Abstract
It was hypothesized that under induced lipid malabsorption/maldigestion conditions, an enriched sn-1(3)-monoacylglycerol (MAG) oil may be a better carrier for n-3 long-chain PUFAs (LC-PUFAs) compared with triacylglycerol (TAG) from fish oil. This monocentric double blinded clinical trial examined the accretion of EPA (500 mg/day) and DHA (300 mg/day) when consumed as TAG or MAG, into the erythrocytes, plasma, and chylomicrons of 45 obese (BMI ≥30 kg/m2 and ≤40 kg/m2) volunteers who were and were not administered Orlistat, an inhibitor of pancreatic lipases. Intake of MAG-enriched oil resulted in higher accretion of LC-PUFAs than with TAG, the concentrations of EPA and DHA in erythrocytes being, respectively, 72 and 24% higher at 21 days (P < 0.001). In addition, MAG increased the plasma concentration of EPA by 56% (P < 0.001) as compared with TAG. In chylomicrons, MAG intake yielded higher levels of EPA with the area under the curve (0–10 h) of EPA being 55% greater (P = 0.012). In conclusion, in obese human subjects with Orlistat-induced lipid maldigestion/malabsorption conditions, LC-PUFA MAG oil increased LC-PUFA levels in erythrocytes, plasma, and chylomicrons to a greater extent than TAG. These results indicate that MAG oil might require minimal enzymatic digestion prior to intestinal uptake and transfer across the epithelial barrier.
Keywords: fatty acid/metabolism, lipase, digestion, diet effects/lipid metabolism, clinical trials, lipid absorption, Orlistat, obese, docosahexaenoic acid, eicosapentaenoic acid, polyunsaturated fatty acid
Dietary fats are not only a source of energy, but also of bioactive nutrients, which are essential in maintaining human health. Among the fats, some FAs are considered essential FAs (EFAs) because they are not de novo synthesized and humans lack the enzymes required for their biosynthesis. Dietary fat absorption involves two main steps: first, lipolysis of dietary triacylglycerol (TAG) by sn-1,3’ specific gastric and (colipase-dependent) pancreatic lipases to release two FFAs, and an sn-2 monoacylglycerol (MAG); and second, the formation of micellar structures (via association of intraluminal conjugated bile salts and lipolytic products), which aid enteral uptake (1, 2). Conventional knowledge indicates that FAs at the sn-1 and -3 positions are hydrolyzed and sn-2 FA is absorbed directly as monoglycerides; as such, the stereospecific position of FAs on the TAG backbone plays an important role in determining the uptake and metabolic fate of a particular FA (especially EFA) (1, 3). FAs such as linoleic acid (LA) and α-linolenic acid (ALA) are EFAs, and dietary ALA can be metabolically converted to other long-chain PUFAs (LC-PUFAs), such as DHA and EPA, with recognized beneficial health effects. However, the efficiency of enzymatic conversion from ALA to DHA/EPA appears to be relatively low in humans (4). Therefore, larger quantities of DHA/EPA may be obtained from dietary sources such as fatty fish for ultimate health benefit. Among their health benefits, EPA and DHA are cardioprotective and have a positive impact on inflammatory conditions by interfering with the production of systemic inflammatory mediators, such as leukotrienes, platelet-activating factor, interleukin-l, and tumor necrosis factor (5–7). Impairment of intestinal uptake of fat in conditions such as maldigestion and malabsorption can occur, for example, when a long-standing inflammation of the pancreas alters the organ’s normal function or when pancreatic lipase concentration is severely reduced causing steatorrhea. The absorption of fat is compromised and, in such conditions, it is of great importance to ensure that EFAs are delivered to the body (8). The above conditions can be caused by different diseases and related risk factors, such as chronic pancreatitis, Crohn’s disease, cystic fibrosis, Shwachman-Diamond syndrome, or history of previous surgery (e.g., ileal resection, bariatric surgery) on the gastrointestinal (GI) tract (9–11). Because of the modified anatomy in some bariatric surgery procedures (e.g., biliopancreatic diversion) or extensive resection of the small bowel, both digestion and absorption of nutrients is altered and can lead to a delayed mixing of fat with pancreatic enzymes and bile salts, thereby lowering levels of some nutrients, such as fat-soluble vitamins (12–14). Another probable reason beyond surgical procedures includes the intentional Orlistat-induced fat malabsorption in obese subjects with the desire to reduce obesity. Orlistat is an inhibitor of pancreatic, gastric, and other lipases (15, 16). It reduces body weight mainly by causing fat malabsorption, which can result in insufficient nutrient (e.g., EFA) uptake and utilization by the GI tract (8, 9, 15–17).
Current strategies to combat lipid malabsorption may include dietary supplements, intestinal enzyme therapy, and treatment of underlying causes such as inflammation. In subjects who have undergone major GI surgery, LC-PUFA supplementation has been shown to reduce concentrations of inflammatory biomarkers and to shorten hospital length of stay (11, 12). According to previous studies, EFAs delivered as MAG have been reported to be better absorbed than EFAs delivered as TAG (18–21). Moreover, previous studies have reported that FAs at the sn-2 position are more efficiently absorbed by the intestine. Therefore, sn-2 molecules present a possible approach to enhance EFA absorption in malabsorption conditions. However, a challenge with sn-2 structures is that they are not very stable and easily undergo isomerization to form sn-1-(3)-MAG structures, which are hydrolyzed by luminal lipases to FFA and a glycerol molecule (19–21).
The objective of the present study was to examine the effect of enriched sn-1(3)-MAG oil versus TAG on the accretion and delivery of LC-PUFAs to the circulatory system of obese females with lipid malabsorption/maldigestion induced by a pancreatic lipase inhibitor (Orlistat). Obese females with lipid malabsorption/maldigestion induced by Orlistat were used as a model population given the known issues with lipid/EFA absorption and the rise in the use of Orlistat as a weight loss aid.
MATERIALS AND METHODS
Subjects
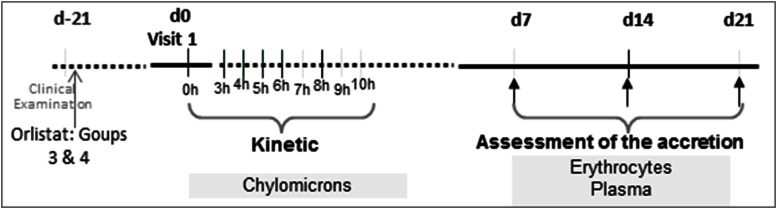
The primary outcome of the study was to evaluate the accretion of LC-PUFAs in erythrocytes for a 21 day period. PUFAs were fed to obese subjects with or without Orlistat-induced malabsorption, either as an oil enriched in sn-1(3)-MAG or TAG [sourced from fish oil (FO)]. The period of 21 days has been previously found to indicate long-term dietary changes of such patients (22). Key secondary outcomes included: 1) assessment of accumulation of LC-PUFAs (EPA/DHA) in plasma when consumed as TAG or MAG in the aforementioned induced malabsorption subjects; and 2) assessment of the bioavailability of LC-PUFAs in the chylomicron compartment after an acute dose, either in the form of TAG or MAG, in a kinetic pattern (0–10 h post prandial) (Fig. 1).
Fig. 1.
Clinical trial design outline.
Study design and test oils
This was a randomized controlled double-blind clinical trial conducted at Centre Hospitalier Universitaire Vaudois (CHUV) in Lausanne, Switzerland. It was approved by the Commission Cantonale d’éthique de la Recherché sur l’être Humain (Cantonale Ethics Committee of Lausanne Switzerland). The clinical study was registered at ClinicalTrials.gov with the identifier NCT01797757.
The trial followed a four-arm parallel 2 × 2 factorial design with double-blinded identity of the tested encapsulated oils; however, the presence or absence of Orlistat was readily distinguishable. The four study groups were as follows: 1) TAG without Orlistat, 2) MAG without Orlistat, 3) TAG with Orlistat, and 4) MAG with Orlistat (Table 1). For groups 3 and 4, Orlistat was administered three times a day for 21 days before oil capsules were supplied, in order to introduce malabsorption conditions. All subjects ingested three capsules per day, containing doses of 500 mg of EPA and 300 mg of DHA per day for 21 days as shown in Table 1. Doses were distributed on a weekly basis and compliance was monitored by accounting for capsules at each subject visit.
TABLE 1.
Study groups and oil dosage
| Group Number | n | Oil Type | Capsules per Day | Total EPA per Day | Total DHA per Day | Orlistat®, 120 mg | |
| 1 | 11 | TAG | 3 | 500 mg | 316 mg | No | — |
| 2 | 11 | MAG | 3 | 505 mg | 300 mg | No | — |
| 3 | 12 | TAG | 3 | 500 mg | 316 mg | Yes | 3 |
| 4 | 11 | MAG | 3 | 505 mg | 300 mg | Yes | 3 |
MAG refers to enriched sn-1(3)-MAG oil; TAG is from FO.
Oils used in this study were provided either as enriched MAG oil, mainly as sn-1(3)-MAG (Cognis GmbH, Illertissen, Germany), or as FO, mainly as TAG (Sofinol S.A., Manno, Switzerland). Oils were encapsulated (1 g oil per capsule) containing 1,500 mg/kg of the mixed tocopherols and 200 mg/kg of ascorbyl palmitate as antioxidants. Oils were prepared such that the EPA and DHA content was similar in both MAG and TAG study groups; the FA profile of the capsules is shown in Table 2. For groups 3 and 4, capsules of 120 mg Orlistat (Xenical® by Roche, Basel, Switzerland), known by its IUPAC name as tetrahydrolipstatin (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3- hexyl-4-oxo-2-oxetanyl] methyl]dodecyl ester], were administered as described above.
TABLE 2.
FA composition of capsules in milligrams per gram of oil before treatment
| FA | MAG (mg/g oil) | TAG (mg/g oil) |
| 14:0 | 0.03 | 6.26 |
| 16:0 | 1.98 | 13.14 |
| 17:0 | 0.06 | 0.32 |
| 18:0 | 2.63 | 2.33 |
| 20:0 | 0.37 | 0.12 |
| 22:0 | 0.48 | 0.12 |
| 24:0 | 0.26 | 0.04 |
| 16:1 n-9 | 0.22 | 7.67 |
| 18:1 n-9 + n-7 | 37.11 | 11.87 |
| 20:1 n-9 | 0.79 | 0.44 |
| 24:1 n-9 | 0.06 | 0.25 |
| 18:2 n-6 | 4.22 | 3.36 |
| 18:3 n-6 | 0.45 | 0.21 |
| 20:3 n-6 | 0.11 | 0.14 |
| 20:4 n-6 | 0.77 | 0.87 |
| 18:3 n-3 | 0.24 | 0.91 |
| 20:3 n-3 | 0.07 | 0.06 |
| 20:5 n-3 | 16.84 | 16.65 |
| 22:6 n-3 | 9.99 | 10.56 |
| Others | 9.73 | 13.27 |
MAG refers to enriched sn-1(3)-MAG oil; TAG is from FO.
Participants and anthropometric measurements
Once the volunteers agreed to participate and were verified for inclusion/exclusion criteria, subjects were exposed to routine dietary evaluation (food records for the last 48 h). Additional routine clinical examinations (vital signs; heart rate, blood pressure) were carried out along with anthropometric measurements (i.e., age, BMI, height, and weight). Exclusion criteria were: medication affecting lipid metabolism, pregnancy, no consumption of fish, or the use of other supplements containing FO, for 3 months before inclusion in the trial and for the duration of the trial. Other exclusion criteria were participation in any weight loss treatment(s); history of metabolic, cardiovascular, hepatic, or renal diseases or diseases that could interfere with intestinal absorption; or history of abdominal/gastric surgery. Subjects to be enrolled in the clinical trial returned to the clinic after confirmation of no abnormalities from the tests listed above. Forty-five subjects (18–65 years of age) with BMI ≥30 and ≤40 kg/m2, were recruited. They provided informed consent before beginning the trial and were randomly assigned to one of the study groups (Table 1).
Acute phase to assess kinetics
At the first visit, blood was drawn after an overnight fast in order to separate plasma, erythrocytes, and chylomicrons. This sampling point was considered the baseline for both the short-term (10 h post prandial) and long-term (21 day) phases of the study. Immediately after the baseline was taken, subjects ingested three capsules containing 1 g of oil each, one capsule of Orlistat (if subjects were randomized to groups 3 and 4), and a standardized breakfast containing 40 g of lipids per serving. No further intake of food was permitted except ad libitum water for 10 h (execution of the short-term phase of the study).
For the postprandial study, blood sampling was performed 3 h after capsule intake and then every hour until 10 h had passed (Fig. 1). Blood samples were fractionated into chylomicrons, erythrocytes, and plasma.
Assessment of accretion
At the first visit, each subject was instructed to orally consume one study capsule immediately before the major meals (three times a day) and, at the same time, one capsule of Orlistat for subjects in groups 3 and 4, for days 1–21. Fasting blood samples (5.5 ml in fasting conditions) were taken at days 7, 14, and 21 to collect erythrocytes and plasma samples for FA analysis (Fig. 1).
Biochemical assessments
Blood was collected after overnight fast before subjects began the intervention. After plasma separation, total cholesterol (Roche CHOD-PAP; Roche Molecular Biochemicals Systems, GmbH, Mannheim, Germany), HDL cholesterol (HDL-C plus, second generation, Roche Diagnostic GmbH, Mannheim, Germany), and triglycerides (TG GPO-PAP; Roche Diagnostic) were measured using an automated biochemistry analyzer (Hitachi 917 Roche apparatus). LDL cholesterol (LDL-C) was then calculated with Friedewald’s formula.
Analysis of the FA composition in blood lipids
Blood was collected in 9 ml EDTA-containing S-Monovette KE (Sarstedt #02.1066.001). Sample preparation for FA methyl ester (FAME) analysis was done as previously described, with some modifications to adjust for the physicochemical characteristics of the matrix (milk vs. plasma vs. erythrocytes vs. chylomicrons) (23). First, erythrocytes were separated by centrifugation (3,600 rpm for 10 min at 4°C) and 200 μl were sampled into 10 ml glass tubes containing 200 μl of erythrocyte lysis buffer, followed by aliquoting of plasma (200 μl) into 10 ml glass tubes containing 200 μl of absolute ethanol. Tubes were vortexed for 10 s and tightly capped. Chylomicrons were isolated (24) from the plasma (at the junction of chylomicron and plasma using a Beckman tube slicer kit #303811) immediately after ultracentrifugation (35 min at 130,000 g at 4°C). An aliquot (200 μl) was mixed with absolute ethanol (200 μl) by vortexing for 10 s. All blood fractions were kept on ice during sample preparation and finally stored at −80°C until FA analysis. FAMEs of erythrocytes were prepared directly in the tubes in which they were aliquoted and stored. Internal standards, FAME 21:0 (1 mg/ml) and phosphatidylcholine 23:0 (0.4 mg/ml), were added (100 μl each) plus 2 ml of methanol, 2 ml of catalyst methanol/HCl (3N), and 1 ml of n-hexane. Test tubes were firmly capped, shaken vigorously, and heated at 100°C for 90 min, followed by occasional shaking. After cooling down, 2 ml of water was added and the n-hexane phase was subjected to GC analysis after separation (1,200 g for 5 min). The same procedure was followed for plasma and chylomicron samples, except that phosphatidylcholine 23:0 was substituted with 100 μl of internal standard TAG 13:0 (0.1 mg/ml) and the transesterification was performed at 100°C for 60 min.
Analysis of total FAMEs was performed by Fast GC, as previously described (25), on a 7890 Agilent gas chromatograph (Agilent Technologies, Palo-Alto, CA) equipped with a fused-silica BPX-70 capillary column (10 m, 0.1 mm inner diameter, 0.2 μm film thickness; SGE, Melbourne, Australia). Split injector (50:1) and flame-ionization detector were operating at 250°C with an oven volume reduced to about 5,400 cm3. Oven temperature was kept at 50°C for 0.2 min, increased to 180°C at a rate of 120°C/min, kept at 180°C for 1 min, and then increased to 220°C at a rate of 20°C/min and then to 250°C at 50°C/min (total run time 4.9 min). Hydrogen was used as carrier gas under constant flow mode at 1 ml/min and the acquisition of the flame-ionization detector signal was performed at 100 Hz.
Statistical analysis
Background knowledge for powering the trial has some limitations, as only animal experimental data are available using the same concept. The sample size was chosen at 11 subjects per group in order to detect a difference of 0.7 in EPA expressed in units of percent total FA; the standard deviation was estimated at 0.5 with a statistical power of 80%.
The 2 × 2 factorial design was applied: TAG versus MAG under induced malabsorption or without induced malabsorption. The factorial design was analyzed by a mixed model, where fixed effects were time (7, 14, and 21 days), treatment (TAG or MAG), and malabsorption (Orlistat or no Orlistat) and the interaction term, treatment × malabsorption. The model had a random intercept for each subject. Once effect modification was demonstrated, the treatment effect was estimated in subjects with induced malabsorption by another mixed model, where fixed effects were baseline, time, and treatment. The same model was repeated in subjects without induced malabsorption. Because malabsorption was already induced before baseline, the model for exploring the interaction effect was not corrected for baseline. Similar models were employed for the other outcome measures. The mixed model was assessed for treatment differences at certain time points by appropriate contrasts. A feasibility analysis was performed before code break and revealed that FAs are approximately log-normally distributed. We took advantage of the property of the (natural) log, dlog = dx/x, and interpreted treatment differences on the log-scale as percentage changes with respect to the grand mean. Although the power calculation was performed for primary outcome, EPA in percent total FA, it turned out that findings in percent were confirmed in milligrams per deciliter. For the ease of interpretation, all results in this report are presented in milligrams per deciliter. Throughout this report, the P values are not corrected for multiplicity and serve as flags indicating interesting results. A small P value (P < 0.05) on the primary outcome will reject the null and hence confirm our planning assumptions. Kinetic characteristics of interest are area under the curve (AUC), Cmax, and Tmax. AUC, Cmax, and Tmax were analyzed by ANCOVA correcting for baseline. AUC and Cmax were approximately log-normally distributed. All statistical analyses were conducted using the software, Statistical Analysis System (SAS, version 9.1).
RESULTS
Clinical and compliance evaluation
Forty-five subjects were screened and recruited, with an average age of 39.5 years, and group characteristics are shown in Table 3. One subject discontinued participation voluntarily at day 7 of treatment after learning that she was pregnant. A second individual left the trial without further explanation and was only randomized. A third withdrawal from the study related to GI events that occurred in the TAG plus Orlistat group due to medication. All other subjects were 100% compliant with the study supplements for the duration of their participation in the study.
TABLE 3.
Study group characteristics
| Study Groups | |||||
| No Orlistat | Plus Orlistat | ||||
| Day | TAG (n = 11) | MAG (n = 11) | TAG (n = 12) | MAG (n = 11) | |
| Age (years) | — | 37.10 ± 7.2 | 42.4 ± 9.8 | 36.45 ± 10.3 | 42.3 ± 9.3 |
| Weight (kg)a | 0 | 91.4 ± 10.9 | 100.3 ± 12.3 | 101.7 ± 9.8 | 96.85 ± 10.1 |
| 7 | 0.1 ± 1.2 | 0.3 ± 0.8 | −0.6 ± 0.9 | −0.05 ± 0.63 | |
| 14 | 0.7 ± 1.2 | 0.1 ± 0.9 | −0.7 ± 1 | 0.03 ± 1.5 | |
| 21 | 0.2 ± 0.06 | 0.2 ± 1.0 | −0.6 ± 1.1 | 0.1 ± 1.4 | |
| BMI (kg/m2)b | 0 | 32.87 ± 3.1 | 36.04 ± 2.6 | 35.97 ± 3.4 | 35.05 ± 2.9 |
| 7 | 0.08 ± 0.5 | 0.1 ± 0.32 | −0.1 ± 0.33 | −0.02 ± 0.23 | |
| 14 | 0.15 ± 0.5 | 0.04 ± 0.36 | −0.16 ± 0.37 | −0.09 ± 0.5 | |
| 21 | 0.14 ± 0.23 | 0.07 ± 0.38 | −0.1 ± 0.4 | 0.04 ± 0.5 | |
| Total cholesterol (mmol/l) | 0 | 5.28 ± 1.0 | 5.3 ± 0.87 | 5.05 ± 0.7 | 4.89 ± 0.81 |
| HDL-C (mmol/l) | 0 | 1.49 ± 0.4 | 1.47 ± 0.2 | 1.67 ± 9.7 | 1.32 ± 28 |
| LDL-C (mmol/l) | 0 | 3.26 ± 0.7 | 3.35 ± 0.7 | 2.86 ± 0.63 | 3.05 ± 0.47 |
| Triglycerides (mmol/l) | 0 | 1.16 ± 0.6 | 1.05 ± 0.3 | 1.14 ± 22 | 1.13 ± 43 |
| EPA intake (mg/3 g oil) | — | 505.2 ± 0.34 | 500 ± 0.75 | 505.2 ± 0.34 | 500 ± 0.75 |
| DHA intake (mg/3 g oil) | — | 316.8 ± 1.32 | 299.7 ± 0.86 | 316.8 ± 1.32 | 299.7 ± 0.86 |
Values represent mean ± SD. MAG refers to enriched sn-1(3)-MAG oil.
Weight change from baseline.
BMI percentage change from baseline.
The provided supplements were generally well-tolerated. Some GI events occurred in a few subjects in the “Orlistat-treated” groups: abdominal pain, diarrhea, fecal urgency, bloating, and oily/liquid stools. For subjects presenting symptoms, most experienced one or two episodes and most GI events were mild to moderate, with none of them reaching severe intensity and all resolving without intervention. Subject characteristics and results for baseline analyses are shown in Table 3. At the beginning of the study, body weight average was 97.5 kg, while BMI average was 35 kg/m2. Body weight and BMI were not significantly different among groups at inclusion and after 21 days, but groups on Orlistat (3, 4) showed a tendency to decrease over time. No significant differences were found among participants regarding total cholesterol, HDL-C, LDL-C, or triglycerides, measured only at baseline. Intake levels of DHA and EPA in both study groups is shown in Table 3.
Incorporation of EPA in erythrocytes and plasma
The primary outcome of the study was to assess the LC-PUFA accretion in erythrocytes when lipase activity is compromised. Hence, through similar doses of supplementation for 21 days, MAG and TAG treatments were compared (Table 2). A summary of the FA profile obtained from erythrocytes is shown in Table 4.
TABLE 4.
FA composition of chylomicrons, erythrocytes, and plasma: summary statistics
| Study Groups | |||||
| No Orlistat | Plus Orlistat | ||||
| TAG | MAG | TAG | MAG | ||
| Chylomicrons | |||||
| 16:0 | Day 1 | 18.86 ± 17.2 | 18.91 ± 7.5 | 19.55 ± 16.4 | 16.94 ± 10.1 |
| 18:1n-9 | Day 1 | 0.88 ± 0.78 | 0.78 ± 0.34 | 1.18 ± 1.02 | 0.80 ± 0.54 |
| 18:2n-6 | Day 1 | 7.88 ± 7.17 | 7.58 ± 2.99 | 8.34 ± 5.57 | 6.68 ± 2.96 |
| 20:3n-6 | Day 1 | 0.46 ± 0.48 | 0.43 ± 0.20 | 0.55 ± 0.42 | 0.47 ± 0.30 |
| 20:4n-6 | Day 1 | 1.54 ± 1.18 | 1.76 ± 0.74 | 2.03 ± 1.45 | 1.60 ± 1.01 |
| 20:5n-3 | Day 1 | 1.03 ± 0.80 | 1.20 ± 0.50 | 0.58 ± 0.72 | 0.81 ±± 0.38 |
| 22:6n-3 | Day 1 | 0.83 ± 0.79 | 0.87 ± 0.36 | 0.75 ± 0.72 | 0.82 ± 0.44 |
| ∑SFA | Day 1 | 20.24 ± 17.95 | 21.48 ± 8.78 | 24.83 ± 22.27 | 19.05 ± 10.90 |
| ∑MUFA | Day 1 | 1.86 ± 1.57 | 2.07 ± 0.85 | 1.33 ± 1.42 | 1.63 ± 0.76 |
| ∑PUFA | Day 1 | 9.88 ± 3.86 | 9.78 ± 3.84 | 10.92 ± 7.25 | 8.75 ± 8.78 |
| Erythrocytes | |||||
| 16:0 | Day 1 | 29.24 ± 5.48 | 28.36 ± 6.8 | 28.77 ± 7.20 | 27.45 ± 4.96 |
| Day 21 | 31.25 ± 4.08 | 35.14 ± 8.3 | 29.37 ± 4.05 | 31.83 ± 5.82 | |
| 18:1n-9 | Day 1 | 1.46 ± 0.27 | 1.34 ± 0.38 | 1.48 ± 0.42 | 1.34 ± 0.35 |
| Day 21 | 1.50 ± 0.26 | 1.78 ± 0.49 | 1.41 ± 0.12 | 1.52 ± 0.34 | |
| 18:2n-6 | Day 1 | 12.67 ± 2.33 | 11.72 ± 3.25 | 11.73 ± 3.51 | 10.69 ± 2.30 |
| Day 21 | 13.37 ± 2.34 | 14.00 ± 3.68 | 11.38 ± 1.98 | 11.74 ± 2.31 | |
| 20:3n-6 | Day 1 | 2.45 ± 0.74 | 2.35 ± 0.66 | 2.35 ± 0.65 | 2.38 ± 0.64 |
| Day 21 | 2.43 ±0.44 | 2.80 ± 0.75 | 2.37 ± 0.54 | 2.76 ± 0.71 | |
| 20:4n-6 | Day 1 | 19.69 ± 4.56 | 19.33 ± 4.30 | 20.89 ± 5.89 | 18.04 ± 3.80 |
| Day 21 | 21.46 ± 3.03 | 25.21 ± 8.20 | 21.37 ± 3.15 | 21.54 ± 3.72 | |
| 20:5n-3 | Day 1 | 0.84 ± 0.28 | 0.96 ± 0.37 | 0.72 ± 0.19 | 0.76 ± 0.43 |
| Day 21 | 1.67 ± 0.49 | 2.11 ± 1.04 | 0.88 ± 0.26 | 1.73 ± 0.42 | |
| 22:6n-3 | Day 1 | 6.20 ± 1.59 | 6.57 ± 2.62 | 6.08 ± 1.43 | 6.10 ± 1.66 |
| Day 21 | 8.47 ± 2.17 | 9.29 ± 2.44 | 7.11 ± 1.40 | 8.89 ± 1.38 | |
| ∑SFA | Day 1 | 29.83 ± 6.30 | 28.71 ± 5.94 | 29.46 ± 8.69 | 28.83 ± 5.74 |
| Day 21 | 33.20 ± 4.91 | 37.12 ± 8.19 | 30.06 ± 3.89 | 34.66 ± 6.49 | |
| ∑MUFA | Day 1 | 7.04 ± 1.60 | 7.53 ± 2.96 | 6.79 ± 1.51 | 6.86 ± 1.88 |
| Day 21 | 10.14 ± 2.56 | 11.40 ± 3.34 | 7.99 ± 1.51 | 10.61 ± 1.68 | |
| ∑PUFA | Day 1 | 34.81 ± 5.76 | 33.41 ± 7.66 | 34.97 ± 9.55 | 31.10 ± 7.21 |
| Day 21 | 37.26 ± 5.58 | 42.01 ± 12.17 | 35.12 ± 5.02 | 36.03 ± 5.33 | |
| Plasma | |||||
| 16:0 | Day 1 | 59.24 ± 21.96 | 51.79 ± 13.36 | 64.83 ± 19.72 | 55.09 ± 18.09 |
| Day 21 | 69.95 ± 21.73 | 63.71 ± 9.63 | 67.09 ± 12.35 | 60.42 ± 19.53 | |
| 18:1n-9 | Day 1 | 4.63 ± 1.66 | 4.05 ± 1.46 | 5.44 ± 1.82 | 4.15 ± 1.10 |
| Day 21 | 4.97 ± 1.47 | 4.68 ± 1.07 | 5.17 ± 1.35 | 4.21 ± 1.39 | |
| 18:2n-6 | Day 1 | 68.01 ± 14.19 | 62.68 ± 14.00 | 65.15 ± 14.96 | 53.24 ± 10.14 |
| Day 21 | 78.38 ± 19.12 | 76.12 ± 11.56 | 68.69 ± 10.35 | 56.74 ± 8.86 | |
| 20:3n-6 | Day 1 | 4.84 ± 1.84 | 4.33 ± 0.92 | 5.26 ± 1.80 | 4.47 ± 0.97 |
| Day 21 | 5.16 ± 1.90 | 4.79 ± 0.70 | 5.72 ± 1.71 | 4.73 ± 1.11 | |
| 20:4n-6 | Day 1 | 17.36 ± 4.33 | 18.09 ± 5.63 | 20.91 ± 5.81 | 16.77 ± 3.29 |
| Day 21 | 19.32 ± 3.60 | 21.26 ± 4.11 | 22.04 ± 4.41 | 17.99 ± 5.41 | |
| 20:5n-3 | Day 1 | 1.69 ± 1.47 | 2.26 ± 1.42 | 1.46 ± 0.63 | 1.77 ± 0.92 |
| Day 21 | 5.25 ± 1.68 | 5.51 ± 1.09 | 2.48 ± 0.83 | 4.48 ± 1.71 | |
| 22:6n-3 | Day 1 | 4.50 ±1.37 | 4.61 ± 2.11 | 4.81 ± 1.34 | 4.36 ± 1.16 |
| Day 21 | 7.28 ± 2.25 | 7.67 ± 1.38 | 6.11 ± 1.56 | 6.76 ± 2.09 | |
| ∑SFA | Day 1 | 78.57 ± 33.09 | 67.14 ± 18.21 | 84.62 ± 31.26 | 71.82 ± 22.97 |
| Day 21 | 87.99 ± 25.55 | 80.61 ± 13.85 | 80.35 ± 19.22 | 76.62 ± 23.34 | |
| ∑MUFA | Day 1 | 6.19 ± 1.75 | 6.87 ± 3.48 | 6.26 ± 1.75 | 6.13 ± 1.97 |
| Day 21 | 12.53 ± 3.72 | 13.18 ± 2.35 | 8.59 ± 1.88 | 11.24 ± 3.52 | |
| ∑PUFA | Day 1 | 90.21 ± 18.99 | 85.11 ± 19.80 | 91.32 ± 19.96 | 74.48 ± 12.37 |
| Day 21 | 102.86 ± 23.35 | 102.17 ± 15.17 | 96.45 ± 14.38 | 79.47 ± 13.23 | |
Values represent mean ± SD in milligrams per deciliter. AUC of FA in chylomicrons was calculated over 10 h after dose (Intention-to-Treat data set). MAG refers to enriched sn-1(3)-MAG oil.
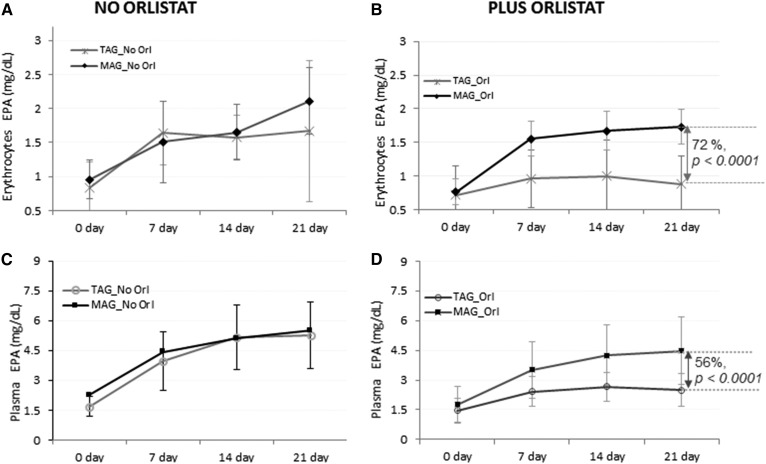
At baseline, the EPA levels in erythrocytes for groups on TAG- or MAG-enriched oil with Orlistat (0.72 and 0.76 mg/dl, respectively) and in groups without Orlistat (0.84 and 0.96 mg/dl) were not significantly different. Incorporation of EPA in erythrocytes at different time points is shown in Fig. 2A, B.
Fig. 2.
Accretion of LC-PUFAs at days 0, 7, 14, and 21. EPA content after MAG-enriched oil and TAG supplementation in erythrocytes (A, B) and plasma (C, D) per treatment group (Intention-to-Treat analysis set). Results are expressed in milligrams per deciliter.
An effect modification was demonstrated at 21 days by Orlistat [Δ = 47%, 95% confidence interval (CI) 10–84%, P = 0.013]. The treatment difference between MAG and TAG at 21 days in the group with Orlistat was significant (Δ = 72%, 95% CI 46–97%, P = <0.0001); the treatment difference between MAG and TAG at 21 days in the group without Orlistat was not significant (Δ = 16%, 95% CI −5 to 38%, P = 0.14). Figure 2A, B shows that the level of EPA in the MAG group was significantly higher over time than in the TAG group when malabsorption conditions were present. Regarding other FAs (Table 4), oleic acid (18:1n9), LA (18:2n6), and total PUFAs also showed a notable increase at day 21, mainly for the groups on MAG-enriched oil with and without Orlistat; however, they increased in proportion to their original MAG and TAG oil composition (Table 2).
A key secondary outcome included the assessment of accumulation of LC-PUFA in plasma.
The EPA level at baseline was not significantly different among groups. Figure 2C, D shows differences in EPA levels between groups fed TAG and MAG oil during the 21 days of the study. Moreover, we could not demonstrate effect modification at 21 days by Orlistat (Δ = 44%, 95% CI −3 to 92%, P = 0.065); the treatment difference of MAG versus TAG at 21 days in the group with Orlistat was significant (Δ = 56%, 95% CI 29–83%, P < 0.0001) where the TAG group showed lower EPA concentrations as early as day 7, compared with the MAG group, which showed increases in EPA concentrations over baseline throughout the time course (Fig. 2C, D). The treatment difference between MAG and TAG at 21 days in the group with no Orlistat was not significant (Δ = 17%, 95% CI −19 to 53%, P = 0.34).
Regarding other FAs (Table 4), as observed with erythrocytes, there were also increases in oleic acid (18:1n9), LA (18:2n6), and total PUFAs observed at day 21, mainly for the groups on MAG-enriched oil with and without Orlistat; however, that would be driven by the difference in original composition of MAG and TAG (Table 2).
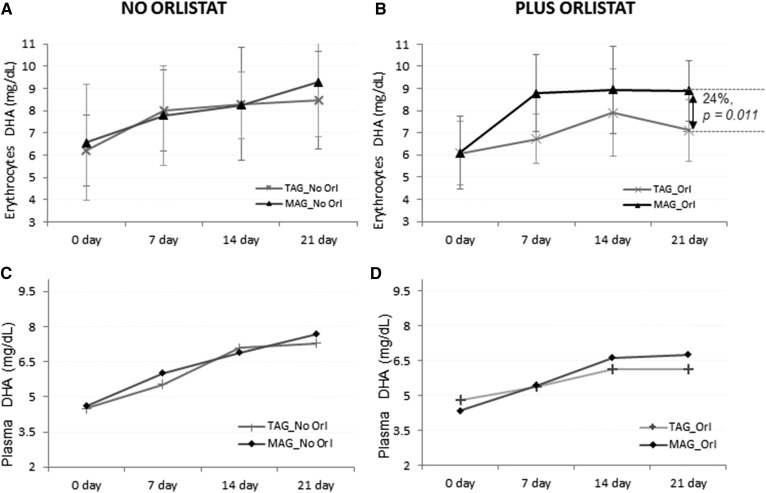
Incorporation of DHA in erythrocytes and plasma
Incorporation of DHA in erythrocytes is shown in Fig. 3A, B. At baseline, groups with no Orlistat showed the same concentration of DHA. Similar concentrations were observed throughout the study with a tendency for DHA content to be higher at day 21 for the MAG-enriched oil group compared with the TAG group.
Fig. 3.
Accretion of LC-PUFAs at days 0, 7, 14, and 21. DHA content after MAG-enriched oil and TAG supplementation in erythrocytes (A, B) and plasma (C, D) per treatment group (Intention-to-Treat analysis set). Results are expressed in milligrams per deciliter.
For groups on Orlistat, a higher concentration of DHA in erythrocytes for the group on MAG was observed throughout the study. The MAG group showed significantly higher concentrations along the trial, including day 21, when compared with the TAG group (Δ = 24%, 95% CI 6–42%, P = 0.011).
The concentration of DHA in plasma lipids was similar between the groups with TAG and MAG with no Orlistat, and both showed an increase through time until the end of the trial at 21 days (Fig. 3C, D). In groups receiving Orlistat, levels of DHA in plasma for TAG and MAG groups were also similar in both groups. The MAG-enriched oil group showed a trend toward higher amounts of DHA after day 7 until day 21, but was not significantly different (P = 0.1).
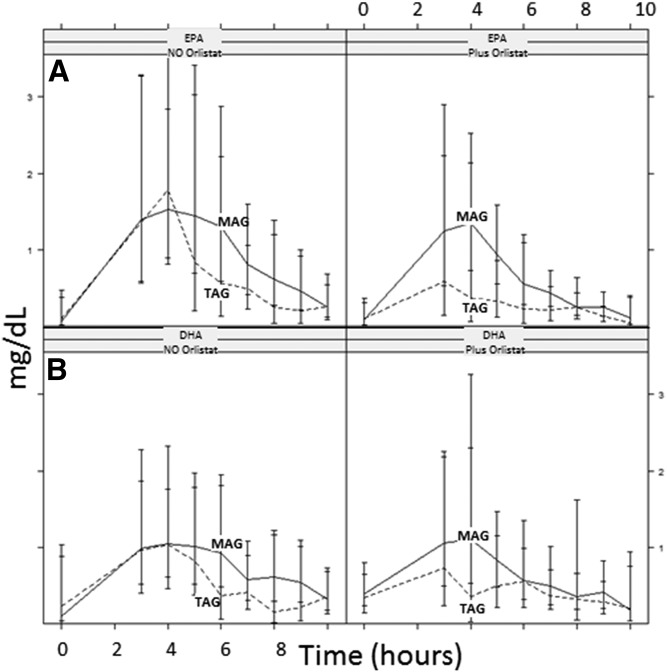
Incorporation of EPA and DHA in chylomicrons: acute phase
Blood samples were taken hourly up to 10 h on the first day and were measured to show how EPA/DHA was incorporated in chylomicrons by MAG intake and how Orlistat was inhibiting this uptake. Kinetic characteristics of interest were AUC, Cmax, and Tmax. Kinetic characteristics were analyzed by ANCOVA correcting for baseline. We could not demonstrate effect modification of MAG by Orlistat (P = 0.56, P = 0.17, and P = 0.69 for AUC, Cmax, and Tmax of EPA, respectively); hence, we pooled the Orlistat and control groups and estimated the MAG versus TAG effect in the pooled group. Intake of MAG was better than that of TAG and resulted in higher AUC (0–10 h) of EPA (0.60 vs. 0.44 AUC in milligrams per deciliter). ANCOVA showed a treatment difference of 55% (95% CI 13–98%, P = 0.012) (Fig. 4A). DHA AUC (Fig. 4B) was also higher (0.62 vs. 0.57 AUC in milligrams per deciliter for the MAG vs. TAG group), with a difference of 41% (95% CI 3–79%, P = 0.035). Cmax demonstrated similar findings to AUC. We were not able to show effects for Tmax. A complete FA profile for chylomicrons was performed and the summary is shown in Table 4. In general, increases for EPA were observed in chylomicrons, but this was less true for DHA, with more obvious and significant differences exhibited when subjects were in the MAG group with and without Orlistat.
Fig. 4.
Acute effect: pharmacokinetic results, EPA (A) and DHA (B) in chylomicrons, AUC over 10 h postprandial. Results are expressed as milligrams per deciliter × hours.
Based on the obtained outcomes, comparable results were observed for acute (chylomicrons) and chronic enrichment (plasma).
DISCUSSION
Due to their long chain-length, digestion/absorption of LC-PUFAs may be not optimal. For that reason, subjects with lipid maldigestion/malabsorption may be at risk of LC-PUFA deficiencies and the exacerbation of inflammatory processes (5–7). Therefore, we studied the efficiency of enriched sn-1(3)-MAG oil on the accretion and delivery of nutritionally important LC-PUFAs (i.e., EPA and DHA) to erythrocytes (26–29). The MAG oil enriched with EPA and DHA was selected, as it would require minimum digestion before crossing the gut barrier and would therefore enhance the bioavailability of LC-PUFAs. FO was used as a reference oil, primarily containing TAG. TAG also happens to make up the bulk of the dietary lipids in routine human consumption.
One of the important aspects of this study was to establish which of the two study groups showed higher LC-PUFA absorption under conditions of induced malabsorption after 21 days. Therefore, it was important that oils were prepared so that the concentrations of EPA and DHA were similar between both treatments. The 500 mg/day EPA and 300 mg/day DHA doses showed a significant increase in erythrocytes throughout the study and both FAs were observed at the highest level at the end of the 21 days of continuous intake when consumed as MAG. We postulate that additional increases may be observed in studies with longer intakes, as we did not observe any plateau in our results. In the absence of induced malabsorption, the subjects fed PUFAs as TAG showed significantly lower increases.
In order to create conditions resembling malabsorption, we also had two groups of subjects consuming Orlistat, a human pancreatic and gastric lipase inhibitor. Early studies demonstrated that Orlistat produced a dose-dependent reduction in dietary fat absorption, which is near maximum, approximately 30% at a dose of 120 mg three times daily (16, 22, 30). Our results clearly indicate that the MAG oil is superior to TAG oil in elevating circulating levels of the LC-PUFAs in these groups of induced malabsorption. These results suggest that the MAG-enriched oil absorption is possible even when Orlistat significantly reduces lipolysis and enables improved EPA and DHA accretion in erythrocytes.
Owing to the preference of digestive enzymes to hydrolyze sn-1(3) positions of a TAG molecule to two FAs and a sn-2-MAG taken up by enterocytes via micelles, it has been assumed that FAs on the sn-2 position will also be taken up efficiently (31–33). However, the role of sn-1(3)-MAG in luminal digestion and uptake is not very clear. The few studies that did report any data failed to characterize the material fed to the subjects, giving us no data on the stereospecific position of the LC-PUFAs on the glycerol molecule, which provides addition challenges toward drawing any conclusions on the influence of sn-2- versus sn-1(3)-MAG positions (31, 34, 35). In this study, we have shown for the first time that the sn-1(3)-MAG is a better carrier than TAG counterparts that contain bioactive PUFAs in humans with induced malabsorption.
In the absence of Orlistat, we can safely assume that digestive enzymes hydrolyze the FAs to yield glycerol, which itself may contribute to better absorption of FAs by two possible channels. First, in the intestinal lumen, it would enhance the contribution to micelles due to its amphiphilic chemistry. Second, after uptake into the enterocyte, it lends all three positions for reesterification of the FAs that are also taken up and secreted into circulation via chylomicrons. However, we observed that MAG-enriched oil also imparted better absorption of LC-PUFAs in the presence of Orlistat, indicating that enzymatic hydrolysis may not be an essential step to efficient absorption of LC-PUFAs. Indeed, our earlier work in rats also showed better absorption and accretion of LC-PUFAs when delivered via MAG-enriched oil (22). We think that further in vitro work might shed more light into the mechanistic aspects of enhanced uptake of LC-PUFAs from MAG versus TAG and whether the step of enzymatic hydrolysis is essential or sn-1(3)-MAG is taken up unaltered. Indeed more work can be done to measure what the statistically significant differences observed in this small study may have on biological implications in real life.
In the present study with induced maldigestion/malabsorption subjects, higher levels of EPA and DHA were found in erythrocytes, which confirmed our previous hypothesis that the enriched sn-1(3)-MAG oil has benefits over traditional TAG-based oils to deliver essential LC-PUFAs.
CONCLUSION
In conclusion, oral supplementation with sn-1(3)-MAG resulted in superior incorporation of n-3 LC-PUFAs into the erythrocyte membrane and improved bioavailability compared with TAG, thus showing the importance of the regiospecific structure of the sn-1(3)-MAG oil for optimal absorption. Our results strongly suggest that sn-1(3)-MAG-enriched oil does not need to be digested prior to absorption. Nutrition therapy with sn-1(3)-MAG-EPA and/or sn-1(3)-MAG-DHA can be considered to guarantee the absorption of necessary amounts of beneficial FAs when used as a treatment for subjects on pharmacologically induced enzyme insufficiency (e.g., apparently healthy obese subjects) causing maldigestion and malabsorption conditions.
While our results cannot be generalized, it is suggested that they may also be applicable to malabsorption conditions with underlying causes other than pancreatic lipase inhibition with Orlistat. Further clinical research is needed to confirm whether sn-1(3)-MAG-EPA and sn-1(3)-MAG-DHA can promote the accretion of LC-PUFAs in humans with disease conditions comprising low lipid digestion due to lipase activity insufficiency. Potential applications of this concept are relevant to an array of disease conditions comprising low lipid digestion due to lipase activity insufficiency (e.g., chronic pancreatitis, Shwachman-Diamond syndrome, diabetes, cystic fibrosis). Other maldigestion/malabsorption conditions that are linked to issues other than lipase insufficiency, such as low bile acid secretion or injury of the gut mucosa, would also require clinical studies to evaluate whether sn-1(3)-MAG-EPA can be successfully absorbed.
Footnotes
Abbreviations:
- ALA
- α-linolenic acid
- AUC
- area under the curve
- CI
- confidence interval
- EFA
- essential FA
- FAME
- fatty acid methyl ester
- FO
- fish oil
- GI
- gastrointestinal
- HDL-C
- HDL cholesterol
- LA
- linoleic acid
- LC-PUFA
- long-chain PUFA
- LDL-C
- LDL cholesterol
- MAG
- monoacylglycerol
- TAG
- triacylglycerol
This work was supported by grants from Nestec (S.d.G., V.G.) and Centre Hospitalier Universitaire Vaudois (S.d.G., V.G., L.T.). C.C-H., FD., S.K.T., L.G., E.W., D.G., C.R., and F.G. report personal fees from Nestec.
REFERENCES
- 1.Amate L., Ramírez M., and Gil A.. 1999. Positional analysis of triglycerides and phospholipids rich in long- chain polyunsaturated fatty acids. Lipids. 34: 865–871. [DOI] [PubMed] [Google Scholar]
- 2.Hayes K. C. 2001. Synthetic and modified glycerides: effects on plasma lipids. Curr. Opin. Lipidol. 12: 55–60. [DOI] [PubMed] [Google Scholar]
- 3.Arterburn L. M., Hall E. B., and Oken H.. 2006. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 83: 1467S–1476S. [DOI] [PubMed] [Google Scholar]
- 4.Calder P. C. 2012. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 56: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 5.Fan C., Zirpoli H., and Qi K.. 2013. n-3 fatty acids modulate adipose tissue inflammation and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care. 16: 124–132. [DOI] [PubMed] [Google Scholar]
- 6.Itariu B. K., Zeyda M., Hochbrugger E. E., Neuhofer A., Prager G., Schindler K., Bohdjalian A., Mascher D., Vangala S., Schranz M., et al. 2012. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am. J. Clin. Nutr. 96: 1137–1149. [DOI] [PubMed] [Google Scholar]
- 7.Anderson B. M., and Ma D. W.. 2009. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 8: 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeppesen P. B., Høy C., and Mortensen P. B.. 1999. Differences in essential fatty acid requirements by enteral and parenteral routes of administration in patients with fat malabsorption. Am. J. Clin. Nutr. 70: 78–84. [DOI] [PubMed] [Google Scholar]
- 9.Siener R., Alteheld B., Terjung B., Junghans B., Bitterlich N., Stehle P., and Metzner C.. 2010. Change in the fatty acid pattern of erythrocyte membrane phospholipids after oral supplementation of specific fatty acids in patients with gastrointestinal diseases. Eur. J. Clin. Nutr. 64: 410–418. [DOI] [PubMed] [Google Scholar]
- 10.Stallings V. A., Mondick J. T., Schall J. I., Barrett J. S., Wilson M., and Mascarenhas M. R.. 2013. Diagnosing malabsorption with systemic lipid profiling: pharmacokinetics of pentadecanoic acid and triheptadecanoic acid following oral administration in healthy subjects and subjects with cystic fibrosis. Int. J. Clin. Pharmacol. Ther. 51: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calder P. C. 2010. Rationale and use of n-3 fatty acids in artificial nutrition. Proc. Nutr. Soc. 69: 565–573. [DOI] [PubMed] [Google Scholar]
- 12.Buchwald H., Avidor Y., Braunwald E., Jensen M. D., Pories W., Fahrbach K., and Schoelles K.. 2004. Bariatric surgery: A systematic review and meta-analysis. JAMA. 292: 1724–1737. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R., Lieske J. C., Collazo-Clavell M. L., Sarr M. G., Olson E. R., Vrtiska T. J., Bergstralh E. J., and Li X.. 2011. Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery. 149: 654–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez-Leite J. I. 2004. Nutrient deficiencies secondary to bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care. 7: 569–575. [DOI] [PubMed] [Google Scholar]
- 15.Drew B. S., Dixon A. F., and Dixon J. B.. 2007. Obesity management: update on orlistat. Vasc. Health Risk Manag. 3: 817–821. [PMC free article] [PubMed] [Google Scholar]
- 16.Carrière F., Renou C., Ransac S., Lopez V., De Caro J., Ferrato F., De Caro A., Fleury A., Sanwald-Ducray P., Lengsfeld H., et al. 2001. Inhibition of gastrointestinal lipolysis by Orlistat during digestion of test meals in healthy volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 281: G16–G28. [DOI] [PubMed] [Google Scholar]
- 17.Murphy J. L., Badaloo A. V., Chambers B., Forrester T. E., Wootton S. A., and Jackson A. A.. 2002. Maldigestion and malabsorption of dietary lipid during severe childhood malnutrition. Arch. Dis. Child. 87: 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mu H., and Høy C. E.. 2004. The digestion of dietary triacylglycerols. Prog. Lipid Res. 43: 105–133. [DOI] [PubMed] [Google Scholar]
- 19.Christensen M. S., Høy C. E., Becker C. C., and Redgrave T. G.. 1995. Intestinal absorption and lymphatic transport of eicosapentaenoic (EPA), docosahexaenoic (DHA), and decanoic acids: dependence on intramolecular triacylglycerol structure. Am. J. Clin. Nutr. 61: 56–61. [DOI] [PubMed] [Google Scholar]
- 20.Innis S. M., and Dyer R.. 1997. Dietary triacylglycerols with palmitic acid (16:0) in the 2-position increase 16:0 in the 2-position of plasma and chylomicron triacylglycerols, but reduce phospholipid arachidonic and docosahexaenoic acids, and alter cholesteryl ester metabolism in formula-Fed piglets. J. Nutr. 127: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 21.Valenzuela A., Valenzuela V., Sanhueza J., and Nieto S.. 2005. Effect of supplementation with docosahexaenoic acid ethyl ester and sn-2 docosahexaenyl monoacylglyceride on plasma and erythrocyte fatty acids in rats. Ann. Nutr. Metab. 49: 49–53. [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Hernandez C., Thakkar S. K., Moulin J., Oliveira M., Masserey-Elmelegy I., Dionisi F., and Destaillats F.. 2012. Benefits of structured and free monoacylglycerols to deliver eicosapentaenoic (EPA) in a model of lipid malabsorption. Nutrients. 4: 1781–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Hernandez C., Goeuriot S., Giuffrida F., Thakkar S. K., and Destaillats F.. 2013. Direct quantification of fatty acids in human milk by gas chromatography. J. Chromatogr. A. 1284: 174–179. [DOI] [PubMed] [Google Scholar]
- 24.Wilcox H. G., Davis D. C., and Heimberg M.. 1971. The isolation of lipoproteins from human plasma by ultracentrifugation in zonal rotors. J. Lipid Res. 12: 160–172. [PubMed] [Google Scholar]
- 25.Destaillats F., and Cruz-Hernandez C.. 2007. Fast analysis by gas–liquid chromatography Perspective on the resolution of complex fatty acid compositions. J. Chromatogr. A. 1169: 175–178. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q., Ma J., Campos H., Hankinson S. E., and Hu F. B.. 2007. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 86: 74–81. [DOI] [PubMed] [Google Scholar]
- 27.Barceló-Coblijn G., Murphy E. J., Othman R., Moghadasian M. H., Kashour T., and Friel J. K.. 2008. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am. J. Clin. Nutr. 88: 801–809. [DOI] [PubMed] [Google Scholar]
- 28.Simoens C. M., Deckelbaum R. J., Massaut J. J., and Carpentier Y. A.. 2008. Inclusion of 10% fish oil in mixed medium-chain triacylglycerol-long-chain triacylglycerol emulsions increases plasma triacylglycerol clearance and induces rapid eicosapentaenoic acid (20:5n-3) incorporation into blood cell phospholipids. Am. J. Clin. Nutr. 88: 282–288. [DOI] [PubMed] [Google Scholar]
- 29.Mori T. A., Burke V., Puddey I. B., Watts G. F., O’Neal D. N., Best J. D., and Beilin L. J.. 2000. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 71: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 30.Anderson J. W. 2007. Orlistat for the management of overweight individuals and obesity: a review of potential for the 60 mg, over-the counter dosage. Expert Opin. Pharmacother. 8: 1733–1742. [DOI] [PubMed] [Google Scholar]
- 31.Sadou H., Léger C. L., Descomps B., Barjon J. N., Monnier L., and Crastes de Paulet A.. 1995. Differential incorporation of fish-oil eicosapentaenoate and docosahexaenoate into lipids of lipoprotein fractions as related to their glyceryl esterification: a short-term (postprandial) and long-term study in healthy humans. Am. J. Clin. Nutr. 62: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 32.Banno F., Doisaki S., Shimizu N., and Fujimoto K.. 2002. Lymphatic absorption of docosahexaenoic acid given as monoglyceride, diglyceride, triglyceride, and ethyl ester in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 48: 30–35. [DOI] [PubMed] [Google Scholar]
- 33.Straarup E. M., and Hoy C.. 2001. Lymphatic transport of fat in rats with normal- and malabsorption following intake of fats made from fish oil and decanoic acid. Effects of triacylglycerol structure. Nutr. Res. 21: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 34.Wakil A., Mir M., Mellor D. D., Mellor S. F., and Atkin S. L.. 2010. The bioavailability of eicosapentaenoic acid from reconstituted triglyceride fish oil is higher than that obtained from the triglyceride and monoglyceride forms. Asia Pac. J. Clin. Nutr. 19: 499–505. [PubMed] [Google Scholar]
- 35.Yli-Jokipii K., Kallio H., Schwab U., Mykkänen H., Kurvinen J. P., Savolainen M. J., and Tahvonen R.. 2001. Effects of palm oil and transesterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response. J. Lipid Res. 42: 1618–1625. [PubMed] [Google Scholar]