Abstract
Whether the cortical processing of nociceptive input relies on the activity of nociceptive-specific neurons or whether it relies on the activity of neurons also involved in processing non-nociceptive sensory input remains a matter of debate. Here, we combined EEG “frequency-tagging” of steady-state evoked-potentials (SS-EPs) with an intermodal selective attention paradigm to test whether the cortical processing of nociceptive input relies on nociceptive-specific neuronal populations that can be selectively modulated by top-down attention. Trains of nociceptive and vibrotactile stimuli (experiment 1) and trains of nociceptive and visual stimuli (experiment 2) were applied concomitantly to the same hand, thus eliciting nociceptive, vibrotactile and visual SS-EPs. In each experiment, a target detection task was used to focus attention towards one of the two concurrent streams of sensory input. We found that selectively attending to nociceptive or vibrotactile somatosensory input indistinctly enhances the magnitude of nociceptive and vibrotactile SS-EPs, whereas selectively attending to nociceptive or visual input independently enhances the magnitude of the SS-EP elicited by the attended sensory input. This differential effect indicates that the processing of nociceptive input involves neuronal populations also involved in the processing of touch, but distinct from the neuronal populations involved in vision.
Keywords: Electroencephalography (EEG), Intermodal attention, Nociception, Somatosensory, Steady-state evoked potentials (SS-EP)
Introduction
Conscious perception of an external stimulus requires encoding by sensory organs, transmission and processing within a dedicated sensory system, and activation of appropriate sensory cortical areas (Treede & Apkarian, 2008). In healthy individuals, it is clearly established that the perception of pain relies on the encoding of nociceptive input by specific receptors, i.e. Aδ and C fiber nociceptors, and on the transmission of this nociceptive input to the thalamus and cortex through specific ascending pathways, i.e. the spinothalamic tracts (McMahon & Wall, 2013). It is also clearly established that transient painful stimuli activate a vast network of cortical areas, including the primary (S1) and secondary (S2) somatosensory cortices, the insula, the posterior parietal cortex, the anterior cingulate cortex (ACC) and parts of the prefrontal cortex (Apkarian, Bushnell, Treede, & Zubieta, 2005; Garcia-Larrea, Frot, & Valeriani, 2003; Peyron, Laurent, & Garcia-Larrea, 2000). However, and contrasting with other sensory systems, there appears to be no spatially segregated cortical area devoted specifically to the processing of nociceptive input, i.e. a cortical area which could be considered as a “primary nociceptive cortex” (Andersson & Rydenhag, 1985; Iannetti & Mouraux, 2010). Indeed, the brain areas responding to nociceptive input are all also involved in other sensory, emotional, cognitive, motor or autonomic functions (Iannetti & Mouraux, 2010; Legrain, Iannetti, Plaghki, & Mouraux, 2011; Treede & Apkarian, 2008).
Whether the cortical processing of nociceptive input relies on the activity of nociceptive-specific neurons, or whether it relies on neurons also processing other types of sensory input – in particular innocuous somatosensory input (Iannetti & Mouraux, 2010) – thus remains a crucial open question in pain neuroscience.
In favor of the view that perceiving pain involves the activity of nociceptive-specific neurons, single-cell recordings in animals have shown the existence of neurons responding specifically to nociceptive stimuli, especially in S1 (Apkarian, et al., 2005; Bushnell et al., 1999; D. R. Kenshalo, Iwata, Sholas, & Thomas, 2000; Whitsel, Favorov, Li, Quibrera, & Tommerdahl, 2009) and the parasylvian cortex (Apkarian, et al., 2005; Treede, Apkarian, Bromm, Greenspan, & Lenz, 2000). Some of these neurons exhibit punctate receptive fields and/or their frequency of discharge appears to encode the intensity of the stimulus, suggesting a specific role in the sensori-discriminative representation of pain (Hofbauer, Rainville, Duncan, & Bushnell, 2001; D. R. Kenshalo, Jr., Chudler, Anton, & Dubner, 1988; Timmermann et al., 2001). Moreover, whereas vibrotactile afferents primarily project to area 3b of S1, neurons responding to nociceptive stimuli have been mainly identified in area 1 and/or area 3a, suggesting that the processing of nociceptive and vibrotactile inputs within S1 may involve spatially-distinct subregions (Baumgartner, Vogel, Ohara, Treede, & Lenz, 2011; D. R. Kenshalo, et al., 2000; Mountcastle, Steinmetz, & Romo, 1990; Tommerdahl et al., 1998; Vierck, Whitsel, Favorov, Brown, & Tommerdahl, 2013; Whitsel, et al., 2009). Furthermore, lesions of the parasylvian cortex, in particular, lesions including the posterior insula, have been reported to cause a deficit in pain perception (Garcia-Larrea, 2012; Greenspan, Lee, & Lenz, 1999), and epileptic activity or direct electrical stimulation of this region can, among other things, cause painful experiences (Ostrowsky et al., 2002), suggesting that parts of the parasylvian cortex may contain neuronal populations preferentially involved in the perception of pain (Greenspan, et al., 1999; Treede & Apkarian, 2008).
However, one main common feature of nociceptive-specific neurons throughout the cortex is their scarcity (reviewed in Iannetti & Mouraux, 2010) Furthermore, because of the intrinsic significance of nociceptive input (Belmonte & Viana, 2008), brain activity that has been interpreted as “nociceptive-specific” could, at least in some cases, reflect the activity of neurons that are unspecific for nociception and, instead, mainly be involved in the detection of salient sensory input regardless of whether that input is conveyed through nociceptive pathways (Legrain, et al., 2011). Supporting this view, several studies have shown the existence of neurons responding to both nociceptive stimuli and stimuli belonging to another sensory modality, especially if the stimuli convey information signaling a potential impact on the body (e.g., a visual stimulus moving towards the body) (Dong, Chudler, Sugiyama, Roberts, & Hayashi, 1994; Hutchison, Davis, Lozano, Tasker, & Dostrovsky, 1999; D. R. Kenshalo & Douglass, 1995).
Nonetheless, one cannot rule out the possibility that nociceptive stimuli activate sparse and intermingled clusters of nociceptive-specific neurons, whose synaptic activity cannot be spatially distinguished from that of non-nociceptive-specific neurons, especially when using conventional functional neuroimaging techniques that sample human brain activity at population level.
For this reason, the present study aimed to explore nociceptive processing in the human cortex using a different approach, referred to as “frequency tagging” with steady-state evoked potentials (SS-EP) (Regan, 1989). It has been shown, for example, that if one presents simultaneously an auditory stimulus modulated at frequency f1 and a visual stimulus modulated at frequency f2, this elicits two distinct peaks in the EEG frequency spectrum at frequencies f1 and f2, respectively tagging the cortical activity elicited by the auditory and visual stimuli (Colon, Legrain, & Mouraux, 2012; de Jong, Toffanin, & Harbers, 2010; Giani et al., 2012; Keitel, Schroger, Saupe, & Muller, 2011; Nozaradan, Peretz, & Mouraux, 2011; Saupe, Schroger, Andersen, & Muller, 2009; Talsma, Doty, Strowd, & Woldorff, 2006). Most interestingly, discrimination between the activities elicited by each of the two streams of sensory input is not dependent on the spatial resolution of the brain sampling technique, as they will be isolated in the frequency domain even if the eliciting neuronal populations are spatially intermingled. Furthermore, using the “frequency tagging” approach, previous studies on intermodal selective attention have shown that selectively attending one of several concurrent streams of sensory input belonging to different sensory modalities increases the magnitude of the SS-EP elicited by the sensory inputs of the attended modality (de Jong, et al., 2010; Giani, et al., 2012; Keitel, et al., 2011; Nozaradan, et al., 2011; Saupe, et al., 2009; Talsma, et al., 2006), probably through a selective enhancement of the responsiveness of the neuronal populations responding to the attended input. The approach has also been used within a given sensory modality. For example, it was shown that selectively attending to a given color selectively increases the magnitude of the visual SS-EP elicited by flickering dots of the attended color, probably through a top down enhancement of the responsiveness of neurons responding preferentially to the attended visual feature (Muller et al., 2006) (see also Hillyard, Vogel, & Luck, 1998); Morgan, Hansen, & Hillyard, 1996). Hence, it can be hypothesized that if the processing of nociceptive and non-nociceptive somatosensory inputs at least partly relies on neuronal populations preferentially responding to one of the two types of somatosensory inputs, SS-EPs elicited by combined nociceptive and non-nociceptive somatosensory stimulation while participants selectively attend one of the two streams of somatosensory input will show a selective enhancement of the SS-EP elicited by the attended input (Legrain, Guerit, Bruyer, & Plaghki, 2002; Legrain et al., 2012). Conversely, if the processing of nociceptive and non-nociceptive somatosensory inputs does not involve neuronal populations selective for nociceptive or non-nociceptive somatosensory input, selectively attending to the noxious or the innocuous stream of somatosensory input will indistinctly enhance the SS-EPs elicited by both types of stimuli.
Finally, a small number of studies have suggested that cortical integration of different streams of sensory input can be revealed by the appearance of additional SS-EPs, appearing at non-linear cross-modulation frequencies corresponding to the sum or differences of the eliciting frequencies or their harmonics (i.e., nf1±mf2, where n and m are integers), and reflecting the activity of multisensory neurons onto which the different sensory inputs converge (Giani, et al., 2012; M. P. Regan, He, & Regan, 1995).
These different hypotheses were tested in the present study, in which we compared the effect of intermodal selective attention on the SS-EPs elicited by concomitant nociceptive and tactile stimulation (experiment 1) and concomitant nociceptive and visual stimulation (experiment 2).
Methods
Participants
Sixteen healthy volunteers (10 males, aged 19 to 35 years, 13 right handed) took part in the nociceptive-tactile experiment (experiment 1). Twelve healthy volunteers (6 males, aged 23 to 33 years, 10 right handed) took part in the nociceptive-visual experiment (experiment 2). All participants had normal or corrected-to-normal vision and no prior history of neurological, psychiatric and chronic pain disorders. Before the experiments, participants were familiarized with the experimental setup and task, and exposed to a small number of test stimuli. Written informed consent was obtained from all participants and they were paid for their participation. The study was approved by the local Ethics Committee and conformed to the latest revision of the Declaration of Helsinki.
Stimuli
Nociceptive somatosensory stimulation
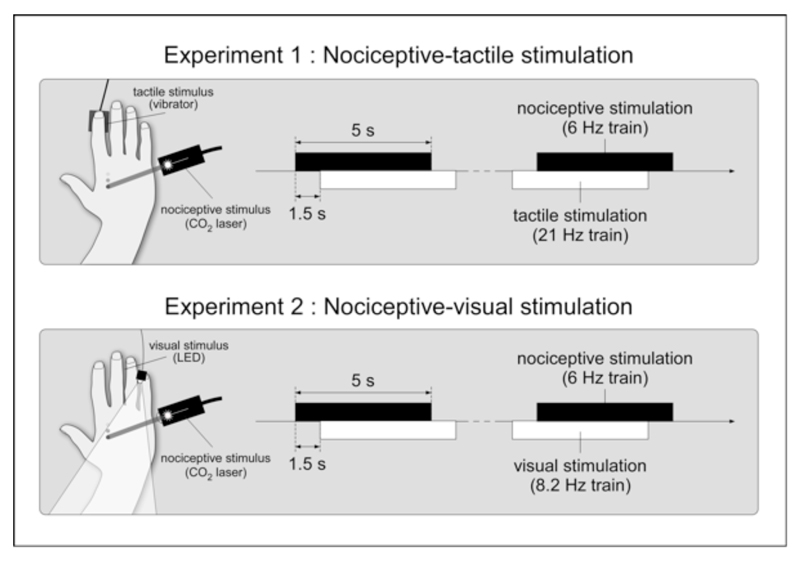
Infrared laser stimulation was used to selectively activate heat sensitive nociceptive free nerve endings of the skin (Plaghki & Mouraux, 2003, 2005). The CO2 laser (wavelength, 10.6 μm) was designed and built in the Department of Physics of the Université catholique de Louvain. Brief (20 ms) and focal (5 mm beam diameter) laser pulses were delivered to the left or right hand dorsum, as follows. The participants were seated in a chair with one of the two hands resting on a table (Figure 1). Before the experimental session, the energy of the laser stimulus was defined for each hand, such as to slightly exceed the thermal activation threshold of Aδ-nociceptors, defined as the energy at which a single laser pulse was detected with a reaction time (RT) shorter than 650 ms (i.e. compatible with the conduction velocity of Aδ-fibers) (Churyukanov, Plaghki, Legrain, & Mouraux, 2012). The average energy density of the stimulus was 12.6 ±2.1 mJ/mm2. This energy density was similar to the energy density used in our previous studies using single stimuli (e.g. (Mouraux & Plaghki, 2007a, 2007b)) or trains of stimuli (e.g. (Mouraux et al., 2011)). Participants described the sensation elicited by these stimuli as a short-lasting pricking/burning sensation. Thirty points were then defined on each hand dorsum, at locations where the laser beam was close to orthogonal relative to the skin surface. The distance between two points was approximately 5 mm. The target of the laser stimulus was displaced using a dual-axis galvanometer mirror positioning system with switching times as short as a few microseconds (LSST-10.6-12-105-8062-3A, Sintec Optronics, Singapore). The distance between the mirrors and the hand dorsum was approximately 35 cm. The stimuli were delivered as trains lasting 5 s and consisting in 30 laser pulses delivered at a rate of 6 Hz to each of the 30 predefined locations. The target of the laser stimulus was displaced after each pulse to avoid skin overheating and possible sensitization or habituation of the activated nociceptors. The displacement followed a zigzag path of points beginning on the left side of the hand dorsum and ending on the right side of the hand dorsum. The frequency of 6 Hz was chosen relative to previous studies using nociceptive SS-EPs (Colon, Nozaradan, Legrain, & Mouraux, 2012; Mouraux, et al., 2011). The stimulation trains were perceived by the participants as a continuous burning/pricking sensation.
Figure 1.
In the nociceptive-tactile experiment (experiment 1), nociceptive and vibrotactile stimuli were applied to the left or right hand dorsum. The nociceptive stimuli consisted in 30 laser pulses delivered at a rate of 6 Hz to predefined locations on the hand. The vibrotactile stimuli consisted in a 128 Hz carrier frequency periodically modulated at 21 Hz, generated by a recoil-type vibrotactile transducer positioned on the index fingertip. Each stimulation train lasted 5 s. A 1.5 s delay separated the onsets of the two trains. In the nociceptive-visual experiment (experiment 2), the vibrotactile stimulus was replaced by a visual stimulus, consisting in light-emitting diode placed close to the fifth finger, flickering at 8.2 Hz.
Vibrotactile stimulation
Vibrotactile stimuli were generated by a recoil-type vibrotactile transducer driven by a standard audio amplifier (Haptuator, Tactile Labs Inc., Canada) and positioned on the left or right index fingertip. The vibrotactile stimulus lasted 5 seconds and consisted in a 128 Hz carrier frequency periodically modulated at 21 Hz (previous studies have shown that the optimal modulation frequency to elicit vibrotactile SS-EPs lies within the range of 20-30 Hz) (Colon, Legrain, et al., 2012; Snyder, 1992). The same maximum output voltage was used in all participants (3V). At this output voltage, the carrier frequency (128 Hz) may be expected to generate forces of up to 0.45 N. The stimulation trains were perceived by the participants as a clear continuous vibration. The vibration was neither qualified as painful or as unpleasant by the participants.
Visual stimulation
Visual stimuli were continuous flashes generated by means of a white light-emitting diode (LED) with a 12 lm luminous flux, a 5.10 cd luminous intensity and a 120° visual angle (GM5BW97333A, Sharp Corporation, Japan). The LED was placed on the fifth finger of the right or left hand, according to the stimulation block. The visual stimuli were delivered as trains lasting 5 seconds during which the LED was switched on and off with a periodicity of 8.2 Hz. The frequency of 8.2 Hz was chosen in relation with the preferred range of stimulation frequencies to elicit visual SS-EPs (Vialatte et al., 2010). The stimulation trains were perceived by the participants as a continuous flashing white light.
Procedures
Both experiments were conducted in a dim and silent room. During the recordings, participants wore protective goggles. White noise was presented continuously through headphones, at a comfortable listening level, to mask any sounds produced by the laser stimulator as well as the vibrotactile stimulator. In the nociceptive-tactile experiment, participants were asked to maintain their gaze on a central fixation cross in front of them. In the nociceptive-visual experiment they were asked to maintain their gaze on a fixation cross placed on the right side of the table when the left hand was stimulated and on the left side of the table when the right hand was stimulated.
In the nociceptive-tactile experiment, participants received nociceptive and tactile stimuli. The two types of stimuli were concomitantly applied to the left hand and to the right hand, in separate blocks. Participants were asked to direct their attention towards one of the two sensory modalities such as to detect the occasional occurrence of a target (change within the stimulation train) in the attended modality, also in separate blocks.
In the nociceptive modality, the target consisted in a jump of stimulus location at the beginning of the train (rather than beginning on the left side of the hand, the predefined zig-zag path began on the middle of the hand dorsum). In the tactile modality, the target consisted in a 70-ms interruption of the vibrator, and could occur at any time during the 5-s stimulation train. These targets were defined after several pilot experiments to ensure that the task was feasible but sufficiently difficult to require attention to be fully focused towards the attended modality. At the end of each trial, participants had to report whether a target was present or not in the attended modality by responding ‘yes’ or ‘no’. No target was presented in the unattended modality. To ease selection of the sensory input belonging to the attended modality, the onsets of the nociceptive and tactile stimulation trains were asynchronous with an inter-onset delay of 1.5 s. Therefore, each stimulation train lasted 5 s but an entire trial lasted 6.5 s. (Figure 1). The order of the trains (nociceptive-tactile, tactile-nociceptive) was randomized across trials. A 10-s inter-trial interval separated each trial. The experiment consisted in 8 blocks of stimulation presented in random order: four different block types according to the selective attention task (attend nociceptive vs. attend tactile) and stimulus location (left hand vs. right hand), repeated two times. Each block contained 12 trials (including 2 or 3 target trials which were discarded from the analysis). Therefore, in each participant, 20 trials were collected for each condition. The entire recording lasted approximately 1.3 hours.
The nociceptive-visual experiment was identical to the nociceptive-tactile experiment, except for the fact that the tactile stimulus was replaced by a visual stimulus. In the visual modality, the change consisted in a 70-ms interruption of the flashing LED, which could occur at any time during the 5-s stimulation train.
Measures
Behavioral measures
For each experiment and each attention condition, behavioral performance, merged for left and right hands, was estimated by an accuracy index computed as follows: (number of true positives + number of true negatives)/(number of true positives + number of true negatives + number of false negatives + number of false positives) (Macmillan, 2005), where true positives are defined as a correct detection of a target stimulus in the attended stream, true negatives as a correct rejection of an attended non-target stimulus, false positives as a false alarm to an attended non-target stimulus and false negatives as a missed response to an attended target stimulus. In each experiment, the accuracy indexes to the two selective attention conditions were compared using a paired t-test (SPSS 18, IBM, USA).
Electrophysiological measures
The EEG was recorded using 64 Ag-AgCl electrodes placed on the scalp according to the international 10/10 system (Waveguard64 cap, Cephalon A/S, Denmark). Electrodes impedances were kept below 10 kΩ. Ocular movements and eye blinks were recorded using two additional surface electrodes placed at the upper-left and the lower-right sides of the right eye. Signals were amplified and digitized using a sampling rate of 1000 Hz and an average reference (64-channel high-speed amplifier, Advanced Neuro Technology, The Netherlands).
Analysis of the EEG data was carried out using Analyzer 1.05 (Brain Products, Germany) and Letswave 5 (http:/nocions.webnode.com/letswave) (see also Mouraux and Iannetti, 2008). The continuous EEG recordings were filtered using a 0.01-0.3 Hz high-pass Butterworth filter to remove slow drifts in the recorded signals. Non-overlapping EEG epochs were then obtained by segmenting the recordings from 0 to 6.5 s relative to the onset of the first stimulation train. Each EEG epoch was demeaned using the time-interval ranging from 0 to 6.5 s. Artefacts due to eye blinks or eye movements were then removed using a validated method based on an independent-component analysis (FastICA algorithm) (Hyvarinen & Oja, 2000). In addition, epochs with amplitude values exceeding ± 500 μV (i.e. epochs likely to be contaminated by an artefact) were rejected. These epochs constituted 4.8 ± 6.6 % of the total number of epochs in the nociceptive-tactile experiment and 3.3 ± 3.3 % of the total number of epochs in the nociceptive-visual experiment. Finally, the 6.5-s epochs were segmented in 5-s epochs relative to the onset of each stimulation train, and separate average waveforms were computed for each modality (nociceptive and tactile in the nociceptive-tactile experiment; nociceptive and visual in the nociceptive-visual experiment), attended condition (attend nociceptive vs. tactile in the nociceptive-tactile experiment; attend nociceptive vs. visual in the nociceptive-visual experiment) and stimulated hand (left vs. right hand). Finally, the obtained average waveforms were transformed in the frequency domain using a discrete Fourier Transform (FFTW) (Frigo & Johnson, 1998), yielding an amplitude spectrum (μV) ranging from 0 to 500 Hz with a frequency resolution of 0.1 Hz (Bach & Meigen, 1999).
Within the obtained frequency spectra, the signal amplitude at 6 Hz (nociceptive stimulus) and 21 Hz (tactile stimulus) in the nociceptive-tactile experiment and, at 6 Hz (nociceptive stimulus) and 8.2 Hz (visual stimulus) in the nociceptive-visual experiment was measured at each EEG electrode. These measures may be expected to correspond to the sum of the stimulus-evoked steady-state response (i.e. the nociceptive, tactile or visual SS-EP) and unrelated residual background noise. Therefore, to obtain valid estimates of the magnitude of the elicited SS-EPs, the contribution of this residual noise was removed by subtracting, at each electrode and at each frequency bin, the average amplitude of the signal measured at neighboring frequencies (± 0.3-0.5 Hz relative to the expected SS-EP frequency) (Mouraux, et al., 2011). In the absence of a steady-state response, this noise-subtracted average signal amplitude may be expected to tend towards zero. Hence, to assess the significance of the responses measured at each frequency, experimental condition and electrode, a t-test against zero was used to determine whether the magnitude of the noise-subtracted signal amplitude was significantly greater than zero (SPSS 18, IBM, USA).
Estimation of SS-EP amplitude
To avoid any bias related to an arbitrary selection of electrodes, the analyses were performed using the estimates of noise-subtracted SS-EP amplitudes averaged across all scalp channels. As there was no statistical difference between the SS-EP amplitude measures obtained from stimulation of the left and right hands in the two experiments and the two conditions (nociceptive-visual experiment: all t(11) > -2.11, p > 0.06; nociceptive-tactile experiment all t(15) > -0.9, p > 0.38), the noise-subtracted SS-EP amplitudes were averaged for left and right hand stimulation to reduce the number of experimental factors and to increase the signal-to-noise ratio.
Effect of selective attention on the magnitude of SS-EPs
To assess whether the magnitude of the nociceptive SS-EP was differently affected by selectively attending to tactile input vs. selectively attending to visual input, an ANOVA with one within-subject factor (‘attention’: attend the nociceptive modality vs. attend another modality) and one between-subject factor (‘experiment’: nociceptive-tactile vs. nociceptive-visual) was performed (SPSS 18, IBM, USA). Next, a repeated-measures ANOVA was used to assess the effect of selective attention on the amplitude of the nociceptive, tactile and visual SS-EPs obtained in each of the two experiments, with the following two factors: ‘modality’ (nociceptive vs. tactile in the nociceptive-tactile experiment; nociceptive vs. visual in the nociceptive-visual experiment) and ‘attention’ (attend nociceptive vs. tactile in the nociceptive-tactile experiment; attend nociceptive vs. visual in the nociceptive-visual experiment). Size effects of ANOVAs were measured with partial Eta-squared (η2p) and α was set at 0.05. When significant, pairwise comparisons were performed using paired-sample t-tests and in this case, α was set at 0.025 in order to correct multiple comparisons using the Bonferroni criterion.
Cross-modulation frequencies
In order to examine the presence of cross-modulation frequencies, the signal amplitude averaged across all scalp channels at 27 Hz and 15 Hz, corresponding to the sum and difference of the frequency of the nociceptive (6 Hz) and tactile (21 Hz) stimuli in the nociceptive-tactile experiment, and at 14.2 Hz and 2.2 Hz, corresponding to the sum and difference of the frequency of the nociceptive (6 Hz) and visual (8.2 Hz) stimuli in the nociceptive-visual experiment was measured. To assess the significance of the responses measured at each frequency, a t-test against zero was used to determine whether the magnitude of the noise-subtracted signal amplitude was significantly greater than zero.
Results
Task Performance
In the nociceptive-tactile experiment, the accuracy index in the ‘attend nociceptive’ condition was 0.86 ± 0.12 and the accuracy index in the ‘attend tactile’ condition was 0.85 ± 0.11. This difference was not significantly different across subjects, t(15) = 0.28, p = 0.79, d = 0.09. In the nociceptive-visual experiment, the accuracy index in the ‘attend nociceptive’ condition was 0.86 ± 0.11 and the accuracy index in the ‘attend visual’ condition was 0.99 ± 0.02. This difference was significantly different across subjects, t(11) = -4.75, p = 0.001, d = 2.4, suggesting that the performance in detecting the visual target was better than the performance in detecting the nociceptive target.
Nociceptive SS-EP
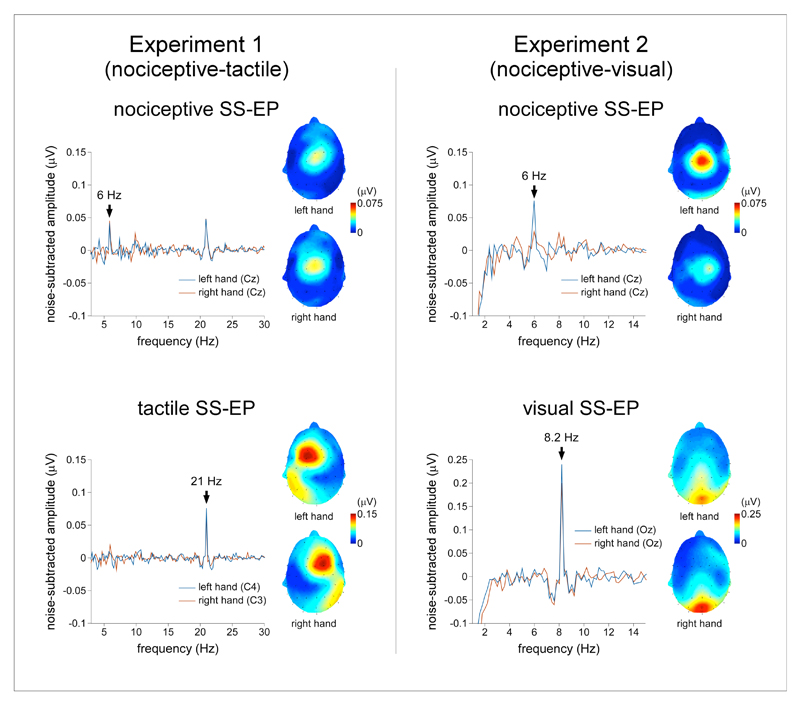
In the nociceptive-tactile experiment, nociceptive stimulation elicited an increase in signal amplitude at 6 Hz in the ‘attend nociceptive’ condition (group-level average of noise-subtracted amplitudes pooled across all scalp channels: 0.02 ± 0.03 μV) as well as in the ‘attend tactile’ condition (0.02 ± 0.03 μV). This increase in signal amplitude was significantly different from zero (‘attend nociceptive’ condition: t(15) = 3.15; p = 0.007, d = 1.6; ‘attend tactile’ condition: t(15) = 2.35, p = 0.03, d = 1.2). The scalp topography of the nociceptive SS-EP was maximal at the scalp vertex and symmetrically distributed over the two hemispheres (Figure 2). In the nociceptive-visual experiment, nociceptive stimulation elicited an increase in signal amplitude at 6 Hz in the ‘attend nociceptive’ condition (0.03 ± 0.04 μV; difference from zero: t(11) = 2.77; p = 0.02, d = 1.67) but not in the ‘attend visual’ condition (0.005 ± 0.02 μV; difference from zero: t(11) = 1.12, p = 0.29, d = 0.68). Such as in the nociceptive-tactile experiment, the scalp topography of the nociceptive SS-EP was maximal at the scalp vertex and symmetrically distributed over the two hemispheres (Figure 2).
Figure 2.
Experiment 1 (nociceptive-tactile). The upper panel represents the noise-subtracted EEG amplitude spectrum (μV) of the nociceptive SS-EP measured at Cz, (group-level average). Nociceptive stimulation elicited a significant increase in signal power at 6 Hz whose scalp topography was maximal over the scalp vertex regardless of the stimulated hand. The lower panel represents the noise-subtracted EEG amplitude spectrum (μV) of the vibrotactile SS-EP measured at the central electrode contralateral to the stimulated hand (left hand: C4; right hand: C3; group-level average). Vibrotactile stimulation elicited a significant increase in signal power at 21 Hz, whose scalp topography was maximal over the parietal region contralateral to the stimulated hand. Experiment 2 (nociceptive-visual). The upper panel represents the noise-subtracted EEG amplitude spectrum (μV) measured at Cz (group-level average). Such as in experiment 1, nociceptive stimulation elicited a significant increase in signal power at 6 Hz whose scalp topography was maximal over the scalp vertex regardless of the stimulated hand. The lower panel represents the noise-subtracted EEG amplitude spectrum (μV) measured at Oz (group-level average). Visual stimulation elicited a significant increase in signal power at 8.2 Hz whose scalp topography was maximal over occipital regions.
Vibrotactile SS-EP
In the nociceptive-tactile experiment, vibrotactile stimulation elicited an increase in signal amplitude at 21 Hz in both the ‘attend nociceptive’ condition (0.05 ± 0.04 μV) and the ‘attend tactile’ condition (0.05 ± 0.04 μV). In both conditions, this increase was significantly different from zero (‘attend nociceptive’ condition: t(15) = 5.61, p < 0.001, d = 2.89; ’attend tactile’ condition: t(15) = 5.86, p < 0.001, d = 3.03). The scalp topography of the vibrotactile SS-EP was maximal over the parietal region contralateral to the stimulated side and markedly different from that of the nociceptive SS-EP (Figure 2).
Visual SS-EP
In the nociceptive-visual experiment, visual stimulation elicited a significant increase in signal power at 8.2 Hz in both the ‘attend nociceptive’ condition (0.07 ± 0.045 μV; difference from zero: t(11) = 5.36, p < 0.001, d = 3.23) and the ‘attend visual’ condition (0.11 ± 0.05 μV; difference from zero: t(11) = 7.18 p < 0.001, d = 4.33). The scalp topography of the visual SS-EP was maximal over occipital regions, and markedly different from that of the nociceptive SS-EP (Figure 2).
Modulation of SS-EP amplitude by intermodal selective attention
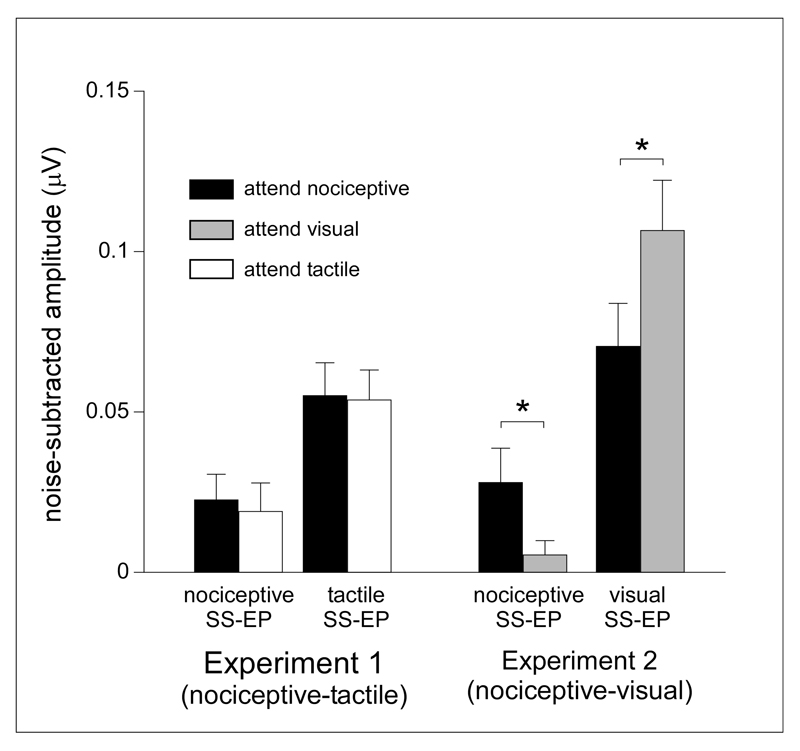
The effect of selectively attending to vibrotactile input (nociceptive-tactile experiment) vs. selectively attending to visual input (nociceptive-visual experiment) on the magnitude of the nociceptive SS-EPs was assessed using an ANOVA with one within-subject factor (‘attention’: attend nociceptive vs. attend other) and one between-subject factor (‘experiment’: nociceptive-tactile vs. nociceptive-visual). This revealed a significant main effect of ‘attention’ (F(1,26) = 7.89, p = 0.009, η2p = 0.23), no main effect of ‘experiment’ (F(1,26) = 0.23, p = 0.64, η2p = 0.009) and, most importantly, a significant interaction between the factors ‘attention’ and ‘experiment’ (F(1,26) = 4.46, p = 0.045, ηp2 = 0.15). This interaction indicates that the effect of selective attention on the magnitude of nociceptive SS-EPs was significantly different between the group of participants having performed the nociceptive-tactile experiment and the group of participants having performed the nociceptive-visual experiment (Figure 5).
Figure 5.
Group-level average (±sd) amplitude of the nociceptive, visual and tactile SS-EPs obtained in the nociceptive-tactile and the nociceptive-visual experiments when attending the nociceptive, visual or tactile stimuli. Note that the magnitude of the nociceptive SS-EP appeared to be enhanced both when attending the nociceptive stimulus and the tactile stimulus in the nociceptive-tactile experiment, whereas it was enhanced only when attending the nociceptive stimulus in the nociceptive-visual experiment. Also note that attending to the nociceptive or visual stimulus significantly modulated the amplitude of the visual SS-EP; whereas attending to the nociceptive or tactile stimulus did not modulate the amplitude of the tactile SS-EP.
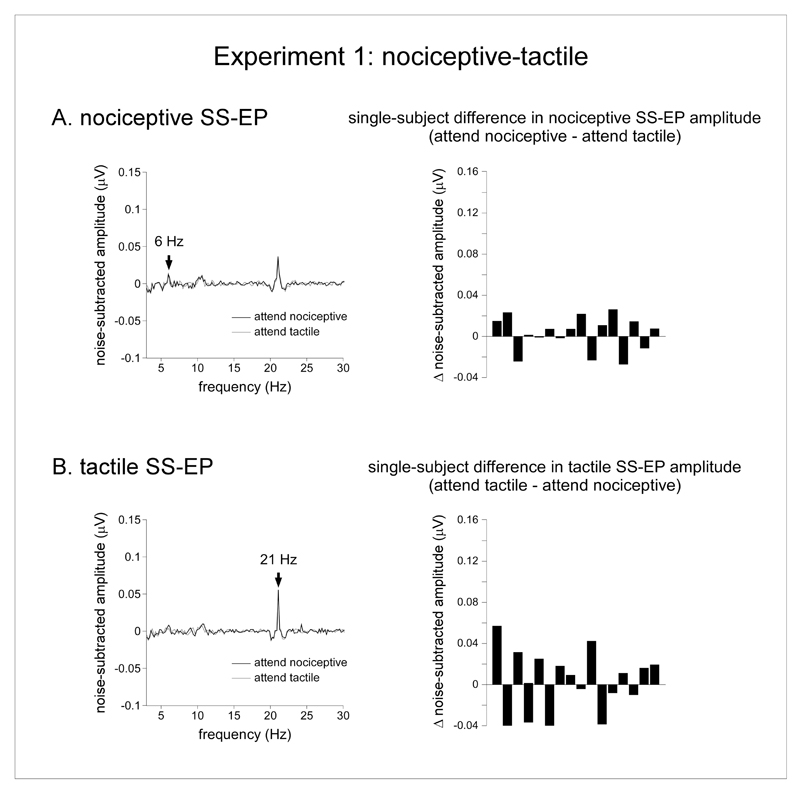
On average, in the nociceptive-tactile experiment, attending to the nociceptive stimulus or attending to the vibrotactile stimulus did not modulate the magnitude of the nociceptive and vibrotactile SS-EPs. Indeed, the magnitudes were highly similar across the two experimental conditions (attend nociceptive vs. attend tactile) (Figure 3). In the nociceptive-tactile experiment, the repeated-measures ANOVA with the factors ‘modality’ (nociceptive vs. tactile) and ‘attention’ (attend nociceptive vs. attend tactile) showed a significant main effect of ‘modality’ (F(1,15) = 9.39 p = 0.008, η2p = 0.39). On average, the magnitude of the vibrotactile SS-EP was greater than the magnitude of the nociceptive SS-EP. There was no main effect of ‘attention’ (F(1,15) = 0.12 p = 0.74, η2p = 0.008) and, most importantly, no significant interaction between the two factors (F(1,15) = 0.07, p = 0.79, η2p = 0.004), suggesting that the magnitude of nociceptive and vibrotactile SS-EPs were not modulated by the focus of attention.
Figure 3.
Effect of selective attention in the nociceptive-tactile experiment (experiment 1). A. The left graph represents the noise-subtracted EEG amplitude spectrum (μV) of the nociceptive SS-EP, averaged across all subjects and all scalp electrodes in the ‘attend nociceptive’ (black waveform) and ‘attend tactile’ (grey waveform) conditions. The right graph displays the single-subject differences between the magnitudes of the nociceptive SS-EPs obtained in the ‘attend nociceptive’ and ‘attend tactile’ conditions (noise-subtracted signal amplitude measured at 6 Hz). Note that its amplitude was not consistently different in the two conditions. B. The left graph represents the noise-subtracted EEG amplitude spectrum (μV) of the tactile SS-EP, averaged across all subjects and all scalp electrodes in the ‘attend nociceptive’ (black waveform) and ‘attend tactile’ (grey waveform) conditions. The right graph displays the single-subject differences between the magnitudes of the vibrotactile SS-EPs obtained in the ‘attend tactile’ and ‘attend nociceptive’ conditions (noise-subtracted signal amplitude measured at 21 Hz). Such as for the nociceptive SS-EP, the amplitude of the vibrotactile SS-EP was not consistently different in the two conditions.
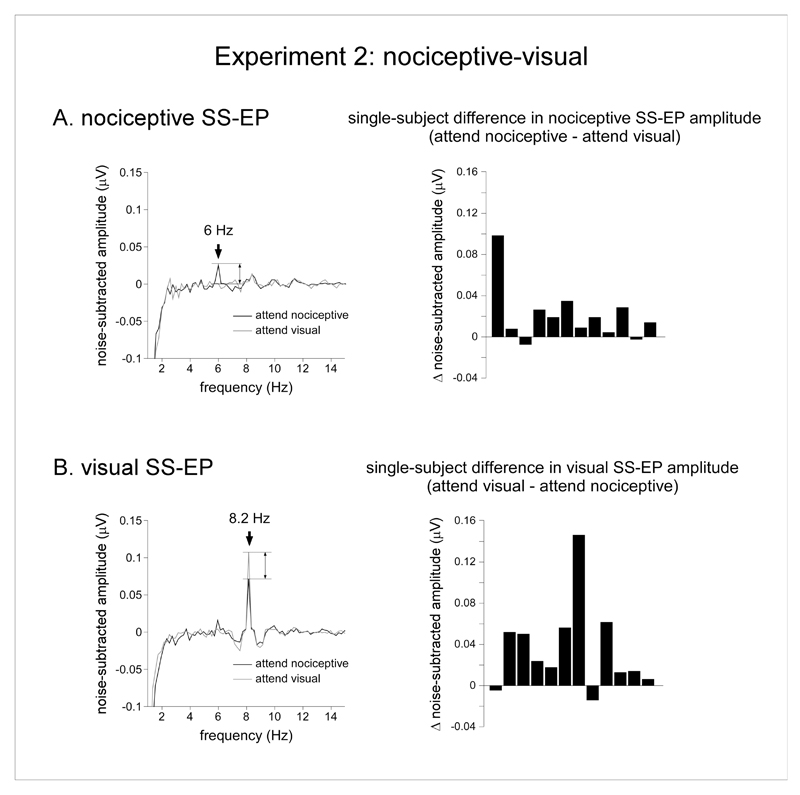
In the nociceptive-visual experiment, the repeated-measures ANOVA with the factors ‘modality’ (nociceptive vs. visual) and ‘attention’ (attend nociceptive vs. attend visual) showed also a significant main effect of ‘modality’ (F(1,11) = 20.6, p = 0.001, η2p = 0.65). On average, the magnitude of the visual SS-EP was greater than the magnitude of the nociceptive SS-EP. There was no main effect of ‘attention’ (F(1,11) = 0.55, p =0.48, η2p = 0.05) . However, and contrasting with the nociceptive-tactile experiment, there was a significant interaction between the factors ‘modality’ and ‘attention’ (F(1,11) = 22.96, p = 0.001, η2p = 0.68). This suggests that attending to the nociceptive stimulus or attending to the visual stimulus modulated the magnitude of the nociceptive and visual SS-EPs. Post-hoc pairwise comparisons showed that the amplitude of the nociceptive SS-EP was greater in the ‘attend nociceptive’ condition as compared to the ‘attend visual’ condition (t(11) = 2.58, p = 0.026, d = 0.79), whereas the amplitude of the visual SS-EP was greater in the ‘attend visual’ condition as compared to the ‘attend nociceptive’ condition (t(11) = -2.92, p = 0.01, d = 0.84). Therefore, the magnitude of the nociceptive SS-EP was greater when attending the nociceptive stimulus as compared to when attending the visual stimulus. Conversely, the magnitude of the visual SS-EP was greater when attending the visual stimulus as compared to when attending the nociceptive stimulus (Figure 4).
Figure 4.
Effect of selective attention in the nociceptive-visual experiment (experiment 2). A. The left graph represents the noise-subtracted EEG amplitude spectrum (μV) of the nociceptive SS-EP, averaged across all subjects and all scalp electrodes in the ‘attend nociceptive’ (black waveform) and ‘attend visual’ (grey waveform) conditions. The right graph displays the single-subject differences between the magnitudes of the nociceptive SS-EPs obtained in the ‘attend nociceptive’ and ‘attend visual’ conditions (noise-subtracted signal amplitude measured at 6 Hz). Note that the amplitude of the nociceptive SS-EP was consistently greater in the ‘attend nociceptive’ condition as compared to the ‘attend visual’ condition. B. The left graph represents the noise-subtracted EEG amplitude spectrum (μV) of the visual SS-EP, averaged across all subjects and all scalp electrodes in the ‘attend nociceptive’ (black waveform) and ‘attend visual’ (grey waveform) conditions. The right graph displays the single-subject differences between the magnitudes of the nociceptive SS-EPs obtained in the ‘attend visual’ and ‘attend nociceptive’ conditions (noise-subtracted signal amplitude measured at 8.2 Hz). Note that the amplitude of the visual SS-EP was consistently greater in the ‘attend visual’ condition as compared to the ‘attend nociceptive’ condition.
Cross-modulation frequencies
In the nociceptive-tactile experiment and in the nociceptive-visual experiment, no significant increase of signal amplitude was identified at frequencies corresponding to potential cross-modulation frequencies (27 Hz and 15 Hz in the nociceptive-tactile experiment, 14.2 Hz and 2.2 Hz in the nociceptive-visual experiment) (Figure 2).
Discussion
The aim of the present study was to test whether the cortical processing of nociceptive input relies on nociceptive-specific neuronal populations, or whether it relies on non-specific neuronal populations also involved in the processing of non-nociceptive sensory input. For this purpose, we used an experimental paradigm combining “frequency tagging” of the cortical activity elicited by different streams of sensory input with a cross-modal selective attention task to examine whether top-down attentional modulation mechanisms selectively increase the responsiveness of neuronal populations processing the attended sensory input and/or increase their phase-locking to the periodicity of the attended input (Nozaradan, et al., 2011). Specifically, we hypothesized that if the cortical processing of nociceptive and non-nociceptive sensory inputs involves distinct neuronal populations, selective attention would selectively increase the magnitude of the SS-EPs elicited by the attended stream of sensory input. Conversely, if the cortical processing of the two sensory inputs involves the same neuronal populations, selective attention would indistinctly increase the magnitude of the responses elicited by the attended and unattended streams of sensory input.
Comparison of the effect of selective attention in the nociceptive-tactile and nociceptive-visual experiments revealed a significant difference in the effect of attending to vibrotactile input as compared to visual input on the magnitude of nociceptive SS-EPs. In the nociceptive-tactile experiment, we found that the magnitude of the nociceptive and tactile SS-EPs were unaffected by the focus of attention: the magnitude of the tactile and nociceptive SS-EPs were similar in both the ‘attend nociceptive’ and the ‘attend tactile’ conditions. This lack of differential effect of selective attention on the responses elicited by nociceptive and non-nociceptive somatosensory input contrasted with the results obtained in the nociceptive-visual experiment, in which the magnitude of the nociceptive SS-EP was greater in the ‘attend nociceptive’ condition as compared to the ‘attend visual’ condition, whereas the visual SS-EP was greater in the ‘attend visual’ condition as compared to the ‘attend nociceptive’ condition. Taken together, these results indicate that nociceptive SS-EPs are differently affected by selectively attending to tactile or to visual input.
Consistent with the results of previous studies, the scalp topographies of nociceptive, vibrotactile and visual SS-EPs were markedly different from one another. In both experiments, the scalp topography of nociceptive SS-EPs was maximal at the scalp vertex and symmetrically distributed over both hemispheres. The brain generators of nociceptive SS-EPs remain largely unknown. It was previously suggested that nociceptive SS-EPs could originate from a midline brain structure such as the cingulate cortex and/or bilateral activity originating from operculo-insular cortices (Colon, Nozaradan, et al., 2012; Mouraux, et al., 2011). However, this should be confirmed using other techniques, such as the recording of local field potentials in patients with implanted electrodes for the presurgical evaluation of intractable epilepsy. Contrasting with the scalp topography of nociceptive SS-EPs, the scalp topography of vibrotactile SS-EPs was maximal over the parietal region contralateral to the stimulated hand, compatible with activity originating mainly from S1 (Colon, Nozaradan, et al., 2012; Giabbiconi, Dancer, Zopf, Gruber, & Muller, 2004; Giabbiconi, Trujillo-Barreto, Gruber, & Muller, 2007; Mouraux, et al., 2011), whereas the scalp topography of visual SS-EPs was maximal over occipital regions compatible with activity originating mainly from visual areas (Saupe, et al., 2009; Vialatte, Maurice, Dauwels, & Cichocki, 2010). These clear differences in topographical distribution indicate that the neuronal populations generating nociceptive SS-EPs reflect the activity of neuronal populations that are distinct from the neuronal populations generating non-nociceptive vibrotactile SS-EPs and visual SS-EPs. Yet, the present results show that selectively attending to nociceptive vs. vibrotactile input does not differentially modulate the responses elicited by nociceptive vs. vibrotactile somatosensory stimulation, whereas selectively attending to nociceptive vs. visual input significantly modulates the responses elicited by nociceptive vs. visual stimulation. This suggests that even though the bulk of nociceptive and non-nociceptive somatosensory SS-EPs reflect activity originating from distinct brain areas, they rely, at least in part, on the activity of shared neuronal populations. The responsiveness of these neurons would be indistinctly increased when selectively attending nociceptive input and when selectively attending vibrotactile input, but not when selectively attending visual input. Compatible with the hypothesis that the processing of nociceptive and non-nociceptive somatosensory inputs relies at least in part on the initial activation of shared neuronal populations, dynamic causal modeling of functional MRI data has recently suggested that the thalamocortical projections of nociceptive and non-nociceptive somatosensory inputs to S1 and S2 are indistinguishable (Liang, Mouraux, & Iannetti, 2011).
Whether other experimental factors could explain the differential results obtained in the nociceptive-tactile and nociceptive-visual experiments should be considered. For example, attending to a stimulus in a given modality delivered at a particular location can enhance the event-related potentials (ERPs) elicited by sensory input of a different modality presented at this attended location (Driver & Spence, 1998; Eimer, 2001). In the present study, all stimuli were delivered on the same hand (left or right) and, most importantly, the distance between the location of the nociceptive and tactile stimuli and the distance between the location of the nociceptive and visual stimuli was strictly identical (Figure 1). In addition, recent studies reported strong crossmodal interaction between nociceptive and visual stimuli when the visual stimul are delivered in the peripersonal space of the stimulated hand (De Paepe, 2014). Therefore, it seems unlikely that differences in spatial location could explain the differential effect of attending to vibrotactile vs. visual input on the magnitude of nociceptive SS-EPs.
A second factor that should be taken into consideration is the relatively low signal-to-noise ratio of nociceptive SS-EPs. The low amplitude of nociceptive SS-EPs as compared, for example, to vibrotactile SS-EPs could be related to the fact that the generation of nociceptive SS-EPs requires initial transduction of the thermal stimuli by heat-sensitive nociceptive afferents, and that this transduction step may be an important source of inter-trial variability, reducing the periodicity of the nociceptive afferent volley. While this could have been an explanation for the lack of effect of selective attention on the magnitude of nociceptive SS-EPs in the nociceptive-tactile experiment, it could not explain the lack of effect of selective attention on the magnitude of the vibrotactile SS-EPs obtained in the same experiment, nor could it explain why selective attention did significantly modulate the magnitude of nociceptive SS-EPs in the nociceptive-visual experiment. Moreover, the low amplitude of the nociceptive SS-EPs obtained in the attend visual condition could be related to the findings that attention to a given modality can result in decreased activity in cortical regions that process inputs belonging to unattended sensory modalities (Mozolic et al., 2008; Shomstein & Yantis, 2004).
A third factor that should be considered is that the visual detection task was easier to perform than the nociceptive and vibrotactile detection tasks, as suggested by the differences in task performance. Assuming that increasing task difficulty would require subjects to focus more their attention towards the task relevant stream of sensory input, one would have expected a stronger modulation by the focus of attention in the nociceptive-tactile experiment as compared to the nociceptive-visual experiment (Berti & Schroger, 2003; Legrain, Bruyer, Guerit, & Plaghki, 2005; Rauss, Pourtois, Vuilleumier, & Schwartz, 2009; Schwent, Hillyard, & Galambos, 1976). Therefore, it seems unlikely that the differences observed in the two experiments could be explained by differences in task difficulty.
Finally, cross-modulation SS-EPs could not be identified in the present study, neither in the nociceptive-tactile experiment, nor in the nociceptive-visual experiment. Had they been present, these cross-modulations SS-EPs would have constituted an index of the activity generated by neuronal populations onto which nociceptive and tactile inputs, or nociceptive and visual inputs, converged (Giani, et al., 2012; M. P. Regan, et al., 1995). Their absence could be related to the fact that participants performed a task which required to specifically focus on one of the two concurrently-presented streams of sensory input. This could have interfered with the multisensory integration of the two inputs into a unified percept. Supporting this view, it has been shown that audio-visual interactions assessed using event-related potentials depend on whether subjects attend to both sensory modalities (Talsma, Doty, & Woldorff, 2007). However, in a recent study, Giani et al. (2012) recorded SS-EPs to concomitant auditory and visual stimulation, with the aim of investigating whether the presence of cross-modulation SS-EPs is dependent on the cognitive state of the subjects. In one of the experiments, the participants were engaged in a target detection task that required simultaneously attending both streams of sensory input. Even in this condition expected to promote multisensory integration, no cross-modulation SS-EPs could be observed. Another possibility that should be examined in future studies is whether nociceptive-tactile cross-modulation SS-EPs could be recorded when the intensity of the tactile stimulus is increased. Indeed, this could be expected if there are neuronal populations onto which nociceptive and non-nociceptive inputs converge if and only if their intensity is sufficient.
Conclusion
By showing that selectively attending to nociceptive or vibrotactile somatosensory input does not selectively increase the magnitude of nociceptive and vibrotactile somatosensory SS-EPs, whereas selectively attending to nociceptive or visual input selectively increase the magnitude of the SS-EP elicited by the attended stream of sensory input, our results suggest that the cortical processing of nociceptive and non-nociceptive somatosensory input relies, at least partly, on shared neuronal populations whose responsiveness is indistinctly increased when selectively attending nociceptive input or vibrotactile input, but not when selectively attending visual input.
Acknowledgments
E.C. is research fellow FSR of the Université catholique de Louvain (Belgium). V.L. is Research Associate from the Fund for Scientific Research of the French-speaking community of Belgium (F.R.S. -FNRS). A.M. has received the support from a Mandat d’Impulsion Scientifique of the Belgian F.R.S. –FNRS and an ERC Starting Grant (336130).
References
- Andersson SA, Rydenhag B. Cortical nociceptive systems. Philos Trans R Soc Lond B Biol Sci. 1985;308(1136):347–359. doi: 10.1098/rstb.1985.0035. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bach M, Meigen T. Do's and don'ts in Fourier analysis of steady-state potentials. Documenta Ophthalmologica. 1999;99(1):69–82. doi: 10.1023/a:1002648202420. [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Vogel H, Ohara S, Treede RD, Lenz F. Dipole source analyses of laser evoked potentials obtained from subdural grid recordings from primary somatic sensory cortex. J Neurophysiol. 2011;106(2):722–730. doi: 10.1152/jn.00135.2011. doi: jn.00135.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Viana F. Molecular and cellular limits to somatosensory specificity. Mol Pain. 2008;4:14. doi: 10.1186/1744-8069-4-14. doi: 1744-8069-4-14 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti S, Schroger E. Working memory controls involuntary attention switching: evidence from an auditory distraction paradigm. Eur J Neurosci. 2003;17(5):1119–1122. doi: 10.1046/j.1460-9568.2003.02527.x. doi: 2527 [pii] [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96(14):7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churyukanov M, Plaghki L, Legrain V, Mouraux A. Thermal detection thresholds of Adelta- and C-fibre afferents activated by brief CO2 laser pulses applied onto the human hairy skin. PLoS One. 2012;7(4):e35817. doi: 10.1371/journal.pone.0035817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon E, Legrain V, Mouraux A. Steady-state evoked potentials to study the processing of tactile and nociceptive somatosensory input in the human brain. Neurophysiol Clin. 2012;42(5):315–323. doi: 10.1016/j.neucli.2012.05.005. doi: S0987-7053(12)00259-6 [pii] [DOI] [PubMed] [Google Scholar]
- Colon E, Nozaradan S, Legrain V, Mouraux A. Steady-state evoked potentials to tag specific components of nociceptive cortical processing. Neuroimage. 2012;60(1):571–581. doi: 10.1016/j.neuroimage.2011.12.015. doi: S1053-8119(11)01417-0 [pii] [DOI] [PubMed] [Google Scholar]
- de Jong R, Toffanin P, Harbers M. Dynamic crossmodal links revealed by steady-state responses in auditory-visual divided attention. International Journal of Psychophysiology. 2010;75:3–15. doi: 10.1016/j.ijpsycho.2009.09.013. [DOI] [PubMed] [Google Scholar]
- De Paepe AL, Crombez G, Spence C, Legrain V. Mapping nociceptive stimuli in a peripersonal fram of reference: Evidence from a temporal order judgement task. Neuropsychologia. 2014 Jan 31;(56C):219–228. doi: 10.1016/j.neuropsychologia.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T. Somatosensory, multisensory, and task-related neurons in cortical area 7b (PF) of unanesthetized monkeys. J Neurophysiol. 1994;72(2):542–564. doi: 10.1152/jn.1994.72.2.542. [DOI] [PubMed] [Google Scholar]
- Driver J, Spence C. Attention and the crossmodal construction of space. Trends in Cognitive Sciences. 1998;2(7):254–262. doi: 10.1016/S1364-6613(98)01188-7. [DOI] [PubMed] [Google Scholar]
- Eimer M. Crossmodal links in spatial attention between vision, audition, and touch: evidence from event-related brain potentials. Neuropsychologia. 2001;39(12):1292–1303. doi: 10.1016/s0028-3932(01)00118-x. [DOI] [PubMed] [Google Scholar]
- Frigo M, Johnson SG. FFTW: an adaptative software architecture for the FFT. Paper presented at the International Conference of Acoustics, Speech, and Signal Processing; Seattle, WA. June; 1998. [Google Scholar]
- Garcia-Larrea L. The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiol Clin. 2012;42(5):299–313. doi: 10.1016/j.neucli.2012.06.001. doi: S0987-7053(12)00260-2 [pii] [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol Clin. 2003;33(6):279–292. doi: 10.1016/j.neucli.2003.10.008. doi: S0987705303000790 [pii] [DOI] [PubMed] [Google Scholar]
- Giabbiconi CM, Dancer C, Zopf R, Gruber T, Muller MM. Selective spatial attention to left or right hand flutter sensation modulates the steady-state somatosensory evoked potential. Brain Res Cogn Brain Res. 2004;20(1):58–66. doi: 10.1016/j.cogbrainres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Giabbiconi CM, Trujillo-Barreto NJ, Gruber T, Muller MM. Sustained spatial attention to vibration is mediated in primary somatosensory cortex. Neuroimage. 2007;35(1):255–262. doi: 10.1016/j.neuroimage.2006.11.022. doi: S1053-8119(06)01158-X [pii] [DOI] [PubMed] [Google Scholar]
- Giani AS, Ortiz E, Belardinelli P, Kleiner M, Preissl H, Noppeney U. Steady-state responses in MEG demonstrate information integration within but not across the auditory and visual senses. Neuroimage. 2012;60(2):1478–1489. doi: 10.1016/j.neuroimage.2012.01.114. doi: S1053-8119(12)00132-2 [pii] [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81(3):273–282. doi: 10.1016/S0304-3959(99)00021-4. doi: S0304395999000214 [pii] [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86(1):402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2(5):403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Networks. 2000;13(4–5):411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Exp Brain Res. 2010;205(1):1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- Keitel C, Schroger E, Saupe K, Muller MM. Sustained selective intermodal attention modulates processing of language-like stimuli. Experimental Brain Research. 2011;213(2–3):321–327. doi: 10.1007/s00221-011-2667-2. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Douglass DK. The role of the cerebral cortex in the experience of pain. In: Bromm B, Desmedt JE, editors. Pain and the brain: from nociception to cognition. New York: Raven Press; 1995. pp. 21–34. [Google Scholar]
- Kenshalo DR, Iwata K, Sholas M, Thomas DA. Response properties and organization of nociceptive neurons in area 1 of monkey primary somatosensory cortex. Journal of Neurophysiology. 2000;84(2):719–729. doi: 10.1152/jn.2000.84.2.719. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Jr, Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which monkeys perceive the intensity of noxious thermal stimulation. Brain Res. 1988;454(1–2):378–382. doi: 10.1016/0006-8993(88)90841-4. doi: 0006-8993(88)90841-4 [pii] [DOI] [PubMed] [Google Scholar]
- Legrain V, Bruyer R, Guerit JM, Plaghki L. Involuntary orientation of attention to unattended deviant nociceptive stimuli is modulated by concomitant visual task difficulty. Evidence from laser evoked potentials. Clin Neurophysiol. 2005;116(9):2165–2174. doi: 10.1016/j.clinph.2005.05.019. doi: S1388-2457(05)00223-3 [pii] [DOI] [PubMed] [Google Scholar]
- Legrain V, Guerit JM, Bruyer R, Plaghki L. Attentional modulation of the nociceptive processing into the human brain: selective spatial attention, probability of stimulus occurrence, and target detection effects on laser evoked potentials. Pain. 2002;99(1–2):21–39. doi: 10.1016/s0304-3959(02)00051-9. doi: S0304395902000519 [pii] [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded A salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. doi: S0301-0082(10)00175-9 [pii] [DOI] [PubMed] [Google Scholar]
- Legrain V, Mancini F, Sambo CF, Torta DM, Ronga I, Valentini E. Cognitive aspects of nociception and pain: bridging neurophysiology with cognitive psychology. Neurophysiol Clin. 2012;42(5):325–336. doi: 10.1016/j.neucli.2012.06.003. doi: S0987-7053(12)00262-6 [pii] [DOI] [PubMed] [Google Scholar]
- Liang M, Mouraux A, Iannetti GD. Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: evidence from dynamic causal modeling of functional magnetic resonance imaging data. J Neurosci. 2011;31(24):8976–8985. doi: 10.1523/JNEUROSCI.6207-10.2011. doi: 31/24/8976 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman DC, editors. Detection theory: a user's guide. Mahwah,New Jersey: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- McMahon SB, Wall PD. Wall and Melzack's textbook of pain. 6th ed. Oxford: Saunders; 2013. [Google Scholar]
- Morgan ST, Hansen JC, Hillyard SA. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proc Natl Acad Sci U S A. 1996;93(10):4770–4774. doi: 10.1073/pnas.93.10.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Steinmetz MA, Romo R. Frequency discrimination in the sense of flutter: psychophysical measurements correlated with postcentral events in behaving monkeys. J Neurosci. 1990;10(9):3032–3044. doi: 10.1523/JNEUROSCI.10-09-03032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Across-trial averaging of event-related EEG responses and beyond. Magn Reson Imaging. 2008;26:1041–1054. doi: 10.1016/j.mri.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD, Colon E, Nozaradan S, Legrain V, Plaghki L. Nociceptive steady-state evoked potentials elicited by rapid periodic thermal stimulation of cutaneous nociceptors. J Neurosci. 2011;31(16):6079–6087. doi: 10.1523/JNEUROSCI.3977-10.2011. doi: 31/16/6079 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Plaghki L. Are laser-evoked brain potentials modulated by attending to first or second pain? Pain. 2007;129(3):321–331. doi: 10.1016/j.pain.2006.10.018. doi: S0304-3959(06)00586-0 [pii] [DOI] [PubMed] [Google Scholar]
- Mouraux A, Plaghki L. Cortical interactions and integration of nociceptive and non-nociceptive somatosensory inputs in humans. Neuroscience. 2007b;150(1):72–81. doi: 10.1016/j.neuroscience.2007.08.035. doi: S0306-4522(07)01089-5 [pii] [DOI] [PubMed] [Google Scholar]
- Mozolic JL, Joyner D, Hugenschmidt CE, Peiffer AM, Kraft RA, Maldjian JA, et al. Cross-modal deactivations during modality-specific selective attention. BMC Neurol. 2008;8:35. doi: 10.1186/1471-2377-8-35. doi: 1471-2377-8-35 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MM, Andersen S, Trujillo NJ, Valdes-Sosa P, Malinowski P, Hillyard SA. Feature-selective attention enhances color signals in early visual areas of the human brain. Proc Natl Acad Sci U S A. 2006;103(38):14250–14254. doi: 10.1073/pnas.0606668103. doi: 0606668103 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S, Peretz I, Mouraux A. Steady-state evoked potentials as an index of multisensory temporal binding. Neuroimage. 2011;60(1):21–28. doi: 10.1016/j.neuroimage.2011.11.065. doi: S1053-8119(11)01347-4 [pii] [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12(4):376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. doi: S0987-7053(00)00227-6 [pii] [DOI] [PubMed] [Google Scholar]
- Plaghki L, Mouraux A. How do we selectively activate skin nociceptors with a high power infrared laser? Physiology and biophysics of laser stimulation. Neurophysiol Clin. 2003;33(6):269–277. doi: 10.1016/j.neucli.2003.10.003. doi: S0987705303000753 [pii] [DOI] [PubMed] [Google Scholar]
- Plaghki L, Mouraux A. EEG and laser stimulation as tools for pain research. Curr Opin Investig Drugs. 2005;6(1):58–64. [PubMed] [Google Scholar]
- Rauss KS, Pourtois G, Vuilleumier P, Schwartz S. Attentional load modifies early activity in human primary visual cortex. Hum Brain Mapp. 2009;30(5):1723–1733. doi: 10.1002/hbm.20636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan, editor. Human brain electrophysiology. Evoked potentials and evoked magnetics fields in science and medicine. New York: Elsevier; 1989. [Google Scholar]
- Regan MP, He P, Regan D. An audio-visual convergence area in the human brain. Exp Brain Res. 1995;106(3):485–487. doi: 10.1007/BF00231071. [DOI] [PubMed] [Google Scholar]
- Saupe K, Schroger E, Andersen SK, Muller MM. Neural mechanisms of intermodal sustained selective attention with concurrently presented auditory and visual stimuli. Frontiers in Human Neuroscience. 2009;3:58. doi: 10.3389/neuro.09.058.2009. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwent VL, Hillyard SA, Galambos R. Selective attention and the auditory vertex potential. Effects of signal intensity and masking noise. Electroencephalogr Clin Neurophysiol. 1976;40(6):615–622. doi: 10.1016/0013-4694(76)90136-x. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. J Neurosci. 2004;24(47):10702–10706. doi: 10.1523/JNEUROSCI.2939-04.2004. doi: 24/47/10702 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AZ. Steady-state vibration evoked potentials: descriptions of technique and characterization of responses. Electroencephalogr Clin Neurophysiol. 1992;84(3):257–268. doi: 10.1016/0168-5597(92)90007-x. [DOI] [PubMed] [Google Scholar]
- Talsma D, Doty TJ, Strowd R, Woldorff MG. Attentional capacity for processing concurrent stimuli is larger across sensory modalities than within a modality. Psychophysiology. 2006;43(6):541–549. doi: 10.1111/j.1469-8986.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- Talsma D, Doty TJ, Woldorff MG. Selective attention and audiovisual integration: is attending to both modalities a prerequisite for early integration? Cereb Cortex. 2007;17(3):679–690. doi: 10.1093/cercor/bhk016. doi: bhk016 [pii] [DOI] [PubMed] [Google Scholar]
- Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, Schnitzler A. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86(3):1499–1503. doi: 10.1152/jn.2001.86.3.1499. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Delemos KA, Favorov OV, Metz CB, Vierck CJ, Jr, Whitsel BL. Response of anterior parietal cortex to different modes of same-site skin stimulation. J Neurophysiol. 1998;80(6):3272–3283. doi: 10.1152/jn.1998.80.6.3272. [DOI] [PubMed] [Google Scholar]
- Treede RD, Apkarian AV. 5.45 - Nociceptive Processing in the Cerebral Cortex. In: Allan IB, Akimichi K, Gordon MS, Gerald W, Thomas DA, Richard HM, Peter D, Donata O, Stuart F, Gary KB, Bushnell MC, et al., editors. The Senses: A Comprehensive Reference. New York: Academic Press; 2008. pp. 669–697. [Google Scholar]
- Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87(2):113–119. doi: 10.1016/S0304-3959(00)00350-X. [DOI] [PubMed] [Google Scholar]
- Vialatte FB, Maurice M, Dauwels J, Cichocki A. Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Prog Neurobiol. 2010;90(4):418–438. doi: 10.1016/j.pneurobio.2009.11.005. doi: S0301-0082(09)00185-3 [pii] [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Whitsel BL, Favorov OV, Brown AW, Tommerdahl M. Role of primary somatosensory cortex in the coding of pain. Pain. 2013;154(3):334–344. doi: 10.1016/j.pain.2012.10.021. doi: S0304-3959(12)00607-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel BL, Favorov OV, Li Y, Quibrera M, Tommerdahl M. Area 3a neuron response to skin nociceptor afferent drive. Cereb Cortex. 2009;19(2):349–366. doi: 10.1093/cercor/bhn086. doi: bhn086 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]