Abstract
The burden caused by familial hypercholesterolemia (FH) varies among countries and ethnic groups. The prevalence and characteristics of FH in Latin American (LA) countries is largely unknown. We present a systematic review (following the PRISMA statement) of FH in LA countries. The epidemiology, genetics, screening, management, and unique challenges encountered in these countries are discussed. Published reports discussing FH in Hispanic or LA groups was considered for analysis. Thirty studies were included representing 10 countries. The bulk of the data was generated in Brazil and Mexico. Few countries have registries and there was little commonality in FH mutations between LA countries. LDL receptor mutations predominate; APOB and PCSK9 mutations are rare. No mutation was found in an FH gene in nearly 50% of cases. In addition, some country-specific mutations have been reported. Scant information exists regarding models of care, cascade screening, cost, treatment effectiveness, morbidity, and mortality. In conclusion, FH is largely underdiagnosed and undertreated in the LA region. The genetic admixture with indigenous populations, producing mestizo’s groups, may influence the mutational findings in Latin America. Potential opportunities to close gaps in knowledge and health care are identified.
Keywords: heterozygous familial hypercholesterolemia, homozygous familial hypercholesterolemia, autosomal recessive hypercholesterolemia
Familial hypercholesterolemia (FH) is a syndrome that causes defective clearance of LDL cholesterol (LDL-C) and results in premature coronary heart disease (CHD) (1–3). Two forms have been reported: autosomal dominant and autosomal recessive. The vast majority of cases have the autosomal dominant pattern of inheritance with 90% penetrance. Autosomal dominant FH is attributed to mutations in three different genes: LDL receptor (LDLR), APOB, and proprotein convertase subtilisin/kexin type 9 (PCSK9) (1, 3–5). Other FH genes have been searched for using exome sequencing without success (2). FH caused by mutations in LDLR adaptor protein (LDLRAP) is known as autosomal recessive FH (2). FH is the most common monogenic disorder leading to premature CHD; despite this fact, it is notoriously underdiagnosed and undertreated worldwide (6).
Homozygous FH (HoFH) is characterized by extremely high levels of LDL-C (460–1,160 mg/dl) and early onset coronary artery disease (typically by the second decade of life) (7). Mean LDL-C concentration in untreated patients is close to 615 mg/dl (7, 8). Patients are classified into two groups based on the level of LDLR activity, either <2% (receptor negative) or 2–25% (receptor defective). Receptor defective patients have a better prognosis than receptor negative cases (9–11).
Heterozygous FH (HeFH) is caused by a single inherited copy of a mutation. The frequency of a heterozygous mutation is >90, 5, and <1% in the LDLR, APOB, and PCSK9 genes, respectively (5). A causal mutation in one of these genes is identified in 60–80% of cases. Affected individuals are characterized by LDL-C levels two to three times greater than normal (190–400 mg/dl). The mean untreated LDL-C concentration is 199.9 mg/dl (12). HeFH is suspected with an elevated LDL-C (LDL-C >190 mg/dl), the presence of xanthomas (tendinous/tuberous), corneal arcus (<45 years old), family history of FH, and either a personal or family history of premature coronary artery disease.
Traditionally, the prevalence of HeFH is considered to be 1 in 500 individuals. This figure is based on the results of a European survey of familial lipoprotein disorders in myocardial infarction survivors (13, 14). However, recent work in a Dutch population estimated a prevalence of 1 in 137 persons (1, 10, 11). In Australia, 1 in 229–353 inhabitants may be affected, while a figure of 1 in 213 has been proposed in a Chinese population (15, 16). Such disparities in prevalence estimates are due to differences in population ancestry and in diagnostic criteria used to define the disease. In populations with founder effect, such as those of South Africa, Quebec, and Lebanon, prevalence estimates are expected to be higher. For example, the prevalence of HeFH in French-Canadians (Quebec) is approximately 1:270; in the rest of Canada, where the population is more genetically heterogeneous, this figure may be closer to 1:500 (14).
The burden caused by FH varies among countries and ethnic groups. The existing information concerning FH has been gathered from European cohorts or countries with national registries. The Netherlands has the best record, with an estimated 71% of cases identified. However, the situation is very different in other parts of the world. Globally, less than 5% of FH individuals have been identified (5). In Latin America, the prevalence of FH is largely unknown. In this part of the world, there is a lack of public awareness regarding FH. In this article, we present a systematic review of FH in LA countries. The epidemiology, genetics, screening, management, and unique challenges encountered in this region are discussed. Armed with this knowledge, LA health systems may implement policies, including national registries, enabling the opportune diagnosis and management of this disease.
METHODS
A systematic review was undertaken to identify all published data regarding FH in LA populations. LA populations are any Spanish or Portuguese speaking nations south of the United States.
A systematic search was conducted following the PRISMA statement (17) in PubMed and SciELO from 1982 to 2016. The aim of the PRISMA statement is to help authors improve the reporting of systematic reviews and meta-analyses. PRISMA is an evidence-based minimum set of items for reporting such documents; it consists of a 27 item checklist and a flowchart explaining the flow of information through the phases of the review. Any type of published report discussing FH epidemiology, FH mutations, and FH public health policy in Hispanic or LA groups was considered for analysis. Articles providing clinical phenotype and/or mutational analysis in FH subjects were selected. Thus, all epidemiological, cross-sectional, cohort, retrospective, longitudinal, observational, comparative, case-control, and case reports were considered containing the following keywords or MeSH terms: familial hypercholesterolemia, heterozygous familial hypercholesterolemia, homozygous familial hypercholesterolemia, type II hyperlipoproteinemia, LDL receptor mutation, apoB mutation, PCSK9 mutation, xanthomas, arcus senilis, aortic valvular lesions and hypercholesterolemia, severe hypercholesterolemia, stroke and hypercholesterolemia, premature cardiac disease and hypercholesterolemia, cascade screening, and founder effect. These terms were crossed with the keywords and MeSH terms for LA ethnicity: minority groups, ethnic groups, Hispanic American, Native American, continental ancestry, American Indian, and all countries that constitute Latin America.
All literature in Spanish, Portuguese, and English was included. In addition, the reference lists of review articles and conference abstracts were also considered. Abstracts were assessed independently by two research associates to identify eligible research reports. If an abstract was not disqualified on the basis of the study methods or data presented in the abstract, a full copy of the report was obtained.
RESULTS
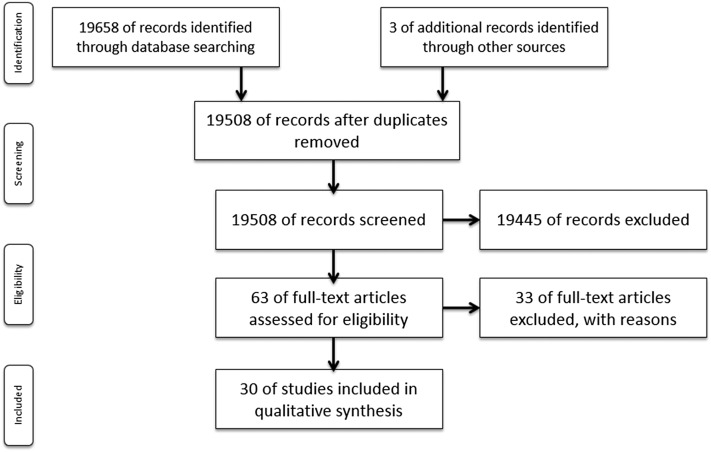
Our search strategy retrieved a total of 19,658 publications. After removing any duplicate documents, a total of 19,508 abstracts were reviewed. Of these, 19,454 articles were excluded as they did not complete the inclusion criteria of the systematic analysis. Finally, 63 relevant full-text articles were assessed in detail for eligibility. Of these, 33 publications were excluded due to incomplete information. As a result, a total of 30 studies were included for the purposes of this article. The PRISMA algorithm is presented in Fig. 1. The characteristics of the 30 articles included in this systematic analysis are shown in Table 1.
Fig. 1.
PRISMA algorithm.
TABLE 1.
Articles identified in the systematic analysis: FH in Latin America
| Country | Reference | HeFH/ HoFH | Study Type | Sample Size | Agea | Male (%) | Premature CHD (%) | Xanthoma (%) | Mean total cholesterola (mg/dl) | Mean LDL-Ca (mg/dl) | FH Mutation | FH Diagnosis |
| Argentina | 18 | HeFH | Retrospective pediatric cohort | 33 | 9.5 | 12% (n = 4) | NR | 0 | 305 ± 47.9 | 232 ± 54.2 | NR | LDL-C levels and family history |
| 19 | HoFH | Case report | 2 (brother and sister) | 8 and 12 | 50% (n = 1) | Sister with bicuspid aortic valves and thickened intima of carotid artery | 50% (n = 1) | 521 | 409 | NR | LDL-C levels and family history | |
| 20 | HoFH | Case report | 1 | 22 | 100% | Bicuspid aortic valve and ischemic cardiomyopathy | NR | 486 | NR | NR | LDL-C levels and family history | |
| Brazil (30) | 21 | HeFH and HoFH | FH cohort (probands) (Ribeirao Preto) | 18 cases from 10 families | HeFH 48.6 | NR | 38.9%HeFH | 17.6% HeFH (n = 3) | HeFH 350.3 | HeFH 271.2 | 9 subjects from 5 families with Lebanese allele (LDLR) (exon 14 Cys681) | Total cholesterol and LDL-C >95th percentile for age and sex and autosomal transmission |
| 17 HeFH | HoFH 34 | 100% HoFH | HoFH 610.2 | HoFH 545.2 | ||||||||
| 1 HoFH | ||||||||||||
| 22 | HeFH and HoFH | FH cohort (probands and relatives)(Ribeirao Preto) | 59 from 31 families (includes 10 families reported previously) | 4–69 | NR | 17.2% HeFH (n = 10) | 8.6% HeFH (n = 5) | HeFH range (197–529.8) | HeFH (range 135-464) | 30% with Lebanese allele (n = 17) + 1 case with 4 kb deletion of the LDL-R exon 12-14 | Total cholesterol and LDL-C >95th percentile for age and sex | |
| 58 HeFH | 100% HoFH | 100% HoFH | HoFH 610.2 | HoFH 545.2 | ||||||||
| 1 HoFH | ||||||||||||
| 23 | HoFH | Case report (Ribeirao Preto) | 1 | 22 | 100% | Cardiac catheterization | NR | 678 | 323 | Compound heterozygote for Lebanese allele exon 14 and c.977C>G exon 7 | Total cholesterol and LDL-C >95th percentile for age and sex | |
| 24 | HeFH | Probands and controls | 35 cases/200 controls | HeFH 50 ± 19 | 28.6% (n = 10) | 57% (n = 20) | 71% | 363.5 ± 62 | 294 ± 66 | 22 of 35 subjects with LDLR mutations (63%) | Total cholesterol >360 mg/dl for age 40+ (or 270 mg/dl in youth) (87) | |
| 59% in mutation positive subjects (n = 13) | ||||||||||||
| 25 | HoFH | Case report | 1 | 20 | 100% | 100% two stents in left anterior descending artery | Tendon xanthomas | 785 | 710 | LDLR exon 11 homozygous A540T | DLCN | |
| 26 | HeFH | Case report | 1 | 46 | 100% | Severe carotid stenosis | Tendon and planar xanthomas | 554 | NR | NR | DLCN | |
| 27 | HeFH and HoFH | FH cohort | 248 index cases (IC) (125+, 123-) | 52.7 IC mutation+ | 40.8% IC mutation + | 12% IC mutation+ | 6.4% IC mutation + | 403 ± 84 IC mutation + | 323 ± 85 IC mutation + | IC: 108 LDLR (97.2%) and 2 in APOB (2.8%) | LDL-C >210 mg/dl in adults and >170 mg/dl in children | |
| 394 relatives (234+, 160-) | 43.9 relatives mutation+ | 40.2% relatives mutation+ | 11.1% relatives+ | 2.6% relatives mutation+ | 362 ± 77 relatives mutation+ | 288 ± 79 relatives mutation+ | (Exon 26: Arg3527Gln Arg 3558Cys) | |||||
| (10 homozygotes: 8 IC and 2 relatives) | ||||||||||||
| 28 | HoFH | Review of HoFH | 14 probands | NR | NR | NR | NR | NR | NR | Homozygous mutations in LDLR | LDL-C levels, clinical phenotype and mutational analysis | |
| Includes LDLR mutation A540T on exón 11 | ||||||||||||
| 29 | HeFH | HeFH cohort | 202 | 51 | 35% | 28.2% | 20.3% | 335 | 257 | 90.6% in LDLR | DLCN or mutational analysis 195 with definitive diagnosis | |
| 24.6% CHD | No APOB or PCSK9 mutations | |||||||||||
| 18.2% without CHD | ||||||||||||
| Chile | 31 | HeFH | Case report | 1 | 46 | 100% | 0 | Tendon xanthomas: hands and ankles | 524 | NR | LDLR G545A | LDL-C levels and mutational analysis |
| 32 | HeFH | Case report | 1 | 24.5 | 100% | Thickening of carotid artery on ultrasound | Tendon xanthomas: hands and ankles | 270 | 184 | LDLR Cys95Gly | LDL-C levels and mutational analysis | |
| Costa Rica | 33 | HeFH | Case report | 2 probands in same family | NR | NR | NR | NR | NR | NR | LDLR c.2056G>T | NR |
| 34 | FH | Case report | 1 | NR | NR | NR | NR | NR | NR | LDLR c.681-699dup (18 bp) | NR | |
| Cuba | 35 | HeFH | Three families with HeFH (cascade screening) | 16 probands from 3 families | 38 ± 20 | 43% | 6% (n = 1) | 75% (n = 12) | 382.8 | 324.4 | LDLR | LDL-C levels and mutational analysis |
| Val408Met-(Afrikanar mutation) | ||||||||||||
| Glu256Lys Va1776Met | ||||||||||||
| Honduras | 36 | HoFH | Case report | 1 | 38 | 100% | Severe coronary artery disease and bilateral carotid stenosis | Tendon xanthomas and xanthelasma | NR | 425 | LDLR intron 7 1061(-1)G>C (skipping of exon 8) | LDL-C levels and clinical phenotype |
| México | 37 | ARH (LDL receptor adaptor protein mutation) | Case report | 2 cases in 1 family | 20- and 27-year-old siblings | 50% | NR | Tendon xanthomas | 677 | NR | ARH IVS4+2T>G | LDL-C levels and mutational analysis |
| 655 | ||||||||||||
| 38 | HoFH | Case report | 1 | 18 | 0% | Aortic valvulopathy | Tendon and tuberous xanthomas | 519 | 463 | LDLR c2271delT | LDL-C levels, clinical phenotype and mutational analysis | |
| 39 | HoFH | Case report | 1 | 8 | 100% | Bilateral atheromatous carotid disease with severe stenosis (52%) of the left internal carotid artery | Tendon and planar xanthomas | 782 | 715 | LDLR Cys352Tyr | LDL-C levels, clinical phenotype and mutational analysis | |
| 40 | HeFH | FH cohort | HeFH | 51 ± 16 | 49% (n = 29) | 29% (n = 17) | 30% (n = 18) | All = 324 ± 57 | All = 247 ± 55 | 21 mutations in LDLR | Total cholesterol and LDL-C >90th percentile for age and sex | |
| n = 59 | Mutation+ = 338 ± 57 | Mutation+ = 267 ± 53 | 1 mutation in APOB (Tyr3560Cys) | |||||||||
| Mutation positive = 38 | Mutation− = 304 ± 50 | Mutation− = 232 ±± 39 | 1 mutation in PCSK9 c.1850C>A (p.Ala617Asp) | |||||||||
| HoFH | HoFH | 21 ± 13 | 33% (n = 1) | 66.6% | 100% (n = 3) | 588 ± 65 | 537 ± 66 | 3 in LDLR | ||||
| n = 3 | 2 of the 3 had CHD | |||||||||||
| Mutation positive = 3 | ||||||||||||
| 41 | HeFH | Case report and cascade screening of family | Proband and 9 family members | 93 | 0% | Atherosclerotic lesions in carotid arteries | Proband and 4 family members | 395 | NR | NR | Total cholesterol and LDL-C >95th percentile for age and sex | |
| 42 | HeFH | FH cohort (46 IC and 68 relatives) | 46 ICs | 43.4 ± 18 Mutation + | NR | NR | 81% (n = 37) | 360 ± 63 Mutation+ = | 287 ± 62 Mutation + = | 17 in LDLR | Total cholesterol and LDL-C >95th percentile for age and sex and clinical phenotype | |
| 17 LDLR mutation+ | 39.2 ± 15.4 Mutation– | 390 ± 48 Mutation− = | 315 ± 46 Mutation− = | |||||||||
| 25 mutation− | 44.3 ± 13.4 | 325 ± 64 | 258 ± 65 | |||||||||
| 43 | HeFH | HeFH cohort | 30 (1 proband with mutation in APOB) | 47 ± 16.2 (APOB mutation + =44) | 13.3% (n = 4) | 13.3% (n = 4) | 100% | 351 ± 61 (APOB mutation += 370) | 374.9 ± 60.4 (APOB mutation +=300) | (APOB mutation+ = Arg3500Gln) | LDL-C levels >160 mg/dl, family history and clinical phenotype | |
| Panama | 44 | HoFH | Case report | 1 | 7 | 100% | NR | Tuberous and tendon xanthomas | 407 | 349 | NR | LDL-C levels and clinical phenotype |
| Paraguay | 45 | HoFH | Case report (subject is of Paraguayan descent) | 1 subject + family | 28 | 0% | Aortic valvulopathy carotid and coronary stenosis | Planar xanthomas | 714.2 | 667 | LDLR homozygous mutation exón 14, g.2051delC | Simon Broom criteria (SBR) |
| Uruguay | 46 | HoFH | Case report and cascade screening of family | 8 HeFH | Mean = 53.4 (range 16–83) | 37.5% (n = 3) | 12.5% (n = 1) | 25% (n = 2) | 289.4 | NR | LDLR 47C>A (promoter region) | LDL-C levels, clinical phenotype + mutational analysis in HoFH proband |
| 3 HoFH | 55,48,45 | 33.3% (n = 1) | 66.6% (n = 2) | 100% (n = 3) | Proband = 714 | Proband = 629 | ||||||
| Mean = 614 | Mean = 543 | |||||||||||
| 47 | HeFH | Cohort/ registry | 71 index cases | 37.7 | 54% (n = 38) | 25% (n = 18) | NR | 338 | NR | LDLR mutations = 67 | MEDPED | |
| APOB mutations = 2 | ||||||||||||
| 48 | HeFH | Case report | 2 | 50-year-old woman | 50% (n = 1) | 100% Both with premature CHD | NR | Woman: 380 | NR | Both were in APOB Arg3500Gln (R3500Q) (reported in registry) | MEDPED | |
| 48-year-old man | Man: 365 |
NR, not reported; IC, index case; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; CHD, coronary heart disease.
Mean or range.
Characteristics of individuals with FH in Latin America: summary
Information regarding the scenario of FH in Latin America was available from 30 published articles (Table 1) (11, 18–48). In total, 10 countries are represented, namely Argentina, Brazil, Chile, Costa Rica, Cuba, Honduras, Mexico, Panama, Paraguay, and Uruguay. Of these, Brazil and Mexico provided the bulk of the information with nine and seven publications, respectively. The types of publications included 18 case reports (often with cascade screening of the family) and 12 reviews of FH cohorts. Data on both homozygous FH (HoFH) and HeFH have been published.
With regard to FH case reports (n = 18), 10 articles identified HoFH subjects (those that report mutational analysis confirm homozygous LDLR mutations), 6 discuss HeFH cases (4 caused by LDLR mutations and 3 due to APOB mutations), and 1 reports the characteristics of a Mexican individual with autosomal recessive hypercholesterolemia (ARH) (due to a LDLRAP mutation). Five case reports do not have mutational analysis (Argentina, 2; Honduras, 1; Panama, 1; and Brazil, 1). In the HoFH case reports (n = 10: Brazil, 2; Mexico, 2; Argentina, 2; Honduras, 1; Panama, 1; Paraguay, 1; and Uruguay, 1), the mean age of adult subjects (n = 9) was 32.9 years. The mean total cholesterol and LDL-C levels were 702.2 mg/dl and 521.1 mg/dl, respectively. The majority of the HoFH cases (83%) had evidence of atherosclerotic disease.
In the HeFH case reports (n = 6: Chile, 2; Brazil, 1; Mexico, 1; and Uruguay, 2), the mean age of subjects was 51.3 years and the mean total cholesterol level was 415 mg/dl (n = 6). Tendon xanthomas and premature coronary artery disease were reported in four and four cases, respectively. Mutational analysis was available in four case reports, three found mutations in the LDLR (Chile and Costa Rica), and one article reported an APOB mutation (Uruguay).
The only case of ARH was reported in two Mexican siblings (aged 20 and 27 years, respectively). The mean total cholesterol and LDL-C concentrations were 677 and 655 mg/dl, respectively. They both had tendinous and tuberous xanthomata, but the presence of atherosclerotic lesions was not recorded.
In addition, we found 11 HeFH cohorts {n = 11: Argentina [1 pediatric cohort without genetic testing (n = 33)]; Brazil [5, of which only 2 are pure HeFH cohorts (n = 35 and n = 202)], Cuba [1 (n = 16)]; Mexico [3, of which 2 are pure HeFH cohorts (n = 46 and n = 29)]; and Uruguay [1 (n = 71)]}. The mean age of adult participants was 46.0 years (range 37.7–52.7 years) and the mean total cholesterol and LDL-C levels were 347 and 303.3 mg/dl, respectively. In the pediatric cohort (aged 2–15.5 years), the mean levels were 305 ± 47.9 mg/dl and 232 ± 54.2 mg/dl, respectively. The percentage of adult HeFH subjects with xanthomas was recorded in nine studies and ranged from 6.4% to 100%; the presence of CHD was mentioned in nine cohorts and ranged from 6% to 59% of the population. The age of diagnosis was late (mean 46 years). Finally, with regard to genetic analysis, the majority of cohorts reported LDLR mutations, with the Lebanese allele predominant in Brazil and an Afrikaner mutation reported in a Cuban family. Mutations in APOB were published in one Brazilian, one Uruguayan, and one Mexican cohort, and one PCSK9 mutation was recorded in a Mexican cohort.
The cohort studies did not contain information to assess the prevalence of atherosclerotic disease. There were no prospective studies with long-term follow up. Information regarding statin use was also incomplete. The diagnostic criteria utilized also varied, underscoring the lack of uniform criteria.
Genetics of FH in Latin America
LDLR mutations.
Table 2 shows the most common LDLR mutations associated with FH in Latin America. The most frequent FH mutations encountered in Latin America are on LDLR (21–54). However, autosomal recessive FH and APOB mutations have also been reported in this region (55–58).
TABLE 2.
Most common LDLR mutations causing FH in Latin America (mutations reported in at least 3 subjects for Brazil and Mexico; or mutations published in case reports for other countries
| Country | Exon | Mutation (amino acid change/nucleotide change) | References Which Report Mutation | |
| Brazil | 1 | G(-20)R (Gly2Arg) | c.4G>C2 | (24) |
| (27) | ||||
| 4 | p.(Asp221Gly) | c.662A>G | (27) | |
| 4 | p.(Asp224_Ser226dup) | c.670_678dupGACAAATCT | (27) | |
| 4 | p.(Ser177Leu) | c.530C>T | (27) | |
| 7 | p.(Arg350) | c.1048C>T | (27) | |
| 7 | p.(Gly343Ser) | c.1027G>A | (24) | |
| (27) | ||||
| (49) | ||||
| 7 | p.(Ser326Cys) (S305C) | c.977C>G | (23) (Ribeirao Preto) | |
| (27) | ||||
| 8 | p.(Gly373Asp) | c.1118G>A | (24) | |
| (27) | ||||
| G352D (Gly → Asp) | (29) | |||
| (50) | ||||
| 8 | A370T (Ala → Thr) | c.1171G>A | (24) | |
| 9 | p.(Ala431Thr) | c.1291G>A | (29) | |
| 11 | p.(Ala540Thr) | c.1618G>A | (25) | |
| (26) | ||||
| 12 | D580H (Asp601His) | c.1801G>C | (24) | |
| (27) | ||||
| 13 | p.(Phe629Tyrfs16) | c.1885_1886insA | (27) | |
| 14 (Lebanese Allele) | p.(Cys681) (C660X) | c.2043C>A | (21) (Ribeirao Preto) | |
| (22) (Ribeirao Preto) | ||||
| (23) (Ribeirao Preto) | ||||
| (24) | ||||
| 14 | C677Y | c.2093G>A | (24) | |
| 14 | p.(Pro699Leu) | c.2096C>T | (29) | |
| Intron 10 | c.1586 + 1G> | (27) | ||
| Intron 3 | c.313 + 1G>A | (27) | ||
| (29) | ||||
| Chile | 3 | p.(Cys95Gly) | (32) | |
| (51) | ||||
| Intron 11 | c.1705 + 1G>A | (31) | ||
| Costa Rica | 4 | p.206 | c.681-699dup (18 bp) | (34) |
| 14 | Q665X | c.2056G>T | (33) | |
| Cuba | 6 | p.(Glu256Lys) | (35) | |
| 9 | p.(Val408Met) | (35) | ||
| (52) (Afrikaner mutation) | ||||
| 16 | p.(Va1776Met) | (35) | ||
| Honduras | Intron 7 | c.1061(-1)G>C (skipping of exon 8) | (36) (FH-Honduras) | |
| Mexico | 4 | p.(Glu113fsX17) | c.338insG | (42) |
| 4 | p.(Glu228Lys) | c.682G>A | (40) (FH-México mutation) | |
| 6 | E256K (substitution of cysteine in position 256) | c.829G/C | (40) | |
| (42) | ||||
| (51) | ||||
| 7 | p.(Cys352Tyr) | c.1055G.A | (39) | |
| (40) | ||||
| (FH-México 2 mutation) | ||||
| 7 | p.(Leu348AlafsX12) | c.1034 1035insA | (40) | |
| 8 | p.(Cys364Arg) | c.1090T>C | (40) | |
| (42) | ||||
| (FH-México 3 mutation) | ||||
| 10 | p.(Gly529Asp) | c.1586G>A | (40) | |
| 15 | p.(Ala755GlyfsX7) | c227delT (c.2264_2273del) | (38) | |
| (40) | ||||
| (42) | ||||
| 15 | Q718X | c.2216C/T | (42) | |
| (53) | ||||
| Intron 4 | c.695 −1G>T | (40) | ||
| Paraguay | 14 | M687X | c.2051delC | (45) |
| Uruguay | Promotor region | c.-47C>A | (46) | |
Mutations reported in at least three subjects for Brazil and Mexico or mutations published in case reports for other countries. Mutations unique to a country (according to the University College of London database or article cited) are FH-Hondurus, FH Mexico 2 and 3. Mutations which do not appear in the University College of London database are shown in italic (we assume they are unique to the country in which they are reported). Nucleotide numbering as suggested by Yamamoto et al. (54). In accordance with LDLR variants reported in LOVD-Select Database (http://www.ucl.ac.uk/ldlr).
In Brazil, in the early 1990s, a common mutation in the LDLR gene was found in FH probands. This was called the “Lebanese allele” (exon 14 Cys681); it is a cause of FH in Arab populations and produces a shortened receptor lacking three domains, resulting in an abnormally slow exit rate from the endoplasmic reticulum (55). Figueiredo et al. (21) searched for the presence of this mutation in 18 FH subjects from 10 unrelated families living in Ribeiro Preto, Brazil. They were able to identify the mutant allele in nine patients from five families (eight heterozygous cases and one homozygous case), all five families confirmed Lebanese ancestry. This was the first report of this mutation in a non-Arab population, indicating an important contribution of this ethnic group to FH in Brazil. Alberto et al. (22) studied 59 FH subjects from the same region of Brazil and confirmed the presence of the Lebanese allele in 30% of the cases. Several years later, Salazar et al. (24) discovered seven novel LDLR mutations in families living in Sao Paolo; however, the Lebanese mutation was not found. They speculated that the frequency of FH mutations may vary from state to state depending on the ethnic background of the inhabitants. Recently, Jannes et al. (27) described 248 index cases; an FH-causing mutation (LDLR, APOB, or PCSK9 genes) was found in 125 subjects (50.4%). After analyzing index cases and mutation-positive relatives (n = 359), 70 different causal mutations in the LDLR (97.2%) and 2 in APOB (2.8%) were found. Only 10 homozygote cases (8 index cases and 2 relatives) and 1 compound heterozygote case were identified. The most common mutations were in exons 14 and 4, with 8.5% of the mutation-positive population carrying the Lebanese allele.
In Mexico City, Robles-Osorio et al. (42) assessed the contributions of the LDLR, APOB, and PCSK9 mutations as causes of FH. Three reference centers participated in this analysis and 46 index cases and 68 affected relatives were identified. All index cases were diagnosed as having heterozygous autosomal dominant FH. LDLR mutations were identified in 17 cases (4 of which were novel) and 1 case had an APOB mutation. The most common LDLR mutation (n = 4) consisted of an insertion in exon 4 (338insG). All of the previously known LDLR mutations had been identified in Spanish FH patients. PCSK9 was sequenced in the remainder of probands with no identified LDLR or APOB defects; however, no PCSK9 mutations were found. The study also included linkage analyses; only one large kindred showed significant linkage to the PCSK9 locus (1p34.1–32) (multipoint LOD score of 3.3). In at least four families, it was possible to exclude the participation of known FH genes (with mutational and linkage analysis), suggesting the existence of unidentified causal genes. In 2011, Vaca et al. (40) reported FH cases in the metropolitan area of Guadalajara, México based on clinical and biochemical phenotype. The 62 index cases and their families underwent mutational analysis. Among probands, 3 were homozygotes, and 59 were heterozygotes. Mutations were identified in 38 (61%) index cases. A total of 25 mutations are described; 24 in LDLR and 1 in APOB. Overall, 21 of the 25 mutations had been previously observed in various countries, including Spain, some occurring in other ethnicities. Of the prevalent mutations in LDLR, three are associated with Mexican ancestry: c.682G>A (referred to as FH-Mexico), c.1055G>A (p.Cys352Tyr, referred to as FH-Mexico 2), and c.1090T>C (p.Cys364Arg, referred to as FH-Mexico 3). In addition, a novel mutation in PCSK9, c.1850C>A (p.Ala617Asp), was also detected, although it was considered noncausal. Also, in Guadalajara, Magaña-Torres et al. (39) reported the case of an 8-year-old boy with homozygous hypercholesterolemia due to the p.Cys352Tyr alteration (FH-Mexico 2). This individual was from Papantla, Veracruz, with apparently unrelated parents. Finally, in Oaxaca, Martinez et al. (38) described a local woman with HoFH; she was found to have an LDLR mutation on exon 15 (c2271delT), which results in a stop codon and a truncated protein. This mutation was originally reported by Robles-Osorio et al. (Mexico City) (42) and subsequently confirmed in the Guadalajara cohort. All three cases originated from a Mixteca community of Oaxaca, within a geographically isolated population.
In Uruguay, a pilot registry reported 71 mutations in the LDLR, but did not catalog these alterations in the publication (47). Only one case report describes a Uruguayan family with a novel mutation in the promotor region of the LDLR gene (c.-47C>A) (46).
Information regarding LDLR mutations is limited to case reports in Argentina (18–20), Chile (31, 32), Costa Rica (33, 34), Cuba (35), Honduras (36), Panama (44), and Paraguay (45). Mutations reported in the majority of the cases were inherited from European or Afrikaner ancestors. Novel mutations were reported in Cuba (35) and Honduras (36). Some of the case reports had atypical presentations, such as the coexistence of FH and cerebrotendinous xanthomatosis (32).
APOB mutations.
With respect to APOB mutations, six cases have been reported in Latin America. Two cases were from Uruguay (48), two probands were from Mexico (40, 43), and two were from Brazil (27, 30). Total cholesterol and LDL-C levels were lower in these subjects than in FH patients with LDLR mutations. All of them had European ancestry. It is important to highlight the absence of APOB mutations in people of Native American ancestry. Three cases have the same mutation (Arg3500Gln). This is an adenine for guanine substitution (57) that results in an arginine for glutamine change at the 3500 amino acid position. This interferes with the apoB-LDLR binding. The majority of affected individuals are heterozygotes for this mutation. The remaining APOB mutations were also located on exon 26, and were very close to the R3500Q site [Tyr3560Cys (Mexico) and Arg3558Cys (Brazil)].
In summary, the information regarding the molecular diagnosis of FH in Latin America is scant. In Brazil and Mexico, the countries with the largest cohorts, LDLR mutations exhibit allelic heterogeneity. The majority of LDLR mutations have previously been encountered in European populations. In general, mutations are found along the entire sequence of the LDLR gene. In Brazil, exons 4, 7, and 14 are the most common sites. The Lebanese allele was identified in Brazil and an Afrikaner mutation in Cuba. In Mexico, six unique mutations have been reported. Novel mutations have also been reported in Honduras (one), Brazil (two), and Cuba (one). It is interesting to note that Brazil and Mexico do not share commonality regarding LDLR mutations (Table 2). Furthermore, mutations in APOB or PCSK9 are unusual throughout Latin America (43). In addition, a significant percentage of FH cases do not have previously known FH mutations. Ahmed et al. (59) were unable to identify a disease-causing mutation in the LDLR, APOB, or PCSK9 genes in 66% of their multi-ethnic autosomal dominant hypercholesterolemia population (53% in Hispanics). Although this could be due to methodological limitations, we must consider other explanations. LA populations have ancestors of Spanish and Portuguese origin; however, the genetic contribution of the indigenous populations has a remarkable effect on the genetic epidemiology of the chronic diseases in Latin America. We have seen that between countries in Latin America there are no common FH-causing mutations. This observation suggests that founder effects are not common among the Amerindian FH cases. Clearly, additional studies are needed to describe the contribution of the Amerindian susceptibility variants in the pathogenesis of FH in LA mestizo populations. The existence of undiscovered FH-causing mutations (59) is an alternative explanation.
Autosomal recessive FH.
Canizales-Quinteros et al. (37) reported the characteristics of two Mexican siblings who were found to have a novel splice site mutation at the 1p35 locus (IVS4+2T>G) of the LDLRAP gene. LDLRAP encodes a protein required for clathrin-mediated internalization of the LDLR in the liver. To date, three loci have been described for ARH (1p35, 15q25-q26, and 13q). The majority of subjects with this disorder are of Mediterranean or Middle Eastern origin, and more than half are from Sardinia (56). To our knowledge, of the 11 ARH mutations described worldwide, this is the only one reported in Latin America.
Epidemiology of hypercholesterolemia in Latin America: national registries
The identification of possible FH cases is a challenge in Latin America. Farzadfar et al. (60) recently analyzed global population trends in serum total cholesterol levels. They reported that Latin America and the Caribbean were among the few regions without sufficient data. Information regarding population lipid levels can be obtained from national health surveys; these have only been carried out in Argentina (61), Brazil (62, 63), Chile (64), Ecuador (65), and Mexico (66). However, these surveys are methodologically dissimilar. Some are only self-reported questionnaires, while others include blood samples from the general population. None of them included the systematic search for tendinous xanthomata and the sample sizes were not large enough to estimate the FH prevalence. Among those surveys in which blood samples were drawn, LDL-C >190 mg/dl was observed in 5% of the sample in Brazil (63) and 11.2% of adults in Mexico (66–68).
Recently, Khera et al. (69) evaluated the diagnostic yield of sequencing FH genes in patients with severe hypercholesterolemia. Using an LDL-C threshold of 190 mg/dl, 7% of their population (n = 1,386) had severe hypercholesterolemia and 2% carried an FH mutation. This information suggests that the FH prevalence is greater than the originally considered (0.14% vs. 0.02%). This interpretation is supported by the prevalence (nearly 0.2%) found in the NHANES report using a modified version of the Dutch Lipid Clinics Network (DLCN). The survey included an analysis by ethnic group. Hispanics and Mexican Americans had a prevalence that was similar to that found in other ethnic groups.
Only a few countries have implemented such national registries. These include The Netherlands, the UK, Norway, Spain, South Africa, Japan, and the USA (12, 70–75). In Latin America, only Brazil and Uruguay have FH registries (see below). The use of registries allows the long-term follow-up of affected individuals (76–81) Last year, the European Atherosclerosis Society FH Studies Collaboration launched a global initiative that, through a consortium of major FH registries worldwide, aims to generate large-scale robust data on FH detection and management to overcome existing gaps in knowledge and care (82). Cascade screening is an essential aspect of identifying cases. Several LA countries have endorsed this proposal; as a result, new FH registries will be available in the region in the next few years.
Diagnosis: cascade screening and genetic testing
Diagnosis is based on clinical phenotype with the application of instruments such as the DLCN, Simon Broome Registry, and US MEDPED (Make Early Diagnosis to Prevent Early Death) criteria, followed by cascade screening to identify affected relatives (83–88) (85–88). As yet, there are no internationally agreed upon diagnostic criteria. The most commonly used instrument is the DLCN; this is because it combines LDL-C levels, family history of hypercholesterolemia, and presence of premature coronary artery disease with the possibility of molecular diagnosis in one score. One of the drawbacks of these criteria is that tendon xanthomas and arcus cornealis are often present only with advanced disease. Furthermore, because LDL-C levels vary with age, using this criterion in a categorical manner, regardless of age, may result in under-diagnosis in younger populations. A new tool to aid in the selection of cases for genetic testing has recently been developed. It tries to take these drawbacks into account and may be useful in differentiating family members with and without FH, but is less good at diagnosing index patients (89). LA studies have selected the DLCN criteria as the most common tool to diagnose FH.
No country has universal population screening; this is precluded by the high costs involved and the possible high numbers of false positives (70). In order to identify new FH cases from an index case, cascade family screening should be used. This is a method for identifying people at risk for a genetic condition by a process of systematic family tracing (83). It is considered the most efficient method for the diagnosis of new FH cases. Countries such as The Netherlands, UK, Norway, Spain, Japan, Australia, Canada, and the USA have established cascade screening programs. For example, the UK’s National Institute for Health and Clinical Excellence (NICE) recommends using cascade screening with genetic testing to detect new FH cases (84). The benefits of cascade screening include early identification of FH patients and increased numbers of patients on cholesterol-lowering therapy resulting in reduced morbidity and mortality. Several types of cascade screening are available; either clinical, genetic, or a combination of both. Following the identification of an index case, family members are assessed for risk. In European countries, genetic cascade screening has been found to be cost-effective; The Netherlands was the first country to institute a competent genetic cascade screening program (90, 91). Here, the FH probands are identified using the DLCN criteria in lipid clinics. DNA samples of patients with a positive clinical diagnosis are analyzed. If a mutation is identified, the patient is used as the index case for family cascade screening. To date, this program has identified around 25,000 FH patients.
LA countries lag behind in the establishment of models of care, national registries, and cascade family screening programs; the early detection of FH patients only occurs in specialized centers (29, 92). There are no standardized protocols for cascade and genetic testing. Only two countries have published the results of their cascade screening programs and have specific clinical guidelines. In Brazil, the results of an FH genetic cascade screening program in Sao Pablo have recently been published. From 125 index cases with confirmed mutations, 394 relatives were genetically screened, resulting in 234 (59.4%) individuals with pathological mutations. Therefore, for every index case, 1.8 affected relatives were identified (27). GENYCO (from “Genes y Colesterol” in Spanish) is the national program for early detection and treatment of FH in Uruguay. It is a centralized national registry providing access to data regarding genetic testing, cascade screening, and the management of FH. It was established in 2012 and may allow the identification of 80% of Uruguayan FH patients over the next 8 years. The program has included 71 index cases and their relatives. In total, 1,024 individuals have been included, 760 of which were alive and 264 deceased. Of these, the at-risk population, estimated using Mendelian transmission, consisted of 335 persons. Analysis of this group resulted in the identification of 294 affected individuals (245 of which were still living). The authors were able to conclude that for every index case they would be able to identify 3.4 additional cases (47, 82).
Individuals with a definite or probable phenotypic diagnosis using DLCN may undergo genetic testing if available. However, genetic mutations may not be encountered in up to 20–70% of subjects with a positive clinical diagnosis (75). A recent study analyzing the performance of the three diagnostic criteria reported that the DLCN criteria identified 62% of mutation-positive individuals; Simon Broome identified 52%; and MEDPED criteria only 23% (89). This same study reported that an LDL-C of >170 mg/dl showed the best sensitivity and specificity (99.5% and 97.5%, respectively) for identifying cases. In Latin America, molecular testing for FH is uncommon and only performed in reference centers. Furthermore, no standardized genetic testing procedures exist. As previously described, a constant finding in the LA series is the large proportion of FH cases in which no mutation is identified. The lack of the characterization of the Amerindian mutations may contribute to the low rate of positive genetic screening in LA patients. Commercially available DNA analysis is available in the region. The test includes the search of variants that have been associated with changes in LDL-C concentrations in Spanish or LA samples (735 for LDLR, 61 for APOB, 255 for PCSK9, and 68 for LDLRAP1). However, it did not consider the genetic variants of unrepresented populations in the published reports (i.e., Amerindian subjects).
Treatment options in Latin America
Treatment options consist of lifestyle changes, drug therapy, and LDL apheresis. In addition to modifications in diet and physical activity, cardiovascular risk factors should be tackled. This includes smoking cessation and management of obesity, diabetes, and blood pressure if present. The assessment of cardiovascular risk utilizing the Framingham tables is not recommended because this instrument underestimates risk in FH patients (93). Such individuals are at considerably higher risk due to a lifelong exposure to elevated cholesterol levels. The chronic disease model is the standard to treat FH, in which knowledge and motivation should result in patient empowerment. FH specific issues (i.e., pregnancy, genetic counseling) should be reviewed by a multidisciplinary team. As a result, the ideal setting to treat this condition is in FH clinics, but this resource exists only in a few reference centers in Latin America.
Statins remain the cornerstone of treatment in FH. The UK NICE guideline and the National Lipid Association guidelines endorse a reduction in LDL-C of at least 50% from baseline level (84, 94). For high-risk patients (including HoFH patients), the National Lipid Association recommends that non-HDL-C and LDL-C levels be lowered to <130 mg/dl and <100 mg/dl, respectively. The latest European guidelines are, likewise, suggesting an LDL-C level <100 mg/dl or a 50% reduction in LDL levels from baseline (if between 100 and 200 mg/dl) (95). The International FH Foundation recommends a more stringent LDL-C target of <70 mg/dl in FH patients with CHD or other major risk factors (96). Statin therapy should be started between the ages of 8 and 10 years in confirmed cases, in order to reduce the effects of LDL-C burden (97). To achieve such targets, moderate to high intensity statin therapy with ezetimibe is often necessary.
For HeFH, in primary prevention, statins reduce cardiovascular mortality by 48%, whereas in secondary prevention this figure is lower at 25% (98). A study in HeFH patients showed that the use of statins resulted in a significant delay (on average 7 years) in the onset of coronary artery disease compared with subjects without statins (99). Wong et al. (100) reported an odds ratio of 13.2 (95% CI, 10.0–17.4) for patients not receiving lipid-lowering therapy and 10.3 (95% CI, 7.8–13.8) for patients receiving cholesterol-lowering medication. A recent report estimated that high-intensity statin therapy would lead to 10% fewer coronary artery disease deaths per 1,000 FH patients treated, compared with no treatment (101). Despite the acknowledged benefits of statin therapy, LDL-C targets are not reached (102). Pijlman et al. (103), in a Dutch cohort, showed that only 21% were at target LDL-C levels and only 21% of patients were on combined therapy. Data from the US CASCADE FH registry showed that only 25% of patients receiving lipid-lowering therapy achieved an LDL-C <100 mg/dl, and 41% achieved a >50% reduction in LDL (104).
Other treatment options for HoFH include portacaval shunt, partial ileal bypass surgery, liver transplantation, and LDL apheresis. Apheresis is offered to HoFH patients on a weekly basis and to HeFH patients on a twice monthly basis in specialized centers. This is the best option for HoFH with receptor-null mutations (105). Apheresis typically results in a 50–70% reduction in LDL-C levels that can last for up to 2 weeks. However, drawbacks include limited availability, high cost, procedure duration, and the need to maintain adequate vascular access. Novel therapeutic options for HoFH include lomitapide and mipomersen (106, 107). Although these drugs are available in several LA countries, the annual cost of mipomersen and lomitapide limits their use ($176,000 and $235,000–295,000 US dollars per year, respectively).
For HoFH (receptor-defective patients) and HeFH, PCSK9 monoclonal antibodies have recently been approved and introduced to the LA market (108). Clinical trials have shown that PCSK9 inhibitors added to high dose statins can further decrease LDL-C by up to 60% in HeFH to produce a decrease in LDL concentrations of 40–70%. The cost efficacy of these new cholesterol-lowering treatments on cardiovascular outcomes needs to be assessed in developing countries. In the US, alirocumab and evolocumab currently cost $14,600 and $14,100 a year, respectively; approximately $40 a day.
In Latin America, because there are no long-term follow up studies of FH patients treated with lipid-lowering therapies, there is little information regarding adherence and responsiveness to therapy. Under-treatment with LDL-C levels above desirable levels is probably common. In addition, the use of statins is not universally covered by healthcare systems and health insurance; it remains an out-of-pocket expense. In Latin America, about 78% of all medicines are paid for out-of-pocket in retail pharmacies. For example, in Mexico, only pravastatin and atorvastatin are available in the national health system. The proportion of FH patients receiving statins in Latin America is unknown. In 2009, Gonzalez et al. (108) carried out a survey to determine the percentage of Mexican patients treated with statins who achieved their therapeutic goal (as determined by the National Cholesterol Education Program Adult Treatment Panel III guidelines) under routine care. Only 20.4% of the hypercholesterolemic patients were adequately treated. Considering risk categories, 48, 20, and 12% of the patients in risk categories I, II, and III, respectively, were on target. Hence, despite the accepted efficacy of statins, in Latin America as in other parts of the world, a significant proportion of patients is not at goal.
In the case of LDL apheresis, this is only available in five LA countries. With regard to newer treatments, mipomersen is available in Argentina, Chile, Brazil, México, Venezuela, and Colombia; whereas, lomitapide is approved in Brazil, Argentina, Colombia, and Mexico. The PCSK9 inhibitors have only recently been approved in Brazil and Mexico. However, insurance companies will cover these therapies in HeFH patients for secondary prevention or statin intolerance; this is because FH is considered a pre-existing condition, as it is present from birth. Thus, patients will not be able to receive treatment as part of a primary prevention strategy.
In addition, all of these novel therapies have been registered as orphan drugs; this means that financial incentives were offered to promote their development. Many pharmaceutical products are tested in Latin America, but, they are subsequently unavailable and/or unaffordable (cost is much greater than average monthly income) to most of the population (109). Public-sector affordability thresholds for new pharmaceutical products should be determined in order to increase the access to the new drugs in developing countries.
Latin America is characterized by socioeconomic inequality. An inefficient provision of healthcare and variability in the quality of care are often a problem in this region. The poorer sections of society may be less aware of disease risk (in particular chronic nontransmissible diseases, which are often asymptomatic) and cultural barriers may also be present (language barriers for indigenous populations).
Burden of FH in Latin America
The burden of a disease is defined as the sum of the direct and indirect impact of a particular health problem in a region (110). It can be considered in terms of financial and social impact caused by morbidity and mortality. Measuring disease burden in FH is important; it can aid in prioritizing actions in FH management and research, implementing preventive strategies, and assessing the performance of healthcare systems. Two parameters that permit comparisons across populations and combine data on mortality and morbidity are quality-adjusted life years (QALYs) and disability-adjusted life years (DALYs). A QALY is the measure of the life expectancy corrected for loss of quality of that life caused by diseases. Whereas a DALY reflects the potential years of life lost due to premature death and equivalent years of “healthy” life lost by virtue of poor health.
Few studies have assessed the burden of disease in FH patients using these parameters. In Latin America, no such assessment has been undertaken. Because FH is under-diagnosed and undertreated in this region, the presence of premature coronary artery disease represents an important economic burden to the healthcare systems. Healthcare economic modeling has demonstrated that identifying and treating FH subjects promptly would result in considerable long-term savings by preventing cardiovascular disease (5). The cost per year of life gained is comparable to that achieved with mammography in breast cancer screening. Nherera et al. (111) carried out an analysis to identify the most cost-effective screening method in FH. They showed that DNA testing, in combination with cascade screening, had an acceptable incremental cost-effectiveness ratio of £3666 per QALY; this is well below the £20,000 per QALY used by NICE as a benchmark for cost effectiveness.
FH is a considerable burden for patients, not only due to physical signs and limitations caused by the disease but because of psychosocial factors, treatment-related issues, and impact on employment (112, 113). In general, FH patients regard themselves as healthy before the occurrence of CVD. Studies have shown that some patients experience temporary anxiety on receiving the diagnosis, others worry more about the risk of CVD in their FH relatives than for themselves, while others are more concerned about passing the disease on to their children (114). Mortensen et al. (115) found that having FH did not have much impact on the quality of life of well-treated patients, but patients who had not reached their treatment goals were concerned about the long-term impact of not being effectively treated, including the risk of premature death and disability.
In conclusion, the assessment of the burden of FH in the LA region is an area of opportunity. National registries capturing socioeconomic information and health-related quality of life data are needed to accomplish this goal.
CONCLUSIONS
This systematic review identified publications discussing the epidemiology, genetics, or public health issues of FH in LA populations. The PRISMA statement was utilized to permit the transparent and complete reporting of this information. However, drawbacks of this process are apparent. The quality of the data retrieved depends on the quality of the information source. The majority of studies were case reports and cohort studies, no prospective randomized controlled trials were available. Publications tended to discuss subjects with severe disease, and often the given data was incomplete (molecular analysis, patient characteristics, follow up, etc.) (Table 1). Furthermore, not all FH cases in Latin America have been published and there were a limited number of populations in whom information was available.
The results of this systematic review identify major knowledge gaps in the study of FH in Latin America. It is evident that there is a general under-diagnosis of this disease in the region. The general unawareness of the medical community maintains the diagnosis of this disease infrequent. As a consequence, prevalence estimates for this region are unavailable. It is evident that the age of diagnosis is late, often at the time of a coronary event. This is particularly alarming, as this is a potential economic burden for each population. In addition, there is a lack of data to estimate the burden of the disease in terms of QALYs and DALYs. Another problem is the lack of universal diagnostic criteria, with centers using distinct methods to identify patients. National registries have not been implemented in the majority of the LA countries. Cascade screening with genetic analysis is only available in specialized centers. A significant proportion of cases have no identifiable mutation. We can speculate, from the limited molecular information available, that there is little overlap with European populations. This is not surprising because the ethno-racial composition of modern-day LA nations is complex and diverse, combining indigenous American populations with influence from Iberian colonizers and African groups, and also recent immigrant groups from all over the world. Ancestry studies show differences between countries and among regions within individual countries. Amerindian ancestry is most prevalent in Meso-America (central Mexico, Belize, Guatemala, El Salvador, Honduras, and Nicaragua), Amazonia, and the Andean regions; whereas, African ancestry is predominantly found in Caribbean regions and Brazil. The Iberian immigrants admixed with both the Amerindians and Africans. Table 2 shows the most common FH mutations reported in LA patients. Several of these mutations have not been reported in individuals from other ethnicities. Ethnic-specific variants have been reported for other conditions in Amerindian populations. This is the case for type 2 diabetes and hypoalphalipoproteinemia in Amerindian individuals (i.e., SLC16A11 variants in type 2 diabetes and the R230C variant of ABCA1). The study of genetic epidemiology of FH in Amerindian populations opens the possibility to continue the search for new genes involved in the pathogenesis of the disease.
We must acknowledge that the limited number of studies in Latin America may have influenced our findings. Further research is necessary to confirm whether certain variants are specific to a particular ethnicity and whether PCSK9 mutations, which have only recently been identified as a cause of FH, are actually rarer in LA groups compared with other populations. In addition, ancestry markers were unavailable in order to confirm whether the mutational findings are specific to the ethnic mix in Latin America.
With regard to disease management, access to lipid-lowering medication is not universal, with out-of-pocket expenditure being the norm; we can speculate that under-treatment is common. LDL apheresis centers are not a possibility in Latin America. Novel therapies show LDL-lowering efficacy, but are not an option for the majority of affected individuals due to their high cost.
National registries need to be created, not only for the follow up of patients but also to stimulate the collaboration with the international community. This includes collaboration between LA countries and with European initiatives, such as the European Atherosclerosis Society FH studies collaboration. In Latin America, the “RED Iberoamericano de hipercolesterolemia familiar” was established on August 22, 2013; Argentina, Brazil, Chile, Spain, México, Portugal, Uruguay, and Columbia are current members (116). The objectives of this network include: promoting education about FH for general physicians and health service providers; development of instruments to permit opportune diagnosis and treatment; improve consciousness of the disease in carriers, family members, and health authorities; and to encourage genetic and clinical investigation specific for LA populations.
National guidelines should also be established for each population. Such recommendations will raise awareness for this disease and permit dependable diagnosis and improved management of patients. Cascade screening with a defined methodology is necessary to aid in the systematic search of affected individuals. Molecular screening has been limited to the three common genes. Genome sequencing may aid in identifying new pathogenic variants; again, this will be possible in only specialized centers. Molecular tools from Spain or Portugal do not include the Amerindian component of the LA populations
The provision of care for persons with FH is inadequate. In Latin America, the establishment of models of care is essential to allow the multidisciplinary management of this disease (96, 106, 117). Models of care involve the development of systems supported by scientific evidence (clinical experience, expert opinion, published evidence, and consultations) for the provision of quality healthcare services to a defined population. This will demand effective cooperation with various stakeholders. These include patient support groups, the public, foundations, universities and academic centers, nongovernment organizations, health economists, policy makers/politicians, and government ministers. Once a model of care is established, regular auditing and economic evaluation is necessary to maintain the standard of care.
Here, we highlight potential opportunities to create new knowledge and identify areas in which health providers should act to assure proper care for FH patients in our region.
Supplementary Material
Footnotes
Abbreviations:
- ARH
- autosomal recessive hypercholesterolemia
- CHD
- coronary heart disease
- DALY
- disability-adjusted life year
- DLCN
- Dutch Lipid Clinics Network
- FH
- familial hypercholesterolemia
- HeFH
- heterozygous familial hypercholesterolemia
- HoFH
- homozygous familial hypercholesterolemia
- LA
- Latin American
- LDL-C
- LDL cholesterol
- LDLR
- LDL receptor
- LDLRAP
- Low density lipoprotein receptor adaptor protein
- NICE
- National Institute for Health and Clinical Excellence
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- QALY
- quality-adjusted life year
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Singh S., and Bittner V.. 2015. Familial hypercholesterolemia–epidemiology, diagnosis, and screening. Curr. Atheroscler. Rep. 17: 482–485. [DOI] [PubMed] [Google Scholar]
- 2.Najam O., and Ray K.. 2015. Familial hypercholesterolemia: a review of the natural history, diagnosis, and management. Cardiol. Ther. 4: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benn M., Watts G., Tybjærg-Hansen A., and Nordestgaard B.. 2016. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur. Heart J. 37: 1384–1394. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros A., Alves A., and Bourbon M.. 2016. Mutational analysis of a cohort with clinical diagnosis of familial hypercholesterolemia: considerations for genetic diagnosis improvement. Genet. Med. 18: 316–324. [DOI] [PubMed] [Google Scholar]
- 5.Nordestgaard B., Chapman M., Humphries S., Ginsberg H., Masana L., Descamps O., Wiklund O., Hegele R., Raal F., Defesche J., et al. 2013. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 34: 3478–3490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rynkiewicz A., Cybulska B., Banach M., Filipiak K., Guzik T., Idzior-Waluś B., Imiela J., Jankowski P., Kłosiewicz-Latoszek L., Limon J., et al. 2013. Management of familial heterozygous hypercholesterolemia: Position Paper of the Polish Lipid Expert Forum. J. Clin. Lipidol. 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 7.Farnier M., and Bruckert E.. 2012. Severe familial hypercholesterolaemia: current and future management. Arch. Cardiovasc. Dis. 105: 656–665. [DOI] [PubMed] [Google Scholar]
- 8.Vogt A. 2015. The genetics of familial hypercholesterolemia and emerging therapies. Appl. Clin. Genet. 8: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito M., and Watts G.. 2015. Challenges in the diagnosis and treatment of homozygous familial hypercholesterolemia. Drugs. 75: 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos R. 2016. Homozygous familial hypercholesterolemia: phenotype rules! Atherosclerosis. 248: 252–254. [DOI] [PubMed] [Google Scholar]
- 11.Bell D. A., and Watts G. F.. 2016. Progress in the care of familial hypercholesterolemia: 2016. Med. J. Aust. 205: 232–236. [DOI] [PubMed] [Google Scholar]
- 12.Besseling J., Kindt I., Hof M., Kastelein J., Hutten B., and Hovingh G.. 2014. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: a study of a cohort of 14,000 mutation carriers. Atherosclerosis. 233: 219–223. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein J., Hazzard W., Schrott H., Bierman E., and Motulsky A.. 1973. Hyperlipidemia in coronary heart disease I. Lipid levels in 500 survivors of myocardial infarction. J. Clin. Invest. 52: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genest J., Hegele R., Bergeron J., Brophy J., Carpentier A., Couture P., Davignon J., Dufour R., Frohlich J., Gaudet D., et al. 2014. Canadian Cardiovascular Society position statement on familial hypercholesterolemia. Can. J. Cardiol. 30: 1471–1481. [DOI] [PubMed] [Google Scholar]
- 15.de Ferranti S., Rodday A., Mendelson M., Wong J., Leslie L., and Sheldrick R.. 2016. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES) clinical perspective. Circulation. 133: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg A., and Gidding S.. 2016. Knowing the prevalence of familial hypercholesterolemia matters. Circulation. 133: 1054–1057. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., and Altman D.. 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araujo M., Botto P., and Mazza C.. 2012. Uso de ezetimibe en el tratamiento de la hipercolesterolemia. An. Pediatr. (Barc.). 77: 37–42. [DOI] [PubMed] [Google Scholar]
- 19.Araujo M. B., Pacce M. S., Bravo M., Pugliese A. M., and Mazza C.. 2011. Severe hypercholesterolemia in children. Presentation of two cases and update of the literature. Arch. Argent. Pediatr. 109: e67–e71. [DOI] [PubMed] [Google Scholar]
- 20.Ahualli L., Stewart-Harris A., Bastianelli G., Radlovachki D., Bartolomé A., Trigo P., Cejas N., Aballay Soteras G., Duek F., Lendoire J., et al. 2007. Combined cardiohepatic transplantation due to severe heterozygous familial hypercholesteremia type II: first case in Argentina–a case report. Transplant. Proc. 39: 2449–2453. [DOI] [PubMed] [Google Scholar]
- 21.Figueiredo M., Dos Santos J., Alberto F., and Zago M.. 1992. High frequency of the Lebanese allele of the LDLr gene among Brazilian patients with familial hypercholesterolaemia. J. Med. Genet. 29: 813–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberto F., Figueiredo M., Zago M., Araújo A., and Dos-Santos J.. 1999. The Lebanese mutation as an important cause of familial hypercholesterolemia in Brazil. Braz. J. Med. Biol. Res. 32: 739–745. [DOI] [PubMed] [Google Scholar]
- 23.van de Kerkhof L., Van Eijk S., Defesche J., and Dos-Santos J.. 2003. Identification of a new mutation, S305C, in exon 7 of the low-density lipoprotein receptor gene in a Brazilian family with homozygous familial hypercholesterolemia. Genet. Test. 7: 77–79. [DOI] [PubMed] [Google Scholar]
- 24.Salazar L., Hirata M., Cavalli S., Nakandakare E., Forti N., Diament J., Giannini S., Bertolami M., and Hirata R.. 2002. Molecular basis of familial hypercholesterolemia in Brazil: identification of seven novel LDLR gene mutations. Hum. Mutat. 19: 462–463. [DOI] [PubMed] [Google Scholar]
- 25.Rocha V., Chacra A., Salgado W., Miname M., Turolla L., Gagliardi A., Ribeiro E., Rocha R., Avila L., Pereira A., et al. 2013. Extensive xanthomas and severe subclinical atherosclerosis in homozygous familial hypercholesterolemia. J. Am. Coll. Cardiol. 61: 2193. [DOI] [PubMed] [Google Scholar]
- 26.Barros M., Ferreira-Fernandes H., Barros I., Barbosa A., and Pinto G.. 2014. A case of severe carotid stenosis in a patient with familial hypercholesterolemia without significant coronary artery disease. Case Rep. Cardiol. 2014: 853921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jannes C., Santos R., de Souza Silva P., Turolla L., Gagliardi A., Marsiglia J., Chacra A., Miname M., Rocha V., Filho W., et al. 2015. Familial hypercholesterolemia in Brazil: cascade screening program, clinical and genetic aspects. Atherosclerosis. 238: 101–107. [DOI] [PubMed] [Google Scholar]
- 28.Santos R. 2014. What are we able to achieve today for our patients with homozygous familial hypercholesterolaemia, and what are the unmet needs? Atheroscler. Suppl. 15: 19–25. [DOI] [PubMed] [Google Scholar]
- 29.Pereira C., Miname M., Makdisse M., Kalil Filho R., and Santos R.. 2014. Association of peripheral arterial and cardiovascular diseases in familial hypercholesterolemia. Arq. Bras. Cardiol. 103: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos R., Gagliardi A., Xavier H., Casella Filho A., Araújo D., Cesena F., Alves R., Pereira A., Lottemberg A., Chacra A., et al. 2012. First Brazilian guidelines for familial hypercholesterolemia. Arq. Bras. Cardiol. 99: 1–28. [DOI] [PubMed] [Google Scholar]
- 31.Arteaga Ll. A., Cuevas M. A., Rigotti R. A., González F., Castillo S., Mata L. P., Alonso K. R.. 2007. Hipercolesterolemia familiar heterocigota: diagnóstico molecular y terapia combinada. Caso clínico. Rev. Med. Chil. 135: 216–220. Spanish. [DOI] [PubMed] [Google Scholar]
- 32.Huijgen R., Stork A., Defeschea J., Petera J., Alonso R., Cuevas A., Kastelein J., Durand M., and Stroes E.. 2012. Extreme xanthomatosis in patients with both familial hypercholesterolemia and cerebrotendinous xanthomatosis. Clin. Genet. 81: 24–28. [DOI] [PubMed] [Google Scholar]
- 33.Thiart R., Loubser O., de Villiers J., Santos M., and Kotze M.. 1997. Novel stop mutation causing familial hypercholesterolemia in a Costa Rican family. Mol. Cell. Probes. 11: 457–458. [DOI] [PubMed] [Google Scholar]
- 34.Kotze M., Thiart R., Loubser O., de Villiers J., Santos M., Vargas M., and Peeters A.. 1996. Mutation analysis reveals an insertional hotspot in exon 4 of the LDL receptor gene. Hum. Genet. 98: 476–478. [DOI] [PubMed] [Google Scholar]
- 35.Pereira E., Ferreira R., Hermelin B., Thomas G., Bernard C., Bertrand V., Nassiff H., Del Castillo D., Bereziat G., and Benlian P.. 1995. Recurrent and novel LDL receptor gene mutations causing heterozygous familial hypercholesterolemia in La Habana. Hum. Genet. 96: 319–322. [DOI] [PubMed] [Google Scholar]
- 36.Yu L., Heere-Ress E., Boucher B., Defesche J., Kastelein J., Lavoie M., and Genest J. Jr. 1999. Familial hypercholesterolemia. Acceptor splice site (G→C) mutation in intron 7 of the LDL-R gene: alternate RNA editing causes exon 8 skipping or a premature stop codon in exon 8. LDL-R(Honduras-1) [LDL-R1061(-1) G→C]. Atherosclerosis. 146: 125–131. [DOI] [PubMed] [Google Scholar]
- 37.Canizales-Quinteros S., Aguilar-Salinas C., Huertas-Vázquez A., Ordóñez-Sánchez M., Rodríguez-Torres M., Venturas-Gallegos J., Riba L., Ramírez-Jimenez S., Salas-Montiel R., Medina-Palacios G., et al. 2005. A novel ARH splice site mutation in a Mexican kindred with autosomal recessive hypercholesterolemia. Hum. Genet. 116: 114–120. [DOI] [PubMed] [Google Scholar]
- 38.Martinez L., Ordoñez Sanchez M., Letona R., Olvera Sumano V., Miguel Guerra M., Tusie-Luna M., and Aguilar-Salinas C.. 2011. Hipercolesterolemia familiar homocigota por la mutación c2271delT del gen del receptor LDL, detectada únicamente en mexicanos. Gac. Med. Mex. 147: 525–527. [PubMed] [Google Scholar]
- 39.Magaña Torres M., Mora-Hernández S., Vázquez Cárdenas N., and González Jaimes A.. 2014. Homozygous familial hypercholesterolemia: the c.1055G>A mutation in the LDLR gene and clinical heterogeneity. J. Clin. Lipidol. 8: 525–527. [DOI] [PubMed] [Google Scholar]
- 40.Vaca G., Vàzquez A., Magaña M., Ramìrez M., Dàvalos I., Martìnez E., Marìn B., and Carrillo G.. 2011. Mutational analysis of the LDL receptor and APOB genes in Mexican individuals with autosomal dominant hypercholesterolemia. Atherosclerosis. 218: 391–396. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar-Salinas C. 2001. Familial Hypercholesterolemia. Rev. Invest. Clin. 53: 254–265. [PubMed] [Google Scholar]
- 42.Robles-Osorio L., Huerta-Zepeda A., Ordóñez M. L., Canizales-Quinteros S., Díaz-Villaseñor A., Gutiérrez-Aguilar R., Riba L., Huertas-Vázquez A., Rodríguez-Torres M., Gómez-Díaz R. A., et al. 2006. Genetic heterogeneity of autosomal dominant hypercholesterolemia in Mexico. Arch. Med. Res. 37: 102–108. [DOI] [PubMed] [Google Scholar]
- 43.Robles-Osorio L., Ordoñez M. L., Aguilar-Salinas C. A., Aurón-Gómez M., Tusié-Luna M. T., Gómez-Pérez F. J., and Rull-Rodrigo J. A.. 2003. Familial hypercholesterolemia due to ligand-defective apolipoprotein B100: first case report in a Mexican family. Arch. Med. Res. 34: 70–75. [DOI] [PubMed] [Google Scholar]
- 44.Burgos A., Aguilar M., and de Arias K.. 2011. Visual vignette. Familial hypercholesterolemia. Endocr. Pract. 17: 154. [DOI] [PubMed] [Google Scholar]
- 45.Cefalù A., Barraco G., Noto D., Valenti V., Barbagallo C., Elisir G., Cuniberti L., Werba J., Libra M., Costa S., et al. 2006. Six novel mutations of the LDL receptor gene in FH kindred of Sicilian and Paraguayan descent. Int. J. Mol. Med. 17: 539–546. [PubMed] [Google Scholar]
- 46.Esperón P., Raggio V., and Stoll M.. 2009. Una nueva mutación en el promotor del gen del receptor de las lipoproteínas de baja densidad asociada a hipercolesterolemia familiar en homocigosis y heterocigosis. Clin. Investig. Arterioscler. 21: 51–55. [Google Scholar]
- 47.Stoll M., Lorenzo M., Raggio V., Esperon P., and Zelarayan M.. 2011. Previniendo el infarto en el adulto joven: GENYCO, un registro nacional de hipercolesterolemia familiar. Revista Uruguaya Cardiologia. 26: 16–26. [Google Scholar]
- 48.Esperon P., Raggio V., Lorenzo M., and Stoll M.. 2013. Mutación en el gen de la apolipoproteina B responsable de hipercolesterolemia familiar: primeros dos casos clínicos reportados en Uruguay. Revista Uruguaya de Cardiología. 28: 182–188. [Google Scholar]
- 49.Hobbs H., Brown M., and Goldstein J.. 1992. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum. Mutat. 1: 445–466. [DOI] [PubMed] [Google Scholar]
- 50.Bertolini S., Cassanelli S., Garuti R., Ghisellini M., Simone M., Rolleri M., Masturzo P., and Calandra S.. 1999. Analysis of LDL receptor gene mutations in Italian patients with homozygous familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 19: 408–418. [DOI] [PubMed] [Google Scholar]
- 51.Cenarro A., Jensen H., Casao E., Civeira F., González-Bonillo J., Rodríguez-Rey J., Gregersen N., and Pocoví M.. 1998. Identification of recurrent and novel mutations in the LDL receptor gene in Spanish patients with familial hypercholesterolemia. Hum. Mutat. 11: 413. [DOI] [PubMed] [Google Scholar]
- 52.Leitersdorf E., Van der Westhuyzen D., Coetzee G., and Hobbs H.. 1989. Two common low density lipoprotein receptor gene mutations cause familial hypercholesterolemia in Afrikaners. J. Clin. Invest. 84: 954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu W., Nohara A., Higashikata T., Lu H., Inazu A., and Mabuchi H.. 2002. Molecular genetic analysis of familial hypercholesterolemia: spectrum and regional difference of LDL receptor gene mutations in Japanese population. Atherosclerosis. 165: 335–342. [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto T., Davis C., Brown M., Schneider W., Casey M., Goldstein J., and Russell D.. 1984. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 39: 27–38. [DOI] [PubMed] [Google Scholar]
- 55.Dos Santos J. 2003. Familial hypercholesterolemia in Brazil. Atheroscler. Suppl. 4: 1–2. [DOI] [PubMed] [Google Scholar]
- 56.Cohen J., Kimmel M., Polanski A., and Hobbs H.. 2003. Molecular mechanisms of autosomal recessive hypercholesterolemia. Curr. Opin. Lipidol. 14: 121–127. [DOI] [PubMed] [Google Scholar]
- 57.Bednarska-Makaruk M., Bisko M., Puławska M., Hoffman-Zacharska D., Rodo M., Roszczynko M., Solik-Tomassi A., Broda G., Polakowska M., Pytlak A., et al. 2001. Familial defective apolipoprotein B-100 in a group of hypercholesterolaemic patients in Poland. Identification of a new mutation Thr3492Ile in the apolipoprotein B gene. Eur. J. Hum. Genet. 9: 836–842. [DOI] [PubMed] [Google Scholar]
- 58.Palacios L., Grandoso L., Cuevas N., Olano-Martín E., Martinez A., Tejedor D., and Stef M.. 2012. Molecular characterization of familial hypercholesterolemia in Spain. Atherosclerosis. 221: 137–142. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad Z., Adams-Huet B., Chen C., and Garg A.. 2012. Low prevalence of mutations in known loci for autosomal dominant hypercholesterolemia in a multiethnic patient cohort. Circ Cardiovasc Genet. 5: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farzadfar F., Finucane M., Danaei G., Pelizzari P., Cowan M., Paciorek C., Singh G., Lin J., Stevens G., Riley L., et al. 2011. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 377: 578–586. [DOI] [PubMed] [Google Scholar]
- 61.Ballesteros M. S., and Freidin B.. 2015. Reflections on the conceptualization and measurement of access to health services in Argentina: the case of the National Survey of Risk Factors 2009. Salud Colect. 11: 523–535. [DOI] [PubMed] [Google Scholar]
- 62.Malta D. C., Stopa S. R., Iser B. P., Bernal R. T., Claro R. M., Nardi A. C., Dos Reis A. A., and Monteiro C. A.. 2015. Risk and protective factors for chronic diseases by telephone survey in capitals of Brazil, Vigitel 2014. Rev. Bras. Epidemiol. 18(Suppl 2): 238–255. [DOI] [PubMed] [Google Scholar]
- 63.Lotufo P., Santos R., Figueiredo R., Pereira A., Mill J., Alvim S., Fonseca M., Almeida M., Molina M., Chor D., et al. 2016. Prevalence, awareness, treatment, and control of high low-density lipoprotein cholesterol in Brazil: Baseline of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). J. Clin. Lipidol. 10: 568–576. [DOI] [PubMed] [Google Scholar]
- 64.Cabrera S., Alvo M., Mindell J. S., and Ferro C.. 2015. The National Health Survey of Chile gives useful information to health policy planning: analysis of diabetic nephropathy as an index of potential saving. Rev. Med. Chil. 143: 679–681. [DOI] [PubMed] [Google Scholar]
- 65.Freire W. B., Belmont P., López-Cevallos D. F., and Waters W.. 2015. Ecuador’s National Health and Nutrition Survey: objectives, design, and methods. Ann. Epidemiol. 25: 877–878. [DOI] [PubMed] [Google Scholar]
- 66.Jiménez-Corona A., Aguilar-Salinas C. A., Rojas-Martínez R., and Hernández-Ávila M.. 2013. Type 2 diabetes and frequency of prevention and control measures. Salud Publica Mex. 55(Suppl 2): S137–S143. [PubMed] [Google Scholar]
- 67.Aguilar-Salinas C., Gómez-Pérez F., Rull J., Villalpando S., Barquera S., and Rojas R.. 2010. Prevalence of dyslipidemias in the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 52: S44–S53. [DOI] [PubMed] [Google Scholar]
- 68.Gómez-Pérez F., Rojas R., Villalpando S., Barquera S., Rull J., and Aguilar-Salinas C.. 2010. Prevention of cardiovascular disease based on lipid lowering treatment: a challenge for the Mexican health system. Salud Publica Mex. 52(Suppl 1): S54–S62. [DOI] [PubMed] [Google Scholar]
- 69.Khera A., Won H., Peloso G., Lawson K., Bartz T., Deng X., van Leeuwen E., Natarajan P., Emdin C., Bick A., et al. 2016. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J. Am. Coll. Cardiol. 67: 2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mabuchi H., Miyamoto S., Ueda K., Oota M., Takegoshi T., Wakasugi T., and Takeda R.. 1986. Causes of death in patients with familial hypercholesterolemia. Atherosclerosis. 61: 1–6. [DOI] [PubMed] [Google Scholar]
- 71.1991. Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ. 303: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohrschladt M., Westendorp R., Gevers Leuven J., and Smelt A.. 2004. Cardiovascular disease and mortality in statin-treated patients with familial hypercholesterolemia. Atherosclerosis. 172: 329–335. [DOI] [PubMed] [Google Scholar]
- 73.Mundal L., Sarancic M., Ose L., Iversen P., Borgan J., Veierod M., Leren T., and Retterstol K.. 2014. Mortality among patients with familial hypercholesterolemia: a registry-based study in Norway, 1992-2010. J. Am. Heart Assoc. 3: e001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mata N., Alonso R., Badimón L., Padró T., Fuentes F., Muñiz O., Perez-Jiménez F., López-Miranda J., Díaz J., Vidal J., et al. 2011. Clinical characteristics and evaluation of LDL-cholesterol treatment of the Spanish Familial Hypercholesterolemia Longitudinal Cohort Study (SAFEHEART). Lipids Health Dis. 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Brien E., Roe M., Fraulo E., Peterson E., Ballantyne C., Genest J., Gidding S., Hammond E., Hemphill L., Hudgins L., et al. 2014. Rationale and design of the familial hypercholesterolemia foundation cascade screening for awareness and detection of familial hypercholesterolemia registry. Am. Heart J. 167: 342–349.e17. [DOI] [PubMed] [Google Scholar]
- 76.Santos R., Frauches T., and Chacra A.. 2015. Cascade screening in familial hypercholesterolemia: advancing forward. J. Atheroscler. Thromb. 22: 869–880. [DOI] [PubMed] [Google Scholar]
- 77.Mundal L., and Retterstl K.. 2016. A systematic review of current studies in patients with familial hypercholesterolemia by use of national familial hypercholesterolemia registries. Curr. Opin. Lipidol. 27: 388–397. [DOI] [PubMed] [Google Scholar]
- 78.1999. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 142: 105–112. [PubMed] [Google Scholar]
- 79.Krogh H., Mundal L., Holven K., and Retterstøl K.. 2016. Patients with familial hypercholesterolaemia are characterized by presence of cardiovascular disease at the time of death. Eur. Heart J. 37: 1398–1405. [DOI] [PubMed] [Google Scholar]
- 80.Alonso R., Mata N., Castillo S., Fuentes F., Saenz P., Muñiz O., Galiana J., Figueras R., Diaz J., Gomez-Enterría P., et al. 2008. Cardiovascular disease in familial hypercholesterolaemia: influence of low-density lipoprotein receptor mutation type and classic risk factors. Atherosclerosis. 200: 315–321. [DOI] [PubMed] [Google Scholar]
- 81.Versmissen J., Botden I., Huijgen R., Oosterveer D., Defesche J., Heil T., Muntz A., Langendonk J., Schinkel A., Kastelein J., et al. 2011. Maternal inheritance of familial hypercholesterolemia caused by the V408M low-density lipoprotein receptor mutation increases mortality. Atherosclerosis. 219: 690–693. [DOI] [PubMed] [Google Scholar]
- 82.Vallejo-Vaz A., Kondapally Seshasai S., Cole D., Hovingh G., Kastelein J., Mata P., Raal F., Santos R., Soran H., Watts G., et al. 2015. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis. 243: 257–259. [DOI] [PubMed] [Google Scholar]
- 83.Ned R., and Sijbrands E.. 2011. Cascade screening for familial hypercholesterolemia (FH). PLoS Curr. 3: RRN1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeMott, K., NhereraL. , ShawEJ. , MinhasR. , HumphriesSE. , KathoriaM. , RitchieG. , NunesV. , DaviesD. , LeeP. , et al. 2008. Clinical Guidelines and Evidence Review for Familial Hypercholesterolaemia: the identification and management of adults and children with familial hypercholesterolaemia. National Collaborating Centre for Primary Care and Royal College of General Practitioners, London.
- 85.Haralambos K., Ashfield-Watt P., and McDowell I.. 2016. Diagnostic scoring for familial hypercholesterolaemia in practice. Curr. Opin. Lipidol. 27: 367–374. [DOI] [PubMed] [Google Scholar]
- 86.1991. Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. BMJ. 303: 893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams R., Hunt S., Schumacher M., Hegele R., Leppert M., Ludwig E., and Hopkins P.. 1993. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am. J. Cardiol. 72: 171–176. [DOI] [PubMed] [Google Scholar]
- 88.WHO Human Genetics Programme. 1999. Familial hypercholesterolaemia (FH): report of a second WHO consultation, Geneva, 4 September 1998. World Health Organization, Geneva. Accessed September 20, 2016, at http://www.who.int/iris/handle/10665/66346.
- 89.Haralambos K., Whatley S. D., Edwards R., Gingell R., Townsend D., Ashfield-Watt P., Lansberg P., Datta D. B., and McDowell I. F.. 2015. Clinical experience of scoring criteria for familial hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis. 240: 190–196. [DOI] [PubMed] [Google Scholar]
- 90.Umans-Eckenhausen M. A., Defesche J. C., Sijbrands E. J., Scheerder R. L., and Kastelein J. J.. 2001. Review of first 5 years of screening for familial hypercholesterolemia in the Netherlands. Lancet. 357: 165–168. [DOI] [PubMed] [Google Scholar]
- 91.Defesche J. 2010. Defining the challenges of FH screening for familial hypercholesterolemia. J. Clin. Lipidol. 4: 338–341. [DOI] [PubMed] [Google Scholar]
- 92.Weng S., Kai J., Andrew Neil H., Humphries S., and Qureshi N.. 2015. Improving identification of familial hypercholesterolaemia in primary care: derivation and validation of the familial hypercholesterolaemia case ascertainment tool (FAMCAT). Atherosclerosis. 238: 336–343. [DOI] [PubMed] [Google Scholar]
- 93.Sharifi M., Rakhit R., Humphries S., and Nair D.. 2016. Cardiovascular risk stratification in familial hypercholesterolaemia. Heart. 102: 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jacobson T. A., Ito M. K., Maki K. C., Orringer C. E., Bays H. E., Jones P. H., McKenney J. M., Grundy S. M., Gill E. A., Wild R. A., et al. 2014. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1—executive summary. J. Clin. Lipidol. 8: 473–488. [DOI] [PubMed] [Google Scholar]
- 95.M. F. Piepoli, A. W. Hoes, S. Agewall, C. Albus, C. Brotons, A. L. Catapano, M. T. Cooney, U. Corrà, B. Cosyns, C. Deaton, et al. 2016. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis. 252: 207–274. [DOI] [PubMed] [Google Scholar]
- 96.Watts G., Gidding S., Wierzbicki A., Toth P., Alonso R., Brown W., Bruckert E., Defesche J., Lin K., Livingston M., et al. 2014. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int. J. Cardiol. 171: 309–325. [DOI] [PubMed] [Google Scholar]
- 97.Vuorio A., Docherty K., Humphries S., Kuoppala J., and Kovanen P.. 2013. Statin treatment of children with familial hypercholesterolemia–trying to balance incomplete evidence of long-term safety and clinical accountability: Are we approaching a consensus? Atherosclerosis. 226: 315–320. [DOI] [PubMed] [Google Scholar]
- 98.Neil A., Cooper J., Betteridge J., Capps N., McDowell I., Durrington P., Seed M., and Humphries S.. 2008. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur. Heart J. 29: 2625–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harada-Shiba M., Sugisawa T., Makino H., Abe M., Tsushima M., Yoshimasa Y., Yamashita T., Miyamoto Y., Yamamoto A., Tomoike H., et al. 2010. Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J. Atheroscler. Thromb. 17: 667–674. [DOI] [PubMed] [Google Scholar]
- 100.Wong B., Kruse G., Kutikova L., Ray K., Mata P., and Bruckert E.. 2016. Cardiovascular disease risk associated with familial hypercholesterolemia: a systematic review of the literature. Clin. Ther. 38: 1696–1709. [DOI] [PubMed] [Google Scholar]
- 101.2010. FH Guideline Implementation Team Toolkit. Heart UK, West Berkshire, UK. [Google Scholar]
- 102.Gencera B., and Nanchen D.. 2016. Identifying familial hypercholesterolemia in acute coronary syndrome. Curr. Opin. Lipidol. 27: 375–381. [DOI] [PubMed] [Google Scholar]
- 103.Pijlman A. H., Huijgen R., Verhagen S. N., Imholz B. P., Liam A. H., Kastelein J. J., Abbink E. J., Stalenhoef A. F., and Visseren F. L.. 2010. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in The Netherlands. Atherosclerosis. 209: 189–194. [DOI] [PubMed] [Google Scholar]
- 104.deGoma E., Ahmad Z., O’Brien E., Kindt I., Shrader P., Newman C., Pokharel Y., Baum S., Hemphill L., Hudgins L., et al. 2016. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States. Circ Cardiovasc Genet. 9: 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson G. 2008. Recommendations for the use of LDL apheresis. Atherosclerosis. 198: 247–255. [DOI] [PubMed] [Google Scholar]
- 106.Gidding S., Ann Champagne M., de Ferranti S., Defesche J., Ito M., Knowles J., McCrindle B., Raal F., Rader D., Santos R., et al. 2015. The agenda for familial hypercholesterolemia. Circulation. 132: 2167–2192. [DOI] [PubMed] [Google Scholar]
- 107.Santos R., Gidding S., Hegele R., Cuchel M., Barter P., Watts G., Baum S., Catapano A., Chapman M., Defesche J., et al. 2016. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 4: 850–861. [DOI] [PubMed] [Google Scholar]
- 108.González C. A., Pavía A., Redding F. J., Zacarías J. L., Ramírez M. A., Alpízar M., Aspe J., Carranza J., Barba R., Ramírez J. C., et al. 2009. Encuesta sobre el manejo de dislipidemia con estatinas en México: Porcentaje de pacientes que alcanzan las metas establecidas por el Programa Nacional de Educación en Colesterol (National Cholesterol Education Program [NCEP]). Rev Mex Cardiol. 20: 18–22. [Google Scholar]
- 109.Homedes N., and Ugalde A.. 2015. Availability and affordability of new medicines in Latin American countries where pivotal clinical trials were conducted. Bull. World Health Organ. 93: 674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pr#xFCss-#xDCst#xFCn A., MathersC. , Corval#xE1nC. , and WoodwardA. 2003. Introduction and methods: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series, No. 1. World Health Organization, Geneva, Switzerland.
- 111.Nherera L., Marks D., Minhas R., Thorogood M., and Humphries S.. 2011. Probabilistic cost-effectiveness analysis of cascade screening for familial hypercholesterolaemia using alternative diagnostic and identification strategies. Heart. 97: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 112.Bruckert E., Saheb S., Bonté J., and Coudray-Omnès C.. 2014. Daily life, experience and needs of persons suffering from homozygous familial hypercholesterolaemia: Insights from a patient survey. Atheroscler. Suppl. 15: 46–51. [DOI] [PubMed] [Google Scholar]
- 113.Mata N., Alonso R., Banegas J., Zambon D., Brea A., and Mata P.. 2014. Quality of life in a cohort of familial hypercholesterolemia patients from the south of Europe. Eur. J. Public Health. 24: 221–225. [DOI] [PubMed] [Google Scholar]
- 114.Ågård A., Bolmsjö I., Hermerén G., and Wahlstöm J.. 2005. Familial hypercholesterolemia: ethical, practical and psychological problems from the perspective of patients. Patient Educ. Couns. 57: 162–167. [DOI] [PubMed] [Google Scholar]
- 115.Mortensen G. L., Madsen I. B., Kruse C., and Bundgaard H.. 2016. Familial hypercholesterolaemia reduces the quality of life of patients not reaching treatment targets. Dan. Med. J. 63: A5224. [PubMed] [Google Scholar]
- 116.Merchán A., Ruiz A. J., Campo R., Prada C. E., Toro J. M., Sánchez R., Gómez J. E., Jaramillo N. I., Molina D. I., Vargas-Uricoechea H. , et al. 2016. Hipercolesterolemia familiar: artículo de revisión. Rev. Colomb. Cardiol. 23(S4): 4–26. [Google Scholar]
- 117.Watts G., Sullivan D., Poplawski N., van Bockxmeer F., Hamilton-Craig I., Clifton P., O’Brien R., Bishop W., George P., Barter P., et al. 2011. Familial hypercholesterolaemia: A model of care for Australasia. Atheroscler. Suppl. 12: 221–263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.