Abstract
The Interagency Emergency Health Kit (IEHK) provides a standard package of medicines and simple medical devices for aid agencies to use in emergencies such as disasters and armed conflicts. Despite the increasing burden of non-communicable diseases (NCDs) in such settings, the IEHK includes few drugs and devices for management of NCDs. Using published data to model the population burden of acute and chronic presentations of NCDs in emergency-prone regions, we estimated the quantity of medications and devices that should be included in the IEHK. NCDs considered were cardiovascular diseases, diabetes, hypertension and chronic respiratory disease. In scenario 1 (the primary scenario), we assumed that resources in the IEHK would only include those needed to manage acute life-threatening conditions. In scenario 2, we included resources required to manage both acute and chronic presentations of NCDs. Drugs and devices that might be required included amlodipine, aspirin, atenolol, beclomethasone, dextrose 50%, enalapril, furosemide, glibenclamide, glyceryl trinitrate, heparin, hydralazine, hydrochlorothiazide, insulin, metformin, prednisone, salbutamol and simvastatin. For scenario 1, the number of units required ranged from 12 (phials of hydralazine) to ∼15 000 (tablets of enalapril). Space and weight requirements were modest and total cost for all drugs and devices was approximately US$2078. As expected, resources required for scenario 2 were much greater. Space and cost requirements increased proportionately: estimated total cost of scenario 2 was $22 208. The resources required to treat acute NCD presentations appear modest, and their inclusion in the IEHK seems feasible.
Key questions.
What is already known about this topic?
The Interagency Emergency Health Kit (IEHK) was developed by the WHO to meet the primary care needs of 10 000 people treated for ∼3 months following natural disasters and other emergencies.
Non-communicable diseases (NCDs) such as cardiovascular disease, diabetes, hypertension and chronic lung diseases are major causes of morbidity and mortality and their prevalence is increasing in areas where natural disasters and other emergencies are common.
The IEHK currently contains few drugs for the management of NCDs, and little information is available on which such drugs should be included.
What are the new findings?
Epidemiological data were used to estimate the quantities of medicines that would be needed to manage acute and chronic presentations of the four key NCDs in postemergency settings under various scenarios.
Space and weight requirements for the required medicines were modest when only acute presentations were considered; costs were modest.
Space and weight requirements were up to 20 times higher when both acute and chronic presentations of NCDs were considered, as compared with acute presentations alone
Recommendations for policy
It appears feasible to include the medicines needed to manage acute presentations of NCDs in the IEHK, and the financial cost of their inclusion is low.
If the IEHK is modified to include these medicines, the responsible agencies should commission a prospective needs assessment that evaluates whether this modification is fit for purpose.
Introduction
Non-communicable diseases (NCDs) such as cardiovascular disease, diabetes, cancer and chronic lung diseases are a key health and development challenge worldwide.1–3 Almost three-quarters of all NCD deaths (28 million), and the majority of premature deaths (82%), occur in low-income and middle-income countries (LMICs).4 5 NCDs are projected to account for more than 50% of all disability-adjusted life-years lost in all regions by 2030 except for Africa,6 and the total economic burden of NCDs in LMIC between 2011 and 2030 is $21.3 trillion.7
Emergencies include natural disasters such as floods, earthquakes and severe meteorological events, but also ‘complex emergencies’ often resulting from armed conflict and its consequences. The frequency and severity of emergencies appear to have increased over the past two decades,8 possibly due to the effects of climate change and exacerbated by poverty, urbanisation and overcrowding. The health component of the humanitarian response to emergencies has traditionally focused on management of acute conditions such as trauma and infectious illnesses. However, NCDs are highly prevalent worldwide—and emergencies can increase the risk of acute NCD exacerbations and decrease the ability of health systems to respond.9–16 In addition, population ageing and globalisation have increased the prevalence of NCDs and their risk factors in LMICs—often without compensatory social changes that have mitigated these adverse trends in wealthier nations.17 Therefore, NCDs already account for a substantial burden of illness in emergency settings, and the prevalence of NCDs among people living in disaster-prone areas has increased in recent years.18
The Interagency Emergency Health Kit (IEHK) was developed by the WHO in the 1980s to provide a standard package of medicines and simple medical devices for aid agencies to use in emergencies.19 The IEHK aims to meet the primary care needs of 10 000 people treated for ∼3 months—after which these needs can be met by local procurement efforts that are targeted to specific needs on the ground. The IEHK can be rapidly deployed (within 48 hours of requisition), and has become an important component of the material resources used in international response to disasters and other emergencies.19 However, the IEHK was not designed with management of NCDs in mind.
The composition of the IEHK is reviewed every 5 years and the next revision is due in 2016.20 Given increasing interest in the management of NCDs in emergencies, it seems appropriate to consider whether NCD-related medicines should be included in the 2016 revision. An important limiting factor is the lack of accurate information about what should be included in the kit. Here, we estimate the requirements for medicines that would be needed for acute and chronic NCD management within predicted deployment scenarios, aiming to inform the 2016 IEHK revision.
Methods
Since this work did not involve primary data collection in humans, it did not require external ethics review or approval.
NCDs considered
Consistent with other WHO priorities for NCD management in primary care, cardiovascular diseases (including myocardial infarction, stroke and heart failure), diabetes and chronic respiratory disease (asthma, chronic obstructive pulmonary disease) were addressed (table 1). Hypertension was also included because of its treatable nature and its close association with cardiovascular disease and diabetes.21 Cancer and kidney failure were not included as they generally cannot be managed by primary care clinicians in LMIC. Mental illnesses are covered by existing emergency response schemes and were not considered here.
Table 1.
Target conditions
| Condition | Presentations treated in scenario 1 | Additional presentations treated in scenario 2 |
|---|---|---|
| Coronary disease |
|
|
| Cerebrovascular disease |
|
|
| Heart failure |
|
|
| Hypertension |
|
|
| Diabetes |
|
|
| Chronic lung disease |
|
|
Scenario 1 includes only acute presentations of NCDs. Scenario 2 includes all the presentations in scenario 1 but also the chronic presentations of NCDs in the table. Conditions were assumed to be present in adults only, except for severe hyperglycaemia, severe hypoglycaemia, asthma exacerbation, diabetes, asthma, which were assumed to be present in adults and children.
COPD, chronic obstructive pulmonary disease; IEHK, Interagency Emergency Health Kit; MI, myocardial infarction; NCD, non-communicable disease.
Types of emergencies considered
Emergencies include a range of circumstances ranging from acute (often temporary) situations following a disaster (eg, a tsunami) to protracted and chronic conditions (such as those following armed conflict, ongoing famines and floods). We focused on large-scale emergencies that would lead to the deployment of an IEHK.18 Examples of recent emergencies that meet this criterion include the 2015 Nepal earthquake, the Ebola epidemic in West Africa and the ongoing conflicts in Ukraine and Syria.
Details of the IEHK
One IEHK (kit) consists of 10 basic units and 1 supplementary unit. The basic unit ‘contains essential equipment that can be used by primary healthcare workers with limited training’22 (eg, amoxicillin, ferrous sulfate, adhesive tape). The supplementary unit contains essential medicines and medical devices for 10 000 people, and is for use by physicians. Its contents do not overlap with the basic unit22 (eg, ketamine, ceftriaxone, Foley catheters). The current manuscript concerns estimate for additions to the supplementary unit.
Which NCD-related conditions should be treated?
Two scenarios were considered: in scenario 1 (the primary scenario), resources in the IEHK are restricted to those needed to manage acute conditions (eg, those that would be life-threatening without immediate treatment). In scenario 2, resource requirements for the IEHK include those that might be required for ongoing management of acute and chronic presentations of NCDs (figure 1). Scenario 2 includes management of people whose primary concern is a stable NCD (eg, a patient who presents seeking ongoing management of chronic, stable hypertension) as well as those in whom the stable NCD is discovered incidentally (eg, a patient who presents with an emergency-related fracture and is subsequently found to have chronic stable hypertension). Primary prevention of NCDs was considered out-of-scope for care in emergency settings, and was not addressed in either scenario.
Figure 1.
Scope of conditions to be included. The grey shaded area represents scenario 1, which includes only acute presentations of NCDs. Scenario 2 includes the grey shaded area as well as the area enclosed by the dotted line, which encompasses acute and chronic presentations of NCDs and requires markedly more resources. (3) ‘Follow-up Rx for survivors of 1 and 2’ is only partially enclosed in the grey area, because only 90 days of follow-up is contemplated as compared with the lifelong treatment, that is, required. DM, diabetes mellitus; MI, myocardial infarction; NCD, non-communicable disease; Rx, management.
Which drugs should be provided in the IEHK to treat NCDs?
All items included in the IEHK (by agreement) must be drawn from the WHO Essential Medicines List.22 Also, the IEHK is already large and heavy (1045 kg; 4.6 m3), but must remain portable for use by staff in the field. The WHO Package of Essential NCD interventions (PEN)23 is a comprehensive list of NCD-relevant essential medicines, and includes simple management protocols.23 The proposed management of NCDs in emergency settings as discussed here was based on the treatments and treatment protocols included in the PEN.
What quantity of drugs will be needed in the revised IEHK?
These quantities were estimated based on simple modelling of the population burden of acute and chronic presentations of NCDs in emergency-prone regions. The prevalence of the target conditions in each region was estimated using data from the Global Burden of Disease project24 where possible, supplemented by other credible sources of data such as national surveys if necessary. Costs were estimated based on 2015 UNICEF costs data in US$ (table 2).
Table 2.
Quantity of medicine supplied per user (scenarios 1 and 2) for a 90-day period
| Medication | Supplied as | Quantity required per user | Cost, US$ |
|---|---|---|---|
| Amlodipine | 5 mg | 90 tablets | 0.006/tablet |
| Aspirin | 81 mg (75–100 mg) | 90 tablets | 0.0089/tablet |
| Atenolol | 50 mg | 90 tablets | 0.0039/tablet |
| Beclomethasone | 100 doses of 100 mcg | 2 inhalers (2 times a day) | 4.80/inhaler |
| Dextrose 50% | 50 mL ampoules | 2 ampoules | 0.675/ampoule |
| Enalapril (heart failure) | 5 mg | 360 tablets | 0.0329/tablet |
| Enalapril (all else) | 5 mg | 180 tablets | 0.0329/tablet |
| Furosemide | 20 mg | 360 tablets | 0.0022/tablet |
| Glibenclamide | 2.5 mg | 90 tablets | 0.0035/tablet |
| Glyceryl trinitrate | 0.3 mg | 15 tablets (5 tablets×3 days) | 0.047/tablet |
| Heparin (10 000 U/mL) | 50 000 U phials | 2 phials (12 500 U SC q12 hours×3 days) | 1.99/phial |
| Hydralazine | 20 mg in 1 mL phials | 4 phials | 20.13/phial |
| Hydrochlorothiazide | 12.5 mg | 90 tablets | 0.0026/tablet |
| Insulin 30/70 (adult DM) | 10 mL of 100 U/mL | 2 phials (average of 60 U/days) | 2.40 per phial |
| Insulin 30/70 (child DM) | 10 mL of 100 U/mL | 2 phials (average of 20 U/days) | 2.40/phial |
| Metformin | 500 mg | 270 tablets | 0.007/tablet |
| Prednisone (adult asthma) | 10 mg | 15 tablets (3 tablets×5 days) | 0.0206/tablet |
| Prednisone (child asthma) | 10 mg | 6 tablets (3 tablets×2 days) | 0.0206/tablet |
| Prednisone (COPD) | 10 mg | 21 tablets (3 tablets×7 days) | 0.0206/tablet |
| Salbutamol (asthma) | 200 doses of 100 μg per inhaler | 1 inhaler (2 times a day) | 3.32/inhaler |
| Salbutamol (COPD) | 200 doses of 100 μg per inhaler | 3 inhalers (6 doses per day) | 3.32/inhaler |
| Simvastatin | 20 mg | 90 tablets | 0.0168/tablet |
DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
Key assumptions
We assumed that all patients with an indication for treatment would receive such treatment, although in reality some would decline due to intolerance or preference, and not all would access health systems despite an appropriate indication. Conversely, existing data may underestimate the prevalence of NCDs in LMIC. Since these two limitations may partially offset one another, estimates of prevalence were not adjusted for underdiagnosis. All NCD drugs in the IEHK were assumed to be oral except triglyceryl nitrate (sublingual), heparin (subcutaneous), beclomethasone (inhaled), hydralazine (intravenous), insulin (intravenous or subcutaneous) and salbutamol (inhaled). The conditions to be treated in scenario 1 vs 2 are shown in table 1. Details of the medicines assumed to be required for management of each presentation are shown in table 2; the associated rationale is presented in the online supplementary appendix.
bmjgh-2016-000128supp_appendix.pdf (372.5KB, pdf)
Countries considered as potential deployment scenarios
Target countries were generated from a list of contexts where emergencies have recently occurred (Bangladesh, Central African Republic, Egypt, Guinea, Indonesia, Iraq, Jordan, Lebanon, Liberia, Philippines, Sierra Leone, Syrian Arab Republic, Turkey, Ukraine).
Methods for calculating incidence, prevalence and number of users
The incidence of asthma exacerbations and the prevalence of stable asthma were calculated separately for adults and children. The incidence and prevalence of all other conditions except those related to diabetes (diabetes, severe hyperglycaemia, hypoglycaemia) were calculated for adults only. For each condition, incidence and prevalence were estimated based on a systematic literature search that prioritised information from WHO reports, reports from other UN agencies, and the Global Burden of Diseases project. If the required data were not available from these sources, a broader search of the peer-reviewed English language literature was carried out.
Incidence rates from these sources were standardised to a 90-day period and then adjusted for the increased incidence rates expected during emergencies.9–16 25 26 Estimates of prevalence assumed that all affected individuals would present to health services during the 90-day period following onset of the emergency. Where possible, country-specific estimates of incidence and prevalence were used. In many cases, country-specific data could not be identified, and the best available estimates were assumed to apply in all countries.
These estimates of incidence and prevalence were then applied to country-specific population data27 that yielded the number of adults (aged ≥18 years) versus children (aged <18 years) among a population of 10 000 people whose health needs were being served by one IEHK.
For example, the 3-month incidence of acute myocardial infarction (AMI) per 10 000 people in Bangladesh was calculated as follows. From online supplementary table S2 in Moran et al,28 we extracted the incidence of AMI for females and males of all ages in South Asia as 155 and 245 per 100 000, respectively, for 2010 calendar year. We assumed a 1:1 distribution of females to males. We estimated the number of AMIs expected in this population over 3 months in normal circumstances, then multiplied the baseline incidence by 3 to account for the increased incidence of AMI typically seen during emergencies (see online supplementary appendix). Estimates were rounded up to the nearest whole number. As described in the online supplementary appendix, AMI will be treated with aspirin, heparin and glyceryl trinitrate as per instructions in the online supplementary appendix (detailed assumptions for treatment). The costs of these treatments are given in table 2. A complete spreadsheet of estimates (users, quantities and costs) is available from the authors on request.
Sensitivity analyses for medicine requirements
In scenario 1, we assumed that everyone (adults and children) presenting with severe hyperglycaemia would require insulin management. For scenario 2, all adults with stable diabetes were assumed to be managed using oral hypoglycaemic agents rather than insulin. In reality, some adults would require insulin, but the quantities of insulin required would overwhelm the limited cold chain space available in the IEHK. Therefore, the quantity of insulin required to manage adults in scenario 2 was not estimated. However, children with diabetes were assumed to require insulin in scenario 2.
Results
Tables 3 and 4 present the estimated number of users with acute and chronic presentations (respectively) of the four NCDs of interest.
Table 3.
Estimated number of users per 10 000 population with an acute presentation of an NCD
| Country | Adults with hypertensive urgency | Adults with hypertensive emergency | Adult with hypertension | Adults with severe hyperglycaemia | Adults with severe hypoglycaemia | Children with severe hyperglycaemia | Children with severe hypoglycaemia | Adults with acute MI | Adults with acute stroke | Adults with heart failure | Adults with asthma attacks | Children with asthma attacks | Adults with COPD exacerbation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bangladesh | 11 | 4 | 15 | 41 | 105 | 1 | 1 | 16 | 9 | 6 | 6 | 8 | 25 |
| Central African Republic | 9 | 3 | 12 | 28 | 71 | 1 | 1 | 15 | 13 | 5 | 7 | 10 | 19 |
| Egypt | 11 | 4 | 15 | 31 | 79 | 1 | 1 | 16 | 10 | 6 | 8 | 8 | 27 |
| Guinea | 9 | 3 | 12 | 30 | 77 | 1 | 1 | 13 | 12 | 5 | 7 | 10 | 19 |
| Indonesia | 11 | 4 | 15 | 30 | 78 | 1 | 1 | 11 | 13 | 6 | 8 | 7 | 29 |
| Iraq | 9 | 3 | 12 | 45 | 116 | 1 | 1 | 16 | 9 | 5 | 7 | 10 | 19 |
| Jordan | 10 | 4 | 14 | 71 | 183 | 1 | 1 | 16 | 9 | 6 | 8 | 8 | 21 |
| Lebanon | 13 | 5 | 18 | 59 | 152 | 1 | 1 | 16 | 9 | 7 | 9 | 6 | 35 |
| Liberia | 9 | 3 | 12 | 30 | 78 | 1 | 1 | 13 | 11 | 5 | 7 | 10 | 18 |
| Philippines | 10 | 4 | 14 | 26 | 68 | 1 | 1 | 11 | 13 | 6 | 9 | 9 | 64 |
| Sierra Leone | 9 | 3 | 12 | 33 | 86 | 1 | 1 | 13 | 12 | 5 | 7 | 10 | 18 |
| Syrian Arab Republic | 10 | 4 | 14 | 50 | 130 | 1 | 1 | 16 | 9 | 6 | 7 | 9 | 21 |
| Turkey | 12 | 4 | 16 | 46 | 119 | 1 | 1 | 16 | 9 | 7 | 9 | 7 | 26 |
| Ukraine | 14 | 5 | 19 | 56 | 145 | 1 | 1 | 23 | 32 | 8 | 7 | 4 | 47 |
Acute NCD presentations include acute coronary syndrome, acute stroke, acute heart failure, hypertensive urgency/emergency, acute severe hyperglycaemia or hypoglycaemia, COPD exacerbation, asthma exacerbation.
COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; NCD, non-communicable disease.
Table 4.
Estimated number of users per 10 000 population with a chronic presentation of an NCD
| Country | Adults with hypertension | Adults with diabetes | Children with diabetes | Adults with coronary disease | Adults with cerebrovascular disease | Adults with heart failure | Adults with asthma | Children with asthma | People with asthma | Adults with COPD |
|---|---|---|---|---|---|---|---|---|---|---|
| Bangladesh | 954 | 610 | 26 | 145 | 47 | 16 | 351 | 290 | 641 | 269 |
| Central African Republic | 1398 | 410 | 18 | 242 | 17 | 10 | 391 | 373 | 764 | 205 |
| Egypt | 1021 | 456 | 19 | 207 | 22 | 31 | 463 | 294 | 757 | 295 |
| Guinea | 1361 | 445 | 19 | 188 | 14 | 7 | 373 | 392 | 765 | 207 |
| Indonesia | 1268 | 451 | 19 | 148 | 40 | 16 | 481 | 274 | 755 | 310 |
| Iraq | 856 | 673 | 29 | 207 | 25 | 31 | 390 | 374 | 764 | 201 |
| Jordan | 703 | 1062 | 45 | 207 | 29 | 31 | 439 | 319 | 758 | 226 |
| Lebanon | 1609 | 883 | 37 | 207 | 39 | 31 | 538 | 212 | 750 | 380 |
| Liberia | 1202 | 449 | 19 | 188 | 14 | 7 | 370 | 395 | 765 | 197 |
| Philippines | 830 | 391 | 17 | 148 | 51 | 16 | 543 | 324 | 867 | 260 |
| Sierra Leone | 1648 | 499 | 21 | 188 | 13 | 7 | 379 | 385 | 764 | 191 |
| Syrian Arab Republic | 990 | 752 | 32 | 207 | 30 | 31 | 428 | 332 | 760 | 229 |
| Turkey | 944 | 689 | 29 | 207 | 26 | 31 | 505 | 247 | 752 | 339 |
| Ukraine | 3484 | 843 | 36 | 285 | 68 | 42 | 389 | 140 | 529 | 513 |
Chronic NCD presentations include management of stable coronary disease, heart failure, hypertension, diabetes, COPD or asthma.
COPD, chronic obstructive pulmonary disease; NCD, non-communicable disease.
In scenario 1, users receive a 90-day course of treatment following the acute event. In scenario 2, users receive a 90-day course of treatment following identification with a stable chronic NCD. Therefore, although the indications for treatment and number of users differ for scenarios 1 and 2, the quantity of medicines required for each user is the same for both scenarios. Unit costs for each medication are also presented. Table 5 presents the estimated medicine requirements for scenarios 1 and 2, in strata representing settings with low, median and high NCD incidence.
Table 5.
Estimated medicine requirements in strata representing settings with low, median and high NCD incidence
| Scenario 1 |
Scenario 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Median | Low | High | Median, rounded* |
Cost, US$ |
Median | Low | High | Median, rounded* | Cost, US$ |
| Amlodipine tablets | 630 | 540 | 810 | 500 | 3.00 | 25 650 | 16 470 | 79 200 | 25 500 | 153.00 |
| Aspirin tablets | 2250 | 2160 | 4950 | 2500 | 22.25 | 23 040 | 19 080 | 36 720 | 23 000 | 204.70 |
| Atenolol tablets | 2520 | 2160 | 3600 | 2500 | 9.75 | 44 145 | 34 200 | 107 640 | 44 500 | 173.55 |
| Beclomethasone inhalers | 76 | 70 | 164 | 75 | 360.00 | 2047 | 1898 | 2418 | 2050 | 9840.00 |
| Dextrose 50% amps | 193 | 138 | 368 | 200 | 135.00 | 193 | 138 | 368 | 200 | 135.00 |
| Enalapril tablets | 8280 | 7560 | 14 940 | 8500 | 279.65 | 130 590 | 100 260 | 332 280 | 130 500 | 4293.45 |
| Furosemide tablets | 2160 | 1800 | 2880 | 2000 | 4.40 | 10 440 | 4320 | 18 000 | 10 500 | 23.10 |
| Glibenclamide tablets | – | – | – | – | – | 8820 | 12 510 | 23 940 | 13 000 | 45.50 |
| Glyceryl trinitrate tablets | 323 | 255 | 465 | 500 | 23.50 | 323 | 255 | 465 | 500 | 23.50 |
| Heparin (10 000 U/ml) phials | 32 | 22 | 46 | 25 | 49.75 | 32 | 22 | 46 | 25 | 49.75 |
| Hydralazine phials | 16 | 12 | 20 | 25 | 503.25 | 16 | 12 | 20 | 25 | 503.25 |
| Hydrochlorothiazide tablets | 1620 | 1530 | 2790 | 1500 | 3.90 | 36 720 | 23 850 | 112 590 | 37 000 | 96.20 |
| Insulin 30/70 phials | 80 | 113 | 215 | 125 | 300.00 | 160 | 114 | 305 | 175 | 420.00 |
| Metformin tablets | – | – | – | – | – | 112 455 | 79 380 | 215 190 | 112 500 | 787.50 |
| Prednisone tablets | 636 | 543 | 1533 | 500 | 10.30 | 636 | 543 | 1533 | 500 | 10.30 |
| Salbutamol inhalers | 84 | 71 | 210 | 100 | 332.00 | 1532 | 1408 | 2220 | 1525 | 5063.00 |
| Simvastatin tablets | 2250 | 2160 | 4950 | 2500 | 42.00 | 23 040 | 19 080 | 36 720 | 23 000 | 386.40 |
| Total | 2078.75 | 22 208.20 | ||||||||
*Rounded to the nearest 500 or 25 units as appropriate.
NCD, non-communicable disease.
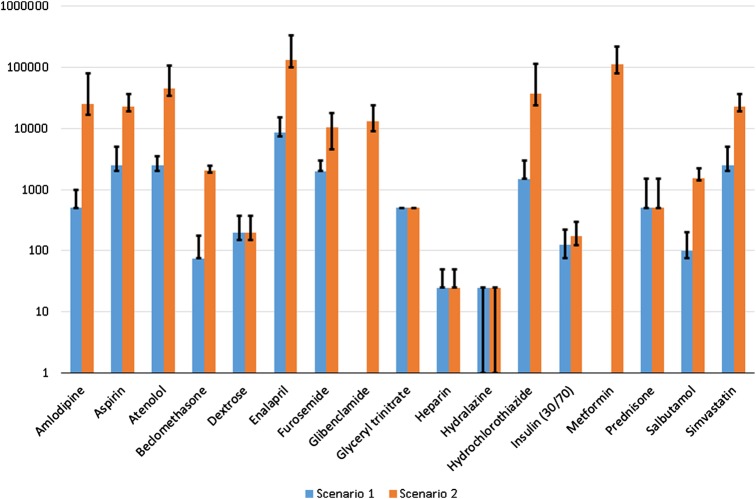
As expected, the quantity of medicines required under scenario 2 is much larger than in scenario 1. The total costs for scenario 2 are 20 times larger than the total costs in scenario 1. Figure 2 compares the total quantity of each medicine required (in the median prevalence setting) for scenarios 1 and 2.
Figure 2.
Medicine requirements by scenario. The Y-axis uses a logarithmic scale to show the median number of units of each medication required for each scenario. The top of the black bars show the maximum number of units required for any one of the included countries. The bottom of the black bars show the minimum number of units required for any one of the included countries.
In both scenarios, requirements for median prevalence settings are generally closer to those in low prevalence settings versus high prevalence settings. The very large quantity of medicines implied by the high prevalence setting suggest that it may be most appropriate to base requirements on the median incidence setting. Figure 2 shows the range of medications implied across the settings considered (minimum for low prevalence; maximum for high prevalence settings).
Discussion
There is increasing interest in improving the care of people with NCDs who are affected by disasters and other emergencies. A potential mechanism for achieving this objective is to modify the IEHK to include medicines that are necessary for the primary care of patients with NCD in LMIC. Here, we have estimated the quantities of medicines that would be needed to achieve this objective under various scenarios, focusing on four key NCDs that account for the majority of NCD-related morbidity and mortality worldwide. We found that the quantities of medicines needed to manage acute NCD complications in emergency settings are modest and inexpensive, suggesting that they warrant serious consideration for inclusion in the 2016 IEHK revision.
There are three key decisions to be made should the IEHK be modified to include medicines for managing the four specified NCDs.
The first and most important is deciding whether or not to include medicines for the management of chronic stable NCDs. The potential health benefits of treating life-threatening exacerbations and manifestations of NCDs appear clear. For example, beginning early statin treatment following myocardial infarction appears to improve cardiovascular outcomes at 30 days compared with placebo.29 Similar but slightly smaller benefits have been observed for β-blockade following myocardial infarction.30 Proponents of proceeding with scenario 2 argue that treatments such as management of stable hypertension are effective and cost-effective; that stable NCDs account for an increasing proportion of presentations to medical services following emergencies; and that it is unethical to deny treatment to the many people affected by these conditions. These facts do not appear to be a convincing rationale for choosing scenario 2 over scenario 1. While treatment of stable chronic NCDs will reduce morbidity and mortality at reasonable costs, these benefits require time to accrue. Although it could be argued that the IEHK should include medicines for management of chronic NCDs in countries where there is a strong pre-existing health system, the IEHK cannot easily be customised for different settings. Equally important, the IEHK is already close to its maximum size and weight—so any additions must be balanced by removal of other materials. Overall, it appears that scenario 1 is more suitable than scenario 2. An option for the future would be to consider a supplementary module that could be deployed only to settings with established capacity for management of chronic NCDs.
The second key decision concerns the quantity of each medicine to provide, given the differences between the country-specific estimates. This presents three options: accept the possibility of oversupply for lower prevalence areas, accept possible undersupply for higher prevalence areas or offer a method for adapting the IEHK to the setting. The latter is not generally regarded as feasible. Given that not all patients with acute presentations of NCDs will seek care and that space/weight within the IEHK are at a premium, accepting possible undersupply in higher prevalence areas appears most prudent.
The third key decision concerns the provision of diagnostic tests (eg, blood troponin and creatinine assays using point of care devices; ECG) in the IEHK. Such tests are included in the WHO PEN under the heading ‘add when resources permit’, so would potentially be in scope for inclusion. However, considerations related to the size and weight of the required equipment preclude its inclusion in the IEHK at present.
It could be argued that an ‘emergency medical kit’ should only include drugs and supplies for urgent conditions. Although the IEHK is deployed to disasters and other emergency situations, it is intended to support primary care—which includes management of urgent and non-urgent conditions. In fact, the IEHK already includes medications to manage such conditions: antacids, oral iron and paracetamol are included in the basic kit, and the supplementary kit includes oral vitamin C and clotrimazole pessaries. Therefore, there does not seem to be a sound rationale for excluding medicines needed for chronic NCD management, such as simvastatin.
In summary, the best approach appears to be resourcing the IEHK to manage acute presentations of NCDs, and accepting the possibility of undersupply in very high prevalence contexts. The recommended quantities should be sufficient to manage the majority of acute NCD presentations during the 90 days following an acute emergency in nearly all settings. However, it is possible that quantities will be insufficient to manage all such patients, especially in countries with a high baseline prevalence of NCDs. In addition, it does not appear feasible to support the management of patients with chronic stable presentations of NCDs using the supplies in the IEHK, or to include simple NCD-related diagnostic tests in the IEHK. These could be longer term objectives, which could perhaps be achieved by developing a module for the IEHK that is specific for NCD management.
Economic considerations
All of the recommended additions are on the WHO Best Buy list, and the focus on acute presentations should yield better value for money than treatment of chronic stable NCDs (since the number needed to treat is lower for the former). The total cost of the recommended additions for scenario 1 is approximately US$2079 per kit, or 10.1% of the total current cost of the IEHK. Costs were∼10 times higher if medicines required to manage scenario 2 were included (table 5).
Ethical considerations
First, survivors of acute presentations of NCDs might appropriately be prescribed lifelong treatment with several medicines—all of which might have to be purchased out of pocket by the patient or his/her family. Therefore, during the transition from the IEHK to local procurement, it will be important to avoid conflicts of interest to ensure that beneficence (rather than inappropriate financial gain) is seen as the primary goal.
Second, treating acute presentations of NCDs could be harmful if withdrawal of the medicines (eg, when the 90 days supply ends) increases the risk of adverse events compared with no treatment. However, we were unable to identify good quality evidence that withdrawal of these medicines is worse than never having initiated them to start with.31 32 Therefore, although it would clearly be preferable to ensure ongoing treatment, uncertainty about whether this goal is realistic should not preclude initiation of therapy.
Third, restricting the scope of the IEHK to acute presentations might raise concerns about lack of distributive justice. However, it is well established that need (in this case, illness acuity) can appropriately be used as a determinant of what aid a given person receives. Another aspect of justice requires consideration in situations where the IEHK is accessible to some people within a region (eg, refugees displaced by conflict33) but not others (eg, local residents). Although this may be acceptable for short periods, it would be desirable to integrate the two populations to avoid inequity and minimise tensions among the different populations.
Practical considerations
An important secondary benefit of the proposed modification to the IEHK is that it may help to engage local physicians in acute and follow-up management of patients with NCD in the postemergency setting. This action will help raise awareness and build capacity for NCD management once the emergency has resolved. Such efforts will be facilitated by WHO's ongoing attempts to strengthen primary care management of NCDs in LMIC outside emergency settings.
The recommended actions in the Sphere guidance34 clarify that the IEHK is simply one component of an appropriate humanitarian response to the burden of NCDs in emergencies. As the baseline (pre-emergency) prevalence of NCDs continues to rise worldwide, practitioners will increasingly encounter patients presenting with an acute emergency-related health need (traumatic injury, acute infection) or social need (bereavement; lack of food, water, shelter) who also have an NCD that requires treatment. The proposed modifications to the IEHK represent a first step towards a more holistic management approach, within the limitations of what is possible in an emergency setting.
Limitations
This work has important limitations that should be considered, many of which are related to the assumptions made in calculating the estimates. First, data on the burden of NCDs in LMICs are limited, and many approximations and extrapolations were necessary. Although we used national estimates of incidence and prevalence where possible, there is substantial within-country variation in some settings, which our analysis does not address. Other sources of uncertainty include lack of data for some countries (requiring extrapolation between countries or regions) and failure to account for differences in sex ratio between countries (we assumed that it was 1:1 in all cases). However, our goal was not to produce country-specific estimates for medication requirements, but rather to assess the plausible range of medication requirements across all possible deployments. We believe that between-country variation across the countries we included is greater than within-country variation. Therefore, lack of regional data within countries should not affect our conclusions about medication requirements. Second, even with accurate epidemiological data about burden of disease, patterns of health system usage during emergencies are difficult to predict. For instance, patients with NCD might delay or forgo visits to health facilities during emergencies because of problems with transport, concern about leaving family members, lack of knowledge about where to seek care or fear of another disaster such as an aftershock. Patients who may be in need of medical support may prioritise the needs of other family members with needs that are considered to be more urgent. Alternatively, patients might migrate to another area, thus leading to higher than expected usage in one region and lower usage in another. Third, given the wide range of agencies that use the IEHK, there is no agreed set of practice guidelines for management of the target conditions, which complicates estimates of medicine requirements. However, the estimates here are based on evidence-based guidelines and rely on the essential medicines for the WHO PEN package. Fourth, space for medication that requires refrigeration is extremely limited in the IEHK. Therefore, we decided to estimate insulin requirements only for those in whom lack of insulin would clearly be fatal (those with type 1 disease), recognising that this may disadvantage some patients with type 2 diabetes who may have previously received insulin. Fortunately this point may become moot, as interesting work suggests that modern insulins may remain potent despite lack of a functional cold chain—potentially allowing larger quantities of insulin to be included in the IEHK.35 Finally, we based our estimates of the quantity of medications required to treat chronic NCDs on the proportion of people in each country who have each condition. One could argue that these estimates should be based on the proportion who are currently receiving treatment for each condition and if so our estimates are higher than truly required. For example, the proportion of people with hypertension who report receiving treatment is 31%, 68% and 80% in Bangladesh, Iraq and Lebanon, respectively.36–38
Despite our best efforts, the estimates will undoubtedly be too low in some emergency settings and too high in others. However, our goal has been to produce estimates that are the best available, accepting that there will be some inaccuracies pending further research. Better information on the incidence and prevalence of NCDs in settings where the IEHK has been deployed should be a very high priority for future studies, recognising that data collection under such circumstances is challenging.
Conclusions
Expansion of the IEHK to include NCD-relevant medicines is a promising potential mechanism for improving the health of people with NCDs. We conclude that the resources required to treat acute NCD presentations are modest, and propose a detailed list of medicines that could be included in the 2016 IEHK revision. Given the growing prevalence of NCDs worldwide and the increasing burden of these conditions in emergency settings, this proposal appears to warrant serious consideration.
Acknowledgments
The authors thank the members of the Interagency Emergency Health Kit committee for their constructive comments on the assumptions underlying the scenarios presented in the article proposal. Portions of this manuscript were submitted by MT as an MSc thesis at Imperial College London. The authors of this report are grateful to Sophanny Tiv and Ghenette Houston for research and administrative assistance.
Footnotes
Handling editor: Seye Abimbola
Contributors: MT conceived and designed the study. MT drafted the manuscript. NW performed the statistical analyses. All authors have made substantial contributions to the development of the manuscript, all have been involved in revising it for important intellectual content and all approved the final version. MT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Extracted data together with calculations are available on request.
References
- 1.World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Secondary Global action plan for the prevention and control of noncommunicable diseases 2013–2020 2013. http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf
- 2.World Health Organization. The Brazzaville declaration on noncommunicable diseases prevention and control in the WHO African region Secondary The Brazzaville declaration on noncommunicable diseases prevention and control in the WHO African region 2011. http://www.who.int/nmh/events/2011/ncds_brazzaville_declaration.pdf
- 3.The NCD Alliance. The NCD Alliance. Secondary The NCD Alliance 2015. http://www.ncdalliance.org
- 4.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013;369:448–57. 10.1056/NEJMra1201534 [DOI] [PubMed] [Google Scholar]
- 5.Beaglehole R, Bonita R, Horton R. Independent global accountability for NCDs. Lancet 2013;381:602–5. 10.1016/S0140-6736(13)60101-4 [DOI] [PubMed] [Google Scholar]
- 6.Nugent R, Feigl AB.2010. Where have all the donors gone? Scarce donor funding for non-communicable diseases working paper 228. Secondary Where have all the donors gone? Scarce donor funding for non-communicable diseases working paper 228. http://www.cgdev.org/publication/where-have-all-donors-gone-scarce-donor-funding-non-communicable-diseases-working-paper.
- 7.Bloom DE, Cafiero ET, Jané-Llopis E, et al. 2012. The global economic burden of noncommunicable diseases. Secondary: the global economic burden of noncommunicable diseases. http://www.hsph.harvard.edu/program-on-the-global-demography-of-aging/WorkingPapers/2012/PGDA_WP_87.pdf.
- 8.Guha-Sapir D, Hoyois P, Below R. Annual Disaster Statistical Review 2013: the numbers and trends. Brussels, Belgium: Centre for Research on the Epidemiology of Disasters, Institute of Health and Society, Universite cathloique de Louvain, 2014. [Google Scholar]
- 9.Peters MN, Moscona JC, Katz MJ et al. Natural disasters and myocardial infarction: the six years after Hurricane Katrina. Mayo Clin Proc 2014;89:472–7. 10.1016/j.mayocp.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 10.Trichopoulos D, Katsouyanni K, Zavitsanos X et al. Psychological stress and fatal heart attack: the Athens (1981) earthquake natural experiment. Lancet 1983;1:441–4. [DOI] [PubMed] [Google Scholar]
- 11.Niiyama M, Tanaka F, Nakajima S et al. Population-based incidence of sudden cardiac and unexpected death before and after the 2011 earthquake and tsunami in Iwate, Northeast Japan. J Am Heart Assoc 2014;3:e000798 10.1161/JAHA.114.000798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen K, Al-Faisal W. Reforms and emerging noncommunicable disease: some challenges facing a conflict-ridden country—the case of the Syrian Arab Republic. Int J Health Plann Manage 2013;28:290–302. 10.1002/hpm.2193 [DOI] [PubMed] [Google Scholar]
- 13.Kenerson JG. Hypertension in Haiti: the challenge of best possible practice. J Clin Hypertens (Greenwich) 2014;16:107–14. 10.1111/jch.12242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S, Hanagama M, Yamanda S et al. Impact of a large-scale natural disaster on patients with chronic obstructive pulmonary disease: the aftermath of the 2011 Great East Japan Earthquake. Respir Investig 2013;51:17–23. 10.1016/j.resinv.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Meisel SR, Kutz I, Dayan KI et al. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet 1991;338:660–1. 10.1016/0140-6736(91)91234-L [DOI] [PubMed] [Google Scholar]
- 16.Itoh T, Nakajima S, Tanaka F et al. Impact of the Japan earthquake disaster with massive Tsunami on emergency coronary intervention and in-hospital mortality in patients with acute ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care 2014;3:195–203. 10.1177/2048872614538388 [DOI] [PubMed] [Google Scholar]
- 17.Engelgau MM, El-Saharty S, Kudesia P, et al. 2011. Capitalizing on the demographic transition: Tackling noncommunicable diseases in South Asia. Secondary Capitalizing on the demographic transition: Tackling noncommunicable diseases in South Asia. http://econpapers.repec.org/paper/esswpaper/id_3a3595.htm.
- 18.International Diabetes Federation. IDF Diabetes Atlas, 6th edition: Key findings 2014. Secondary IDF Diabetes Atlas, 6th edition: Key findings 2014 2014. http://www.idf.org/diabetesatlas/update-2014
- 19.World Health Organization. The Interagency Emergency Health Kit 2011. Secondary The Interagency Emergency Health Kit 2011 2011. http://www.who.int/medicines/publications/emergencyhealthkit2011/en/
- 20.Laroche SA. Quick overview of the WHO kits. World Health Organization, 2015. [Google Scholar]
- 21.World Health Organization. Global status report on noncommunicable diseases 2014. Secondary Global status report on noncommunicable diseases 2014 2014. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1
- 22.van Ommeren M, Barbui C, de Jong K et al. If you could only choose five psychotropic medicines: updating the interagency emergency health kit. PLoS Med 2011;8:e1001030 10.1371/journal.pmed.1001030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Package of essential noncommunicable (PEN) disease interventions for primary health care in low-resource settings. Secondary Package of essential noncommunicable (PEN) disease interventions for primary health care in low-resource settings 2010. http://apps.who.int/iris/bitstream/10665/44260/1/9789241598996_eng.pdf
- 24.Institute for Health Metrics and Evaluation. Global burden of disease (GBD). Secondary Global burden of disease (GBD) 2015. http://www.healthdata.org/gbd
- 25.Nakamura M, Tanaka F, Nakajima S et al. Comparison of the Incidence of acute decompensated heart failure before and after the major tsunami in Northeast Japan. Am J Cardiol 2012;110:1856–60. 10.1016/j.amjcard.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Takahashi J, Fukumoto Y et al. Effect of the Great East Japan Earthquake on cardiovascular diseases—report from the 10 hospitals in the disaster area. Circ J 2013;77:490–3. 10.1253/circj.CJ-12-1594 [DOI] [PubMed] [Google Scholar]
- 27.United Nations Department of Economic and Social Affairs Population Division. World Population Prospects, the 2015 revision. Secondary World Population Prospects, the 2015 revision 2015. http://esa.un.org/unpd/wpp/Download/Standard/Population/
- 28.Moran AE, Forouzanfar MH, Roth GA et al. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation 2014;129:1493–501. 10.1161/CIRCULATIONAHA.113.004046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ray KK, Cannon CP, McCabe CH et al. Early and late benefits of high-dose atorvastatin in patients with acute coronary syndromes: results from the PROVE IT-TIMI 22 trial. J Am Coll Cardiol 2005;46:1405–10. 10.1016/j.jacc.2005.03.077 [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee S, Chaudhuri D, Vedanthan R et al. Early intravenous beta-blockers in patients with acute coronary syndrome—a meta-analysis of randomized trials. Int J Cardiol 2013;168:915–21. 10.1016/j.ijcard.2012.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari E, Menabue L, Saladini M. Characterization and metal affinity of Tirofiban, a pharmaceutical compound used in acute coronary syndromes. Biometals 2004;17:145–55. 10.1023/B:BIOM.0000018376.52169.40 [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Morimoto T, Natsuaki M et al. Antiplatelet therapy discontinuation and the risk of serious cardiovascular events after coronary stenting: observations from the CREDO-Kyoto Registry Cohort-2. PLoS ONE 2015;10:e0124314 10.1371/journal.pone.0124314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegel PB, Checchi F, Colombo S et al. Health-care needs of people affected by conflict: future trends and changing frameworks. Lancet 2010;375:341–5. 10.1016/S0140-6736(09)61873-0 [DOI] [PubMed] [Google Scholar]
- 34.Sphere Project. Humanitarian Charter and Minimum Standards in Disaster Response. Secondary Humanitarian Charter and Minimum Standards in Disaster Response 2011. http://www.ifrc.org/PageFiles/95530/The-Sphere-Project-Handbook-20111.pdf
- 35.Kaufmann B.2015. A potential revolution in diabetes type 1 care: Heat stability of insulin in tropical conditions. Secondary A potential revolution in diabetes type 1 care: Heat stability of insulin in tropical conditions 7 May 2015. http://f1000research.com/slides/1000061.
- 36.Bangladesh Society of Medicine, World Health Organization, NCD Directorate General of Health Services, et al. Non-Communicable Disease Risk Factor Survey, Bangladesh 2010. Secondary Non-Communicable Disease Risk Factor Survey, Bangladesh 2010. http://www.who.int/chp/steps/2010_STEPS_Report_Bangladesh.pdf?ua=1
- 37.. Ministry of Health Directorate of Public Health and Primary Health Care, Ministry of Planning and Development Cooperation Central Organization for Statistics and Information, World Health Organization . Chronic Non-Communicable Diseases Risk Factors Survey in Iraq 2006. Secondary Chronic Non-Communicable Diseases Risk Factors Survey in Iraq 2006. http://www.who.int/chp/steps/IraqSTEPSReport2006.pdf?ua=1.
- 38.American University of Beirut, World Health Organization. Chronic Disease Risk Factor Surveillance Data Book for Lebanon. Secondary Chronic Disease Risk Factor Surveillance Data Book for Lebanon 2010. http://www.who.int/chp/steps/2008_STEPS_Lebanon.pdf?ua=1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2016-000128supp_appendix.pdf (372.5KB, pdf)