. R., Frederick D. W., et al. . 2008. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 135: 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eros G., Varga G., Varadi R., Czobel M., Kaszaki J., Ghyczy M., and Boros M.. 2009. Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur. Surg. Res. 42: 40–48. [DOI] [PubMed] [Google Scholar]
- 161.Shiratsuchi A., Ichiki M., Okamoto Y., Ueda N., Sugimoto N., Takuwa Y., and Nakanishi Y.. 2009. Inhibitory effect of N-palmitoylphosphatidylethanolamine on macrophage phagocytosis through inhibition of Rac1 and Cdc42. J. Biochem. 145: 43–50. [DOI] [PubMed] [Google Scholar]
- 162.Cao H., Gerhold K., Mayers J. R., Wiest M. M., Watkins S. M., and Hotamisligil G. S.. 2008. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 134: 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chan K. L., Pillon N. J., Sivaloganathan D. M., Costford S. R., Liu Z., Theret M., Chazaud B., and Klip A.. 2015. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK). J. Biol. Chem. 290: 16979–16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Talbot N. A., Wheeler-Jones C. P., and Cleasby M. E.. 2014. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell. Endocrinol. 393: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yore M. M., Syed I., Moraes-Vieira P. M., Zhang T., Herman M. A., Homan E. A., Patel R. T., Lee J., Chen S., Peroni O. D., et al. . 2014. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 159: 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., and Olefsky J. M.. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ichimura A., Hirasawa A., Poulain-Godefroy O., Bonnefond A., Hara T., Yengo L., Kimura I., Leloire A., Liu N., Iida K., et al. . 2012. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 483: 350–354. [DOI] [PubMed] [Google Scholar]
- 168.González-Périz A., Horrillo R., Ferré N., Gronert K., Dong B., Morán-Salvador E., Titos E., Martínez-Clemente M., López-Parra M., Arroyo V., et al. . 2009. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 23: 1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Spite M., Claria J., and Serhan C. N.. 2014. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19: 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Soria-Gómez E., Bellocchio L., Reguero L., Lepousez G., Martin C., Bendahmane M., Ruehle S., Remmers F., Desprez T., Matias I., et al. . 2014. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 17: 407–415. [DOI] [PubMed] [Google Scholar]
- 171.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Batkai S., Harvey-White J., Mackie K., Offertaler L., Wang L., et al. . 2005. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 115: 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Osei-Hyiaman D., Liu J., Zhou L., Godlewski G., Harvey-White J., Jeong W. I., Batkai S., Marsicano G., Lutz B., Buettner C., et al. . 2008. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Invest. 118: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schmitz K., Mangels N., Haussler A., Ferreiros N., Fleming I., and Tegeder I.. 2016. Pro-inflammatory obesity in aged cannabinoid-2 receptor-deficient mice. Int. J. Obes. (Lond). 40: 366–379. [DOI] [PubMed] [Google Scholar]
- 174.Han K. H., Lim S., Ryu J., Lee C. W., Kim Y., Kang J. H., Kang S. S., Ahn Y. K., Park C. S., and Kim J. J.. 2009. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 84: 378–386. [DOI] [PubMed] [Google Scholar]
- 175.Grygiel-Górniak B. 2014. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr. J. 13: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., and Piomelli D.. 2005. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 67: 15–19. [DOI] [PubMed] [Google Scholar]
- 177.Kang K., Reilly S. M., Karabacak V., Gangl M. R., Fitzgerald K., Hatano B., and Lee C. H.. 2008. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosen E. D., and Spiegelman B. M.. 2001. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J. Biol. Chem. 276: 37731–37734. [DOI] [PubMed] [Google Scholar]
- 179.Rong X., Albert C. J., Hong C., Duerr M. A., Chamberlain B. T., Tarling E. J., Ito A., Gao J., Wang B., Edwards P. A., et al. . 2013. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 18: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zelcer N., and Tontonoz P.. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Y. D., Chen W. D., Yu D., Forman B. M., and Huang W.. 2011. The G-protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology. 54: 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schaap F. G., Trauner M., and Jansen P. L.. 2014. Bile acid receptors as targets for drug development. Nat. Rev. Gastroenterol. Hepatol. 11: 55–67. [DOI] [PubMed] [Google Scholar]
- 183.Zhang S., Wang J., Liu Q., and Harnish D. C.. 2009. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 51: 380–388. [DOI] [PubMed] [Google Scholar]
- 184.Hammarstedt A., Sopasakis V. R., Gogg S., Jansson P. A., and Smith U.. 2005. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia. 48: 96–104. [DOI] [PubMed] [Google Scholar]
- 185.Belfort R., Harrison S. A., Brown K., Darland C., Finch J., Hardies J., Balas B., Gastaldelli A., Tio F., Pulcini J., et al. . 2006. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N. Engl. J. Med. 355: 2297–2307. [DOI] [PubMed] [Google Scholar]
- 186.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. . 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 187.Kralisch S., Ebert T., Lossner U., Jessnitzer B., Stumvoll M., and Fasshauer M.. 2014. Adipocyte fatty acid-binding protein is released from adipocytes by a non-conventional mechanism. Int. J. Obes. (Lond). 38: 1251–1254. [DOI] [PubMed] [Google Scholar]
- 188.Mita T., Furuhashi M., Hiramitsu S., Ishii J., Hoshina K., Ishimura S., Fuseya T., Watanabe Y., Tanaka M., Ohno K., et al. . 2015. FABP4 is secreted from adipocytes by adenyl cyclase-PKA- and guanylyl cyclase-PKG-dependent lipolytic mechanisms. Obesity (Silver Spring). 23: 359–367. [DOI] [PubMed] [Google Scholar]
- 189.Kim Y. C., Cho Y. K., Lee W. Y., Kim H. J., Park J. H., Park D. I., Sohn C. I., Jeon W. K., Kim B. I., Park S. E., et al. . 2011. Serum adipocyte-specific fatty acid-binding protein is associated with nonalcoholic fatty liver disease in apparently healthy subjects. J. Nutr. Biochem. 22: 289–292. [DOI] [PubMed] [Google Scholar]
- 190.Terra X., Quintero Y., Auguet T., Porras J. A., Hernandez M., Sabench F., Aguilar C., Luna A. M., Del Castillo D., and Richart C.. 2011. FABP 4 is associated with inflammatory markers and metabolic syndrome in morbidly obese women. Eur. J. Endocrinol. 164: 539–547. [DOI] [PubMed] [Google Scholar]
- 191.von Eynatten M., Breitling L. P., Roos M., Baumann M., Rothenbacher D., and Brenner H.. 2012. Circulating adipocyte fatty acid-binding protein levels and cardiovascular morbidity and mortality in patients with coronary heart disease: a 10-year prospective study. Arterioscler. Thromb. Vasc. Biol. 32: 2327–2335. [DOI] [PubMed] [Google Scholar]
- 192.Ishimura S., Furuhashi M., Watanabe Y., Hoshina K., Fuseya T., Mita T., Okazaki Y., Koyama M., Tanaka M., Akasaka H., et al. . 2013. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS One. 8: e81318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu M., Zhou M., Bao Y., Xu Z., Li H., Zhang H., Zhu W., Zhang J., Xu A., Wei M., et al. . 2013. Circulating adipocyte fatty acid-binding protein levels are independently associated with heart failure. Clin. Sci. (Lond.). 124: 115–122. [DOI] [PubMed] [Google Scholar]
- 194.Bao Y., Lu Z., Zhou M., Li H., Wang Y., Gao M., Wei M., and Jia W.. 2011. Serum levels of adipocyte fatty acid-binding protein are associated with the severity of coronary artery disease in Chinese women. PLoS One. 6: e19115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hsu B. G., Chen Y. C., Lee R. P., Lee C. C., Lee C. J., and Wang J. H.. 2010. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ. J. 74: 327–331. [DOI] [PubMed] [Google Scholar]
- 196.Furuhashi M., Ishimura S., Ota H., Hayashi M., Nishitani T., Tanaka M., Yoshida H., Shimamoto K., Hotamisligil G. S., and Miura T.. 2011. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 6: e27356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yoo H. J., Kim S., Park M. S., Choi H. Y., Yang S. J., Seo J. A., Kim S. G., Kim N. H., Baik S. H., Choi D. S., et al. . 2011. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. J. Clin. Endocrinol. Metab. 96: E488–E492. [DOI] [PubMed] [Google Scholar]
- 198.Xu A., Wang Y., Xu J. Y., Stejskal D., Tam S., Zhang J., Wat N. M., Wong W. K., and Lam K. S.. 2006. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 52: 405–413. [DOI] [PubMed] [Google Scholar]
- 199.Miyoshi T., Onoue G., Hirohata A., Hirohata S., Usui S., Hina K., Kawamura H., Doi M., Kusano K. F., Kusachi S., et al. . 2010. Serum adipocyte fatty acid-binding protein is independently associated with coronary atherosclerotic burden measured by intravascular ultrasound. Atherosclerosis. 211: 164–169. [DOI] [PubMed] [Google Scholar]
- 200.Lamounier-Zepter V., Look C., Alvarez J., Christ T., Ravens U., Schunck W. H., Ehrhart-Bornstein M., Bornstein S. R., and Morano I.. 2009. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ. Res. 105: 326–334. [DOI] [PubMed] [Google Scholar]
- 201.Wu L. E., Samocha-Bonet D., Whitworth P. T., Fazakerley D. J., Turner N., Biden T. J., James D. E., and Cantley J.. 2014. Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol. Metab. 3: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Burak M. F., Inouye K. E., White A., Lee A., Tuncman G., Calay E. S., Sekiya M., Tirosh A., Eguchi K., Birrane G., et al. . 2015. Development of a therapeutic monoclonal antibody that targets secreted fatty acid-binding protein aP2 to treat type 2 diabetes. Sci. Transl. Med. 7: 319ra205. [DOI] [PubMed] [Google Scholar]
- 203.Hotamisligil G. S., and Bernlohr D. A.. 2015. Metabolic functions of FABPs–mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 11: 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arguello G., Balboa E., Arrese M., and Zanlungo S.. 2015. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta. 1852: 1765–1778. [DOI] [PubMed] [Google Scholar]
- 205.Van Rooyen D. M., Gan L. T., Yeh M. M., Haigh W. G., Larter C. Z., Ioannou G., Teoh N. C., and Farrell G. C.. 2013. Pharmacological cholesterol lowering reverses fibrotic NASH in obese, diabetic mice with metabolic syndrome. J. Hepatol. 59: 144–152. [DOI] [PubMed] [Google Scholar]
- 206.Antonopoulos A. S., Margaritis M., Lee R., Channon K., and Antoniades C.. 2012. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 18: 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Micha R., Imamura F., Wyler von Ballmoos M., Solomon D. H., Hernan M. A., Ridker P. M., and Mozaffarian D.. 2011. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 108: 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nidorf S. M., Eikelboom J. W., Budgeon C. A., and Thompson P. L.. 2013. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 61: 404–410. [DOI] [PubMed] [Google Scholar]
- 209.Yin M. J., Yamamoto Y., and Gaynor R. B.. 1998. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 396: 77–80. [DOI] [PubMed] [Google Scholar]
- 210.Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z. W., Karin M., and Shoelson S. E.. 2001. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 293: 1673–1677. [DOI] [PubMed] [Google Scholar]
- 211.Hotamisligil G. S., Shargill N. S., and Spiegelman B. M.. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 212.Uysal K. T., Wiesbrock S. M., Marino M. W., and Hotamisligil G. S.. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 389: 610–614. [DOI] [PubMed] [Google Scholar]
- 213.Hotamisligil G. S. 1999. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J. Intern. Med. 245: 621–625. [DOI] [PubMed] [Google Scholar]
- 214.Uysal K. T., Wiesbrock S. M., and Hotamisligil G. S.. 1998. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology. 139: 4832–4838. [DOI] [PubMed] [Google Scholar]
- 215.Ventre J., Doebber T., Wu M., MacNaul K., Stevens K., Pasparakis M., Kollias G., and Moller D. E.. 1997. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 46: 1526–1531. [DOI] [PubMed] [Google Scholar]
- 216.De Taeye B. M., Novitskaya T., McGuinness O. P., Gleaves L., Medda M., Covington J. W., and Vaughan D. E.. 2007. Macrophage TNF-alpha contributes to insulin resistance and hepatic steatosis in diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 293: E713–E725. [DOI] [PubMed] [Google Scholar]
- 217.Salles J., Tardif N., Landrier J. F., Mothe-Satney I., Guillet C., Boue-Vaysse C., Combaret L., Giraudet C., Patrac V., Bertrand-Michel J., et al. . 2012. TNFalpha gene knockout differentially affects lipid deposition in liver and skeletal muscle of high-fat-diet mice. J. Nutr. Biochem. 23: 1685–1693. [DOI] [PubMed] [Google Scholar]
- 218.Bouter B., Geary N., Langhans W., and Asarian L.. 2010. Diet-genotype interactions in the early development of obesity and insulin resistance in mice with a genetic deficiency in tumor necrosis factor-alpha. Metabolism. 59: 1065–1073. [DOI] [PubMed] [Google Scholar]
- 219.Aouadi M., Tencerova M., Vangala P., Yawe J. C., Nicoloro S. M., Amano S. U., Cohen J. L., and Czech M. P.. 2013. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc. Natl. Acad. Sci. USA. 110: 8278–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hadad N., Burgazliev O., Elgazar-Carmon V., Solomonov Y., Wueest S., Item F., Konrad D., Rudich A., and Levy R.. 2013. Induction of cytosolic phospholipase a2alpha is required for adipose neutrophil infiltration and hepatic insulin resistance early in the course of high-fat feeding. Diabetes. 62: 3053–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Koulmanda M., Bhasin M., Awdeh Z., Qipo A., Fan Z., Hanidziar D., Putheti P., Shi H., Csizuadia E., Libermann T. A., et al. . 2012. The role of TNF-alpha in mice with type 1- and 2- diabetes. PLoS One. 7: e33254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Araújo E. P., De Souza C. T., Ueno M., Cintra D. E., Bertolo M. B., Carvalheira J. B., Saad M. J., and Velloso L. A.. 2007. Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology. 148: 5991–5997. [DOI] [PubMed] [Google Scholar]
- 223.Ishikawa K., Takahashi K., Bujo H., Hashimoto N., Yagui K., and Saito Y.. 2006. Subcutaneous fat modulates insulin sensitivity in mice by regulating TNF-alpha expression in visceral fat. Horm. Metab. Res. 38: 631–638. [DOI] [PubMed] [Google Scholar]
- 224.Borst S. E., and Bagby G. J.. 2002. Neutralization of tumor necrosis factor reverses age-induced impairment of insulin responsiveness in skeletal muscle of Sprague-Dawley rats. Metabolism. 51: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 225.Li Z., Yang S., Lin H., Huang J., Watkins P. A., Moser A. B., Desimone C., Song X. Y., and Diehl A. M.. 2003. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 37: 343–350. [DOI] [PubMed] [Google Scholar]
- 226.Borst S. E., Lee Y., Conover C. F., Shek E. W., and Bagby G. J.. 2004. Neutralization of tumor necrosis factor-alpha reverses insulin resistance in skeletal muscle but not adipose tissue. Am. J. Physiol. Endocrinol. Metab. 287: E934–E938. [DOI] [PubMed] [Google Scholar]
- 227.Cheung A. T., Ree D., Kolls J. K., Fuselier J., Coy D. H., and Bryer-Ash M.. 1998. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology. 139: 4928–4935. [DOI] [PubMed] [Google Scholar]
- 228.Liang H., Yin B., Zhang H., Zhang S., Zeng Q., Wang J., Jiang X., Yuan L., Wang C. Y., and Li Z.. 2008. Blockade of tumor necrosis factor (TNF) receptor type 1-mediated TNF-alpha signaling protected Wistar rats from diet-induced obesity and insulin resistance. Endocrinology. 149: 2943–2951. [DOI] [PubMed] [Google Scholar]
- 229.Esser N., Paquot N., and Scheen A. J.. 2015. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin. Investig. Drugs. 24: 283–307. [DOI] [PubMed] [Google Scholar]
- 230.Donath M. Y. 2014. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 13: 465–476. [DOI] [PubMed] [Google Scholar]
- 231.Lu Z., Zhang X., Li Y., Lopes-Virella M. F., and Huang Y.. 2015. TLR4 antagonist attenuates atherogenesis in LDL receptor-deficient mice with diet-induced type 2 diabetes. Immunobiology. 220: 1246–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Nakamura T., Arduini A., Baccaro B., Furuhashi M., and Hotamisligil G. S.. 2014. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes. 63: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z., and Hotamisligil G. S.. 2006. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 313: 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang J. S., Kim J. T., Jeon J., Park H. S., Kang G. H., Park K. S., Lee H. K., Kim S., and Cho Y. M.. 2010. Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice. PLoS One. 5: e13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Caglieris S., Giannini E., Dardano G., Mondello L., Valente U., and Testa R.. 2000. Tauroursodeoxycholic acid administration as adjuvant therapy in cirrhotic patients on transplantation waiting lists. Hepatogastroenterology. 47: 1045–1047. [PubMed] [Google Scholar]
- 236.Seyhun E., Malo A., Schafer C., Moskaluk C. A., Hoffmann R. T., Goke B., and Kubisch C. H.. 2011. Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, acinar cell damage, and systemic inflammation in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 301: G773–G782. [DOI] [PubMed] [Google Scholar]
- 237.Ben Mosbah I., Alfany-Fernandez I., Martel C., Zaouali M. A., Bintanel-Morcillo M., Rimola A., Rodes J., Brenner C., Rosello-Catafau J., and Peralta C.. 2010. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 1: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kars M., Yang L., Gregor M. F., Mohammed B. S., Pietka T. A., Finck B. N., Patterson B. W., Horton J. D., Mittendorfer B., Hotamisligil G. S., et al. . 2010. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 59: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gentile C. L., and Pagliassotti M. J.. 2008. The endoplasmic reticulum as a potential therapeutic target in nonalcoholic fatty liver disease. Curr. Opin. Investig. Drugs. 9: 1084–1088. [PMC free article] [PubMed] [Google Scholar]
- 240.Talukdar S., Olefsky J. M., and Osborn O.. 2011. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 32: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu H. D., Wang W. B., Xu Z. G., Liu C. H., He D. F., Du L. P., Li M. Y., Yu X., and Sun J. P.. 2015. FFA4 receptor (GPR120): A hot target for the development of anti-diabetic therapies. Eur. J. Pharmacol. 763: 160–168. [DOI] [PubMed] [Google Scholar]
- 242.Neuhofer A., Zeyda M., Mascher D., Itariu B. K., Murano I., Leitner L., Hochbrugger E. E., Fraisl P., Cinti S., Serhan C. N., et al. . 2013. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 62: 1945–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Andreasen A. S., Larsen N., Pedersen-Skovsgaard T., Berg R. M., Moller K., Svendsen K. D., Jakobsen M., and Pedersen B. K.. 2010. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 104: 1831–1838. [DOI] [PubMed] [Google Scholar]
- 244.Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y., Okano M., Kagoshima M., and Tsuchida T.. 2010. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 64: 636–643. [DOI] [PubMed] [Google Scholar]