Abstract
Disorders of thyroid function are common, and screening, diagnosis, and management are often performed by primary care providers. While management of significant biochemical abnormalities is reasonably straight forward, laboratory tests only slightly outside, or even within, the normal range are becoming more difficult to appropriately manage. A large part of this increasing difficulty in appropriate management is caused by patients requesting, and even demanding, certain tests or treatments that may not be indicated. Symptoms of thyroid dysfunction are non-specific and extremely prevalent in the general population. This, along with a growing body of information available to patients via the lay press and internet suggesting that traditional thyroid function testing is not reliable, has fostered some degree of patient mistrust. Increasingly, when a physician informs a patient that their thyroid is not the cause of their symptoms, the patient is dissatisfied and even angry. This review aims to clarify the interpretation of normal and mild abnormalities of thyroid function tests by describing pituitary-thyroid physiology and through an in depth review of, arguably, the three most important biochemical tests of thyroid function: TSH, free T4, and anti-TPO antibodies. It is important for primary care providers to have an understanding of the shortcomings and proper interpretation of these tests to be better able to discuss thyroid function with their patients.
Keywords: Thyroid disease, TSH, Primary care
Functional disorders of the thyroid (hypothyroidism and hyperthyroidism) are common and, in many cases, managed by primary care providers. In addition to diagnosed cases, there are many patients who present to their provider seeking evaluation of their thyroid status as a possible cause of a variety of complaints including obesity, mood changes, hair loss, and fatigue. There is an ever-growing body of literature in the public domain, whether in print or internet-based, suggesting that thyroid conditions are under-diagnosed by physicians and that standard thyroid function tests are unreliable. Primary care providers are often the first to evaluate these patients and order biochemical testing. This has become a more complex process, with many patients requesting and even demanding certain biochemical tests that may not be indicated. This review aims to describe three important biochemical tests of thyroid status (thyroid stimulating hormone [TSH], free thyroxine [free T4], and anti-thyroid peroxidase antibodies [anti-TPO ABs]) the primary care provider should be comfortable not only ordering and interpreting, but also not ordering in many circumstances. Discussion will include the indications, utility, and potential short-comings of these tests in relation to the scrutiny that has been placed on their accuracy and validity by a growing number of patients.
OVERVIEW OF NORMAL THYROID PHYSIOLOGY
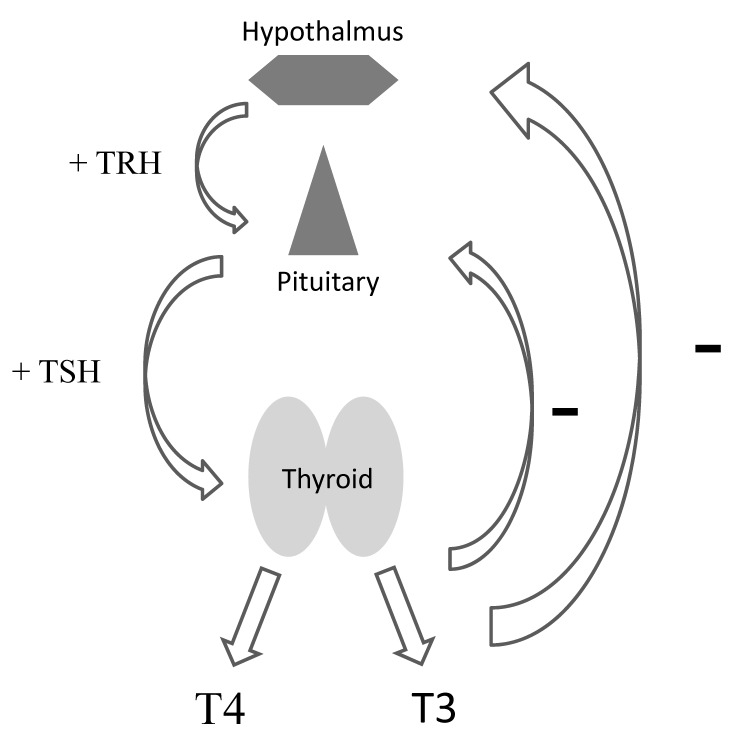
The proper interpretation of thyroid function tests requires an understanding of thyroid physiology. Thyroid function is regulated by a relatively straightforward relationship between the hypothalamus, pituitary, and the thyroid gland itself (figure 1). Thyrotropin releasing hormone (TRH) from the hypothalamus stimulates the release of TSH from the pituitary gland which, in turn, regulates a variety of steps in the production of thyroid hormones from the uptake of iodine to the regulation of enzymatic steps in the process. The majority of thyroid hormone released by the gland (~ 85%) is thyroxine (T4), while a smaller proportion (~15%) is tri-iodothyronine (T3). These thyroid hormones are highly protein-bound (99.8%), with only the free components (free T3 and free T4) having the ability to bind to their respective receptors. The active thyroid hormone is free T3, and there is tissue-specific regulation of the conversion of T4 to T3 by a set of deiodinase enzymes peripherally allowing each tissue to, in a sense, self-regulate its exposure to free T3. This is crucial, because different tissues require different levels of T3. This conversion of T4 to T3 is how treatment of hypothyroidism with levothyroxine (T4 only) still allows for adequate, tissue-specific, T3 exposure.
Figure 1.
Hypothalamic-pituitary-thyroid axis (TRH: Thyrotropin releasing hormone, TSH: Thyroid stimulating hormone, T3: tri-iodothyronine and T4: thyroxine).
Next, it is essential to appreciate the negative feedback of free T3 and free T4 at the level of the hypothalamus and pituitary (see figure 1). Also, the relationship between these thyroid hormones and TSH is not linear but log-linear, such that very small changes in free T3 and/or free T4 will result in very large changes in TSH. Conversely, very small changes in TSH reflect extremely minute changes in free T3 and free T4. For instance, a 2-fold change in free T4 will result in a 100-fold change in TSH. Thus, a free T4 change from 1.0 ng/dL to 0.5 ng/dL will result in a TSH rise from 0.5 mIU/mL to 50 mIU/mL. On the other hand, a rise in TSH from 1.0 mIU/mL to 5.0 mIU/mL reflects a drop in free T4 from 1.0 ng/dL to just 0.9 ng/dL. It is also important to note that each individual has a set point for their own free T3 and free T4 level that is quite stable in the absence of disease. Therefore, changes in any given patient’s free T3 and/or free T4 within the normal range will result in an abnormal TSH value. This supports the role of TSH, in the absence of hypothalamic/pituitary disease, as the most sensitive marker of thyroid function. Table 1 lists common patterns of thyroid function tests and their interpretation, assuming an intact hypothalamic-pituitary-thyroid axis and the absence of significant non-thyroidal illness. These interpretations will be valid in the vast majority of non-hospitalized patients presenting to the primary care provider.
Table 1.
Interpretation of common patterns of thyroid function tests
| TSH | Free T3 and/or Free T4 | Diagnosis |
|---|---|---|
| → | → | Euthyroidism |
| ↑* | → | Subclinical hypothyroidism |
| ↑† | ↓ | Overt hypothyroidism |
| ↓‡ | → | Subclinical hyperthyroidism |
| ↓§ | ↑ | Overt hyperthyroidism |
→ Within normal range; ↑above normal range; ↓below normal range
generally < 8.0 mIU/mL;
generally ≥ 8.0 mIU/mL;
generally > 0.1 mIU/mL;
generally < 0.1 mIU/mL
NON-THYROIDAL ILLNESS
As mentioned previously, thyroid function tests can be difficult to reliably interpret in patients who are acutely ill, and the severity of the illness plays a role as well. As such, thyroid function tests should be interpreted with extreme caution in hospitalized patients and in those recently discharged from the hospital. The term used to describe these non-specific effects on thyroid function tests is non-thyroidal illness (previously termed euthyroid sick syndrome). An in depth discussion of the pathophysiology of non-thyroidal illness is beyond the scope of this review, but the interested reader is referred to a recent summary by Farwell.1 Most important to this discussion is that the TSH may be mildly suppressed in acute illness and mildly elevated during recovery from acute illness. However, TSH values are typically not suppressed to < 0.1 mIU/mL or elevated to > 10 mIU/mL in these circumstances. Thus, a patient seen by their primary care provider with a minimally abnormal TSH in, or recently discharged from, the hospital should simply have the thyroid function tests repeated in several weeks. This review will, however, focus on the non-hospitalized patient.
THYROID STIMULATING HORMONE (TSH)
Assessment of TSH is the single most useful test of thyroid function in the vast majority of patients. Primary care providers should seldom need to order any other biochemical thyroid test. In most cases the TSH will be within the normal range, and no further testing is indicated. However, providers should be aware of several important issues in the interpretation of a TSH value. The importance of these issues is mainly that any clinical decision should not be made (in a non-pregnant patient) based on a single TSH value if it is within or close to the normal range.
Physiologic Issues in Assessing TSH Values
Normal Range
Considerable literature exists regarding what the normal range for TSH really should be, and this topic is covered at length in recent reviews2,3 and in clinical guidelines for the diagnosis and management of hypothyroidism.4,5 Indeed, even though the normal range for TSH is generally listed at between 0.35 mIU/mL and 4.50 mIU/mL, it is likely that the “most normal” range is between 0.5 mIU/mL and 2.50 mIU/mL. It is for this reason that the target TSH in the management of hypothyroidism is within this latter range.4,5 Note that the goal range for replacement therapy is different than the range at which hypothyroidism is diagnosed (ie, while a TSH of 4.2 mIU/mL may warrant an increase in levothyroxine (L-T4) dose, it does not warrant initiation of replacement therapy in a non-pregnant patient). The mean inter-assay precision for the TSH measurement is generally good. The method used at our institution has a precision of ~3.2%, indicating that variations in the result over time in a single patient are not likely to be related to the assay itself but rather to other factors (see below). Lastly, it is important to understand that normal ranges are calculated based on the 2.5th to 97.5th percentiles of the distribution of values measured in the population tested. Therefore, 2.5% of people with completely normal thyroid function will have a TSH slightly below the listed normal range (and 2.5% slightly above the normal range).
While there may be slight differences in TSH reference ranges based on race,6 the effect of age on normal range appears to be more important, especially in advanced age.7 In an analysis of the National Health and Nutrition Examination Survey (NHANES) III database, the upper limit of normal for TSH at the 97.5th percentile was 3.5 mIU/mL for 20–29 year olds increasing to 4.5 mIU/mL for 50–70 year olds and 7.5 mIU/mL for those over the age of 80 years. This age-related increase in TSH may be an adaptive mechanism, as there is evidence showing increased mortality in advanced age as TSH declines within the normal range.8
Pregnancy is the one circumstance wherein initiation or adjustment of replacement therapy with L-T4 is indicated for a TSH within the upper normal range.4 While several articles about this very important issue are available for the interested reader,9–11 most, if not all, of these patients should be managed by an endocrinologist.
Circadian Variability
It is not generally appreciated by primary care providers that TSH secretion follows a circadian rhythm, with maximal levels seen in the early morning and a nadir in the late afternoon to mid-evening.12 While generally not resulting in TSH values outside the normal range, this circadian rhythm may cause a variation in TSH by a mean of between 0.95 mIU/mL to 2.0 mIU/mL,13 which could affect treatment decisions. Also, this circadian rhythm appears to remain intact in patients with subclinical hypothyroidism, and in one small study 50% of patients with a mildly elevated TSH between 8:00–9:00 am had a normal TSH (< 4.0 mIU/mL) when assessed between 2:00–4:00 pm.14
Individual Variation
Also underappreciated is the individual variation in TSH that occurs for no apparent reason. In a study assessing TSH values monthly for one year in healthy men, this apparent random variation occurred with a mean TSH of 0.75 mIU/mL and a range of 0.2–1.6 mIU/mL.15 Thus, current guidelines note that a variation in TSH within the normal range of up to 40–50% does not necessarily indicate a change in thyroid function or status.4 Lastly, as with the circadian rhythm previously noted, this intra-individual variation in TSH appears to also be present in patients with subclinical hypothyroidism.16
Subclinical Hypothyroidism
The definition of subclinical hypothyroidism is a mildly elevated TSH (4.6–8.0 mIU/mL) in the setting of a normal free T4. This biochemical finding may or may not be accompanied by mild symptoms of hypothyroidism. The difficultly in determining which, if any, symptoms are truly related is the non-specific nature of the symptoms of hypothyroidism and the high prevalence of many of these same complaints in the general population. Indeed, approximately 67% of the U.S. population is overweight or obese,17 about 9% suffer from depression;18 over 30% of women have female pattern hair loss,19 and 22%–37% of patients report fatigue.20,21 In the author’s opinion, therefore, greater weight should be placed on the laboratory results versus any particular symptoms that may be present. Furthermore, when discussing these issues with any given patient in the context of their symptoms, it should be explained that if/when the thyroid gland becomes completely non-functional, the TSH rises dramatically to levels often exceeding 100 mIU/mL. Common sense, therefore, needs to be used when attributing any given symptom to a patient’s thyroid status. That is, a TSH of 6.7 mIU/mL is not the cause of extreme fatigue or mood changes, whereas a TSH of 40.0 mIU/mL may indeed explain these same symptoms.
That being said, the prevalence of subclinical hypothyroidism is quite high at between 3.9% and 8.5% (versus the 0.2%–0.4% prevalence of overt hypothyroidism).6,22 The primary care provider will, therefore, encounter many patients with a mildly elevated TSH and a normal free T4, and it is important that they have a clear sense of how to approach these patients. First, reassessment and not immediate replacement therapy is the best initial step in management. Indeed, in one study, spontaneous normalization occurred in 52% of patients with an initial TSH of 5.0–9.9 mIU/mL, while only 5.6% developed overt hypothyroidism over a mean follow-up of 31.7 months.23 It is extremely useful to have an understanding of the 20-year follow-up data of the Whickham survey in this regard.24 In that study, subjects with an elevated TSH (> 6.0 mIU/mL) alone developed overt hypothyroidism at a rate of just 2.6% per year; or 33% over the 20 years of follow-up.24 The presence of anti-TPO antibodies in addition to an elevated TSH imparted a higher risk of 4.3% per year or 55% at the end of follow-up. Therefore, it appears that many patients with a mildly elevated TSH can be followed without L-T4 treatment. This option, however, has to involve a careful discussion with the patient. It is important for the provider to acknowledge the patient’s symptoms, but also to put the severity of those symptoms in the context of the degree of TSH abnormality. While the author does not recommend it, some providers consider a 3-month trial of low dose L-T4 therapy in patients with persistent mild elevation of TSH, then continuing therapy in those with improved symptoms and stopping it in those with no improvement. Lastly, as mentioned earlier, it is likely that elderly patients are a group best to follow without treatment for subclinical hypothyroidism. The interested reader is directed to two excellent reviews of subclinical hypothyroidism.8,25
Subclinical Hyperthyroidism
Subclinical hyperthyroidism is defined as a mildly suppressed TSH (generally still > 0.1 mIU/mL) in a patient without overt symptoms of hyperthyroidism. The primary care provider will see fewer of these patients owing to the lower prevalence of between 0.2–0.9%.6,22,26 As is the case with subclinical hypothyroidism, the most important first step in the management of a mildly suppressed TSH is reassessment over time. The natural history of subclinical hyperthyroidism was shown nicely in the Thyroid Epidemiology, Audit, and Research Study (TEARS).26 In a large cohort of 1507 patients with a baseline TSH 0.1–0.4 mIU/mL followed over time, the progression to overt hyperthyroidism was just 0.5%, 0.7%, and 0% at 2, 5, and 7 years, respectively.26 The majority of patients continued with a mildly suppressed TSH during the same follow-up time periods (71.8%, 55%, and 50.2%), while a significant number spontaneously resolved to normal (16.7%, 31.6%, and 37.6%). In contrast to subclinical hypothyroidism, it is the elderly who likely warrant the most aggressive evaluation and management for subclinical hyperthyroidism, owing to a greater risk of adverse health outcomes.8 Indeed, persistent subclinical hyperthyroidism in an elderly patient or a patient with heart disease or osteoporosis may warrant referral to endocrinology.
When Normal TSH Does Not Reflect Euthyroidism
As mentioned earlier, while there may be some controversy about where the normal range for TSH should lie,2,3 current clinical guidelines are clear that a TSH in the upper normal range does not reflect hypothyroidism.4,5 The potential unreliability of a normal TSH to accurately reflect true thyroid status is an ever-increasing question raised by patients who suffer from a myriad of non-specific complaints. As mentioned previously, many times these complaints are extremely prevalent: overweight (~33%), obesity (~33%), depression (~9%), hair loss (~30%), and fatigue (~30%). The quest for a reason and eventual relief of these burdensome symptoms is understandable and, as such, a normal TSH result may lead to patient dissatisfaction and mistrust of their physician, whom they see as relying more on a biochemical test than their symptoms. Indeed, much of the print and internet-based literature fosters this mistrust of standard biochemical testing and provider reliance on such tests. An understanding of the actual prevalence for a truly unreliable TSH is, therefore, extremely helpful for primary providers as they discuss a normal TSH value with a sometimes skeptical patient.
Hypothalamic and/or Pituitary Disease
As already described, the reliable interpretation of thyroid function tests requires an intact hypothalamic-pituitary-thyroid axis. Thankfully, disruption of this hormonal axis is uncommon to rare and, when present is usually already diagnosed (ie, a patient with a history of a pituitary macroadenoma). The main concern of the primary care provider, then, is to know the prevalence of undiagnosed hypothalamic/pituitary disease causing hypothyroidism. Population-based data on this subject is limited, but Regal et al27 reported a prevalence of hypothyroidism due to hypopituitarism of just 19–29/100,000 (perhaps 1 case per 4355 patients) in an adult Caucasian population in northwestern Spain. This prevalence is likely an underestimate based on flaws in case identification inherent in population-based studies. As such, two possible etiologies deserve further discussion: pituitary macroadenoma and empty sella.
While pituitary microadenoma is quite common (~10% of the population), the vast majority of these small tumors are not large enough to adversely affect normal pituitary function. The prevalence of pituitary macroadenoma, based on data from magnetic resonance imaging (MRI) studies showing incidentally discovered lesions, has been estimated at between 0.16–0.2%.28,29 Importantly, however, not all pituitary macroadenomas adversely affect normal pituitary function, and when they do, limitation of growth hormone secretion and gonadal function are more common than an effect on thyroid function. In three recent studies (totaling ~330 patients) assessing pituitary function in patients with macroadenoma, the prevalence of central hypothyroidism was 13.6–39%.30–32 Based on these data, an estimate of approximately 0.05% (1 per 2000) can then be made as to the prevalence of hypothyroidism related to undiagnosed pituitary macroadenoma.
The prevalence of empty sella can also be estimated based on incidental discovery on MRI imaging. In a study of 500 consecutive subjects undergoing MRI of the brain, Foresti et al33 found 12.4% to have a total empty sella, and an additional 15.6% had a partially empty sella. Looking at radiological and autopsy data on the whole, the prevalence of empty sella is between 5.5% and 35%.34 Just as with pituitary macroadenoma, not all patients with empty sella will have central hypothyroidism, and the data is limited. In five studies involving a total of 343 patients with empty sella, the prevalence of central hypothyroidism was 0–22.2%.34–38 However, it should be noted that the studies with the highest prevalence of central hypothyroidism (14.3% and 22.2%, respectively) were either a retrospective analysis34 or analyzed patients admitted to the hospital38 and, thus, may overestimate the prevalence. The other three studies involving a total of just 87 patients showed a combined prevalence of central hypothyroidism of just 3.4%.35–37 Based on these data (5.5–35% prevalence of empty sella and an estimated prevalence of resultant hypothyroidism of 3.4%), central hypothyroidism due to empty sella would appear to be more common than that due to pituitary macroadenoma: between 1 per 83 and 1 per 526 patients. However, these may still be overestimates because in the population-based data only ~5.5% of cases of hypopituitarism were caused by empty sella versus ~60% being related to pituitary tumors.27
As has been demonstrated, the prevalence of previously undiagnosed central hypothyroidism causing a normal TSH is impossible to estimate reliably. All things considered, perhaps 0.05–0.1% (about 1 case per 1500 patients) may be a reasonable approximation. While price varies widely, a free T4 level may cost between $55 US to $108 US. Assuming proper diagnosis could be based on a single free T4 level documented below the normal range, it would cost between $82,500 US and $162,000 US to identify a single case of undiagnosed central hypothyroidism. This brings up significant issues in terms of the cost-effectiveness of adding a free T4 to confirm the reliability of a normal TSH result.
Other conditions
There are several other possible situations in which a normal TSH may not reflect euthyroidism. These conditions are all quite rare and, thus, will not be discussed at length. The presence of heterophile antibodies (produced as a result of close contact with animals) can potentially interfere with the TSH assay, causing either a falsely high or falsely low result.39 Therefore, it is plausible that a truly elevated TSH could be falsely lowered into the normal range by the presence of heterophile antibodies. Resistance to thyroid hormone (RTH) is a rare condition generally caused by a mutation in the thyroid hormone receptor β gene resulting in elevated levels of free T3 and free T4, but a normal (or slightly elevated) TSH.40 The hallmarks of this condition are goiter, sinus tachycardia, and attention deficit hyperactivity disorder. The presence of a TSH-secreting pituitary adenoma can have laboratory results indistinguishable from RTH and may present with a normal TSH in the setting of hyperthyroidism.41 There are several other fleetingly rare genetic disorders of thyroid hormone transport, metabolism, or action that can result in a falsely normal TSH, but only a handful of cases have thus far been described in the literature.42 Though each of these conditions could possibly result in a normal TSH despite abnormal levels of free T3 and free T4, they are mostly of academic interest. Table 2 lists the pattern of thyroid function tests and prevalence of the conditions that might result in a falsely normal TSH.
Table 2.
Conditions that might result in a falsely normal TSH
| Condition | TSH* | Free T3 and/or Free T4 |
Prevalence |
|---|---|---|---|
| Hypothalamic/pituitary disease | → | ↓ | ~ 1:1500 |
| Heterophile antibodies | → | ↓or ↑ | ~ 1:3000† |
| Resistance to thyroid hormone β | → | ↑ | ~ 1:40,000 |
| TSH-secreting adenoma | → | ↑ | ~ 1:350,000 |
→Within normal range;↑above normal range;↓below normal range
The TSH is not always normal in any of these conditions but for the sake of this discussion it is listed as normal;
While the overall prevalence of heterophile antibodies is ~ 30% only about 0.033% affect the TSH result39
FREE THYROXINE (FREE T4)
Beyond the TSH, assessment of free T4 is the most commonly ordered thyroid function test. In the United States alone, approximately 18 million free T4 tests were performed in 2008 compared to approximately 59 million TSH tests.43 Based on the previous discussions, one could argue that one free T4 test for every three TSH tests likely indicates that far too many free T4 levels are being ordered. Again, the TSH is seldom unreliable in ruling out hypothyroidism, the prevalence of subclinical or overt hyperthyroidism is perhaps just 0.2–0.9%, and the assessment of free T4 is unnecessary in the follow-up of thyroid hormone replacement therapy in the vast majority of patients.4 Having said that, the assessment of free T4 is required in the diagnosis of subclinical hypothyroidism in the setting of a mildly elevated TSH.
An important point to make about the assessment of free T4 (beyond whether it is even indicated) is the reliability of the result. The accuracy of free T4 is highly dependent upon the assay employed, and unfortunately, the assay used in the vast majority of laboratories may not be terribly reliable. While the inter-assay precision of free T4 assays is generally good (~4.3% in our laboratory), the accuracy of that result may be poor. Indeed, in one survey of 13 free T4 methods, four of them had more than 50% of the results NOT meeting the allowable inaccuracy criteria.44 To address this important issue, a working group for the international standardization of the free T4 assay has been formed.45–47 Thus, if the assessment of free T4 may not be necessary in many cases (low pre-test probability), and the reliability is limited, there is likely to be a substantial number of false-positive results.
Most laboratories utilize the direct analog immunoassay (IA) for the measurement of free T4, and again the validity of the results are debated and poorly standardized.43,44,47 Measurement of free T4 by equilibrium dialysis is considered the gold standard but, it is of limited availability to most providers. There is good correlation of free T4 by equilibrium dialysis and free T4 by liquid chromatography-tandem mass spectrometry (LC-MS/MS).48 In a comparison of the IA and LC-MS/MS method in 109 patients, the correlation between free T4 and TSH was quite poor for the IA method and substantially better for LC-MS/MS.49 Indeed, when the TSH was above or below the normal range, the inverse log-linear Pearson correlation was 0.45 for IA and 0.84 for LC-MS/MS. Importantly, when the TSH was in the normal range, the IA method fared even worse: inverse log-linear Pearson correlation just 0.20 (versus 0.74 for LC-MS/MS). For reference, a correlation of ≥ 0.70 signifies a very strong relationship, whereas a value ≤ 0.19 signifies a negligible relationship. Thus, making clinical decisions based on a free T4 in the setting of a normal TSH in the vast majority of instances is fraught with difficulties. Unfortunately, many patients and providers alike question the meaning of a free T4 in the lower half of the normal range in the setting of a normal TSH, especially in the presence of non-specific complaints. These data further support the need to trust the TSH in most cases. Table 3 lists the limited indications for which a free T4 test should be ordered.
Table 3.
Indications for ordering a free T4
| • Assess the degree of hyperthyroidism when TSH is suppressed |
| • To confirm the diagnosis of subclinical hypothyroidism in the setting of a mildly elevated TSH |
| • Monitor response to anti-thyroid drug therapy and radioiodine (TSH may not be reliable in the initial months after therapy) |
| • Monitor L-thyroxine therapy in patients with known pituitary disease (TSH is not reliable) |
| • Single assessment to assure TSH is a reliable indicator of thyroid function? |
ANTI-THYROID PEROXIDASE ANTIBODIES (ANTI-TPO ABS)
Thyroid peroxidase is one of the key enzymes involved in the synthesis of T3 and T4, catalyzing several steps in the process. The presence of anti-TPO ABs is a hallmark of autoimmune thyroid disease, especially Hashimoto’s thyroiditis, but also being highly prevalent in postpartum thyroiditis and Graves’ disease.50 In addition, the presence of anti-TPO ABs is common in euthyroid subjects. For instance, in the NHANES III study, 14.6% and 8.0% of euthyroid females and males, respectively, had such antibodies.6 As mentioned previously, the presence of anti-TPO ABs predicts the risk of developing overt hypothyroidism in both euthyroid subjects and those with subclinical hypothyroidism. Indeed, the Whickham survey demonstrated a rate of progression to overt hypothyroidism of 2.1% per year based on just the presence of anti-TPO ABs (with a normal TSH) with an increase in that risk to 4.3% per year with a combination of a TSH > 6.0 and positive anti-TPO ABs.24 Therefore, it is arguable whether the assessment of anti-TPO ABs is of any clinical utility in the evaluation of subclinical hypothyroidism beyond predicting the rate or risk of progression to overt hypothyroidism. There is also evidence that the development of anti-TPO ABs is the result of lymphocytic infiltration of the thyroid gland as opposed to the cause of that infiltration or damage.50,51 Thus, there is really nothing to be done about the presence of these antibodies. As such, recent guidelines give this practice a grade “B,” as it is only predictive in nature.4 In the author’s opinion, the assessment of anti-TPO ABs should be discouraged in the assessment of subclinical hypothyroidism and should never be performed when the TSH is normal (except in the circumstances involving pregnancy as described in following paragraphs). Not enough can be said as to the possible adverse psychological effect on a patient of a positive anti-TPO AB level. If found to be anti-TPO AB positive, some patients will feel themselves as having a condition or “disease” for which nothing is being done and may continually blame non-specific symptoms on the presence of the antibodies themselves. The assessment of anti-TPO AB is completely unnecessary in patients with overt hypothyroidism, as management will not change based on the presence or absence of antibodies. Furthermore, virtually all hypothyroid patients in iodine-sufficient areas of the world without a prior history of thyroid surgery or radioactive iodine therapy will have Hashimoto’s thyroiditis, making the assessment of anti-TPO ABs to determine the etiology of overt hypothyroidism superfluous.
One instance where assessment of anti-TPO AB is recommended (even when the TSH is normal) is in some women in relation to pregnancy or planned pregnancy. Because of the importance of maintaining euthyroidism in pregnancy, the pre-conception identification of women at risk for hypothyroidism is essential. However, this does not mean that all women should have anti-TPO ABs testing preconception. Rather, just those women at higher risk for autoimmune thyroid disease, such as those with a family history of thyroid disease or a personal history of other autoimmune disease (such as type 1 diabetes or Addison’s disease), should be tested. Another instance in which assessment of anti-TPO ABs is recommended is in the setting of infertility and/or recurrent miscarriage—grade “A” in clinical guidelines.4 Many endocrinologists and gynecologists will recommend treatment with low-dose L-T4 therapy in anti-TPO ABs positive, euthyroid patients with a history of infertility and/or recurrent miscarriage. However, the efficacy of this practice is not fully proven with some,52 but not all,53 studies showing benefit.
OTHER THYROID FUNCTION TESTS
There are many other tests of thyroid status that can be ordered by providers beyond the three discussed in this review. However, it should be noted that these remaining tests are seldom needed, even by endocrinologists outside of very well-defined clinical scenarios. Again, the only test of thyroid function needed by the vast majority of patients seen in primary care is the TSH, despite what patients themselves may request or demand. Table 4 lists these other thyroid tests and their main clinical use.
Table 4.
Other tests of thyroid function/status
| Thyroid test | Clinical utility |
|---|---|
| Free T3 | To determine the degree of hyperthyroidism when TSH is suppressed (sometimes used in conjunction with free T4) |
| Reverse T3 | No clinical utility (elevated in non-thyroidal illness) |
| Free thyroxine index | Evaluation and management of hyperthyroidism during pregnancy |
| Thyroid stimulating immunoglobulin & TSH receptor antibodies | Evaluation of the cause of hyperthyroidism (used in conjunction with thyroid uptake and scan and/or at times when radioiodine scanning cannot be performed (i.e. pregnancy)) |
| Thyroglobulin | Follow-up of differentiated thyroid cancer |
| Anti-thyroglobulin antibodies | Only useful in conjunction with thyroglobulin (to assure reliability of thyroglobulin result) |
| Calcitonin | Diagnosis and follow-up of medullary thyroid cancer |
| 24 hour urine iodine | To assure excess iodine from amiodarone or i.v. contrast has dissipated from the body |
| Total T3 and T4 | No clinical utility with the availability of assays for free hormone levels |
| Free T4 by equilibrium dialysis | Considered the gold standard for free T4 measurement (seldom ordered as the test is costly and not widely available) |
CONCLUSION
Primary hypothyroidism is one of the most common endocrine disorders encountered and managed by primary care providers. Unfortunately, the symptoms of hypothyroidism are extremely non-specific and otherwise highly prevalent in the population. Therefore, providers need to rely on biochemical testing to confirm or rule-out the diagnosis of hypothyroidism. This long-standing reliance on the TSH has come under increased scrutiny in the public domain, and many alternative and traditional medicine providers are now questioning the reliability of standard biochemical testing of thyroid function. Many patients struggle with a multitude of these non-specific complaints, and in their quest for answers become upset when they are told their thyroid function is normal. As reviewed, true hypothyroidism in the setting of a normal TSH is highly unlikely, with an estimated prevalence of perhaps 1 case per 1500 patients. It is uncertain, therefore, whether the assessment of a single free T4 is cost-effective in the assessment of a patient’s thyroid status. If a free T4 test is obtained, the limitations of the assay method employed need to be considered. Lastly, the assessment of anti-TPO ABs should be avoided in non-pregnant patients with a normal TSH, as treatment decisions based on the presence or absence of these antibodies is not supported by current clinical guidelines. The increasingly maligned TSH is still the best, and often only, thyroid function test that is needed in the assessment of most patients.
REFERENCES
- 1.Farwell AP. Nonthyroidal illness syndrome. Curr Opin Endocrinol Diabetes Obes 2013;20:478–484. [DOI] [PubMed] [Google Scholar]
- 2.Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 2005;90:5483–5488. [DOI] [PubMed] [Google Scholar]
- 3.Surks MI, Goswami G, Daniels GH. The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab 2005;90:5489–5496. [DOI] [PubMed] [Google Scholar]
- 4.Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, Pessah-Pollack R, Singer PA, Woeber KA; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012;18:988–1028. [DOI] [PubMed] [Google Scholar]
- 5.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014;24:1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–499. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B. The normal TSH reference range: what has changed in the last decade? J Clin Endocrinol Metab 2013;98:3584–3587. [DOI] [PubMed] [Google Scholar]
- 8.Franklyn JA. The thyroid–too much and too little across the ages. The consequences of subclinical thyroid dysfunction. Clin Endocrinol 2013;78:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med 2004;351:241–249. [DOI] [PubMed] [Google Scholar]
- 10.Abalovich M, Vazquez A, Alcaraz G, Kitaigrodsky A, Szuman G, Calabrese C, Astarita G, Frydman M, Gutiérrez S. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancy. Thyroid 2013;23:1479–1483. [DOI] [PubMed] [Google Scholar]
- 11.Teng W, Shan Z, Patil-Sisodia K, Cooper DS. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol 2013;1:228–237. [DOI] [PubMed] [Google Scholar]
- 12.Persani L, Terzolo M, Asteria C, Orlandi F, Angeli A, Beck-Peccoz P. Circadian variations of thyrotropin bioactivity in normal subjects and patients with primary hypothyroidism. J Clin Endocrinol Metab 1995;80:2722–2728. [DOI] [PubMed] [Google Scholar]
- 13.Keffer JH. Preanalytical considerations in testing thyroid function. Clin Chem 1996;42:125134. [PubMed] [Google Scholar]
- 14.Sviridonova MA, Fadeyev VV, Sych YP, Melnichenko GA. Clinical significance of TSH circadian variability in patients with hypothyroidism. Endocr Res 2013;38:24–31. [DOI] [PubMed] [Google Scholar]
- 15.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 2002;87:1068–1072. [DOI] [PubMed] [Google Scholar]
- 16.Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function tests in patients with stable untreated subclinical hypothyroidism. Thyroid 2008;18:303–308. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatric Dis Treat 2011;7:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atanaskova Mesinkovska N, Bergfeld WF. Hair: what is new in diagnosis and management? Female pattern hair loss update: diagnosis and treatment. Dermatol Clin 2013;31:119127. [DOI] [PubMed] [Google Scholar]
- 20.Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res 1998;45:53–65. [DOI] [PubMed] [Google Scholar]
- 21.Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA. Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce. J Occup Environ Med 2007;49:672–679. [DOI] [PubMed] [Google Scholar]
- 22.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000;160:526–534. [DOI] [PubMed] [Google Scholar]
- 23.Diez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab 2004;89:4890–4987. [DOI] [PubMed] [Google Scholar]
- 24.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. [DOI] [PubMed] [Google Scholar]
- 25.Fatourechi V. Subclinical hypothyroidism: an update for primary care physicians. Mayo Clin Proc 2009;84:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): the natural history of endogenous subclinical hyperthyroidism. J Clin Endocrinol Metab 2011;96:E1–8. [DOI] [PubMed] [Google Scholar]
- 27.Regal M, Paramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol (Oxf) 2001;55:735–740. [DOI] [PubMed] [Google Scholar]
- 28.Nammour GM, Ybarra J, Naheedy MH, Romeo JH, Aron DC. Incidental pituitary macroadenoma: a population-based study. Am J Med Sci 1997;314:287–291. [DOI] [PubMed] [Google Scholar]
- 29.Yue NC, Longstreth WT, Jr, Elster AD, Jungreis CA, O’Leary DH, Poirier VC. Clinically serious abnormalities found incidentally at MR imaging of the brain: data from the Cardiovascular Health Study. Radiology 1997;202:41–46. [DOI] [PubMed] [Google Scholar]
- 30.Cury ML, Fernandes JC, Machado HR, Elias LL, Moreira AC, Castro M. Non-functioning pituitary adenomas: clinical feature, laboratorial and imaging assessment, therapeutic management and outcome. Arq Bras Endocrinol Metabol 2009;53:31–39. [DOI] [PubMed] [Google Scholar]
- 31.Caputo C, Sutherland T, Farish S, McNeill P, Ng KW, Inder WJ. Gender differences in presentation and outcome of nonfunctioning pituitary macroadenomas. Clin Endocrinol (Oxf) 2013;78:564–570. [DOI] [PubMed] [Google Scholar]
- 32.Robenshtok E, Benbassat CA, Hirsch D, Tzvetov G, Cohen ZR, Iraqi HM, Gorshtein A, Toledano Y, Shimon I. Clinical course and outcome of nonfunctioning pituitary adenomas in the elderly compared with younger age groups. Endocr Pract 2014;20:159164. [DOI] [PubMed] [Google Scholar]
- 33.Foresti M, Guidali A, Susanna P. [Primary empty sella. Incidence in 500 asymptomatic subjects examined with magnetic resonance]. Radiol Med 1991;81:803–807. Italian. [PubMed] [Google Scholar]
- 34.Guitelman M, Garcia Basavilbaso N, Vitale M, Chervin A, Katz D, Miragaya K, Herrera J, Cornalo D, Servidio M, Boero L, Manavela M, Danilowicz K, Alfieri A, Stalldecker G, Glerean M, Fainstein Day P, Ballarino C, Mallea Gil MS, Rogozinski A. Primary empty sella (PES): a review of 175 cases. Pituitary 2013;16:270–274. [DOI] [PubMed] [Google Scholar]
- 35.Del Monte P, Foppiani L, Cafferata C, Marugo A, Bernasconi D. Primary “empty sella” in adults: endocrine findings. Endocr J 2006;53:803–809. [DOI] [PubMed] [Google Scholar]
- 36.Cannavo S, Curto L, Venturino M, Squadrito S, Almoto B, Narbone MC, Rao R, Trimarchi F. Abnormalities of hypothalamic-pituitary-thyroid axis in patients with primary empty sella. Journal Endocrinol Invest 2002;25:236–239. [DOI] [PubMed] [Google Scholar]
- 37.Ghatnatti V, Sarma D, Saikia U. Empty sella syndrome - beyond being an incidental finding. Indian J Endocrinol Metab 2012;16:S321–S323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuhur SS, Kuzu I, Ozturk FY, Uysal E, Altuntas Y. Anterior pituitary hormone deficiency in subjects with total and partial primary empty sella: do all cases need endocrinological evaluation? Turk Neurosurg 2014;24:374–379. [DOI] [PubMed] [Google Scholar]
- 39.Stockigt JR, Lim CF. Medications that distort in vitro tests of thyroid function, with particular reference to estimates of serum free thyroxine. Best Pract Res Clin Endocrinol Metab 2009;23:753–767. [DOI] [PubMed] [Google Scholar]
- 40.Onigata K, Szinnai G. Resistance to thyroid hormone. Endocr Dev 2014;26:118–129. [DOI] [PubMed] [Google Scholar]
- 41.Onnestam L, Berinder K, Burman P, Dahlqvist P, Engström BE, Wahlberg J, Nyström HF. National incidence and prevalence of TSH-secreting pituitary adenomas in Sweden. J Clin Endocrinol Metab 2013;98:626–635. [DOI] [PubMed] [Google Scholar]
- 42.Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab 2013;27:745–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thienpont LM, Van Uytfanghe K, Poppe K, Velkeniers B. Determination of free thyroid hormones. Best Pract Res Clin Endocrinol Metab 2013;27:689–700. [DOI] [PubMed] [Google Scholar]
- 44.Steel BW, Wang E, Klee GC, Thienpont LM, Soldin SJ, Sokoll LJ, Winter W, Fuhrman SA, Elin RJ. Analytic bias of thyroid function tests. Arch Pathol Lab Med 2005;129:310317. [DOI] [PubMed] [Google Scholar]
- 45.Thienpont LM, Beastall G, Christofides ND, Faix JD, Leiri T, Miller WG, Miller R, Nelson JC, Ross HA, Ronin C, Rottmann M, Thijssen JH, Toussaint B. International federation of clinical chemistry and laboratory medicine (IFCC), Scientific division working group for standardization of thyroid function tests (WG-STFT). Measurement of free thyroxine in laboratory medicine: proposal of measurand definition. Clin Chem Lab Med 2007;45:563–564. [DOI] [PubMed] [Google Scholar]
- 46.Thienpont LM, Beastall G, Christofides ND, Faix ID, leiri T, Jarrige V, Miller WG, Nelson JC, Ronin C, Ross HA, Rottmann M, Thijssen JH, Toussaint B. IFCC scientific division working group for standardization of thyroid function tests (WG-STFT). Proposal of a candidate international conventional reference measurement procedure for free thyroxine in serum. Clin Chem Lab Med 2007;45:934–936. [DOI] [PubMed] [Google Scholar]
- 47.Thienpont LM, Van Uytfanghe K, Beastall G, Faix JD, Ieiri T, Miller WG, Nelson JC, Ronin C, Ross HA, Thijssen JH, Toussaint B; IFCC Working Group on Standardization of Thyroid Function Tests. Report of the IFCC Working Group for Standardization of Thyroid Function Tests; part 2: free thyroxine and free triiodothyronine. Clin Chem 2010;56:912–920. [DOI] [PubMed] [Google Scholar]
- 48.Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta 2005;358:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem 2011;57:122–127. [DOI] [PubMed] [Google Scholar]
- 50.Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab 2005;19:1–15. [DOI] [PubMed] [Google Scholar]
- 51.Raber W, Gessl A, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hunder fifty-one subjects. Thyroid 2002;12:715–731. [DOI] [PubMed] [Google Scholar]
- 52.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab 2006;91:2587–2591. [DOI] [PubMed] [Google Scholar]
- 53.Yan J, Sripada S, Saravelos SH, Chen ZJ, Egner W, Li TC. Thyroid peroxidase antibody in women with unexplained recurrent miscarriage: prevalence, prognostic value, and response to empirical thyroxine therapy. Fertil Steril 2012;98:378–382. [DOI] [PubMed] [Google Scholar]