Abstract
Multisite phosphorylation regulates many transcription factors, including the Serum Response Factor partner Elk-1. Phosphorylation of the transcriptional activation domain (TAD) of Elk-1 by the protein kinase ERK at multiple sites potentiates recruitment of the Mediator transcriptional coactivator complex and transcriptional activation, but the roles of individual phosphorylation events remained unclear. Using time-resolved nuclear magnetic resonance spectroscopy, we found that ERK2 phosphorylation proceeds at markedly different rates at eight TAD sites in vitro, which we classified as fast, intermediate and slow. Mutagenesis experiments showed that phosphorylation of fast and intermediate sites promoted Mediator interaction and transcriptional activation, whereas modification of slow sites counteracted both functions, thereby limiting Elk-1 output. Progressive Elk-1 phosphorylation thus ensures a self-limiting response to ERK activation, which occurs independently of antagonizing phosphatase activity.
Results and Discussion
Multisite protein phosphorylation increases the complexity of functional signaling outputs that can be generated from single protein kinase inputs. It can set thresholds for activity, or transform graded signals into switch-like responses (1–4). Many transcription factors and their interacting regulatory proteins are subject to multisite phosphorylation, which allows distinct aspects of protein function, including protein turnover, nuclear import and export, and specific protein interactions, to be controlled independently (5). However, in general, the dynamics and functional roles of individual phosphorylation events are incompletely understood.
The ternary complex factor (TCF) subfamily of Ets-domain transcription factors, consisting of Elk-1, SAP-1, and Net, provides an example of multisite phosphorylation in transcriptional activation. TCFs, together with their partner protein SRF, function in many biological processes by coupling SRF target genes to mitogen-activated protein kinase (MAP kinase) signaling (5). Mitogenic and stress stimuli induce phosphorylation of TCF C-terminal transcriptional activation domains (TADs) at multiple S/T-P (Ser- or Thr-Pro) phosphorylation sequences, of which eight are conserved across the family (Fig. 1A; fig. S1) (6–11). Two MAP kinase-docking sites, the D-box and the Phe-Gln-Phe-Pro (FQFP) motif, control phosphorylation of these sites (12–15). Multisite phosphorylation triggers transcriptional activation by TCFs, facilitating their interaction with the Mediator transcriptional co-activator complex (16–19) but the kinetics with which the different sites are phosphorylated, and whether they serve distinct functions, remain unclear.
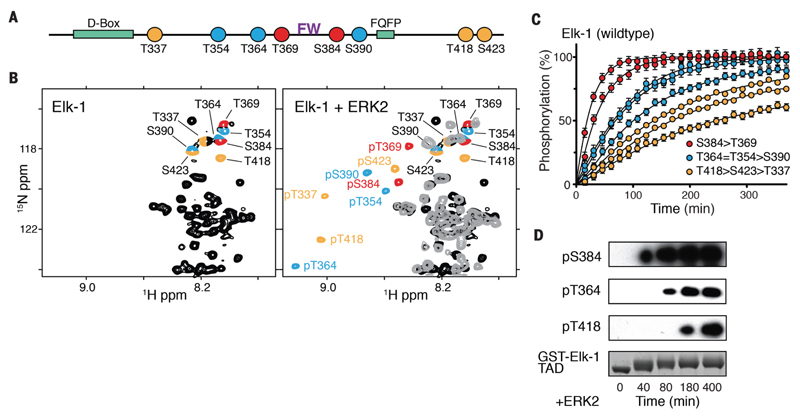
Figure 1. Multisite phosphorylation of Elk-1 TAD.
(A) Linear outline of Elk-1 fast (red), intermediate (blue), and slow (yellow) S/T-P phosphorylation sites. Kinase docking motifs are shown in green; the FW residues (purple) are essential for Mediator association. (B) NMR analysis of Elk-1 TAD (amino acids 309 to 429) phosphorylation with recombinant ERK2. Left: 2D 1H-15N NMR spectrum of unphosphorylated Elk-1 (black), with phosphorylation-site signals color-coded as in (A). Right: overlay of 2D NMR spectra of phosphorylated [grey & color-coded as in (A)] and unmodified Elk-1 (black). (C) Time-resolved modification curves of individual Elk-1 sites upon phosphorylation with ERK2; error bars denote differences between replicate experiments on two independent samples. (D) Time-course Western blot of GST-Elk-1 TAD vphosphorylation and phosphorlyation-site-specific antibody detection.
To obtain atomic-resolution insights into phosphorylation of the Elk-1 TAD, we used nuclear magnetic resonance (NMR) spectroscopy (20) to monitor its modification by recombinant ERK2 in vitro (Fig. 1B; fig. S2A). Time-resolved NMR experiments revealed that each phosphorylation proceeded efficiently, but at markedly different rates. Phosphorylation of Thr369 and Ser384, which flank the central Phe–Trp (FW) motif implicated in Mediator interaction (18), occurred faster than modification of Thr364, Thr354 and Ser390, whereas residues Thr418, Ser423 and Thr337 were modified more slowly (Fig. 1C), which we confirmed by immunoblotting (Fig. 1D). Chemical shift analysis (Cα, Cβ) showed no stable secondary structure elements in unmodified or phosphorylated Elk-1 TAD (fig. S2B).
As a first step towards understanding the basis for the phosphorylation sites' differential kinetic behavior, we devised a reaction model based on Michaelis-Menten enzyme kinetics. To simplify the mathematical treatment, we grouped Elk-1 sites into three classes: fast (Thr369 and Ser384), intermediate (Thr354, Thr364, and Ser390) and slow (Thr337, Thr418, and Ser423). We assumed that ERK2 phosphorylation is distributive, that enzymatic rate constants (kcat) are similar for all sites, and that the different sites have relative affinities for ERK2 modeled by increasing Michaelis-Menten constants (KMFast < KMInt < KMSlow)(Fig. 2A, fig. S3A, 20). This model, which recapitulated the measured kinetics of in vitro Elk-1 phosphorylation well (Fig. 2B) predicted that removal of fast or intermediate sites should increase the phosphorylation rates of other sites. To test this idea, we analyzed the phosphorylation kinetics of Elk-1 TAD mutants in which we substituted all fast or intermediate phosphoacceptor residues with alanines (Elk-1F: Thr369➔Ala, Ser384➔Ala; Elk-1I: Thr354➔Ala, Thr364➔Ala, and Ser390➔Ala)(Fig. 2A). In the fast site mutant Elk-1F, phosphorylation rates of intermediate and slow sites increased, whereas those of the fast and slow sites increased in the intermediate-site mutant Elk-1I; in both cases the altered kinetics fit well with those predicted by the model (Fig 2B, fig. S3B). Thus, even the fast sites are not phosphorylated at the maximum possible rate in the wildtype protein. Moreover, phosphorylation of an Elk-1 TAD mutant in which Thr369 and Ser384 were replaced with aspartates was similar to Elk-1F, excluding the possibility that fast-site phosphorylation primes later modification events (fig. S3B).
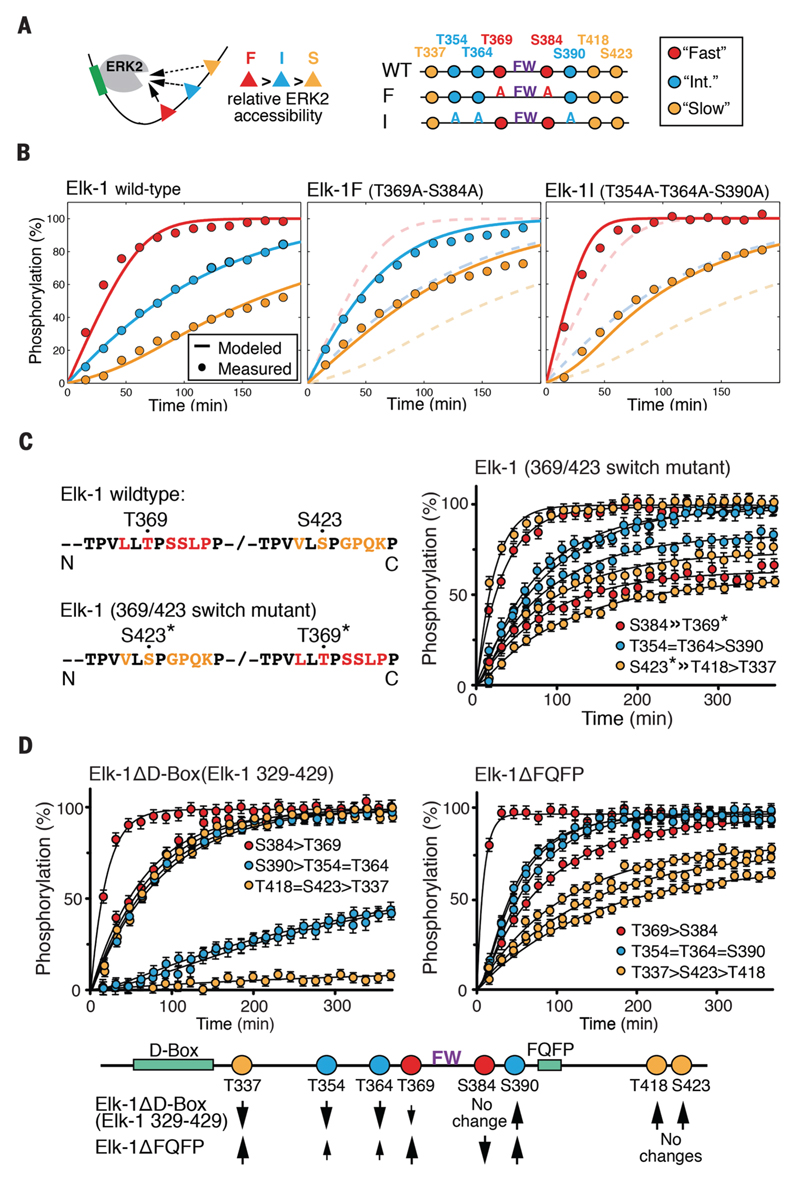
Figure 2. Phosphorylation kinetics of Elk-1 TAD mutants.
(A) Outline of analyzed Elk-1 TAD mutants. Substrate sites were classified as fast (red), intermediate (blue) or slow (yellow) for model calculations. (B) Left: comparison of averaged measured data points of wild-type Elk-1 TAD fast-, intermediate- and slow-site phosphorylation by ERK2 (circles) with calculated rates according to the competitive inhibition model (solid lines). Center: fast-site alanine-substituted Elk-1 TAD (Elk-1F); dashed lines show wildtype Elk-1 TAD for comparison. Right: intermediate-site alanine-substituted Elk-1 TAD (Elk-1I); dashed lines show wildtype Elk-1 TAD for comparison. (C) Time-resolved modification curves (right) of the Elk-1 369/423 switch mutant (left). Error bars denote differences between replicate experiments on two independent samples. (D) Time-resolved modification curves of ERK docking-site mutants Elk-1ΔD-box (left) and Elk-1ΔFQFP (right), presented as in (C). Effects of D-box and FQFP site deletions on Elk-1 TAD phosphorylation rates are summarised below.
To gain more insight into the factors affecting individual sites' phosphorylation kinetics, we assessed the role played by primary sequence. To do this, we exchanged the sequences surrounding the fast Thr369 and slow Ser423 sites. This also effectively exchanged their reactivities, which suggests that these sites' phosphorylation rates reflect their position relative to ERK docking sequences, rather than intrinsic differences in reactivity (Fig. 2C). We therefore examined the contributions of the D-box and FQFP ERK docking motifs to each site's phosphorylation kinetics. Deletion of the D-box decreased the rates of Thr337, Thr354, Thr364 and Thr369 phosphorylation, but increased the rates of Ser390, Thr418, and Ser423 modification (Fig. 2D). In contrast, deletion of the FQFP motif decreased the rate of Ser384 phosphorylation, but enhanced modification of intermediate sites, including adjacent Ser390, with no effect on the C-terminal sites (Fig. 2D). Thus, the ERK docking motifs differentially affect each phosphorylation site's competitive behavior. Previous studies showed that Elk-1 TAD phosphorylation by JNK and p38 MAP kinase differs from phosphorylation by ERK (10, 21–24), and that this reflects differences in their docking interactions (12, 14, 15, 25). Indeed, these kinases exhibited site preferences and phosphorylation rates that were distinct from that of ERK2 (fig. S3C). Taken together, our results show that the different rates of Elk-1 TAD phosphorylation by ERK2 follow a competition mechanism that is governed by the position of individual Elk-1 substrate sites relative to ERK2 docking interactions.
To test whether the different kinetic classes of Elk-1 TAD phosphorylation sites are functionally equivalent, we expressed mouse Elk-1 mutants in fibroblasts derived from TCF-deficient (Elk1-/-;Elk3δ/δ;Elk4-/-) triple-knockout mouse embryos (TKO MEFs; fig. S4, A-C). In these cells, immediate-early (IE) gene expression is defective, but expression of wild-type mouse Elk-1 restored the IE transcriptional activation seen in wild-type MEFs after activation of ERK by treatment with TPA (12-O-tetradecanoylphorbol-13-acetate) (fig. S4D). As expected, alanine substitutions of fast and/or intermediate sites, or of the FW motif, greatly diminished or abolished the ability of Elk-1 to activate TCF-SRF target gene transcription after TPA stimulation (Fig. 3A; fig. S4D). Surprisingly, however, mutation of the slow sites substantially enhanced Elk-1-mediated activation of TCF-SRF target genes (Fig. 3A). Alanine substitutions at individual slow sites also increased Elk-1 activity, with Thr418 exhibiting the greatest effect (Fig. 3B, fig. S4, E-G). TCF-SRF signaling is important for cellular proliferation (26, 27), and TKO MEFs proliferate more slowly than wildtype MEFs. The reconstituted TKO MEFs exhibited enhanced proliferation rates, which correlated with the ability of each mutant to promote transcriptional activation (Fig. 3C).
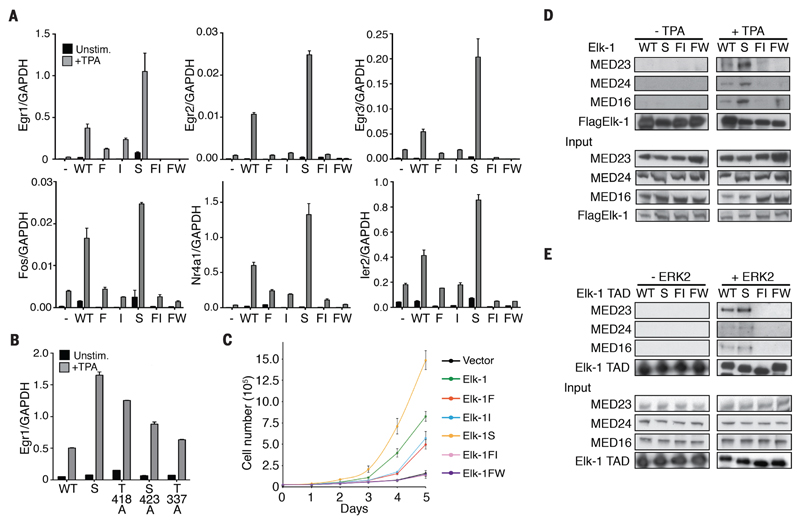
Figure 3. Effects of Elk-1 TAD mutations on TCF target gene expression, cell proliferation and Mediator binding.
(A) Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis of TCF target gene transcription in reconstituted TKO MEFs. Cells were reconstituted with wild-type mouse Elk-1 (WT) or fast-site (F), intermediate-site (I), slow-site (S), fast- and intermediate-site (FI) or alanine-substituted FW motif (FW) mutants. (B) Effects of individual slow-site alanine substitutions on Egr1 expression. In (A) and (B) cells were stimulated with 50 ng/ml TPA where indicated. RNA levels are quantified relative to GAPDH; data are means ± SEM, n = 3. (C) Proliferation of wild-type and mutant Elk-1 TKO MEFs. Data are means ± SEM, n = 3. (D) Co-immunoprecipitation of Mediator with wildtype or mutant Flag-tagged Elk-1 from NIH3T3 cell extracts. Antibodies to Mediator subunits MED23, MED24 and MED16 were used for immunoblotting. (E) Mediator co-precipitation from unstimulated NIH3T3 cell extracts using wild-type and mutant GST-tagged Elk-1 TAD proteins with and without prior ERK2 phosphorylation.
Phosphorylation of Elk-1 promotes transcriptional activation by facilitating its MED23-dependent interaction with the Mediator complex (16–18). We therefore investigated whether the different transcriptional activities of the Elk-1 mutants reflected alterations in Mediator binding. We prepared extracts of TKO cells expressing wildtype or mutant Elk-1 proteins and assessed Elk-1 assocation with Mediator by co-immunoprecipitation of the MED23, MED24 and MED16 subunits. Consistent with the transcription experiments, Elk-1–Mediator interaction was induced by TPA stimulation and dependent on the FW motif; it was abolished by alanine substitutions of fast and intermediate sites, and was increased in the slow-site Elk-1 mutant (Fig. 3D). We obtained similar results when we used glutathione S-transferase (GST)-Elk-1 TAD proteins to recover Mediator proteins from unstimulated NIH3T3 cell extracts (Fig. 3E). In this assay, ERK2 phosphorylation time-course experiments showed that Mediator recovery by the wildtype Elk-1 TAD was most efficient prior to modifications of the slow sites (fig. S5, A and B). Taken together, these data show that according to the sites involved, ERK2 phosphorylation promotes or inhibits transcriptional activation by Elk-1, which reflects alterations in Elk-1–Mediator interactions.
Next, we investigated Elk-1 TAD phosphorylation kinetics in vivo. Previous studies were unable to distinguish the progressive phosphorylation of fast and slow Elk-1 sites (6). However, by incubating cells at 25° C to slow down reactions, we confirmed that phosphorylation rates can be ranked in the order Ser384>Thr364>Thr418, and that different site classes exhibited a similar competitive behavior, as seen in vitro (fig. S6A). Reasoning that phosphorylation of the Elk-1 TAD might be sensitive to kinetic effects at limiting signal strengths, we titrated ERK activity using increasing amounts of TPA. This both increased the maximal extent of ERK activation and advanced the time at which it occurred (fig. S6B). At low TPA concentrations, Elk-1 fast-site (Thr384) and slow-site (Thr418)-site modifications accumulated slowly over 1 hour, whereas at a saturating TPA dose they were maximal by 10 minutes. Both phosphorylations declined at late times, presumably owing to the action of Elk-1 phosphatases (Fig. 4A)(6, 28).
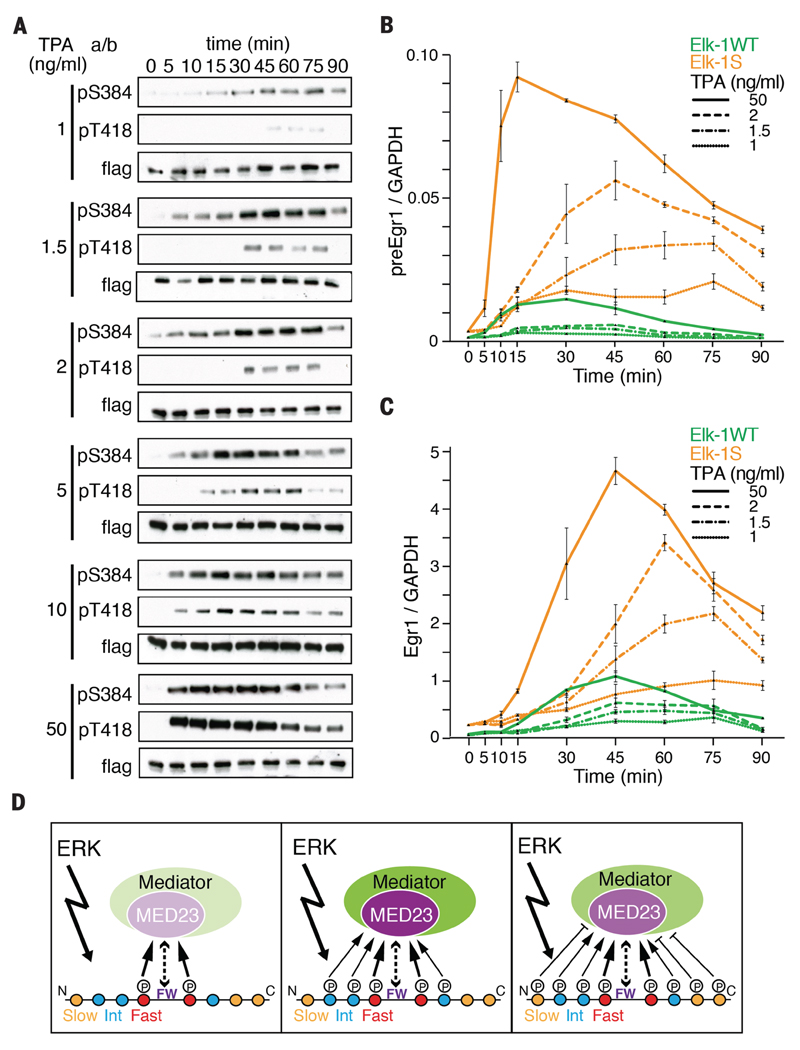
Figure 4. Multisite phosphorylation of Elk-1 shapes the transcriptional response to ERK activation.
(A) Kinetics of Elk-1 fast- and slow-site phosphorylation in cells treated with increasing concentrations of TPA. (B) Transcription rate of the TCF-SRF target gene Egr1 in TKO MEFs expressing wildtype Elk-1 or mutant Elk-1S. Precursor RNA was monitored by qRT-PCR after stimulation with different concentrations of TPA. Data are mean ± SEM; n=3. (C) Kinetics of Egr1 mRNA accumulation in cells as in (B) monitored by qRT-PCR (D) Progressive Elk-1 phosphorylation by ERK has both activating (left and center) and inhibitory (right) effects on Mediator recruitment, as suggested by shading densities. A strong signal will rapidly reach the attenuated state shown at the right, a weak signal may reach this state only if sustained.
Having established that Elk-1 phosphorylation kinetics are tuned by signal strength, we investigated their relationship to transcriptional activation. We compared the ability of wildtype Elk-1 and the slow-site mutant Elk-1S to activate transcription in response to signals of differing strengths. At saturating TPA concentrations, both proteins activated Egr1 transcription with similar transient kinetics, although Elk-1S was much more active, reflecting the loss of the inhibitory sites (Fig. 4, B and C). At limiting TPA doses, however, their behaviors were markedly different. Whereas the activity of wildtype Elk-1 was almost maximal by 15 minutes, that of Elk-1S increased substantially beyond this time (Fig. 4B), resulting in prolonged Egr1 mRNA accumulation (Fig. 4C). Thus, progressive phosphorylation of the Elk-1 TAD by a single kinase, ERK, attenuates the transcriptional response of Elk-1, shaping it according to the strength and kinetics of ERK activation.
Our results show that phosphorylation of the Elk-1 TAD by a single kinase, ERK, can either promote or inhibit Mediator interaction, depending on the sites involved, thereby modulating transcriptional activation. Given that the TAD sequences are conserved in the other TCFs, our findings may also apply to them. The more rapidly phosphorylated sites are located in the substantially conserved central core of the TAD, and are essential for transcriptional activation, lying close to the FW hydrophobic motif required for Elk-1–Mediator interaction (10, 18). Multisite phosphorylation of these residues might stabilize this interaction, and perhaps also set a signaling threshold for it, similar to the way that multisite phosphorylation sets a threshold for the Sic1–Cdc4 interaction (29). In contrast, slowly phosphorylated sites located N- and C-terminal of the conserved TAD core act negatively. Their phosphorylation inhibits Mediator recruitment and limits transcriptional activation (Fig 4D), and may also facilitate recruitment of negative regulators of Elk-1 activity. Together these properties ensure that ERK phosphorylation of the Elk-1 TAD is self-limiting, whereby phosphorylation of slow sites attenuates TCF-SRF target gene expression under conditions of strong or sustained ERK signaling (Fig. 4D). Our results challenge the common assumption that multisite modification events act unidirectionally and can only be reversed or limited by antagonistic enzymes. Given the prevalence of such events in different biological processes, we expect that similar mechanisms may govern other regulatory interactions.
Supplementary Materials
Acknowledgements
Work in the Treisman group was supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001-190), the UK Medical Research Council (FC001-190) and the Wellcome Trust (FC001-190); and by European Research Council (ERC) advanced grant 268690 ACTINonSRF. Work in the Selenko group is funded by the ERC consolidator grant #647474 NeuroInCellNMR. F.-X.T. is supported by Agence Nationale pour la Recherche grant ANR14-ACHN-0015-01. The authors have no conflicts of interest. AM conceived the project; A.M., C.F., and F.M. designed and performed molecular and cell biology experiments; F.X.-T., T.C. and P.B. developed the competitive inhibition model; F.-X.T. and P.S. designed, executed and interpreted the NMR experiments, and A.M., P.S. and R.T. designed and interpreted experiments and wrote the paper. We thank F. Gualdrini, C. Esnault and P. Costello for characterising gene regulation in reconstituted TKO MEFs, sharing their unpublished data, and generating reconstituted TKO MEF cells; the Crick Genomics and Flow Cytometry technology platforms for technical support; and A. Behrens, V. Calleja, A. Chakraborty, M. Diefenbacher, C. Duellberg, R. Nicolas, P. Riou, M. Skehel, and our group members for advice and discussions.
References
- 1.Gunawardena J. Multisite protein phosphorylation makes a good threshold but can be a poor switch. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14617–14622. doi: 10.1073/pnas.0507322102. published online EpubOct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunawardena J. Distributivity and processivity in multisite phosphorylation can be distinguished through steady-state invariants. Biophysical journal. 2007;93:3828–3834. doi: 10.1529/biophysj.107.110866. published online EpubDec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salazar C, Hofer T. Multisite protein phosphorylation--from molecular mechanisms to kinetic models. The FEBS journal. 2009;276:3177–3198. doi: 10.1111/j.1742-4658.2009.07027.x. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Bardwell L, Nie Q. A combination of multisite phosphorylation and substrate sequestration produces switchlike responses. Biophysical journal. 2010;98:1396–1407. doi: 10.1016/j.bpj.2009.12.4307. published online EpubApr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Current biology : CB. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 6.Cruzalegui FH, Cano E, Treisman R. ERK activation induces phosphorylation of Elk-1 at multiple S/T-P motifs to high stoichiometry. Oncogene. 1999;18:7948–7957. doi: 10.1038/sj.onc.1203362. published online EpubDec 23. [DOI] [PubMed] [Google Scholar]
- 7.Janknecht R, Ernst WH, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. The EMBO journal. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 9.Janknecht R, Ernst WH, Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995;10:1209–1216. [PubMed] [Google Scholar]
- 10.Price MA, Rogers AE, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) The EMBO journal. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. The EMBO journal. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantz DA, Jacobs D, Glossip D, Kornfeld K. Docking sites on substrate proteins direct extracellular signal-regulated kinase to phosphorylate specific residues. The Journal of biological chemistry. 2001;276:27256–27265. doi: 10.1074/jbc.M102512200. published online EpubJul 20. [DOI] [PubMed] [Google Scholar]
- 13.Yang SH, Yates PR, Whitmarsh AJ, Davis RJ, Sharrocks AD. The Elk-1 ETS-domain transcription factor contains a mitogen-activated protein kinase targeting motif. Molecular and cellular biology. 1998;18:710–720. doi: 10.1128/mcb.18.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SH, Whitmarsh AJ, Davis RJ, Sharrocks AD. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. The EMBO journal. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. published online EpubMar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs D, Glossip D, Xing H, Muslin AJ, Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes & development. 1999;13:163–175. [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens JL, Cantin GT, Wang G, Shevchenko A, Shevchenko A, Berk AJ. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science. 2002;296:755–758. doi: 10.1126/science.1068943. published online EpubApr 26. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Balamotis MA, Stevens JL, Yamaguchi Y, Handa H, Berk AJ. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Molecular cell. 2005;17:683–694. doi: 10.1016/j.molcel.2005.02.010. published online EpubMar 4. [DOI] [PubMed] [Google Scholar]
- 18.Balamotis MA, Pennella MA, Stevens JL, Wasylyk B, Belmont AS, Berk AJ. Complexity in transcription control at the activation domain-mediator interface. Science signaling. 2009;2:ra20. doi: 10.1126/scisignal.1164302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Huang L, Huang Y, Yin JW, Berk AJ, Friedman JM, Wang G. Mediator MED23 links insulin signaling to the adipogenesis transcription cascade. Developmental cell. 2009;16:764–771. doi: 10.1016/j.devcel.2009.04.006. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theillet FX, Rose HM, Liokatis S, Binolfi A, Thongwichian R, Stuiver M, Selenko P. Site-specific NMR mapping and time-resolved monitoring of serine and threonine phosphorylation in reconstituted kinase reactions and mammalian cell extracts. Nature protocols. 2013;8:1416–1432. doi: 10.1038/nprot.2013.083. [DOI] [PubMed] [Google Scholar]
- 21.Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. The EMBO journal. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gille H, Strahl T, Shaw PE. Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Current biology : CB. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- 23.Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 24.Price MA, Cruzalegui FH, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate Ternary Complex Factors and c-fos transcription in response to UV light. The EMBO journal. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 25.Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. The Journal of biological chemistry. 2008;283:19511–19520. doi: 10.1074/jbc.M801074200. published online EpubJul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickers ER, Kasza A, Kurnaz IA, Seifert A, Zeef LA, O'Donnell A, Hayes A, Sharrocks AD. Ternary complex factor-serum response factor complex-regulated gene activity is required for cellular proliferation and inhibition of apoptotic cell death. Molecular and cellular biology. 2004;24:10340–10351. doi: 10.1128/MCB.24.23.10340-10351.2004. published online EpubDec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wozniak MA, Cheng CQ, Shen CJ, Gao L, Olarerin-George AO, Won KJ, Hogenesch JB, Chen CS. Adhesion regulates MAP kinase/ternary complex factor exchange to control a proliferative transcriptional switch. Current biology : CB. 2012;22:2017–2026. doi: 10.1016/j.cub.2012.08.050. published online EpubNov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugimoto T, Stewart S, Guan KL. The calcium/calmodulin-dependent protein phosphatase calcineurin is the major Elk-1 phosphatase. The Journal of biological chemistry. 1997;272:29415–29418. doi: 10.1074/jbc.272.47.29415. [DOI] [PubMed] [Google Scholar]
- 29.Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–521. doi: 10.1038/35107009. published online EpubNov 29. [DOI] [PubMed] [Google Scholar]
- 30.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science. 2007;316:1749–1752. doi: 10.1126/science.1141084. published online EpubJun 22. [DOI] [PubMed] [Google Scholar]
- 31.Ayed A, Mulder FA, Yi GS, Lu Y, Kay LE, Arrowsmith CH. Latent and active p53 are identical in conformation. Nature structural biology. 2001;8:756–760. doi: 10.1038/nsb0901-756. published online EpubSep. [DOI] [PubMed] [Google Scholar]
- 32.Tamiola K, Acar B, Mulder FA. Sequence-specific random coil chemical shifts of intrinsically disordered proteins. Journal of the American Chemical Society. 2010;132:18000–18003. doi: 10.1021/ja105656t. published online EpubDec 29. [DOI] [PubMed] [Google Scholar]
- 33.Tamiola K, Mulder FA. Using NMR chemical shifts to calculate the propensity for structural order and disorder in proteins. Biochemical Society transactions. 2012;40:1014–1020. doi: 10.1042/BST20120171. published online EpubOct. [DOI] [PubMed] [Google Scholar]
- 34.Schwarzinger S, Kroon GJ, Foss TR, Chung J, Wright PE, Dyson HJ. Sequence-dependent correction of random coil NMR chemical shifts. Journal of the American Chemical Society. 2001;123:2970–2978. doi: 10.1021/ja003760i. [DOI] [PubMed] [Google Scholar]
- 35.Bienkiewicz EA, Lumb KJ. Random-coil chemical shifts of phosphorylated amino acids. Journal of biomolecular NMR. 1999;15:203–206. doi: 10.1023/a:1008375029746. [DOI] [PubMed] [Google Scholar]
- 36.Platzer G, Okon M, McIntosh LP. pH-dependent random coil (1)H, (13)C, and (15)N chemical shifts of the ionizable amino acids: a guide for protein pK a measurements. Journal of biomolecular NMR. 2014;60:109–129. doi: 10.1007/s10858-014-9862-y. published online EpubNov. [DOI] [PubMed] [Google Scholar]
- 37.Patwardhan P, Miller WT. Processive phosphorylation: mechanism and biological importance. Cellular signalling. 2007;19:2218–2226. doi: 10.1016/j.cellsig.2007.06.006. published online EpubNov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Putz M, Lacrama A, Ostafe V. Full Analytic Progress Curves of Enzymic Reactions in Vitro. Int J Mol Sci. 2006;7:469–484. doi: 10.3390/i7110469. [DOI] [Google Scholar]
- 39.Theillet FX, Rose HM, Liokatis S, Binolfi A, Thongwichian R, Stuiver M, Selenko P. Corrigendum: Site-specific NMR mapping and time-resolved monitoring of serine and threonine phosphorylation in reconstituted kinase reactions and mammalian cell extracts. Nature protocols. 2016;11:192. doi: 10.1038/nprot0116-192a. published online EpubJan. [DOI] [PubMed] [Google Scholar]
- 40.Costello P, Nicolas R, Willoughby J, Wasylyk B, Nordheim A, Treisman R. Ternary complex factors SAP-1 and Elk-1, but not net, are functionally equivalent in thymocyte development. Journal of immunology. 2010;185:1082–1092. doi: 10.4049/jimmunol.1000472. published online EpubJul 15. [DOI] [PubMed] [Google Scholar]
- 41.Mylona A, Nicolas R, Maurice D, Sargent M, Tuil D, Daegelen D, Treisman R, Costello P. The essential function for serum response factor in T-cell development reflects its specific coupling to extracellular signal-regulated kinase signaling. Molecular and cellular biology. 2011;31:267–276. doi: 10.1128/MCB.01058-10. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.